Abstract
The Forkhead family of transcription factors participates in the induction of death-related genes. In NMuMG and 4T1 mammary epithelial cells, transforming growth factor β (TGFβ) induced phosphorylation and cytoplasmic retention of the Forkhead factor FKHRL1, while reducing FHKRL1-dependent transcriptional activity. TGFβ-induced FKHRL1 phosphorylation and nuclear exclusion were inhibited by LY294002, an inhibitor of phosphatidylinositol-3 kinase. A triple mutant of FKHRL1, in which all three Akt phosphorylation sites have been mutated (TM-FKHRL1), did not translocate to the cytoplasm in response to TGFβ. In HaCaT keratinocytes, expression of dominant-negative Akt prevented TGFβ-induced 1) reduction of Forkhead-dependent transcription, 2) FKHRL1 phosphorylation, and 3) nuclear exclusion of FKRHL1. Forced expression of either wild-type (WT) or TM-FKHRL1, but not a FKHRL1 mutant with deletion of the transactivation domain, resulted in NMuMG mammary cell apoptosis. Evidence of nuclear fragmentation colocalized to cells with expression of WT- or TM-FKHRL1. The apoptotic effect of WT-FKHRL1 but not TM-FKHRL1 was prevented by exogenous TGFβ. Serum starvation-induced apoptosis was also inhibited by TGFβ in NMuMG and HaCaT cells. Finally, dominant-negative Akt abrogated the antiapoptotic effect of TGFβ. Taken together, these data suggest that TGFβ may play a role in epithelial cell survival via Akt-dependent regulation of FKHRL1.
INTRODUCTION
Transforming growth factor β (TGFβ) is involved in various cellular processes, including cell division, differentiation, motility, adhesion, and apoptosis (Massagué, 1998). TGFβ stimulates the proliferation of mesenchymal cells while inhibiting the growth of most normal epithelial cells (Massagué and Chen, 2000; Massagué and Wotton, 2000). TGFβ signals are transmitted through a heterodimeric complex of two transmembrane serine/threonine kinases, the type I and II TGFβ receptors (Massagué, 1998; Massagué and Wotton, 2000). Receptor-associated Smads are intracellular signal transducers that associate with TβRI, become phosphorylated, and translocate to the nucleus where they regulate transcription of TGFβ target genes (Lagna et al., 1996; Massagué and Chen, 2000). TGFβ modulates several signaling pathways in mammalian cells. The c-Jun NH2-terminal kinase (JNK) can either be activated (Atfi et al., 1997) or inhibited by TGFβ (Imai et al., 1999; Huang et al., 2000). Rapid activation of extracellular signal-regulated kinase by TGFβ has been reported in epithelial cells (Hartsough et al., 1996). We previously reported that TGFβ phosphorylates Akt in a phosphatidylinositol-3 kinase (PI3K)-dependent manner, response that was required for TGFβ-mediated epithelial-to-mesenchymal transition and migration of mammary cells (Bakin et al., 2000).
The Akt kinase is activated by phosphorylation at Thr308 and Ser473 mediated by 3-phosphoinositide–dependent protein kinase 1 and 2, respectively (Alessi et al., 1997; Stokoe et al., 1997; Stephens et al., 1998). Akt phosphorylates and inactivates glycogen synthase kinase3-β, an enzyme that regulates glycogen biosynthesis (Cross et al., 1995). In addition to regulating cellular metabolism, Akt can promote enhanced cell survival (Dudek et al., 1997; Kauffmann-Zeh et al., 1997; Datta et al., 1999). Bad was the first reported proapoptotic factor directly phosphorylated and inactivated by Akt (del Peso et al., 1997). Akt also phosphorylates the proapoptotic molecule caspase 9 at Ser196 (Cardone et al., 1998), which results in suppression of caspase 9-induced apoptosis in 293 cells (Datta et al., 1999).
The role of Akt in gene transcription was first discovered by studies performed in Caenorhabditis elegans. DAF-16, a Forkhead transcription factor in C. elegans, is negatively regulated by Akt. DAF-16 is activated by DAF-2 and DAF-23, where DAF-2 is an insulin receptor-like protein, and DAF-23, a PI3K-like protein (Kops and Burgering, 1999). The mammalian orthologs of DAF-16 are AFX, Forkhead response element (FKHR), and FKHRL1. In C. elegans, mutations of daf2 synergize with mutations of daf1, a type I TGFβ receptor, in inducing dauer formation (Ogg et al., 1997), suggesting that TGFβ can interact with the DAF-2/DAF-16 pathway. All three DAF-16 mammalian homologs share a Forkhead 100-amino acid core domain, responsible for binding to DNA. AFX, FKHR, and FKHRL1, each contain three Akt phosphorylation sites (Datta et al., 1999), which can be phosphorylated by Akt in mammalian cells (Brunet et al., 1999; Guo et al., 1999; Kops et al., 1999; Nakae et al., 1999). On phosphorylation by Akt, Forkhead factors translocate from the nucleus to the cytoplasm (Biggs et al., 1999; Brunet et al., 1999), where 14-3-3 proteins may sequester them and prevent their function (Brunet et al., 1999). In their unphosphorylated state, Forkhead factors predominantly localize in the nucleus where they bind to insulin response elements and/or the Fas ligand (FasL) promoter and activate transcription of target genes that may induce cell death (Brunet et al., 1999; Kops and Burgering, 1999; Tang et al., 1999). Accordingly, overexpression of either FKHR or FKHRL1 results in apoptosis (Brunet et al., 1999; Tang et al., 1999). So far, FasL is the only known candidate gene to mediate FKHRL1-induced apoptosis (Brunet et al., 1999). Therefore, by phosphorylating FKHRL1 and excluding it from the nucleus, the PI3K target Akt may prevent the transcriptional engagement of FKHRL1, inhibit Forkhead-induced apoptosis, and contribute to cell survival.
In this report, we show that treatment with TGFβ results in phosphorylation and nuclear exclusion of endogenous and ectopic FKHRL1 in mammary epithelial cells and skin keratinocytes. This effect required PI3K and Akt function as inhibitors of PI3K or expression of dominant-negative Akt (dn Akt) prevented TGFβ-mediated inhibition of FKHRL1. Moreover, both Forkhead-dependent transcription and cell death induced by either serum starvation or forced expression of FKHRL1 was partially blocked by TGFβ. These results suggest that TGFβ, via activation of Akt, may induce cytoplasmic retention of FKHRL1 and thus act as an antiapoptotic factor in epithelial cells. These mechanisms may be biologically relevant to TGFβ-mediated tumor progression.
