Abstract
During the last few decades there has been an enormous increase in the usage of cell phones as these are one of the most convenient gadgets and provide excellent mode of communication without evoking any hindrance to movement. However, these are significantly adding to the electromagnetic field radiations (EMF-r) in the environment and thus, are required to be analysed for their impacts on living beings. The present study investigated the role of cell phone EMF-r in inciting oxidative damage in onion (Allium cepa) roots at a frequency of 2100 MHz. Onion roots were exposed to continuous wave homogenous EMF-r for 1, 2 and 4 h for single day and generation of reactive oxygen species (ROS) in terms of malondialdehyde (MDA), hydrogen peroxide (H2O2) and superoxide anion (O2•−) content and changes in the activities of antioxidant enzymes- superoxide dismutases (SOD) and catalases (CAT) were measured. The results showed that EMF-r exposure enhanced the content of MDA, H2O2 and O2•−. Also, there was an upregulation in the activity of antioxidant enzymes− SOD and CAT− in onion roots. The study concluded that 2100 MHz cell phone EMF-r incite oxidative damage in onion roots by altering the oxidative metabolism.
Keywords: Cellphone radiations, Oxidative damage, Lipid peroxidation, In situ detection, Antioxidant enzymes
1. Introduction
Last couple of decades have witnessed unprecedented growth in communication sector with an exponential increase in the number of wireless devices based on non-ionizing radiations [1]. Of the various wireless devices, mobile phones are the ones that are being most widely used and have become part and parcel of modern day life. According to International Telecommunication Union (ITU) report, there are about 7.377 billion mobile cellular telephone subscriptions globally, with an average of 99.7 subscriptions per 100 inhabitants [2]. With an upsurge in the usage of such devices there has been a tremendous increase in the non-ionizing radio frequency electromagnetic fields in the environment and hence, an enhanced level of exposure of electromagnetic field radiations (EMF-r) to the living beings [3]. Notwithstanding, the accelerated and widespread use of these gadgets, it has become a topic of utmost concern among the scientists owing to the health implications of EMF-r on living beings [3,4]. Studies have documented various biological effects like induction of lesions in vital organs [5], and alterations in gene expression [6], cell cycle [7], enzyme activity [8], protein expression [9,10], hormone levels [11] oxidative metabolism [12], cell membrane permeability [13], and genotoxicity [14] on exposure to EMF-r. However, most of these findings have been derived from studies on animal system and less attention has been paid to explore the effect on plants [4]. Moreover, amongst the scanty publications on plants, few have reported EMF-r to enhance plant growth [15,16,17], whereas, others have documented the inhibitory effects of EMF-r. For example, EMF-r cause reduction in seed germination [18,19], impair root growth, early seedling growth and rhizogenesis [19,20,21] and induce biochemical changes [20,21] in plants. EMF-r at 900 MHz affect transcription, translation, calcium transport and energy charge in tomato (Lycopersicon esculentum Mill.) [22]. Monselise et al. [23] reported production of alanine (as a metabolic response to stress) in duckweed plants (Spirodela oligorrhiza) exposed to low-frequency electromagnetic fields, whereas, no accumulation was reported in unexposed control. EMF-r alter mitosis, and increase mitotic index, frequency of mitotic and chromosomal abnormalities, and micronucleus frequency [19,24,25,26]. Of late, 900 EMF-r have been reported to induce ROS-mediated oxidative damage in plant roots [27,28].
With the ongoing increase of the electromagnetic field radiations in the environment and the advent of new technology like 3G and 4G, it is pertinent to evaluate the impact of high frequency (2100 MHz) EMF-r exposure. Till date, most of the studies have evaluated the impacts of 900–1800 MHz EMF-r on plants; however, not much is known about biological impacts of 2100 MHz EMF-r in plants. With this background, we hypothesized that 2100 MHz EMF-r may also inhibit plant growth and induce abiotic stress. We, therefore, investigated the role of 2100 MHz EMF-r in inciting oxidative damage in Allium cepa (onion) roots. For this, we evaluated reactive oxygen species (ROS) generation both, biochemically as well as histochemically, along with the alterations in the activities of antioxidant enzymes.
