Abstract
Self-reported pain intensity assessments are central to chronic pain research. Ecological Momentary Assessment (EMA) methodologies are uniquely positioned to collect these data, and are indeed being utilized in the field. However, EMA protocols are complex, and many decisions are necessary in the design of EMA research studies. A systematic literature review identified 105 articles drawing from 62 quantitative EMA research projects examining pain intensity in adult chronic pain patients. Study characteristics were tabulated in order to summarize and describe the use of EMA, with an emphasis placed on various dimensions of decision-making involved in executing EMA methodologies. Most identified studies considered within-person relationships between pain and other variables, and a few examined interventions on chronic pain. There was a trend toward the use of smartphones as EMA data collection devices more recently, and completion rates were not reported in nearly one-third of studies. Pain intensity items varied widely with respect to number of scale points, anchor labels, and length of reporting period; most used numeric rating scales. Recommendations are provided for reporting to improve reproducibility, comparability, and interpretation of results, and for opportunities to clarify the importance of design decisions.
Keywords: Ecological momentary assessment, experience sampling, electronic diaries, self-report, chronic pain
Introduction
Pain intensity represents the primary outcome in most clinical trials of pain disorders and is nearly universally assessed in chronic pain research32, 79. Chronic pain affects over 11% of the population of the United States90, and there is an undisputed need for the accurate and reliable assessment of pain. Although alternatives to self-reported pain intensity (e.g., observation of pain behaviors64) have previously been considered, self-reports presently constitute the gold standard of pain assessment because they are able to reflect the subjectivity inherent in the pain experience32.
Within the family of self-report methodologies, Ecological Momentary Assessment (EMA) is uniquely positioned to assess a patient’s pain experience with high precision. EMA involves momentary data collection in participants’ natural environments at multiple points in time, and its advantages follow from these three central aspects112. First, momentary measurement reduces recall biases by capturing present pain experiences rather than pain beliefs or summary ratings based on memory. Second, EMA occurs in patients’ natural environments and social contexts, thus increasing the ecological validity of the assessment. Third, multiple repeated assessments occur over time, providing potentially fine-grained information about pain experiences. Whereas pain research is often based on cross-sectional snapshots, EMA methodologies provide rich data that facilitate the examination of short-term shifts, temporal dynamics, and the effects of specific contexts on the pain experience. In addition, Ecological Momentary Interventions, also known as Just-in-Time Adaptive Interventions, become possible with EMA57, 91.
The importance ascribed to the advantages of EMA by organizations that drive protocol design for chronic pain research [e.g., Food and Drug Administration (FDA) guidelines108, Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations32], along with the increased availability of associated technologies, has naturally translated into an increase in the use of EMA in pain research. Nevertheless, it remains a specialized approach, and knowledge of its implementation is still relatively fragmented.
It is a central tenet of research design that the way in which data are collected influences the type of data collected. In a study comprised of a single assessment, the collection of pain intensity data upon waking from one group and in the middle of the day from another group would certainly be relevant to the interpretation of results. EMA methodology can similarly introduce bias, and, because of its complexity, can harbor many potential means of doing so. For example, contextual factors (e.g. location, social environment) may be associated with pain, which makes it important to consider the timing and frequency of EMA sampling128. If sampling times are too sparse, the design may brush over symptom exacerbations or contextual influences may be overlooked, whereas too frequent sampling may be burdensome and may negatively impact data quality. Similarly, reporting decisions, such as whether compliance with momentary pain assessments is reported before or after exclusion of dropouts, can impact the appearance of study results. The importance of the various design and reporting decisions that are implicated in EMA studies necessitates detailed and comprehensive documentation.
This systematic review aims to summarize and describe the use of EMA in chronic pain research while emphasizing the various domains of decision-making involved in EMA methodologies. Since there is large variation among studies, this review is primarily descriptive. The main purpose was to examine characteristics pertaining to study populations and sampling procedures, the rationale for using EMA, data input modalities, pain assessment instrumentation, EMA completion rates, and statistical reporting. We recommend thorough reporting with respect to these domains to improve reproducibility, understanding of comparability across studies, and accuracy of interpretation of study results; we also note opportunities for future research to clarify the importance of design decisions.
Methods
Search strategy
A systematic literature search was conducted through PubMed and Web of Science databases with the following search terms: [("Ecological Momentary Assessment" or "Experience Sampling" or "Electronic Diary" or "Electronic Diaries" or "Electronic Interview" or "Electronic Interviews" or "Interactive Voice Response" or "Intensive Diaries" or "Ambulatory Monitoring" or "Ambulatory Assessment") and "Pain"]. The goal of the search was to include studies that specifically reference EMA methodologies, and we recognize the possibility that articles presenting dynamic data without specifically referring to the methodologies used to obtain the data were unintentionally excluded from the review. The search was conducted in October 2016 and therefore includes only articles published prior to that point; no other restrictions were placed on publication date.
This review focuses on quantitative EMA studies of pain intensity in adult chronic pain patients. As such, any studies that (1) did not present empirical data, (2) did not measure pain intensity with EMA, (3) did not consider a chronic (non-cancer) pain sample, or (4) did not consider a sample of adults, as well as any (5) case or qualitative studies, were excluded from the review. EMA involves the contemporary assessment of variables in participants’ natural environments; therefore, studies asking participants to recall pain experienced across the last day, as is typical in daily diary studies, as well as laboratory studies taking place outside of participants’ natural environments (e.g., studies of procedural pain), were excluded. Both paper and electronic methods of EMA data collection were acceptable for inclusion. Pain items were required to be specific to pain intensity (excluding, e.g., pain quality) and dichotomous pain variables describing the presence or absence of pain were not in and of themselves considered to be pain intensity items. Further, patient populations for whom the pain history, mechanisms, or treatment strategies would take on different forms (e.g., acute pain, cancer pain, children)50, 143 were also excluded. There were no other disease or design restrictions, and methodological, observational, feasibility, and intervention studies were all deemed relevant and therefore included.
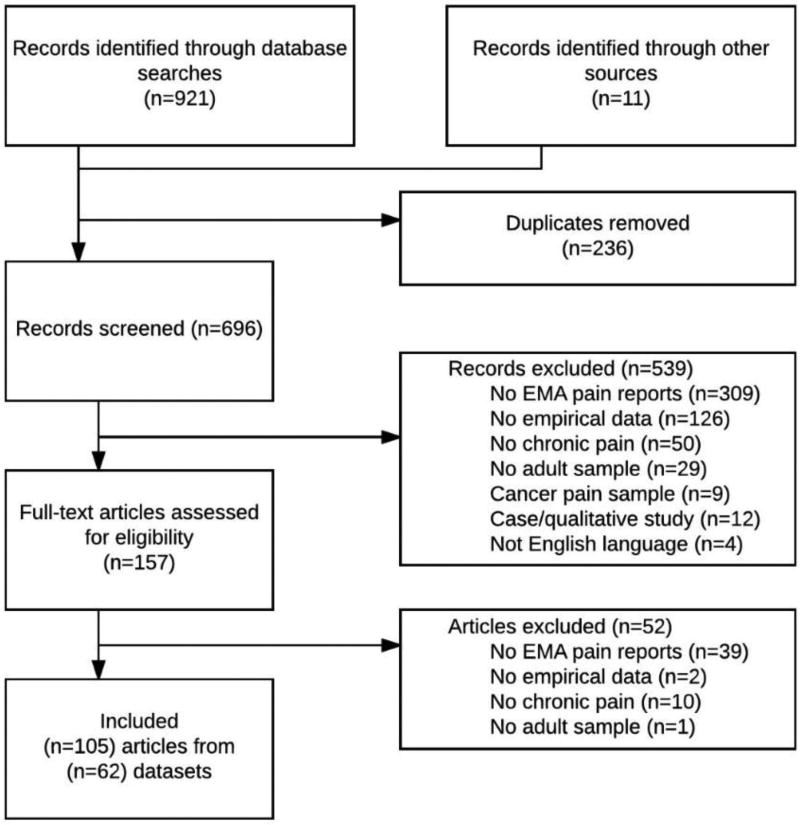
The search identified 685 unique articles, and 11 articles were additionally identified through other sources (e.g., by consulting the reference lists of articles identified by the database search). Articles were considered for inclusion in a two-step process (see Figure 1): first, the abstract of each article was reviewed, and any that met the exclusion criteria were removed from the sample; second, full-text versions of all remaining publications were examined, and articles were again excluded on the basis of the aforementioned exclusion criteria. A total of 105 articles drawing from 62 unique research projects were included in the present review.
Fig. 1.
PRISMA flowchart describing the identification of articles
Extraction of study characteristics
We extracted and tabulated study characteristics from each of the articles (see Table 1). If an article referenced another article for additional protocol details, data were extracted from both articles, even if the second article was not part of the review. Review characteristics were grouped according to the following general themes: sample and design characteristics; rationale for using EMA methodologies; EMA sampling approach; data input modality; pain intensity item; EMA completion rates; statistical reporting. Two of the authors (MM and MO) created a coding manual for the review categories, which were drawn from existing guidelines for the reporting of EMA data74, 111, 131. Each article was coded twice, and discrepancies between the two coders were resolved after a joint review and discussion.
Table 1.
