Abstract
BACKGROUND
Criteria for identification of anatomic ventricular tachycardia (VT) substrates in arrhythmogenic right ventricular cardiomyopathy (ARVC) on late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) are unclear.
OBJECTIVE
We sought to define a) the association of regional RV epicardial voltage amplitude with the distribution of LGE, and b) appropriate image signal intensity (SI) thresholds for VT substrate identification, in ARVC.
METHODS
Pre-procedural LGE-CMR and epicardial electrogram mapping were performed in 10 ARVC patients. The location of epicardial electrogram map points, obtained during sinus rhythm with intrinsic conduction or RV pacing, were retrospectively registered to the corresponding LGE image regions. Standardized SI z-scores (standard deviation distance from the mean) were calculated for each 10-mm region surrounding map points.
RESULTS
In patient-clustered, generalized estimating equations models that included 3205 epicardial EAM points and corresponding SI measures, bipolar (−1.43 mV/z-score, P < 0.001) and unipolar voltage amplitude (−1.22 mV/z-score, P < 0.001) were associated with regional SI z-scores. In contrast to the QRS-LP interval (P =0.362), the LP activation index (LPAI) defined as electrogram duration divided by QRS-LP was associated with regional SI z-scores (P < 0.001). SI z-score thresholds of >0.05 [95% confidence interval (CI), −0.05–0.15] and < −0.16 (95% CI, −0.26–0.06) corresponded to bipolar voltage measures <0.5 and >1.0 mV, respectively.
CONCLUSION
Increased RV gadolinium uptake is associated with lower epicardial bipolar and unipolar electrogram voltage amplitude. Standardized LGE-CMR SI z-scores may augment pre-procedural planning for identification of low voltage zones and abnormal myocardium in ARVC.
Keywords: Arrhythmogenic right ventricular cardiomyopathy/dysplasia, Scar, Epicardial Mapping, Ventricular tachycardia, Late gadolinium delayed enhancement, Magnetic resonance imaging
Introduction
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) is characterized by fibro-fatty replacement of myocardium that primarily involves the right ventricle (RV).1, 2 This infiltrative process occurs more extensively in the RV epicardium and is heterogeneous, thus creating channels of viable but slow conductive myocardium as the substrate for reentrant ventricular tachycardia (VT).1–4 Prior studies have shown that catheter ablation using a combined endocardial and epicardial approach targeting late potentials (LP), which represent slow conducting channels within non-conductive tissue, results in improved acute and long-term results.3–10 Careful endocardial and epicardial electro-anatomic mapping (EAM) to delineate the three-dimensional distribution of non-conductive fibro-fatty infiltrates as well as conductive channels is critical to guide entrainment maneuvers and ablation. However, the EAM process is often limited by sampling density limitations due to papillary muscles and trabeculae on the endocardium and adherent (not intra-myocardial) fat on the epicardium. Therefore, development of techniques to visualize non-conductive infiltrates in the RV myocardium would provide clinical value.
The thin profile of the RV has limited the image based visualization of VT substrates in ARVC. Recent improvements in cardiac magnetic resonance (CMR) late gadolinium enhancement (LGE), however, have resulted in renewed interest in myocardial characterization in the setting of ARVC.11, 12 The objective of this study was to define a) the association of regional RV bipolar and unipolar voltage amplitude with the distribution of LGE, and b) appropriate image signal intensity (SI) thresholds for VT substrate identification, in ARVC patients referred for ablation. Due to the known preponderance of electrogram abnormalities on the RV epicardium compared to the endocardium,3 we focused on the association of epicardial voltage amplitude with LGE findings.
Methods
Patient Cohort
The institutional VT ablation database was screened to identify consecutive ARVC patients that underwent epicardial mapping and VT ablation at the Hospital of the University of Pennsylvania using the Carto 3 mapping system (Biosense-Webster, Diamond Bar, CA) and pre-procedural LGE-CMR using a contemporary high-resolution 3-dimensional (3D) ECG and respiratory gated sequence. Ten patients met these criteria during the period from 2011–2014, and formed the study cohort (Table 1). All patients met the 2010 International Task force criteria for ARVC.13 The study was approved by the institutional committee on human research at the University of Pennsylvania Health System.
Table 1.
