Abstract
Diffuse intrinsic pontine glioma is a lethal brain cancer that arises in the pons of children. The median survival for children with diffuse intrinsic pontine glioma is less than 1 year from diagnosis, and no improvement in survival has been realized in more than 30 years. Currently, the standard of care for diffuse intrinsic pontine glioma is focal radiation therapy, which provides only temporary relief. Recent genomic analysis of tumors from biopsies and autopsies, have resulted in the discovery of K27M H3.3/H3. 1 mutations in 80% and ACVRI mutations in 25% of diffuse intrinsic pontine gliomas, providing renewed hope for future success in identifying effective therapies. In addition, as stereotactic tumor biopsies at diagnosis at specialized centers have been demonstrated to be safe, biopsies have now been incorporated into several prospective clinical trials. This article summarizes the epidemiology, clinical presentation, diagnosis, prognosis, molecular genetics, current treatment, and future therapeutic directions for diffuse intrinsic pontine glioma.
Keywords: diffuse intrinsic pontine glioma, brainstem glioma, ACVRI, K27M
Diffuse intrinsic pontine glioma is a fatal brain cancer that originates in the pons of mostly children. Despite numerous efforts to improve treatment, prognosis remains poor, with more than 90% of children dying within 2 years of diagnosis, making it one of the major causes of brain-related death in children.1–3 Radiation therapy remains the only treatment with proven but temporary benefit.4 Genomic analysis of diffuse intrinsic pontine glioma tissue obtained both at diagnosis and at postmortem has unraveled the genomic landscape including the identification of novel drivers of diffuse intrinsic pontine glioma pathogenesis.5–10 In particular, the discovery of the gain of function K27M histone mutations in H3.3 or H3.1 found in approximately 80% of human diffuse intrinsic pontine gliomas (as well as other midline gliomas) suggest that finding a way to reverse the mechanism(s) by which these mutations promote diffuse intrinsic pontine glioma pathogenesis may lead to the identification of improved therapies.5,6,11,12 This article summarizes the epidemiology, clinical presentation, diagnosis, prognosis, molecular genetics, and treatment of diffuse intrinsic pontine glioma. Table 1 outlines the highlights of this article. The 2 patient cases illustrate the classic presenting symptoms of diffuse intrinsic pontine glioma, the corresponding neuroimaging findings, and current treatment options as part of ongoing clinical trials (case 2). Lastly we discuss future therapeutic directions.
Table 1.
Article Highlights.
| • | DIPG is a fatal brain cancer that arises in the brainstem of children. |
| • | Can occur in all ages, but predominates in children. Median age is approximately 7 years. |
| • | Dismal prognosis. Median survival is less than 1 year from diagnosis. |
| • | Diagnosis criteria typically accepted include symptom duration less than 6 months, at least 2 or 3 symptoms related to brainstem dysfunction, and pontine enlargement with evidence of diffusely infiltrative tumor centered in and involving greater than 50%−66% of the pons. |
| • | Radiation therapy is the standard of care but provides only temporary relief. |
| • | Stereotactic biopsies at specialized centers can be performed safely although still not the standard of care in the United States. |
| • | The discovery of K27M histone H3 and ACVRI mutations has led to new hope for effective targeted treatments. |
| • | Clinical trials are ongoing with hopes to discover a treatment regime that will improve overall survival. |
Abbreviation: DIPG, diffuse intrinsic pontine glioma.
Epidemiology
Brain tumors are the largest group of solid tumors and the leading cause of cancer-related deaths in childhood.8,13 Brainstem gliomas comprise 12.5% of primary brain and central nervous system tumors in children aged 0 to 14.14 Approximately 80% of pediatric brainstem tumors arise in the pons. Diffuse intrinsic pontine glioma can arise in all ages, but it predominates in children, with approximately 200 to 300 new diagnoses in the United States each year.15,16 Most diffuse intrinsic pontine glioma patients are of school age (median age is approximately 7 years). Diffuse intrinsic pontine gliomas do not show a predilection for either sex.17
Clinical Presentation
The classic symptom triad includes cranial nerve palsies (especially sixth and seventh nerve palsies), long tract signs (hyperreflexia, clonus, increased tone, presence of a Babinski reflex), and ataxia.4,16,18 The first symptom is typically a sixth nerve palsy resulting in esotropia and diplopia, followed by other commonly reported symptoms including an asymmetric smile, clumsiness, difficulty walking, loss of balance, and weakness. 16 Signs and symptoms of increased intracranial pressure occur in approximately one-third of patients at the time of diagnosis due to obstructive hydrocephalus resulting from expansion of the pons.16 Patients with diffuse intrinsic pontine gliomas typically have a rapid onset of symptoms and are usually diagnosed in less than 3 months from onset of symptoms. Symptom duration greater than 6 months prior to presentation should trigger an investigation of an alternative diagnosis.4
Case 1
An 8-year-old girl presented with a 2-week history of diplopia, left-sided ptosis, drooling, dysarthria, ataxia, and headaches for several months. A computed tomography head scan obtained in the emergency room showed a mass involving the brainstem with compression of the fourth ventricle and the cerebral aqueduct resulting in mild hydrocephalus. She was started on Dex-amethasone with some improvement of neurologic symptoms. Magnetic resonance imaging (MRI) of the brain showed a large mass expanding the pons (Figure 1A-C). Because of the location of the mass, surgical resection was not recommended. Secondary to the poor prognosis and toxicities associated with radiation therapy and chemotherapy, the family decided against any treatment and chose to maximize her quality of life. She continued with symptomatic management and passed away 4 months after the diagnosis.
