Abstract
Although there are many applications of silver nanoparticles (Ag-NPs) in human activities, there is still little known about their potential environmental toxicity, particularly to fish. In the present study, the effects of Ag-NPs on African catfish (Clarias gariepinus) were studied using melanomacrophage centers as immunohistological biomarkers. Fish were exposed to 25 mg/L, 50 mg/L and 75 mg/L 100-nm Ag-NPs. We studied the effects on the size and number of melanomacrophage centers in all target tissues. Many histopathological alterations in those tissues were observed. The histological changes were represented as dislocation of the epithelium, dilatation of central veins associated with inflammatory leukocytic infiltration, necrosis, and pyknotic nuclei of hepatocytes. There was shrinkage of Malpighian corpuscles, dislocation of nuclei of convoluted tubules, cellular degeneration, and dispersed infiltration of leukocytes in kidney tissue. Examination of spleen sections after exposure to Ag-NPs showed rupture within the red pulp and hemorrhage, dislocation of nuclei, accumulation of inflammatory leukocytes, and congestion in blood vessels. In conclusion, exposure to Ag-NPs induced alterations in tissues, suggesting a possible increase in oxidative stress in those tissues.
Keywords: Clarias gariepinus, DNA, liver, melanomacrophage, silver nanoparticles
1. Introduction
As a result of the rapid development of the nanotechnology industry, there has been concern regarding its potential toxicity and ecotoxicology. Although there are many applications of nanoparticles (NPs), their effects within the environment and their interactions with living organisms are not fully known.
Toxicity of silver NPs (Ag-NPs) on rat, mouse, and fish models has been investigated [1,2,3]. Histological biomarkers were used for the detection of Ag-NPs in some fish [1,2], indicating the severe damage of those NPs. Neurotoxicity and cytotoxicity of Ag-NPs were observed in several studies [3,4]. It has been reported that the effects of Ag-NPs may be dependent on the cell types and species used [5]. Many studies have indicated oxidative stress as a response to exposure to Ag-NPs [3,5].
Melanomacrophages of fish were identified as pigment-containing cells and a prominent feature of hematopoietic tissue [6]. As a relationship between the breakdown product of hemoglobin in the red blood cells of fishes and the pigment inside them, the study of the changes of melano-macrophages (size and distribution) in target organs after exposure to Ag-NPs. Also, this study will confirm our previous publications about the genotoxicity in RBCs after Ag-NPs exposure [3]. Melanomacrophages in fish play a role in immunological protection as phagocytosis and they have been used as biomarkers for environmental pollution [7,8].
Several studies have investigated the melanomacrophage centers (MMCs) in a wide range of fish [7,9,10,11,12,13,14,15]. There are several reports on the immunotoxic effects of starvation, potassium dichromate, ulcer disease, cadmium chloride, foreign bodies, toxic plants, and heavy metals on MMCs in several fish species [6,9,13,14,16,17,18].
The purposes of our present study were twofold: (1) to focus on the effect of Ag-NPs on the occurrence of MMCs in liver, spleen, and kidney as target organs of one of the most economically important fish in Egypt, African catfish (Clarias gariepinus); and (2) to investigate the histopathological alterations in target organs after Ag-NP exposure.
2. Materials and Methods
2.1. C. gariepinus
Adult male C. gariepinus (Burchell, 1822) were collected from the River Nile in July 2012. Fish were kept at ~28°C with a 12 h/12 h light/dark cycle and held in a closed recirculating system (300 L) for 6 months to acclimatize to laboratory conditions. The adaption conditions, feeding, and fish body size have been described previously [3].
2.2. Experimental design
The experimental design has been described previously [3]. All information about the preparation, characterization of Ag-NPs, and determination of Ag bioaccumulation have also been reported previously.
2.3. Histopathological alterations
After 14 days Ag-NP exposure, the specimens from the selected organs (spleen, kidney, and liver) were rapidly excised, and cut into small pieces, which were used for histopathological examination. Tissue pieces were fixed in 10% of neutral-buffered formalin (pH 7.2), dehydrated in an ascending series of ethanol, cleared in methyl benzoate, and embedded in paraffin wax. Paraffin sections of 7 μm thickness were prepared and stained with Harris's hematoxylin and eosin. Twenty randomly selected sections of six fish from each experimental group were chosen to indicate each histopathological parameter as (mild, + < 25% area of section; moderate, ++ 25–50% area of section; and severe, +++ > 50% area of section). Finally, tissues were examined and imaged using an Omax advanced trinocular biological microscope with a 14MP USB Digital Camera (CS-M837ZFLR-C140U, U.S.).