2. Material and methods
2.1. Materials
Onion bulbs of equal size were procured from local market and scrapped to remove old and dry scales and roots so that the apices of root primordia were exposed. The bulbs were placed in coplin jars with their basal ends dipped in distilled water, and were set for rooting at room temperature (25 ± 1°C). All the reagents and chemicals used in the study were of analytical grade purchased from Sisco Research Laboratory Pvt. Ltd., India; Sigma Co., St. Louis, USA; Merck Ltd., India; and Loba-Chemie Pvt., Ltd., India.
2.2. EMF-r treatment
The exposure system consisted of RF signal generator (Agilent N9310A; Keysight Technologies, USA) that generates homogenous EMF-r, similar to mobile phone frequency in a range of 9KHz − 3 GHz. It was further attached with an RF power amplifier (Model No. ZHL-5W-2GX+; Minicircuits, USA) and a DC regulated power supply. The exposure system was placed in an empty room coated with YSHIELD® (HSF54) radiofrequency shielding paint, free from any external source of EMF-r. Once the roots attained the length of 3–4 cm, they were subjected to exposure. No EMF-r treatment was given to group 1 and group 2, which served as control and Sham control, respectively. Group 1 was placed in an empty room similar to the one of exposure system without any EMF-r source. Group 2 was placed in the exposure room only but without any EMF-r exposure. Group 3,4 and 5 were exposed to EMF-r for 1, 2 and 4 h. The output power density was measured using ScanEM®-C Probe (Model No. CTK015; 3 M Technologies, USA) attached to RF power density meter (Spectran, HF-4060, range 100 MHz to 6 GHz; Aaronia AG, Germany). The average power density received at a distance of 5 cm from antenna was 489.7 ± 18.15 mW m−2 with a specific absorption rate (SAR) of 2.82 ± 0.12 × 10−1 W kg−1. SAR value was determined roughly as it is relatively difficult to measure SAR values on exposed tissues directly [29]. It was calculated by taking the values of electrical conductivity (σ) and tissue density (ρ) for the dielectric properties of tissue at 2100 MHz (σ = 1.57 S m−1 and ρ = 1030 kg cm−3) from IFAC (Institute of Applied Physics, Sesto Fiorentino, Italy) database [30].
2.3. Appraisal of the oxidative stress in terms of ROS generation
EMF-r induced oxidative stress was measured in terms of lipid peroxidation and ROS– hydrogen peroxide (H2O2) and superoxide anions (O2•−)– accumulation in the onion roots.
Lipid or membrane peroxidation was estimated in terms of malondialdehyde (MDA) content [31]. Briefly, 100 mg of roots were homogenized in 10 ml of 0.1% (w/v) trichloroacetic acid and centrifuged at 12,000g for 15 min at 4°C. To 1 ml of supernatant, 4 ml of 0.5% of thiobarbituric acid (prepared in 20% trichloroacetic acid, TCA) was added. The reaction mixture was then heated at 95°C for 30 min, cooled immediately in an ice bath and then again centrifuged at 10,000g for 10 min. The absorbance of the supernatant thus obtained was read at 532 nm and corrected for non-specific absorbance at 600 nm. The concentration of MDA was calculated using extinction coefficient (ε) of 155 mM−1 cm−1 and expressed as nanomoles per gram fresh weight (nM g−1 f. wt.).
H2O2 content was determined as per the method described by Velikova et al. [32]. The roots (100 mg) were homogenized in 0.1% TCA (10 ml) and centrifuged at 12,000g for 15 min. To 0.5 ml of the supernatant, 0.5 ml of 10 mM PO43– buffer (pH 7) and 1 ml of 1 M potassium iodide were added and absorbance of the reaction mixture was read at 390 nm. The H2O2 content was quantified using ε = 0.28 μM−1 cm−1 and expressed as nM g−1 f. wt. [28].
