Abstract
Opportunities for restorative sleep and optimal sleep-wake schedules are becoming luxuries in industrialized cultures, yet accumulating research has revealed multiple adverse health effects of disruptions in sleep and circadian rhythms, including increased risk of breast cancer. The literature on breast cancer risk has focused largely on adverse effects of night shift work and exposure to light at night (LAN), without considering potential effects of associated sleep disruptions. As it stands, studies on breast cancer risk have not considered the impact of both sleep and circadian disruption, and the possible interaction of the two through bidirectional pathways, on breast cancer risk in the population at large. We review and synthesize this literature, including: 1) studies of circadian disruption and incident breast cancer; 2) evidence for bidirectional interactions between sleep and circadian systems; 3) studies of sleep and incident breast cancer; and 4) potential mechanistic pathways by which interrelated sleep and circadian disruption may contribute to the etiology of breast cancer.
Keywords: Breast cancer, Circadian disruption, Sleep disruption, Sleep, Sleep duration, Light at night, Night shift work, LAN, Mammary oncogenesis, Circadian rhythms, Cancer
1. Introduction
Breast cancer is the most common cancer in women and the principal cause of death from cancer among women worldwide (DeSantis et al., 2014). Recently, the National Cancer Institute projected that the total number of breast cancer cases in the United States will be 50% greater by 2030 than it was in 2011 (Rosenberg et al., 2015). Given the increasing personal and global health burden posed by this disease, there is a need to identify potentially modifiable behavioral risk factors that may illuminate new opportunities for cancer prevention and targets for intervention.
Researchers have identified a number of risk factors for breast cancer, including penetrant gene mutations (Walsh et al., 2016) and genetic polymorphisms (Dunning et al., 1999), race (Chlebowski et al., 2005), age (Smigal et al., 2006), and breast density (McCormack and dos SS, 2006). Many of the identified risk factors are non-modifiable, however, and thus far, only a small percentage of breast cancer risk has been successfully linked to genetic inheritance (Oldenburg et al., 2007; Ghoussaini et al., 2013). Considerable research has investigated the associations between daytime behaviors, such as alcohol consumption (Smith-Warner et al., 1998), smoking (Xue et al., 2011), diet (Boyd, 2003), and physical activity (Vainio et al., 2002), and individual risk for breast cancer. However, much less attention has been paid to the one-third of our daily experience that occurs during sleep. The research that has been conducted to date on nocturnal behaviors and incident cancer has focused largely on exposure to light at night (LAN) and night shift workers, a population with high LAN exposure. There is considerable evidence supporting a role for LAN in cancer risk, particularly in night shift workers, and this evidence has been thoroughly reviewed elsewhere (Haus and Smolensky, 2013; Kochan and Kovalchuk, 2015; Stevens et al., 2014). However, we propose that the LAN model of circadian disruption for oncogenesis is incomplete without considering the contributions of a densely interrelated process: sleep.
The present review therefore builds upon the framework established in these previous models in three innovative and important ways: 1) we present converging trans-disciplinary empirical evidence to support expanding the LAN model of cancer risk to include sleep disruption, above and beyond night shift work; 2) we propose that sleep disruption and circadian disruption may be risk factors for all individuals, regardless of shift work status; and 3) we focus our review specifically on incident breast cancer, given that the heterogeneity of cancers makes broader generalizations and associations challenging to support.
Three points are integral to this review: First, the theoretical mechanisms of LAN exposure for cancer risk are not limited to night shift workers. The average human in industrialized societies today is regularly exposed to a significant degree of LAN and chronodisruption, regardless of shift work status, particularly in relation to the recent surge in technological availability and nighttime use (Vijakkhana et al., 2015; Levenson et al., 2016) and overall country-level LAN (Kloog et al., 2010; Stevens and Zhu, 2015). Furthermore, in all humans, changes in circadian rhythms are often accompanied by changes in sleep duration, continuity, and/or timing. Adaptive sleep behaviors and sleep-wake rhythms are foundational for maintaining optimal functioning (Buysse et al., 2015), and disruptions in these systems are associated with adverse health outcomes (see Cappuccio et al., 2011; Gallicchio and Kalesan, 2009 for reviews).
Second, the sleep-wake system is strongly and bidirectionally associated with the circadian system, and changes in one affect the other. For example, Borbély’s (1982) two-process model of sleep-wake regulation posits that periods of sleep and wakefulness are determined by the relationship between an endogenous circadian rhythm and a homeostatic sleep drive. Given the growing body of evidence supporting the carcinogenic effects of circadian desynchronization (e.g., Haus and Smolensky, 2013; He et al., 2015a), the influence of the sleep-wake system on circadian functioning should not be overlooked in mechanistic models. Endogenous and exogenous cues that entrain the circadian system are necessary but insufficient for the generation of sleep periods; behavioral, psychological, and social factors also influence the availability and timing of sleep periods and thereby influence circadian rhythms.
Finally, sleep is a critical biobehavioral mechanism for maintaining optimal immune (Irwin, 2002; Lange et al., 2010), cellular (Tononi and Cirelli, 2006; Inoue et al., 1995a), metabolic (Knutson et al., 2007), and endocrine (Leproult and Van, 2010) functioning, although many of these studies fail to account for the potentially mediating effects of concurrent changes in circadian rhythms. In turn, dysfunction in each of these neurophysiological systems is implicated in the oncogenic pathway (Pevet and Challet, 2011a). It is therefore plausible that disruption of the sleep system may represent a direct and largely unexplored risk factor for mammary oncogenesis.
The current paper is an evidence-based review of the associations between circadian disruption, sleep disruption, and breast cancer etiology within the framework of our proposed model (Fig. 1). First, we briefly review the state-of-science on circadian disruption and breast cancer risk (for a longer review, see (Haus and Smolensky, 2013)). Next, we present evidence that supports the bidirectional associations between the circadian and sleep-wake systems. Next, we review the literature on associations between sleep disruption and incident breast cancer. Finally, we explore potential mechanistic pathways linking mammary oncogenesis to circadian disruption and sleep disruption, focusing on the roles of cellular damage via oxidative stress, melatonin, inflammation, and metabolic function.
Fig. 1.
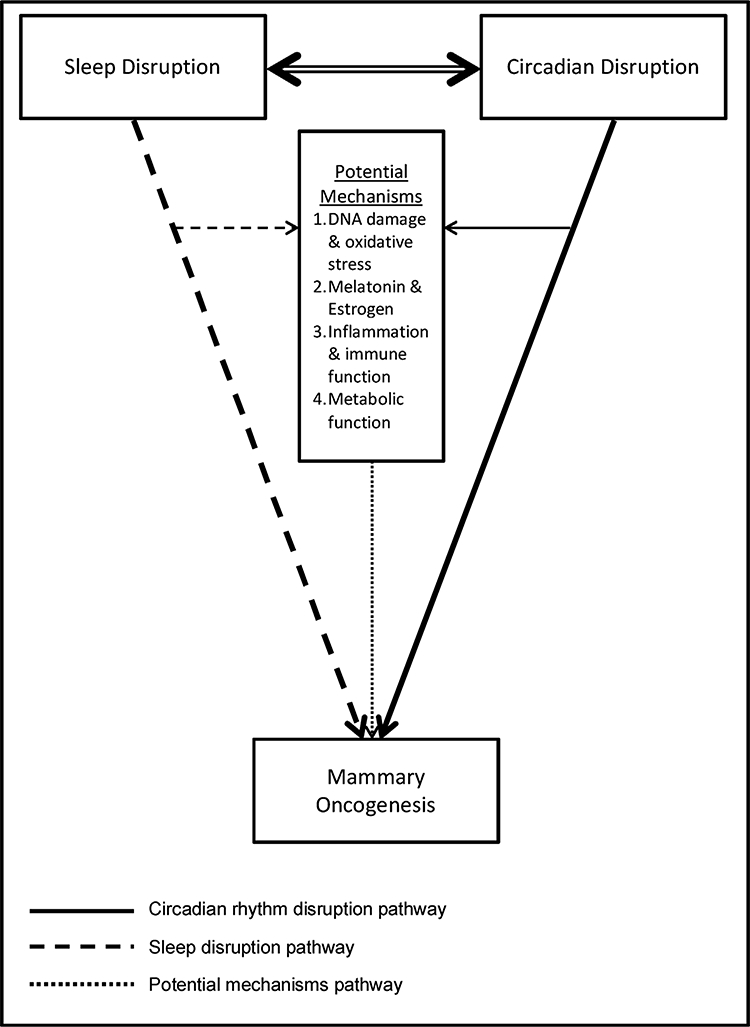
Biopsychosocial Model of Circadian and Sleep Disruption and Mammary Oncogenesis.
1.1. Literature search criteria
To be included in this review, studies had to investigate: 1) sleep disruption (“sleep”, “sleep duration”, “sleep disruption”) and/or 2) circadian disruption (“circadian rhythms”, “circadian disruption”, “circadian dysregulation”, “light at night”, “shift work”) and 3) incident breast cancer (“breast cancer risk”, “breast cancer prevalence”) and/or mechanisms implicated in mammary oncogenesis, including cellular damage, the melatonin-estrogen pathway, inflammation, and neuroendocrine and metabolic function. A literature search was conducted in PubMed and Google Scholar that included all combinations of these keywords. References of selected original and review publications were reviewed for further inclusion. Abstracts and editorials were excluded.
2. The circadian disruption and breast cancer pathogenesis model
Nearly three decades ago, research demonstrated that women reporting a history of night shift work had greater risk of developing breast cancer (Madigan et al., 1995). These findings led to the consideration of light at night (LAN) as both a hallmark of industrialized countries and a plausible pathway to mammary cancer via the pineal gland and melatonin, a LAN pathway model of breast cancer pathogenesis pioneered by Richard Stevens (Stevens et al., 2014; Stevens et al., 1992).
2.1. Overview of the circadian system
The circadian system is a hierarchical organization of endogenous, oscillating clocks throughout the body that are regulated by the su-prachiasmatic nuclei (SCN), located in the hypothalamus (Fuller et al., 2006). The SCN receive input from what are known as zeitgebers, external cues that entrain the internal circadian clock in the SCN to the external 24-h light/dark cycle. For the central clock, the most potent zeitgeber is light input via light sensitive cells located in the retina (Altimus et al., 2008). Importantly, the SCN has diverse projections that allow it to coordinate autonomic, neuroendocrine, and reproductive processes displaying biological rhythms throughout the body, which are implicated in mammary oncogenesis (Kalsbeek and Buijs, 2002). The SCN also projects to the gonadotropin-releasing hormone (GnRH) system in the hypothalamus, allowing for circadian control of the hypothalamic-pituitary-gonadal (HPG) axis (Tonsfeldt and Chappell, 2012). Due to the diversity of SCN outputs, disruption of the SCN results in disruption of numerous coordinated systems, which is the foundational argument underlying the circadian disruption hypothesis in breast cancer.
The output of the SCN results in coordinated, endogenous biological rhythms that are entrained to the 24-h solar light-dark cycle. Early interest in understanding how organisms are entrained to the 24-h solar light-dark cycle revealed the existence of molecular oscillators within single neurons. These “circadian clocks” are encoded by an auto-regulatory transcriptional-translational feedback loop that relies on a cycle of positive and negative feedback co-regulated by CLOCK:BMALl heterodimers within the cell (Ko and Takahashi, 2006). In the positive feedback loop, these heterodimers activate rhythmic transcription of core clock genes, including Period (Per1 and Per2 in humans) and Cryptochrome (Cry1 and Cry2) genes (Reppert and Weaver, 2002). When these PER:CRY heterodimers translocate back into the nucleus, they act on the CLOCK:BMALl complex to repress their own transcription. Circadian oscillation is further regulated by CLOCK:BMALl heterodimer transcription activation of retinoic acid-related orphan nuclear receptors (ROR), Rev-erbα and Rorα. REV-ERBs and RORs competitively bind to ROR response elements in the Bmal1 promoter region to repress or activate transcription of Bmal1, respectively, thus feeding back on the CLOCK:BMALl heterodimer. These autoregulatory feedback loops constitute the circadian molecular clock and take approximately 24 h to complete one cycle (Gachon et al., 2004). Importantly, the transcriptional factors and targets that comprise the molecular oscillator are found not only in the central nervous system but in peripheral tissues, including the breast (Schibler and Sassone-Corsi, 2002). Additionally, core clock genes regulate clock-controlled genes (CCGs), many of which are also integral cell cycle regulators implicated in tumorigenesis (Sahar and Sassone-Corsi, 2007; Panda et al., 2002), including c-Myc (Lee, 2006), Wee1 (Yokoe et al., 2003), and cyclin D1 (Roy and Thompson, 2006), although the effects of these cell cycle regulators are outside the scope of this review.