2.4. MMC frequency
According to the method of Kranz and Gercken [18], histological evaluation of spleen, kidney, and liver cross-sections included counting of MMCs/1 cm organ and measurement of MMCs in defined organ areas were done. For comparison of the results, the frequency of MMCs per defined organ area, the average size of MMCs per 1 cm organ, and the percentage of MMCs area per 1 cm organ area were calculated using ImageJ software (ImageJ/Fiji 1.46).
2.5. Statistical analysis
The basic statistics (means, standard divisions, and ranges) were calculated. One-way analysis of variance using the SPSS package at the 0.00001 was used.
2.6. Ethical statement
All experiments were carried out in accordance with the Egyptian laws and University guidelines for the care of experimental animals. All procedures were approved by the Committee of the Faculty of Science of Assiut University, Egypt.
3. Results
3.1. Frequency, size, and proportion of MMCs
In the spleen, size and average number of MMCs per 1 cm/organ was the highest value compared with other organs. In kidney and liver, MMCs were noted in all groups. The average number of MMCs per organ size in all tissues of exposed fish increased when compared with the controls. There was a significant (p < 0.00001) difference in the size and the frequency of MMCs between the control and treated groups (Table 1, Figure 1).
Table 1.
Changes in number and size of MMCs/cm organ after exposure to Ag-NPs in adult Clarias gariepinus (n = 6).
| Item | No. of MMCs/cm organ | Size of MMC/cm organ (μm) | ||||
|---|---|---|---|---|---|---|
| Group/Organ | Spleen | Kidney | Liver | Spleen | Kidney | Liver |
| Control | 6 ± 1.06 | 4 ± 0.37 | 2 ± 0.26 | 1.13 ± 0.21 | 0.7 ± 0.09 | 0.72 ± 0.07 |
| (2–9)c | (3–5)c | (1–3)c | (0.7–2.1)b | (0.5–1.1)c | (0.5–0.9)c | |
| 25 mg/L Ag-NPs | 8.33 ±0.71 | 4.67 ± 0.5 | 3.83 ±1.08 | 2.65 ± 0.22 | 2.65 ± 0.4 | 1.05 ±0.08 |
| (5–10)c | (3–6)c | (1–8)bc | (2.1–3.4)b | (1.6–3.7)c | (0.8–1.3)c | |
| 50 mg/L Ag-NPs | 18.67 ± 1.52 | 7.5 ± 0.85 | 7.67 ± 1.17 | 11.95 ± 2.4 | 5.48 ± 0.65 | 2.12 ± 0.14 |
| (14–24)b | (5–10)b | (3–11)ab | (6.5–22.5)a | (3.6–7.8)b | (1.7–2.6)b | |
| 75 mg/L Ag-NPs | 28.17 ± 1.01 | 15 ± 0.89 | 11.17 ± 1.35 | 10.22 ± 0.72 | 8.35 ± 0.63 | 2.97 ± 0.14 |
| (24–31)a | (12–18)a | (7–16)a | (7.8–12.1)a | (6.7–10.1)a | (2.4–3.4)a | |
sig.=0.00001 between groups of all organs (analysis of variance)
Fig. 1.
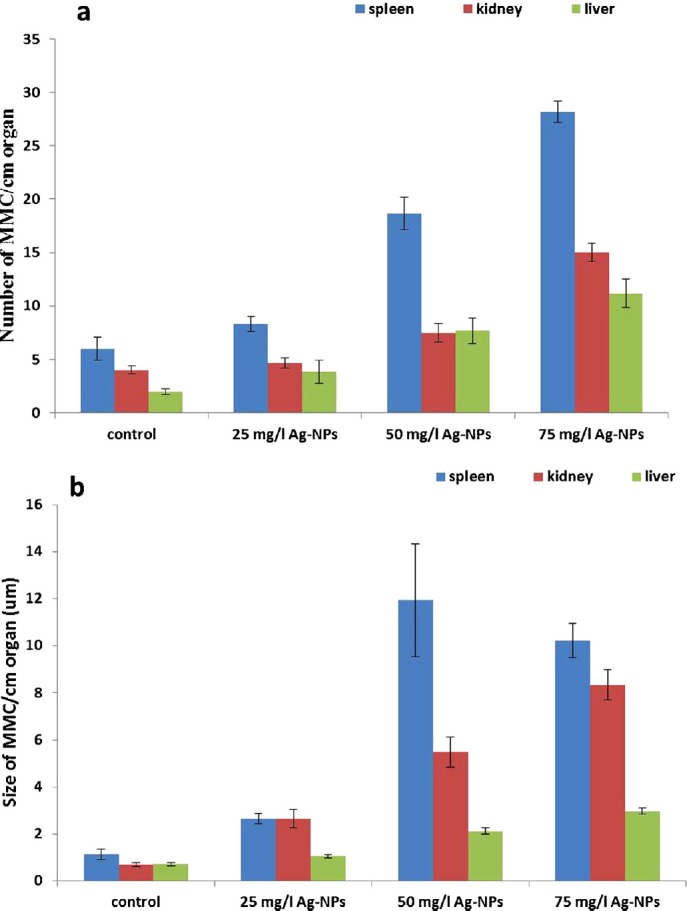
Changes in the number and size of melanomacrophages centers (MMCs)/cm in each organ after exposure to silver nanoparticles in adult African catfish (Clarias gariepinus) (n = 6).