O2•− content was estimated according to the method given by Misra and Fridovich [33]. The root tissue (100 mg) was homogenized in 10 ml of 0.1 M PO43− buffer (pH 7), and centrifuged at 12,000g for 15 min at 4°C. To 1.8 ml of 1 mM adrenalin solution (prepared in 75 mM PO43– buffer; pH 7.4), 0.2 ml of supernatant was added. The change in the absorbance was read at 480 nm over a time period of 5 min and O2•− content was calculated using ε = 4.02 mM−1 cm−1 and expressed as μM g−1 f. wt.
2.4. In situ ROS detection
In situ ROS accumulation in onion roots was determined histochemically in terms of lipid peroxidation, O2•−, H2O2 and loss of membrane integrity. Histochemical detection of lipid peroxidation was performed using Schiff’s reagent [34]. Freshly excised root tips were stained in Schiff’s reagent for 1 h, followed by rinsing with potassium metabisulphite solution (0.5%, w/v). Loss of membrane integrity was determined using Evans blue as per Yamamoto et al. [35]. Freshly excised root tips were stained in Evan’s blue solution (0.025%, w/v, in 100 μM CaCl2, pH 5.6) for 30 min. Diaminobenzidine (DAB) solution was used for the histochemical detection of H2O2 [36]. Freshly harvested roots were stained in DAB solution (0.3 mg/ml) for 10 min, followed with rinsing with distilled water. Root tips were then boiled in 90% ethanol (v/v) for 10 min for decolorization. In situ localization of O2•− was done as per the method given by Doke [37]. Freshly cut roots were incubated in nitrobluetetrazolium (NBT; 0.05%, w/v, prepared in 100 mM PO43– buffer, pH 7.8) for 20 min, then transferred to 96% ethanol for 30 min to stop the reaction. The excess stain was washed out by immersing the root tips in distilled water, followed by observation under the Trinocular Stereo Zoom microscope. In situ ROS detection in roots was done on the basis of colour intensity.
2.5. Assays of antioxidant enzymes
For the estimation of activities of antioxidant enzymes, root tissue (100 mg) was homogenized in 10 ml of 100 mM PO43− buffer (pH 7) and centrifuged for 25 min at 15,000 g. The supernatant thus obtained was used for the assay of activities of superoxide dismutases (SOD; EC 1.15.1.1) and catalases (CAT; EC 1.11.1.6). SOD was assayed for its ability to inhibit the photochemical reduction of NBT at 560 nm [38]. The amount of enzyme required to inhibit the photoreduction of NBT by 50% at 25°C was defined as one unit of SOD. CAT activity was determined in terms of disappearance rate of H2O2 at 240 nm and calculated using ε = 2.8 mM−1 cm−1 [39]. One unit of CAT is the amount of enzyme required to decompose 1 μM of H2O2 per min at 25°C.
2.6. Statistical analysis
The experiments were conducted in a completely randomized design. There were five replications for each treatment, with each replication consisting of single onion bulb. For biochemical analysis, there were five replicated (independent) tissue samples. The data were analysed by one-way ANOVA followed by the comparison of mean values at p ≤ 0.05 using post hoc Tukey’s test. The statistical analyses were done using SPSS ver. 16.