In mammals, the timing and regularity of light exposure are critical for optimal circadian rhythmicity. Prior to the invention of artificial light, environmental light exposure was confined to the day, resulting in alignment of the solar light/dark cycle and endogenous circadian rhythms. Exposure to light at night (LAN) results in misalignment between the central clock and the solar light-dark cycle, with downstream effects on neuroendocrine and behavioral processes, such as melatonin suppression (Thapan et al., 2001) and sleep disruption (de et al., 2012). Variability in exposure to LAN results in perturbations of circadian synchrony and can alter the phase of physiological processes under circadian control, including sleep (Czeisler et al., 1999).
2.2. The “light-gated” pathway of circadian disruption and breast cancer
Circadian disruption is hypothesized to increase risk for breast cancer via disruption of central and peripheral clocks, resulting in widespread dysrégulation of clock-controlled biological processes and melatonin suppression, processes that have been thoroughly reviewed elsewhere (Haus and Smolensky, 2013). Clock genes and circadian regulation are involved in virtually all cellular processes underlying cancer initiation, including cell cycle regulation, DNA damage and repair, cellular apoptosis and survival, and cell proliferation (Truong et al., 2014). In order to understand why exposure to LAN can have such potent downstream—and ultimately carcinogenic—effects, it is critical to first understand circadian gating of light. In humans, synchrony between light exposure and the solar light-dark cycle is necessary for optimal expression of numerous physiological and behavioral processes. However, the roughly 24.18-h endogenous human circadian period is slightly longer than the 24-h solar period (Czeisler et al., 1999) and therefore requires “resetting” each day to in order to re-align with the solar photoperiod (Czeisler et al., 1986). To achieve this, the circadian system must be highly sensitized to and able to integrate photic and temporal information in order to anticipate and adapt to environmental changes. For diurnal animals such as humans, light exposure is expected during the middle of the subjective or solar day and therefore has little effect on altering circadian rhythms. Light exposure during the solar night and near dawn or dusk, however, is a potent entrainment cue for the circadian system and is effective in phase shifting the central clock, as well as initiating a cascade of neuroendocrine processes under circadian control. This “light-gated” model of circadian disruption begins with photic initiation of the core clock transcriptional-translational feedback loop out of phase with the solar light-dark cycle, for example due to LAN exposure from shift work or during nocturnal awakenings from sleep. Light at night suppresses melatonin (N-acetyl-5-methoxy-trypamine), a potent pleiotropic hormone released from the pineal gland that displays a diurnal pattern with maximal expression at night (Brainard et al., 2001; Buman et al., 2015). Melatonin is both an output of the central circadian system and a key regulator of central and peripheral oscillators, including oscillators found in breast tissue (Stehle et al., 2003), and has been identified as a critical modulator in breast cancer development.
2.3. The circadian system and melatonin
Melatonin entrains peripheral clocks to the SCN in organs that express the receptors melatonin 1 (MT1) and melatonin 2 (MT2); (Slominski et al., 2012). Suppression of melatonin is influenced by light duration, irradiance, and wavelength (Reiter, 1985). Melatonin suppression is dose-responsive, with maximal suppression at 3000 lx, and wavelength-dependent, maximally affected by blue wavelength (440–460λ); (Thapan et al., 2001). Recent evidence has demonstrated that exposure to irradiance of as little as 1 lx of 440–160 wavelength light can lower melatonin expression by ∼8% (Glickman et al., 2002). The low levels of light to which we are exposed in the evening, from TV, phone, and computer screens and incandescent light bulbs, are sufficient to cause phase delays in the circadian system (Gooley et al., 2011; Chellappa et al., 2011).
Melatonin plays a regulatory role in the HPG axis, suppressing ovarian estrogen synthesis (Bondi et al., 2014). Melatonin is also a potent repressor of estrogen-induced estrogen-receptor-α (ERα) transcriptional activity (Ram et al., 2002), an action that is attenuated by LAN exposure. Elevated levels of ovarian hormones contribute to the development of breast cancers (e.g., (Shafie and Grantham, 1981; Ziegler et al., 2015), and high levels of endogenous estrogen are associated with increased risk of incident breast cancer in both pre- and post-menopausal women (Key et al., 2013), respectively). These findings highlight the importance of an estrogen-suppressing mechanism such as melatonin in a model of breast cancer pathogenesis.
2.4. Classification of light at night and/or shift work as carcinogenic to humans
In 2007, the International Agency for Research on Cancer (IARC) Working Group concluded that “shift-work that involves circadian disruption is probably carcinogenic to humans” (group 2A) on the basis of “sufficient evidence in experimental animals for the carcinogenicity of light during the daily dark period (biological night)” and “limited evidence in humans for the carcinogenicity of shift-work that involves night work”. In 2012, the American Medical Association House of Delegates adopted a similar policy regarding light at night. In their Executive Summary (Blask et al., 2012), the ΑΜΑ report states that nighttime lighting includes “potential carcinogenic effects related to melatonin suppression, especially breast cancer”. The AMA report cites evidence from laboratory models of cancer of the role of melatonin as an anticancer and tumor suppressor agent, as well as limited epidemiological studies on LAN and/or disruption of circadian rhythm on breast cancer risk. While the IARC Working Group statement emphasizes the risks to night shift workers, the AMA statement broadens the risk to all persons exposed to nighttime lighting.
Importantly, the IARC published a follow-up paper (Stevens et al.,2011) advocating a movement toward greater specificity in the definition of night shift work as a cancer risk factor. The working group identified several domains that may contribute to variability in cancer risk: “shift system”, comprised of timing of shift work, length of shift work per 24-h period, permanent or rotating, and regularity of shift work; cumulative shift work exposure in years over the individual’s lifetime; and intensity of shift work, calculated as time off between shift work days. Significant variability in the operational definition and characterization of NSW across studies may account for some of the discrepancies in risk observed across prospective studies, as described below.
2.5. Associations among circadian disruption, light at night, melatonin, and breast cancer
2.5.1. Melatonin: in vivo and in vitro studies
Melatonin has the distinction of being the first identified nocturnal anticancer signal in humans that links the central circadian clock with breast carcinogenesis (Blask, 2009). Melatonin release is dependent on both the pineal gland and the SCN, with nocturnal release of this hormone gated by the SCN (Pevet and Challet, 2011b; Shah et al., 1984), suggesting that disruptions in the SCN, either via sleep and/or circadian disruption, may also alter melatonin release.
2.5.2. Reproductive hormones: in vivo and in vitro studies
The central circadian system exerts multiple modulatory effects on the synthesis and release of reproductive hormones via the hypothalamic-pituitary-gonadal axis and expression of hormone receptors. There is evidence that ERα expression is directly modulated by the core clock gene Per2, and the transcriptional capability of ERα in healthy and cancerous breast cells displays periodicity (Tonsfeldt and Chappell,. 2012). Deregulation of Per1, Per2, and Per3 genes in breast cancer cells has also been detected (Chen et al., 2005). Clock genes regulate the HPG axis and affect reproductive viability. Clock mutant mice are subfertile and have lengthened estrous cycles (Chappell et al., 2003; Miller et al., 2004), and Bmal1 knockout mice are infertile (Boden et al., 2010; Ratajczak et al., 2009). Furthermore, the LH surge shows a circadian release pattern in the presence of estradiol (Christian et al., 2005), while SCN lesions abolish this (Schwartz and Zimmerman, 1991). These data support the role of the central clock in coordinating reproductive rhythms. Mechanisms that disrupt the circadian system are therefore capable of dysregulating the HPG axis.
2.5.3. Case-control and cohort studies
Converging evidence suggests that sources of circadian disruption, such as LAN and shift work, similarly disrupt melatonin in humans and have oncogenic effects. Blask’s group (Blask et al., 2005a) extended their animal research findings by testing the blood of human volunteers exposed to bright ( ~ 2800 lx) LAN, finding that LAN abolished the tumor suppressive effects of melatonin and that these effects were restored by the addition of melatonin to the blood. In a prospective case-control study from the Nurses’ Health Study cohort of 18,643 cancer-free women, those with the highest morning melatonin, as measured by levels of the primary metabolite 6-sulfatoxymelatonin (aMT6s) in first morning urine, had the lowest risk of incident breast cancer (Schernhammer and Hankinson, 2009). A related prospective case-control study from the Nurses’ Health Study II cohort of 147 women with invasive breast cancer and 291 matched controls found that women with the highest levels of aMT6 s also had the lowest risk of invasive breast cancer (Schernhammer and Hankinson, 2005). A retrospective case-control study found that breast cancer risk was increased in women who reported frequently not sleeping during the time of night when melatonin levels peak ( ~ 1:00 a.m.) in the 10 years prior to diagnosis (Davis et al., 2001). A meta-analysis of five prospective case-control studies indicated an overall inverse association between incident breast cancer risk and higher levels of urinary aMT6s (Basler et al., 2014).
Researchers have also investigated associations between other sources of LAN and incident breast cancer. One case-control study found that keeping the lights on while sleeping, not drawing the shades while sleeping at night, and sleeping in the daytime were all associated with slight but non-significant increases in breast cancer risk (Li et al., 2010). More compelling evidence for the risks of LAN comes from a case-control study of 1679 women (Kloog et al., 2011), where greater bedroom-light intensity while sleeping was associated with increased breast cancer risk (OR = 1.22, 95% CI = 1.12–1.31). Recently, it has been demonstrated that ipRGCs are able to receive and transmit photic input even through closed eyelids, and brief flashes of light administered to sleeping individuals induced both shifts in circadian phase and melatonin suppression (Figueiro et al., 2013). It is therefore plausible that exposure to ambient bedroom LAN could induce sufficient circadian disruption to be potentially oncogenic.
There is strong evidence to suggest that women working night shifts are at increased risk for incident breast cancer. In a comparison of Danish night and day shift workers, women working night shifts had significantly decreased urinary aMT6s concentrations than day shift workers (Marie et al., 2006). A case-control study in France found that there was an increased rate of breast cancer in women who had ever worked night shifts (Menegaux et al., 2013). Women employed in night work for more than 4 years prior to their first pregnancies had the highest rates of incident breast cancer (OR = 1.95, 95% CI = 1.13–3.35). These finding corroborate a Danish nurse case-control study (Hansen and Stevens, 2012), where night shifts workers had increased risk for breast cancer (OR = 1.8; CI 1.2–2.8). Permanent night shift work was associated with the greatest risk. A population-based case-control study in Australia found a 22% increase in breast cancer risk with frequently rotating night shift schedules, and a trend for increased risk for women ever working 12:00–5:00 a.m. (Smith et al., 2013). However, in a large, prospective Dutch cohort study of incident breast cancer, neither occasional nor regular night work was associated with hospital admission for breast cancer during the 7-year follow-up (Koppes et al., 2014). While these findings certainly run contrary to the previous studies, the study is limited by using hospital admission as its primary outcome measure.
