Abstract
The purpose of the study was to investigate the effects of pulsed digital electromagnetic radiation emitted by mobile phones on the central nervous system of the adult Wistar albino rats. The study evaluated structural and functional impacts of four treatment arms: electromagnetic field (EMF) exposed; EMF exposed + melatonin treated group (EMF + Mel); EMF exposed + omega-3 (ω3) treated group (EMF + ω3); and control group (Cont). The 12-weeks-old rats were exposed to 900 MHz EMF for 60 min/day (4:00–5:00 p.m.) for 15 days. Stereological, biochemical and electrophysiological techniques were applied to evaluate protective effects of Mel and ω3. Significant cell loss in the CA1 and CA2 regions of hippocampus were observed in the EMF compared to other groups (p < 0.01). In the CA3 region of the EMF + ω3, a significant cell increase was found compared to other groups (p < 0.01). Granular cell loss was observed in the dentate gyrus of the EMF compared to the Cont (p < 0.01). EMF + ω3 has more granular cells in the cerebellum than the Cont, EMF + Mel (p < 0.01). Significant Purkinje cell loss was found in the cerebellum of EMF group compared to the other (p < 0.01). EMF + Mel and EMF + ω3 showed the same protection compared to the Cont (p > 0.05). The passive avoidance test showed that entrance latency into the dark compartment was significantly shorter in the EMF (p < 0.05). Additionally, EMF had a higher serum enzyme activity than the other groups (p < 0.01). In conclusion, our analyses confirm that EMF may lead to cellular damage in the hippocampus and the cerebellum, and that Mel and ω3 may have neuroprotective effects.
Keywords: Hippocampus, Cerebellum, Neuronal loss, Optical dissector, Neurogenesis
1. Introduction
Mobile phones were first introduced into the market in the 1970s with no pre-market safety testing [1]. As most users were adult male medical and military personnel, the average call was assumed to last six minutes and was judged safe if it resulted in no increase in temperature from the weak non-ionizing radiation emitted. Today, very young persons or those with much smaller brains and bodies in comparison to the 175 cm tall, 88 kg male phantom against which phones were first tested for their capacity to change temperature, use more than half of the world’s nearly 7 billion phones. Because they are often held directly next to the head and pulsed digital radiation is absorbed about 2 cm into the brain, the effects of mobile phones particularly on the central nervous system may be remarkable. Biological agents can be thought of as having two distinct types of impacts: those that constitute direct physical impacts such as change in structure, cell count, proteomics, etc.; and those that constitute functional or behavioral effects such as change in response time, memory, or other standardized measure of performance that have been well characterized through a battery of tests [2,3,4].
Previous investigations have confirmed a number of important structural impacts of EMF. A decrease in the number of Purkinje cells in the rats exposed to a 900 MHz electromagnetic field (EMF) was previously reported [5]. Additionally, a significant decrease in the number of granular and pyramidal cells of the hippocampus, which play a key role in memory-related functions, was shown by stereological techniques that allow 3-dimensional imaging of location [5,6,7]. Other destructive effects have also been determined by different methods. Damage to the cortex, cerebellum, hippocampus, and basal ganglia have resulted from neuronal damage induced by exposure to a 900 MHz EMF [8].
Other studies have demonstrated critical functional changes after EMF exposures. The effect of EMF exposure on increasing permeability of the blood-brain barrier (BBB) has been well documented for more than four decades [9,10]. This increased permeability affects homeostasis and may cause leakage of serum albumin into brain tissue. This process also leads to neuronal degeneration in the brain [11]. Currently, this property of EMF/RF is being used for enhanced uptake of chemotherapy in the treatment of brain cancer, while amplitude modulated current is being employed as part of a treatment involving tumor-treating fields (TTF) that can interfere with post-mitotic spindle formation and increase survival of seriously ill brain and pancreas cancer patients [12,13].
Exogenous influences such as extra-low-frequency electromagnetic field (EMF) have been shown to effect pain and inflammation by modulating G-protein receptors, down-regulating cyclooxygenase-2 activity, and affecting the calcium/calmodulin/nitric oxide pathway. Investigators have reported changes in opioid receptors and second messengers, such as cyclic adenosine monophosphate (cAMP), in opiate tolerance and dependence by showing how repeated exposure to morphine decreases adenylate cyclase activity causing cAMP to return to control levels in the tolerant state, and increase above control levels during withdrawal [14].
In addition, other functional impacts of EMF exposure may affect neuronal membranes and organelles, such as lysosomes and mitochondria; consequently, heavy metals and reactive oxygen species (ROS) may reach high levels in intracellular areas [15,16]. Thus, electromagnetic radiation-induced tissue damage may be associated with ROS. In this context, one of the several molecular pathways involved in EMF exposure-induced neuronal damage is the caspase3-dependent pathway [17]. The extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) pathway also plays a crucial role in signal transduction regarding cell growth and differentiation [18,19]. Friedman et al. have effectively shown that MAP-kinase are also affected by low levels of EMF/RF and thus electromagnetic radiation emitted from mobile phones may induce the ERK cascade and the other cellular processes [20].
Neuronal ultrastructural effects of EMF exposure on these pathways have been investigated using proper immunohistochemical and electron microscopic analyses in a study conducted by Tang et al. [21]. The hippocampus and parietal cortex revealed tight and regular formation of neurons with neuroglia cells in the non-exposed group. Electron microscopic analysis in the group exposed to 900 MHz EMF for 28 days showed many empty and inadequate areas surrounded by vessels and neurons [22]. Moreover, this is consistent with many studies showing the harmful effects of EMF on the developing nervous system during prenatal and postnatal periods [23]. In particular, EMF exposure can lead to neuronal death and inhibits transformation of neuronal stem cells into neurons [23]. The structural damage has produced many functional disorders, such as cognitive impairment [5,6,7,22]. Oxidative stress is a type of disequilibrium between antioxidants and free radicals. After electromagnetic radiation (EMR), the presence of increased free radicals causes the deterioration of membrane integrity by acting on proteins and nucleic acids. This also leads to some gene mutations and antioxidant defenses, which occur to counter these effects [24,25]. Measurement of ROS to determine these effects is very difficult due to their high reactivity, short half-life, and high concentrations. The intracellular damage activates superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) [26,27]. The first enzymatic defense against to the ROS is SOD [28,29]. CAT produces a peroxidative reaction in the presence of low levels of H2O2 and high concentrations of free electron donors [30]. Normally, the main enzyme responsible for the detoxification of H2O2 is GSH-Px, and it inhibits lipid peroxidation. GSH-Px produces H2O2 from water by reducing GSH [28,29]. By means of this reaction, GSH becomes oxidized glutathione (GSSG) [31].
Many studies have examined both the neuroprotective effects of some antioxidants as well as the damaging impacts of EMF. In term of this point, the efficiency of melatonin (Mel) and omega-3 (ω3) to induce repair or otherwise interfere with the damaging effects of EMF is noteworthy. Research shows that Mel is an antioxidant agent, as well as ω3 [32]. As a free radical scavenger, Mel has a high degree of lipophilicity and therefore does not require any binding receptor for its effects [33,34,35]. Additionally, ω3 polyunsaturated fatty acids (ω3 PUFAs) are an essential type of fatty acids and play a crucial role in neurological function because they are integral membrane components and energy substrates [36].
A few studies have been done using stereological techniques to evaluate the effect of EMF exposure on the CNS. In addition, structural changes after prenatal exposures to a 900 MHz EMF have been investigated by several studies [5,6]. However, there are no enough studies regarding evaluation of EMF exposure on the cellular number in the brain and cerebellum of the rats using quantitative techniques. In this context, the present study was designed to investigate the structural and functional impacts of EMF radiation on the brain. In addition, the possible neuroprotective effects of Mel and ω3 have also been searched against EMF exposure.
2. Material and methods
The main purpose of this study was to investigate the effects of 900 MHz EMF exposure and the possible neuroprotective effects of the Mel and ω3 on the granular and pyramidal cells in the hippocampus and Purkinje and granular cells in the cerebellum of adult rats. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are two important fatty acids that can be found in fish oil [37], and for this reason fish oil is an important nutritional source. To obtain reliable data for EMF exposure and the neuroprotective ability of these substances, the optical fractionator method – which is a type of unbiased stereological probing technique – paired with cognitive tests and biochemical parameters of oxidative stress. Electrophysiological analysis may inform us about sensitive alterations of interactions in the brain. In this context, electrophysiological assessment was also used in the present study.