MATERIALS AND METHODS
Cell Lines, Inhibitors, and Antibodies
NMuMG nontumorigenic mouse mammary epithelial cells were purchased from the American Type Culture Collection (Manassas, VA) and cultured in DMEM supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml insulin. 4T1 breast tumor cells, kindly provided by F. Miller (Karmanos Cancer Center, Detroit, MI), were maintained in DMEM with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin. HaCaT keratinocytes, stably transfected with a dn Akt mutant vector or vector alone (mock cells), have been described previously (Jost et al., 2001). The HaCaT cells were maintained in DMEM supplemented with 10% FCS, 0.1 mg/ml hygromycin B, 2 μg/ml tetracycline (Tet), 100 U/ml penicillin, and 100 μg/ml streptomycin. To induce expression of the dn Akt protein, cells were washed two times with Tet-free medium and kept in Tet-free medium for 48 h. TGFβ1 was obtained from R & D Systems (Minneapolis, MN). Antibodies to FKHRL1, phospho-Ser-253 FKHRL1 and C-terminal phospho Smad2 were from Upstate Biotechnology (Lake Placid, NY) to Smad2 from Santa Cruz Biotechnology (Santa Cruz, CA). Texas Red-conjugated anti-mouse IgG secondary antibody was purchased from Molecular Probes (Eugene, OR) and anti-hemagglutinin (HA)-fluorescein mouse monoclonal antibody from Roche Molecular Biochemicals (Indianapolis, IN). Akt and phospho-Ser-473 Akt polyclonal antibodies were from New England Biolabs (Beverly, MA). LY294002 was from Calbiochem (San Diego, CA)
Immunoblot Analysis
After washes with phosphate buffered saline (PBS), cell monolayers were lysed in a buffer containing 20 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 20 mM NaF, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, and 2 μg/ml leupeptin. Equal amount of protein in whole cell lyates [as measured by Bradford (1976) method] were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% skim milk in Tris-buffered saline-Tween 20 (TBST) containing 20 mM Tris, pH 7.6, 137 mM NaCl, 0.1% Tween 20 (v/v) for 1 h at ambient temperature and then incubated overnight with primary antibodies in TBST in 1% skim milk at 4°C. After washing membranes with TBST three times, they were incubated with a 1:5000 dilution of horseradish peroxidase-linked secondary antibody in TBST for 1 h, followed by three washes in TBST. Immunoreactive bands were visualized by enhanced chemiluminescence (Pierce Chemical, Rockford, IL).
Immunocytochemistry and Transfections
Cells were grown in DMEM/10% FCS to ∼60% confluence on glass coverslips in 12-well plates, washed with serum-free medium, incubated in serum-free medium for 24 h, and then stimulated with 2 ng/ml TGFβ for 1 h in the absence or presence of 20 μM LY294002, an inhibitor of PI3K (Vlahos et al., 1994). In experiments involving ectopic FKHRL1 expression, cells in 60-mm dishes (106 cells/dish) were transfected with 10 μg of either WT-FKHRL1 or triple mutant (TM)-FKHRL1, each for 16 h with the use of FUGENE 6 (Roche Molecular Biochemicals). Cells were then transferred to coverslips on 12-well plates, incubated in serum-free medium for 24 h, fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, and then permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. Coverslips were next blocked with 3% skim milk in PBS for 30 min and incubated with primary antibodies diluted in 1% skim milk/PBS (1:500 for FKHRL1 and P-Ser253 FKHRL1; 1:200 for anti-HA fluorescein). After three washes with PBS, samples were incubated with fluorescent secondary antibodies diluted in PBS (1:500) for 1 h at room temperature. Coverslips were mounted on glass slides with AquaPolyMount (Polysciences, Warrington, PA) and examined by laser scanning confocal microscopy (LSM 410; Carl Zeiss, Thornwood, NY). For detection of apoptotic nuclei, cells were incubated in 1 μg/ml Hoechst 33258 (Sigma, St. Louis, MO) in PBS for 10 min after incubation with secondary antibody. Fluorescent images of Hoechst-stained nuclei or HA-stained samples were recorded with a Zeiss Axiophot upright microscope.
Transcriptional Reporter Assays
Cells were seeded at the density of 105 cells/well (12-well plates). After 24 h, the cells were transfected with 0.5 μg/well of either WT-FKHRL-HA or TM-FKHRL1-HA, each with 0.5 μg/well of Forkhead-responsive element (FHRE)-Luc and 0.005 μg/well of pCMV-Rl (Promega, Madison, WI) with the use of 3 μl/well of FUGENE6 reagent for 16 h. Transfected cells were then subjected to serum starvation either in the presence or absence of 2 ng/ml TGFβ for 24 h. Firefly luciferase and Renilla reniformis luciferase activities in cell lysates were determined with the use of the Dual Luciferase Reporter Assay System (Promega) according to the manufacturer's protocol in a Monolight 2001 luminometer (Analytical Luminescence Laboratory). Firefly luciferase activity was normalized to R. reniformis luciferase activity and presented as relative luciferase units.
Apoptosis Assays
Cells were seeded at the density of 5 × 105 cells/well in six-well dishes. The following day, the medium was changed to serum-free medium with or without 2 ng/ml TGFβ. Both floating cells and adherent cells were harvested 72 h later. Pooled cells were washed with PBS and then subjected to Apo-5-bromo-2′-deoxyuridine (BrdU) analysis with the use of an Apo-BrdU assay kit (Phoenix Flow Systems, San Diego, CA) according to the manufacturer's protocol in a FACS/Calibur Flow Cytometer (BD Biosciences, Mansfield, MA). For evaluation of DNA fragmentation, 106 cells/dish in 60-mm dishes were incubated in serum-free medium ± 2 ng/ml TGFβ. After 72 h, floating and adherent cells were harvested, washed with PBS, and resuspended in 200 μl of cytosolic DNA extraction buffer (5 mM Tris, pH 7.4, 20 mM EDTA, 0.5% Triton X-100) followed by vortexing for 1 min. After centrifugation for 15 min at 12,000 rpm at 4°C, the supernatants were transferred into new tubes and subjected to phenol/chloroform extraction. The DNA fragments were pelleted by adding 3 M sodium acetate, washed with ethanol, resuspended in TE containing 200 μg/ml RNase, and separated by 1.5% agarose gel electrophoresis.
Cell Cycle Analysis by Flow Cytometry
Cells were harvested by trypsinization, fixed in ethanol, and labeled with 50 μg/ml propidium iodide (Sigma) containing 125 U/ml protease-free RNase (Calbiochem) as described previously (Busse et al., 2000). Cells were filtered through a 95-μm pore size nylon mesh (Small Parts, Miami Lakes, FL) and a total of 15,000 stained nuclei was analyzed in a FACS/Calibur Flow Cytometer (BD Biosciences).
RESULTS
TGFβ Induces Phosphorylation and Nuclear Exclusion of Endogenous FKHRL1
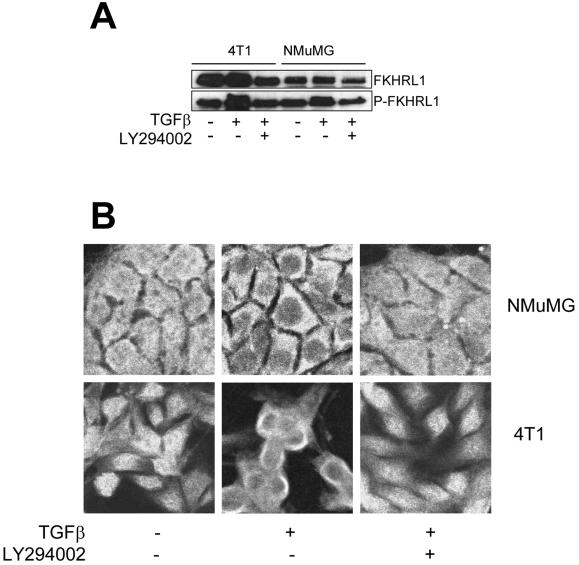
We have previously observed that TGFβ can phosphorylate and activate Akt (Bakin et al., 2000), which is known to phosphorylate FKHRL1 both in vivo and in vitro (Brunet et al., 1999). Thus, we first determined whether TGFβ can induce FKHRL1 phosphorylation. As shown in Figure 1A, treatment with 2 ng/ml TGFβ for 30 min increased the phosphorylation of FKHRL1 in 4T1 and NMuMG cells as determined by immunoblot with a P-Ser253 FKHRL1 antibody. Akt can phosphorylate FKHRL1 at Thr32, Ser253, and Ser315. Brunet et al. (1999) showed that the Akt-dependent shift in FKHRL1 mobility on SDS-PAGE is primarily due to phosphorylation at Ser315, suggesting that the slower migrating band in the lysates from TGFβ-treated cells (Figure 1A) may represent P-Ser315 FKHRL1 also recognized by the P-Ser253 FKHRL1 antibody. Cotreatment with LY294002 inhibited TGFβ-mediated phosphorylation of FKHRL1, suggesting that this effect required PI3K function.