Baseline Characteristics of Patients
| Patient# | Age(y) | Sex | History of cardiac arrest or syncope | Task Force criteria | MRI Completed before ICD Implanted (4/10) | Antiarrhythmic Drug Failed | Endocardial ablation before | SI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Structural | Depolarization | Repolarization | Arrhythmia | Family History | ||||||||
| 1 | 45 | M | N | minor | NA | minor | major | No | No | metoprolol | No | 100.1±35.4 |
| 2 | 53 | M | N | major | major | major | major | No | Yes | satalol and amiodarone | No | 39.3±14.3 |
| 3 | 28 | F | N | No | No | major | major | No | No | satalol, propafenone, mexilitine | No | 101.6±36.3 |
| 4 | 38 | F | N | major | No | major | minor | No | Yes | amiodarone | No | 91.8±18.4 |
| 5 | 27 | M | N | minor | major | minor | major | Yes | No | amiodarone | No | 18.6±8.0 |
| 6 | 14 | M | Y | major | minor | major | minor | No | No | Satalol and amiodarone | No | 22.4±4.4 |
| 7 | 28 | F | Y | No | major | minor | minor | No | No | Satalol, propafenone, mexilitine | No | 96.2±42.6 |
| 8 | 30 | M | Y | major | minor | No | minor | No | Yes | Yes | 102.3±43.6 | |
| 9 | 39 | M | N | No | minor | No | minor | Yes (major) | Yes | Soltalol, metoprol | Yes | 92.4±26.0 |
| 10 | 49 | M | N | major | No | No | major | No | No | Satalol and amiodarone | No | 90.2±32.0 |
M, male; F, female; and N/A, not applicable (patient with paced ECG complex), SI, signal intensity
Cardiovascular Magnetic Resonance Acquisition and Image Analysis
CMR was performed on a 1.5 T Siemens Sonata or Avanto (Siemens, Erlangen, Germany) magnetic resonance scanner and a 6-channel phased array body coil in combination with a 6-channel spine matrix coil. LGE-MRI scans were acquired 10 minutes following IV administration of 0.1 mmol/kg Gadolinium-DTPA (Schering, Berlin, Germany) using a fat-saturated 3D inversion recovery prepared fast spoiled gradient recalled echo sequence with respiratory navigation and ECG-gating, echo time of 1.52ms, repetition time of 3.8ms, in-plane resolution of 1.3 × 1.3, slice thickness of 2.0 mm, and flip angle of 10 degrees. Trigger time for 3D LGE-CMR images was optimized to acquire imaging data during ventricular diastole as dictated by inspection of the cine images. The optimal inversion time (TI) was identified with a TI scout scan to maximize nulling of left ventricular myocardium.
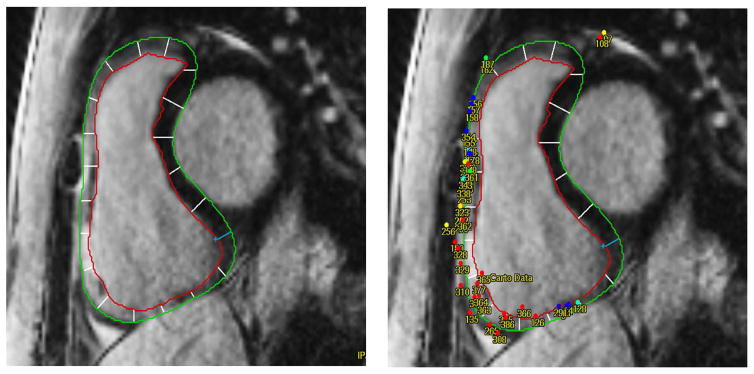
A 3D right ventricular reconstruction was obtained by using Mass Research (Version 7.2, Leiden University Medical Center, Leiden, Netherlands). Right ventricular endocardial/epicardial contours were manually defined on contiguous short-axis planes as demonstrated in Figure 1. In thin walled regions, comparison with steady state free precession cine or black blood images was utilized to accurately identify endocardial and epicardial boundaries. The RV myocardium visualized in each short-axis image plane was divided into 20 sectors, with clockwise numbering starting from a reference point placed at the posterior RV insertion into the septum (Figure 1). Following previously described methodology,14 and using Mass Research software (Version 7.2, Leiden University Medical Center, Leiden, Netherlands) electro-anatomic map point and intra-cardiac echocardiogram coordinates were co-registered with LGE image planes. Right ventricular apex, pulmonary artery, and aortic cusp 3D anatomic reconstructions from SoundStar 3D diagnostic ultrasound catheter (Biosense Webster, Inc., Diamond, Bar, CA) and Cartosound mapping system were registered to corresponding contours on the MR image. In all cases, the His electrogram was confirmed to localize to the anteroseptal tricuspid valve on the MR image. Figure 1 illustrates an example of electrogram to image registration. The surrounding 10 mm SI and SI z score for each electro-anatomic map point were exported for data analysis. The SI z-score for each map region (10 mm diameter surrounding each map point) was calculated using the mean (μ) and standard deviation (SD) of the SI distribution for all RV sectors for each patient, using the following formula:
Figure 1.