We employed two overall methods to evaluate both structural and functional impacts on the young animal brain and their subsequent performance [16,38,39]. Prior to sacrifice, animal behaviors were evaluated by the passive avoidance test [16], where animals were trained to avoid a noxious stimulant such as an electric shock for a food reward. In this behavioral test, three parts were conducted, including an exploration test (1), a learning test (2) and a retention test (3). The exploration test was conducted in three trials. The rats were kept in the center of the light compartment and the door was kept open for 3 min. The total time to enter the dark compartment was noted for each trial. When the rats entered the dark compartment, the door between the compartments was closed and 0.5 mA electric shock was given for 3 s. Then, the ceiling was opened and the rats were returned to the home cage. Each rat received retention tests that were applied after the 24 and 48 h of 0.5 mA electric shock. During this period of rest, the sliding door was open. The latency time, which is the time-required for rats to enter the dark compartment, was noted. The latency time to enter the dark compartment was 3 min for the rats. Positive memory retention was shown by absence of entry into the dark compartment.
After the behavioral experiments were completed, we evaluated structural impacts on rat brain transcardial perfused with 60 ml of 0.9% heparinized saline (1 unit heparin/ml) and neutral formalin. After perfusion, the routine histological procedures were applied for the brain and cerebellum, which had been removed from the cranium [39,40]. Numbers of cells in the stained sections were estimated using the optical fractionator technique, which permits identification of both cell number and membrane integrity.
2.1. Animals and experimental design
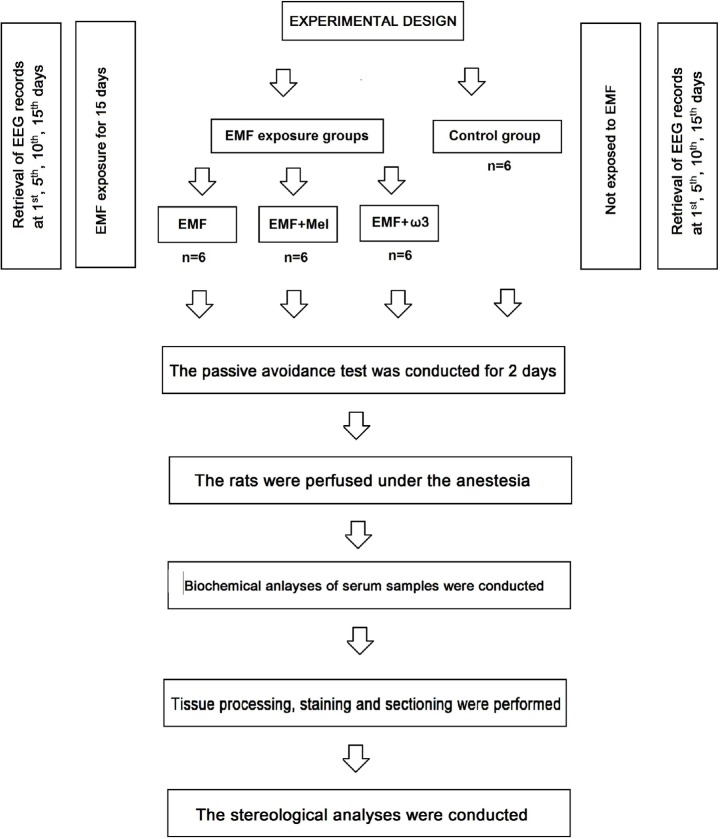
Experiments in the present study were approved by the Experimental Animal Studies Ethics Committee of Ondokuz Mayis University (2013/20; 05.06.2013). The adult male Wistar albino rats (n = 24), 12 week-old, and 230–280 g, were separately housed in special cages consisting of glass ceilings and plastic floors. During the study, rats were maintained at room temperature (22 ± 2°C), with 40%–50% relative humidity under a 12/12-h light/dark cycle. The rats were fed tap water and standard rat chow. This study had four groups that consist of electromagnetic field (EMF) exposed group (n = 6), electromagnetic field exposed + melatonin treated group (EMF + Mel) (n = 6), electromagnetic field exposed + omega-3 (ω3) (n = 6) treated group (EMF + ω3) and control group (Cont) (n = 6). The Cont group was not exposed to any procedure, the EMF group was exposed to a 900 MHz EMF for 1 h/day, the EMF + Mel group was given 50 mg/kg/day Mel (Sigma-Aldrich Comp., St. Louis, MO, USA) injected intraperitoneally (i.p.) while receiving 900 MHz EMF exposure [41] and the EMF + ω3 animals were fed with standard feed plus an additional 10% of Ω3 substances (Sigma Chemical Co., St. Louis, MO, USA) while receiving the same EMF exposure. Rats were first exposed at 12 weeks of age and weighed 230–280 g on the first day of exposure (Fig. 1). In the laboratory conditions, the technicians were advised to keep their mobile phones turned off during the experimental process. The rats were placed in a Faraday cage, which blocks electric fields and electromagnetic waves.
Fig. 1.
A flowchart that is showing the experimental design and trial protocols.
2.2.1. Exposure system
The EMF exposure system included a round plastic cage with 16 separate parts (diameter 5.5 cm, length 12 cm), an EMF meter, and a monopole antenna. The signal generator was operated at 2 W (Electromagnetic Compatibility Laboratory, Süleyman Demirel University, Isparta, Turkey), and the heads of rats were placed in close proximity to the probe. The rats of EMF (n = 6), EMF + Mel (n = 6) and EMF + ω3 (n = 6) groups were passively constrained to keep their heads near the exposure, while 900 MHz of continuously modulated wave electromagnetic energy (peak specific absorption rate [SAR] 2 W/kg, average power density 1 ± 0.4 mW/cm2) was emitted [42,43,44]. The specific absorption rate was obtained from the estimated numerical calculations [45]. The rats were exposed to 900 MHz of EMF for 60 min/day (4:00 p.m. to 5:00 p.m.) for 15 days.
2.3. Cardiac perfusion and tissue preparation
Following the exposure period and passive avoidance tests, the rats were anesthetized with ketamine (5 mg/kg i.p.) and Xylazin (2 mg/kg i.p.). Cardiac perfusion was performed with 10% neutral buffered formalin while the heartbeat continued. Following the perfusion, the brain and cerebellum were processed in graded alcohols (70% to 100%) and xylene, and embedded in paraffin for sectioning. Sections of the hippocampus and cerebellum were taken with a rotary microtome (Leica RM 2135; Leica Instruments, Nussloch, Germany). Next, using systematic random sampling, 20 μm thick serial sections were obtained from paraffin-embedded blocks in the sagittal plane. One out of every six sections was selected for analysis, placed on coated slides, and stained with cresyl violet.
2.4. Stereological analysis
The total numbers of pyramidal and granule cells in the hippocampus, and Purkinje and granular cells in the cerebellum were estimated using the optical fractionator technique, in conjunction with a stereo investigator analyzing system (Stereoinvestigator 9.0 MicroBrieldField; Colchester, USA) and a light microscope (Leica M 4000 B; Germany) with a digital color camera attachment (Microbrightfield, Williston, VT, USA) [38]. The coefficient of error (CE) for a subject was considered ≤ 0.05 for appropriate sampling. CE and coefficient variation (CV) values are important for the appropriate estimation. The first section in the series was chosen at random from the first six sections, and then every sixth section was collected in a systematic manner. Therefore, the section-sampling fraction was given as 1/6. A total of 16–23 sections were obtained from each brain and cerebellum for adequate estimation with the optical fractionator [46,47]. For analyzing the number of granular cells in the dentate gyrus of hippocampus (DG), the counting-frame size was 100 μm2 and the sampling grid area was 22.500 μm2. For analyzing the number of pyramidal cells in the hippocampus, the counting frame was 1600 μm2 and the sampling grid area was 22.500 μm2. For analyzing the number of granular cells in the cerebellum, the counting frame was 625 μm2 and the sampling grid area was 90.000 μm2. Finally, for analyzing the number of Purkinje cells in the cerebellum, the counting frame was 1600 μm2 and the grid sampling area was 22.500 μm2.
2.5. Passive avoidance apparatus and tasks
The passive avoidance test apparatus consisted of two compartments − dark and light. A 7 × 7 cm guillotine separated these areas. The light compartment’s dimensions were 30 × 20 × 20 cm, and the walls were made of Plexiglas. The floor of the dark compartment was furnished with conducting wires and connected to a 5 mA, 12 V shock device [16]. Before the passive avoidance test, the animals were allowed to acclimate to the conditions, and following each test, the cage was cleaned. After resting in the light compartment, a 0.5 mA electric shock was administered to the rat for 3 s via the conducting wire when they entered the dark compartment. After being placed back into the light compartment, the latency time before reentering the dark compartment was recorded. The rats exposed to electrical shocks were returned to their cages within 10 s. This section of the test was called the learning period. Twenty-four hours after the learning period, the rats were returned to the light compartment and the duration before entering the dark compartment was again recorded. This period was called the retention time.