2.5.4. Meta-Analyses
Overall, the majority of results from nine published meta-analyses of the associations between circadian disruption and incident breast cancer support the circadian disruption model of breast cancer pathogenesis (see Table 2). Eight of the meta-analyses reported an overall increased incidence of breast cancer (ranging from 5 to 48%) in women exposed to some form of circadian disruption, including lifetime exposure to night shift work and LAN (He et al., 2015a; Megdal et al., 2005; Jia et al., 2013; Kamdar et al., 2013; Wang et al., 2013; Ijaz et al., 2013; Yang et al., 2014; Lin et al., 2015). Furthermore, six of the metaanalyses conducted sub-analyses on years and nights of exposure, with converging evidence from five of the six studies suggesting that circadian disruption is associated with increased risk in a dose-dependent manner (Wang et al., 2013; Ijaz et al., 2013; Yang et al., 2014; Lin et al., 2015; He et al., 2015b). Kamdar and colleagues (Kamdar et al., 2013) did not find that participants working 8 or more years of NSW were at greater risk for developing breast cancer compared to non-shift workers, and Jia and colleagues (Jia et al., 2013) found that women working 15 or more years of NSW were not at increased risk above and beyond women who reported ever working night shift. The most recent meta-analysis (Travis et al., 2016) which included previously unpublished data from three prospective studies along with data from 7 published studies, found no significant associations among NSW and incident breast cancer risk (RR = 0.99, 95% CI = 0.95–1.03). However, as with previous meta-analyses, there was significant heterogeneity in the characterization of NSW as a risk factor across the included studies. Moreover, as was identified in a recently published response to the Travis et al. meta-analysis, the baseline age of women in the paper’s 3 prospective studies ranged from early-50 s to upper-60 s with follow-up periods of only 2–3 years (Travis et al., 2016; Schernhammer, 2017). This makes it challenging to support the authors’ claim that the “melatonin hypothesis” for breast cancer risk lacks merit, but gives credence to the IARC Working Group’s {IARC, 2009} argument for establishing greater consistency in operationally defining NSW as a risk factor.
Table 2.
Meta-analyses of circadian disruption and incident breast cancer risk.
| Citation | Number of studies | Study Types | Predictor Variables | Overall Odds Ratio (OR) or Relative Risk (RR) |
|---|---|---|---|---|
| Koppes et al. (2014) | 6 | 4 cohort, 2 case-control | NSW | 1.48 (95% Cl = 1.36–1.61) |
| Megdal et al. (2005) | 13 | 5 cohort, 8 case-control | NSW | 1.20 (95% Cl = 1.08–1.33) |
| Kamdar et al. (2013) | 10 | 3 cohort, 5 case-control | NSW | 1.19 (95% Cl = 1.05–1.35) |
| Wang et al. (2013) | 12 | 1 cohort, 11 case-control | NSW | 1.05 (95% Cl = 1.01–1.10) for 5-year NSW |
| Jia et al. (2013) | 15 | 5 cohort, 10 case-control | NSW | 1.21 (95% Cl = 1.00–1.47) |
| Ijaz et al. (2013) | 5 | 5 case-control | LAN exposure | LAN: 1.17 (95% Cl = 1.11–1.24) |
| He et al. (2015a) | 18 (n = 12 NSW; n = 3 LAN; n = 3 NSW and LAN) | NSW: 5 cohort, 7 case-control; LAN: 3 case-control; both: 3 case-control | NSW; LAN exposure; both | NSW: 1.19 (95% Cl = 1.08–1.32); LAN: 1.12 (95% Cl = 1.119–1.121) |
| Yang et al. (2014) | 6 | 6 cohort | NSW | 1.057 (95% Cl = 1.014–1.102) |
| Travis et al. (2016) | 10 | 3 prospective, 7 cohort | NSW | 0.99 (95% Cl = 0.95–1.03) |
Note: NSW = night shift work; LAN = light at night.
While the overall findings from these meta-analyses suggest that circadian disruption is a significant predictor of breast cancer, results should be interpreted with some degree of caution. As mentioned above, many of the meta-analyses combined samples potentially too heterogeneous to be included in one statistical model, including samples of mixed menopausal status or occupation, such as flight attendants, who are exposed to different circadian disruptions than other NSWs, including frequent time zone changes and increased radiation exposure, which may predispose them to increased rates of breast cancer (Rafnsson et al., 2001). Furthermore, latitude and season were not accounted for in these analyses, introducing error due to shortened or lengthened light-dark cycles. However, these results are promising, and suggest that circadian disruption from LAN exposure and night shift work is likely carcinogenic and in a time-dependent manner.
3. Sleep disruption and breast cancer oncogenesis: expanding the model
3.1. Bidirectional associations among sleep and circadian systems
The sleep and circadian systems are yoked, with sleep demonstrating circadian rhythmicity in all vertebrate and invertebrate species in which it has been investigated (Siegel, 2011; Cirelli and Bushey, 2008), including mature humans, who typically spontaneously enter into sleep and wake periods once per day in concordance with the solar light-dark cycle. Sleep behavior is an integrated function of both an innate homeostatic sleep drive and endogenous circadian rhythms, and changes in one system effect changes in the other. Many of these effects are mediated by light, which affects sleep by synchronizing the clock via specialized light-sensitive cells in the retina that project to the SCN (Hankins et al., 2008; Moore and Lenn, 1972). In turn, the SCN has multisynaptic projections to brain areas involved in sleep-wake regulation, including the ventrolateral preoptic (VLPO) and lateral hypothalamus (LH; Fuller et al., 2006). These two systems are therefore inextricably linked, as demonstrated by experimental findings in animals with variants in core circadian clock genes, which show altered sleep phenotypes. For example, Clock mutant mice have shorter sleep periods than wild type (WT) mice (Naylor et al., 2000). Animals with double knockouts of cryptochrome 1 and 2 (Cry1−/−/Cry2−/−) exhibit changes in sleep micro- and macro-architecture, including increased non-REM (NREM) sleep (Wisor et al., 2002). Emall mutant mice show increased sleep fragmentation and duration (Turek et al., 2005). These findings may be mediated by changes in the rhythmicity of postulated wake and sleep agents, orexin and adenosine, which appear to be under a degree of circadian regulation. Orexin activity is increased during wake periods (Estabrooke et al., 2001; Espana et al., 2002), while adenosine is maximally expressed at night in dark conditions (Ribelayga and Mangel, 2005). While they are interrelated, it is important to note that sleep and circadian rhythms are distinct processes. Experimental evidence demonstrates that the sleep-wake rhythm is preserved in the absence of the circadian clock through Opn4-mediated photic input that activates neurons in the VLPO (Pruessner et al., 2008).
Converging empirical evidence suggests that changes in sleep elicit changes in circadian rhythms. For instance, a partial sleep deprivation condition in Syrian hamsters under constant dark conditions induced large circadian phase advances of up to 4 h, suggesting that the neurophysiological effects of sleep deprivation are sufficient to reset the circadian clock (Antle and Mistlberger, 2000). In a follow-up study, adenosine agonist administration induced dose-dependent phase shifts similar to the sleep deprivation condition (Antle et al., 2001). Importantly, these findings were independent of light-dark effects; both the sleep deprivation and AD agonist administration conditions were sufficient to inhibit light-induced phase shifts, above and beyond resetting of the circadian clock. On a molecular level, experimental sleep deprivation affects transcriptional activity of core clock genes bmal1, clock, and cry2 (Wisor et al., 2008). Interpreting these results in the context of a human model, it is plausible that sleep deprivation may disrupt the circadian clock independently of LAN exposure, triggering the pro-oncogenic downstream effects of circadian disruption.
3.2. Systematic review of associations between sleep disruption and incident breast cancer
To date, twelve studies have been published on the relationships between one or more index of sleep and incident breast cancer, with mixed results (see Table 3). Of these, 5 were case-control studies and 7 were cohort studies; the studies were evenly split between prospective and retrospective designs.
Table 3.
Studies of sleep disruption and incident breast cancer risk.
| Citation | Study Type | Number of Participants & Cases | Sample | Sleep Variables | Incident Breast Cancer Risk | Findings |
|---|---|---|---|---|---|---|
| Verkasalo et al. (2005) | Prospective, population-based cohort | N = 12,222; Incident cases n = 242 | Finnish women born before 1958 | Self-reported sleep duration assessed at 1975 and 1981; steady LS (≥9h at both times) | HR = 0.28 (95% Cl 0.09–0.88) steady LS vs. steady 7–8 h sleepers | LS ↓ risk |
| McElroy et al. (2006) | Retrospective, population-based case-control | N = 9347; BCa patients n = 4033 | Invasive breast cancer patients and community controls | Self-reported sleep duration over past 2 yrs.; LS (≥9h) | OR = 1.13 (95% Cl 0.93–1.37) LS vs. 7–8 h sleepers | LS ↑ risk (trend) |
| Pinheiro et al. (2006a,b) | Prospective, cohort | N = 77,418; Incident cases n = 4223 | Nurses’ Health Study | Self-reported habitual sleep duration reported at 1986 and 2000; SS (≤ 5 h); LS (≥ 9 h); steady LS (≥ 9 h at both times) | HR = 0.93 (95% Cl 0.79–1.09) SS vs. 7 h sleepers; HR = 0.95 (95% Cl 0.82–1.11) LS vs. 7 h sleepers Linear trend for sleep duration as continuous variable (p = 0.07) |
≠ LS ↑ risk (trend, continuous sleep predictor) |
| Kakizaki et al. (2008a,b) | Prospective, population-based cohort | N = 23,995; Incident cases n = 143 | Ohsaki National Health Insurance Cohort Study | Self-reported habitual sleep duration; SS (≤ 6 h) | HR = 1.62 (95% Cl 1.05–2.50) SS vs. 7-h sleepers Linear trend for sleep duration as continuous variable (p = 0.03) |
SS ↑ risk |
| Wu et al. (2008) | Prospective, population-based cohort | N = 33,528; Incident cases n = 525 | Singapore Chinese Health Study | Self-reported habitual sleep duration; LS (≥ 9 h) | RR = 0.67 (95% Cl 0.4–1.1) LS vs. <6h sleepers | LS ↓ risk |
| Cairns et al. (2012) | Prospective, cohort | N = 795,238; Incident cases n = 20,058 | The Million Women Study, UK | Self-reported daytime napping frequency (never, sometimes, usually) | RR = 1.06 (95% Cl 1.04–1.08) sometimes nap vs. never; RR = 1.11 (95% Cl 1.07–1.15) usually nap vs. never |
Naps ↑ risk |
| Fritschi et al. (2013) | Retrospective, case-control | N = 2994; BCa patients n = 1205 | Western Australia women | Self-reported “sleep disruption” composite measure | OR = 1.21 (95% Cl 0.95–1.55) “ever” sleep disruption vs. never | Sleep disruption ↑ risk (trend) |
| Wu et al. (2013) | Nested case-control study | N = 991; BCa patients n = 248 | Singapore Chinese Health Study | Self-reported habitual sleep duration | OR = 0.89 (95% Cl 0.64–1.22) LS vs. ≤6 h sleepers | ≠ |
| Girschik et al. (2013a,b) | Retrospective, case-control | N = 2994; BCa patients n = 1205 | Breast Cancer Environment and Employment Study | Self-reported habitual sleep duration; SS (< 6 h); LS (> 8h) | OR = 1.05 (95% Cl 0.82–1.33) SS vs. 7–8 h sleepers; OR = 1.10 (95% Cl 0.87–1.39) LS vs. 7–8 h sleepers |
≠ |
| Vogtmann et al. (2013) | Prospective, cohort | N = 110,011; Incident cases n = 5149 | Women’s Health Initiative | Self-reported sleep duration, sleep quality, sleep disturbance; SS (≤ 5 h); LS (≥ 9 h) | HR = 0.95 (95% Cl 0.85–1.07) SS vs. 7 h sleepers; HR = 1.03 (95% Cl 0.90–1.18) LS vs. 7 h sleepers |
≠ |
| Wang et al. (2015) | Retrospective, case-control | N = 1454; BCa patients n = 712 | Chinese breast cancer patients and age-matched controls | Self-reported 10-year habitual sleep duration; SS (≤ 6 h); LS (≥ 9 h); daytime napping | OR = 1.53 (95% Cl 1.10–2.12) SS vs. 6.1–8.9 h; OR = 1.59 (95% Cl 1.17–2.17) LS vs. 6.1–8.9 h OR = 0.57 (95% Cl 0.36–0.90) napping NSW vs. non-napping NSW |
SS ↑ risk LS ↑ risk Naps ↓ risk in NSW |
| Qian et al. (2015) | Prospective, cohort | N = 40,013; Incident cases n = 1846 | Breast Cancer Detection Demonstration Project | Self-reported weekday and weekend sleep duration; SS (< 6 h); LS (≥ 9 h) | RR = 0.87 (95% Cl 0.70–1.22) SS vs. 8–9 h sleepers; RR = 1.00 (95% ci 0.84–1.19) LS vs. 8–9 h sleepers | ≠ |
Notes: SS = short sleepers; LS = long sleepers; NSW = night shift workers. Italics indicate trend approaching statistical significance.