EMA studies of chronic pain
| First Author | Year | Project | Use of EMA |
Design | Chronic Pain n |
Diagnosis | Data input |
Duration | Intensity | % Completion |
Pain Item |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alschuler10 | 2011 | 1 | Btwn | O | 20 | B/NSP | Watch | 5.0 | 4 | NR | NRS; 11-pt; M |
| Alschuler11 | 2011 | ||||||||||
| Bruehl19 | 2012 | 2 | Wthn | O | 48 | B/NSP | Palm | 7.0 | 4 | 99.0 | VAS; 101-pt; M |
| Burns20 | 2015 | 3 | Wthn | O | 105 | B/NSP | Palm | 14.0 | 5 | 83.6 | NRS; 9-pt; C |
| Burns21 | 2013 | ||||||||||
| Dhingra31 | 2014 | 4 | Wthn | O | 36 | CP | Palm | 7.0 | 6 | NR | NRS; 11-pt; M |
| Fischer35 | 2016 | 5 | Wthn | O | 32 | R | S-Phone | 14.0 | 6 | 85.0 | VAS; 101-pt; M |
| Focht36 | 2002 | 6 | Wthn | O | 32 | R | Paper | 6.0 | 5.5 | 91.3 | NRS; 11-pt; M |
| Focht37 | 2004 | ||||||||||
| Garcia-Palacios40 | 2014 | 7 | Met | E | 47 | R | S-Phone | 14.0 | 3 | 69.8 | NRS; 11-pt; M |
| Geisser41 | 1995 | 8 | Wthn | O | 21 | B/NSP | Paper | 3.0 | NR | NR | VAS; 11-pt; M |
| Graham-Engeland52 | 2016 | 9 | Wthn | O | 31 | R | Palm | 7.0 | 5 | NR | NRS; 7-pt; M |
| Russell100 | 2016 | ||||||||||
| Smyth116 | 2014 | ||||||||||
| Hallman53 | 2014 | 10 | Btwn | O | 29 | B/NSP | S-Phone | 3.0 | 8 | NR | NRS; 11-pt; M |
| Hamilton55 | 2008 | 11 | Btwn, Wthn | O | 89 | R | Palm | 30.0 | 3 | 98.9 | NRS; 7-pt; C |
| Tennen136 | 2006 | ||||||||||
| Affleck4 | 2000 | ||||||||||
| Affleck7 | 1996 | ||||||||||
| Affleck5 | 1998 | ||||||||||
| Affleck6 | 2001 | ||||||||||
| Zautra147 (Study 2) | 2001 | ||||||||||
| Hamilton54 | 2007 | 12 | Wthn | O | 49 | R | Paper | 2.0 | 7 | NR | NRS; 7-pt; NR |
| Honkoop59 | 1999 | 13 | Wthn | O | 56 | H/M | Palm | 70.0 | 6 | 80.0 | NR |
| Kinne68 | 1999 | 14 | Btwn | O | 30 | Other | Palm | 1.0 | 5 | 84.0 | VAS; 20-pt; M |
| Linnemann75 | 2015 | 15 | Wthn | O | 30 | R | S-Phone | 14.0 | 5 | 89.7 | VAS; 101-pt; M |
| Liszka-Hackzell76 | 2004 | 16 | Wthn | O | 18 | B/NSP | Palm | 21.0 | 9 | NR | NRS; 11-pt; M |
| Liszka-Hackzell77 | 2005 | ||||||||||
| Lousberg83 | 1997 | 17 | Wthn, Met | O | 57 | CP | Paper | 6.0 | 10 | 88.3 | NRS; 7-pt; NR |
| Vendrig139 | 1997 | ||||||||||
| Mujagic86 | 2015 | 18 | Met | O | 26 | IB | S-Phone | 7.0 | 10 | 76.8 | NRS; 7-pt; NR |
| Glaros43 | 2005 | 19 | Btwn, Wthn | O | 171 | TMD | Paper | 7.0 | NR | NR | NRS; 11-pt; M |
| Glaros47 | 2005 | ||||||||||
| Glaros48 | 2008 | ||||||||||
| Glaros49 | 2005 | ||||||||||
| Glaros44 | 2016 | ||||||||||
| Glaros45 | 2007 | 20 | Intv | E (CT) | 14 | TMD | Paper | 14.0 | NR | NR | NRS; 11-pt; NR |
| Glaros42 | 2014 | 21 | Intv, Btwn | E (CT) | 23 | H/M | Paper | 7.0 | NR | NR | NRS; 11-pt; NR |
| Glaros46 | 2007 | ||||||||||
| Odawara92 | 2015 | 22 | Intv | E (CT) | 27 | H/M | Palm | 56.0 | 5 | 82.4 | VAS; 101-pt; M |
| Okifuji93 | 2011 | 23 | Wthn | O | 81 | R | Palm | 30.0 | 4 | 80.2 | NRS; 7-pt; M |
| Robinson97 | 2012 | 24 | Btwn | O | 30 | CP | Palm | 7.0 | 7 | 49.6 | NRS; 11-pt; M |
| Weinland141 | 2011 | 25 | Btwn | O | 58 | IB | Palm | 14.0 | 7 | 74.0 | OTH; 11-pt; M |
| Murphy87 | 2012 | 26 | Wthn | O | 44 | R | Watch | 14.0 | 5 | NR | NRS; 11-pt; M |
| Viane140 (Study 2) | 2004 | 27 | Btwn, Wthn | O | 62 | CP | Palm | 14.0 | 8 | 88.0 | NRS; 7-pt; M |
| Crombez28 | 2013 | ||||||||||
| Roelofs99 | 2006 | 28 | Btwn, Wthn, Intv | Q-E | 79 | B/NSP | Palm | 14.0 | 8 | 72.7 | NRS; 7-pt; M |
| Roelofs98 | 2004 | ||||||||||
| Huijnen62 | 2009 | ||||||||||
| Huijnen61 | 2011 | ||||||||||
| Smith112 | 2016 | 29 | Wthn | O | 120 | R | Call | 7.0 | 4 | 80.8 | NRS; 5-pt; NR |
| Sorbi118 | 2007 | 30 | Intv | O | 5 | H/M | Palm | 8.5 | 8 | 82.7 | VAS; NR; M |
| Peters94 | 2000 | 31 | Wthn, Met | O | 80 | CP | Palm | 28.0 | 4 | 82.9 | NRS; 7-pt; M |
| Sorbi119 | 2006 | ||||||||||
| Sorbi120 | 2006 | ||||||||||
| Kop69 | 2005 | 32 | Wthn | O | 38 | R | Watch | 5.0 | 5 | NR | NRS; 10-pt; NR |
| Lewis73 | 1995 | 33 | Met, Intv | O | 36 | R | Palm | 70.0 | 4 | NR | NRS; 7-pt; C |
| Lewis72 | 1994 | ||||||||||
| Litt82 | 2009 | 34 | Intv | E (CT) | 54 | TMD | Call | 21.0 | 4 | 71.5 | NRS; 7-pt; M |
| Litt81 | 2004 | 35 | Wthn | O | 30 | TMD | Palm | 7.0 | 4 | 81.0 | NRS; 11-pt; M |
| Murphy88 | 2010 | 36 | Btwn, Wthn | O | 40 | R | Watch | 5.0 | 6 | NR | NRS; 5-pt; NR |
| Murphy89 | 2008 | ||||||||||
| Alsaadi9 | 2014 | 37 | Wthn | O | 80 | B/NSP | Paper | 7.0 | 1 | NR | NRS; 11-pt; M |
| Christian24 | 2015 | 38 | Wthn | O | 90 | CP | S-Phone | 15.0 | 1 | 79.3 | NRS; 6-pt; M |
| Houtveen60 | 2013 | 39 | Wthn | O | 87 | H/M | S-Phone | 42.0 | 4 | 89.5 | NRS; 3-pt; M |
| Kikuchi66 | 2006 | 40 | Wthn, Met | O | 40 | H/M | Watch | 7.0 | 6 | 97.0 | VAS; 101-pt; M |
| Kikuchi67 | 2007 | ||||||||||
| Kikuchi65 | 2015 | ||||||||||
| Aaron1 | 2004 | 41 | Wthn, Met, Intv | E (CT) | 158 | TMD | Palm | 56.0 | 3 | 98.0 | VAS; 11-pt; C |
| Aaron2 | 2005 | ||||||||||
| Aaron3 | 2005 | ||||||||||
| Turner137 | 2004 | ||||||||||
| Wig143 | 2004 | ||||||||||
| Turner138 | 2005 | ||||||||||
| Allen8 | 2009 | 42 | Wthn | O | 157 | R | Palm | 2.0 | 7 | NR | VAS; 101-pt; NR |
| Clauw25 | 2008 | 43 | Intv | E (CT) | 1196 | R | Palm | 28.0 | 5 | NR | VAS; 101-pt; M |
| Harris56 | 2005 | 44 | Intv | E (CT) | 125 | R | Palm | 28.0 | 3.4 | NR | OTH; 124-pt; M |
| Smith114 | 1993 | 45 | Met | E | 30 | CP | Palm | 7.0 | 6.1 | N/A | VAS; 11-pt; NR |
| Stone129 | 2003 | 46 | Wthn, Met | E | 68 | R | Palm | 14.0 | 12 | 94.5 | VAS; 101-pt; M |
| Stone130 | 2004 | ||||||||||
| Litcher-Kelly80 | 2004 | ||||||||||
| Rasell 96 | 2007 | ||||||||||
| Stone131 | 2005 | ||||||||||
| Stone127 | 1997 | 47 | Wthn, Met | O | 35 | R | Paper | 7.0 | 7 | 94.2 | NRS; 7-pt; M |
| Cruise29 | 1996 | ||||||||||
| Broderick18 | 2008 | 48 | Wthn, Met | O | 106 | R | Palm | 30.0 | 7 | 96.0 | VAS; 101-pt; M |
| Broderick17 | 2008 | ||||||||||
| Broderick15 | 2009 | ||||||||||
| Schneider103 | 2011 | ||||||||||
| Stone128 (Study 1) | 2012 | ||||||||||
| Stone126 | 2010 | 49 | Met | O | 128 | R | Palm | 14.0 | 9 | 85.0 | VAS; 101-pt; M |
| Stone134 | 2003 | 50 | Met | E | 80 | CP | Palm | 21.0 | 3 | 52.3 | NR |
| Stone133 | 2002 | ||||||||||
| Litcher-Kelly78 | 2007 | 51 | Met | O | 16 | IB | Palm | 21.0 | 12 | 79.0 | NR |
| Broderick16 | 2003 | 52 | Met | Q-E | 27 | CP | Paper | 24.0 | 3 | 29.1 | NR |
| Williams145 | 2004 | 53 | Met | O | 14 | R | Palm | 84.0 | 6 | 85.0 | NR |
| Wolf146 | 2015 | 54 | Wthn | O | 220 | R | S-Phone | 21.0 | 1 | 83.0 | NRS; 101-pt; C |
| Zia148 | 2016 | 55 | Met | O | 11 | IB | S-Phone | 14.0 | NR | 78.0 | VAS; 101-pt; NR |
| Sterling125 | 2010 | 56 | Wthn | O | 32 | Other | Palm | 2.0 | 4 | 85.0 | VRS; 12-pt; M |
| Lacy71 | 2013 | 57 | Intv | E (CT) | 175 | Other | Palm | 2.0 | 10 | NR | NRS; 11-pt; M |
| Evans34 | 2007 | 58 | Intv | E (CT) | 229 | Other | Palm | NR | 6 | 90.9 | OTH; 13-pt; M |
| Jamison63 | 2006 | 59 | Met | O | 21 | B/NSP | Palm | 371.0 | 1 | NR | VAS; 11-pt; M |
| Eich33 | 1985 | 60 | Met | O | 57 | H/M | Paper | NR | 24 | NR | NRS; 11-pt; M |
| McLean84 | 2005 | 61 | Wthn | O | 28 | R | Call | 2.0 | 5 | 98.1 | NRS; 101-pt; C |
| Sheftell109 | 2005 | 62 | Intv | E (CT) | 2696 | H/M | Palm | NR | 4 | NR | VRS; 4-pt; NR |
Note: NR = Not Reported.
Btwn = Between-person study of pain, Wthn = Within-person study of pain, Met = Methodological study of pain measurement, Intv = Intervention to reduce pain.
O = Observational, E = Experimental, CT = Clinical Trial, Q-E = Quasi-Experimental.
B/NSP = Back/Neck-Shoulder Pain, CP = Chronic Pain (unspecified), R = Rheumatological disorder(s), H/M = Headache/Migraine, TMD = Temporomandibular Disorder, IB = Irritable Bowel Disease/Syndrome.
Palm = Palmtop Computer, S-Phone = Smartphone, Call = Telephone Call.
NRS = Numeric Rating Scale, VAS = Visual Analogue Scale, VRS = Verbal Rating Scale, OTH = Other, M = Momentary, C = Coverage.