3. Results and discussion
In the present study, exposure to cell phone EMF-r incited a ROS-mediated oxidative stress in the onion roots. Upon exposure, the levels of ROS like MDA, H2O2 and O2•− were enhanced as compared to the control in a time-dependent manner. To quantify the EMF-r induced damage to the membranes, lipid peroxidation analysis was done. Exposure to 2100 MHz EMF-r enhanced MDA content (by ~23–73%), a measure of lipid peroxidation, as compared to the control (Table 1). MDA is a free radical generated as a byproduct of lipid peroxidation and enhanced levels of MDA upon exposure advocates that EMF-r increased lipid peroxidation, caused damage to the membrane and induced oxidative stress in the roots. It was confirmed by staining of roots with Evans blue that showed the damage to the membrane in a time-dependent manner (Fig. 1a). Enhanced lipid peroxidation upon EMF-r exposure was confirmed by the in situ histochemical localization with Schiff’s reagent, wherein the roots exposed to cell phone EMF-r stained darker than those of the control (Fig. 1b). Parallel to MDA accumulation, H2O2 and O2•− levels were increased upon exposure to EMF-r (Table 1). H2O2 content increased by ~5.1 times upon exposure for 4 h, compared to the control (Table 1). In situ detection of H2O2 with DAB also supported the greater accumulation of H2O2, exhibiting greater colour intensity with increase in the exposure duration (Fig. 1c). O2•− content was enhanced by ~2.3 times over the control on EMF-r exposure for 4 h (Table 1). It was confirmed by in situ detection with NBT (Fig. 1d). The observations made in the present study are corroborated by earlier reports that EMF-r act as an abiotic stress for plants [23,40,41], and induce ROS (H2O2, O2•−) generation, resulting in enhanced lipid peroxidation and damage to membranes [27,28,42]. Monselise et al. [23] reported that low intensity magnetic field exposure cause oxidative stress in duck weed plants, as evidenced by accumulation of alanine, another stress signal.
Table 1.
Effect of cell phone EMF-r at 2100 MHz on the contents of (a) MDA, (b) H2O2, and (c) O2•− in onion roots.