2.6. Biochemical analysis
At the end of the experimental process, 1 ml of intra-cardiac blood was collected into tubes during the cardiac perfusion. The samples were centrifuged at 4°C at 2000 rpm for 15 min (Hettich EB 20 centrifuge, Hettich, Tuttlingen, Germany). The serum samples from each rat were stored at −80°C. The serum enzyme activity of GSH, CAT and SOD was measured using a Cayman Kit (Cayman Chemical Company). Additionally, GSH, CAT and SOD enzyme activity was determined at 410 nm, 540 nm and 450 nm, respectively.
2.7. Electrophysiological assessment
Under anesthesia (mixture of ketamine HCl 9 mg/kg i.p and 1 mg/kg xylazine i.p.), the cranium of each rat was positioned in a stereotactic device (Harvard Stereotaxic Instruments, Massachusetts, USA), and a 3 cm rostrocaudal incision was made in the scalp. Next, four small holes (each approximately 1 mm in diameter) were drilled 4 mm anterior and posterior to the bregma, and left and right laterally at 3 mm from the midline. Four electrodes were fixed to the skull and isolated with dental cement. After surgery, each animal was allowed to recover for seven days in a separate cage. The basal activity signal from the electrodes was amplified with a BioAmp (ADInstruments, Australia), and transferred to PowerLab 4/SP (ADInstruments Pty Ltd., Castle Hill, Australia). Brain electrical activity was analyzed with LabChart v 7.3.7 software (ADInstruments). The power spectrum of each sample was segmented into six frequency bands: delta (1–4 Hz), theta (4.1–8 Hz), alpha (8.1–15 Hz), beta (16–31 Hz), gamma-1 (32–45 Hz) and gamma-2 (55–100 Hz). All electroencephalographic (EEG) analyses were performed on the first, fifth, tenth and fifteenth days before and after exposure for each group.
2.8. Statistical analysis
Results from each group were compared using a one-way ANOVA related to the normality of variable distribution, and the Bonferonni and Tukey post-hoc tests were performed. Also, statistical comparisons between the EEG bands before and after exposure for each group were determined using the Student t-test. Mean ± SEM. P values of less than 0.05 were considered significant, and a value less than 0.01 was considered highly significant. Statistical analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Structural analysis pyramidal cell number in the hippocampus
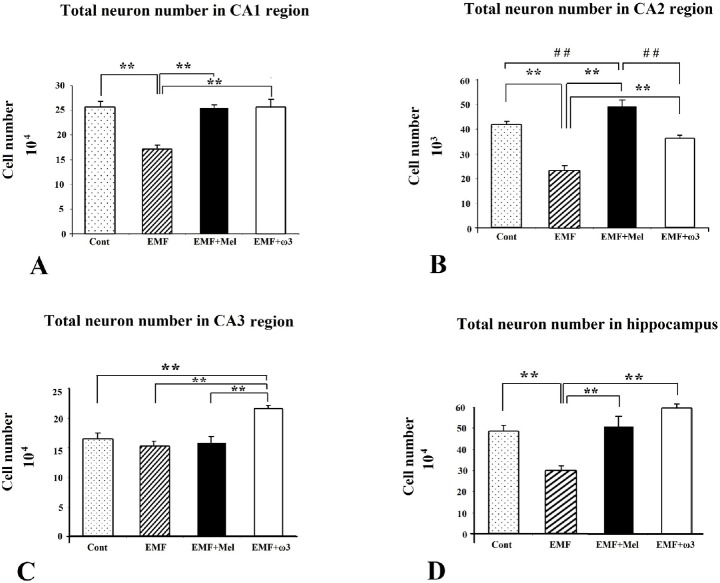
Pyramidal cell numbers were estimated using the optical fractionator technique [47]. Significant cell loss was observed in the EMF group compared to the other study groups (p < 0.01). Protective effects were evident in that there were no significant differences between the Cont and the EMF + Mel, or between the Cont and the EMF + ω3 in terms of the number of pyramidal cells in the cornu ammonis (CA) CA1 and CA2 regions (p > 0.05). On the other hand, there was no significant difference among the cell numbers for the EMF, the Cont, and the EMF + Mel in terms of the number of pyramidal neurons in the CA3 region. In contrast, a significantly increased cell number was found within the CA3 region in the EMF + ω3 group compared to the other groups (p < 0.01) (Figs. 2 and 3). The CE and CV values were within acceptable ranges (Table 1).
Fig. 2.
The neuron numbers in CA regions at (A–C) and total neuron number in hippocampus at (D) are shown by graphs for each group. **, ## represent p<0.01. Data are presented as mean ± SEM.
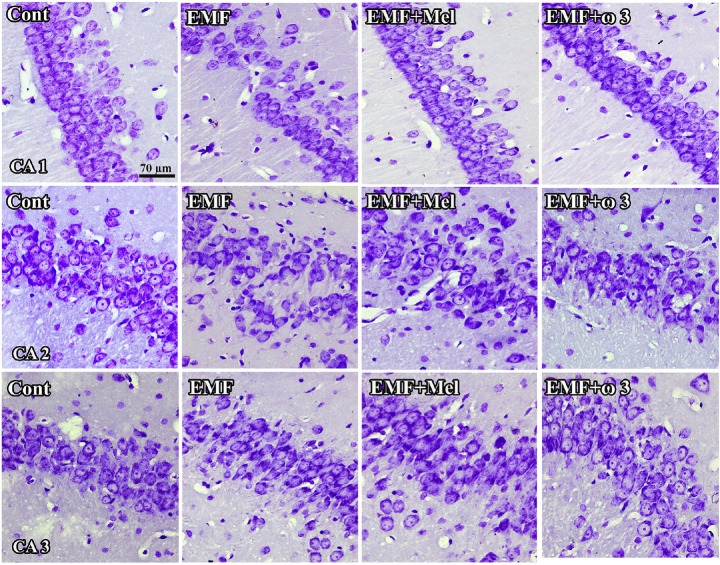
Fig. 3.
CA1, CA2, and CA3 regions in hippocampus are respectively shown in the cresyl violet stained sections. Especially, neuronal cell loss of EMF groups in CA1 and CA2 regions was shown in the cresyl violet stained sections. It should be noted that the view of images may be changed by the tissue shrinkage or swelling and section plane taken. Scale bars: 70 µm.
Table 1.
The mean CE and CV values of stereological analysis of pyramidal and granular cell number in the hippocampus, DG and cerebellum for the all groups.
| Groups | Hippocampus Pyramidal cell number | Granular cell number | Purkinje cell number | Granular cell number | |||
|---|---|---|---|---|---|---|---|
| CA1 | CA2 | CA3 | DG | ||||
| Cont | CE | 0.05 | 0.03 | 0.03 | 0.02 | 0.05 | 0.02 |
| CV | 0.11 | 0.12 | 0.08 | 0.07 | 0.16 | 0.13 | |
| EMF | CE | 0.05 | 0.03 | 0.05 | 0.03 | 0.04 | 0.02 |
| CV | 0.1 | 0.09 | 0.08 | 0.06 | 0.11 | 0.09 | |
| EMF + Mel | CE | 0.05 | 0.05 | 0.04 | 0.03 | 0.05 | 0.03 |
| CV | 0.07 | 0.06 | 0.13 | 0.11 | 0.19 | 0.12 | |
| EMF + ω3 | CE | 0.04 | 0.02 | 0.03 | 0.02 | 0.05 | 0.03 |
| CV | 0.09 | 0.08 | 0.07 | 0.09 | 0.15 | 0.14 | |
3.2. Granular cell number in the DG
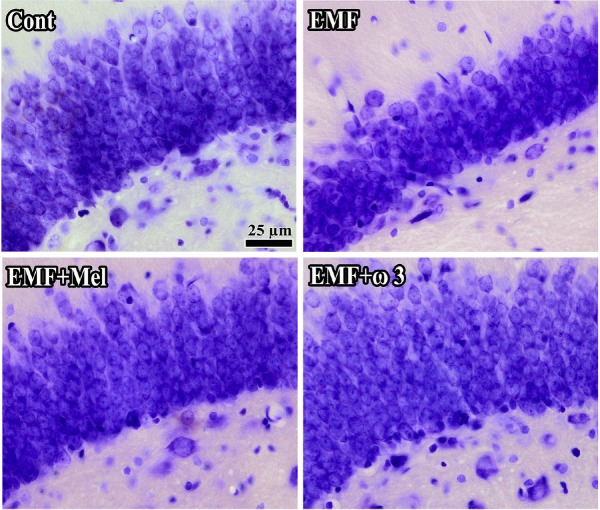
In terms of granular cell number, the granular cell number was significantly decreased in the EMF group compared with the Cont group (p < 0.01). Additionally, there was highly significant cell lost in the EMF group compared to EMF + ω3 and EMF + Mel groups (p < 0.05). Furthermore, the EMF + ω3 group showed an increased number of granular cells in comparison to the Cont and the EMF + Mel groups (p < 0.01) (Figs. 4 and 5). Evidence for a protective effect was evident in that there was no significant difference between the Cont and the EMF + Mel (p > 0.05). The CE and CV values were within acceptable ranges (Table 1).