A single data collection project sometimes yields multiple research articles, and this complicates the summary of information in systematic reviews. Careful consideration of this fact is essential in order to avoid biasing results towards datasets that yield a high number of publications58, 85. Information from all publications drawing from the same dataset was therefore aggregated into a single project-based entry (see Table 1), although we recognize the possibility that additional publications stemming from these projects were not identified by the present review. The manner of aggregation employed for each variable is described in greater detail below, and cases in which it was deemed advantageous to consider publications belonging to the same project independently from one another (e.g., in consideration of the reporting of completion rates) are specifically noted.
Sample and design characteristics
Data about project sample sizes were drawn from the abstract or methods section of each publication, and were intended to reflect the number of individuals who engaged in the protocol, rather than the number of individuals with whose data the final analyses were conducted. Tabulated sample size values reflect only patient groups, although studies were additionally classified into those that considered only individuals with chronic pain, those that included individuals with chronic pain and a healthy comparison group, and those that included individuals with chronic pain and other specialized comparison groups.
Information about the specific chronic pain conditions included in the sample was taken from the methods section and subdivided into rheumatological diseases, headache or migraine, back or neck-shoulder pain, temporomandibular disorder, irritable bowel disease or syndrome, other specific chronic pain, and general chronic pain. Based on protocol characteristics, the study design was classified as observational, experimental (clinical trial), experimental (non-clinical trial), or quasi-experimental.
In order to consider research projects rather than individual publications, the highest sample size and the greatest number of groups described across relevant publications were taken to represent the project sample. When both experimental and non-experimental publications stemmed from the same project, the project was considered to be experimental in nature.
Rationale for using EMA methodologies
Research articles were described according to the main reason for their use of EMA methodologies. EMA is often a single component of a multifaceted study, and the rationale for the use of EMA is therefore not necessarily synonymous with the study design presented in a particular article. Articles drawing from the same project were coded separately since the purpose of EMA varies by research question. Four categories emerged: (1) within-person studies of pain, (2) between-person studies of pain, (3) methodological studies of pain measurement, and (4) interventions to reduce pain. Although it was possible for an article to pursue more than one research question, articles were reviewed for their primary purpose and assigned to only one of the four themes. Within each theme, articles were further categorized in terms of the content or constructs examined, into subcategories that varied across themes.
EMA sampling approach
The completion of repeated daily assessments places a burden on study participants, and careful consideration of the specific EMA sampling approach is, therefore, essential. EMA protocols can be broadly described as either event-based or time-based, with the former referring to assessments of concrete episodes or behaviors and the latter including assessments that are scheduled randomly or in regularly spaced or fixed intervals111. Random schedules can be administered completely at random or randomized within specific blocks of the day, while fixed schedules occur at pre-defined time points throughout the day. Event-based approaches often rely on the participant to make an entry after the occurrence of a specific event. Each project was coded for (1) the type of sampling schedule, (2) the intensity of assessments (i.e., the number of scheduled or targeted prompts per day), and (3) the total duration of the EMA protocol (i.e., the number of days). Where multiple articles from the same project differed with respect to information regarding sampling approaches, the greatest intensity and longest duration of the EMA protocol as described across publications were taken to represent the corresponding project.
Data input modality
Since the advent of EMA, devices and technologies utilized for data input have changed dramatically. The projects identified by this review were categorized into five groups that roughly trace the evolution of EMA data input modalities: (1) paper booklets, often coupled with signaling devices (e.g., pagers), (2) manually- or digitally-initiated telephone calls, (3) watches or wrist-worn data input devices, (4) palmtop computers, and (5) smartphones. Paper diaries were the earliest modality of EMA data collection; this particular methodology, however, is vulnerable to issues like backfilling, which compromise data quality132. To ensure the accurate recording of response times, technologies with automated time-stamping of assessment entries are now being utilized. Although the fourth and fifth groups of input devices share similar functionalities, smartphones possess greater flexibility than palmtop computers and allow for the integration of EMA with passive, unobtrusive sensor technology13.
Projects were additionally categorized based on the flexibility to engage with the EMA protocol that was afforded by a particular data input technology. Whether or not convenience features like delaying or suspending prompts were available was noted. In this case and with respect to data input devices, the most complete information from any particular article was taken to represent the corresponding project.
Pain intensity item
Although an early adaptation of EMA in pain research traces back to the 1980s33, there are no established rules or standards for the construction of an effective EMA pain item. To summarize the field’s current practices, both item characteristics and response scale characteristics were considered.
Although EMA generally uses very short reporting periods, researchers have been taking different approaches to the characterization of “momentary” pain experiences by varying the definition of the reporting period. These approaches were broadly categorized into two models of EMA item design: (1) a momentary model and (2) a coverage model27. The momentary model intends to capture immediate experience by asking about one’s status “right now” or “right before the prompt”. The coverage model intends to capture a brief yet extended period of one’s experience, ranging from “in the last half hour” to “since the last prompt”; by extending the reporting period, this model covers a fuller range of within-day momentary experiences.
Items utilized in the identified studies also vary with respect to the specification of location(s) of pain sensation. Patients’ pain experiences vary depending on chronic pain diagnosis, with some diagnoses involving pain sensation in one location and others involving pain sensation in multiple locations. Depending on the scope of the study, specifying pain location(s) within an item may be essential to avoid criterion contamination in pain ratings (i.e., consideration of pain that is irrelevant to the diagnosis of concern).
The final item characteristic considered was whether researchers assessed pain intensity with a single rating or took a two-stage approach to isolate pain frequency and pain intensity102. In the latter approach, researchers first ask participants about the presence or absence of their pain, and then, if pain is present, follow up by asking about the intensity of pain. Although “no pain” is often included as a response option in one-stage approach items, Schneider and Stone103 have demonstrated the utility of examining frequency and intensity separately.
Projects were additionally classified according to the type of response scale, the number of response scale points, and the wording of response scale anchors. Response scales encountered in this review included numeric rating scales (NRS), verbal rating scales (VRS), visual analogue scales (VAS), as well as visual ordinal and Gracely51 scales. A standard approach to recall-based pain items commonly administered in clinics is the use of an 11-point scale that ranges from “no pain” to “worst possible pain”32, though whether this practice is common within the EMA pain literature was unclear prior to this review. A related feature with respect to response scales is how each or some of the scale points are verbally anchored. Due to a broad consensus regarding the lowest level of pain (i.e., “no pain”), anchoring descriptors were examined only for the maximum response option. Descriptors often convey both the domain (i.e., pain) and the subjective degree of momentary experiences; this is in line with general recommendations that the sensation to be observed should be defined as part of the response scale 39. Once again, the most complete information from any particular article was taken to represent the corresponding project with respect to the pain intensity item.
EMA completion rates
The conceptualization and presentation of completion rates in research publications varies widely. Completion was defined as the percentage of non-self-initiated, momentary pain intensity item prompts that participants responded to. In some instances, completion rates for pain items could not be disentangled from completion rates for non-pain items, or only completion rates for non-pain items (e.g., fatigue items) were reported. In other instances, completion rates for momentary prompts could not be separated from completion rates for nonmomentary prompts (e.g., end of day diaries). In all of these as well as in related cases, any available data regarding completion rates were taken to reflect the completion rates of non-self-initiated, momentary pain intensity items. If multiple completion rates were presented for different aspects of the protocol (e.g., paper diary completion vs. electronic diary completion), the average of these values was taken.
Publications that reported completion rate data were classified according to five additional, non-mutually-exclusive categories. In line with recommendations from Stone and Shiffman131, the presentation of completion rates, ranges of completion rates, and/or distributions of completion rates (e.g., by time of day) was noted. Whether completion data were presented only for participants who exceeded a set completion rate threshold and whether information about the handling of missing data (e.g., due to suspend functions or technological issues) was presented was also indicated.
Whether and how completion rates were reported, as described above, was considered on the basis of articles. Actual completion rates of EMA protocols were considered at the project level. If completion rates differed across articles belonging to a project, which was common due to variations in the sample considered, the highest reported completion rate was extracted. To calculate an average completion rate across projects, the completion rates from each project were arcsine transformed and weighted by sample size, and the average of the transformed, weighted values was back-transformed into a percentage value.
Statistical reporting
Data obtained from EMA studies are multilevel in nature105. When assessing pain intensity on a momentary basis, variance components can be calculated at both within- and between-person levels. These components can be used to compute an intraclass correlation coefficient (ICC), which describes the proportion of the variance that is due to between-person differences, with the remainder of the proportion due to within-person differences and error. Reporting ICCs or multi-level variance estimates is important when examining within-person relationships, as it provides information about the origin of variance. It is also important when aggregating pain scores to examine between-person relationships, in which case it can be considered as a measure of reliability. For each article, whether the ICC or between- or within-person variance estimates were presented was noted. Because statistical reporting was considered on the basis of articles, data were not aggregated by projects.
Results
Sample and design characteristics
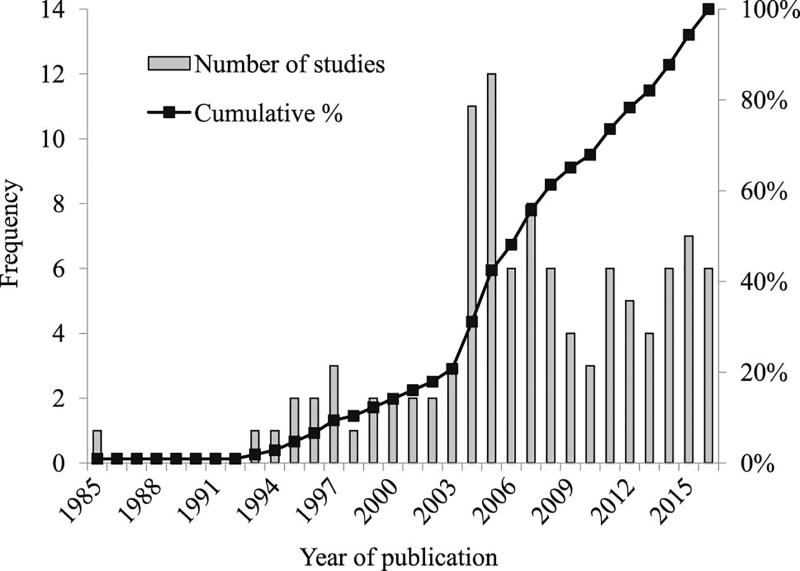
A total of 105 articles met the inclusion criteria for the present review. The articles draw from 62 individual research projects. Of the 62 projects, 42 resulted in a single publication, 11 resulted in two publications, and the remaining 9 resulted in three, four, five, six, or seven publications. Few articles were published before the year 2000, and the rate of publication increased sharply in subsequent years (see Figure 2). Forty-six (74.2%) projects were observational studies, 10 (16.1%) were experimental (clinical trials), 4 (6.5%) were experimental (non-clinical trials), and 2 (3.2%) were quasi-experimental studies.
Fig. 2.