Example of LGE-CMR and EAM registration. A, Right ventricular endocardial/epicardial contours were manually defined on contiguous short-axis image planes. Each plane was divided into 20 sectors (with clockwise numbering from the reference point. B, Registration of EAM points to RV by using the location land mark of tricuspid annulus.
Electrophysiological Study and Substrate-based strategy for VT ablation
Epicardial access and electrogram mapping was performed using previously described techniques.3, 15 Briefly, an 8F sheath was introduced into the pericardial space, and the ablation catheter (n=9, 3.5 mm tip, 2 mm body electrodes, 2-5-2 spacing, Thermo-Cool, Biosense Webster, Diamond Bar, CA) or DECANAV (n=1, 2 mm tip electrode, 1 mm body electrodes, 2-8-2 mm spacing, Biosense Webster, Diamond Bar, CA) was advanced through the sheath for mapping. Detailed epicardial voltage mapping focused on the entire RV and extended over the left ventricular (LV) surface (Figure 1) during sinus rhythm with intrinsic conduction or RV pacing. Valve planes and per-valvular tissues were confirmed by intra-cardiac echocardiography (ICE, Acuson, Siemens Healthcare, Erlangen, Germany) and were given a “location only” tag to avoid downward bias upon the voltage map due to structural barriers. A bipolar signal amplitude of ≥ 1.0 mV was categorized as normal, and a threshold of <0.5 mV was defined as “dense scar”, based upon prior comparison of data from patients with and without cardiomyopathy.3 Electrogram points with voltage < 1.0 mV with underlying epicardial fat as identified by ultrasound (primarily from annular sites), or normal electrogram duration (<100 ms) were deleted during mapping.
Surface electrocardiograms and intra-cardiac and epicardial signals were recorded and analyzed with the use of the Prucka Cardiolab recording system (Houston, TX). Detailed voltage mapping was performed, with attention to tagging the location of signals with LPs and localization of conductive channels within low-voltage region. The QRS-LP interval was measured as the time interval from the onset of surface QRS to the latest LP electrogram component.16 LP activation index (LPAI) was defined as the ratio of electrogram duration divided by QRS-LP interval. VTs were induced using a programmed ventricular stimulation protocol with up to triple extra-stimuli from ≥ 2 ventricular sites following ≥ 2 drive cycle lengths. The combination of LPs, a long stimulus-QRS interval during pace mapping, and 12/12 pace-map QRS match suggested the likelihood of proximity to VT origin/circuit when a substrate-based ablation strategy was used.17, 18 When hemodynamically stable VT was inducible, entrainment maneuvers were used to target circuit sites. Radiofrequency energy was applied using an open irrigated ablation catheter (Thermo-Cool, Biosense Webster, Diamond Bar, CA) with power of 20–50 W, targeting a maximum temperature of 42°, and a maximum impedance drop of 12–15 Ohms. Acute procedural success was defined as total if all VTs were rendered non-inducible, partial if the clinical VT was non-inducible but other VT morphologies remained inducible, and failure if the clinical VT remained inducible at the end of the procedure.19
Follow-Up
Patients were followed in clinic 6 weeks following the procedure and every 6 months thereafter, in addition to symptom or device therapy prompted visits. When longitudinal office follow-up visits were unavailable, telephone interviews were performed with patients and/or referring physicians at 6- month intervals to confirm the absence of arrhythmias.