| Treatment/Exposure Period (h) | MDA content (nM g−1 f. wt.) | H2O2 content (nM g−1 f.wt.) | Superoxide anions (mM g−1 f.wt.) |
|---|---|---|---|
| Control | 11.2 ± 1.14 a | 3.57 ± 0.41 a | 0.30 ± 0.03 a |
| Sham Control | 13.8 ±0.57 a | 5.24 ±0.63 ab | 0.35 ± 0.03 a |
| 1 h | 14.4 ± 0.42 b | 7.86 ± 0.41 b | 0.41 ± 0.04 a |
| 2 h | 15.5 ± 0.37 b | 10.48 ± 0.63 c | 0.61 ± 0.04 b |
| 4 h | 19.4 ± 0.99 c | 18.33 ± 0.86 d | 0.70 ± 0.03 c |
Different letters in a column represent significant difference at p ≤ 0.05 according to Tukey's Post hoc test.
Fig. 1.
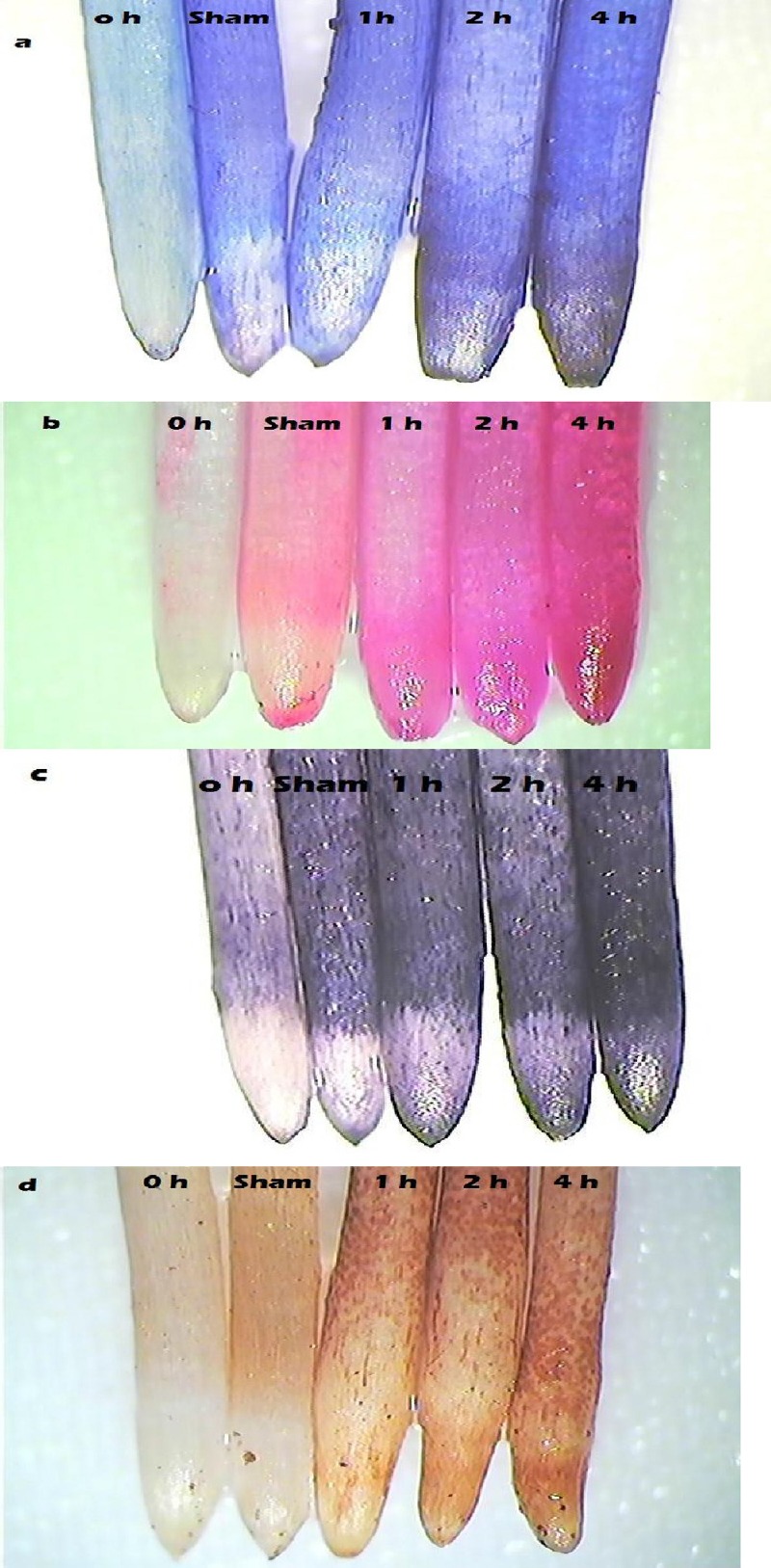
Photographs showing in situ histochemical localization depicting (a) loss of membrane integrity, (b) lipid peroxidation, and accumulation of (c) H2O2 and (d) O2•− in Allium cepa roots after exposure to 2100 MHz EMF-r.
The cells have an inherent mechanism to overcome stress induced by overproduction of ROS and one such strategy is the upregulation/greater production of antioxidant enzymes that scavenge free radicles [12,43]. In the present study, an upregulation in the activities of antioxidant enzymes- SOD and CAT- in a dose-dependent manner was also observed upon EMF-r exposure (Table 2). EMF-r exposure for 1 h enhanced the SOD activity by ~1.17 times, which further increased by ~1.5 and 2.42 times over control, on exposure for 2 h and 4 h, respectively (Table 2). Likewise, CAT activity increased by ~1.66 and ~3.5 times as compared to the control on exposure for 2 h and 4 h, respectively (Table 2).
Table 2.
Effect of cell phone EMF-r at 2100 MHz on the specific activities of (a) SOD and (b) CAT in onion roots.
| Treatment/Exposure Period (h) | SOD activity (EU mg−1 protein) | CAT activity (EU mg−1 protein) |
|---|---|---|
| Control | 3.51 ±0.09 a | 1.28 ±0.04 a |
| Sham Control | 3.90 ±0.02 ab | 1.44 ±0.08 ab |
| 1 h | 4.12 ±0.06 b | 1.73 ± 0.03 b |
| 2 h | 5.21 ± 0.13 c | 2.13 ± 0.06 c |
| 4 h | 8.51 ± 0.41 d | 3.17 ± 0.15 d |
Different letters in a column represent significant difference at p ≤ 0.05 according to Tukey's Post hoc test.