Figure 1.
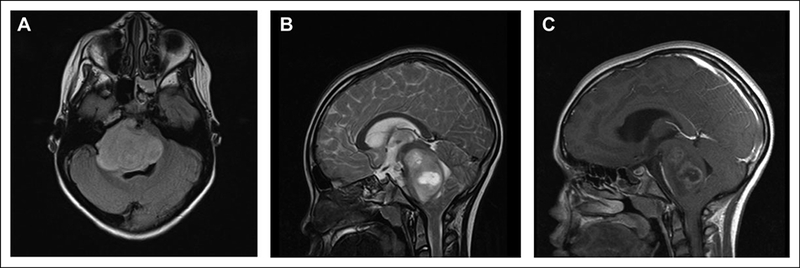
Imaging of the patient in case 1. Magnetic resonance imaging (MRI) of diffuse intrinsic pontine glioma. (A) Axial fluid-attenuated inversion recovery and (B) sagittal T2-weighted images show a large mass expanding the pons with portions extending into the middle cerebellar peduncles, midbrain, and medulla. (C) Sagittal T1-weighted post-contrast image illustrates a focal heterogeneous component with peripheral enhancement within the central portions of the pontine mass.
Case 2
A 2-year-old boy presented with ataxia and left lower extremity weakness for 2 weeks and right sixth cranial nerve palsy for 1 week. An MRI of the brain demonstrated an infiltrating mass expanding the pons, primarily centered in the right pons with extension to the right middle cerebellar peduncle (Figure 2A and B). He was enrolled in an ongoing phase II clinical trial through the Pediatric Brain Tumor Consortium (PBTC) evaluating whether veliparib, an oral poly ADP-ribose polymerase (PARP) inhibitor administered with concurrent radiation therapy, followed by veliparib in combination temozolomide (an FDA-approved drug for glioblastoma in adults but not children) can prolong the survival of children with diffuse intrinsic pontine glioma. He received a total of 54 Gray (Gy) in 180 centigray (cGy) fractions daily, Monday through Friday, over 6 weeks. Brain MRI obtained 1 month after the completion of radiation therapy showed a significant decrease in the size of the infiltrative pontine mass. He continued maintenance chemotherapy with veliparib and temozolomide and had a subsequent stable MRI scan 2 months later. Within the following 2 months, he then began to have worsening ataxia, worsening right sixth cranial nerve palsy, headaches, decreased appetite, emotional lability, and difficulty sleeping. A brain MRI showed a stable mass in the right pons with a new area of enhancement (Figure 2C). He was taken off the trial because of clinical and radiologic evidence of progressive disease. Interestingly, the new enhancement seen on MRI may have been induced by the radiation, as it was not present on a follow-up scan 2 months later. At 1.5 years following initial diagnosis, he began experiencing further worsening of ataxia with frequent falls, worsening left lower extremity weakness, new left arm weakness, right-sided facial droop, drooling, dysarthria, worsening right sixth cranial nerve palsy, and dysphagia and subsequently passed away less than 1 month later.
Figure 2.
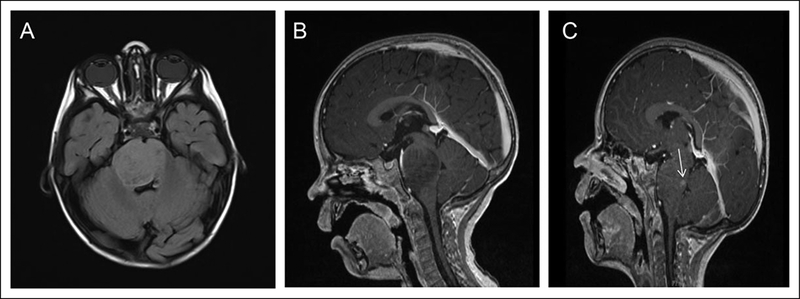
Imaging of the patient in case 2. Magnetic resonance imaging (MRI) of diffuse intrinsic pontine glioma. (A) Axial fluid-attenuated inversion recovery showing a mass like abnormal signal involving the pons and right middle cerebellar peduncle with mild effacement of the fourth ventricle. (B) Sagittal T1 + contrast image showing a nonenhancing infiltrating mass expanding the pons. (C) Sagittal T1 + contrast image illustrating a new area of enhancement in the pons posteriorly (white arrow).