Statistical Analysis
Continuous variables are expressed as mean ± SD, and categorical variables are expressed as percentages. Due to the left skewed nature of bipolar voltage data, log transformation was used to accommodate modeling within a linear framework. Sequential generalized estimating equation models with an exchangeable working correlation structure, Gaussian family and identity link function, were used to examine the association between bipolar voltage, unipolar voltage, QRS-LP interval, LPAI as dependent variables and Z scores as the independent variable, while accounting for data clustering within patients. Based upon these models, Z score thresholds for a) typical voltage thresholds assigned to epicardial abnormal voltage and “dense scar”, and b) identification of delayed potential sites were determined. Receiver Operator Characteristics Regression with 1000 bootstrap resamples and data clustering per patient was performed to characterize the diagnostic accuracy of SI z-score thresholds for identification of delayed potential sites. Statistical analyses were performed using Stata software (version 12, StataCorp, College Station, TX).
Results
Patient Characteristics
Table 1 tabulates the baseline characteristics on a per patient basis. There were 4 (40%) women and the mean age was 43±15 years. All patients presented with drug refractory recurrent VT and underwent epicardial mapping and ablation. Two patients had undergone a prior failed endocardial ablation attempt. The CMR examination was performed prior to ICD implantation in 4 patients and following ICD implantation in the remaining 6 patients, and 52±67 days prior to mapping and ablation in all cases. All patients exhibited regional RV LGE. The endocardial voltage was normal in 6 patients, which displayed extensive voltage abnormalities (<1.0 mV) corresponding to regions with LGE upon epicardial mapping. The remaining 4 patients, displayed voltage abnormalities corresponding to LGE regions in both the endocardium and epicardium.
Association of SI on LGE-CMR with Bipolar and Unipolar Epicardial Voltage
A total of 2,340 sectors from 117 LGE short axis image planes were quantitatively analyzed. Overall, 3312 EAM points were collected. Of these EAM points, 107 points were excluded from the study owing to image artifact at adjacent image sectors, lack of a stable contact signal, or poor approximation between the point and corresponding ventricular epicardial myocardium (generally near valve planes). The remaining 3205 EAM points obtained from the RV epicardium were included for analysis and registered to the corresponding LGE-MRI 10-mm regions. The mean and standard deviation for SI of all regions per patient were calculated and summarized in Table 1.
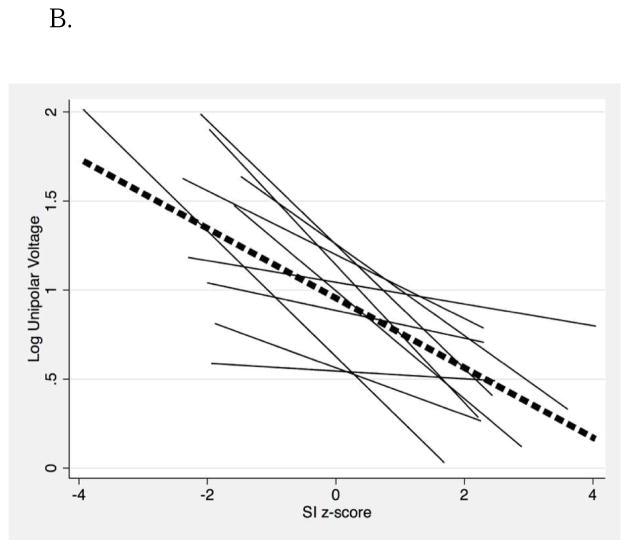
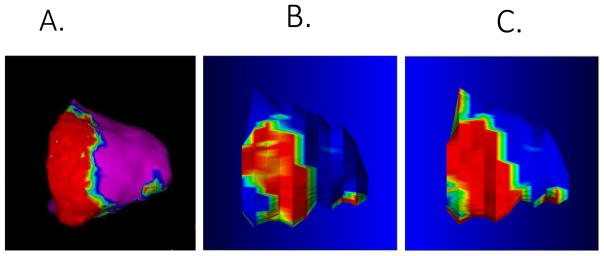
In patient-clustered, generalized estimating equations linear models, bipolar (−1.43 mV/z-score, P < 0.001) and unipolar voltage amplitude (−1.22 mV/z-score, P < 0.001) were associated with regional SI z-scores (Figure 2). Based on these models, SI z-score thresholds of > 0.05 [95% confidence interval (CI), −0.05–0.15] and < −0.16 (95% CI, −0.26–0.06) corresponded to bipolar voltage measures <0.5 and >1.0 mV, respectively. Figure 3 illustrates EAM voltage (Panel A), non-invasive scar estimation based upon SI z-score thresholds (Panel B), and non-invasive scar estimation based upon a routinely used threshold at 6 SD (Panel C) above the mean intensity of normal myocardium, in a representative patient with ARVC. Scar size based upon SI z-score thresholds on LGE-CMR and electrogram mapping were strongly correlated (Spearman’s rho 0.867, P = 0.001).