Fig. 4.
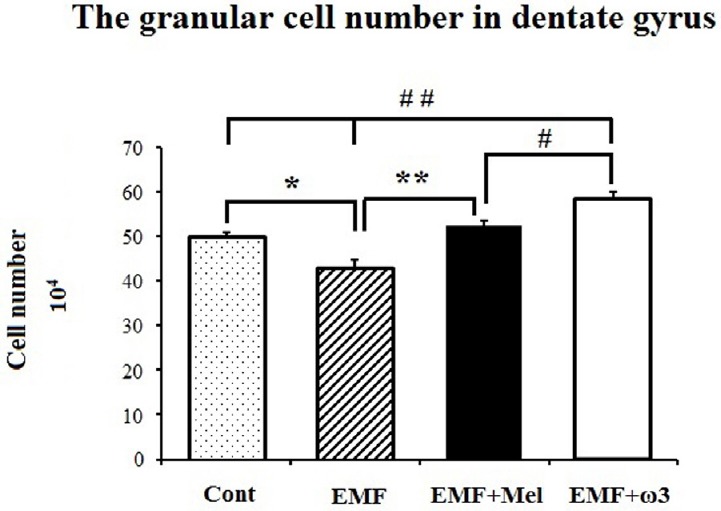
The granular cell number in DG is shown by graph for each group. **, ## represent p < 0.01 and *,# represent the p < 0.05. Data are presented as mean ± SEM.
Fig. 5.
Representative images indicated the alterations in cell structure and loss for each group. It should be noted that the view of images might be changed by the tissue shrinkage or swelling and section plane taken. Bars: 25 µm.
3.3. Purkinje cell number in the cerebellum
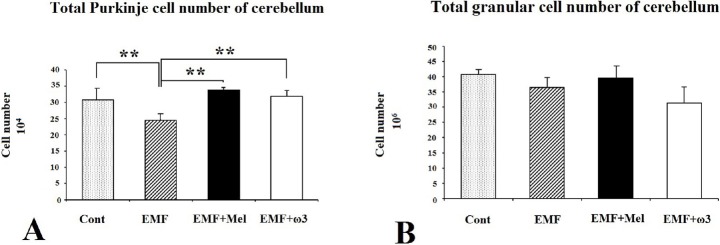
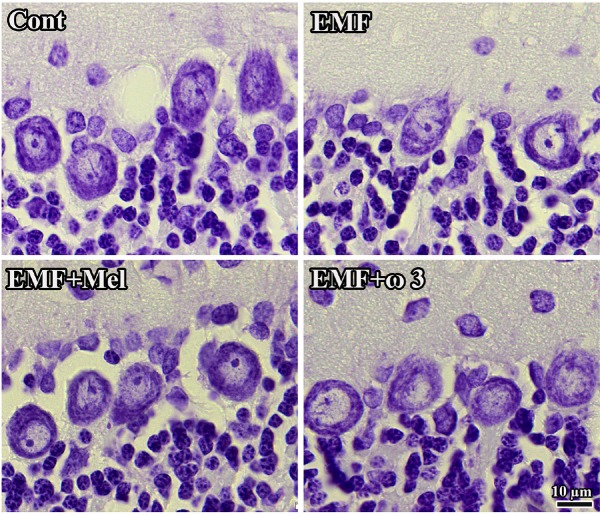
When the Purkinje cell numbers within each group were estimated, it was found that the EMF group contained fewer Purkinje cells in the cerebellum in comparison to other groups (p < 0.01). It was observed that melatonin has a neuroprotective effect on Purkinje cells due to the absence of any difference between the EMF + Mel and the Cont groups. The same neuroprotective effects were seen in the ω3-exposed group, i.e. the EMF + ω3 and the Cont (p > 0.05) (Figs. 6A and 7). The CE and CV values were within acceptable ranges (Table 1).
Fig. 6.
The Purkinje cell number in cerebellum at (A) and the granular cell number in cerebellum at (B) are shown by graph for each group. ** represent p <0.01.
Fig. 7.
Histological images show the cellular organization of each group. It should be noted that the view of images might be changed by the tissue shrinkage or swelling and section plane taken. Scale bars: 10 µm.
3.4. Granular cell number in the cerebellum
In terms of granular cell numbers for each group, there were no significant differences between the EMF + Mel and the Cont, the EMF + ω3 and the Cont, or the EMF and the Cont (p > 0.05). Similarly, there was no significant difference between the EMF + ω3 and EMF + Mel groups (Figs. 6B and 7). The CV and CE values were within acceptable ranges (Table 1).
3.5. Evaluation of cognitive test
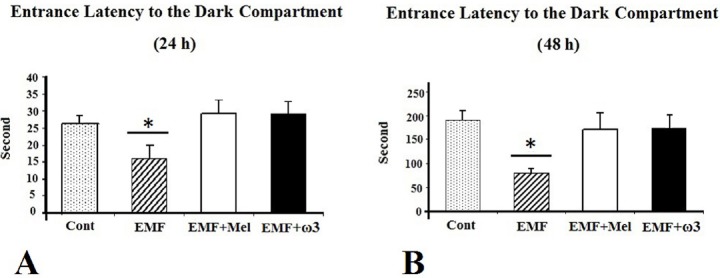
For the passive avoidance test performed after 24 h, the entrance latency into the dark compartment was significantly shorter in the EMF than in the EMF + Mel, the EMF + ω3, and the Cont groups (p < 0.05). However, there were no significant differences among the EMF + Mel, the EMF + ω3, and the Cont groups. Additionally, during the memory-retention test performed 48 h after the shock trial, the entrance latency into the dark compartment was significantly shorter in the 900 MHz-exposed EMF rats when compared to the Cont, the EMF + ω3, and the EMF + Mel groups (p < 0.05) (Fig. 8).
Fig. 8.
Effect of 900 MHz EMF on latency to enter the dark compartment 24 h (A) and 48 h (B) after the shock trial. Data are presented as mean ±SEM. *: p <0.05.
3.6. Assessment of the oxidative stress parameters
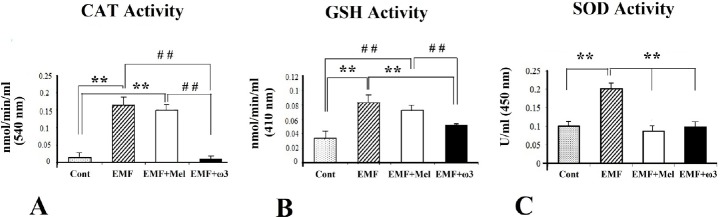
The serum activities of CAT, total GSH and SOD were analyzed in each group. There was no significant difference between the Cont and the EMF + ω3 groups in terms of CAT and total GSH activity. In addition, serum SOD activity did not show significant difference among the Cont, the EMF + Mel and the EMF + ω3 groups (p > 0.05). Serum enzyme activity in the EMF group was significantly increased in comparison to the other groups (p < 0.01). The serum CAT and total GSH activity of the EMF + Mel group was increased in comparison to the Cont and the EMF + ω3 groups (p < 0.01) (Fig. 9).
Fig. 9.
The graphs show the comparison of the serum levels of CAT, total GSH, and SOD among EMF, Cont, EMF + Mel, EMF + ω3 (A-C). **, ##: P < 0.01. U/ml: units per milliliter. Data are presented as mean ± SEM.
3.7. Electrophysiological assessment
Electroencephalograms (EEGs) were analyzed to determine the changes in the delta, theta, alpha, beta, gamma-1 and gamma-2 bands. Band powers were obtained by 1-min Fast Fourier Transformation (FFT) analysis for representative traces from continuous digital EEG recordings, and then used to calculate the relative band power for each band (percentage of the total power 0.1–50 Hz). Statistical analysis revealed no significant differences between the total power values for the delta, theta, alpha, beta and gamma-2 bands among the groups (p > 0.05). There were significant differences between the groups in terms of gamma-1 relative power. Compared to the Cont group, a significant decrease was observed in the EMF group 15 days before exposure and one and five days after EMF exposure (p < 0.05). In addition, a significant decrease was observed in the EMF group in comparison to the EMF + Mel group 10 and 15 days before exposure and 15 days after exposure (p < 0.05).