Figure 1.
TGFβ induces cytoplasmic retention and phosphorylation of FKHRL1 in Ser253. (A) Serum-starved, subconfluent 4T1 and NMuMG cells were treated with 2 ng/ml TGFβ for 30 min with or without 20 μM LY294002. After lysis, protein extracts (50 μg/lane) were subjected to SDS-PAGE followed by immunoblot procedures as described in MATERIALS AND METHODS. Dilution ratio for primary antibodies is 1:500 for both FKHRL1 and P-Ser253 FKHRL1. (B) 4T1 and NMuMG cells grown to 60% confluence on coverslips in 12-well plates, serum-starved for 24 h, and then treated with 2 ng/ml TGFβ for 30 min with or without 20 μM LY294002. Cells were washed, fixed as described in MATERIALS AND METHODS, and stained with antibodies against FKHRL1 (1:500). Texas Red-conjugated anti-rabbit IgG (1:500) was used as secondary antibody. Fluorescent images were captured with the use of a laser scanning confocal microscope.
Others have reported that Forkhead transcription factors translocate from the nucleus to the cytoplasm after Akt-mediated phosphosphorylation (Biggs et al., 1999; Brunet et al., 1999; del Peso et al., 1999; Takaishi et al., 1999). To determine whether TGFβ can induce translocation of endogenous FKHRL1, we stimulated serum-starved cells with 2 ng/ml TGFβ for 30 min and then performed immunofluorescence analysis. Figure 1B shows FKHRL1 staining in cytoplasm and nucleus in both NMuMG and 4T1 cells. TGFβ treatment results in exclusion of the nuclear FKHRL1 staining, which was prevented by LY294002. These results suggest that TGFβ-mediated phosphorylation and subsequent nuclear exclusion of FKHRL1 are both PI3K-dependent.
TGFβ Inhibits Nuclear Translocation of Exogenous FKHRL1 and Forkhead-dependent Transcription
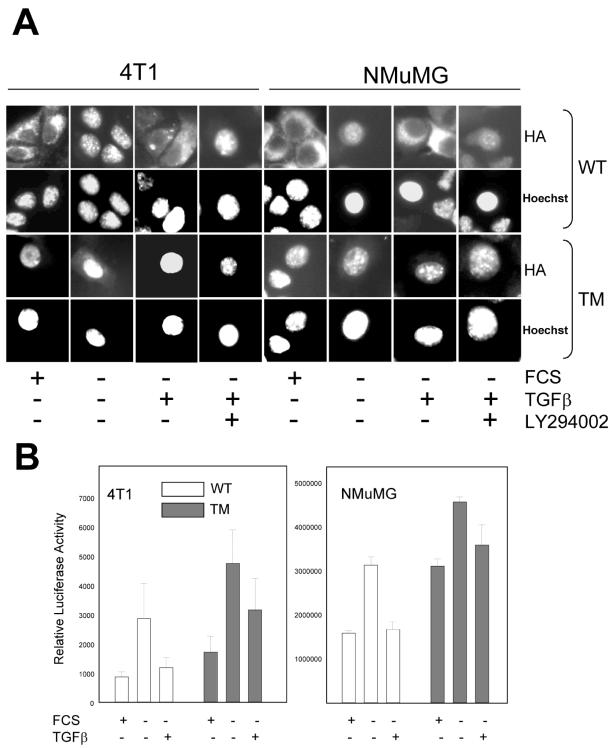
We next determined whether TGFβ-mediated FKHRL1 regulation required function of the PI3K effector kinase Akt. Cells were transfected with HA-tagged WT-FKHRL1 or a triple mutant TM-FKHRL1, in which all three Akt phosphorylation sites (Thr32, Ser253, and Ser315) (Brunet et al., 1999) had been mutated to alanine, and examined the subcellular localization of ectopic FKHRL1 under various conditions. In serum-containing medium and in both 4T1 and NMuMG mammary cells, WT-FKHRL1 primarily localized in the cytoplasm (Figure 2A, first column of each cell line). On removal of serum for 24 h, WT-FKHRL1 protein localized predominantly in the nucleus. Treatment with TGFβ promoted WT-FKHRL1 translocation to the cytoplasm, which was prevented by cotreatment with the PI3K inhibitor LY294002. 4T1 and NMuMG cells transfected with the TM-FKHRL1 construct exhibited exclusive nuclear localization of the HA-tagged mutant under any experimental condition (Figure 2A, third row). The results imply that the phosphorylation status of the three Akt sites determines the subcellular distribution of FKHRL1: unphosphorylated FKHRL1 is localized mainly in the nucleus; phosphorylation of the three Akt-consensus sites upon the addition of TGFβ results in nuclear exclusion of FKHRL1.
Figure 2.
TGFβ inhibits nuclear translocation of exogenous FKHRL1 and Forkhead-dependent transcription. (A) Cells seeded in 60-mm dishes (106 cells/dish) were transfected with 10 μg/dish of either WT- or TM-FKHRL1-HA for 16 h and transferred to coverslips on 12-well plates. The cells were then incubated in serum-free medium for 24 h followed by stimulation with 2 ng/ml TGFβ for 30 min with or without 20 μM LY294002. Fixation and staining were performed as described in MATERIALS AND METHODS. Fluorescence intensities associated with anti-HA fluorescein and Hoechst 33258 were observed with a Zeiss Aiophot upright microscope. (B) Cells were transfected with WT-or TM-FKHRL1, each with FHRE-Luc and pCMV-Rl vectors followed, where indicated, with removal of serum and the addition or not of 2 ng/ml TGFβ for 24 h. Dual luciferase assay was performed as described in MATERIALS AND METHODS. Relative luciferase units represents firefly luciferase activity normalized to Renilla luciferase activity. Each data point represents mean ± SD of four wells.
We next asked whether Forkhead-dependent transcription was regulated by TGFβ signaling. We cotransfected cells with either a WT- or TM-FKHRL1 constructs and a FHRE-Luc vector in which a Forkhead response element is linked to a luciferase reporter gene. FHRE contains the binding site of the FasL promoter and FKHRL1 is known to bind this site and enhance transcription of the FasL gene (Brunet et al., 1999). Withdrawal of serum increased FKHRL1-dependent luciferase expression in both NMuMG and 4T1 cells (Figure 2B). However, the addition of TGFβ reduced luciferase activity to levels obtained in the presence of serum, suggesting that TGFβ down-regulates Forkhead-dependent transcription possibly by a mechanism involving nuclear exclusion of FKHRL1. In cells transfected with the Akt-insensitive TM-FKHRL1, transcriptional activity was higher than in cells transfected with WT-FKHRL1. Although to a lesser degree than in cells tranfected with the WT construct, TGFβ also reduced the transcriptional activity mediated by TM-FKHRL1 (Figure 2B).