3.2. Histopathological alterations
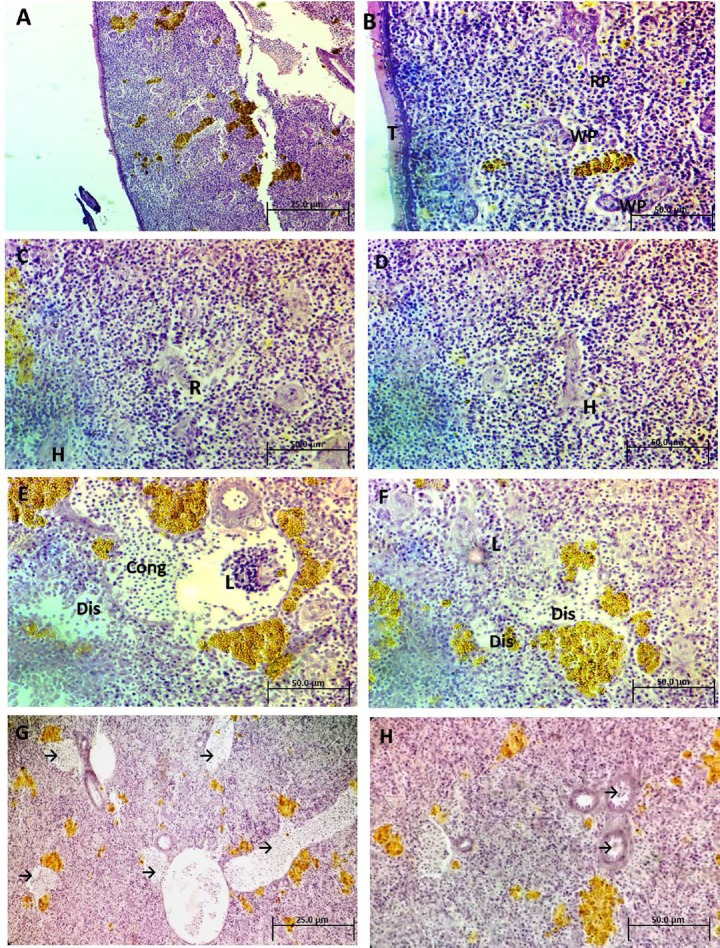
Table 2 summarizes the tissue lesion score according to severity. Figures 2A and 2B illustrate the normal architecture of the spleen as red and white pulp. White pulp typically surrounds the arterioles. The thin pale bands are trabeculae (supporting collagen bundles). Spleen sections after exposure to 25 mg/L Ag-NPs for 2 weeks (Figures 2C and 2D) showed rupture within the white pulp and hemorrhage. Spleen sections after exposure to 50 mg/L Ag-NPs for 2 weeks showed different morphological changes, such as a dislocation of nuclei and accumulation of inflammatory leukocytes, and congestion in blood vessels (Figures 2E and 2F). Spleen sections after exposure to 75 mg/L Ag-NPs for 2 weeks showed severe congestion in the blood vessels (arrows), and different types of degeneration throughout the tissue (Figures 2G and 2H).
Table 2.
Histopathological changes in adult Clarias gariepinus exposed to Ag-NPs for 14 days.
| Organ | Parameters | Control | 25 mg/L Ag-NPs | 50 mg/L Ag-NPs | 75 mg/LAg-NPs |
|---|---|---|---|---|---|
| Spleen | Irregular architecture | – | + | + | + |
| Red pulp rupture | – | + | + | ++ | |
| Hemorrhage | – | + | – | – | |
| Nuclei dislocation | – | – | ++ | ++ | |
| Leukocytic infiltration | – | – | + | + | |
| Blood vessel congestion | – | – | + | ++ | |
| Kidney | Irregular architecture | – | + | + | + |
| Malpighian corpuscle shrinkage | – | + | + | + | |
| Nuclei dislocation | – | + | + | + | |
| Cellular degeneration | – | + | ++ | +++ | |
| Leukocytic infiltration | – | + | ++ | ||
| Liver | Irregular architecture | – | + | + | + |
| Epithelium dislocation | – | + | + | ++ | |
| Blood vessel dilation | – | + | + | + | |
| Leukocytic infiltration | – | + | + | ++ | |
| Pyknotic nuclei | – | – | + | + | |
| Necrotic areas | – | – | – | + | |
| Connective tissue fibers | – | – | – | + | |
| Congestion | – | – | ++ | – |
Lesions were scored based on their severity as (– none, + mild, ++ moderate, +++ severe). Ag-NP = silver nanoparticle.