SOD is a first line of defence of plants against the elevated levels of ROS and mitigates the excessive O2•− generation. Likewise, CAT also plays an important role in plants under stress and decompose H2O2 into H2O and O2 [42]. Hence, the upregulated activities of SOD and CAT could be attributed to excessive ROS generation upon EMF-r exposure. These observations are in agreement with the earlier studies documenting the elevated activities of SOD and CAT in response to EMF-r [12,27,28,44].
4. Conclusions
The study concludes that 2100 MHz EMF-r alter the oxidative metabolism in plants. It incites the enhancement of ROS generation and alters the activities of antioxidant enzymes in a dose-dependent manner. This study implies that plants perceive EMF-r as an abiotic stress and it induces oxidative damage to the plants. Nevertheless, further studies are required to explore the mechanism of action of EMF-r on plants at the molecular levels to understand impacts of EMF-r on cellular metabolism and develop certain strategies to undermine its harmful effects.
Conflict of interest
Authors declare that they have no conflicts of interest.
Acknowledgement
Authors are thankful to Science and Engineering Research Board, Department of Science and Technology, India, for financial support.
References
- [1].Dasdag S, Akdag MZ. The link between radiofrequencies emitted from wireless technologies and oxidative stress. J Chem Neuroanat. 2016;75:85–93. doi: 10.1016/j.jchemneu.2015.09.001. [DOI] [PubMed] [Google Scholar]
- [2].ITU (International Telecommunication Union) [Accessed on 01.06.2017]. http://www.itu.int/en/ITU-D/Statistics/Pages/stat/default.aspx .
- [3].Redlarski G, Lewczuk B, Zak A, Koncicki A, Krawczuk M, Piechocki J, et al. The influence of electromagnetic pollution on living organisms: historical trends and forecasting changes. BioMed Res Int. 2015. pp. 1–18. Article ID 234098. http://dx.doi.org/10.1155/2015/234098 . [DOI] [PMC free article] [PubMed]
- [4].Vian A, Davies E, Gendraud M, Bonnet P. Plant responses to high frequency electromagnetic fields. BioMed Res Int. 2016. pp. 1–13. http://dx.doi.org/10.1155/2016/1830262 . Article ID 1830262. [DOI] [PMC free article] [PubMed]
- [5].Sepehrimanesh M, Azarpira N, Saeb M, Nazifi S, Kazemipour N, Koohi O. Pathological changes associated with experimental 900-MHz electromagnetic wave exposure in rats. Comp Clin Path. 2014;23(5):1629–31. [Google Scholar]
- [6].Lee S, Johnson D, Dunbar K, Dong H, Ge X, Kim YC, et al. 2.45 GHz radiofrequency fields alter gene expression in cultured human cells. FEBS Lett. 2005;579(21):4829–36. doi: 10.1016/j.febslet.2005.07.063. [DOI] [PubMed] [Google Scholar]
- [7].Cleary SF, Cao G, Liu LM. Effects of isothermal 2.45 GHz microwave radiation on the mammalian cell cycle: comparison with effects of isothermal 27 MHz radiofrequency radiation exposure. Bioelectrochem Bioenergy. 1996;39(2):167–73. [Google Scholar]
- [8].Paulraj R, Behari J. The effect of low level continuous 2.45 GHz waves on enzymes of developing rat brain. Electromagn Biol Med. 2002;21(3):221–31. [Google Scholar]
- [9].Sepehrimanesh M, Kazemipour N, Saeb M, Nazifi S. Analysis of rat testicular proteome following 30-day exposure to 900 MHz electromagnetic field radiation. Electrophoresis. 2014;35(23):3331–8. doi: 10.1002/elps.201400273. [DOI] [PubMed] [Google Scholar]
- [10].Sepehrimanesh M, Kazemipour N, Saeb M, Nazifi S, Davis DL. Proteomic analysis of continuous 900-MHz radiofrequency electromagnetic field exposure in testicular tissue: a rat model of human cell phone exposure. Environ Sci Pollut Res. 2017;1015(24):13666–73. doi: 10.1007/s11356-017-8882-z. [DOI] [PubMed] [Google Scholar]