Diagnosis
Although universally accepted diagnostic criteria have not been defined,2,4,19 criteriatypically acceptedinclude symptom duration less than 6 months, at least 2 or 3 symptoms related to brainstem dysfunction, and pontine enlargement with evidence of diffusely infiltrative tumor centered in and involving greater than 50% to 66% of the pons.2,4,20 Diffuse intrinsic pontine glioma classically manifests as a large expansile brainstem mass involving the majority of the pons. Infiltration into the midbrain, bilateral tha-lami, and cerebellar peduncles is common. The medulla, however, is typically not involved at presentation; hence, a clear pontomedullary demarcation on sagittal imaging is a classic finding.4
On computed tomography (CT) head scan, diffuse intrinsic pontine glioma appears isodense or hypodense, without calcifications. On brain MRI, diffuse intrinsic pontine glioma is typically hypointense on T1 and shows diffuse bright signal on T2-weighted and fluid-attenuated inversion recovery imaging. Contrast enhancement is variable in both modalities, and frequently does not significantly enhance at diagnosis.16,17 Engulfment or displacement of the basilar artery by the engorged pons is common.4 Magnetic resonance spectroscopy shows a modest increase in choline levels and a decrease in N-acetylaspartate levels.15 Petechial hemorrhages are common but are rarely neurologically significant and are best identified by susceptibility-weighted imaging.17,21 Necrosis can be present. Obstructive hydrocephalus can occur, which may require a ventriculoperitoneal shunt or an endoscopic third ventriculostomy. On pathologic examination either at biopsy or autopsy, diffuse intrinsic pontine gliomas range from World Health Organization (WHO) grade II to IV. However, these tumors are almost always highly malignant, and WHO grade classification in this disease has no prognostic significance.17,22
Neuraxis dissemination at diagnosis can occur but is uncommon. Patients should have spinal imaging at diagnosis if there are any clinical symptoms to suggest disseminated disease.17,23 By contrast, pathologic analysis at autopsy demonstrates that neuroaxis dissemination is quite common.22,24 It is not clear if microscopic neuroaxis dissemination outside of the pons is present at diagnosis or arises as part of the disease course. In the United States, biopsies are not the standard of care in the management of children with diffuse intrinsic pontine glioma unless atypical signs are present. Interestingly, the rates of biopsies performed on diffuse intrinsic pontine gliomas have increased since the initial report demonstrating that biopsies can be safely performed,25 and now brainstem biopsies have been incorporated into several prospective clinical trials.4 Several studies have revealed a high success rate and low complication rate of biopsy in centers with expertise in perorming the procedure.17,26–29 Areas of T2 hypointensity that are contrast-enhancing and demonstrate restricted diffusion may represent areas of focal anaplasia that are good targets for stereotactic biopsy.17,30
The differential diagnosis for diffuse intrinsic pontine gliomas includes other brainstem gliomas: focal midbrain, dorsally exophytic, or cervicomedullary. Growth patterns vary according to location and anatomic barriers, with focal midbrain tumors usually remaining circumscribed in the tegmentum and tectal plate, dorsal exophytic tumors growing into the fourth ventricle, and cervicomedullary tumors originating in the upper cervical cord with caudal cylindrical growth and rostral growth toward the obex.31,32 Additionally, postmortem evaluation has revealed a histologic diagnosis of primitive neuroectodermal tumors in as many as 22% of patients thought to have diffuse intrinsic pontine glioma.4,33 Other diseases, including demyelinating, vascular, or infectious diseases (such as infectious rhombencephalitis), can involve predominately the pons, with imaging features that may mimic diffuse intrinsic pontine glioma.4,34
Prognosis
The prognosis for children with diffuse intrinsic pontine gliomas is significantly worse than that of other brainstem tumors and is near 100% fatal.8,16 Diffuse intrinsic pontine gliomas are nowthe main cause of brain tumor-related death in children.8 Without radiation, median survival is approximately 4 months.4,35 Subsequent tumor progression is almost universal, with median survival between 8 and 11 months, and overall survival of approximately 30% at 1 year and less than 10% at 2 years.2,4,20 Rare (2%−3%) long-term survival has been reported, usually associated with atypical imaging and clinical features at presentation.4,19,36 Pathologic grade does not affect prognosis.17,22 Among findings on conventional MRI, only focal contrast enhancement has been found to hold prognostic significance, predicting poorer overall survival, in some but not all studies.17,37,38 Other MRI techniques may also help with prognostication. Apparent diffusion coefficient values from diffusion-weighted MRI may identify prognos-tically distinct subgroups of diffuse intrinsic pontine glioma.39 Magnetic resonance spectroscopy detection of lactate and high choline/N-acetylaspartate ratio were found to be poor prognostic factors at diagnosis.40,41 Lastly, retrospective analysis has demonstrated that the presence of the histone mutation is a poor prognostic factor, but this has not been validated prospectively.11,22
Molecular Genetics
The importance of understanding the biology of diffuse intrinsic pontine glioma has been brought to the forefront with the successful development of effective molecularly targeted agents for other cancers.16 Historically, biopsies were not regularly performed on diffuse intrinsic pontine glioma, because diagnosis on MRI was found to be reliable and a histological diagnosis would not change therapy for the individual patient.42 Initially, the primary source of tissue for biologic investigation has been postmortem autopsy samples, which may not accurately reflect the biology of these tumors at diagnosis.4 Although the potential for disparity between the molecular characteristics of primary untreated tumor and the characteristics of posttreatment terminal disease exists as a result of the accumulation of additional mutations induced by radiation and chemotherapy, an extensive analysis of a large number of matched samples (pretreatment and postmortem) have not been performed in diffuse intrinsic pontine glioma.4,43,44 