3.3. Case-control studies
The findings from the case-control studies are mixed. In the earliest study, McElroy and colleagues (McElroy et al., 2006) found that women reporting ≥ 9 h of sleep per night had a 6% increase in risk for every additional hour of sleep, compared to women sleeping 7.0–7.9 h. This study relied on the retrospective self-report of invasive breast cancer patients and community controls. Additionally, while the authors reported that shorter sleep duration was not associated with increased risk, their cutoff of < 7 h differs from the conventional threshold of ≤ 5 h or ≤ 6 h often used in research on the health outcomes of short sleep (e.g. Kripke et al., 2002). Fritschi and colleagues (Fritschi et al.,2013), created a composite variable of self-reported “sleep disturbance” that included either short ( < 6 h) or long ( ≥ 9 h) sleep duration, poor sleep quality, and frequent difficulty falling or staying asleep. They found that greater sleep disturbance was not associated with increased incidence of breast cancer, although the odds ratio approached significance (OR = 1.21, 95% CI 0.95–1.55, “ever” having sleep disruption vs. never). It is worth noting that the authors did not investigate breast cancer risk for each sleep variable separately, and they also failed to find any associations between either night shift work or LAN and breast cancer in their sample. This same group published a follow-up paper that did investigate sleep duration individually in this same case-control sample (Girschik et al., 2013a); they found no associations between self-reported short ( < 6 h) or long ( > 8 h) sleepers and breast cancer. Like the McElroy et al. (2006) study, this study sample was a retrospective case-control examination of the self-reported sleep of women recently diagnosed with incident invasive breast cancer (Girschik et al., 2013a). It is possible that the retrospective nature of the report, after first cancer diagnosis, introduces some element of reporting bias. Yet another case-control study, a nested design within the larger prospective Singapore Chinese Health Study cohort, did not find that self-reported sleep duration was associated with breast cancer risk (Wu et al., 2013). In this study, they compared long sleepers against short sleepers; it is unclear whether conventionally short ( ≤ 6 h) or long ( ≥ 9 h) sleepers were at risk compared to average (7–8 h) sleepers. Most recently, Wang et al. (2015) found that women who reported both short (≤6 h) and long (≥9 h) habitual sleep over the past 10 years had 53% and 59% increased risk, respectively. Interestingly, this group also looked at history of night shift work and daytime napping and found that napping appeared to confer some degree of protection against breast cancer, but only in NSW. This finding of a dynamic association among shift work as a risk factor and sleep as a protective mechanism supports the importance of assessing both sleep and circadian variables in research studies.
The findings of the 6 published prospective cohort studies are also mixed. One study of 23,995 Japanese women found that shorter sleep duration was associated with higher risk of incident breast cancer (Kakizaki et al., 2008a); compared to women sleeping 7 h, women who reported sleeping ≤ 6 h had higher rates of incident breast cancer (HR = 1.62, 95% Cl = 1.05–2.50), with associations most pronounced in women under 60 years, who had a 200% increase in risk (HR = 2.01, CI = 1.12–3.59). Two prospective, population-based cohort studies found that longer sleep duration may have a protective effect against incident breast cancer (Verkasalo et al., 2005; Wu et al., 2008). In a population study of Finnish women born before 1958, sleep was assessed by self-reported questionnaires administered in 1975 and 1981. Compared to women who reported habitually sleeping 7–8 h, women who reported sleeping ≥9 h at both times had a 72% decreased risk of incident breast cancer (HR = 0.28, 95% Cl = 0.09–0.88) (Wu et al., 2013). A study of incident breast cancer in postmenopausal women from the Singapore Chinese Health Study (Wu et al., 2008) found that women reporting ≥ 9 h of sleep showed a trend for reduced risk of incident breast cancer an average of 11 years later (RR = 0.67, 95% CI = 0.4–1.1, p = 0.047) compared to < 6 h sleepers. Urinary aMT6 s levels were positively associated with hours of self-reported sleep (p = 0.035) and were 42% higher in women sleeping ≥9 h compared to those reporting ≤ 6 h. These findings suggest that sleep duration may gate melatonin secretion; the oncostatic effect of melatonin may be one mechanism by which prolonged sleep duration protects against breast cancer.
Finally, three prospective studies found no associations between sleep duration and breast cancer (Girschik et al., 2013a; Pinheiro et al., 2006a; Vogtmann et al., 2013). The first, using prospective data from the Nurses’ Health Study, found that habitual self-reported short or long sleep duration was not associated with self-reported incident breast cancer over the 16 year follow-up (Pinheiro et al., 2006a), although there was a linear trend for longer sleep duration and increasing risk (p = 0.07). More recently, Vogtmann et al. (2013) also reported no associations between self-reported sleep duration, sleep quality, insomnia, or sleep disturbance and incident breast cancer in a sample of 110,011 postmenopausal women in the Women’s Health Initiative cohort. Similar null findings were published from the Breast Cancer Detection Demonstration Project, which failed to find that self-reported short or long sleep was a risk factor in their sample (Qian et al., 2015). Finally, one study examined self-reported daytime napping frequency in the Million Women Study (Cairns et al., 2012), finding 6% increased breast cancer risk in women who napped “sometimes” and 11% increased risk in women who “usually” napped, compared to non-nap-pers. These data suggest that, if there is a beneficial effect to sleep, it may be optimal only when the sleep period is optimally aligned with circadian rhythms and the exogenous light-dark cycle, although further research is needed.
Critical factors may underlie the discrepant findings of the studies on sleep and breast cancer. First, it appears that none of these studies were specifically designed to test associations between measures of sleep and incident breast cancer, and this is reflected by the general paucity of sleep measures. Second, all studies relied on a self-reported measure of sleep, often assessed with a single question. Third, only half of these studies were able to collect data prospectively, introducing retrospective reporting bias. Fourth, there was significant heterogeneity between the samples in these studies, particularly in percentages of pre-and post-menopausal status, an independent predictor of breast cancer risk, and in types of breast cancers, which also displays great heterogeneity. Finally, very few of the studies included concurrent measures on night shift work or LAN exposure, making it difficult to determine the relative contributions of the circadian and sleep systems.
3.4. Meta-analyses of sleep duration and breast cancer risk
The first meta-analysis of the associations between sleep and cancer included studies examining all types of cancers (Lu et al., 2013). Ten prospective studies were included, with a combined sample of 555,678 participants and 8392 incident cancer cases. The authors found that neither short (RR = 1.05, 95% CI = 0.90–1.24) nor long (RR = 0.92, 95% Cl = 0.76–1.12) sleep duration was associated with increased all cancer or breast cancer risk. However, these analyses included only 3 studies of breast cancer, which have been previously discussed in this review (Kakizaki et al., 2008a; Verkasalo et al., 2005; Pinheiro et al., 2006b).
Two more recent meta-analyses focusing exclusively on breast cancer found no evidence that sleep duration was associated with increased incidence (Yang et al., 2014; Qin et al., 2014). Both reviewed the same six studies, all that had been published on sleep duration and breast cancer through 2013. Yang and colleagues (Yang et al., 2014) analyzed the summed relative risks across all 6 studies for 1 h increase in sleep per night (RR = 1.00, CI = 0.995–1.01) and longest versus shortest sleepers (RR = 0.96, CI = 0.77–1.19). Qin and colleagues grouped their analyses by short and long sleep versus reference, neither of which were significant predictors (RR = 1.01, CI = 0.90–1.14 and RR = 0.95, CI = 0.86–1.04, respectively). However, the discrepancy between study definitions of short and long sleep is worth noting. While Wu et al. (2013), Girschik et al. (2013b), and Kakizaki et al. (2008b) used a threshold of ≤ 6 h, Pinheiro et al. (2006a) and McElroy et al. (2006) used ≤5 h and Verkasalo et al. (2005) ≤4. For long sleep, most studies used a threshold of ≥9h except for Girschik (Girschik et al., 2013b; ≥8h) and Verkasalo (Verkasalo et al., 2005; ≥ 10 h). Both meta-analyses included retrospective studies, and all studies used self-reported sleep measures, limiting our interpretability of their null findings. Well-designed, prospective studies using objective measures of sleep are needed to better address the question of whether any indices of sleep are associated with incident breast cancer.
4. Potentially interrelated mechanistic pathways between circadian and sleep disruption and mammary oncogenesis
Circadian and sleep disruption may act on immunologic, molecular, cellular, neuroendocrine, and metabolic levels in ways that contribute to breast cancer development. Table 1 provides an overview of the potential mechanistic pathways, noting the strength of the experimental and/or epidemiological evidence for each pathway.
Table 1.
Potential mechanistic pathways between circadian and sleep disruption and mammary oncogenesis.
| Mechanistic Pathway | Evidence for Effects of Circadian Disruption | Evidence for Effects of Sleep Disruption |
|---|---|---|
| 1. DNA DAMAGE & OXIDATIVE STRESS | ||
| Increased oxidative stress, oxidative DNA damage | Limited | ✓✓ |
| Decreased antioxidant effects | ✓ | Theoretical |
| 2. MELATONIN & ESTROGEN | ||
| Decreased melatonin release | ✓✓ | Limited |
| Increased mammotropic hormone production and/or release | ✓✓ | ✓ |
| 3. INFLAMMATION & IMMUNE FUNCTION | ||
| Decreased NK cell count & cytotoxicity | ✓✓ | |
| Shift to Th-2 cytokine production & tumor cell survival | ✓ | |
| Decreased Th-1 cytokine production (e.g., IL-2) | ✓✓ | ✓✓ |
| Chronic inflammation via activation of pro-inflammatory pathways | ✓ | ✓✓ |
| Increased repeated SNS activation | ✓✓ (especially via SDB) | |
| Changes in glucocorticoid production & feedback | Limited | ✓ |
| 4. METABOLIC FUNCTION | ||
| Increased adiposity & obesity | ✓✓ | ✓✓ |
| Metabolic disruption (e.g. insulin, glucose, leptin, ghrelin) | ✓✓ | ✓✓ (especially via SDB) |
Notes: ✓✓ = strong research support; ✓ = some/mixed research support; “limited” = support from 1 to 2 studies; “theoretical” = no known support, compelling theoretical association; SDB = sleep disordered breathing
Mammary carcinogenesis begins with the mutation of a healthy cell through a process that includes alterations in cellular and immune processes, including oxidative nuclear DNA damage (Loft and Poulsen, 1996), changes in cellular apoptosis (Abedin et al., 2007), and changes in innate immune function (Herberman and Ortaldo, 1981). Mutated cells are able to survive in the face of compromised innate tumor suppressor mechanisms, such as inactivation of the p53 gene (Ozbun and Butel, 1995), which can result in decreased apoptosis of aberrant cells (Raulet and Guerra, 2009). The growth of human breast cells is regulated by steroid hormones, and the role of estrogen as an oncogenic agent is well-established (Pike et al., 1993; Pietras et al., 1995). Sleep disruption and circadian disruption negatively impact the timing and availability of melatonin, an oncostatic agent and potent estrogen suppressor. Changes in the immune system also predispose the system to oncogenesis. Persistent inflammation can induce genetic instability that may lead to cell mutations. Downregulation of anti-tumor immune responses and upregulation of pro-angiogenic immune factors contribute to survival of mutated cells and tumorigenesis. Disruptions in sleep and circadian systems also affect metabolic processes and predispose the organism to increased adiposity, which is associated with increased breast cancer risk.