4. Discussion
Previous studies reported that as a result of EMF exposure to levels comparable to current cell phones, the numbers of hippocampal pyramidal neurons and granule cells in the DG of the EMF group were decreased when compared to the control group [48]. Terzi et al. [48] more recently noted that sensitivity to EMF exposure could lead to neurodegenerative disorders as well as cancer and other degenerative chronic illnesses. Furthermore, neuroendocrinological changes may be seen after pronounced EMF exposure and this can cause cognitive effects that cannot easily be monitored in the human population. Despite these important considerations, little is known about the potential structural or functional impacts of EMF, nor of ways to mitigate such impacts through the use of melatonin, polyphenols or other antioxidants. While calcium channels appear to be critical pathways, mechanisms of neurobehavioral and other functional impacts of EMF exposure remain unclear [48]. Intriguing hypotheses involving the opioid receptor have been elucidated previously. Naloxone is used to block heroin overdoses because of its capacity to interfere with the opioid receptor. Animal studies have found that after EMF stimulation, rodents previously conditioned to respond for a food reward will not seek that food reward when injected with naloxone. Thus, indicating that EMF can have a direct physiological impact on addictive behaviors [49,50,51].
The hippocampus is critical for controlling some behavioral and cognitive functions, which includes storing and retaining information during and after learning. According to Global System for Mobile Communications (GSM), the most popular standard for mobile phones is 900 MHz [6]. Thus, 900-MHz wave frequencies were chosen for this study. Harmful environmental conditions may deeply affect neurogenesis in the brain that occurs during the prenatal period, and such conditions may have increased neurobiological or concurrent behavioral defects [6,52].
Others have also previously shown that weakly powered digital EMF can have both structural and functional impacts on the brain and behavior. Odaci et al. reported a decrease in the number of granule neurons in the DG of rats exposed to 900 MHz EMF during the prenatal period when compared with the control group [6]. Furthermore, a great number of morphological deteriorations have been shown to occur in addition to a decrease in cell number [7]. Using a design similar to this study, Bas et al. found that four week old rats which had been prenatally exposed to 900 MHz EMF experienced a decrease in the total number of pyramidal neurons in the hippocampus when compared with controls [7]. In their study, Sonmez et al. reported that no change occurred in the number of Purkinje cells in the cerebellum of adult female rats, which had been exposed, to an hour of EMF for 28 days; however, there was an obvious decrease in the Purkinje numbers when compared with the control group and the sham group [5]. In another study by Bas et al., researchers found a significant decrease in the pyramidal neuron numbers in the hippocampus of adult females exposed to an hour of EMF daily for a period of 28 days [7] as compared with sham and other controls. Lai and Singh have reported that exposure to radiofrequency waves induced DNA fragmentation in rat brain cells [53]. In line with this finding, Robison et al. also observed that EMF exposure in HL-60 and HL-60R cells is associated with decreased DNA repair rates [54].
In considering pathological processes that occur in biological systems due to EMF exposure, ROS and the action of free radicals in the cells appears critical. Lipids also play a role in intercellular signal transmission since they are critical to multiple unsaturated fatty acids and amino acids membrane structure that are easily damaged by ROS. When hydrogen is removed from unsaturated chains in the membrane, carbon radicals form and react with oxygen to produce highly reactive and unstable peroxyl radicals. Some antioxidant enzymes have the capacity to scavenge free radicals and provide chemoprotection. Low levels of these enzymes in the brain and high levels of naturally protective fatty acids that can be easily oxidized in neuron membranes are proof of the brain’s predisposition for oxidative damage [55,56,57].
Due to the rapid cellular traffic between neuronal membranes, brain tissue is quite sensitive to oxidative stress. When the rate of ROS increases in an organism, calcium is released and becomes free. This increase in free calcium causes membrane structure and enzyme activities to deteriorate; as a result, nitric oxide radical formation is increased, resulting in concomitant cell damage and can directly or indirectly induce apoptosis [26]. Morphologically, EMF-exposed cells are characterized by a decrease in cell volume, deterioration in membrane integrity, and nuclear fragmentation. Studies have confirmed the oxidative damage of a great number of apoptotic proteins, such as caspase [58,59]. Additionally, İkinci et al. reported that continuous exposure to 900 MHz EMF can affect the development of the human fetus spinal cord in the gestational period. They observed histopathological changes in the spinal cord exposed to EMF such as vacuolization, atrophy, and thickening. They also found significant changes in MDA, SOD and CAT levels of exposed spinal cord tissues [60]
It is known that the cerebellum is critically involved in learning, balance, impulse control, memory and the formation of synapses. Purkinje cells form many synapses with the dendrites of granule cells. A number of studies have reported that EMF affects the number of granule cell numbers in the cerebellum and have implicated changes in the DNA structure as the underlying cause [61]. Investigations of Martin Pall (ref) have shown that EMF alters the activity of calcium channels and hypothesized that this affects membrane conductivity. Thus, changes in voltage may lead to granule cells necrosis or apoptosis [62,63,64]. Yet other investigators have reported no such results regarding a decrease or an increase in the number of granule cells due to non-ionizing radiation. In that regard, our study did not find a statistically significant difference between the EMF-exposed and other treatment arms in terms of the number of granule cells in the cerebellum [65]. We obtained similar results when we compared the EMF and control groups of our young adult rats. Clearly, the impact on granular cell formation of the hippocampus is a topic that merits further investigations with special attention to be paid to the age of exposure.
It is noteworthy that oxidative stress-induced loss, which was seen in the cerebellar granule cells exposed during the prenatal period, was not observed in those exposed as adult rats. This situation raises the question of whether there are critical windows of exposure during which prenatal exposure can affect cerebellar development, but adult exposures have no such impact. EMF exposure does not only result in a loss of granular cells, but also definitely influences granule cell migration [61,66]. Molecular studies that include the prenatal period should therefore be conducted to clarify whether critical periods of vulnerability exist for EMF as they do for other well-known pollutants such as lead or other heavy metals.
The originality of the present study is based on our investigation and confirmation of both the damaging effect of pulsed digital EMF as well as the neuroprotective effects of Mel and ω3 s on the EMF-exposed hippocampal pyramidal neurons and granule cell numbers in the DG. Mel, which is secreted from the pineal gland, is a direct radical scavenger and antioxidant [67]. Since it is highly lipophilic, Mel can be easily transferred to all tissues. Thus, studies have shown that it can decrease the oxidative stress in the central nervous system [68]. Due to this characteristic, it can easily enter all cellular components and offer a neuroprotective effect. Another study showed that Mel significantly decreased brain damage caused by ischemic reperfusion. Thus, the antioxidant efficiency of Mel and its free radical scavenging activities are undeniable [67,69]. A study by Sutcu et al. found that in addition to the anticancer and antioxidant activity of Mel, it also increased the NMDA (N-methyl-D -aspartic acid) receptor activity in the hippocampus [70].
In addition to showing the structurally protective effects of Mel and ω3, our work also demonstrates the protective effects of ω3 and Mel after EMF exposure on functional outcomes such as learning and memory through the results of the passive avoidance tests. Animals exposed to their neuroprotective agents in addition to EMF experienced no decrease in performance as compared to those with EMF exposure alone. In general, Mel is initially given exogenously, and then physiological Mel release continued [71,72,73].
The other protective compound that we evaluated here is ω3–a fatty acid that has become a subject of a great number of studies, especially due to its protective effects on the cardiovascular system. Although the present study indicates a protective effect of ω3 after EMF exposure, it will be important to explore further studies i.e. electron microscopic, immunohistochemical and biochemical studies.
Biochemical analysis of this study also shows that EMF exposure has a negative effect on the biochemical parameters and this effect can be ameliorated by the application of Mel and ω3. Free radicals, which occur due to EMF exposure, are reactive and unstable substances. The balance between oxidants and antioxidants can be disrupted with the increase in free radicals, and this disruption causes oxidative stress in the biological system [74].