Figure 2.
The association of regional RV epicardial voltage with SI z-scores on LGE-CMR in patients with ARVC. Voltage measures have been log-transformed to enable a linear analysis. A, bipolar voltage; B, unipolar voltage.
Figure 3.
Comparison of bipolar electrogram map with image predicted scar distribution. A, Bipolar electrogram map (0.5 mV–1.0 mV); B, SI z-scores based upon data in this study; and C, 6 SD > mean as a commonly applied threshold for quantification of scar in NICM.
Analyzed Standardized Regional SI on LGE-CMR and LP Points
A total of 650 (20.3%) of the sampled points exhibited a LP. The median SI z-score of regions that displayed LP was significantly higher than those without LP (0.36, IQR: −0.05 to 0.85; versus −0.10, IQR: −0.80 to 0.56, P<0.001). This was also true when the comparison was restricted to points within the low voltage <1.0 mV range (0.31, IQR: −0.12 to 0.85; versus 0.12, IQR: −0.52 to 0.72, P<0.001). While LP activation index (LPAI) was associated with SI z-scores (P < 0.001), QRS-LP interval was not (P =0.362). The C statistic for identification of sites exhibiting delayed potentials based upon SI z-scores was 0.660±0.038 (95% CI 0.586–0.734). At a threshold z-score of > 0.05, the sensitivity is 72%, and specificity 56%, for identification of delayed potentials in the RV epicardium.
Follow-up
During a mean follow-up of 35±19 (range 3 to 60) months, 8 (80%) patients remained free of any episodes of VT despite discontinuation of anti-arrhythmic drugs. There were no deaths during the follow-up period. Of the two patients who experienced VT recurrence, one patient had recurrent VT 3 months after incomplete epicardial ablation (procedure terminated prematurely due to hypotension), while the second patient had recurrent VT 36 months following ablation and was subsequently treated with repeat endocardial RF ablation.
Discussion
The main finding of this study is that in patients with ARVC referred for epicardial VT ablation, the epicardial voltage distribution is associated with the regional SI z-score on LGE-CMR. LGE-CMR imaging is a validated technique for quantification of fibrosis in the setting of ischemic/nonischemic cardiaomyopathy. Prior studies have demonstrated the presence of LGE in the RV with overall agreement with histologic fibrosis when regions with LGE were targeted for biopsy.20 Autopsy studies in patients with ARVC have shown that fibrofatty scar tissue progresses from the epicardium toward the endocardium and predominantly involves the right ventricular free wall.2, 21 A prior study has suggested that relatively low resolution (2D) LGE-MRI can identify areas of RV scar in most ARVC patients, albeit with lower sensitivity than endocardial voltage mapping.22 In our study of patients referred for VT ablation, RV LGE was ubiquitous and matched the distribution of epicardial scar. These seemingly discordant findings can be reconciled by several factors. First, the 3D respiratory navigated sequence utilized in the present study provides significantly higher spatial resolution than that employed by prior LGE studies to identify fibro-fatty replacement in ARVC. Thus, volume-averaging artifacts, which may have led to false identification of LGE, are less likely to have occurred in our study. Second, due to a) the intervening tricuspid valve apparatus and acute angle from the RV inlet to the endocardial basal lateral RV, and b) predilection for epicardial involvement, endocardial voltage mapping of the basal right ventricle is often inconclusive and epicardial mapping is essential for accurate identification of voltage abnormalities in ARVC. Our data suggest that regions of the RV epicardium which exhibit increased gadolinium uptake or slow washout on LGE-CMR exhibit lower regional bipolar and unipolar voltage amplitude on epicardial EAM. Importantly, there is a predictable association with voltage, which may enable non-invasive localization of important VT substrates. In this study, we have also quantitated SI z-score thresholds of > 0.05 and < −0.16 corresponding to bipolar voltages of < 0.5 mV and >1.0 mV, respectively. The availability of these thresholds has important clinical implications because differentiation between scar versus epicardial fat can be difficult with epicardial voltage mapping.23, 24
Late Potentials and LGE-MRI in the setting of ARVC