4.1. Inflammation and the immune system
Repeated disruptions of the sleep and circadian systems can affect both innate and acquired immune function. Several studies have demonstrated that a single night of sleep deprivation is associated with decreased numbers of circulating NK cells (Born et al., 1997) and NK cytotoxicity (Irwin et al., 1994). NK cells are enhanced by cytokines such as interferon-gamma (IFN-γ) and interleukin-2 (IL-2); (Herberman and Ortaldo, 1981), which are also affected by sleep duration (Born et al., 1997). Sleep disruption may lead to a shift in cytokine production balance from Th-1 cytokines to Th-2 cytokines, including IL-10 (Dimitrov et al., 2004), which can allow tumor cells to escape from immune surveillance (Marincola et al., 2000) and which is associated with increased breast cancer risk (Langsenlehner et al., 2005). Healthy sleep is associated with production of pre-myeloid dendritic cells, which produce IL-12 (Dimitrov et al., 2007), a cytokine associated with the delay of tumor onset and decreased tumor multiplicity (Boggio et al., 1998).
Chronic inflammation is also implicated in the oncogenic pathway, and both sleep disruption and circadian disruption have been demonstrated to have pro-inflammatory effects (Irwin et al., 2015; Castanon-Cervantes et al., 2010). In addition, melatonin has been shown to enhance IL-2 and IL-6 production via nuclear receptor-mediated mechanisms (RORas; Garcia-Maurino et al., 2000). It was recently discovered that certain lymphocytes are capable of producing melatonin in a time-dependent manner (Carrillo-Vico et al., 2004) and, in turn, melatonin has been shown to moderate the role of many immune functions, including IL-2 production (Carrillo-Vico et al., 2004; Carrillo-Vico et al., 2006). Repeated disruptions in melatonin synthesis resulting from nightly circadian and sleep disruption may have potent negative effects on healthy immune functioning.
Sleep fragmentation is also associated with repeated transient increases in sympathetic nervous system (SNS) activation, and experimental research indicates that beta-adrenergic signaling regulates a number of cellular processes underlying oncogenesis, including compromised DNA repair and cellular immune responses (Cole and Sood, 2012). Sleep disruption is associated with increased glucocorticoid production (Spath-Schwalbe et al., 1992), and numerous studies have shown that short sleep duration (Spath-Schwalbe et al., 1992) and sleep deprivation (Spath-Schwalbe et al., 1991) are associated with altered cortisol profiles and elevated evening cortisol levels (Miller et al., 2009). Glucocorticoid feedback from the adrenal cortex has suppressive effects on the amplitude of circadian rhythms within the hypothalamus and pituitary (Koyanagi et al., 2006; Liu et al., 2006). These studies demonstrate yet another pathway by which sleep disruption may have both direct effects on physiological processes and indirect effects via circadian disruption leading to increased risk for breast cancer.
4.2. Oxidative stress, reactive oxygen species (ROS), and DNA damage
Oxidative stress is known to cause DNA damage and is associated with increased breast cancer risk (Kang, 2002); conversely, antioxidants reduce the amount of DNA damage. Oxidative stress-induced DNA damage may be a primary pathway by which sleep disruption may have greater direct mechanistic effects on mammary oncogenesis than circadian disruption. Sleep is theorized to have antioxidant effects, providing an opportunity for the body to remove oxidants produced during wakefulness (Nakata et al., 2005; Inoue et al., 1995b). Several experimental studies have demonstrated that disrupting sleep increases reactive oxygen species (ROS) (Ramanathan et al., 2002; Silva et al., 2004; Nair et al., 2011). ROS can lead to DNA damage by binding to neighboring molecules with unpaired electrons, causing strand breaks (Boonstra and Post, 2004). ROS formation also may be a link between sleep deprivation and circadian dysrégulation. Sleep deprivation-induced ROS formation and DNA damage has been shown to phase advance the mammalian circadian clock both in vivo and in vitro (Oklejewicz et al., 2008). While the mechanism is poorly understood, this research supports one pathway by which sleep disruption may contribute to oncogenesis directly, through cellular damage, and indirectly, by disrupting the circadian system.
Oxidative stress is also as a mediator of cellular apoptosis (Buttke and Sandstrom, 1994); sleep deprivation-induced oxidative stress may allow mutated cells to escape apoptosis. Circadian mechanisms are also implicated in this pathway. In vitro studies have shown that inhibition of Per1 in human cancer cells blunted DNA damage-induced apoptosis (Gery et al., 2006). Cell samples from human cancer patients show reduced Per1 levels. Dysregulation in the clock gene Per2, which acts as a tumor suppressor, has been associated with increased DNA damage (Fu et al., 2002).
The circadian orphan nuclear receptor RORα also appears to play a role in the cellular stress response and human tumorigenesis. Increase in RORα expression inhibits cellular growth and progression, and experimental evidence testing the effects of various stressors on RORα expression (UV light, MMS, H202) suggests that this is an adaptive response to stress (Zhu et al., 2006). Hypoxia, such as that caused by sleep disordered breathing, also increases the amount of RORα transcripts (Chauvet et al., 2004). However, transcriptional levels of RORα have been found to be downregulated in breast cancer samples (Zhu et al., 2006), indicating dysfunction in the circadian molecular oscillator.
4.3. Melatonin and estrogen
It has been proposed that the circadian system developed as a means of counteracting oxidative damage caused by radiation (Hardeland et al., 1993; Reiter, 1993). Melatonin is a potent free radical scavenger that impacts quinine reductases to reduce oxidative damage by ROS in tissues (Poeggeler et al., 1993). Administration of physiological levels of melatonin has been shown to induce the expression of antioxidants glutathione and glutathione-S-transferase (Blask et al., 1997).
Early in vivo experimental work on the role of melatonin demonstrated that animals whose melatonin production was disrupted by pineal or SCN ablation were prone to increased rates of spontaneous mammary carcinogenesis (Shah et al., 1984; Tamarkin et al., 1981). The most commonly used in vivo model of human breast cancer is a murine mammary tumor induced by 7,12-dimethylbenz [a] anthracene (DMBA), whose pathogenesis is similar to that in humans (Medina, 1996). Experimental studies on the effects of constant light on DMBA-induced murine mammary tumor development demonstrate that melatonin suppression is associated with increased tumorigenesis (Vinogradova et al., 2009) and hyperplastic processes in the mammary gland (Ird, 1966; Anisimov, 1971). Conversely, rodents administered melatonin during DMBA-induced mammary tumorigenesis show decreased tumor incidence and number compared to controls (Tamarkin et al., 1981; Blask et al., 1986), and melatonin administration to rodents with constant light-induced spontaneous mammary hyperplasia resulted in normalization of tissue (Lazarev et al., 1976). In pine-alectomized rats, melatonin administration inhibits mammary carcinogenesis, even in a constant illumination (LL) regimen (Anisimov, 2003).
More recently, Blask and colleagues provided compelling evidence that melatonin is the active factor in LAN-induced mammary tumorigenesis (Blask et al., 2005a). Human breast cancer xenografts in immunodeficient rats perfused with melatonin-rich blood collected at night from female subjects resulted in significantly suppressed tumor proliferative activity and linoleic acid (LA) uptake. Administration of a melatonin receptor blocker negated these effects (Blask et al., 2005a). The suppressive effects on LA uptake may be another oncostatic property of melatonin, as LA demonstrates oncogenic effects via upre-gulation of gene expression involved in ERa expression, G-protein signaling, and cell cycle progression (Blask et al., 2005b). However, the associations between LA and incident breast cancer remain unclear (Zock and Katan, 1998).
Mammotropic hormones, including estrogens, progesterone, and prolactin, impact breast cancer etiology (Adami et al., 1998). Melatonin has an inhibitory effect on estrogen receptors and can counteract the tumor-promoting effects of estrogens (Cos et al., 2006). Melatonin can down-regulate estrogen receptor-α (ERa) protein and mRNA expression in a time- and dose-dependent manner (Molis et al., 1994) and can modulate estrogen-related proteins and growth factors, including c-myc (Cos and Blask, 1994). Melatonin administered at physiological nocturnal doses increases the expression of p53 in MCF-7 cells, a human breast cancer cell line expressing 17p-estradiol receptors (Brooks et al., 1973), inducing apoptosis (Mediavilla et al., 1999). Melatonin can temporarily increase the duration of the cell cycle by more than three hours and arrest progression (Cos et al., 1996). These actions oppose the mechanisms of estradiol, which stimulates cell proliferation and cell progression (Lippman et al., 1976).
Sleep also impacts hormone levels. Sleep deprivation in female rats is associated with higher levels of progesterone (Antunes et al., 2006) and increased levels of prolactin (Machado et al., 2008). Partial sleep deprivation in healthy young women results in increases in LH and estradiol (Baumgartner et al., 1993), and elevated prolactin levels have been observed in humans following sleep deprivation nights (von et al., 1996). In humans, objectively-assessed sleep duration has been shown to be inversely associated with levels of 17p-estradiol (Wang and Phang, 1995). These data suggest that repeated nightly short sleep or sleep disruption may be associated with chronically elevated estrogen levels contributing over time to breast cancer risk (Fig. 2).
Fig. 2.
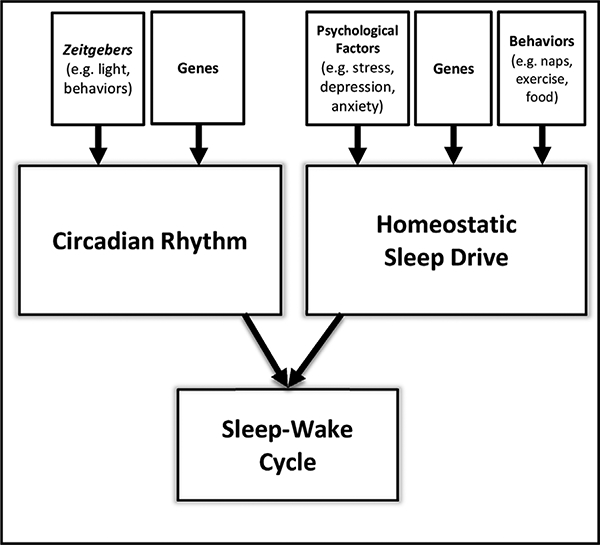
Two-Process Model of Sleep-Wake Cycle Regulation.
In the Wu et al. (2008) study previously described, women who reported sleeping ≥9 h had 42% higher levels of aMT6s and decreased risk of incident breast cancer compared to women sleeping 7 h. While another study found no association between hours of sleep and levels of urinary aMT6s, they did find that women awake at night had higher serum concentrations of estradiol (Nagata et al., 2008).
4.4. Metabolic function
It is plausible that sleep disruption and circadian disruption may contribute to mammary oncogenesis through other physiological mechanisms. One potential pathway may be altered metabolic function. The obesogenic properties of both circadian and sleep disruption have received support from experimental and observational studies. As previously discussed, melatonin suppresses the uptake of linoleic acid, an oncogenic substance associated with rapid development of mammary tumors in mice (Fischer et al., 1992). In humans, experimental circadian misalignment and short sleep duration both result in impaired glucose tolerance and decreased leptin (Scheer et al., 2009; Taheri et al., 2004) and increased adiposity (Chaput et al., 2007). Meta-analysis has established short sleep duration as an independent risk factor for childhood and adult obesity (Cappuccio et al., 2008).
5. Summary
Overall, the experimental data supporting a circadian and, to a lesser extent, sleep disruption model for mammary oncogenesis are promising (Table 1). Future studies are needed to prospectively, objectively, and comprehensively assess indices of sleep disruption and circadian rhythms, including melatonin, LAN exposure, shift work, and nocturnal eating, to determine the relative contributions of these disruptions to mammary oncogenesis. Forced desynchrony studies, whereby the circadian and sleep systems are effectively separated, are needed to disambiguate circadian and sleep effects on biomarkers of cancer development. Observational research that is prospective, longitudinal, and population-based is needed to determine whether sleep disruption, not just sleep duration, is prospectively associated with incident breast cancer cases. Experimental research is needed to test and refine the proposed model to better understand the potential mechanistic pathways by which sleep disruption and circadian disruption may contribute to increased risk. Finally, we must consider the real world context. Future research should seek to understand the relative and combined contributions of susceptibility factors from all sources—biological, environmental, behavioral, and psychosocial—in the development of breast cancers. Ultimately, these future research questions and avenues will allow us to develop a more ecologically valid and complete understanding of risk factors for breast cancer pathogenesis from a biopsychosocial perspective.