Number of studies (n = 105) by publication year
Fifty-two projects (83.9%) considered only chronic pain patients, 7 projects (11.3%) considered chronic pain patients and a healthy comparison group, and 3 projects (4.8%) considered chronic pain patients and an additional specialized comparison group (spouses, participants with asthma, participants with acute pain). Rheumatological diseases were the most common type of chronic pain studied (n = 23, 37.10%), and they included fibromyalgia (n = 11), osteoarthritis (n = 6), rheumatoid arthritis (n = 2), and mixed rheumatological diseases (n = 4). Back or neck-shoulder pain samples were included in 9 projects (14.5%), headache or migraine samples in 8 projects (12.9%), temporomandibular disorder samples in 5 projects (8.1%), irritable bowel disease or syndrome samples in 4 projects (6.5%), and other specific types of chronic pain in 4 projects [sensory neuropathy (1.6%), whiplash-associated disorder (1.6%), coronary heart disease (1.6%), abdominal pain (1.6%)]. Unspecified chronic pain samples were considered in 9 projects (14.5%).
Patient sample sizes ranged from 5 to 2,696 (mean = 123.5, SD = 365.4, median = 45.5). Two of the projects were pharmaceutical clinical trials with sample sizes over 1000. When these were removed, the average sample size was 62.7 (SD = 51.4, range: 5–229, median = 42.0).
Rationale for using EMA methodologies
Articles were categorized according to the main purpose of their use of EMA methodologies. These categorizations are presented in Table 2.
Table 2.
Use of EMA methodology in chronic pain research
| Totala
|
Subcategoriesb
|
|||
|---|---|---|---|---|
| Purpose | n | % | n | % |
| Within-person studies of pain | 50 | 47.6% | ||
| And emotion | 16 | 15.2% | ||
| And coping | 8 | 7.6% | ||
| And fatigue/bodily symptoms | 7 | 6.7% | ||
| And stress/cortisol | 10 | 9.5% | ||
| And physical activity | 9 | 8.6% | ||
| And sleep | 5 | 4.8% | ||
| And health behavior | 3 | 2.9% | ||
| And pain related functioning | 6 | 5.7% | ||
| Temporal characteristics of pain | 7 | 6.7% | ||
| Between-person studies of pain | 15 | 14.3% | ||
| Comparisons with healthy control groups | 6 | 5.7% | ||
| Relationships with psychosocial and physical functioning | 9 | 8.6% | ||
| Methodological studies of pain measurement | 27 | 25.7% | ||
| Acceptability of EMA / completion rates | 8 | 7.6% | ||
| Reactivity to EMA | 5 | 4.8% | ||
| Reliability of EMA | 2 | 1.9% | ||
| EMA used to examine recall bias | 16 | 15.2% | ||
| Interventions to reduce pain | 13 | 12.4% | ||
| EMA as outcome measure | 11 | 10.5% | ||
| Ecological momentary intervention | 2 | 1.9% | ||
Note:
Assignments of articles to major categories are mutually exclusive and sum to the total number of articles.
Assignments to subcategories are not mutually exclusive.
Within-person studies of pain
Almost half of the articles (n = 50, 47.6%) examined concurrent or sequential (i.e., lagged) within-person relationships between pain intensity and other momentary constructs to gain insight into contexts, predictors, or consequences of momentary changes and fluctuations in pain. A variety of momentary constructs related to pain were examined (see Table 2): emotional states (e.g., anger, depressive mood, positive affect), health behaviors (e.g., medication use, smoking), coping (e.g., state catastrophizing, momentary attention to pain), fatigue, stress, physical activity, sleep (e.g., duration, quality), and pain-related functioning (e.g., activity limitations). Few studies utilized other forms of ambulatory monitoring in addition to EMA, such as physiological (n = 3, 2.8%; cortisol as an indicator of stress) or behavioral/activity-based (n = 6, 5.6%; accelerometry, electromyography) ambulatory assessments. In addition, several studies examined temporal patterns of pain, most notably cyclic changes of pain, throughout the day.
Between-person studies of pain
In about one-seventh (n = 15, 14.3%) of the articles, aggregates of multiple EMA ratings over time were used to examine between-person differences in average pain levels and relationships with other measures of disability. Out of these, the majority of articles examined relationships between patients’ pain and psychosocial (e.g., affective distress, social support) and physical functioning. Other articles reported comparisons of pain intensity and disability levels between specific chronic pain conditions and healthy comparison groups.
Methodological studies of pain measurement
Roughly a quarter (n = 27, 25.7%) of the articles were methodological in nature, and these can be further subdivided into two groups. One group of studies addressed the feasibility and quality of EMA data collection in patients with chronic pain; primary foci of the articles were EMA completion rates (e.g., comparing paper diaries and electronic diaries), reactive arrangements due to repeated momentary sampling, and the reliability of momentary assessments as outcome measures. The second group of studies employed EMA methodology to examine the accuracy of traditional questionnaire assessments and to evaluate biases in recall measures.
Interventions to reduce pain
Intervention studies were relatively infrequent in this review of the literature (n = 13, 12.4%). In the identified intervention studies, EMA was primarily used as an outcome measure after the completion of an intervention. Only 2 studies capitalized on the unique features of EMA (densely repeated measures) to test the effects of short-term momentary intervention strategies (i.e., ecological momentary interventions).
EMA sampling approach
Projects were categorized according to sampling schedule as well as intensity (i.e., number of prompts per day) and duration (i.e., number of days) of assessments. The majority of projects (n = 51, 82.3%) employed a time-based sampling approach consisting of either fixed (n = 23, 37.1%) or random/stratified random (n = 28, 45.2%) assessment schedules. A single project relied exclusively on event-based sampling (n = 1, 1.6%), and the remaining projects utilized a combination approach including both random and event-based assessments (n = 10, 16.0%). Across 58 projects, the median number of EMA assessments per day was 5.0 (mean = 5.9, SD = 3.5, range: 1–24); four projects did not report on the scheduled or targeted intensity of EMA assessments. Across 59 projects, the median duration of EMA sampling was 14.0 days (mean = 23.4 days, SD = 49.4, range: 1- 371), with durations of one week (22.0%) and two weeks (20.3%) being most common; 3 projects did not specify the duration of the EMA protocol.
Data input modality
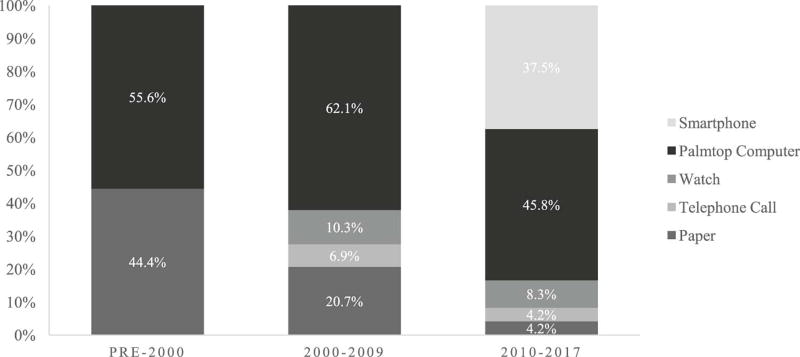
Over half (n = 34, 54.8%) of the projects described in this review used palmtop computers to capture momentary pain. The remaining projects utilized paper booklets (n = 11, 17.7%; either alone or in combination with a rating in a paper booklet), smartphones (n = 9, 14.5%), watches (n = 5, 8.1%), or phone calls (n = 3, 4.8%). Paper booklets and palmtop computers were utilized approximately equally prior to the year 2000 (see Figure 3). Between 2000 and 2009, the proportion of palmtop computers increased while the proportion of paper booklets decreased. The use of smartphones only appears after 2010, following the emergence of mobile-specific operating systems like Android in 2008 and iOS in 2007; it partially replaces the use of palmtop computers for EMA data collection, although there are unique issues that accompany respondents’ use of their own smartphones for EMA protocols. The use of paper booklets has continued to decrease since 2010; paper-based assessments were infrequently utilized in recent studies.
Fig. 3.
Distribution of data input modalities by decade of publication
Fewer than half of the projects reported on device features that enhance participants’ flexibility to engage in momentary assessments (n = 24, 38.7%). These included nap, suspend, or delay features that participants could utilize during times of the day inconvenient for prompting (n = 17, 27.4%) as well as extended completion timeframes potentially accompanied by reminder prompts (n = 7, 11.3%). It is possible that some of the studies not reporting these features did include them, but simply did not mention them in the article.
Pain intensity item
Characteristics of the items used to assess pain intensity are summarized in Table 3. Of the 62 EMA projects, 15 (24.2%) did not report information regarding pain intensity item reporting period. The majority of the projects that did report this information utilized a momentary (n = 41, 66.1%) rather than a coverage (n = 6, 9.7%) approach. Reporting periods for coverage models ranged from 30 to approximately 180 minutes. About 65% (n = 40) of the projects did not specify pain location, whereas 25.8% (n = 16) asked about pain in specific locations (e.g., jaw, head, abdominal). A couple (n = 2, 3.2%) of the projects combined these two approaches by, for instance, averaging multiple location-specific pain ratings to form overall scores29, 126. Lastly, most projects (n = 53, 85.5%) used a single pain intensity item, and only 4 (6.5%) used a two-stage approach, separating questions about the presence and intensity of pain.
Table 3.
Reporting recommendations for EMA studies of pain intensity
| Introduction | Report the following: |
| Rationale |
|
|
| |
| Methods | Report the following: |
| Data input |
|
| Sampling approach |
|
| Pain intensity item |
|
|
| |
| Results | Report the following: |
| Completion rates |
|
| Statistical reporting |
|
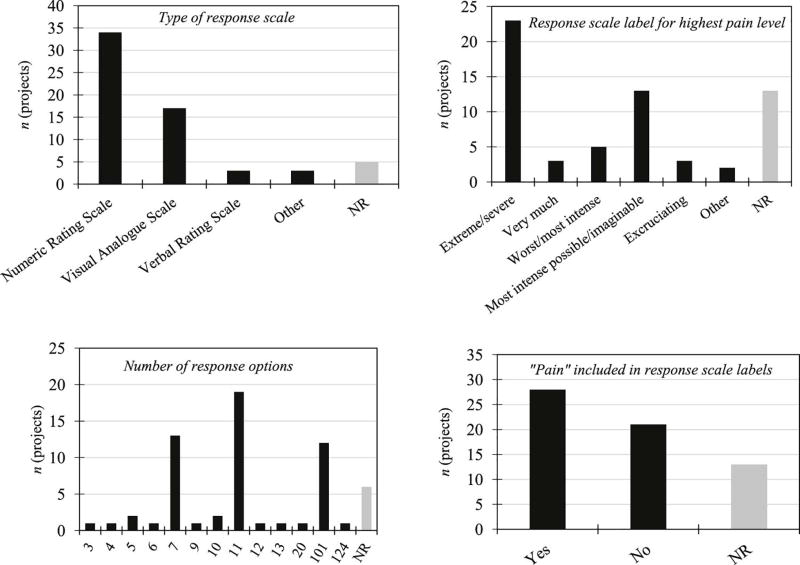
With respect to response scales, more than half (n = 34, 54.8%) of the projects utilized an NRS and approximately one quarter (n = 17, 27.4%) used a VAS. The pain rating scales varied widely in their response option labels and in the descriptors used to anchor the highest pain intensity level (see Table 3). Only about half (n = 28, 45.2%) of the projects utilized response scales that included the word “pain” in the anchoring descriptors. Finally, there was substantial variation in the number of response options, which ranged from 3 to 124 points (see Table 3). Most commonly used were 11-point scales (n = 19, 30.6%), 7-point scales (n = 13, 21.0%), and 101-point scales (n = 12, 19.4%).