Interestingly, Taylor et al45 observed that while the mutational spectrum of the untreated biopsy samples was not significantly different from that of the autopsies, the treatment-naïve samples had a significantly lower overall mutation rate (a mean of 14.8 single nucleotide variants per sample) relative to that observed in radiation-treated autopsy cases (a mean of 32 single nucleotide variants per sample). The potential of molecular analysis as a guide to personalized medicine have resulted in new efforts to obtain pretreatment samples at the time of diagnosis. Recently, specialized centers have demonstrated that, with current techniques, surgical biopsy is safe and feasible, allowing procurement of adequate tissue for meaningful analysis with low morbidity.4,46 One potential limitation of a biopsy is that it is a small sample and may not accurately represent the entire tumor because of the inherent heterogeneity of genetic alterations in diffuse intrinsic pontine glioma tumor cells.43
With the advent of exome sequencing, studies have identified highly recurrent mutations in genes encoding the histone variants H3.3 (H3F3A) and H3.1 (HIST1H3B) in approximately 80% of human diffuse intrinsic pontine gliomas. These mutations result in an amino acid substitution conferring a change in lysine to methionine at position 27 on the histone tail (K27M).5,6,11 This lysine-to-methionine substitution confers global loss of trimethylation at lysine 27 of histone H3 (H3K27me3), which profoundly alters gene expression as H3K27me3 is a histone mark, which negatively correlates with gene expression. Elegant studies have demonstrated that the K27M mutation inhibits enhancer of zeste homolog 2 (EZH2), the catalytic subunit of the Polycomb repressive complex 2 (PRC2), an H3K27 methyltransferase complex.47–49 More recently, 4 independent groups identified recurrent somatic activating mutations in the activin A receptor, type 1 (ACVR1) gene in 20%−32% of diffuse intrinsic pontine gliomas. The mutations were found in either the glycine-serine-rich domain (R206H, Q207E) or protein kinase domain (R258G, G328E/V/ W, G356D) of ACVR1. This provides strong evidence that the ACVR1 gene, which encodes a receptor in the bone morphogenetic protein (BMP) pathway, is an oncogenic driver in this disease.7–9,45 Patients harboring ACVR1 mutations were predominantly female (approximately 2:1) and had a younger age of onset (approximately 5 years) and longer overall survival time (approximately 15 months) compared with wild-type tumors.7,9,10,45 ACVR1 mutations also strongly cosegregated with K27M mutations in the gene encoding histone H3.1 (HIST1H3B), which represent approximately 20% of diffuse intrinsic pontine glioma.10
Some of the somatic mutations in ACVR1 (also called ALK2) identified in diffuse intrinsic pontine glioma50 are identical to mutations found in the germ line of individuals with the congenital childhood developmental disorder fibrodyspla-sia ossificans progressiva (FOP) (MIM 135100).45,51 This debilitating disease is characterized by heterotopic ossification of soft tissue resulting in several skeletal abnormalities.45,52 These mutations have been shown to constitutively activate the BMP pathway in the absence of ligand binding, as evidenced by increased SMAD1 and SMAD5 phosphorylation.45,53–55 Interestingly, the most common ACVR1 mutation identified in 28% of diffuse intrinsic pontine gliomas (G328V) has not been reported in FOP.10 In addition, there are no known reports of coincident FOP and diffuse intrinsic pontine glioma, suggesting that mutant ACVR1 is not sufficient to induce diffuse intrinsic pontine glioma. However, in experimental models of FOP, ACVR1 has been reported to be a key regulator of cell fate decisions, which are also critical in cancer.45,51
In addition to the recently identified mutations in histone 3 and ACVR1, diffuse intrinsic pontine glioma also harbors abnormalities in drivers of gliomagenesis that are common between adult and childhood gliomas. These include mutations in the DNA damage response in approximately two-thirds of tumors (p53 mutations or PPM1D mutations), mutations in the Receptor Tyrosine Kinase-RAS-Phosphoinositide 3-Kinase (RTK-RAS-PI3K) pathways in two-thirds of tumors with PDGFR-A as the most commonly altered RTK, and abnormalities in cell cycle regulation in 30% (amplifications of CDK4/6 and/or cyclins D1–3).7–9,43,45 Interestingly, deletion of the CDKN2a locus (locus for ink4a and arf)—a common genetic alteration in adult gliomas and pediatric high-grade gliomas that arise in the cerebral cortex—is extremely rare in diffuse intrinsic pontine glioma.43 This may be due to K27M mutations, which appear to be mostly mutually exclusive of CDKN2a deletions and may alter the levels of Ink4a through an epigenetic mechanism.48,56
Another aspect of molecular genetics is whether the genomic studies suggest that diffuse intrinsic pontine glioma is one disease or composed of several subtypes. Interestingly, several groups have reported that diffuse intrinsic pontine glioma is not one homogeneous disease but composed of several subtypes. The first publication of diffuse intrinsic pontine glioma subgrouping used unsupervised hierarchic clustering of expression profiling data of mostly postmortem samples and noted the existence of 3 expression subgroups that were labeled as mesenchymal, proliferative, and proneural subgroups because of the similarity to prior classifications in adult and pediatric high-grade gliomas.43 By contrast, another study also used an unsupervised approach of the expression profiles of pretreatment samples and classified diffuse intrinsic pontine gliomas into an oligodendroglial (expression profile most similar to proneural group of adult GBM) subgroup and mesenchymal subgroup.57 The oligodendroglial subgroup, which was enriched for PDGFRA alterations, had a significantly shorter survival. Other groups used proteomics and DNA methylation arrays to subgroup diffuse intrinsic pontine gliomas. Unsupervised proteomics analysis subdivided diffuse intrinsic pontine gliomas into a Myc and hedgehog subgroups whereas unsupervised analysis of CpG island methylation analysis subgrouped diffuse intrinsic pontine gliomas into a MycN, silent, and H3-K27M subgroups.8,58 In conclusion, there is no consensus yet on diffuse intrinsic pontine glioma subgrouping, and from a translational standpoint, subgrouping that yield biomarkers for prognosis or therapeutic interventions would likely be most useful.