Dominant-Negative Akt Inhibits TGFβ-induced FKHRL1 Phosphorylation, Nuclear Exclusion of FKHRL1, and Forkhead-dependent Transcription
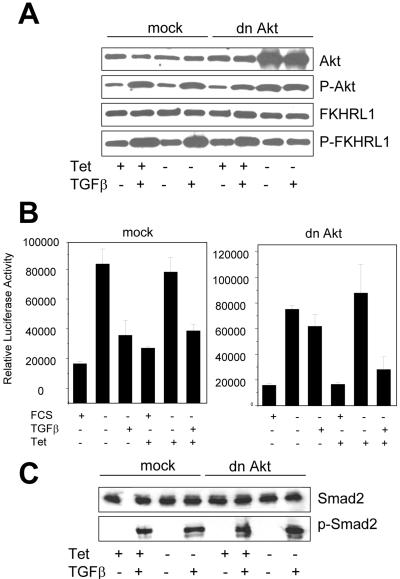
The causal role of Akt in TGFβ-mediated regulation of FKHRL1 was investigated with the use of HaCaT keratinocytes expressing Tet-suppressible dn Akt (Jost et al., 2001). The construct used in this system encodes a kinase-inactive version of Akt in which Lys179 in the catalytic domain has been mutated to Met (Dudek et al., 1997). Withdrawal of Tet from the cell culture medium for 48 h induced expression of dn Akt (Figure 3A). Treatment with TGFβ also induced Akt activity, as measured by P-Ser473 Akt and P-Ser253 FKHRL1 immunoblot analyses in both mock HaCaT cells ± Tet and in dn Akt HaCaT cells in the presence of Tet (Figure 3A). However, when the dn Akt transgene was expressed by withdrawing Tet from dn Akt HaCaT cell culture medium for 48 h, TGFβ-induced phosphorylation at Ser253 of FKHRL1, Ser473 of Akt (Figure 3A) were all reduced. Consistent with transcriptional reporter activity data from NMuMG and 4T1 cells, in mock-transfected HaCaT cells ± Tet and in dn Akt HaCaT cells treated with Tet, TGFβ reduced FKHRL1-dependent transcription (Figure 3B, left). In dn Akt HaCaT cells, however, removal of Tet blocked the inhibitory effect of added TGFβ on FKHRL1-induced reporter activity (Figure 3B, right), suggesting that Akt is indeed responsible for mediating this TGFβ response. To exclude the possibility that dn Akt may be blocking TGFβ effects on FKHRL1 via inhibition of the TGFβ type I receptor (TβRI) kinase, we tested the effect of dn Akt on phosphorylation of Smad2. As shown in Figure 3C, TGFβ-mediated C-terminal phosphorylation of Smad2 in HaCaT cells was not altered by dn Akt.
Figure 3.
Dominant-negative Akt blocks TGFβ-induced FKHRL1 phosphorylation and Forkhead-dependent transcription but not phosphorylation of Smad2. (A) Mock-transfected and dn Akt-transfected HaCaT cells were incubated in medium with or without 2 μg/ml Tet for 48 h. For the last 24 h of incubation, medium was replaced by serum-free medium ± Tet. The cells were then treated with 2 ng/ml TGFβ for 30 min, harvested, and lysed. Protein extracts (30 μg/lane) were subjected to SDS-PAGE followed by immunoblot as described in MATERIALS AND METHODS. Dilution ratios for primary antibodies are 1:500 for P-Ser473 Akt, FKHRL1, and P-Ser253 FKHRL1, and 1:1000 for total Akt. (B) HaCaT cells were transfected with WT- or TM-FKHRL1, each with FHRE-Luc and pCMV-Rl vectors, and incubated in medium with or without 2 μg/ml Tet for 48 h. For the last 24 h where indicated, medium was replaced with serum-free DMEM ± Tet with or without 2 ng/ml TGFβ. Dual luciferase assay was performed as described in MATERIALS AND METHODS. Each data point represents mean ± SD of four wells. (C) In the presence or absence of Tet, cells were incubated with 2 ng/ml TGFβ for 30 min and lysed as in Figure 3A. Cell lysates were resolved by SDS-PAGE and subjected to immunoblot procedures for total and phosphorylated Smad2. Each lane contains 50 μg of protein. Dilution ratios for primary antibodies are 1:1000 for total Smad2 and 1:500 for P-Smad2.
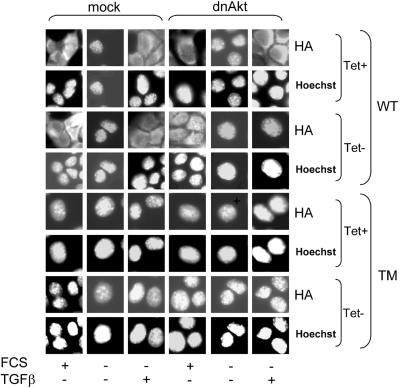
Immunocytochemical analysis (Figure 4) shows that induction of dn Akt can block nuclear exclusion of exogenously expressed FKHRL1 in HaCaT keratinocytes. In mock HaCaT cells, serum starvation resulted in nuclear localization of WT-FKHRL1; treatment with TGFβ for 30 min promoted translocation of WT-FKHRL1 from the nucleus to the cytosol regardless of the presence of Tet. Expression of kinase-inactive Akt by withdrawal of Tet from culture medium, blocked the ability of TGFβ to induce nuclear exclusion of WT-FKHRL1. As expected, TM-FKHRL1 localized in the nucleus of both dn Akt and control cells regardless of the presence of Tet (Figure 4).
Figure 4.
Dominant-negative Akt blocks TGFβ-induced nuclear exclusion of FKHRL1. Mock and dn Akt HaCaT cells seeded in 60-mm dishes (106 cells/dish) ± Tet were transfected with 10 μg/dish of either WT- or TM-FKHRL1 for 16 h and next transferred to coverslips on 12-well plates. In some cases, the cells were serum-starved for 24 h followed by the addition of 2 ng/ml TGFβ for 30 min. Fixation and staining were performed as described in MATERIALS AND METHODS. Fluorescence intensities associated with anti-HA fluorescein and Hoechst 33258 were measured as described in the legend of Figure 2A.