The mechanism of VT in patients with structural heart disease is predominantly scar-related reentry with slow and anisotropic conduction.25 In patients with ARVC, RV fibro-fatty infiltration resulting in scar can serve as the arrhythmogenic substrate for VT.20, 26 Since impulse propagation in diseased myocardium results in local conduction delay with fractionated electrical activity, LPs can be recorded during sinus rhythm or RV pacing.16 It has been shown that elimination of LPs following surgical subendocardial resection or catheter ablation can result in VT noninducibility.27, 28 Using the same strategy of targeting epicardial LPs with catheter ablation, 8 out of 10 patients with ARVC remained free of any episodes of VT during long follow-up. The results of our study are consistent with prior reports of VT ablation in patients with ARVC targeting epicardial LP abolition.29 We showed in this study that the presence of LP was closely associated with RV epicardial SI z-score in patients with ARVC. Thus, similar to the co-localization of LPs with myocardial infarct scar,30 LPs in the RV epicardium of patients with ARVC are also highly specific for regions with the highest gadolinium uptake and dense fibro-fatty changes.
The QRS-LP interval is used to measure the degree of local conduction delay, which is significantly longer near the entrance and isthmus compared to exit sites in ischemic and nonischemic cardiomyopathy.16 We found in our study that the QRS-LP interval was not associated with the SI z-scores of LGE-CMR. However, the LPAI was strongly associated with SI z-scores. The QRS duration affects the measurement of QRS-LP interval, but the use of LPAI overcomes this disadvantage and may better identify critical isthmus sites during VT ablation.
Limitations
This was a retrospective single-center study with a small sample size and larger prospective studies are required to confirm our findings. Mass research software has been previously validated to provide acceptable registration error.31, 32 In the current study, the average distance of each point to epicardial surface was unobtainable due to software limitations; however, the position of the His electrogram and agreement of scar size between images and electrogram mapping suggested adequate registration. Although this study demonstrated an association between the regional epicardial voltage amplitude, and the distribution of LGE-CMR SI in patients with ARVC, it is impossible to distinguish myocardial infiltration with mixed fat and fibrosis from either in isolation. A prior study suggested that the optimal TI for RV myocardial signal suppression may be shorter than for the LV when using a 2D LGE sequence.33 The mechanisms for the aforesaid observation, such as partial volume averaging with fat or blood pool (due to increased trabeculation) in the RV are less likely to confound the optimal RV TI in 3D LGE sequences such as used in the present study.34 Thus, while LV myocardial signal suppression was optimized in the present study, adequate RV signal distribution was observed to distinguish differences between regions with versus without abnormal electrogram characteristics. Some patients in this study had undergone ICD implantation prior to their pre-procedural LGE-CMR and some artifact was observed, requiring the exclusion of affected regions (2%). However, this weakness improves the generalizability of our results to the real-world setting, where many patients present for VT ablation following ICD implantation. Two different mapping catheters were used for the study. However, sensitivity analysis by exclusion of DECANAV data (n=1 patient, 224 matched electrogram and image data), resulted in no significant change in the association of bipolar (−1.41 mV/z-score, P < 0.001) and unipolar voltage amplitude (−1.19 mV/z-score, P < 0.001) with regional SI z-scores. Finally, genetic testing was not routinely performed and the sample size was insufficient to examine the effect of gene positivity upon imaging phenotypes.
Conclusion
Increased RV gadolinium uptake is associated with lower epicardial bipolar and unipolar electrogram voltage amplitude. Standardized LGE-CMR SI z-scores may augment pre-procedural planning for identification of low voltage zones and abnormal myocardium in ARVC.
Acknowledgments
Funding: The study was funded by the National Institutes of Health (R01HL116280) as well as by a Biosense Webster grant to Dr. Nazarian.