Various antioxidant enzymatic and non-enzymatic systems can block the effects of free radicals. Kim and Rhee found that rats exposed to 2.45 GHz radiation for 6 days, 15 min per day showed evidenced of increased oxidative stress indicators, such as superoxide radicals, lipid peroxidase, oxidized proteins, and lipofuscin levels in heart tissue [75]. İlhan et al. reported that EMF produced by mobile phones decreased GPx activity in brain tissue [76]. Bediz et al. determined that 50 Hz EMF decreased glutathione in the erythrocyte and brains of rats [77] and that EMF emitted by mobile phones decreased antioxidant enzyme activities in the brain tissues of subjects [78].
The results of the present study are consistent with other investigations that have found a protective effect of omega 3 fatty acids. The discovery that an increase in CAT and GSH activities within the EMF group was at the same level with the Mel-injected group indicated that the antioxidant effect of ω3 s was stronger when the Mel free radical scavenger group was compared with the group whose feed contained fish oil ω3. Our results underline the point that DHA is one of the most sensitive components of lipid peroxidation [79]. GSH is an important scavenger of reductase and peroxidase enzymes. GSSG is also formed by the oxidation of reduced GSH. Under oxidative stress, GSH levels decrease, but GSSG levels increase. In normal circumstances, GPx is responsible for the detoxification of hydrogen peroxide in the cell. GPx allows for the degradation to water by binding hydrogen peroxide to reduced GSH. During this reaction, GSH becomes GSSG [31]. In our study, the reason that high amounts of serum GSH were obtained from the EMF and EMF + Mel groups was GSH’s inability to be transformed to GSSG and to bind with hydrogen peroxide. In this case, low amounts of GSH and high amounts of GSSG in the control and EMF + ω3 groups have indicated that the free radical scavenger activity of ω3 was more effective when compared with Mel.
In this study, a significant increase of the SOD activity in the EMF exposed group was observed compared to the control and other groups, EMF + Mel and EMF + ω3 groups. SOD enzyme activity may increase after oxidative stress to scavenge superoxides. Additionally, CAT plays a major role along with GPx in scavenging intracellular hydrogen peroxide. This enzyme is especially higher in the kidney, liver, and erythrocytes than in other organs [80]. An increased amount of SOD, CAT, and GSH in the hippocampal tissues exposed to EMF can be seen in other studies [77,78]. In addition, high CAT and GSH activities in the EMF + Mel and EMF groups may indicate that the level of free radicals is very high, stimulating high amounts of antioxidant enzymes.
Our study also found that a varying duration of a 900-MHz EMF application resulted in no significant difference in most of the EEG waves, with the notable exception of the gamma-1 band; other bands remained unchanged in terms of relative power in response to magnetic field application. The gamma band of the mammalian brain encompasses the fastest frequencies [81] and is generally related to cross-modal sensory processing and short-term memory matching of recognized objects, sounds, or sensations. In this regard, a decrease in gamma bands can be interpreted as a sign of cognitive decline [82,83]. We find an important and significant decline in the EMF exposed groups in general, especially after 15 days of regular applications. Interestingly, it appears that both Mel and ω3 applications block these effects so that results are comparable to those of the Cont group. Specific attention must be also directed to the changes in faster EEG bands in order to evaluate brain effects of such EMF exposure.
In addition, many studies have shown a cell loss in the hippocampus, DG, and cerebellum because of being exposed to a 900 MHz EMF for an hour every day for 15 days of period [1,2,3]. Our study has importantly found neuroprotective effects of Mel and ω3 based on the antioxidant defense system as evidenced through histological and biochemical analyses. Thus, consistent with a number of previous investigations our study finds that EMF from mobile phones induces degenerative effects on neurons of critical components of the brain and that Mel and ω3 separately provide important neuroprotective effects against these damaging impacts through increasing antioxidants.
Declaration of interest
The authors declare that there is no conflict of interest.
Acknowledgements
We would like to thanks to Sarina Scott for language edition. This work was supported by Ondokuz Mayıs University, project management office (PYO. TIP.1904.13.023) and by Environmental Health Trust, USA.
References
- [1].Ferranti M. Father of Cell Phone Eyes a Revolution. Vol. 14. New York Bureau: IDG News Service; 1999. p. 31. [Google Scholar]
- [2].Davis DL, Axelrod D, Bailey L, Gaynor M, Sasco AJ. Rethinking breast cancer risk and the environment: The case for the precautionary principle. Environ Health Persp. 1998;106:523–9. doi: 10.1289/ehp.98106523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bouji M, Lecomte A, Hode Y, de Seze R, Villegier AS. Effects of 900 MHz radiofrequency on corticosterone, emotional memory and neuroinflammation in middle-aged rats. Exp Gerontol. 2012;47:444–51. doi: 10.1016/j.exger.2012.03.015. [DOI] [PubMed] [Google Scholar]
- [4].Cook CM, Saucier DM, Thomas AW, Prato FS. Exposure to ELF magnetic and ELF-modulated radiofrequency fields: the time course of physiological and cognitive effects observed in recent studies (2001–2005) Bioelectromagnetics. 2006;27:613–27. doi: 10.1002/bem.20247. [DOI] [PubMed] [Google Scholar]
- [5].Sonmez OF, Odaci E, Bas O, Kaplan S. Purkinje cell number decreases in the adult female rat cerebellum following exposure to 900 MHz electromagnetic field. Brain Res. 2010;1356:95–101. doi: 10.1016/j.brainres.2010.07.103. [DOI] [PubMed] [Google Scholar]
- [6].Odaci E, Bas O, Kaplan S. Effects of prenatal exposure to a 900 MHz electromagnetic field on the dentate gyrus of rats: a stereological and histopathological study. Brain Res. 2008;1238:224–9. doi: 10.1016/j.brainres.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [7].Bas O, Odaci E, Mollaoglu H, Ucok K, Kaplan S. Chronic prenatal exposure to the 900 megahertz electromagnetic field induces pyramidal cell loss in the hippocampus of newborn rats. Toxicol Ind Health. 2009;25:377–84. doi: 10.1177/0748233709106442. [DOI] [PubMed] [Google Scholar]
- [8].Maskey DKM, Aryal B, Pradhan J, Choi IY, Park KS, Son T, et al. Effect of 835 MHz radio frequency radiation exposure on calcium binding proteins in the hippocampus of the mouse brain. Brain Res Mol Brain Res. 2010;1313:232–41. doi: 10.1016/j.brainres.2009.11.079. [DOI] [PubMed] [Google Scholar]
- [9].Mausset AL, de Seze R, Montpeyroux F, Privat A. Effects of radiofrequency exposure on the GABAergic system in the rat cerebellum: clues from semi-quantitative immunohistochemistry. Brain Res. 2001;912:33–46. doi: 10.1016/s0006-8993(01)02599-9. [DOI] [PubMed] [Google Scholar]
- [10].Nittby H, Brun A, Eberhardt J, Malmgren L, Persson BR, Salford LG. Increased blood-brain barrier permeability in mammalian brain 7 days after exposure to the radiation from a GSM-900 mobile phone. Pathophysiology. 2009;16:103–12. doi: 10.1016/j.pathophys.2009.01.001. [DOI] [PubMed] [Google Scholar]
- [11].Iwasaki Y, Ito S, Suzuki M, Nagahori T, Yamamoto T, Konno H. Forebrain ischemia induced by temporary bilateral common carotid occlusion in normotensive rats. J Neurol Sci. 1989;90:155–65. doi: 10.1016/0022-510x(89)90098-1. [DOI] [PubMed] [Google Scholar]