Treatment
Surgical resection of diffuse intrinsic pontine gliomas is not possible because of their anatomic location, as diffuse intrinsic pontine glioma tumor cells infiltrate around pontine nuclei that are critical for survival. The only treatment that can prolong survival but is not curative is radiation therapy. Currently, the standard of care is focal radiation therapy to the tumor, plus a 1-to 2-cm surrounding margin. A total of 54 to 60 Gy in 180- to 200-cGy fractions daily, Monday through Friday, is administered over 6 weeks, which results in clinical improvement in up to 70% and objective tumor response in 40% to 60% 4,42 Radiation therapy appears to control tumor growth for a short period of time, prolonging survival by a mean of approximately 3 months.16,59 Glucocorticoids are frequently administered in an effort to reduce and control edema associated with the tumor and radiation treatment.16 Because diffuse intrinsic pontine glioma is rapidly fatal, most children do not survive for an extended period to manifest long-term complications of radia-tion.15 Higher doses of radiation, up to 7000 cGy, have resulted in increased toxicity without any apparent improvement in out-come.4,60 No benefit has been found with hyperfractionation versus conventional radiation.4,61 Conversely, hypofractionation (over 3–4 weeks) may be used to decrease toxicity without compromising overall survival.15,62,63 Within 3 to 8 months after the completion of radiation therapy, most children with diffuse intrinsic pontine glioma will have clinical or radiographic evidence of disease progression. The pattern of failure is generally local.16 In one study, 25% of cases with disease progression involved the irradiated volume, whereas 75% involved the margin of the radiation field.16,64
Various chemotherapeutic strategies, including preradiation therapy chemotherapy, have been employed in the hope of improving survival. Trials have included cytotoxic agents in various combinations, with varying dose intensities, including myeloablative chemotherapy with stem cell res-cue.15,65,66 Unfortunately, no agent or regimen to date has improved survival over radiation therapy alone.2,15 Chemotherapies such as platinoids, etoposide, and nitrosoureas have been investigated but have not increased long-term overall survival.15,67,68 The lack of efficacy of systemic chemotherapy or targeted agents may be due to the inherent resistance of diffuse intrinsic pontine glioma cells to such treatments, or alternatively due to inadequate delivery of these agents into the tumor because of the relatively intact blood-brain barrier, as evident by the minimal contrast enhancement with magnetic resonance imaging.1 There is no standard therapy for children with diffuse intrinsic pontine glioma once they progress following radiation therapy as clinical studies have failed to identify agents that are effective.15
There are currently 22 open clinical trials for children with diffuse intrinsic pontine glioma listed on ClinicalTrials.gov. Because of space limitations, we will not be able to discuss all of the studies but instead highlight several ongoing studies. The scientific rationale for the PARP inhibitor study that the patient in case 2 enrolled on is that a subset of human diffuse intrinsic pontine gliomas have been reported to have low-level gains in PARP-1, an enzyme that detects and signals the single strand breaks in DNA induced by radiation and chemotherapy for repair.69 Veliparib is a potent PARP-1 and PARP-2 inhibitor that may increase the sensitivity of diffuse intrinsic pontine glioma tumor cells to radiation and chemotherapy by blocking DNA repair. Another trial, a molecularly determined clinical trial for newly diagnosed diffuse intrinsic pontine glioma patients (clinicaltrials.gov; NCT01182350) uses an image-guided stereotactic biopsy to obtain tumor tissue, which is then tested for molecular markers. The treatment cohort is then designated according to 2 biomarkers, O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status and epidermal growth factor receptor (EGFR) expression: cohort 1, bevacizumab (an antibody targeting VEGF-A) plus irradiation (promoter methylation negative, no EGFR overexpression); cohort 2: bevacizumab plus irradiation plus erlotinib (promoter methylation negative, EGFR overexpression); cohort 3: bevacizumab plus irradiation plus temozolomide (promoter methylation positive, no EGFR overexpression); cohort 4: bevacizumab plus irradiation plus erlotinib plus temo-zolomide (promoter methylation positive, EGFR overexpression). This clinical trial is a step forward for the field even though the drugs used in this study have already been evaluated in children with diffuse intrinsic pontine glioma as single agents in combination with radiation and each of them failed to significantly prolong survival.70–72
