A 33-year-old woman with a 2-year history of documented human immunodeficiency virus (HIV) infection presented to the emergency room at San Francisco General Hospital with a new-onset seizure disorder that had recurred over the past month. Physical examination was notable only for generalized weakness and cognitive deficits. The patient had also recently been treated for disseminated mycobacterium avium complex plus herpes simplex viral infections and notably had been non-adherent to anti-retroviral therapy during the past 2 years. The HIV viral load at the time of seizure presentation was 506,444 copies/mL with a peripheral CD4 count of only 1 cell/mm3. Magnetic resonance imaging of the brain demonstrated ring-enhancing lesions in the right frontal, parietal and occipital lobes. After admission and neurosurgical consultation, a stereotactic brain biopsy was performed and Epstein–Barr virus (EBV)+ diffuse large B-cell lymphoma (DLBCL) was diagnosed. Staging evaluation for systemic lymphoma, including computed tomography (CT) body imaging and bone marrow biopsy, was negative. The patient was started on an antiretroviral regimen of nucleoside reverse-transcriptase inhibitors tenofovir/ emtricitabine (Truvada) plus the non-nucleoside reverse-transcriptase inhibitor etravine, and began inpatient chemotherapy for AIDS-related primary CNS lymphoma.
Given concerns regarding the long-term neurotoxicity of whole-brain radiotherapy [1], as well as the pilot data of Jacomet et al. [2], intravenous high-dose methotrexate (3 g/m2) with leucovorin rescue was administered as monotherapy. The patient received five cycles of high-dose methotrexate every 2 weeks without adverse event. However, after an initial minor CD4 response to 2 months of concomitant antiretroviral therapy (CD4 count increased from 1 to 13 cells/mm3), HIV resistance was suspected due to a rising HIV viral load in peripheral blood and was confirmed by a drug resistance assay (Figure 1). A second-line antiretroviral regimen was provided that added ritonavir-boosted darunavir plus the HIV viral integrase antagonist dolutegravir, in combination with the original backbone of tenofovir and emtracitabine; etravirine was discontinued.
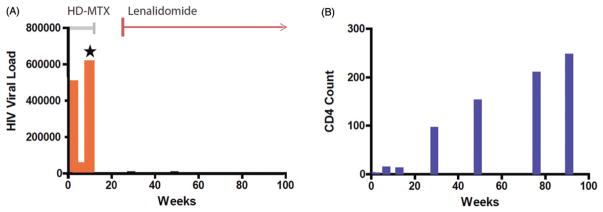
Figure 1.
Second-line cART regimen is effective with lenalidomide. Orange bars (A): viral load (copies/ml); blue bars (B): CD4 count (cells/microliter). Grey line (A): duration of HD-MTX-based therapy. Red line (A): duration of lenalidomide therapy. After 2 months of cART, acquired resistance to the first-line antiretroviral regimen consisting of nucleoside reverse transcriptase inhibitors, tenofovir and emtracitabine plus the non-nucleoside reverse transcriptase inhibitor etravirine, was demonstrated by HIV resistance assay and rising HIV viral load (Star). A second-line, five-drug antiretroviral regimen was provided that added ritonavir-boosted darunavir plus the viral integrase antagonist dolutegravir in combination with the backbone of tenofovir and emtracitabine; etravirine was discontinued. This regimen, administered with lenalidomide (10 mg/d) reduced the HIV viral load in peripheral blood and yielded an increase (B): in the CD4 count from 1 to 206. There was no additive toxicity with combination lenalidomide plus cART, and a complete radiographic response was achieved.
Because progressive CNS lymphoma was detected on MRI at 1 month after initiation of this second-line combination anti-retroviral therapy (cART) regimen, and given recent evidence for efficacy of the immunomodulatory drugs (IMiDs) as monotherapy in CNS lymphomas [3–5], a trial of lenalidomide (10 mg/d, 21 day cycle), was administered, without glucocorticoids and in lieu of additional HD-MTX and/or salvage WBRT. Within 6 weeks, there was marked lymphoma regression on MRI and ultimately a complete radiographic response was achieved (Figure 2). The patient’s Karnofsky’s performance status increased from a baseline of 40 to 70. Moreover, co-administration of lenalidomide did not have a deleterious impact on the CD4 count, which increased from 12 to 246 cells/mm3 in response to second-line cART (Figure 1). The patient continued lenalidomide maintenance therapy plus cART for greater than 3 years and, based on neuroimaging repeated 4 years after diagnosis, the patient remains in remission from primary CNS lymphoma.