- [12].Kirson ED, Schneiderman RS, Dbaly V, Tovarys F, Vymazal J, Itzhaki A, et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields) BMC Med Phys. 2009;9:1. doi: 10.1186/1756-6649-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wong ET, Lok E, Swanson KD, Gautam S, Engelhard HH, Lieberman F, et al. Response assessment of NovoTTF-100A versus best physician’s choice chemotherapy in recurrent glioblastoma. Cancer Med. 2014;3:592–602. doi: 10.1002/cam4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ross CL, Teli T, Harrison BS. Effect of electromagnetic field on cyclic adenosine monophosphate (cAMP) in a human mu-opioid receptor cell model. Electromagn Biol Med. 2016;35:206–13. doi: 10.3109/15368378.2015.1043556. [DOI] [PubMed] [Google Scholar]
- [15].Eberhardt JL, Persson BR, Brun AE, Salford LG, Malmgren LO. Blood-brain barrier permeability and nerve cell damage in rat brain 14 and 28 days after exposure to microwaves from GSM mobile phones. Electromagn Biol Med. 2008;27:215–29. doi: 10.1080/15368370802344037. [DOI] [PubMed] [Google Scholar]
- [16].Narayanan SN, Kumar RS, Potu BK, Nayak S, Bhat PG, Mailankot M. Effect of radio-frequency electromagnetic radiations (RF-EMR) on passive avoidance behaviour and hippocampal morphology in Wistar rats. Upsala J Med Sci. 2010;115:91–6. doi: 10.3109/03009730903552661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu YX, Tai JL, Li GQ, Zhang ZW, Xue JH, Liu HS, et al. Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS One. 2012;7:e42332. doi: 10.1371/journal.pone.0042332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- [19].Gorostizaga A, Mori Sequeiros Garcia MM, Acquier A, Gomez NV, Maloberti PM, Mendez CF, et al. Modulation of albumin-induced endoplasmic reticulum stress in renal proximal tubule cells by upregulation of mapk phosphatase-1. Chem Biol Interact. 2013;206:47–54. doi: 10.1016/j.cbi.2013.08.009. [DOI] [PubMed] [Google Scholar]
- [20].Friedman J, Kraus S, Hauptman Y, Schiff Y, Seger R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem J. 2007;405:559–68. doi: 10.1042/BJ20061653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tang J, Zhang Y, Yang L, Chen Q, Tan L, Zuo S, et al. Exposure to 900 MHz electromagnetic fields activates the mkp-1/ERK pathway and causes blood-brain barrier damage and cognitive impairment in rats. Brain Res. 2015;1601:92–101. doi: 10.1016/j.brainres.2015.01.019. [DOI] [PubMed] [Google Scholar]
- [22].Tang JZY, Yang L, Chen Q, Tan L, Zuo S, Feng H, et al. Exposure to 900 MHz electromagnetic fields activates the mkp1/ERK pathway and causes blood brain barrier damage and cognitive impairment in rats. Brain Res. 2015;1601:92–101. doi: 10.1016/j.brainres.2015.01.019. [DOI] [PubMed] [Google Scholar]
- [23].Kaplan S, Deniz OG, Onger ME, Turkmen AP, Yurt KK, Aydin I, et al. Electromagnetic field and brain development. J Chem Neuroanat. 2016;75(Pt B):52–61. doi: 10.1016/j.jchemneu.2015.11.005. [DOI] [PubMed] [Google Scholar]
- [24].Yu BP. Approaches to anti-aging intervention: the promises and the uncertainties. Mech Ageing Dev. 1999;111:73–87. doi: 10.1016/s0047-6374(99)00072-x. [DOI] [PubMed] [Google Scholar]
- [25].Sefidbakht Y, Moosavi-Movahedi AA, Hosseinkhani S, Khodagholi F, Torkzadeh-Mahani M, Fooladd F, et al. Effects of 940 MHz EMF on bioluminescence and oxidative response of stable luciferase producing HEK cells. Photochem Photobiol Sci. 2014;13:1082–92. doi: 10.1039/c3pp50451d. [DOI] [PubMed] [Google Scholar]
- [26].Halliwell B, Gutteridge JMC. Oxygen free-radicals and iron in relation to biology and medicine–some problems and concepts. Arch Biochem Biophys. 1986;246:501–14. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- [27].Kamath U, Rao G, Raghothama C, Rai L, Rao P. Erythrocyte indicators of oxidative stress in gestational diabetes. Acta Paediatr. 1998;87:676–9. doi: 10.1080/080352598750014102. [DOI] [PubMed] [Google Scholar]
- [28].Sinclair AJ, Barnett AH, Lunec J. Free-radicals and antioxidant systems in health and disease. Brit J Hosp Med. 1990;43:334. [PubMed] [Google Scholar]
- [29].Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–28. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- [30].Kono Y, Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus-plantarum – a new manganese-containing enzyme. J Biol Chem. 1983;258:6015–9. [PubMed] [Google Scholar]
- [31].Pajovic SB, Saicic ZS, Spasic MB, Petrovic VM, Martinovic JV. Effects of progesterone and estradiol benzoate on glutathione dependent antioxidant enzyme activities in the brain of female rats. Gen Physiol Biophys. 1999;18:35–44. [PubMed] [Google Scholar]
- [32].Longoni B, Salgo MG, Pryor WA, Marchiafava PL. Effects of melatonin on lipid peroxidation induced by oxygen radicals. Life Sci. 1998;62:853–9. doi: 10.1016/s0024-3205(98)00002-2. [DOI] [PubMed] [Google Scholar]
- [33].Hataya Y, Akamizu T, Takaya K, Kanamoto N, Ariyasu H, Saijo M, et al. A low dose of ghrelin stimulates growth hormone (GH) release synergistically with GH-releasing hormone in humans. J Clin Endocr Metab. 2001;86:4552–5. doi: 10.1210/jcem.86.9.8002. [DOI] [PubMed] [Google Scholar]
- [34].Reiter RJ. Functional aspects of the pineal hormone melatonin in combating cell and tissue damage induced by free radicals. Eur J Endocrinol. 1996;134:412–20. doi: 10.1530/eje.0.1340412. [DOI] [PubMed] [Google Scholar]
- [35].Ianas O, Olinescu R, Badescu I. Melatonin involvement in oxidative processes. Endocrinologie. 1991;29:147–53. [PubMed] [Google Scholar]
- [36].Zhang WT, Li PY, Hu XM, Zhang F, Chen J, Gao YQ. Omega-3 polyunsaturated fatty acids in the brain: metabolism and neuroprotection. Front Biosci-Landmrk. 2011;16:2653–70. doi: 10.2741/3878. [DOI] [PubMed] [Google Scholar]
- [37].Bousquet M, Calon F, Cicchetti F. Impact of omega-3 fatty acids in Parkinson’s disease. Ageing Res Rev. 2011;10:453–63. doi: 10.1016/j.arr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- [38].West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- [39].Sonmez OF, Odaci E, Bas O, Kaplan S. Purkinje cell number decreases in the adult female rat cerebellum following exposure to 900 MHz electromagnetic field. Brain Res. 2010;1356:95–101. doi: 10.1016/j.brainres.2010.07.103. [DOI] [PubMed] [Google Scholar]
- [40].Odaci E, Bas O, Kaplan S. Effects of prenatal exposure to a 900 MH zelectromagnetic field on the dentate gyrus of rats: a stereological and histopathological study. Brain Res. 2008;1238:224–9. doi: 10.1016/j.brainres.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [41].Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci. 1999;50:271–9. doi: 10.1093/toxsci/50.2.271. [DOI] [PubMed] [Google Scholar]
- [42].Koyu A, Ozguner F, Cesur G, Gokalp O, Mollaglu H, Caliskan S, et al. No effects of 900 MHz and 1800 MHz electromagnetic field emitted from cellular phone on nocturnal serum melatonin levels in rats. Toxicol Ind Health. 2005;21:27–31. doi: 10.1191/0748233705th212oa. [DOI] [PubMed] [Google Scholar]
- [43].Ozguner F, Altinbas A, Ozaydin M, Dogan A, Vural H, Kisioglu AN, et al. Mobile phone-induced myocardial oxidative stress: protection by a novel antioxidant agent caffeic acid phenethyl ester. Toxicol Ind Health. 2005;21:223–30. doi: 10.1191/0748233705th228oa. [DOI] [PubMed] [Google Scholar]
- [44].Yildiz M, Cicek E, Cerci SS, Cerci C, Oral B, Koyu A. Influence of electromagnetic fields and protective effect of CAPE on bone mineral density in rats. Arch Med Res. 2006;37:818–21. doi: 10.1016/j.arcmed.2006.03.006. [DOI] [PubMed] [Google Scholar]
- [45].Sirav B, Seyhan N. Effects of radiofrequency radiation exposure on blood-brain barrier permeability in male and female rats. Electromagn Biol Med. 2011;30:253–60. doi: 10.3109/15368378.2011.600167. [DOI] [PubMed] [Google Scholar]
- [46].Gundersen HJG. Stereology of arbitrary particles–a review of unbiased number and size estimators and the presentation of some new ones, in memory of Thompson, William R. J Microsc-Oxford. 1986;143:3–45. [PubMed] [Google Scholar]
- [47].Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- [48].Terzi M, Ozberk B, Deniz OG, Kaplan S. The role of electromagnetic fields in neurological disorders. J Chem Neuroanat. 2016;75(Pt B):77–84. doi: 10.1016/j.jchemneu.2016.04.003. [DOI] [PubMed] [Google Scholar]