Acknowledgments
Funding sources
This work was supported by the National Institute of General Medical Sciences [grant number R01GM113243] and the National Cancer Institute [grant number P30 CA047904].
Footnotes
Conflict of interest
The authors attest that they have no conflicts of interest.
References
- Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A, 2007. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 14 (March), 500–510. [DOI] [PubMed] [Google Scholar]
- Adami HO, Signorello LB, Trichopoulos D, 1998. Towards an understanding of breast cancer etiology. Semin. Cancer Biol. 8 (August), 255–262. [DOI] [PubMed] [Google Scholar]
- Altimus CM, Guler AD, Villa KL, McNeill DS, Legates TA, Hattar S, 2008. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc. Natl. Acad. Sci. U. S. A. 105 (December), 19998–20003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, 1971. Blastomogenesis in rats with persistent estrus. Vopr. Onkol. 17, 67–75. [PubMed] [Google Scholar]
- Anisimov VN, 2003. The role of pineal gland in breast cancer development. Crit. Rev. Oncol. Hematol 46 (June), 221–234. [DOI] [PubMed] [Google Scholar]
- Antle MC, Mistlberger RE, 2000. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J. Neurosci. 20 (December), 9326–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Steen NM, Mistlberger RE, 2001. Adenosine and caffeine modulate circadian rhythms in the Syrian hamster. Neuroreport 12 (September), 2901–2905. [DOI] [PubMed] [Google Scholar]
- Antunes IB, Andersen ML, Baracat EC, Tufik S, 2006. The effects of paradoxical sleep deprivation on estrous cycles of the female rats. Horm. Behav. 49 (April), 433–440. [DOI] [PubMed] [Google Scholar]
- Basler M, letter A, Fink D, Seifert B, Kullak-Ublick GA, Trojan A, 2014. Urinary excretion of melatonin and association with breast cancer: meta-analysis and review of the literature. Breast Care (Basel) 9 (July), 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner A, Dietzel M, Saletu B, Wolf R, Campos-Barros A, Graf KJ, et al. , 1993. Influence of partial sleep deprivation on the secretion of thyrotropin, thyroid hormones, growth hormone, prolactin, luteinizing hormone, follicle stimulating hormone, and estradiol in healthy young women. Psychiatry Res 48 (August), 153–178. [DOI] [PubMed] [Google Scholar]
- Blask DE, Hill SM, Orstead KM, Massa JS, 1986. Inhibitory effects of the pineal hormone melatonin and underfeeding during the promotional phase of 7,12-di-methylbenzanthracene-(DMBA)-induced mammary tumorigenesis. J. Neural Transm. 67, 125–138. [DOI] [PubMed] [Google Scholar]
- Blask DE, Wilson ST, Zalatan F, 1997. Physiological melatonin inhibition of human breast cancer cell growth in vitro: evidence for a glutathione-mediated pathway. Cancer Res. 57 (May), 1909–1914. [PubMed] [Google Scholar]
- Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, et al. , 2005a. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 65 (December), 11174–11184. [DOI] [PubMed] [Google Scholar]
- Blask DE Dauchy RT, Sauer LA, 2005b. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine 27 (July), 179–188. [DOI] [PubMed] [Google Scholar]
- Blask DE, Brainard GC, Gibbons R, Lockley SW, Stevens RG, Motta ME, 2012. Light pollution adverse health effects of nighttime lighting. Report 4 of the Council on Science and Public Health (A-12). AMERICAN MEDICAL ASSOCIATION, pp. 1–25. [Google Scholar]
- Blask DE, 2009. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 13 (August), 257–264. [DOI] [PubMed] [Google Scholar]
- Boden MJ, Varcoe TJ, Voultsios A, Kennaway DJ, 2010. Reproductive biology of female Bmall null mice. Reproduction 139 (June), 1077–1090. [DOI] [PubMed] [Google Scholar]
- Boggio K, Nicoletti G, Di CE, Cavallo F, Landuzzi L,, Melani C, et al. , 1998. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J. Exp. Med. 188 (August), 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CD, Alonso-Gonzalez C, Clafshenkel WP, Kotlarczyk MP, Dodda BR, Sanchez-Barcelo E, et al. , 2014. The effect of estradiol, progesterone, and melatonin on estrous cycling and ovarian aromatase expression in intact female mice. Eur. J. Obstet. Gynecol. Reprod. Biol 174 (March), 80–85. [DOI] [PubMed] [Google Scholar]
- Boonstra J, Post JA, 2004. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337 (August), 1–13. [DOI] [PubMed] [Google Scholar]
- Borbely AA, 1982. A two process model of sleep regulation. Hum. Neurobiol. 1, 195–204. [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, Fehm HL, 1997. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 158 (May), 4454–4464. [PubMed] [Google Scholar]
- Boyd DB, 2003. Insulin and cancer. Integr. Cancer Ther. 2 (December), 315–329. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. , 2001. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J. Neurosci. 21 (August), 6405–6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SC, Locke ER, Soule HD, 1973. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J. Biol. Chem. 248 (September), 6251–6253. [PubMed] [Google Scholar]
- Buman MP, Kline CE, Youngstedt SD, Phillips B, deMello T, Hirshkowitz M, 2015. Sitting and television viewing: novel risk factors for sleep disturbances and apnea risk? Results from the 2013 national sleep foundation sleep in America poll. Chest 147 (March), 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttke TM, Sandstrom PA, 1994. Oxidative stress as a mediator of apoptosis. Immunol. Today 15 (January), 7–10. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Soehner A, Abbott S, Kapur VK, Mahowald MW, Parker KP, et al. , 2015. Sleep and sleep-wake disorders In: Tasman A, Kay J, Lieberman JA, First MB, Riba MB (Eds.), Psychiatry, 4 ed. Wiley Blackwell, Hoboken, pp. 1264–1310. [Google Scholar]
- Cairns BJ, Travis RC, Wang XS, Reeves GK, Green J, Beral V, 2012. A short-term increase in cancer risk associated with daytime napping is likely to reflect pre-clinical disease: prospective cohort study. Br. J. Cancer 107 (July), 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. , 2008. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 31 (May), 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA, 2011. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32 (June), 1484–1492. [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, Calvo JR, Abreu P, Lardone PJ, Garcia-Maurino S, Reiter RJ, et al. , 2004. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: possible role as intracrine, autocrine, and/or paracrine substance. FASEB J 18 (March), 537–539. [DOI] [PubMed] [Google Scholar]
- Carrillo-Vico A, Reiter RJ, Lardone PJ, Herrera JL, Fernandez-Montesinos R, Guerrero JM, et al. , 2006. The modulatory role of melatonin on immune responsiveness. Curr. Opin. Investig. Drugs 7 (May), 423–431. [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, et al. , 2010. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 185 (November), 5796–5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PE, White RS, Mellon PL, 2003. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1–7 cell line. J. Neurosci. 23 (December), 11202–11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput JP, Despres JP, Bouchard C, Tremblay A, 2007. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 15 (January), 253–261. [DOI] [PubMed] [Google Scholar]
- Chauvet C, Bois-Joyeux B, Berra E, Pouyssegur J, Danan JL, 2004. The gene encoding human retinoic acid-receptor-related orphan receptor alpha is a target for hypoxia-inducible factor 1. Biochem. J. 384 (November), 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa SL, Steiner R, Blattner P, Oelhafen P, Gotz T, Cajochen C, 2011. Nonvisual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS One 6, el6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ, Chang JG, 2005. Deregulated expression of the PERI, PER2 and PER3 genes in breast cancers. Carcinogenesis 26 (July), 1241–1246. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, et al. , 2005. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J. Natl. Cancer Inst. 97 (March), 439–448. [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM, 2005. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc. Natl. Acad. Sei. U. S. A 102 (October), 15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, 2008. Sleep and wakefulness in Drosophila melanogaster. Ann. N. Y. Acad. Sei 1129, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Sood AK, 2012. Molecular pathways: beta-adrenergic signaling in cancer. Clin. Cancer Res. 18 (March), 1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cos S, Blask DE, 1994. Melatonin modulates growth factor activity in MCF-7 human breast cancer cells. J. Pineal Res. 17 (August), 25–32. [DOI] [PubMed] [Google Scholar]
- Cos S, Redo J, Sanchez-Barcelo EJ, 1996. Modulation of the length of the cell cycle time of MCF-7 human breast cancer cells by melatonin. Life Sei. 58, 811–816. [DOI] [PubMed] [Google Scholar]
- Cos S, Gonzalez A, Martinez-Campa C, Mediavilla MD, Alonso-Gonzalez C, Sanchez-Barcelo EJ, 2006. Estrogen-signaling pathway: a link between breast cancer and melatonin oncostatic actions. Cancer Detect. Prev. 30, 118–128. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, et al. , 1986. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science 233, 667–670. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. , 1999. Stability, precision, and near-24-hr period of the human circadian pacemaker. Science 284, 2181. [DOI] [PubMed] [Google Scholar]
- Davis S, Mirick DK, Stevens RG, 2001. Night shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 93 (October), 1557–1562. [DOI] [PubMed] [Google Scholar]
- de LL, Carter ME, Adamantidis A, 2012. Shining light on wakefulness and arousal. Biol. Psychiatry 71 (June), 1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis C, Ma J, Bryan L, Jemal A, 2014. Breast cancer statistics, 2013. CA. Cancer J. Clin. 64 (January), 52–62. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Tieken S, Fehm HL, Born J, 2004. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav. Immun. 18 (July), 341–348. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Nohroudi K, Born J, 2007. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep 30 (April), 401–411. [DOI] [PubMed] [Google Scholar]
- Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF, 1999. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol. Biomarkers Prev. 8 (October), 843–854. [PubMed] [Google Scholar]
- Espana RA, Plahn S, Berridge CW, 2002. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 943 (July), 224–236. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. , 2001. Fos expression in orexin neurons varies with behavioral state. J. Neurosci. 21 (March), 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiro MG, Bierman A, Rea MS, 2013. A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nat. Sei. Sleep 5, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SM, Conti CJ, Locniskar M, Belury MA, Maldve RE, Lee ML, et al. , 1992. The effect of dietary fat on the rapid development of mammary tumors induced by 7, 12-dimethylbenz(a)anthracene in SENCAR mice. Cancer Res. 52 (February), 662–666. [PubMed] [Google Scholar]
- Fritschi L, Erren TC, Glass DC, Girschik J, Thomson AK, Saunders C, et al. , 2013. The association between different night shiftwork factors and breast cancer: a case-control study. Br. J. Cancer 109 (October), 2472–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P,, Lee, C., 2002. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111 (October), 41–50. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, Saper CB, 2006. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J. Biol. Rhythms 21 (December), 482–493. [DOI] [PubMed] [Google Scholar]
- Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibier U, 2004. The mammalian circadian timing system: from gene expression to physiology. Chromosoma 113 (September), 103–112. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Kalesan B, 2009. Sleep duration and mortality: a systematic review and meta-analysis. J. Sleep Res. 18 (June), 148–158. [DOI] [PubMed] [Google Scholar]
- Garcia-Maurino S, Pozo D, Calvo JR, Guerrero JM, 2000. Correlation between nuclear melatonin receptor expression and enhanced cytokine production in human lymphocytic and monocytic cell lines. J. Pineal Res. 29 (October), 129–137. [DOI] [PubMed] [Google Scholar]
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP, 2006. The circadian gene perl plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell 22 (May), 375–382. [DOI] [PubMed] [Google Scholar]
- Ghoussaini M, Pharoah PD, Easton DF, 2013. Inherited genetic susceptibility to breast cancer: the beginning of the end or the end of the beginning? Am. J. Pathol. 183 (October), 1038–1051. [DOI] [PubMed] [Google Scholar]