TGFβ Partially Suppresses Apoptosis Induced by Serum Starvation or by Forced Expression of WT-FKHRL1 but not by TM-FKHRL1
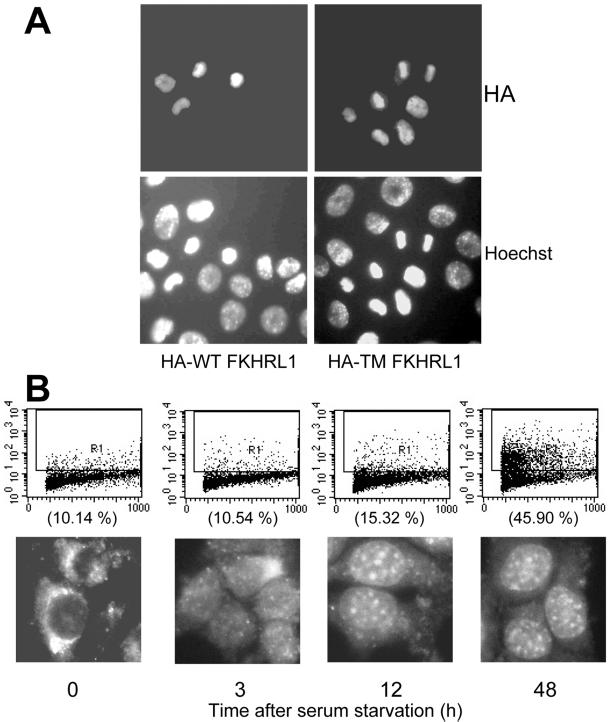
Because TGFβ can induce phosphorylation and subsequent nuclear exclusion of FKHRL1, which is known to be responsible for the expression of death-related genes (Brunet et al., 1999; Kops and Burgering, 1999), we investigated whether expression of ectopic FKHRL1 and/or serum starvation can induce apoptosis in NMuMG cells and whether TGFβ can reverse this process. As shown in Figure 5A, forced expression of either WT- or TM-FKHRL1 resulted in NMuMG cell apoptosis. Cells transfected with either construct were double-labeled with the nuclear stain Hoechst 33258 and an HA antibody. The same cells expressing WT- or TM-FKHRL1, as measured by HA staining, exhibited fragmented nuclei, suggesting that FKHRL1 causes NMuMG cell death. Of ∼150 HA-positive nuclei, 45% of WT-FKHRL1- and 67% TM-FKHRL1-expressing nuclei were apoptotic.
Figure 5.
Forced expression of FKHRL1 or serum starvation induce apoptosis of NMuMG cells. (A) NMuMG cells in 60-mm dishes (106 cells/dish) were transfected with 10 μg/dish of either WT- or TM-FKHRL1-HA for 16 h and transferred to coverslips on 12-well plates. After 24-h incubation, the cells were stained with anti-HA fluorescein and Hoechst 33258. The samples were examined in a Zeiss Axiophot microscope. (B) NMuMG cells in 60-mm dishes (106 cells/dish) were transfected with 0.2 μg/ml WT-FKHRL1-HA for 16 h and transferred to coverslips on 12-well plates. After 24 h, the medium was changed with serum-free medium. At the indicated times, the cells were harvested and assayed for evidence of apoptosis by Apo-BrdU analysis (top row) or stained with anti-Ha flourescein and observed with a Zeiss Axiphot microscope. The percentages of fluorescein isothiocyanate-positive (apoptotic) cells are shown in parentheses.
To further determine whether nuclear localization of FKHRL1 was causal to apoptosis, we examined the temporal correlation of nuclear localization of FKHRL1 with the onset of DNA double-strand breaks as measured by Apo-BrdU assay. Initial experiments showed that ≥2 μg/ml exogenous WT-FKHRL1 was required to induce apoptosis of NMuMG cells (our unpublished results). Therefore, to minimize a possible contribution of the ectopic FKHRL1 to NMuMG cell apoptosis, we used a >10-fold lower concentration (0.2 μg/ml) of HA-tagged WT-FKHRL1. Nuclear localization of FKHRL1 was first evident at 3 h after serum starvation, whereas a low level of apoptosis above baseline was first detectable at 12 h reaching a maximum at 48 h, implying that the nuclear localization of FKHRL1 was not secondary to the onset of apoptosis.
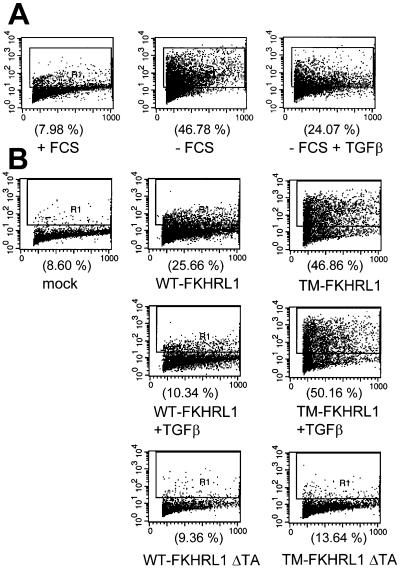
We next investigated whether addition of exogenous TGFβ could rescue apoptosis induced by serum starvation or forced expression of FKHRL1. Removal of FCS for 72 h increased the proportion of apoptotic cells from 7.98 to 46.78%. The latter was reduced to 24.07% by the addition of 2 ng/ml TGFβ (Figure 6A). Transfection of 5 μg/ml WT- or TM-FKHRL1 increased the proportion of apoptotic cells from 8.60% to 25.66 or 46.86%, respectively (Figure 6B). Notably, expression of FKHRL1 mutant constructs with a deletion of the transactivating Forkhead domain (FKHRL1 ΔTA and TM-FKHRL1 ΔTA) did not induce apoptosis above baseline, implying that the transactivating function of FKHRL1 was required for the induction of cell death. Addition of TGFβ markedly inhibited the apoptosis induced by WT-FKHRL1 but not by TM-FKHRL1 (Figure 6B), further suggesting that the protective effect of TGFβ depended on Akt-mediated phosphorylation of FKHRL1.
Figure 6.
TGFβ inhibits NMuMG cells apoptosis induced by serum starvation or by forced expression of FKHRL1. (A) NMuMG cells seeded in serum-free medium at the density of 5 × 105 cells/well in six-well dishes were treated with or without 2 ng/ml TGFβ. Seventy-two hours later, the cells were harvested and evaluated for apoptosis by Apo-BrdU analysis as described in MATERIALS AND METHODS. (B) NMuMG cells seeded at the density of 5 × 105 cells/well in six-well dishes were transfected with 5 μg/ml the indicated WT- and TM-FKHRL1 constructs for 16 h. After transfection, cells were incubated for 72 h in the presence or absence of 2 ng/ml TGFβ and finally subjected to Apo-BrdU analysis.
Dominant-Negative Akt Abolishes Survival Effect of TGFβ
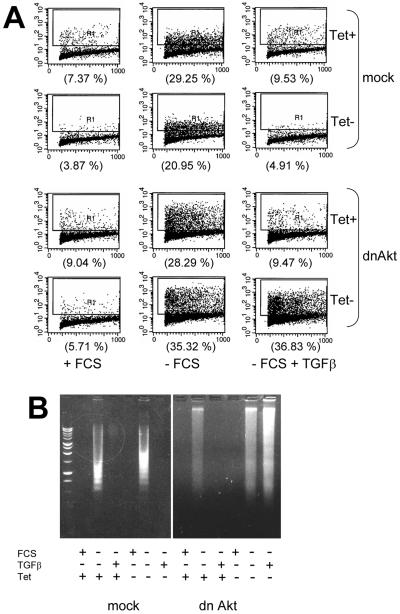
To obtain direct evidence that Akt may play a role in TGFβ-induced cell survival, we used HaCaT cells in which dn Akt was conditionally expressed. As measured by Apo-BrdU assay, a large proportion of mock-transfected HaCaT cells (>20%) became apoptotic upon withdrawal of serum for 72 h. In the presence or absence of Tet, exogenous TGFβ completely prevented apoptosis in control (mock) HaCaT cells and in dn Akt cells treated with Tet (Figure 7A, second column). On the other hand, in dn Akt HaCaT cells, removal of Tet and hence induction of kinase-dead Akt, blocked the ability of TGFβ to prevent the apoptosis induced by serum starvation. Similar data were obtained in DNA fragmentation assays. Serum starvation induced internucleosomal DNA fragmentation in both control and dn Akt cells. TGFβ abolished DNA fragmentation except in dn Akt cells in the absence of Tet (Fg. 7B), implying Akt is causal to the protection from cell death mediated by TGFβ.