Footnotes
Conflict of Interest Disclosure: Dr. Nazarian is a scientific advisor to CardioSolv, Abbott Medical, Siemens Healthcare, and Biosense Webster as well as a principal investigator for research funding from Biosense Webster.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: A report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Tabib A, Loire R, Chalabreysse L, Meyronnet D, Miras A, Malicier D, Thivolet F, Chevalier P, Bouvagnet P. Circumstances of death and gross and microscopic observations in a series of 200 cases of sudden death associated with arrhythmogenic right ventricular cardiomyopathy and/or dysplasia. Circulation. 2003;108:3000–3005. doi: 10.1161/01.CIR.0000108396.65446.21. [DOI] [PubMed] [Google Scholar]
- 3.Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366–375. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Kilicaslan F, Schweikert RA, et al. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005;111:3209–3216. doi: 10.1161/CIRCULATIONAHA.104.510503. [DOI] [PubMed] [Google Scholar]
- 5.Bai R, Di Biase L, Shivkumar K, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: Arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011;4:478–485. doi: 10.1161/CIRCEP.111.963066. [DOI] [PubMed] [Google Scholar]
- 6.Berruezo A, Fernandez-Armenta J, Mont L, Zeljko H, Andreu D, Herczku C, Boussy T, Tolosana JM, Arbelo E, Brugada J. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012;5:111–121. doi: 10.1161/CIRCEP.110.960740. [DOI] [PubMed] [Google Scholar]
- 7.Dalal D, Jain R, Tandri H, et al. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007;50:432–440. doi: 10.1016/j.jacc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Philips B, Madhavan S, James C, et al. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:499–505. doi: 10.1161/CIRCEP.111.968677. [DOI] [PubMed] [Google Scholar]
- 9.Santangeli P, Zado ES, Supple GE, Haqqani HM, Garcia FC, Tschabrunn CM, Callans DJ, Lin D, Dixit S, Hutchinson MD, Riley MP, Marchlinski FE. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2015;8:1413–1421. doi: 10.1161/CIRCEP.115.003562. [DOI] [PubMed] [Google Scholar]
- 10.Wei W, Liao H, Xue Y, et al. Long-term outcomes of radio-frequency catheter ablation on ventricular tachycardias due to arrhythmogenic right ventricular cardiomyopathy: A single center experience. PLoS One. 2017;12:e0169863. doi: 10.1371/journal.pone.0169863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aquaro GD, Barison A, Todiere G, Grigoratos C, Ait Ali L, Di Bella G, Emdin M, Festa P. Usefulness of combined functional assessment by cardiac magnetic resonance and tissue characterization versus task force criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2016;118:1730–1736. doi: 10.1016/j.amjcard.2016.08.056. [DOI] [PubMed] [Google Scholar]
- 12.Deac M, Alpendurada F, Fanaie F, et al. Prognostic value of cardiovascular magnetic resonance in patients with suspected arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. 2013;168:3514–3521. doi: 10.1016/j.ijcard.2013.04.208. [DOI] [PubMed] [Google Scholar]
- 13.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki T, Miller CF, Hansford R, et al. Myocardial structural associations with local electrograms: A study of postinfarct ventricular tachycardia pathophysiology and magnetic resonance-based noninvasive mapping. Circ Arrhythm Electrophysiol. 2012;5:1081–1090. doi: 10.1161/CIRCEP.112.970699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 16.Hsia HH, Lin D, Sauer WH, Callans DJ, Marchlinski FE. Relationship of late potentials to the ventricular tachycardia circuit defined by entrainment. J Interv Card Electrophysiol. 2009;26:21–29. doi: 10.1007/s10840-009-9421-8. [DOI] [PubMed] [Google Scholar]