- Girschik J, Heyworth J, Fritschi L, 2013. a. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. Am. J. Epidemiol. 177 (Febraury), 316–327. [DOI] [PubMed] [Google Scholar]
- Girschik J, Heyworth J, Fritschi L, 2013. b. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. Am. J. Epidemiol. 177 (February), 316–327. [DOI] [PubMed] [Google Scholar]
- Glickman G, Levin R, Brainard GC, 2002. Ocular input for human melatonin regulation: relevance to breast cancer. Neuro Endocrinol. Lett. 23 (July (Suppl. 2)), 17–22. [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van RE, et al. , 2011. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 96 (March), E463–E472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins MW, Peirson SN, Foster RG, 2008. Melanopsin: an exciting photopigment. Trends Neurosci. 31 (January), 27–36. [DOI] [PubMed] [Google Scholar]
- Hansen J, Stevens RG, 2012. Case-control study of shift-work and breast cancer risk in Danish nurses: impact of shift systems. Eur. J. Cancer 48 (July), 1722–1729. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Reiter RJ, Poeggeler B, Tan DX, 1993. The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci. Biobehav. Rev. 17, 347–357. [DOI] [PubMed] [Google Scholar]
- Haus EL, Smolensky MH, 2013. Shiftwork and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev 17 (August), 273–284. [DOI] [PubMed] [Google Scholar]
- He C, Anand ST, Ebell MH, Vena JE, Robb SW, 2015a. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int. Arch. Occup. Environ. Health 88 (July), 533–547. [DOI] [PubMed] [Google Scholar]
- He C, Anand ST, Ebell MH, Vena JE, Robb SW, 2015b. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int. Arch. Occup. Environ. Health 88 (July), 533–547. [DOI] [PubMed] [Google Scholar]
- Herberman RB, Ortaldo JR, 1981. Natural killer cells: their roles in defenses against disease. Science 214, 24–30. [DOI] [PubMed] [Google Scholar]
- Ijaz S, Verbeek J, Seidler A, Lindbohm ML, Ojajarvi A, Orsini N, et al. , 2013. Night-shift work and breast cancer-a systematic review and meta-analysis. Scand. J. Work. Environ. Health 39 (September), 431–447. [DOI] [PubMed] [Google Scholar]
- Inoue S, Honda K, Komoda Y, 1995a. Sleep as neuronal detoxification and restitution. Behav. Brain Res. 69 (July), 91–96. [DOI] [PubMed] [Google Scholar]
- Inoue S, Honda K, Komoda Y, 1995b. Sleep as neuronal detoxification and restitution. Behav. Brain Res. 69 (July), 91–96. [DOI] [PubMed] [Google Scholar]
- Ird EA, 1966. The effect of experimental hypothyroidism on the development of mastopathia and tumors of the mammary gland in rats. Probl. Endokrinol. Gormonoter. 12 (January), 99–102. [PubMed] [Google Scholar]
- Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL, 1994. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom. Med. 56 (November), 493–498. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Carroll JE, 2015. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol. Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, 2002. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav. Immun. 16 (October), 503–512. [DOI] [PubMed] [Google Scholar]
- Jia Y, Lu Y, Wu K, Lin Q, Shen W, Zhu M, et al. , 2013. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 37 (June), 197–206. [DOI] [PubMed] [Google Scholar]
- Kakizaki M, Kuriyama S, Sone T, Ohmori-Matsuda K, Hozawa A, Nakaya N, et al. , 2008a. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br. J. Cancer 99 (November), 1502–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki M, Kuriyama S, Sone T, Ohmori-Matsuda K, Hozawa A, Nakaya N, et al. , 2008. b. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br. J. Cancer 99 (November), 1502–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM, 2002. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 309 (July), 109–118. [DOI] [PubMed] [Google Scholar]
- Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J, 2013. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res. Treat. 138 (February), 291–301. [DOI] [PubMed] [Google Scholar]
- Kang DH, 2002. Oxidative stress, DNA damage, and breast cancer. AACN Clin. Issues 13 (November), 540–549. [DOI] [PubMed] [Google Scholar]
- Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, et al. , 2013. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 14 (September), 1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Stevens RG, Haim A, Portnov BA, 2010. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 21 (December), 2059–2068. [DOI] [PubMed] [Google Scholar]
- Kloog I, Portnov BA, Rennert HS, Haim A, 2011. Does the modern urbanized sleeping habitat pose a breast cancer risk? Chronobiol. Int. 28 (February), 76–80. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, VanCauter E, 2007. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 11 (June), 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS, 2006. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 15 (October), R271–R277 (Spec No 2). [DOI] [PubMed] [Google Scholar]
- Kochan DZ, Kovalchuk O, 2015. Circadian disruption and breast cancer: an epigenetic link? Oncotarget 6 (July), 16866–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes LL, Geuskens GA, Pronk A, Vermeulen RC, de Vroome EM, 2014. Night work and breast cancer risk in a general population prospective cohort study in The Netherlands. Eur. J. Epidemiol. 29 (August), 577–584. [DOI] [PubMed] [Google Scholar]
- Koyanagi S, Suyama H, Kuramoto Y, Matsunaga N, Takane H, Soeda S, et al. , 2006. Glucocorticoid regulation of 24-hour oscillation in interferon receptor gene expression in mouse liver. Endocrinology 147 (November), 5034–5040. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR, 2002. Mortality associated with sleep duration and insomnia. Arch. Gen. Psychiatry 59 (February), 131–136. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J, 2010. Effects of sleep and circadian rhythm on the human immune system. Ann. N. Y. Acad. Sei. 1193 (April), 48–59. [DOI] [PubMed] [Google Scholar]
- Langsenlehner U, Krippl P, Renner W, Yazdani-Biuki B, Eder T, Koppel H, et al. , 2005. Interleukin-10 promoter polymorphism is associated with decreased breast cancer risk. Breast Cancer Res. Treat. 90 (March), 113–115. [DOI] [PubMed] [Google Scholar]
- Lazarev NI, Ird EA, Smirnova IO, 1976. Experimental Models of Endocrine Gynecological Diseases. Meditsina, Moscow. [Google Scholar]
- Lee CC, 2006. Tumor suppression by the mammalian Period genes. Cancer Causes Control 17 (May), 525–530. [DOI] [PubMed] [Google Scholar]
- Leproult R, Van CE, 2010. Role of sleep and sleep loss in hormonal release and metabolism. Endocr. Dev. 17, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JC, Shensa A, Sidani JE, Colditz JB, Primack BA, 2016. The association between social media use and sleep disturbance among young adults. Prev. Med. 85 (April), 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zheng T, Holford TR, Boyle P, Zhang Y, Dai M, 2010. Light at night and breast cancer risk: results from a population-based case-control study in Connecticut, USA. Cancer Causes Control 21 (December), 2281–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Chen W, Wei F, Ying M, Wei W, Xie X, 2015. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 16 (November), 1381–1387. [DOI] [PubMed] [Google Scholar]
- Lippman M, Bolan G, Huff K, 1976. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 36 (December), 4595–4601. [PubMed] [Google Scholar]
- Liu RY, Unmehopa UA, Zhou JN, Swaab DF, 2006. Glucocorticoids suppress vasopressin gene expression in human suprachiasmatic nucleus. J. Steroid Biochem. Mol. Biol 98 (March), 248–253. [DOI] [PubMed] [Google Scholar]
- Loft S, Poulsen HE, 1996. Cancer risk and oxidative DNA damage in man. J. Mol. Med. (Berl) 74 (June), 297–312. [DOI] [PubMed] [Google Scholar]
- Lu Y, Tian N, Yin J, Shi Y,, Huang Z, 2013. Association between sleep duration and cancer risk: a meta-analysis of prospective cohort studies. PLoS One 8, e74723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado RB, Tufik S, Suchecki D, 2008. Chronic stress during paradoxical sleep deprivation increases paradoxical sleep rebound: association with prolactin plasma levels and brain serotonin content. Psychoneuroendocrinology 33 (October), 1211–1224. [DOI] [PubMed] [Google Scholar]
- Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN, 1995. Proportion of breast cancer cases in the United States explained by well-established risk factors. J. Natl. Cancer Inst. 87 (November), 1681–1685. [DOI] [PubMed] [Google Scholar]
- Marie HA, Helene GA, Hansen J, 2006. Diurnal urinary 6-sulfatoxymelatonin levels among healthy Danish nurses during work and leisure time. Chronobiol. Int. 23, 1203–1215. [DOI] [PubMed] [Google Scholar]
- Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S, 2000. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74, 181–273. [DOI] [PubMed] [Google Scholar]
- McCormack VA, dos SS I, 2006. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 15 (June), 1159–1169. [DOI] [PubMed] [Google Scholar]
- McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM, 2006. Duration of sleep and breast cancer risk in a large population-based case-control study. J. Sleep Res. 15 (September), 241–249. [DOI] [PubMed] [Google Scholar]
- Mediavilla MD, Cos S, Sanchez-Barcelo EJ, 1999. Melatonin increases p53 and p21WAFl expression in MCF-7 human breast cancer cells in vitro. Life Sei. 65, 415–420. [DOI] [PubMed] [Google Scholar]
- Medina D, 1996. The mammary gland: a unique organ for the study of development and tumorigenesis. J. Mammary Gland Biol. Neoplasia 1 (January), 5–19. [DOI] [PubMed] [Google Scholar]
- Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES, 2005. Night work and breast cancer risk: a systematic review and meta-analysis. Eur. J. Cancer 41 (September), 2023–2032. [DOI] [PubMed] [Google Scholar]
- Menegaux F, Truong T, Anger A, Cordina-Duverger E, Lamkarkach F, Arveux P, et al. , 2013. Night work and breast cancer: a population-based case-control study in France (the CECILE study). Int. J. Cancer 132 (February), 924–931. [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS, 2004. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr. Biol. 14 (August), 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Kandala NB, Kivimaki M, Kumari M, Brunner EJ, Lowe GD, et al. , 2009. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep 32 (July), 857–864. [PMC free article] [PubMed] [Google Scholar]
- Molis TM, Spriggs LL, Hill SM, 1994. Modulation of estrogen receptor mRNA expression by melatonin in MCF-7 human breast cancer cells. Mol. Endocrinol. 8 (December), 1681–1690. [DOI] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ, 1972. A retinohypothalamic projection in the rat. J. Comp. Neurol. 146 (September), 1–14. [DOI] [PubMed] [Google Scholar]
- Nagata C, Nagao Y, Yamamoto S, Shibuya C, Kashiki Y, Shimizu H, 2008. Light exposure at night, urinary 6-sulfatoxymelatonin, and serum estrogens and androgens in postmenopausal Japanese women. Cancer Epidemiol. Biomark. Prev. 17 (June), 1418–1423. [DOI] [PubMed] [Google Scholar]
- Nair D, Zhang SX, Ramesh V, Hakim F, Kaushal N, Wang Y, et al. , 2011. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am. J. Respir. Crit. Care Med. 184 (December), 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata A, Ikeda T, Takahashi M, Haratani T, Fujioka Y, Fukui S, et al. , 2005. Sleep-related risk of occupational injuries in Japanese small and medium-scale enterprises. Ind. Health 43 (January), 89–97. [DOI] [PubMed] [Google Scholar]
- Naylor E, Penev PD, Orbeta L, Janssen I, Ortiz R, Colecchia EF, et al. , 2000. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep 23 (February), 87–95. [PubMed] [Google Scholar]
- Oklejewicz M, Destici E, Tamanini F, Hut RA, Janssens R, van Der Horst GT, 2008. Phase resetting of the mammalian circadian clock by DNA damage. Curr. Biol. 18 (February), 286–291. [DOI] [PubMed] [Google Scholar]
- Oldenburg RA, Meijers-Heijboer H, Cornelisse CJ, Devilee P, 2007. Genetic susceptibility for breast cancer: how many more genes to be found? Crit. Rev. Oncol. Hematol. 63 (August), 125–149. [DOI] [PubMed] [Google Scholar]
- Ozbun MA, Butel JS, 1995. Tumor suppressor p53 mutations and breast cancer: a critical analysis. Adv. Cancer Res. 66, 71–141. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. , 2002. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109 (May), 307–320. [DOI] [PubMed] [Google Scholar]
- Pevet P, Challet E, 2011. a. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J. Physiol. Paris 105 (December), 170–182. [DOI] [PubMed] [Google Scholar]
- Pevet P, Challet E, 2011. b. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J. Physiol. Paris 105 (December), 170–182. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, et al. , 1995. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene 10 (June), 2435–2446. [PubMed] [Google Scholar]