Figure 7.
Expression of dominant-negative Akt blocks the antiapoptotic effect of TGFβ1. (A) Control (mock) and dn Akt HaCaT keratinocytes were incubated in serum-containing or serum-free medium ± Tet for 72 h in the presence or absence of 2 ng/ml TGFβ. The proportion of apoptotic cells was assessed by Apo-BrdU analysis and is indicated in parentheses. (B) Mock and dn Akt HaCaT cells (106 cells/dish) on 60-mm dishes were incubated in medium containing FCS or in serum-free medium ± 2 ng/ml TGFβ for 72 h. To suppress dn Akt, Tet was added during the incubation period where indicated. Adherent and floating cells were pooled, their DNA was collected, and next evaluated for evidence of internucleosomal fragmentation in 1.5% agarose gels as described in MATERIALS AND METHODS.
Survival Effects of TGFβ Are Independent of Antiproliferative Effects
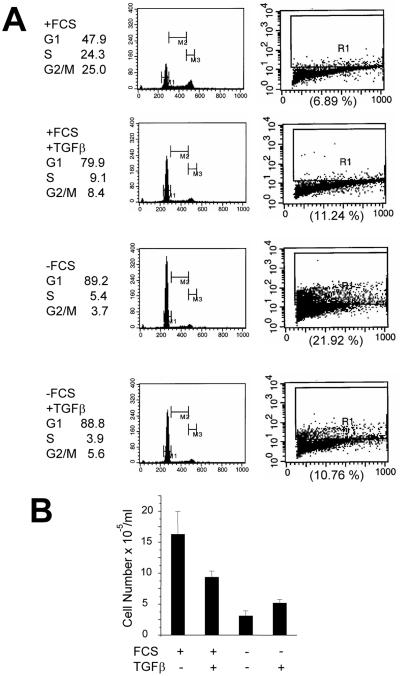
Addition of TGFβ to proliferating NMuMG cells induces growth arrest. These studies have been done with exponentially growing NMuMG cells in serum-containing medium (Miettinen et al., 1994; Piek et al., 1999), conditions under which FKHRL1 localizes mainly in the cytosol and FKHRL1-mediated transcription is low (Figure 2). In serum-containing medium, NMuMG displayed a robust S phase (24.3%) and a low level of apoptosis. A 24-h treatment with TGFβ resulted in G1 arrest and marked reduction in S phase without a significant effect on the low level of apoptosis (6.9 vs. 11.2%; Figure 8A). Under these serum-containing conditions, TGFβ induced a 44% reduction in cell number after 72 h (Figure 8B), consistent with the delay in cell cycle progression. On the other hand, serum withdrawal (for 24 h) per se resulted in G1 arrest (89.2%), whereas 21.9% of cells exhibited evidence of apoptosis. Addition of TGFβ reduced in half the apoptosis induced by serum deprivation (21.9 vs. 10.8%) but had no detectable effect on NMuMG cell cycle distribution as measured by flow cytometry (Figure 8A). Consistent with its antiapoptotic effect, these results, addition of TGFβ to serum-deprived cells increased cell number 90% above untreated control cells after 72 h (Figure 8B). These data suggest that TGFβ-mediated signals that result in growth inhibition may be independent from those involved in the blockade of apoptosis and enhanced survival.
Figure 8.
TGFβ inhibits cell cycle progression of proliferating NMuMG cells but protects serum-starved NMuMG cells from apoptosis. (A) NMuMG cells seeded at the density of 106 cells/dish in 60-mm dishes were incubated in DMEM/10% FCS or serum-free DMEM, each ± 2 ng/ml TGFβ. After 24 h, cells were harvested and analyzed for cell cycle and apoptosis as described in MATERIALS AND METHODS. (B) NMuMG cells (106 cells/dish) in 60-mm dishes were incubated in DMEM/10% FCS or serum-free DMEM, each ± 2 ng/ml TGFβ for 72 h. At this time, the monolayers were washed with PBS and the adherent cells were trypsinized and counted with the use of a Coulter counter. Each bar represents the mean ± SD of four dishes.
DISCUSSION
Results presented herein with mammary epithelial cells and skin keratinocytes indicate that TGFβ induced phosphorylation and nuclear exclusion of both the endogenous and transfected Forkhead transcription factor FKHRL1. LY294002, a small molecule inhibitor of the p110 catalytic subunit of PI3K, blocked these effects, suggesting that TGFβ-mediated regulation of FKHRL1 required PI3K function. LY294002 did not inhibit TGFβ-induced PAI-1 luciferase reporter activity in Mink lung epithelial cells (our unpublished data). This suggests that the blockade of TGFβ effects on FKHRL1 by LY294002 was not due to inhibition of TGFβ type I receptor (TβRI) kinase activity. Moreover, dn Akt did not prevent TGFβ-induced C-terminal phosphorylation of Smad2 in HaCaT cells (Figure 3C) nor in NMuMG cells (Bakin et al., 2000), implying further that blockade of TGFβ action on FKHRL1 by dn Akt did not involve an effect on TβRI kinase activity.
Several results implicated activation of Akt as an effector mechanisms for FKHRL1 regulation by TGFβ. First, a mutant of Forkhead in which all three Akt phosphorylation sites have been replaced with Ala localized exclusively in the nucleus of three cell lines (NMuMG, 4T1, and HaCaT) and was insensitive to TGFβ-mediated retention in the cytosol. Second, in HaCaT cells, inducible kinase-inactive Akt blocked the effects of TGFβ on FKHRL1 Ser253 phosphorylation, nuclear exclusion, and transcriptional activity. Third, in serum-starved NMuMG and 4T1 cells, the transcriptional activity of TM-FKHRL1 was higher than that of WT-FKHRL1. However, the TM-FKHRL1-mediated transcription was still inhibited by TGFβ, suggesting that the mutant construct was unable to override endogenous Forkhead factors potentially regulated by the addition of TGFβ. Thus, we speculate that the reduction in reporter activity mediated by TGFβ in TM-FKHRL1-expressing cells (Figure 2B) might be conferred by the endogenous FKHRL1, which is still subjected to Akt-mediated phosphorylation. Finally, the apoptotic effect of WT-but not TM-FKHRL1 was abrogated by TGFβ as long as Akt was functional. Nonetheless, transfection of a FKHRL1 constructs in which the DNA-binding domain was mutated (H212R) did not prevent TGFβ-induced protection from cell death (our unpublished data). The inability of this ectopic protein with intact Akt phosphorylation sites to reduce the antiapoptotic effect of TGFβ implied that Akt-mediated phosphorylation of FKHRL1 may not be a saturable process. Although the induced dn Akt was effective in blocking TGFβ-induced nuclear exclusion and cytosolic retention of FKHRL1, it only partially induced nuclear localization of FKHRL1 in the presence of serum without added TGFβ (Figure 4). It is conceivable that other activities not affected by dn Akt, such as serum- and glucocorticoid-induced kinases (SGKs; Brunet et al. 2001), may also regulate FKHRL1 localization and/or function in these cells, potentially explaining the cytosolic retention of FKHRL1 despite expression of dn Akt shown in Figure 4. This possibility will require further experiments beyond the scope of this report.