Other ongoing trials for children with diffuse intrinsic pontine glioma are geared to attack cancer targets that are not unique to diffuse intrinsic pontine glioma such as components of the RTK-RAS-PI3K pathway. For example, there is an ongoing clinical trial at St Jude’s hospital (NCT01644773) that uses a combination of 2 multikinase inhibitors, dasatinib (PDGFRA and SRC inhibitor) and crizotinib (c-MET and ALK inhibitor). While in Europe, there is a French upfront biopsy study (NCT02233049) that is testing 3 single drug randomizations depending on the expression of 2 biomarkers, EGFR expression and PTEN loss. The first randomization is between erlotinib (EGFR inhibitor) and dasatinib for tumors that overexpress EGFR without PTEN loss. The second randomization is between everolimus (mTOR inhibitor) and dasatinib for tumors that have PTEN loss without EGFR overexpression. Finally, the third randomization is between dasatinib, erlotinib, and everolimus for tumors that show both EGFR overexpression and PTEN loss or for tumors with an inconclusive biopsy. Again, biopsy driven studies are a step in the right direction for children with diffuse intrinsic pontine glioma although it is hard to imagine that a single targeted agent can have a potent enough effect to prolong survival given the known heterogeneity of this disease.43,57
The use of small molecules to treat diffuse intrinsic pontine glioma faces challenges. Penetration of drugs in diffuse intrinsic pontine glioma seems to be poor, probably because of its relatively intact blood-brain barrier. It is worth noting that there is no objective data to back up this statement as none of the clinical trials for diffuse intrinsic pontine glioma thus far have measured drug levels or target inhibition during therapy. Nonetheless, direct delivery of antineoplastic agents to tumor via convention-enhanced delivery is a novel approach that is currently being explored in diffuse intrinsic pontine glioma.4 Convention-enhanced delivery delivers agents directly into the tumor under continuous low pressure via a catheter placed directly in the tumor or tumor bed.16 One such trial for DIPG patients combined an experimental agent, IL13-PE (IL-13-Pseudomonas exotoxin), with an MRI contrast agent (gadolinium DTPA) to monitor drug delivery (clinicaltrials.gov; NCT00880061). Initial results from the first 4 patients enrolled on the study demonstrate that this approach can be performed safely and coinfusion of the MRI contrast agent provides real-time feedback on drug distribution.73 Another clinical trial is testing convention-enhanced delivery to deliver an agent called 124I-8H9 (a radiolabeled antibody whose target is B7-H3). The agent 124I-8H9 binds to B7-H3 expressed on a subset of diffuse intrinsic pontine glioma tumor cells74 and may induce the tumor cells to die from radiation. At this time, the research team is investigating the safety of 124I-8H9 given by convention-enhanced delivery at different dose levels (clinicaltrials.gov; NCT01502917).
Future Therapies
One area of research in the diffuse intrinsic pontine glioma field is to find a way to target the mutant histone (with the assumption that it is required for tumor maintenance, although this has not been demonstrated). Even though the K27M mutations were just discovered in 2012, there have already been 3 recent publications identifying potentially promising ways to target the mutant histone. One identified an H3K27 demethy-lating agent called GSK-J475 as a promising agent for evaluation in a future clinical trial. A second study identified panobinostat, a histone deacetylase inhibitor as a potent drug and demonstrated that it synergizes with GSK-J4.76 Panobino-stat will be evaluated in a clinical trial through the Pediatric Brain Tumor Consortium in the near future. A third study identified a promising compound targeting menin using a novel diffuse intrinsic pontine glioma model derived from human embryonic stem cells.77 Although the role of menin in diffuse intrinsic pontine glioma is not known, this study suggests that it is a therapeutic target in K27M mutant diffuse intrinsic pontine gliomas.
Another area of research is finding a way to target the newly found ACVR1 mutations in 20% to 30% of diffuse intrinsic pontine gliomas using small-molecule inhibitors although it remains to be determined exactly how these mutations contribute to diffuse intrinsic pontine glioma pathogenesis. Treatment with nonselective ALK2 inhibitors such as dorso-morphin and LDN-193189 has been shown to inhibit the BMP pathway and to reduce heterotypic ossification in FOP models. With more selective ALK2 inhibitors on the horizon, assessment of their ability to get across the blood-brain barrier will be critical.45,78
Lastly, immunotherapy has recently gained momentum as a bona fide treatment for cancer with the approval of checkpoint inhibitors targeting CTLA4, PD1, and PD-L1. In addition to the convention-enhanced delivery trial with the radiolabeled antibody as one form of immunotherapy, another immunothera-peutic strategy is vaccination. One recently published vaccine study evaluated a peptide vaccine of 3 glioma-associated antigens (EphA2, Survivin, and IL13-R α2) in children with diffuse intrinsic pontine glioma and demonstrated preliminary evidence of immunologic and clinical responses.79 Of the 22 open clinical trials for children with diffuse intrinsic pontine glioma, there is 1 open vaccine study (clinicaltrials.gov NCT01400672) and 2 checkpoint inhibitor studies (clinicaltrials.gov NCT02359565 andNCT01952769). In summary, there are several exciting novel approaches against diffuse intrinsic pontine glioma on the horizon and there is reason for cautious optimism against this disease.