Fig. 2.
(A and B) Photomicrograph of spleen sections of control Clarias gariepinus illustrating the normal architecture of the spleen as red pulp (RP) and white pulp (WP). The thin pale bands are trabeculae (T), (H&E, 200× and 400×). (C and D) Photomicrograph of spleen sections of treated C. gariepinus after exposure to 25 mg/L Ag-NPs showing rupture (R) within the WP and little hemorrhages (H) (H&E, 400×). (E and F) Photomicrograph of spleen sections of treated C. gariepinus after exposure to 50 mg/L Ag-NPs showing different features of morphological changes such as dislocation of nuclei (Dis), accumulated inflammatory leukocytes (L), and congestion in blood vessels (cong) (H&E, 400×). (G and H) Photomicrograph of spleen sections of treated C. gariepinus after exposure to 75 mg/L Ag-NPs showing severe congestion in blood vessels (arrows) and different types of degeneration throughout the tissue (H&E, 200× and 400×). Ag-NP = silver nanoparticle; H&E = hematoxylin and eosin.
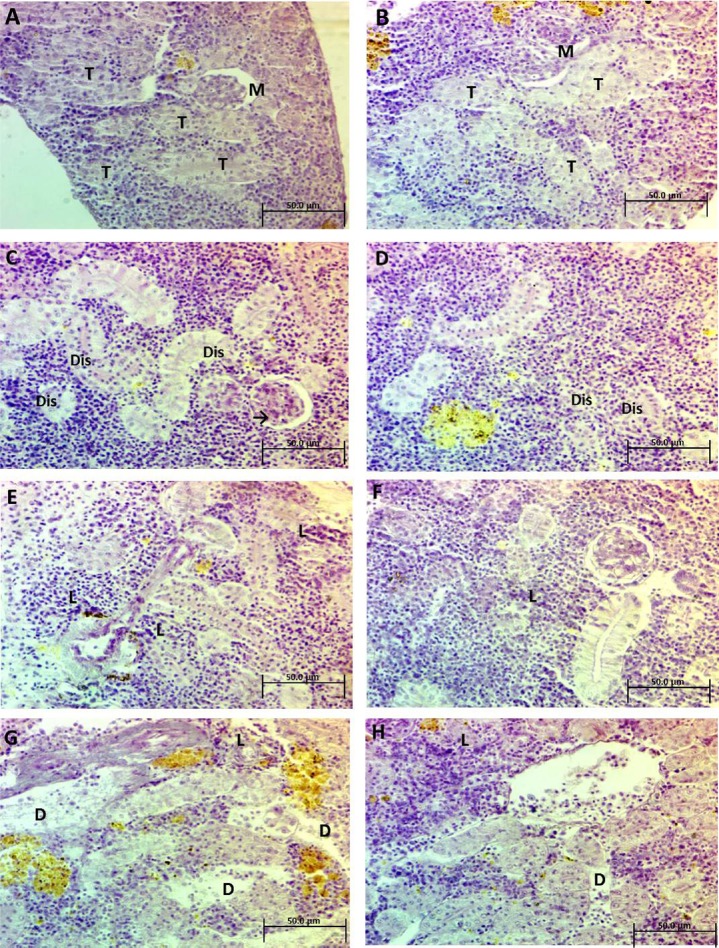
Figures 3A and 3B illustrate the normal architecture of the kidney with Malpighian corpuscles and renal convoluted tubules. Kidney sections after exposure to 25 mg/L Ag-NPs for 2 weeks (Figures 3C and 3D) showed shrinkage of Malpighian corpuscles and dislocation of nuclei in the convoluted tubules. Kidney sections after exposure to 50 mg/L Ag-NPs for 2 weeks showed cellular degeneration that led to disorganization of the convoluted tubules and dispersed infiltration of leukocytes (Figures 3E and 3F). Kidney sections after exposure to 75 mg/L Ag-NPs for 2 weeks showed degeneration that caused many spaces and proliferation of inflammatory leukocytes (Figures 3G and 3H).
Fig. 3.