The potent antiapoptotic effect of Akt and its disabling effect of FKHRL1 function suggested that FKHRL1 may induce apoptosis in the epithelial cells used in our studies. Indeed, overexpression of both WT- and TM-FKHRL1 induced NMuMG cell death as implied by nuclear fragmentation in cells also expressing either the ectopic WT- or TM-FKHRL1 construct (Figure 5A). Apoptosis was not observed with transient transfection of a mutant FKHRL1 that lacked DNA-transactivating function. Notably, transient transfection efficiencies were consistently low (≅3%) with the WT and TM constructs but much higher (≅15%) with FKHRL1 ΔTA, further supporting a causal association between FKHRL1 expression and apoptosis. Moreover, the nuclear localization of FKHRL1 in serum-starved NMuMG cells preceded the onset of apoptosis, implying that cell death is subsequent to the nuclear localization of FKHRL1 and that the latter was not due to an indirect effect of the apoptotic program on nuclear export. Supporting this possibility is the fact that, despite inducing high levels of apoptosis, TM-FKHRL1 was consistently localized in the nucleus (Figures 2A, 4, and 5A). Consistent with the transcriptional reporter activity driven by an FHRE (Figure 2B), the proportion of apoptotic cells was higher in NMuMG cells transfected with TM- than with WT-FKHRL1 (Figure 5A; see RESULTS), implying that a low level of basal Akt activity is able to ameliorate the cell death induced by WT-FKHRL1. It is conceivable that inhibition of other proapoptotic molecules beyond the scope of this report, such as BAD, caspase 9, or IκKs (Datta et al., 1999), are involved in Akt-mediated protection from cell death. However, in preliminary experiments we have been unable to detect increased caspase 9 activity in serum-starved NMuMG cells.
Our data concur with those in other reports. Tang et al. (1999) showed that another Forkhead ortholog (FKHR), in which all three Akt phophorylation sites have been mutated to Ala (TM-FKHR), induced features of apoptosis as membrane blebbing and DNA fragmentation 48 h after transfection into 293T cells. Cells transfected with WT-FKHR showed minimal evidence of apoptosis despite expressing higher level of WT-FKHR than TM-FKHR. In addition, a mutation in the DNA-binding domain of FKHR reduced the ability of this expression vector to induce apoptosis (Tang et al., 1999). In this report (Figure 6B), a mutant FKHLRL1 lacking its transactivation domain failed to elicit apoptosis, implying that both DNA binding and activation of transcription are required for the occurrence of Forkhead-induced apoptosis. Brunet et al. (1999) reported induction of apoptotic cell death by a triple-Akt-sites-mutant of FKHRL1 in cerebellar neurons, CCL39 fibroblasts, and Jurkat T cells. They also provided some evidence that FKHRL1-induced apoptosis was mediated in part by its ability to induce transcription of the FasL gene. Recently, it was reported that TGFβ could decrease apoptosis of human T cells while inhibiting the expression of FasL (Genestier et al., 1999). In PC12 cells, removal of nerve growth factor results in increased JNK activation, enhanced FasL expression, and neuronal cell death (Le-Niculescu et al., 1999). In addition, studies in T lymphocytes have shown that forced expression of FKHRL1 up-regulates the anti-Bcl-2 molecule Bim concomitant with the induction of apoptosis (Dijkers et al., 2000b). Both Forkhead factors AFX and FKHRL1 also have been shown to induce transcription of the cyclin-dependent kinase inhibitor p27Kip1(Dijkers et al., 2000a). This allows for effectors of PI3K, via phosphorylation of Forkhead factors and inhibition of p27 gene transcription, to regulate cell proliferation in addition to cell survival. However, because we did not observe changes in cell cycle distribution in TGFβ-protected cells, which would have been expected from down-regulation of p27, we did not pursue the role of this cyclin-dependent kinase inhibitor on TGFβ-mediated enhanced survival.
The effect of TGFβ on apoptosis has been investigated in different cell systems. In some cells, TGFβ is a potent inducer of apoptosis (Selvakumaran et al., 1994; Lømo et al., 1995; Sánchez et al., 1996), whereas in others it can effectively inhibit apoptosis (Sachsenmeier et al., 1996; Chin et al., 1999; Saile et al., 1999; Huang et al., 2000). In human keratinocytes, the apoptotic cell death induced by loss of anchorage is attenuated by both endogenous and exogenous TGFβ (Sachsenmeier et al., 1996). In this study, TGFβ-neutralizing antibodies enhanced DNA fragmentation after cell suspension, indicating that endogenous TGFβ, via autocrine signaling, may mediate the enhanced survival. The apoptosis precipitated by serum starvation in macrophages is also prevented by exogenous TGFβ via mitogen-activated protein kinase (Chin et al., 1999). In A549 lung adenocarcinoma cells, the antiapoptotic effect of TGFβ requires modulation of JNK activity and phosphorylation of c-Jun (Huang et al., 2000). In addition, TGFβ was reported to act as a survival factor to prevent c-Myc induced cell death in Rat-1 fibroblasts and this response was independent of any effect on cell cycle progression. Expression of dominant-negative forms of various components of the JNK signaling pathway, including Rac1, Cdc42, MKK4, and c-Jun abolished TGFβ-induced survival (Mazars et al., 2000). In our studies with NMuMG cells, the survival effect of TGFβ was clearly dissociated from its antimitogenic effect (Figure 8). Although it is still conceivable that the same Smad-mediated transcriptional responses that induce epithelial cell cytostasis mediate the antiapoptosis effect of TGFβ, a requirement of Smad signaling for the regulation of FKHRL1 and the prevention of cell death in epithelial cells requires further investigation. In a recent study, however, overexpression of Smad2 almost completely abrogated the JNK-dependent survival effect of TGFβ (Mazars et al., 2000), suggesting that Smad signaling was independent and antagonistic of the latter cellular response.
In summary, our results suggest that FKHRL1-dependent transcription may play a role in inducing apoptotic epithelial cell death and that TGFβ partially reverses this effect by mechanism(s) involving Akt-dependent phosphorylation and nuclear exclusion of FKHRL1. We recently reported that PI3K function and its effector kinase Akt are required for TGFβ-mediated fibroblastic transition and cell migration in epithelial cells (Bakin et al., 2000), events involved in the metastatic progression of carcinomas. Transfection of dominant-negative TβRII constructs that disable autocrine TGFβ have been shown to inhibit this mesenchymal transdifferentiation and reduce tumor cell invasiveness and metastases (Oft et al., 1998; Portella et al. 1998; Yin et al., 1999; McEarchern et al., 2001) suggesting that via subversion of an epithelial phenotype, autocrine/paracrine TGFβ can contribute to the metastatic progression of epithelial cancers. Based on the data presented, we propose that down-regulation of Forkhead-dependent transcription and its subsequent positive effect on epithelial cell survival is an integral part of a multisignaling program by which TGFβ contributes to epithelial transformation and tumor progression.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants R01-CA62212 (to C.L.A.) and R01-CA81088 (to U.R.), Department of Defense U.S. Army grant DAMD17-98-1-8262 (to C.L.A.), a Clinical Investigator Award from the Department of Veteran Affairs (to C.L.A.), and Vanderbilt-Ingram Cancer Center National Cancer Institute support grant CA68485.