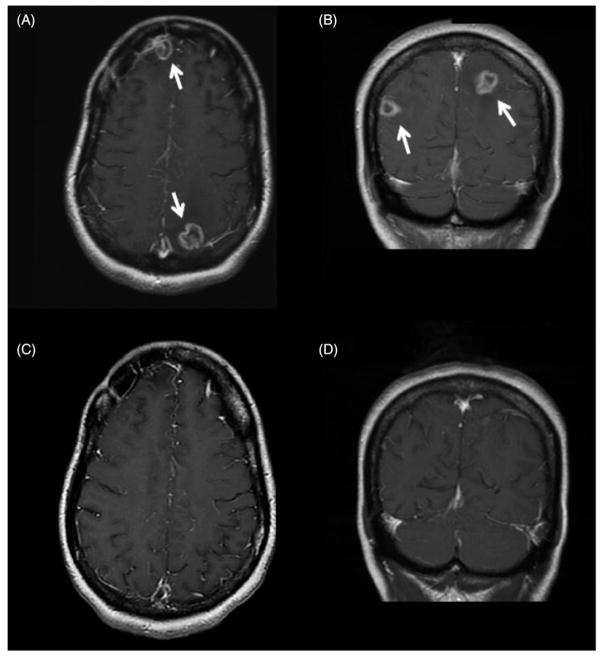
Figure 2.
Radiographic response of methotrexate-resistant AR-PCNSL to lenalidomide in combination with cART. Characteristic multifocal ring-enhancing lesions of AIDS-related primary CNS lymphoma (AR-PCNSL; arrows) in frontal and occipital lobes are demonstrated in T1 axial (A) and T1 coronal (B) images at diagnosis of AR-PCNSL. While the CNS lymphoma was refractory to five cycles of HD-MTX (3 g/m2) plus the first-line cART regimen, there was a complete radiographic response (C,D) with the second-line cART regimen administered with lenalidomide. The remission has been durable, lasting >3 years.
AIDS-related primary CNS lymphoma is typically an end-stage manifestation of HIV infection that is associated with CD4+ cell counts <50 cells/mm3 [6]. These lymphomas display a uniform EBV+, activated B-cell immunophenotype and, in general, the prognosis of AIDS-related primary CNS lymphoma is markedly inferior to that of primary CNS lymphoma arising among patients with an intact immune system. Historically, the median survival of AIDS-related primary CNS lymphoma rarely exceeds 3 months and whole-brain radiotherapy has been considered a standard front-line intervention [7,8]. While the incidence of AIDS-related primary CNS lymphoma declined substantially with the availability of effective combination anti-retroviral therapy, sporadic cases of this highly aggressive brain tumor continue to challenge clinicians, particularly in urban and poverty-stricken areas with higher HIV prevalence and in which there may be barriers to distribution of anti-retroviral medications [9,10]. We recently demonstrated that combination anti-retroviral therapies can be administered in conjunction with high-dose methotrexate-based induction therapy for newly-diagnosed AIDS-related primary CNS lymphoma, yielding long-term survival outcomes that are similar to those obtained in primary CNS lymphoma among the immunocompetent [11–13]. While it is possible that enhanced HIV suppression as well as immune reconstitution contributed to the response, to our knowledge this is the first report of the successful complete regression and durable remission of AIDS-related primary CNS lymphoma with lenalidomide as monotherapy. Importantly, lenalidomide has been demonstrated to markedly enhance T-cell functions, including chemotaxis to S1P and CCL21, as well as IL-2 production, in T cells isolated from HIV-infected patients [14], raising the possibility that lenalidomide might be synergistic with combination anti-retroviral therapy. The outcome in this case is particularly notable given the refractoriness of the CNS lymphoma to intravenous high-dose methotrexate chemotherapy. Of note, there was no clinical evidence of delayed EBV reactivation with ongoing lenalidomide maintenance although repeat viral load measurements of EBV pre- and post-initiation of lenalidomide were not performed.
The role of lenalidomide, and potentially other IMIDs, in AIDS-related primary CNS lymphoma deserves further evaluation in combination with cART. Lenalidomide plus rituximab may also be considered in this setting, given evidence for the efficacy of this combination, provided recently by Soussain et al. [15] in relapsed primary CNS lymphoma. Strategies based on lenalidomide, and potentially other IMiD’s, provide an attractive alternative to whole-brain radiotherapy, as well as to chemotherapy for AR-PCNSL, particularly given its efficacy in the stimulation of T-cell responses [16–18].