EMA completion rates
One of the 105 publications described an entirely event-based (i.e., self-initiated) protocol, for which the reporting of completion rates as defined by the authors (i.e., the percentage of non-self-initiated, momentary pain intensity item prompts that participants responded to) is inapplicable. All values noted in this section therefore reflect a full sample of 104 articles. Out of 104 publications, 33 (31.7%) did not report any completion data. The remaining 71 publications were classified according to five non-mutually-exclusive categories. The majority of articles (n = 66, 63.5%) provided mean completion rates (that is, provided either a mean completion rate value, a range of completion rate values for particular variables or combinations of variables, or information that allowed for the calculation of a mean completion rate). Data on completion distributions, e.g., completion rates by time of day or the number of participants who completed a certain number of prompts, were included in 15 articles (14.4%). Information on types of missing data (describing participants’ use of convenience features like suspend or delay functions, the amount of data missing due to technological issues, or how data missing due to the convenience features or technological issues were accounted for in calculation of the completion rate) was included in 8 publications (7.7%). Completion data were presented only for those participants who exceeded a certain completion-related threshold (e.g., those who completed >50% of prompts for >75% of weeks in the protocol8) in 7 publications (6.7%). The range (i.e., minimum and maximum) of completion rates was included in 5 articles (4.8%).
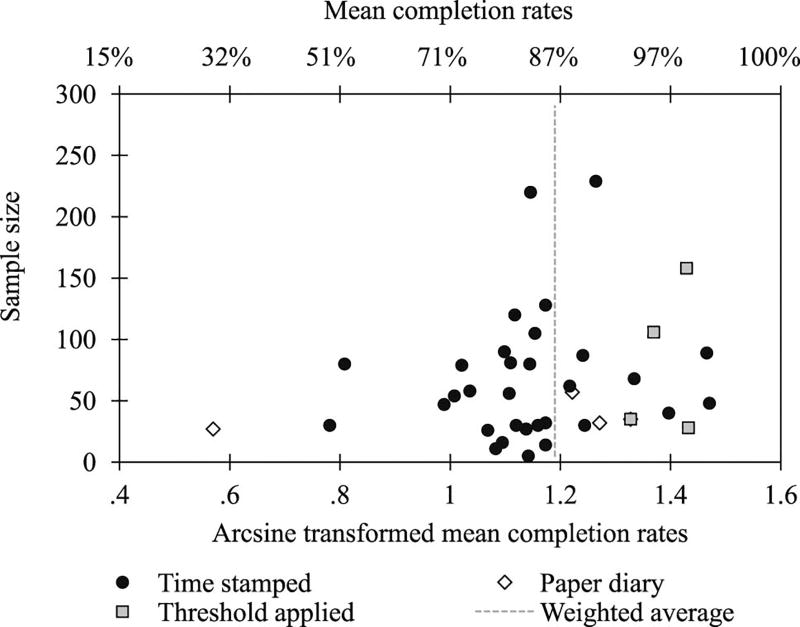
Completion data were available for 39 projects (62.9%), and the distribution of completion rates by sample size is shown in Figure 5. The average completion rate across projects was 86.0%; however, there was substantial variation between studies. Mean completion rates of individual projects ranged from 29.1% to 99.0%, and the lowest completion rate was drawn from a project that used an electronic signaling procedure for paper diary data collection in order to verify self-reported completion rates16. The average of the completion rate values drawn from the other projects utilizing paper diaries was 91.3%. Additionally, as is shown in the figure, completion rates were high when thresholds for minimally acceptable completion were applied to calculate completion rates. Given that the use of paper diaries and the aforementioned thresholds poses a potential risk for biased EMA completion estimates, we examined the observed mean completion rate across projects when these studies were excluded from the analysis. This strategy yielded an average completion rate of 84.1%.
Fig. 5.
Funnel plot of mean EMA completion rates Studies applying a threshold for minimally acceptable completion before calculation of completion rates are shown as squares, paper diaries are shown as diamonds
Statistical reporting
There were 10 articles (9.5%) that reported ICCs or multilevel variance estimates for the pain variable. This may be a conservative estimate of this type of statistical reporting, however, because some articles were not intended to demonstrate relationships between variables (e.g., feasibility studies) or had different outcomes of interest (e.g., fatigue). In these cases, reporting ICCs or multilevel variance estimates for pain intensity may not be essential.
Discussion
The volume of studies identified by the present review suggests that EMA methodologies have been accepted as a sound approach to data collection in chronic pain research. The heterogeneity of this type of research is also evident, and the studies considered here vary widely in various aspects of their design. EMA methodologies are complex, and a multitude of decisions is required for their implementation. We highlight some essential decisions involved in conducting EMA studies and focus on the importance of reporting them (see Table 4 for a summary of recommended reporting practices for EMA study decisions in chronic pain research). Ambiguities regarding the consequences of decision-making that would benefit from further investigation are also noted.
Rationale for using EMA methodologies
EMA methodologies are uniquely suited to examine temporal relationships between pain and affective, cognitive, and behavioral factors. The reviewed studies reflect this notion in that nearly half of the articles examined concurrent or lagged associations between pain and other momentary constructs. While this indicates that EMA is well accepted in observational research, EMA has been very infrequently used to construct outcome measures in chronic pain intervention research, with less than one-eighth of reviewed articles falling into this category. This is surprising in light of the potential of EMA to provide ecologically valid estimates of treatment effects and assay sensitivity in clinical trials. We recommend the use of EMA for outcome measures in clinical trials to measure changes in actual pain experiences that are not confounded by changes in beliefs about pain and symptom recollections. EMA also provides the ability to construct new pain intensity metrics, including pain variability, the proportion of time spent in high or low pain, and the frequency of acute shifts in pain that may uniquely enhance understanding of clinical trial outcomes127. In addition, ecological momentary interventions, which afford real-time treatment in patients’ natural contexts38, 57, 70, offer immense potential for optimizing treatment benefits57. Future research should continue to explore these directions in the assessment and treatment of chronic pain.
EMA sampling approach
The intensive nature of momentary sampling, which offers a fine-grained and nuanced perspective on the assessed constructs, places a burden on study participants. EMA research has often utilized a schedule of three to five assessments per day to minimize burden, and the present review is in line with this; the median number of EMA prompts across projects was 5.0 per day and the median duration of EMA sampling was 14 days. The particular sampling density typically depends on the research question111, 116; while relatively few assessments may suffice when the goal is to examine group differences in average pain levels, many assessments per day are typically required to adequately capture dynamic within-person processes. We expect that future research will continue to balance the duration and intensity of EMA sampling with regard to participant burden and saturation of the constructs under study, but an accepted calculus for achieving this balance does not yet exist.
Data input modality
This review observed a shift in the data input mode of EMA sampling over time: from paper diaries to palmtop computers to smartphones. Notably, completion rates in our sample were over 7% higher in studies that did not use electronic timestamping than in those that did use electronic timestamping and did not employ thresholds for calculating completion. We call for a moratorium of the use of paper diaries given the well-known problems (e.g., back-filling, forward-filling) that can undermine the validity of assessments. Some research protocols utilizing smartphones allow participants the option of bringing their own device into the study, but it is unclear whether this feature is associated with increased or decreased participant engagement. Less than half of the reviewed projects reported on convenience features that allowed for flexibility in the completion of EMA prompts, including nap and delay options. While these features may be advantageous for keeping participants engaged in repeated assessments, they also complicate interpretations of the intended approach to data capture (particularly for random sampling approaches) and the calculations of participant completion of the study protocol78. New technologies involving passive sensors (e.g., GPS) could allay these concerns and allow for the optimization of EMA assessment time points122, 123.
Pain intensity item
Limited attention has been paid to the psychometric properties of EMA pain ratings and the characteristics of EMA pain rating scales. In fact, there was substantial variation between identified studies with respect to the type of rating scale (NRS, VRS, VAS, Other), the number of scale points (ranging from 3 to 124), the labels used to anchor the response scale, the joint assessment of pain frequency and intensity (one- versus two-stage approach), and the length of the reporting period (momentary versus coverage models), with little or no rationale provided for the specific choices made for each study. The need for standardized and normed instruments is increasingly acknowledged in many areas of patient-reported outcomes research12, 23, 32, including in the measurement of pain in clinical trials79, 114, and these issues are similarly important in EMA research. We recommend that EMA pain intensity items clearly indicate the reporting period to the respondent (e.g., current pain, pain before the prompt), specify any pain location of interest, and that the item be clearly worded to ensure that participants exactly understand what is being rated. Additionally, scales with less than five response options should be avoided in that they likely yield poor psychometric properties95.
Even ostensibly minor differences in the decisions regarding EMA items are likely to have non-trivial consequences. For example, varying the wording of pain scale anchors and the number of scale points has been shown to affect response distributions and participants’ interpretations of the rating scale30, 95, 106, 107, 109. In addition, asking participants to rate their pain in the present moment or over the past several hours potentially evokes different mental processes; while momentary ratings presumably involve direct introspection, the use of even short recall periods is likely to capture pain experiences from episodic memory, thereby potentially introducing episodic memory biases such as peak-and-end effects26, 104. However, it could also be argued that self-report covering several hours provides a more reliable depiction of pain states compared to the “spot-checks” involved in momentary pain reports134. In either case, it is important to note that the appropriateness of a particular recall period is also driven by the clinical and theoretical needs of the study in question.
Given that precise and accurate assessment is foundational to EMA methodology, we believe that it is important for future research to identify exactly how EMA items should be designed in order to best reflect the reality of patients’ pain experiences. This includes empirical examination and identification of item characteristics that impact sensitivity to the detection of momentary fluctuations and longer-term changes in pain; convergent and predictive validity of the items with respect to other relevant phenomena should also be established. Further, potential adaptations for older and cognitively impaired patients might be considered22. At present, empirical data on which to base further recommendations for EMA item design and administration are scarce. Lacking these data, a consensus among researchers with respect to standardization would serve as a foundation that would increase comparability across studies
EMA completion rates
The success of EMA protocols hinges on high participant completion of repeated momentary assessments. Because data are usually missing systematically (e.g., response rates might be lower on weekends), low completion rates affect the representativeness of the data and limit the validity and generalizability of study findings118, 141. Recognizing the importance of participant completion in EMA designs, Stone and Shiffman131 emphasized that completion data should be reported in detail. However, in nearly one-third of the studies considered in this review, completion rates were not reported at all. Even when completion data were reported, publications rarely went into detail about the range and distributions of completion rates.