Abbreviations used:
- dn
dominant-negative
- FasL
Fas ligand
- FCS
fetal calf serum
- FHRE
Forkhead response element
- JNK
c-Jun NH2-terminal kinase
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol 3 kinase
- TβRI
TGFβ type I receptor
- Tet
tetracycline
- TGFβ
transforming growth factor β
- TM
triple mutant
- WT
wild-type
REFERENCES
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates, and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases, and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition, and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the Forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse D, Doughty RS, Ramsey TT, Russell WE, Price JO, Flanagan WM, Shawver LK, Arteaga CL. Reversible G1 arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27KIP1 independent of MAPK activity. J Biol Chem. 2000;275:6987–6995. doi: 10.1074/jbc.275.10.6987. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chin BY, Petrache I, Choi A M, K, Choi ME. Transforming growth factor β1 rescues serum deprivation-induced apoptosis via the mitogen-activated protein kinase (MAPK) pathway in macrophages. J Biol Chem. 1999;274:11362–11368. doi: 10.1074/jbc.274.16.11362. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- del Peso L, González VM, Hernández R, Barr FG, Núñez G. Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene. 1999;18:7328–7333. doi: 10.1038/sj.onc.1203159. [DOI] [PubMed] [Google Scholar]
- del Peso L, González-García M, Page C, Herrera R, Núñez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NSB, Lam EW-F, Burgering BMT, Raaijmakers JAM, Lammers J-WJ, Koenderman L, Coffer PJ. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27KIP1. Mol Cell Biol. 2000a;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers PF, Medemadagger RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHRL1. Curr Biol. 2000b;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Genestier L, Kasibhatla S, Brunner T, Green DR. Transforming growth factor β1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med. 1999;189:231–239. doi: 10.1084/jem.189.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR, and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- Hartsough MT, Frey RS, Zipfel PA, Buard A, Cook SJ, McCormick F, Mulder KM. Altered transforming growth factor β signaling in epithelial cells when ras activation is blocked. J Biol Chem. 1996;271:22368–22375. doi: 10.1074/jbc.271.37.22368. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hutter D, Liu Y, Wang X, Sheikh MS, Chan AM-L, Holbrook NJ. Transforming growth factor-β1 suppresses serum deprivation-induced death of A549 cells through differential effects on c-Jun, and JNK activities. J Biol Chem. 2000;275:18234–18242. doi: 10.1074/jbc.M909431199. [DOI] [PubMed] [Google Scholar]
- Imai K, Takeshita A, Hanazawa S. TGF-β inhibits lipopolysaccharide-stimulated activity of c-Jun N-terminal kinase in mouse macrophages. FEBS Lett. 1999;456:375–378. doi: 10.1016/s0014-5793(99)00988-6. [DOI] [PubMed] [Google Scholar]
- Jost M, Hugget TM, Kari C, Boise LH, Rodeck U. Epidermal growth factor receptor-dependent control of keratinocyte survival, and Bcl-xL expression through a MEK-dependent pathway. J Biol Chem. 2001;276:6320–6326. doi: 10.1074/jbc.M008210200. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signaling through PI(3)K, and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Burgering BM. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med. 1999;77:656–665. doi: 10.1007/s001099900050. [DOI] [PubMed] [Google Scholar]
- Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4, and SMAD proteins in TGFβ signaling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Bonfoco E, Kasuya Y, Claret F-X, Green DR, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction, and cell death. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo J, Blomhoff HK, Beiske K, Stokke T, Smeland EB. TGF-β1 and cyclic AMP promote apoptosis in resting human B lymphocytes. J Immunol. 1995;154:1634–1643. [PubMed] [Google Scholar]
- Massagué J. TGFβ signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen Y-G. Controlling TGF-β signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Massagué J, Wotton D. Transcriptional control by the TGFβ/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazars A, Tournigand C, Mollat P, Pruiner C, Ferrand N, Bourgeade M-F, Gespach C, Atfi A. Differential roles of JNK and Smad2 signaling pathways in the inhibition of c-Myc-induced cell death by TGFβ. Oncogene. 2000;19:1277–1287. doi: 10.1038/sj.onc.1203420. [DOI] [PubMed] [Google Scholar]
- McEarchern, et al. Invasion and metastasis of a mammary tumor involves TGFβ signaling. Int J Cancer. 2001;91:76–82. doi: 10.1002/1097-0215(20010101)91:1<76::aid-ijc1012>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck RJ. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- Oft M, Heider K-H, Beug H. TGFβ signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol. 1998;8:1243–1252. doi: 10.1016/s0960-9822(07)00533-7. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Forkhead transcription factor DAF-16 transduces insulin-like metabolic, and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Piek E, Moustakas A, Kurisaki A, Heldin C-H, ten Dijke P. TGF-β type I receptor/ALK-5, and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112:4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- Portella G, Cumming SA, Liddell J, Cui W, Ireland H, Akhurst RJ, Balmain A. Transforming growth factor β is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ. 1998;9:393–404. [PubMed] [Google Scholar]
- Sachsenmeier KF, Sheibani N, Schlosser SJ, Allen-Hoffmann BL. Transforming growth factor-β1 inhibits nucleosomal fragmentation in human keratinocytes following loss of adhesion. J Biol Chem. 1996;271:5–8. doi: 10.1074/jbc.271.1.5. [DOI] [PubMed] [Google Scholar]
- Saile B, Matthes N, Knittel T, Ramadori G. Transforming growth factor β and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology. 1999;30:196–202. doi: 10.1002/hep.510300144. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Alvarez AM, Benito M, Fabregat I. Apoptosis induced by transforming growth factor-β in fetal hepatocyte primary cultures: involvement of reactive oxygen intermediates. J Biol Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- Selvakumaran M, Reed JC, Liebermann D, Hoffmann B. Progression of the myeloid differentiation program is dominant to transforming growth factor β1-induced apoptosis in M1 myeloid leukemic cells. Blood. 1994;84:1036–1042. [PubMed] [Google Scholar]
- Stephens L, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PRJ, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-triphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Takaishi H, Konishi H, Matsuzaki H, Ono Y, Shirai Y, Saito N, Kitamura T, Ogawa W, Kasuga M, Kikkawa U, Nishizuka Y. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc Natl Acad Sci USA. 1999;96:11836–11841. doi: 10.1073/pnas.96.21.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang ED, Núñez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Yin, J.J., Selander, K,.Chirgwin, J.M., Dallas, M., Grubbs, B.G., Wieser, R., Massagué J., Mundy, G.R., and Guise, T.A. (1999). TGFβ signaling blockade inhibits PTHrP secretion by breast cancer cells, and bone metastases development. J. Clin. Invest. 103, 197–206. [DOI] [PMC free article] [PubMed]