- Pike MC, Spicer DV, Dahmoush L, Press MF, 1993. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol. Rev 15, 17–35. [DOI] [PubMed] [Google Scholar]
- Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB, 2006. a. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 66 (May), 5521–5525. [DOI] [PubMed] [Google Scholar]
- Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB, 2006. b. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 66 (May), 5521–5525. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Reiter RJ, Tan DX, Chen LD, Manchester LC, 1993. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J. Pineal Res. 14 (May), 151–168. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. , 2008. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatry 63 (January), 234–240. [DOI] [PubMed] [Google Scholar]
- Qian X, Brinton LA, Schairer C, Matthews CE, 2015. Sleep duration and breast cancer risk in the Breast Cancer Detection Demonstration Project follow-up cohort. Br. J. Cancer 112 (February), 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zhou Y, Zhang X, Wei X, He J, 2014. Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int. J. Cancer 134 (March), 1166–1173. [DOI] [PubMed] [Google Scholar]
- Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J, 2001. Risk of breast cancer in female flight attendants: a population-based study (Iceland). Cancer Causes Control 12 (February), 95–101. [DOI] [PubMed] [Google Scholar]
- Ram PT, Dai J, Yuan L, Dong C, Kiefer TL, Lai L, et al. , 2002. Involvement of the mtl melatonin receptor in human breast cancer. Cancer Lett. 179 (May), 141–150. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Gulyani S, Nienhuis R, Siegel JM, 2002. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport 13 (August), 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak CK, Boehle KL, Muglia LJ, 2009. Impaired steroidogenesis and implantation failure in Bmal1-/- mice. Endocrinology 150 (April), 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH, Guerra N, 2009. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat. Rev. Immunol. 9 (August), 568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, 1985. Action spectra, dose-response relationships, and temporal aspects of light’s effects on the pineal gland. Ann. N. Y. Acad. Sei. 453, 215–230. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, 1993. Interactions of the pineal hormone melatonin with oxygen-centered free radicals: a brief review. Braz. J. Med. Biol. Res. 26 (November), 1141–1155. [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, 2002. Coordination of circadian timing in mammals. Nature 418 (August), 935–941. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC, 2005. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J. Neurosci. 25 (January), 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PS, Barker KA, Anderson WF, 2015. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J. Natl. Cancer Inst. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy PG, Thompson AM, 2006. Cyclin D1 and breast cancer. Breast 15 (December), 718–727. [DOI] [PubMed] [Google Scholar]
- Sahar S, Sassone-Corsi P, 2007. Circadian clock and breast cancer: a molecular link. ABBV Cell Cycle 6 (June), 1329–1331. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA, 2009. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sei. U. S. A. 106 (March). 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer ES, Hankinson SE, 2005. Urinary melatonin levels and breast cancer risk. J. Natl. Cancer Inst. 97 (July), 1084–1087. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Hankinson SE, 2009. Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses’ Health Study cohort. Cancer Epidemiol. Biomark. Prev 18 (January), 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernhammer ES, 2017. RE: night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J. Natl. Cancer Inst. 109 (April), 4. [DOI] [PubMed] [Google Scholar]
- Schibier U, Sassone-Corsi P, 2002. A web of circadian pacemakers. Cell 111 (December), 919–922. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P, 1991. Lesions of the suprachiasmatic nucleus disrupt circadian locomotor rhythms in the mouse. Physiol. Behav. 49 (June), 1283–1287. [DOI] [PubMed] [Google Scholar]
- Shafie SM, Grantham FH, 1981. Role of hormones in the growth and regression of human breast cancer cells (MCF-7) transplanted into athymie nude mice. J. Natl. Cancer Inst. 67 (July), 51–56. [PubMed] [Google Scholar]
- Shah PN, Mhatre MC, Kothari LS, 1984. Effect of melatonin on mammary carcinogenesis in intact and pinealectomized rats in varying photoperiods. Cancer Res. 44 (August), 3403–3407. [PubMed] [Google Scholar]
- Siegel JM, 2011. Sleep mechanisms and phylogeny In: Kryger MH, Roth T, Dement WC (Eds.), Principles and Practices of Sleep Medicine, fifth ed. Elsevier, St. Louis. [Google Scholar]
- Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, et al. , 2004. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 46 (May), 895–903. [DOI] [PubMed] [Google Scholar]
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT, 2012. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 351 (April), 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, et al. , 2006. Trends in breast cancer by race and ethnicity: update 2006. CA. Cancer J. Clin. 56 (May), 168–183. [DOI] [PubMed] [Google Scholar]
- Smith P, Fritschi L, Reid A, Mustard C, 2013. The relationship between shift work and body mass index among Canadian nurses. Appl. Nurs. Res. 26 (February), 24–31. [DOI] [PubMed] [Google Scholar]
- Smith-Warner SA, Spiegelman D, Yaun SS, van den Brandt PA, Folsom AR, Goldbohm RA, et al. , 1998. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 279 (February), 535–540. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL, 1991. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol. Psychiatry 29, 575–584. [DOI] [PubMed] [Google Scholar]
- Spath-Schwalbe E, Scholler T, Kern W, Fehm HL, Born J, 1992. Nocturnal adre-nocorticotropin and cortisol secretion depends on sleep duration and decreases in association with spontaneous awakening in the morning. J. Clin. Endocrinol. Metab. 75 (December), 1431–1435. [DOI] [PubMed] [Google Scholar]
- Stehle JH, von GC, Korf HW, 2003. Melatonin: a clock-output, a clock-input. J. Neuroendocrinol. 15 (April), 383–389. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Zhu Y, 2015. Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Philos. Trans. R Soc. Lond. B Biol. Sei. 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RG, Davis S, Thomas DB, Anderson LE, Wilson BW, 1992. Electric power, pineal function, and the risk of breast cancer. FASEB J. 6 (February), 853–860. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Hansen J, Costa G, Haus E, Kauppinen T, Aronson KJ, Castano-Vinyals G, Davis S, Frings-Dresen MH, Fritschi L, Kogevinas M, Kogi K, Lie JA, Lowden A, Peplonska B, Pesch B, Pukkala E, Schernhammer E, Travis RC, Vermeulen R, Zheng T, Cogliano V, Straif K, 2011. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup. Environ. Med. 68 (February (2)), 154–162. [DOI] [PubMed] [Google Scholar]
- Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME, 2014. Breast cancer and circadian disruption from electric lighting in the modern world. CA. Cancer J. Clin. 64 (May), 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E, 2004. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 1 (December), e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarkin L, Cohen M, Roselle D, Reichert C, Lippman M, Chabner B, 1981. Melatonin inhibition and pinealectomy enhancement of 7, 12-dimethylbenz(a)an-thracene-induced mammary tumors in the rat. Cancer Res. 41 (November), 4432–4436. [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ, 2001. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J. Physiol. 535 (August), 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2006. Sleep function and synaptic homeostasis. Sleep Med. Rev. 10 (February), 49–62. [DOI] [PubMed] [Google Scholar]
- Tonsfeldt KJ, Chappell PE, 2012. Clocks on top: the role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Mol. Cell. Endocrinol. 349 (February), 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang XS, Roddam AW, Gathani T, Peto R, Green J, Key TJ, Beral V, 2016. Night shift work and breast cancer incidence: three prospective studies and meta-analysis of published studies. J. Natl. Cancer Inst. 108 (October), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong T, Liquet B, Menegaux F, Plancoulaine S, Laurent-Puig P, Mulot C, et al. , 2014. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr. Relat. Cancer 21 (August). 629–638. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. , 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308 (May), 1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio H, Kaaks R, Bianchini F, 2002. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur. J. Cancer Prev. 11 (August (Suppl. 2)), S94–100. [PubMed] [Google Scholar]
- Verkasalo PK, Lillberg K, Stevens RG, Hublin C, Partinen M, Koskenvuo M,, et al. , 2005. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 65 (October), 9595–9600. [DOI] [PubMed] [Google Scholar]
- Vijakkhana N, Wilaisakditipakorn T, Ruedeekhajorn K, Pruksananonda C, Chonchaiya W, 2015. Evening media exposure reduces night-time sleep. Acta Paediatr. 104 (March), 306–312. [DOI] [PubMed] [Google Scholar]
- Vinogradova IA, Anisimov VN, Bukalev AV, Semenchenko AV, Zabezhinski MA, 2009. Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in rats. Aging (Albany NY) 1 (October), 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtmann E, Levitan EB, Hale L, Shikany JM, Shah NA, Endeshaw Y, et al. , 2013. Association between sleep and breast cancer incidence among postmenopausal women in the Women’s Health Initiative. Sleep 36 (October), 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von TK, Norman TR, Armstrong SM, 1996. Overnight human plasma melatonin, cortisol, prolactin, TSH, under conditions of normal sleep, sleep deprivation, and sleep recovery. J. Pineal Res. 20 (January), 7–14. [DOI] [PubMed] [Google Scholar]
- Walsh MF, Nathanson KL, Couch FJ, Offit K, 2016. Genomic biomarkers for breast cancer risk. Adv. Exp. Med. Biol. 882, 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Phang JM, 1995. Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res. 55 (June), 2487–2489. [PubMed] [Google Scholar]
- Wang F, Yeung KL, Chan WC, Kwok CC, Leung SL, Wu C, et al. , 2013. A metaanalysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 24 (November), 2724–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Ren FM, Lin Y, Su FX, Jia WH, Su XF, et al. , 2015. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 16 (April), 462–468. [DOI] [PubMed] [Google Scholar]
- Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, et al. , 2002. A role for cryptochromes in sleep regulation. BMC Neurosci. 3 (December), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Pasumarthi RK, Gerashchenko D, Thompson CL, Pathak S, Sancar A, et al. , 2008. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalograph!c differences among mouse strains. J. Neurosci. 28 (July), 7193–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC, 2008. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis 29 (June), 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH, Stanczyk FZ, Wang R, Koh WP, Yuan JM, Yu MC, 2013. Sleep duration, spot urinary 6-sulfatoxymelatonin levels and risk of breast cancer among Chinese women in Singapore. Int. J. Cancer 132 (February), 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB, 2011. Cigarette smoking and the incidence of breast cancer. Arch. Intern. Med. 171 (January), 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Deng Q, Fan WY, Wang WY, Wang X, 2014. Light exposure at night, sleep duration, melatonin, and breast cancer: a dose-response analysis of observational studies. Eur. J. Cancer Prev. 23 (July), 269–276. [DOI] [PubMed] [Google Scholar]
- Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. , 2003. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107 (March), 1129–1134. [DOI] [PubMed] [Google Scholar]
- Zhu Y, McAvoy S, Kuhn R, Smith DI, 2006. RORA, a large common fragile site gene, is involved in cellular stress response. Oncogene 25 (May), 2901–2908. [DOI] [PubMed] [Google Scholar]
- Ziegler RG, Fuhrman BJ, Moore SC, Matthews CE, 2015. Epidemiologic studies of estrogen metabolism and breast cancer. Steroids 99 (July), 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zock PL, Katan MB, 1998. Linoleic acid intake and cancer risk: a review and metaanalysis. Am. J. Clin. Nutr. 68 (July), 142–153. [DOI] [PubMed] [Google Scholar]