- [49].Lai H. Spatial learning deficit in the rat after exposure to a 60 Hz magnetic field. Bioelectromagnetics. 1996;17:494–6. doi: 10.1002/(SICI)1521-186X(1996)17:6<494::AID-BEM9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [50].Lai H, Carino M. Intracerebroventricular injection of mu- and delta-opiate receptor antagonists block 60 Hz magnetic field-induced decreases in cholinergic activity in the frontal cortex and hippocampus of the rat. Bioelectromagnetics. 1998;19:432–7. [PubMed] [Google Scholar]
- [51].Lai H, Carino MA, Horita A, Guy AW. Opioid receptor subtypes that mediate a microwave-induced decrease in central cholinergic activity in the rat. Bioelectromagnetics. 1992;13:237–46. doi: 10.1002/bem.2250130308. [DOI] [PubMed] [Google Scholar]
- [52].Aldad TS, Gan G, Gao XB, Taylor HS. Fetal radiofrequency radiation exposure from 800-1900MHz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep. 2012;2:312. doi: 10.1038/srep00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lai H, Singh NP. Melatonin and a spin-trap compound block radiofrequency electromagnetic radiation-induced DNA strand breaks in rat brain cells. Bioelectromagnetics. 1997;18:446–54. doi: 10.1002/(sici)1521-186x(1997)18:6<446::aid-bem7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [54].Robison JG, Pendleton AR, Monson KO, Murray BK, O’Neill KL. Decreased DNA repair rates and protection from heat induced apoptosis mediated by electromagnetic field exposure. Bioelectromagnetics. 2002;23:106–12. doi: 10.1002/bem.103. [DOI] [PubMed] [Google Scholar]
- [55].Boulton AA. Symptomatic and neuroprotective properties of the aliphatic propargylamines. Mech Ageing Dev. 1999;111:201–9. doi: 10.1016/s0047-6374(99)00073-1. [DOI] [PubMed] [Google Scholar]
- [56].Kovacic P, Pozos RS. Cell signaling (mechanism and reproductive toxicity): redox chains, radicals, electrons, relays, conduit, electrochemistry, and other medical implications. Birth Defects Res C Embryo Today. 2006;78:333–44. doi: 10.1002/bdrc.20083. [DOI] [PubMed] [Google Scholar]
- [57].Hashish AH, El-Missiry MA, Abdelkader HI, Abou-Saleh RH. Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol Environ Saf. 2008;71:895–902. doi: 10.1016/j.ecoenv.2007.10.002. [DOI] [PubMed] [Google Scholar]
- [58].Franco R, Sanchez-Olea R, Reyes-Reyes EM, Panayiotidis MI. Environmental toxicity, oxidative stress and apoptosis: menage a trois. Mutat Res. 2009;674:3–22. doi: 10.1016/j.mrgentox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- [59].Vejux A, Lizard G. Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Aspects Med. 2009;30:153–70. doi: 10.1016/j.mam.2009.02.006. [DOI] [PubMed] [Google Scholar]
- [60].Ikinci A, Mercantepe T, Unal D, Erol HS, Sahin A, Aslan A, et al. Morphological and antioxidant impairments in the spinal cord of male offspring rats following exposure to a continuous 900 MHz electromagnetic field during early and mid-adolescence. J Chem Neuroanat. 2016;75(Pt B):99–104. doi: 10.1016/j.jchemneu.2015.11.006. [DOI] [PubMed] [Google Scholar]
- [61].Bolbanabad HM, KMR, Fatehi D, Rostamzadeh A. Effects of cell phone radiation on migration of granule cells in rat cerebellum. J Bas Res Med Sci. 2014;1:15–22. [Google Scholar]
- [62].He YL, Liu DD, Fang YJ, Zhan XQ, Yao JJ, Mei YA. Exposure to extremely low-frequency electromagnetic fields modulates Na+ currents in rat cerebellar granule cells through increase of AA/PGE(2) and EP receptor-Mediated cAMP/PKA pathway. PLoS One. 2013:8. doi: 10.1371/journal.pone.0054376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–65. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ramundo-Orlando A, Morbiducci U, Mossa G, D’ Inzeo G. Effect of low frequency, low amplitude magnetic fields on the permeability of cationic liposomes entrapping carbonic anhydrase I. Evidence for charged lipid involvement. Bioelectromagnetics. 2000;21:491–8. doi: 10.1002/1521-186x(200010)21:7<491::aid-bem2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- [65].Ragbetli MC, Aydinlioglu A, Koyun N, Ragbetli C, Bektas S, Ozdemir S. The effect of mobile phone on the number of Purkinje cells: a stereological study. Int J Radiat Biol. 2010;86:548–54. doi: 10.3109/09553001003734527. [DOI] [PubMed] [Google Scholar]
- [66].Stamatakis A, Barbas H, Dermon CR. Late granule cell genesis in quail cerebellum. J Comp Neurol. 2004;474:173–89. doi: 10.1002/cne.20066. [DOI] [PubMed] [Google Scholar]
- [67].Kus I, Akpolat N, Ozen OA, Songur A, Kavakli A, Sarsilmaz M. Effects of melatonin on Leydig cells in pinealectomized rat: an immunohistochemical study. Acta Histochem. 2002;104:93–7. doi: 10.1078/0065-1281-00618. [DOI] [PubMed] [Google Scholar]
- [68].Reiter RJ. Oxidative processes and antioxidative defense-Mechanisms in the aging brain. FASEB J. 1995;9:526–33. [PubMed] [Google Scholar]
- [69].Kavakli A, Sahna E, Parlakpinar H, Yahsi S, Ogeturk M, Acet A. The effects of melatonin on focal cerebral ischemia-reperfusion model. Saudi Med J. 2004;25:1751–2. [PubMed] [Google Scholar]
- [70].Sutcu R, Yonden Z, Yilmaz A, Delibas N. Melatonin increases NMDA receptor subunits 2A and 2B concentrations in rat hippocampus. Mol Cell Biochem. 2006;283:101–5. doi: 10.1007/s11010-006-2385-4. [DOI] [PubMed] [Google Scholar]
- [71].Rison RA, Stanton PK. Long-term potentiation and N-methyl-D-aspartate receptors-foundations of memory and neurologic disease. Neurosci Biobehav R. 1995;19:533–52. doi: 10.1016/0149-7634(95)00017-8. [DOI] [PubMed] [Google Scholar]
- [72].Stanton PK. LTD, LTP, and the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6:35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [73].Marti SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- [74].Moustafa YM, Moustafa RM, Belacy A, Abou-El-Ela SH, Ali FM. Effects of acute exposure to the radiofrequency fields of cellular phones on plasma lipid peroxide and antioxidase activities in human erythrocytes. J Pharmaceut Biomed. 2001;26:605–8. doi: 10.1016/s0731-7085(01)00492-7. [DOI] [PubMed] [Google Scholar]
- [75].Kim MJ, Rhee SJ. Green tea catechins protect rats from microwave-induced oxidative damage to heart tissue. J Med Food. 2004;7:299–304. doi: 10.1089/jmf.2004.7.299. [DOI] [PubMed] [Google Scholar]
- [76].Ilhan A, Gurel A, Armutcu F, Kamisli S, Iraz M, Akyol O, et al. Ginkgo biloba prevents mobile phone-induced oxidative stress in rat brain. Clin Chim Acta. 2004;340:153–62. doi: 10.1016/j.cccn.2003.10.012. [DOI] [PubMed] [Google Scholar]
- [77].Meral I, Mert H, Mert N, Deger Y, Yoruk I, Yetkin A, et al. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res. 2007;1169:120–4. doi: 10.1016/j.brainres.2007.07.015. [DOI] [PubMed] [Google Scholar]
- [78].Bediz CS, Baltaci AK, Mogulkoc R, Oztekin E. Zinc supplementation ameliorates electromagnetic field-induced lipid peroxidation in the rat brain. Tohoku J Exp Med. 2006;208:133–40. doi: 10.1620/tjem.208.133. [DOI] [PubMed] [Google Scholar]
- [79].Gutteridg JMC. Lipid-peroxidation and antioxidants as biomarkers of tissue-damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- [80].Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med. 2006;36:327–58. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- [81].Pockett S, Bold GEJ, Freeman WJ. EEG synchrony during a perceptual-cognitive task: widespread phase synchrony at all frequencies. Clin Neurophysiol. 2009;120:695–708. doi: 10.1016/j.clinph.2008.12.044. [DOI] [PubMed] [Google Scholar]
- [82].Kisley MA, Cornwell ZM. Gamma and beta neural activity evoked during a sensory gating paradigm: effects of auditory, somatosensory and cross-modal stimulation. Clin Neurophysiol. 2006;117:2549–63. doi: 10.1016/j.clinph.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kanayama NSA, Ohira H. Cross modal effect with rubber hand illusion and gamma-band activity. Psychophysiology. 2007;44:392–402. doi: 10.1111/j.1469-8986.2007.00511.x. [DOI] [PubMed] [Google Scholar]