- [11].Sepehrimanesh M, Saeb M, Nazifi S, Kazemipour N, Jelodar G, Saeb S. Impact of 900 MHz electromagnetic field exposure on main male reproductive hormone levels: a Rattus norvegicus model. Int J Biometeorol. 2014;58(7):1657–63. doi: 10.1007/s00484-013-0771-7. [DOI] [PubMed] [Google Scholar]
- [12].Sepehrimanesh M, Nazifi S, Saeb M, Kazemipour N. Effect of 900 MHz radiofrequency electromagnetic field exposure on serum and testicular tissue antioxidant enzymes of rat. Online J Vet Res. 2016;20(9):617–24. [Google Scholar]
- [13].Apollonio F, D’Inzeo G, Tarricone L. Modelling of neuronal cells exposed to RF fields from mobile telecommunication equipment. Bioelectrochem Bioenergy. 1998;47(2):199–205. [Google Scholar]
- [14].Ruediger HW. Genotoxic effects of radiofrequency electromagnetic fields. Pathophysiology. 2009;16(2):89–102. doi: 10.1016/j.pathophys.2008.11.004. [DOI] [PubMed] [Google Scholar]
- [15].Ponomarev L, Dolgodvorov V, Popov V, Rodin O, Roman A. The effect of low-intensity electromagnetic microwave field on seed germination. Proc Timiryazev Agric Acad. 1996;2:42–6. [Google Scholar]
- [16].Atak Ç, Emiroğlu Ö, Alikamanoğlu S, Rzakoulieva A. Stimulation of regeneration by magnetic field in soybean (Glycine max L. Merrill) tissue cultures. J Cell Mol Biol. 2003;2(2):113–9. [Google Scholar]
- [17].Jakubowski T. The impact of microwave radiation at different frequencies on weight of seed potato germs and crop of potato tubers. Agric Eng. 2010;14:57–64. [Google Scholar]
- [18].Akbal A, Kiran Y, Turgut-Balik D, Sahin A, Balik HH. Effects of electromagnetic waves emitted by mobile phones on germination, root growth, and root tip cell mitotic division of Lens culinaris Medik. Polish J Environ Stud. 2012;21:23–9. [Google Scholar]
- [19].Tkalec M, Malarić K, Pavlica M, Pevalek-Kozlina B, Vidaković-Cifrek Ž. Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat Res. 2009;672(2):76–81. doi: 10.1016/j.mrgentox.2008.09.022. [DOI] [PubMed] [Google Scholar]
- [20].Sharma VP, Singh HP, Batish DR, Kohli RK. Cell phone radiations affect early growth of Vigna radiata (mung bean) through biochemical alterations. Z Naturforsch C. 2010;65(1–2):66–72. doi: 10.1515/znc-2010-1-212. [DOI] [PubMed] [Google Scholar]
- [21].Singh HP, Sharma VP, Batish DR, Kohli RK. Cell phone electromagnetic field radiations affect rhizogenesis through impairment of biochemical processes. Environ Monit Assess. 2012;184(4):1813–21. doi: 10.1007/s10661-011-2080-0. [DOI] [PubMed] [Google Scholar]
- [22].Roux D, Vian A, Girard S, Bonnet P, Paladian F, Davies E, et al. High frequency (900 MHz) low amplitude (5 V m-1) electromagnetic field: a genuine environmental stimulus that affects transcription, translation, calcium and energy charge in tomato. Planta. 2008;227(4):883–91. doi: 10.1007/s00425-007-0664-2. [DOI] [PubMed] [Google Scholar]
- [23].Monselise EBI, Parola AH, Kost D. Low-frequency electromagnetic fields induce a stress effect upon higher plants, as evident by the universal stress signal, alanine. Biochem Biophys Res Commun. 2003;302(2):427–34. doi: 10.1016/s0006-291x(03)00194-3. [DOI] [PubMed] [Google Scholar]
- [24].Racuciu M. Effects of radiofrequency radiation on root tip cells of Zea mays. Rom Biotech Lett. 2009;14:4365–9. [Google Scholar]
- [25].Pesnya DS, Romanovsky AV. Comparison of cytotoxic and genotoxic effects of plutonium-239 alpha particles and mobile phone GSM 900 radiation in the Allium cepa test. Mutat Res. 2013;750(1):27–33. doi: 10.1016/j.mrgentox.2012.08.010. [DOI] [PubMed] [Google Scholar]