(A and B) Photomicrograph of kidney sections of control Clarias gariepinus illustrates the normal architecture of the kidney as Malpighian corpuscles (M) and renal convoluted tubules (T), (H&E, 400×). (C and D) Photomicrograph of kidney sections of treated C. gariepinus after exposure to 25 mg/L Ag-NPs showing shrinkage of Malpighian corpuscle (arrows) and dislocation of nuclei in convoluted tubules (Dis) (H&E, 400×). (E and F) Photomicrograph of kidney sections of treated C. gariepinus, after exposure to 50 mg/L Ag-NPs showing cellular degeneration and dispersed infiltration of leukocytes (L). (H&E, 400×). (G and H) Photomicrograph of kidney sections of treated C. gariepinus, after exposure to 75 mg/L Ag-NPs showing degeneration (D), causing many spaces and proliferation of inflammatory leukocytes (L). (H&E, 400×) Ag-NP = silver nanoparticle; H&E = hematoxylin and eosin.
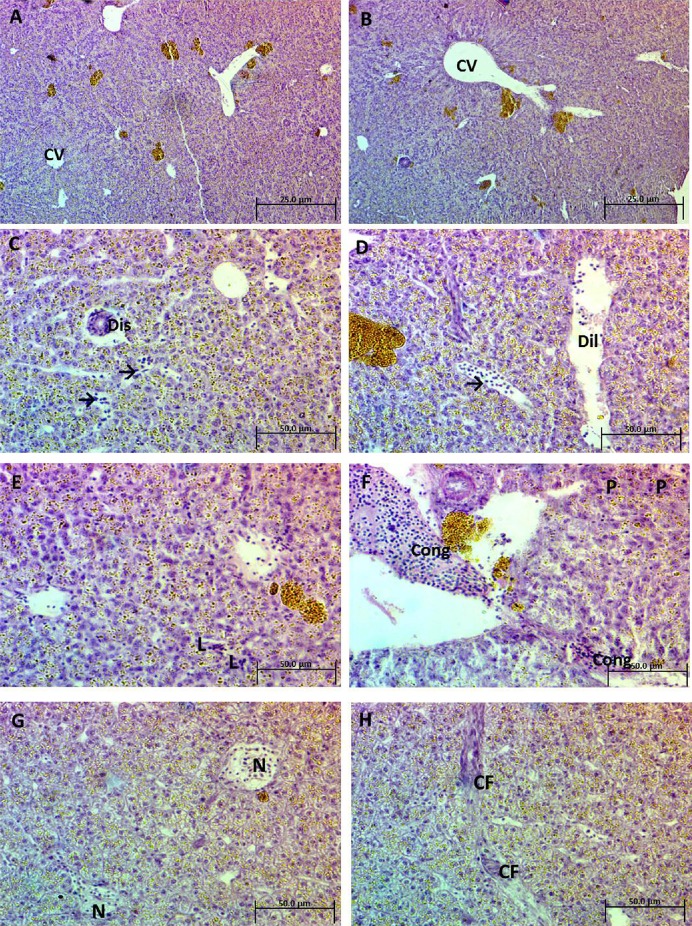
The normal architecture of the liver is shown in Figures 4A and 4B. No signs of histopathological changes were observed in the control group, where the hepatocytes were arranged more or less regularly in columns extending radially from the central veins to the periphery of the lobules. Liver sections after exposure to 25 mg/L Ag-NPs for 2 weeks showed different morphological changes including dislocation of epithelium and dilatation of blood vessels associated with inflammatory leukocytic infiltration (Figures 4C and 4D). Liver sections after exposure to 50 mg/L Ag-NPs for 2 weeks showed mild inflammatory leukocytic infiltration, pyknotic nuclei of hepatocytes, and congestion (Figures 4E and 4F). Liver sections after exposure to 75 mg/L Ag-NPs for 2 weeks in showed necrotic areas and noticeable connective tissue fibers (Figures 4G and 4H).
Fig. 4.
(A and B) Photomicrograph of a liver section of control Clarias gariepinus showing normal architecture of hepatic tissue and the central vein (CV) (H&E, 200×). (C and D) Photomicrograph of liver sections of treated C. gariepinus after exposure to 25 mg/L Ag-NPs showing different features of morphological changes such as dislocation of epithelium (Dis) and dilatation of blood vessels (Dil), associated with inflammatory leukocytic infiltration (arrows) (H&E, 400×). (E and F) Photomicrograph of liver sections of treated C. gariepinus after exposure to 50 mg/L Ag-NPs showing mild inflammatory leukocytic infiltration (L), pyknotic nuclei of hepatocytes (P), and congestion (Cong) (H&E, 400×). (G and H) Photomicrograph of liver sections of treated C. gariepinus after exposure to 75 mg/L Ag-NPs showing necrotic areas (N) and noticeable connective tissue fibers (CF). (H&E, 400×). Ag-NP = silver nanoparticle; H&E = hematoxylin and eosin.