- 17.Arenal A, Glez-Torrecilla E, Ortiz M, Villacastin J, Fdez-Portales J, Sousa E, del Castillo S, Perez de Isla L, Jimenez J, Almendral J. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 18.Cano O, Hutchinson M, Lin D, Garcia F, Zado E, Bala R, Riley M, Cooper J, Dixit S, Gerstenfeld E, Callans D, Marchlinski FE. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Frankel DS, Mountantonakis SE, Zado ES, et al. Noninvasive programmed ventricular stimulation early after ventricular tachycardia ablation to predict risk of late recurrence. J Am Coll Cardiol. 2012;59:1529–1535. doi: 10.1016/j.jacc.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Tandri H, Saranathan M, Rodriguez ER, Martinez C, Bomma C, Nasir K, Rosen B, Lima JA, Calkins H, Bluemke DA. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 21.Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 22.Marra MP, Leoni L, Bauce B, et al. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: Comparison of 3d standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol. 2012;5:91–100. doi: 10.1161/CIRCEP.111.964635. [DOI] [PubMed] [Google Scholar]
- 23.Dixit S, Narula N, Callans DJ, Marchlinski FE. Electroanatomic mapping of human heart: Epicardial fat can mimic scar. J Cardiovasc Electrophysiol. 2003;14:1128. doi: 10.1046/j.1540-8167.2003.03138.x. [DOI] [PubMed] [Google Scholar]
- 24.Reddy VY, Wrobleski D, Houghtaling C, Josephson ME, Ruskin JN. Combined epicardial and endocardial electroanatomic mapping in a porcine model of healed myocardial infarction. Circulation. 2003;107:3236–3242. doi: 10.1161/01.CIR.0000074280.62478.E1. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson WG, Delacretaz E. Strategies for catheter ablation of scar-related ventricular tachycardia. Curr Cardiol Rep. 2000;2:537–544. doi: 10.1007/s11886-000-0039-9. [DOI] [PubMed] [Google Scholar]
- 26.Turrini P, Angelini A, Thiene G, Buja G, Daliento L, Rizzoli G, Nava A. Late potentials and ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 1999;83:1214–1219. doi: 10.1016/s0002-9149(99)00062-4. [DOI] [PubMed] [Google Scholar]
- 27.Josephson ME, Harken AH, Horowitz LN. Endocardial excision: A new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979;60:1430–1439. doi: 10.1161/01.cir.60.7.1430. [DOI] [PubMed] [Google Scholar]
- 28.Silberbauer J, Oloriz T, Maccabelli G, et al. Noninducibility and late potential abolition: A novel combined prognostic procedural end point for catheter ablation of postinfarction ventricular tachycardia. Circ Arrhythm Electrophysiol. 2014;7:424–435. doi: 10.1161/CIRCEP.113.001239. [DOI] [PubMed] [Google Scholar]
- 29.Kirubakaran S, Bisceglia C, Silberbauer J, Oloriz T, Santagostino G, Yamase M, Maccabelli G, Trevisi N, Della Bella P. Characterization of the arrhythmogenic substrate in patients with arrhythmogenic right ventricular cardiomyopathy undergoing ventricular tachycardia ablation. Europace. 2017;19:1049–1062. doi: 10.1093/europace/euw062. [DOI] [PubMed] [Google Scholar]
- 30.Tung R, Nakahara S, Ramirez R, Lai C, Fishbein MC, Shivkumar K. Distinguishing epicardial fat from scar: Analysis of electrograms using high-density electroanatomic mapping in a novel porcine infarct model. Heart Rhythm. 2010;7:389–395. doi: 10.1016/j.hrthm.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piers SR, Tao Q, de Riva Silva M, Siebelink HM, Schalij MJ, van der Geest RJ, Zeppenfeld K. Cmr-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc Imaging. 2014;7:774–784. doi: 10.1016/j.jcmg.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Venlet J, Piers SRD, Kapel GFL, de Riva M, Pauli PFG, van der Geest RJ, Zeppenfeld K. Unipolar endocardial voltage mapping in the right ventricle: Optimal cutoff values correcting for computed tomography-derived epicardial fat thickness and their clinical value for substrate delineation. Circ Arrhythm Electrophysiol. 2017:10. doi: 10.1161/CIRCEP.117.005175. [DOI] [PubMed] [Google Scholar]
- 33.Desai MY, Gupta S, Bomma C, Tandri H, Foo TK, Lima JA, Bluemke DA. The apparent inversion time for optimal delayed enhancement magnetic resonance imaging differs between the right and left ventricles. J Cardiovasc Magn Reson. 2005;7:475–479. doi: 10.1081/jcmr-200053534. [DOI] [PubMed] [Google Scholar]
- 34.Grosse-Wortmann L, Macgowan CK, Vidarsson L, Yoo SJ. Late gadolinium enhancement of the right ventricular myocardium: Is it really different from the left ? J Cardiovasc Magn Reson. 2008;10:20. doi: 10.1186/1532-429X-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]