- [26].Gustavino B, Carboni G, Petrillo R, Paoluzzi G, Santovetti E, Rizzoni M. Exposure to 915 MHz radiation induces micronuclei in Vicia faba root tips. Mutagenesis. 2016;31(2):187–92. doi: 10.1093/mutage/gev071. [DOI] [PubMed] [Google Scholar]
- [27].Tkalec M, Malarić K, Pevalek-Kozlina B. Exposure to radiofrequency radiation induces oxidative stress in duckweed Lemna minor L. Sci Total Environ. 2007;388(1):78–89. doi: 10.1016/j.scitotenv.2007.07.052. [DOI] [PubMed] [Google Scholar]
- [28].Sharma VP, Singh HP, Kohli RK, Batish DR. Mobile phone radiation inhibits Vigna radiata (mung bean) root growth by inducing oxidative stress. Sci Total Environ. 2009;407(21):5543–7. doi: 10.1016/j.scitotenv.2009.07.006. [DOI] [PubMed] [Google Scholar]
- [29].Çenesiz M, Atakijşi O, Akar A, Önbilgin G, Ormanci N. Effects of 900 and 1800 MHz electromagnetic field application on electrocardiogram, nitric oxide, total antioxidant capacity, total oxidant capacity, total protein, albumin and globulin levels in guinea pigs. J Fac Vet Med Univ Kafkas. 2011;17(3):357–62. [Google Scholar]
- [30].Andreuccetti D, Fossi R, Petrucci C. An Internet resource for the calculationof the dielectric properties of body tissues in the frequency range10 Hz–100 GHz, IFAC–CNR, Florence, Italy. Based on data published by C. Gabriel, et al. in 1996. 2017. Available online at http://niremf.ifac.cnr.it/tissprop/
- [31].Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: i. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- [32].Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. [Google Scholar]
- [33].Misra HP, Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972;247(1):188–92. [PubMed] [Google Scholar]
- [34].Pompella A, Maellaro E, Casini AF, Comporti M. Histochemical detection of lipid peroxidation in the liver of bromobenzene-poisoned mice. Am J Pathol. 1987;129(2):295–301. [PMC free article] [PubMed] [Google Scholar]
- [35].Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001;125:199–208. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11(6):1187–94. [Google Scholar]
- [37].Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol. 1983;23(3):345–57. [Google Scholar]
- [38].Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- [39].Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992;98(4):1222–7. doi: 10.1104/pp.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Soran ML, Stan M, Niinemets U, Copolovici L. Influence of microwave frequency electromagnetic radiation on terpene emission and content in aromatic plants. J Plant Physiol. 2014;171(15):1436–43. doi: 10.1016/j.jplph.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lung I, Soran ML, Opriş O, Truşcă MRC, Niinemets U, Copolovici L. Induction of stress volatiles and changes in essential oil content and composition upon microwave exposure in the aromatic plant Ocimum basilicum. Sci Total Environ. 2016;569:489–95. doi: 10.1016/j.scitotenv.2016.06.147. [DOI] [PubMed] [Google Scholar]
- [42].Yao Y, Li Y, Yang Y, Li C. Effect of seed pretreatment by magnetic field on the sensitivity of cucumber (Cucumis sativus) seedlings to ultraviolet-B radiation. Environ Exp Bot. 2005;54:286–94. [Google Scholar]
- [43].Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- [44].Krishnan R, Murugan K. Effect of mobile phone radiation on antioxidant machinery in roots of onion (Allium cepa) Asian J Exp Biol Sci. 2011;2:667–72. [Google Scholar]