The average completion rate across projects that reported completion data was 86.0%, indicating that participants are willing to take on the burden of frequent assessment necessitated by EMA. Excluding studies with paper diaries and those that implemented a threshold for minimally acceptable completion slightly reduced the completion rate to 84.1%. These values are comparable to the 83% completion rate documented in other reviews of the chronic pain literature85 and in other research areas74, 141. However, it is important to note the possibility that these reviews as well as the present undertaking were impacted by publication bias, and that completion rates may therefore be generally overestimated. Since a large proportion of non-significant findings generally remain in a file drawer100, unpublished, it is well possible that studies with low completion rates also remain unpublished.
In addition to under-reporting and publication bias, thresholds are further consideration likely to influence completion rates. Although thresholds were indicated in only 6.7% of articles considered in this review, it is possible that other studies used thresholds without explicitly stating this in the article. We recommend that researchers wishing to implement thresholds in their studies calculate and present completion rates for the full study sample and those participants that were included in the analyses.
Statistical reporting
Multilevel variance estimates, or ICCs, provide essential information about the reliability of EMA pain ratings as well as about statistical power14. Without considering these values, for example, it is possible to create an elaborate model to explain momentary pain without recognizing that the within-person variance is minimal, and that a larger portion of the variance actually stems from between-person differences. This would compromise the utility of the tested model. Nevertheless, multilevel variance estimates were infrequently presented in the studies identified by this review. The reporting of multilevel variance estimates should be customary in EMA research to document the adequacy of statistical model assumptions and to facilitate power analyses for the design of future studies.
Conclusion
The present endeavor constitutes the first systematic review to describe EMA methodologies in chronic pain research. It is constrained by limitations common to systematic reviews, namely that it is possible that articles relevant to the review were not identified and therefore excluded, and that other relevant studies were never published and therefore also excluded. Despite these limitations, this paper provides a thorough overview of the current state of chronic pain literature with respect to EMA methodologies, illustrating the importance of reporting protocol design decisions.
The field of EMA in chronic pain research is still young and relatively disaggregated, and the choices made by investigators when designing a particular study are not always clear. Thorough descriptions of these decisions and the rationale that underlies them would inform reproducibility, comparability across studies, and the interpretation of study results, and guide future researchers in similar decision-making situations. Consideration of variability in these design decisions naturally begs the question of whether or not the decisions, some of them seemingly trivial, actually come to bear on study results. In and of itself, this provides vast opportunities for future research to determine which design aspects are critical and whether studies that employed different strategies can nevertheless be considered comparable.
Fig. 4.
Characteristics of momentary response scales NR=Not reported
Highlights.
-
◦
105 articles from 62 research projects were identified and included
-
◦
Protocol design features of reviewed studies varied widely and were not consistently reported
-
◦
Careful selection of design features and thorough reporting are essential to EMA
Perspective.
Studies that utilize Ecological Momentary Assessment methodologies to assess pain intensity are heterogeneous. Aspects of protocol design, including data input modality and pain item construction, have the potential to influence the data collected. Thorough reporting on design features and completion rates therefore facilitates reproducibility, comparability, and interpretation of study results.
Acknowledgments
Disclosures
This work was supported in part by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR066200). A.A.S. is a Senior Scientist with the Gallup Organization and a consultant with Adelphi Values, inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaron LA, Mancl L, Turner JA, Sawchuk CN, Klein KM. Reasons for missing interviews in the daily electronic assessment of pain, mood, and stress. Pain. 2004;109:389–398. doi: 10.1016/j.pain.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Aaron LA, Turner JA, Mancl L, Brister H, Sawchuk CN. Electronic diary assessment of pain-related variables: Is reactivity a problem? J Pain. 2005;6:107–115. doi: 10.1016/j.jpain.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Aaron LA, Turner JA, Mancl LA, Sawchuk CN, Huggins KH, Truelove EL. Daily pain coping among patients with chronic temporomandibular disorder pain: An electronic diary study. J Orofac Pain. 2005;20:125–137. [PubMed] [Google Scholar]
- 4.Affleck G, Tennen H, Urrows S, Higgins P, Abeles M. Downward comparisons in daily life with chronic pain: Dynamic relations with pain intensity and mood. J Soc Clin Psychol. 2000;19:499–518. [Google Scholar]
- 5.Affleck G, Tennen H, Urrows S, Higgins P, Abeles M, Hall C, Karoly P, Newton C. Fibromyalgia and women's pursuit of personal goals: A daily process analysis. Health Psychol. 1998;17:40–47. doi: 10.1037//0278-6133.17.1.40. [DOI] [PubMed] [Google Scholar]
- 6.Affleck G, Tennen H, Zautra A, Urrows S, Abeles M, Karoly P. Women's pursuit of personal goals in daily life with fibromyalgia: A value-expectancy analysis. J Consult Clin Psychol. 2001;69:587–596. doi: 10.1037//0022-006x.69.4.587. [DOI] [PubMed] [Google Scholar]
- 7.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 8.Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1275–1282. doi: 10.1016/j.joca.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Alsaadi SM, McAuley JH, Hush JM, Lo S, Bartlett DJ, Grunstein RR, Maher CG. The bidirectional relationship between pain intensity and sleep disturbance/quality in patients with low back pain. Clin J Pain. 2014;30:755–765. doi: 10.1097/AJP.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 10.Alschuler KN, Hoodin F, Murphy SL, Geisser ME. Ambulatory monitoring as a measure of disability in chronic low back pain populations. Clin J Pain. 2011;27:707–715. doi: 10.1097/AJP.0b013e318217b7d0. [DOI] [PubMed] [Google Scholar]
- 11.Alschuler KN, Hoodin F, Murphy SL, Rice J, Geisser ME. Factors contributing to physical activity in a chronic low back pain clinical sample: A comprehensive analysis using continuous ambulatory monitoring. Pain. 2011;152:2521–2527. doi: 10.1016/j.pain.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Amtmann D, Cook KF, Jensen MP, Chen W-H, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asselbergs J, Ruwaard J, Ejdys M, Schrader N, Sijbrandij M, Riper H. Mobile phone-based unobtrusive ecological momentary assessment of day-to-day mood: An explorative study. J Med Internet Res. 2016;18:e72. doi: 10.2196/jmir.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolger N, Laurenceau J-P. Intensive longitudinal methods: An introduction to diary and experience sampling research. New York: The Guilford Press; 2013. [Google Scholar]
- 15.Broderick JE, Schwartz JE, Schneider S, Stone AA. Can end-of-day reports replace momentary assessment of pain and fatigue? J Pain. 2009;10:274–281. doi: 10.1016/j.jpain.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick JE, Schwartz JE, Shiffman S, Hufford MR, Stone AA. Signaling does not adequately improve diary compliance. Ann Behav Med. 2003;26:139–148. doi: 10.1207/S15324796ABM2602_06. [DOI] [PubMed] [Google Scholar]
- 17.Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139:146–157. doi: 10.1016/j.pain.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broderick JE, Vikingstad G. Frequent assessment of negative symptoms does not induce depressed mood. J Clin Psychol Med Settings. 2008;15:296–300. doi: 10.1007/s10880-008-9127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruehl S, Liu X, Burns JW, Chont M, Jamison RN. Associations between daily chronic pain intensity, daily anger expression, and trait anger expressiveness: An ecological momentary assessment study. Pain. 2012;153:2352–2358. doi: 10.1016/j.pain.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns JW, Gerhart JI, Bruehl S, Peterson KM, Smith DA, Porter LS, Schuster E, Kinner E, Buvanendran A, Fras AM, Keefe FJ. Anger arousal and behavioral anger regulation in everyday life among patients with chronic low back pain: Relationships to patient pain and function. Health Psychol. 2015;34:547–555. doi: 10.1037/hea0000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns JW, Peterson KM, Smith DA, Keefe FJ, Porter LS, Schuster E, Kinner E. Temporal associations between spouse criticism/hostility and pain among patients with chronic pain: A within-couple daily diary study. Pain. 2013;154:2715–2721. doi: 10.1016/j.pain.2013.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cain AE, Depp CA, Jeste DV. Ecological momentary assessment in aging research: A critical review. J Psychiatr Res. 2009;43:987–996. doi: 10.1016/j.jpsychires.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christian MS, Eisenkraft N, Kapadia C. Dynamic associations among somatic complaints, human energy, and discretionary behaviors: Experiences with pain fluctuations at work. Adm Sci Q. 2015;60:66–102. [Google Scholar]
- 25.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: A 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30:1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Conner TS, Barrett LF. Trends in ambulatory self-report: The role of momentary experience in psychosomatic medicine. Psychosom Med. 2012;74:327–337. doi: 10.1097/PSY.0b013e3182546f18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Council NR. In: Subjective Well-Being: Measuring Happiness, Suffering, and Other Dimensions of Experience. Stone AA, Mackie C, editors. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 28.Crombez G, Viane I, Eccleston C, Devulder J, Goubert L. Attention to pain and fear of pain in patients with chronic pain. J Behav Med. 2013;36:371–378. doi: 10.1007/s10865-012-9433-1. [DOI] [PubMed] [Google Scholar]
- 29.Cruise CE, Broderick J, Porter L, Kaell A, Stone AA. Reactive effects of diary self-assessment in chronic pain patients. Pain. 1996;67:253–258. doi: 10.1016/0304-3959(96)03125-9. [DOI] [PubMed] [Google Scholar]