Acknowledgments
We would like to acknowledge all the physicians and staff at Duke University Medical Center who cared for the 2 children who were illustrated in the case presentations. OJB is a Damon Runyon Clinical Investigator, Rory David Deutsch Scholar and is supported by K02 NS086917–01.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Schroeder KM, Hoeman CM, Becher OJ. Children are not just little adults: recent advances in understanding of diffuse intrinsic pontine glioma biology. Pediatr Res. 2014;75:205–209. [DOI] [PubMed] [Google Scholar]
- 2.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7: 241–248. [DOI] [PubMed] [Google Scholar]
- 3.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40:265–271. [DOI] [PubMed] [Google Scholar]
- 4.Robison NJ, Kieran MW. Diffuse intrinsic pontine glioma: a reassessment. JNeurooncol. 2014;119:7–15. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartzentrnber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. [DOI] [PubMed] [Google Scholar]
- 7.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem highgrade glioma. Nat Genet. 2014;46:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline highgrade astrocytoma. Nat Genet. 2014;46:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor KR, Vinci M, Bullock AN, Jones C. ACVR1 mutations in DIPG: lessons learned from FOP. Cancer Res. 2014;74: 4565–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27 M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–437. [DOI] [PubMed] [Google Scholar]
- 13.Stiller CA, Kroll ME, Pritchard-Jones K. Population survival from childhood cancer in Britain during 1978–2005 by eras of entry to clinical trials. Ann Oncol 2012;23:2464–2469. [DOI] [PubMed] [Google Scholar]
- 14.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 2014; 16(suppl 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm SA, Chamberlain MC. Brainstem glioma: a review. Curr Neurol Neurosci Rep. 2013;13:346. [DOI] [PubMed] [Google Scholar]
- 16.Warren KE. Diffuse intrinsic pontine glioma: poised for progress. Front Oncol. 2012;2:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green AL, Kieran MW. Pediatric brainstem gliomas: new understanding leads to potential new treatments for two very different tumors. Curr Oncol Rep. 2015;17:436. [DOI] [PubMed] [Google Scholar]
- 18.Lassiter KR, Alexander E Jr, Davis CH Jr, Kelly DL Jr. Surgical treatment of brain stem gliomas. J Neurosurg. 1971;34: 719–725. [DOI] [PubMed] [Google Scholar]
- 19.Jackson S, Patay Z, Howarth R, et al. Clinico-radiologic characteristics of long-term survivors of diffuse intrinsic pontine glioma. JNeurooncol. 2013;114:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley KA, Zhou T, McNall-Knapp RY, et al. Motexafingadolinium and involved field radiation therapy for intrinsic pontine glioma of childhood: a children’s oncology group phase 2 study. Int JRadiat Oncol Biol Phys 2013;85:e55–e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobel U, Sedlacik J, Sabin ND, et al. Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology. 2010;52:1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buczkowicz P, Bartels U, Bouffet E, et al. Histopathological spectrum ofpaediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol. 2014;128: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sethi R, Allen J, Donahue B, et al. Prospective neuraxis MRI surveillance reveals a high risk of leptomeningeal dissemination in diffuse intrinsic pontine glioma. JNeurooncol. 2011;102:121–127. [DOI] [PubMed] [Google Scholar]
- 24.Caretti V, Bugiani M, Freret M, et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 2014;128: 605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107: 1–4. [DOI] [PubMed] [Google Scholar]
- 26.Ogiwara H, Morota N. The efficacy of a biopsy of intrinsic brainstem lesions for decision making of the treatments. Childs Nerv Syst. 2013;29:833–837. [DOI] [PubMed] [Google Scholar]
- 27.Rajshekhar V, Moorthy RK. Status of stereotactic biopsy in children with brain stem masses: insights from a series of 106 patients. Stereotact Funct Neurosurg 2010;88:360–366. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZJ, Rao L, Bhambhani K, et al. Diffuse intrinsic pontine glioma biopsy: a single institution experience. Pediatr Blood Cancer. 2015;62:163–165. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Gomez JL, Rodriguez-Alvarez CA, Marhx-Bracho A, Rueda-Franco F. Stereotactic biopsy for brainstem tumors in pediatric patients. Childs Nerv Syst. 2010;26:29–34. [DOI] [PubMed] [Google Scholar]
- 30.Lobel U, Sedlacik J, Reddick WE, et al. Quantitative diffusion-weighted and dynamic susceptibility-weighted contrastenhanced perfusion MR imaging analysis of T2 hypointense lesion components in pediatric diffuse intrinsic pontine glioma. AJNRAm JNeuroradiol. 2011;32:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells EM, Packer RJ. Pediatric brain tumors. Continuum (Minneap Minn). 2015;21:373–396. [DOI] [PubMed] [Google Scholar]
- 32.Epstein FJ, Farmer JP. Brain-stem glioma growth patterns. JNeu rosurg 1993;78:408–412. [DOI] [PubMed] [Google Scholar]
- 33.Angelini P, Hawkins C, Laperriere N, et al. Post mortem examinations in diffuse intrinsic pontine glioma: challenges and chances. J Neurooncol. 2011;101:75–81. [DOI] [PubMed] [Google Scholar]
- 34.Giussani C, Poliakov A, Ferri RT, et al. DTI fiber tracking to differentiate demyelinating diseases from diffuse brain stem glioma. Neuroimage. 2010;52:217–223. [DOI] [PubMed] [Google Scholar]
- 35.Lassman LP, Arjona VE. Pontine gliomas of childhood. Lancet. 1967;1:913–915. [DOI] [PubMed] [Google Scholar]
- 36.Pollack IF, Stewart CF, Kocak M, et al. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011;13:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poussaint TY, Kocak M, Vajapeyam S, et al. MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro Oncol 2011;13:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hargrave D, Chuang N, Bouffet E. Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. JNeurooncol. 2008;86:313–319. [DOI] [PubMed] [Google Scholar]
- 39.Lober RM, Cho YJ, Tang Y, et al. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J Neurooncol. 2014;117:175–182. [DOI] [PubMed] [Google Scholar]