4. Discussion
Although a lot of progress has been made in the science of nanotoxicology, the fate, behavior, and biological effects of NPs in freshwater and marine environments have received little attention [19]. No study to date has investigated the effects of exposure to Ag-NPs in African catfish, especially using MMCs as biomarkers for immunotoxicology [3].
As a result of their small size, the NPs are able to bypass barriers and enter the cells of living organisms, passing through cell membranes and junctions and causing adverse effects such as apoptosis and generation of reactive oxygen species [5]. Liver histopathological lesions have been used as markers of environmental pollution [20,21]. Hepatic histopathological alterations in fish exposed to NPs have been reported [4,22]. Ag-NPs were one of the two most toxic of 31 types of NPs to zebrafish [23]. The concentrations of Ag-NPs used in the present study were higher than those used by Ostaszewska et al [1], and this was because of the annual increase in Ag-NPs needed to reach the lethal levels for aquatic organisms [2,24].
The present study is believed to be the first to report the effects of Ag-NPs on African catfish using MMCs as a biomarker for immunotoxicity. Histopathological analysis revealed that lesions caused by Ag-NPs in the spleen, kidney, and liver, were the most severe alterations recorded in fish exposed to 25 mg/L and 75 mg/L of Ag-NPs, and the frequency and severity of these alterations increased with the concentration of Ag-NPs.
The alterations in RBCs recorded in our previous study [3] and the current alterations in the tissues may be a result of the stressful conditions that affected the fish physiology [2]. Also, tissue-damaging enzymes, including acid phosphatase (ACP), alkaline phosphatase, glutathione S-transferase, superoxide dismutase, and catalase have been found after oral administration of Ag-NPs. The mechanism of this damage is illustrated by the ability of NPs to produce reactive oxygen species, leading to oxidative stress [2,25].
Exposure of Siberian sturgeon to Ag-NPs (0.1 mg/L, 0.5 mg/L, and 1.5 mg/L) caused karyopyknosis, eosinophilic bodies, sinusoidal space dilation, blood cell aggregation in blood vessels, hepatocyte vacuolization, and hepatocyte shrinkage [1]. Rajkumar et al [2] reported vacuolar degeneration in the liver, and necrosis after Ag-NP exposure, and our current results were in agreement with those results. Govindasamy and Rahuman [22] found dilation of sinusoid space in the liver of Mozambique tilapia (Oreochromis mossambicus) treated with Ag-NPs. Those alterations indicated apoptosis and degeneration of hepatocytes caused by the toxic action of NPs [26]. Alterations in spleen, kidney, and liver of Nile tilapia Oreochromis niloticus after exposure to mercury have been reported [27]. Kaewamatawong et al. [27] reported similar alterations in kidney, spleen, and liver of O. niloticus after exposure to mercury, where they observed degeneration and massive necrosis in the kidney, degeneration of hepatocytes with vacuolization, and increased number of MMCs in the spleen in experimental fish compared to the controls.
The role of MMCs is to destroy and detoxify endogenous and exogenous substances, and response to foreign bodies has been reported [7,28], Although MMCs are used as bioindicators of aquatic pollution, stress, environmental impact, and chronic inflammation [15,29], the changes in MMCs are related to age, gender, species, hormonal condition, and other factors [20,30].
In the present study, there was a significant frequency and size of MMCs recorded in the spleen, kidney, and liver of catfish exposed to Ag-NPs. These results were consistent with some previous studies [6,31,32] but contrasted with Jovanović et al [33], who recorded a reduction of MMCs in the kidney after exposure to TiO2 NPs. The increase in MMCs may have resulted from their role in detoxification processes [7], and involvement in innate and adaptive immunity [8].
Increases in the numbers and size of MMCs have been observed after environmental pollution [7,34], heavy metal contamination in fish [27,31], water quality [13], bacterial infection of fish [35], fish diseases [36,37], exposure to toxic plant extracts [16], after starvation [9], after exposure to potassium dichromate [18], fish ulcers [14], and fish parasites [38]. Usually, the increase in MMCs associated with histopathological alterations, indicating the oxidative stress that leads to aggregation of lymphocytes suggesting the immune response of MMCs [9,18].
In conclusion, this study demonstrated that Ag-NPs are toxic to African catfish. Fish exposed to Ag-NPs showed histopathological alterations in the spleen, kidney, and liver, and the severity of this damage depended on the NP concentration. Also, the number and size of MMCs were increased in all target organs, indicating the immune role of the MMCs.
References
- [1].Ostaszewska T, Chojnacki M, Kamaszewski M, Sawosz-Chwalibóg E. Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environ Sci Pollut Res Int. 2016;23:1621–33. doi: 10.1007/s11356-015-5391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rajkumar SK, Kanipandian N, Thirumurugan R. Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci. 2016;6:19–29. [Google Scholar]
- [3].Sayed AH. Genotoxicity detection following exposure to silver nanoparticles in African catfish (Clarias gariepinus) Int J Nanopart. 2016. http://www.inderscience.com/info/ingeneral/forthcoming.php?jcode=ijnp .