Acknowledgments
We are grateful to the dedicated physicians and nurses at San Francisco General Hospital. We are particularly grateful to Judith Luce, MD and Donald Abrams, MD, Division of Hematology/Oncology, San Francisco General Hospital.
Designed research: N.K.G., C.C.W. and J.L.R. Performed research and collected data: N.K.G., C.C.W., G.N.M., J.-P.J.Y. and J.L.R. Analyzed and interpreted the data: N.K.G., C.C.W., G.N.M., J.-P.J.Y. and J.L.R. Wrote/reviewed and approved the article: N.K.G., C.C.W., G.N.M., J.-P.J.Y. and J.L.R.
Funding
Research funding for this work was from Celgene and Genentech (J.L.R.). This work was supported by the NIH R01CA139-83-01A1, and by the Leukemia and Lymphoma Society (J.L.R.).
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at http://dx.doi.org/10.1080/10428194.2017.1312374.
References
- 1.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 2.Jacomet C, Girard PM, Lebrette MG, et al. Intravenous methotrexate for primary central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS. 1997;11:1725–1730. doi: 10.1097/00002030-199714000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein JL, Treseler PA, Stewart PJ. Regression of refractory intraocular large B-cell lymphoma with lenalidomide monotherapy. J Clin Oncol. 2011;29:e595–e597. doi: 10.1200/JCO.2011.34.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox MC, Mannino G, Lionetto L, et al. Lenalidomide for aggressive B-cell lymphoma involving the central nervous system? Am J Hematol. 2011;86:957. doi: 10.1002/ajh.22148. [DOI] [PubMed] [Google Scholar]
- 5.Rubenstein JL, Fraser E, Formaker P, et al. Phase I investigation of lenalidomide plus rituximab and outcomes of lenalidomide maintenance in recurrent CNS lymphoma. J Clin Oncol. 2016:34. (abstr. 7502) [Google Scholar]
- 6.Levine AM. Acquired immunodeficiency syndrome-related lymphoma. Blood. 1992;80:8–20. [PubMed] [Google Scholar]
- 7.Forsyth PA, DeAngelis LM. Biology and management of AIDS-associated primary CNS lymphomas. Hematol Oncol Clin North Am. 1996;10:1125–1134. doi: 10.1016/s0889-8588(05)70388-9. [DOI] [PubMed] [Google Scholar]
- 8.Raez LE, Patel P, Feun L, et al. Natural history and prognostic factors for survival in patients with acquired immune deficiency syndrome (AIDS)-related primary central nervous system lymphoma (PCNSL) Crit Rev Oncog. 1998;9:199–208. [PubMed] [Google Scholar]
- 9.Silverberg MJ, Leyden W, Quesenberry CP, Jr, et al. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med. 2009;24:1065–1072. doi: 10.1007/s11606-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha S, Jacobs EA, Moore RD, et al. Trust in physicians and racial disparities in HIV care. AIDS Patient Care STDs. 2010;24:415–420. doi: 10.1089/apc.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202) J Clin Oncol. 2013;31:3061–8. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3:e217–e227. doi: 10.1016/S2352-3026(16)00036-3. [DOI] [PubMed] [Google Scholar]
- 13.Gupta NK, Nolan A, Omuro A, et al. Long-term survival in AIDS-related primary central nervous system lymphoma. Neuro Oncol. 2017;19:99–108. doi: 10.1093/neuonc/now155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim H, Kane L, Schwartz JB, et al. Lenalidomide enhancement of human T cell functions in human immunodeficiency virus (HIV)-infected and HIV-negative CD4 T lymphocytopenic patients. Clin Exp Immunol. 2012;169:182–189. doi: 10.1111/j.1365-2249.2012.04603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghesquieres H, Houillier C, Chinot O, et al. Rituximab-lenalidomide (REVRI) in relapse or refractory primary central nervous system (PCNSL) or vitreo retinal lymphoma (PVRL): results of a “proof of concept” phase II study of the French LOC Network 2016 Proc. American Society of Hematology. 58th Annual Meeting and Exposition; San Diego. December 3–6, 2016. [Google Scholar]
- 16.Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4 (CRBN.) Br J Haematol. 2014;164:811–821. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BN, Gao H, Cohen EN. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117:3999–4008. doi: 10.1002/cncr.25983. [DOI] [PMC free article] [PubMed] [Google Scholar]