- 30.Dannecker EA, George SZ, Robinson ME. Influence and stability of pain scale anchors for an investigation of cold pressor pain tolerance. J Pain. 2007;8:476–482. doi: 10.1016/j.jpain.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhingra LK, Homel P, Grossman B, Chen J, Scharaga E, Calamita S, Shin J, Portenoy R. Ecological momentary assessment of smoking behavior in persistent pain patients. Clin J Pain. 2014;30:205–213. doi: 10.1097/AJP.0b013e31829821c7. [DOI] [PubMed] [Google Scholar]
- 32.Dworkin RH, Turk DC, Farrar JT, Haythornwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Eich E, Reeves JL, Jaeger B, Graff-Radford SB. Memory for pain: Relation between past and present pain intensity. Pain. 1985;23:375–379. doi: 10.1016/0304-3959(85)90007-7. [DOI] [PubMed] [Google Scholar]
- 34.Evans SR, Simpson DM, Kitch DW, King A, Clifford DB, Cohen BA, McArthur JC, Neurologic ARC, Grp ACT A randomized trial evaluating Prosaptide (TM) for HIV-associated sensory neuropathies: Use of an electronic diary to record neuropathic pain. PLoS Clin Trials. 2007;2:9. doi: 10.1371/journal.pone.0000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer S, Doerr JM, Strahler J, Mewes R, Thieme K, Nater UM. Stress exacerbates pain in the everyday lives of women with fibromyalgia syndrome-The role of cortisol and alpha-amylase. Psychoneuroendocrinology. 2016;63:68–77. doi: 10.1016/j.psyneuen.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Focht BC, Ewing V, Gauvin L, Rejeski WJ. The unique and transient impact of acute exercise on pain perception in older, overweight, or obese adults with knee osteoarthritis. Ann Behav Med. 2002;24:201–210. doi: 10.1207/S15324796ABM2403_05. [DOI] [PubMed] [Google Scholar]
- 37.Focht BC, Gauvin L, Rejeski WJ. The contribution of daily experiences and acute exercise to fluctuations in daily feeling states among older, obese adults with knee osteoarthritis. J Behav Med. 2004;27:101–121. doi: 10.1023/b:jobm.0000019847.80315.4d. [DOI] [PubMed] [Google Scholar]
- 38.Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freyd M. The graphic rating scale. Journal of Educational Psychology. 1923;43:83–102. [Google Scholar]
- 40.Garcia-Palacios A, Herrero R, Belmonte MA, Castilla D, Guixeres J, Molinari G, Banos RM. Ecological momentary assessment for chronic pain in fibromyalgia using a smartphone: a randomized crossover study. Eur J Pain. 2014;18:862–872. doi: 10.1002/j.1532-2149.2013.00425.x. [DOI] [PubMed] [Google Scholar]
- 41.Geisser ME, Robinson ME, Richardson C. A time series analysis of the relationship between ambulatory EMG, pain, and stress in chronic low back pain. Biofeedback Self Regul. 1995;20:339–355. doi: 10.1007/BF01543789. [DOI] [PubMed] [Google Scholar]
- 42.Glaros AG, Hanson AH, Ryen CC. Headache and oral parafunctional behaviors. App Psychophysiol Biofeedback. 2014;39:59–66. doi: 10.1007/s10484-014-9242-0. [DOI] [PubMed] [Google Scholar]
- 43.Glaros AG, Lumley MA. Alexithymia and pain in temporomandibular disorder. J Psychosom Res. 2005;59:85–88. doi: 10.1016/j.jpsychores.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Glaros AG, Marszalek JM, Williams KB. Longitudinal multilevel modeling of facial pain, muscle tension, and stress. J Dent Res. 2016;95:416–422. doi: 10.1177/0022034515625216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glaros AG, Owais Z, Lausten L. Reduction in parafunctional activity: A potential mechanism for the effectiveness of splint therapy. J Oral Rehabil. 2007;34:97–104. doi: 10.1111/j.1365-2842.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- 46.Glaros AG, Urban D, Locke J. Headache and temporomandibular disorders: Evidence for diagnostic and behavioural overlap. Cephalalgia. 2007;27:542–549. doi: 10.1111/j.1468-2982.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 47.Glaros AG, Williams K, Lausten L. The role of parafunctions, emotions and stress in predicting facial pain. J Am Dent Assoc. 2005;136:451–458. doi: 10.14219/jada.archive.2005.0200. [DOI] [PubMed] [Google Scholar]
- 48.Glaros AG, Williams K, Lausten L. Diurnal variation in pain reports in temporomandibular disorder patients and control subjects. J Orofac Pain. 2008;22:115–121. [PubMed] [Google Scholar]
- 49.Glaros AG, Williams K, Lausten L, Friesen LR. Tooth contact in patients with temporomandibular disorders. Cranio. 2005;23:188–193. doi: 10.1179/crn.2005.027. [DOI] [PubMed] [Google Scholar]
- 50.Gordon DB, Dahl JL, Miaskowski C, McCarberg B, Todd KH, Paice JA, Lipman AG, Bookbinder M, Sanders SH, Turk DC, Carr DB. American Pain Society recommendations for improving the quality of acute and cancer pain management: American Pain Society Quality of Care Task Force. Arch Intern Med. 2005;165:1574–1580. doi: 10.1001/archinte.165.14.1574. [DOI] [PubMed] [Google Scholar]
- 51.Gracely RH, Kwilosz DM. The Descriptor Differential Scale: Applying psychophysical principles to clinical pain assessment. Pain. 1988;35:279–288. doi: 10.1016/0304-3959(88)90138-8. [DOI] [PubMed] [Google Scholar]
- 52.Graham-Engeland JE, Zawadzki MJ, Slavish DC, Smyth JM. Depressive symptoms and momentary mood predict momentary pain among rheumatoid arthritis patients. Ann Behav Med. 2016;50:12–23. doi: 10.1007/s12160-015-9723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallman DM, Ekman AH, Lyskov E. Changes in physical activity and heart rate variability in chronic neck-shoulder pain: monitoring during work and leisure time. Int Arch Occup Environ Health. 2014;87:735–744. doi: 10.1007/s00420-013-0917-2. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton NA, Catley D, Karlson C. Sleep and the affective response to stress and pain. Health Psychol. 2007;26:288–295. doi: 10.1037/0278-6133.26.3.288. [DOI] [PubMed] [Google Scholar]
- 55.Hamilton NA, Karlson C, Luxton D, Preacher KJ, Templin JL, Affleck G, Tennen H. Fibromyalgia: The role of sleep in affect and in negative event reactivity and recovery. Health Psychol. 2008;27:490–497. doi: 10.1037/0278-6133.27.4.490. [DOI] [PubMed] [Google Scholar]
- 56.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum. 2005;52:3670–3674. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- 57.Heron KE, Smyth JM. Ecological momentary interventions: Incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15:1–39. doi: 10.1348/135910709X466063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.0. 2008. [Google Scholar]
- 59.Honkoop PC, Sorbi MJ, Godaert GL, Spierings EL. High-density assessment of the IHS classification criteria for migraine without aura: A prospective study. Cephalalgia. 1999;19:201–206. doi: 10.1046/j.1468-2982.1999.019004201.x. [DOI] [PubMed] [Google Scholar]
- 60.Houtveen JH, Sorbi MJ. Prodromal functioning of migraine patients relative to their interictal state: An Ecological Momentary Assessment study. PloS One. 2013;8 doi: 10.1371/journal.pone.0072827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huijnen IP, Verbunt JA, Peters ML, Smeets RJ, Kindermans HP, Roelofs J, Goossens M, Seelen HA. Differences in activity-related behaviour among patients with chronic low back pain. Eur J Pain. 2011;15:748–755. doi: 10.1016/j.ejpain.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Huijnen IP, Verbunt JA, Roelofs J, Goossens M, Peters M. The disabling role of fluctuations in physical activity in patients with chronic low back pain. Eur J Pain. 2009;13:1076–1079. doi: 10.1016/j.ejpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Jamison RN, Raymond SA, Slawsby EA, McHugo GJ, Baird JC. Pain assessment in patients with low back pain: comparison of weekly recall and momentary electronic data. J Pain. 2006;7:192–199. doi: 10.1016/j.jpain.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Keefe FJ. Development of an observation method for assessing pain behavior in chronic low back patients. Behav Ther. 1982;13:363–375. [Google Scholar]
- 65.Kikuchi H, Yoshiuchi K, Ando T, Yamamoto Y. Influence of psychological factors on acute exacerbation of tension-type headache: Investigation by ecological momentary assessment. J Psychosom Res. 2015;79:239–242. doi: 10.1016/j.jpsychores.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Kikuchi H, Yoshiuchi K, Miyasaka N, Ohashi K, Yamamoto Y, Kumano H, Kuboki T, Akabayashi A. Reliability of recalled self-report on headache intensity: investigation using ecological momentary assessment technique. Cephalalgia. 2006;26:1335–1343. doi: 10.1111/j.1468-2982.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 67.Kikuchi H, Yoshiuchi K, Ohashi K, Yamamoto Y, Akabayashi A. Tension-type headache and physical activity: An actigraphic study. Cephalalgia. 2007;27:1236–1243. doi: 10.1111/j.1468-2982.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 68.Kinne G, Droste C, Fahrenberg J, Roskamm H. Symptomatic myocardial ischemia and everyday life: Implications for clinical use of interactive monitoring. J Psychosom Res. 1999;46:369–377. doi: 10.1016/s0022-3999(98)00096-8. [DOI] [PubMed] [Google Scholar]
- 69.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 70.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: A systematic review. Telemed J E Health. 2009;15:231–240. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 71.Lacy BE, Wang F, Bhowal S, Schaefer E. On-demand hyoscine butylbromide for the treatment of self-reported functional cramping abdominal pain. Scand J Gastroenterol. 2013;48:926–935. doi: 10.3109/00365521.2013.804117. [DOI] [PubMed] [Google Scholar]
- 72.Lewis B, Lewis D, Cumming G. The comparative analgesic efficacy of transcutaneous electrical nerve stimulation and a non-steroidal anti-inflammatory drug for painful osteoarthritis. Br J Rheumatol. 1994;33:455–460. doi: 10.1093/rheumatology/33.5.455. [DOI] [PubMed] [Google Scholar]
- 73.Lewis B, Lewis D, Cumming G. Frequent measurement of chronic pain: An electronic diary and empirical findings. Pain. 1995;60:341–347. doi: 10.1016/0304-3959(94)00143-3. [DOI] [PubMed] [Google Scholar]
- 74.Liao Y, Skelton K, Dunton G, Bruening M. A systematic review of methods and procedures used in ecological momentary assessments of diet and physical activity research in youth: An adapted STROBE checklist for reporting EMA studies (CREMAS) J Med Internet Res. 2016;18:e151. doi: 10.2196/jmir.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linnemann A, Kappert MB, Fischer S, Doerr JM, Strahler J, Nater UM. The effects of music listening on pain and stress in the daily life of patients with fibromyalgia syndrome. Front Hum Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liszka-Hackzell JJ, Martin DP. An analysis of the relationship between activity and pain in chronic and acute low back pain. Anesth Analg. 2004;99:477–481. doi: 10.1213/01.ANE.0000132696.15310.DD. [DOI] [PubMed] [Google Scholar]
- 77.Liszka-Hackzell JJ, Martin DP. Analysis of nighttime activity and daytime pain in patients with chronic back pain using a self-organizing map neural network. J Clin Monit Comput. 2005;19:411–414. doi: 10.1007/s10877-005-0392-8. [DOI] [PubMed] [Google Scholar]
- 78.Litcher-Kelly L, Kellerman Q, Hanauer SB, Stone AA. Feasibility and utility of an electronic diary to assess self-report symptoms in patients with inflammatory bowel disease. Ann Behav Med. 2007;33:207–212. doi: 10.1007/BF02879902. [DOI] [PubMed] [Google Scholar]