- [4].Al-Bairuty GA, Shaw BJ, Handy RD, Henry TB. Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2013;126:104–15. doi: 10.1016/j.aquatox.2012.10.005. [DOI] [PubMed] [Google Scholar]
- [5].Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett. 2008;179:93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- [6].Suresh N. Effect of cadmium chloride on liver, spleen and kidney melano macrophage centres in Tilapia mossambica. J Environ Biol. 2009;30:505–8. [PubMed] [Google Scholar]
- [7].Herraez MP, Zapata AG. Structure and function of the melanomacrophage centres of the goldfish Carassius auratus. Vet Immunol Immunopathol. 1986;12:117–26. doi: 10.1016/0165-2427(86)90116-9. [DOI] [PubMed] [Google Scholar]
- [8].Wolke RE. Piscine macrophage aggregates: a review. Ann Rev Fish Dis. 1992;2:91–108. [Google Scholar]
- [9].Agius C. Preliminary studies on the ontogeny of the melanomacrophages of teleost haemopoietic tissues and age-related changes. Dev Comp Immunol. 1981;5:597–606. doi: 10.1016/s0145-305x(81)80034-1. [DOI] [PubMed] [Google Scholar]
- [10].Agius C. The melano-macrophage centres in fish: a review. In: Manning MJ, Tatner MF, editors. Fish immunology. London: Academic Press; 1985. pp. 85–105. [Google Scholar]
- [11].Agius C, Agbede SA. An electron microscopical study on the genesis of lipofuscin, melanin and haemosiderin in the haemopoietic tissues of fish. J Fish Biol. 1984;24:471–88. [Google Scholar]
- [12].Agius C, Roberts RJ. Effects of starvation on the melano-macrophage centres of fish. J Fish Biol. 1981;19:161–9. [Google Scholar]
- [13].Agius C, Roberts RJ. Melano-macrophage centres and their role in fish pathology. J Fish Dis. 2003;26:499–509. doi: 10.1046/j.1365-2761.2003.00485.x. [DOI] [PubMed] [Google Scholar]
- [14].Kranz H. Changes in splenic melano-macrophage centres of dab Limanda limanda during and after infection with ulcer disease. Dis Aquat Org. 1989;6:167–73. [Google Scholar]
- [15].Manrique WG, da Silva Claudiano G, Petrillo TR, Pardi de Castro M, Pereira Figueiredo MA, de Andrade Belo MA, et al. Response of splenic melanomacrophage centers of Oreochromis niloticus (Linnaeus, 1758) to inflammatory stimuli by BCG and foreign bodies. J Appl Ichthyol. 2014;30:1001–6. [Google Scholar]
- [16].Fafioye OO, Adebisi AA, Fagade SO. Toxicity of Parkia biglobosa and Raphia vinifera extracts on Clarias gariepinus juveniles. Afr J Biotechnol. 2004;3:627–30. [Google Scholar]
- [17].Kaewamatawong T, Shimada A, Okajima M, Inoue H, Morita T, Inoue K, et al. Acute and subacute pulmonary toxicity of low dose of ultra-fine colloidal silica particles in mice after intratracheal instillation. Toxicol Pathol. 2006;34:958–65. doi: 10.1080/01926230601094552. [DOI] [PubMed] [Google Scholar]
- [18].Kranz H, Gercken J. Effects of sublethal concentrations of potassium dichromate on the occurrence of splenic melano-macrophage centres in juvenile plaice, Pleuronectes platessa, L. J Fish Biol. 1987;31:75–80. [Google Scholar]
- [19].Scown TM, Santos EM, Johnston BD, Gaiser B, Baalousha M, Mitov S, et al. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci. 2010;115:521–34. doi: 10.1093/toxsci/kfq076. [DOI] [PubMed] [Google Scholar]
- [20].Dabrowska H, Ostaszewska T, Kamaszewski M, Antoniak A, Napora-Rutkowski L, Kopko O, et al. Histopathological, histomorphometrical, and immunohistochemical biomarkers in flounder (Platichthys flesus) from the southern Baltic Sea. Ecotoxicol Environ Saf. 2012;78:14–21. doi: 10.1016/j.ecoenv.2011.10.025. [DOI] [PubMed] [Google Scholar]
- [21].Sayed AH, Abdel-Tawab HS, Abdel Hakeem SS, Mekkawy IA. The protective role of quince leaf extract against the adverse impacts of ultraviolet-A radiation on some tissues of Clarias gariepinus (Burchell, 1822) J Photochem Photobiol B. 2013;119:9–14. doi: 10.1016/j.jphotobiol.2012.11.006. [DOI] [PubMed] [Google Scholar]
- [22].Govindasamy R, Abdul Rahuman A. Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus) J Environ Sci. 2012;24:1091–8. doi: 10.1016/s1001-0742(11)60845-0. [DOI] [PubMed] [Google Scholar]