- 40.Steffen-Smith EA, Shih JH, Hipp SJ, et al. Proton magnetic resonance spectroscopy predicts survival in children with diffuse intrinsic pontine glioma. J Neurooncol. 2011;105:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamasaki F, Kurisu K, Kajiwara Y, et al. Magnetic resonance spectroscopic detection of lactate is predictive of a poor prognosis in patients with diffuse intrinsic pontine glioma. Neuro Oncol. 2011;13:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38:27–35. [DOI] [PubMed] [Google Scholar]
- 43.Paugh BS, Broniscer A, Qu C, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol. 2011;29:3999–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrow J, Adamowicz-Brice M, Cartmill M, et al. Homozygous loss of ADAM3A revealed by genome-wide analysis of pediatric high-grade glioma and diffuse intrinsic pontine gliomas. Neuro Oncol 2011;13:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor KR, Mackay A, Truffaux N, et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. 2014;46:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cage TA, Samagh SP, Mueller S, et al. Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Childs Nerv Syst. 2013;29:1313–1319. [DOI] [PubMed] [Google Scholar]
- 47.Lewis PW, Muller MM, Koletsky MS, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan KM, Fang D, Gan H, et al. The histone H3.3K27 M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bender S, Tang Y, Lindroth AM, et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27 M mutant pediatric high-grade gliomas. Cancer Cell. 2013; 24:660–672. [DOI] [PubMed] [Google Scholar]
- 50.Song GA, Kim HJ, Woo KM, et al. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542–22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shore EM. Fibrodysplasia ossificans progressiva: a human genetic disorder of extraskeletal bone formation, or—how does one tissue become another? Wiley InterdiscipRev Dev Biol. 2012;1:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukuda T, Kanomata K, Nojima J, et al. A unique mutation of ALK2, G356D, found in a patient with fibrodysplasia ossificans progressiva is a moderately activated BMP type I receptor. Bio-chem Biophys Res Commun. 2008;377:905–909. [DOI] [PubMed] [Google Scholar]
- 54.Kaplan FS, Xu M, Seemann P, et al. Classic and atypical fibrodys-plasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaikuad A, Alfano I, Kerr G, et al. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem. 2012;287:36990–36998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korshunov A, Ryzhova M, Hovestadt V, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129:669–678. [DOI] [PubMed] [Google Scholar]
- 57.Puget S, Philippe C, Bax DA, et al. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One. 2012;7:e30313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saratsis AM, Kambhampati M, Snyder K, et al. Comparative multidimensional molecular analyses of pediatric diffuse intrinsic pontine glioma reveals distinct molecular subtypes. Acta Neuro-pathol. 2014;127:881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas-Kogan DA, Baneijee A, Kocak M, et al. Phase I trial of tipi-farnib in children with newly diagnosed intrinsic diffuse brainstem glioma. Neuro Oncol 2008;10:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Children’s Cancer Group Phase I/II Trial. Cancer. 1994;74:1827–1834. [DOI] [PubMed] [Google Scholar]
- 61.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int JRadiat Oncol Biol Phys. 1999;43:959–964. [DOI] [PubMed] [Google Scholar]
- 62.Janssens GO, Jansen MH, Lauwers SJ, et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys. 2013;85:315–320. [DOI] [PubMed] [Google Scholar]
- 63.Janssens GO, Gidding CE, Van Lindert EJ, et al. The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: a pilot study. Int J Radiat Oncol Biol Phys. 2009;73:722–726. [DOI] [PubMed] [Google Scholar]
- 64.Grigsby PW, Garcia DM, Ghiselli R. Analysis of autopsy findings in patients treated with irradiation for thalamic and brain stem tumors. Am J Clin Oncol. 1989;12:255–258. [DOI] [PubMed] [Google Scholar]
- 65.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. [DOI] [PubMed] [Google Scholar]
- 66.Bouffet E, Raquin M, Doz F, et al. Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer. 2000;88:685–692. [DOI] [PubMed] [Google Scholar]
- 67.Freeman CR, Kepner J, Kun LE, et al. A detrimental effect of a combined chemotherapy-radiotherapy approach in children with diffuse intrinsic brain stem gliomas? Int J Radiat Oncol Biol Phys. 2000;47:561–564. [DOI] [PubMed] [Google Scholar]
- 68.Doz F, Neuenschwander S, Bouffet E, et al. Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: a study by the Societe Francaise d’Oncologie Pediatrique. Eur J Cancer 2002;38:815–819. [DOI] [PubMed] [Google Scholar]
- 69.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights plateletderived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 2010;28: 1337–1344. [DOI] [PubMed] [Google Scholar]
- 70.Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28: 3069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geoerger B, Hargrave D, Thomas F, et al. Innovative Therapies for Children with Cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol. 2011;13:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chittiboina P, Heiss JD, Warren KE, Lonser RR. Magnetic resonance imaging properties of convective delivery in diffuse intrinsic pontine gliomas. JNeurosurg Pediatr. 2014;13:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Z, Luther N, Ibrahim GM, et al. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J Neurooncol. 2013;111:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashizume R, Andor N, Ihara Y, et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med. 2014;20:1394–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015; 21:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Funato K, Major T, Lewis PW, et al. Use of human embryonic stem cells to model pediatric gliomas with H3.3K27 M histone mutation. Science. 2014;346:1529–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohedas AH, Wang Y, Sanvitale CE, et al. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. J Med Chem.2014;57:7900–7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pollack IF, Jakacki RI, Butterfield LH, et al. Antigenspecific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol. 2014;32:2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]