- [23].Kovrižnych JA, Sotníková R, Zeljenková D, Rollerová E, Szabová E, Wimmerová S. Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages-comparative study. Interdiscip Toxicol. 2013;6:67–73. doi: 10.2478/intox-2013-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shaw BJ, Handy RD. Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions. Environ Int. 2011;37:1083–97. doi: 10.1016/j.envint.2011.03.009. [DOI] [PubMed] [Google Scholar]
- [25].Jia HY, Liu Y, Zhang XJ, Han L, Du LB, Tian Q, et al. Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J Am Chem Soc. 2009;131:40–1. doi: 10.1021/ja808033w. [DOI] [PubMed] [Google Scholar]
- [26].Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, et al. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol. 2010;100:151–9. doi: 10.1016/j.aquatox.2009.12.012. [DOI] [PubMed] [Google Scholar]
- [27].Kaewamatawong T, Rattanapinyopituk K, Ponpornpisit A, Pirarat N, Ruangwises S, Rungsipipat A. Short-term exposure of Nile Tilapia (Oreochromis niloticus) to mercury: histopathological changes, mercury bioaccumulation, and protective role of metallothioneins in different exposure routes. Toxicol Pathol. 2013;41:470–9. doi: 10.1177/0192623312457269. [DOI] [PubMed] [Google Scholar]
- [28].Ellis AE, de Sousa M. Phylogeny of the lymphoid system. I. A study of the fate of circulating lymphocytes in plaice. Eur J Immunol. 1974;4:338–43. doi: 10.1002/eji.1830040505. [DOI] [PubMed] [Google Scholar]
- [29].Fournie JW, Summers JK, Courtney LA, Engle VD, Blazer VS. Utility of splenic macrophage aggregates as an indicator of fish exposure to degraded environments. J Aquat Anim Health. 2001;13:105–16. [Google Scholar]
- [30].Abdel-Aziz el-SH, Abdu SB, El-Sayed Ali T, Fouad HF. Haemopoiesis in the head kidney of tilapia, Oreochromis niloticus (Teleostei: Cichlidae): a morphological (optical and ultrastructural) study. Fish Physiol Biochem. 2009;36:323–36. doi: 10.1007/s10695-008-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pulsford AL, Ryan KP, Nott JA. Metals and melanomacrophages in flounder, Platichthys flesus, spleen and kidney. J Marine Biol Assoc UK. 1992;72:483–98. [Google Scholar]
- [32].Suresh N, Veeraraghavan K. Effects of sublethal concentration of distillery effluent on the occurrence of kidney melano macrophage centres in Mystus vittatus (Bloch) J Natcon. 1998;10:203–7. [Google Scholar]
- [33].Jovanović B, Whitley EM, Kimura K, Crumpton A, Palić D. Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens. Environ Pollut. 2015;203:153–64. doi: 10.1016/j.envpol.2015.04.003. [DOI] [PubMed] [Google Scholar]
- [34].Wolke RE, Murchelano RA, Dickstein CD, George CJ. Preliminary evaluation of the use of macrophage aggregates (MA) as fish health monitors. Bull Environ Contam Toxicol. 1985;35:222–7. doi: 10.1007/BF01636502. [DOI] [PubMed] [Google Scholar]
- [35].Press CM, Evensen O, Reitan LJ, Landsverk T. Retention of furunculosis vaccine components in Atlantic salmon, Salmo salar L., following different routes of vaccine administration. J Fish Dis. 1996;19:215–24. [Google Scholar]
- [36].Loumbourdis NS, Danscher G. Autometallographic tracing of mercury in frog liver. Environ Pollut. 2004;129:299–304. doi: 10.1016/j.envpol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- [37].Pronina SV, Batueva MD-D, Pronin NM. Characteristics of melanomacrophage centers in the liver and spleen of the roach Rutilus rutilus (Cypriniformes: Cyprinidae) in Lake Kotokel during the Haff disease outbreak. J Ichthyol. 2014;54:104–10. [Google Scholar]
- [38].De Vico G, Cataldi M, Carella F, Marino F, Passantino A. Histological, histochemical and morphometric changes of splenic melanomacrophage centers (SMMCs) in Sparicotyle-infected cultured sea breams (Sparus aurata) Immunopharmacol Immunotoxicol. 2008;30:27–35. doi: 10.1080/08923970701812290. [DOI] [PubMed] [Google Scholar]