- 79.Litcher-Kelly L, Martino SA, Broderick JE, Stone AA. A systematic review of measures used to assess chronic musculoskeletal pain in clinical and randomized controlled clinical trials. J Pain. 2007;8:906–913. doi: 10.1016/j.jpain.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Litcher-Kelly L, Stone AA, Broderick JE, Schwartz JE. Associations among pain intensity, sensory characteristics, affective qualities, and activity limitations in patients with chronic pain: A momentary, within-person perspective. J Pain. 2004;5:433–439. doi: 10.1016/j.jpain.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Litt MD, Shafer D, Napolitano C. Momentary mood and coping processes in TMD pain. Health Psychol. 2004;23:354–362. doi: 10.1037/0278-6133.23.4.354. [DOI] [PubMed] [Google Scholar]
- 82.Litt MD, Shafer DM, Ibanez CR, Kreutzer DL, Tawfik-Yonkers Z. Momentary pain and coping in temporomandibular disorder pain: Exploring mechanisms of cognitive behavioral treatment for chronic pain. Pain. 2009;145:160–168. doi: 10.1016/j.pain.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lousberg R, Schmidt AJ, Groenman NH, Vendrig L, Dijkman-Caes CI. Validating the MPI-DLV using experience sampling data. J Behav Med. 1997;20:195–206. doi: 10.1023/a:1025534812341. [DOI] [PubMed] [Google Scholar]
- 84.McLean SA, Williams DA, Harris RE, Kop WJ, Groner KH, Ambrose K, Lyden AK, Gracely RH, Crofford LJ, Geisser ME, Sen A, Biswas P, Clauw DJ. Momentary relationship between cortisol secretion and symptoms in patients with fibromyalgia. Arthritis Rheum. 2005;52:3550–3669. doi: 10.1002/art.21372. [DOI] [PubMed] [Google Scholar]
- 85.Morren M, van Dulmen S, Ouwerkerk J, Bensing J. Compliance with momentary pain measurement using electronic diaries: A systematic review. Eur J Pain. 2009;13:354–365. doi: 10.1016/j.ejpain.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 86.Mujagic Z, Leue C, Vork L, Lousberg R, Jonkers D, Keszthelyi D, Hesselink MA, van Schagen TJC, Van Os J, Masclee AAM, Kruimel JW. The Experience Sampling Method: A new digital tool for momentary symptom assessment in IBS: an exploratory study. Neurogastroenterol Motil. 2015;27:1295–1302. doi: 10.1111/nmo.12624. [DOI] [PubMed] [Google Scholar]
- 87.Murphy S, Buckle P, Stubbs D. The use of the portable ergonomic observation method (PEO) to monitor the sitting posture of schoolchildren in the classroom. Appl Ergon. 2002;33:365–370. doi: 10.1016/s0003-6870(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 88.Murphy SL, Smith DM. Ecological Measurement of Fatigue and Fatigability in Older Adults With Osteoarthritis. J Gerontol A Biol Sci Med Sci. 2010;65:184–189. doi: 10.1093/gerona/glp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy SL, Smith DM, Clauw DJ, Alexander NB. The impact of momentary pain and fatigue on physical activity in women with osteoarthritis. Arthritis Rheum. 2008;59:849–856. doi: 10.1002/art.23710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16:769–780. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, Murphy SA. Just-in-Time Adaptive Interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Ann Behav Med. 2016 doi: 10.1007/s12160-016-9830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Odawara M, Hashizume M, Yoshiuchi K, Tsuboi K. Real-time assessment of the effect of biofeedback therapy with migraine: A pilot study. Int J Behav Med. 2015;22:748–754. doi: 10.1007/s12529-015-9469-z. [DOI] [PubMed] [Google Scholar]
- 93.Okifuji A, Bradshaw DH, Donaldson GW, Turkt DC. Sequential analyses of daily symptoms in women with fibromyalgia syndrome. J Pain. 2011;12:84–93. doi: 10.1016/j.jpain.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peters ML, Sorbi MJ, Kruise DA, Kerssens JJ, Verhaak PF, Bensing JM. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain. 2000;84:181–192. doi: 10.1016/s0304-3959(99)00206-7. [DOI] [PubMed] [Google Scholar]
- 95.Preston CC, Colman AM. Optimal number of response categories in rating scales: reliability, validity, discriminating power, and respondent preferences. Acta Psychol. 2000;104:1–15. doi: 10.1016/s0001-6918(99)00050-5. [DOI] [PubMed] [Google Scholar]
- 96.Raselli C, Broderick JE. The association of depression and neuroticism with pain reports: A comparison of momentary and recalled pain assessment. J Psychosom Res. 2007;62:313–320. doi: 10.1016/j.jpsychores.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 97.Robinson K, Kennedy N, Harmon D. The flow experiences of people with chronic pain. OTJR (Thorofare N J) 2012;32:104–112. [Google Scholar]
- 98.Roelofs J, Peters ML, Patijn J, Schouten EG, Vlaeyen JW. Electronic diary assessment of pain-related fear, attention to pain, and pain intensity in chronic low back pain patients. Pain. 2004;112:335–342. doi: 10.1016/j.pain.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 99.Roelofs J, Peters ML, Patijn J, Schouten EG, Vlaeyen JW. An electronic diary assessment of the effects of distraction and attentional focusing on pain intensity in chronic low back pain patients. Br J Health Psychol. 2006;11:595–606. doi: 10.1348/135910705X74819. [DOI] [PubMed] [Google Scholar]
- 100.Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 101.Russell MA, Smith TW, Smyth JM. Anger expression, momentary anger, and symptom severity in patients with chronic disease. Ann Behav Med. 2016;50:259–271. doi: 10.1007/s12160-015-9747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schimmack U, Diener E. Affect intensity: Separating intensity and frequency in repeatedly measured affect. J Pers Soc Psychol. 1997;73:1313–1329. [Google Scholar]
- 103.Schneider S, Stone AA. Distinguishing between frequency and intensity of health-related symptoms from diary assessments. J Psychosom Res. 2014;77:205–212. doi: 10.1016/j.jpsychores.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schneider S, Stone AA, Schwartz JE, Broderick JE. Peak and end effects in patients' daily recall of pain and fatigue: A within-subjects analysis. J Pain. 2011;12:228–235. doi: 10.1016/j.jpain.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment data. Health Psychol. 1998;17:6–16. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- 106.Schwarz N, Hippler H-J, Deutsch B, Strack F. Response scales: Effects of category range on reported behavior and comparative judgments. Public Opin Q. 1985;49:388–395. [Google Scholar]
- 107.Schwarz N, Knäuper B, Hippler H-J, Noelle-Neumann E, Clark L. Rating scales numeric values may change the meaning of scale labels. Public Opin Q. 1991;55:570–582. [Google Scholar]
- 108.Services USDoHaH, Administration FaD, Research CfDEa, Research CfBEa, Health CfDaR. Guidance for industry: Patient-Reported Outcome measures: Use in medical product development to support labeling claims, Food and Drug Administration. 2009. [Google Scholar]
- 109.Seymour RA, Simpson JM, Charlton JE, Phillips ME. An evaluation of length and end-phrase of visual analogue scales in dental pain. Pain. 1985;21:177–185. doi: 10.1016/0304-3959(85)90287-8. [DOI] [PubMed] [Google Scholar]
- 110.Sheftell FF, Dahlöf CGH, Brandes JL, Agosti R, Jones MW, Barrett PS. Two replicate randomized, double-blind, placebo-controlled trials of the time to onset of pain relief in the acute treatment of migraine with a fast-disintegrating/rapid-release formulation of Sumatripan tablets. Clin Ther. 2005;27:407–417. doi: 10.1016/j.clinthera.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 111.Shiffman S. Designing protocols for Ecological Momentary Assessment. In: Stone AA, Shiffman S, Atienza A, Nebeling L, editors. The Science of Real-Time Data Capture: Self-Reports in Health Research. New York: Oxford University Press; 2007. pp. 27–53. [Google Scholar]
- 112.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 113.Smith DM, Parmelee PA. Within-day variability of fatigue and pain among African Americans and non-Hispanic Whites with osteoarthritis of the knee. Arthritis Care Res. 2016;68:115–122. doi: 10.1002/acr.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith SM, Hunsinger M, McKeown A, Parkhurst M, Allen R, Kopko S, Lu Y, Wilson HD, Burke LB, Desjardins P. Quality of pain intensity assessment reporting: ACTTION systematic review and recommendations. J Pain. 2015;16:299–305. doi: 10.1016/j.jpain.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 115.Smith WB, Safer MA. Effects of present pain level on recall of chronic pain and medication use. Pain. 1993;55:355–361. doi: 10.1016/0304-3959(93)90011-D. [DOI] [PubMed] [Google Scholar]
- 116.Smyth JM, Stone AA. Ecological Momentary Assessment research in behavioral medicine. J Happiness Stud. 2003;4:35–52. [Google Scholar]
- 117.Smyth JM, Zawadzki MJ, Santuzzi AM, Filipkowski KB. Examining the effects of perceived social support on momentary mood and symptom reports in asthma and arthritis patients. Psychol Health. 2014;29:813–831. doi: 10.1080/08870446.2014.889139. [DOI] [PubMed] [Google Scholar]
- 118.Sokolovsky AW, Mermelstein RJ, Hedeker D. Factors predicting compliance to Ecological Momentary Assessment among adolescent smokers. Nicotine Tob Res. 2014;16:351–358. doi: 10.1093/ntr/ntt154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sorbi MJ, Mak SB, Houtveen H, Kleiboer AM, van Doornen LJP. Mobile web-based monitoring and coaching: Feasibility in chronic migraine. J Med Internet Res. 2007;9 doi: 10.2196/jmir.9.5.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sorbi MJ, Peters ML, Kruise DA, Maas CJ, Kerssens JJ, Verhaak PF, Bensing JM. Electronic momentary assessment in chronic pain I: Psychological pain responses as predictors of pain intensity. Clin J Pain. 2006;22:55–66. doi: 10.1097/01.ajp.0000148624.46756.fa. [DOI] [PubMed] [Google Scholar]
- 121.Sorbi MJ, Peters ML, Kruise DA, Maas CJ, Kerssens JJ, Verhaak PF, Bensing JM. Electronic momentary assessment in chronic pain II: Pain and psychological pain responses as predictors of pain disability. Clin J Pain. 2006;22:67–81. doi: 10.1097/01.ajp.0000148625.84874.48. [DOI] [PubMed] [Google Scholar]
- 122.Steinhubl SR, Muse ED, Topol EJ. Can mobile health technologies transform health care? J Am Med Assoc. 2013;310:2395–2396. doi: 10.1001/jama.2013.281078. [DOI] [PubMed] [Google Scholar]
- 123.Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci Transl Med. 2015;7:283rv283–283rv283. doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sterling M, Chadwick BJ. Psychologic processes in daily life with chronic whiplash: relations of posttraumatic stress symptoms and fear-of-pain to hourly pain and uptime. Clin J Pain. 2010;26:573–582. doi: 10.1097/AJP.0b013e3181e5c25e. [DOI] [PubMed] [Google Scholar]
- 125.Stone AA, Broderick JE, Kaell AT. Single momentary assessments are not reliable outcomes for clinical trials. Contemp Clin Trials. 2010;31:466–472. doi: 10.1016/j.cct.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stone AA, Broderick JE, Porter LS, Kaell AT. The experience of rheumatoid arthritis pain and fatigue: Examining momentary reports and correlates over one week. Arthritis Care Res. 1997;10:185–193. doi: 10.1002/art.1790100306. [DOI] [PubMed] [Google Scholar]
- 127.Stone AA, Broderick JE, Schneider S, Schwartz JE. Expanding options for developing outcome measures from momentary assessment data. Psychosom Med. 2012;74:387–397. doi: 10.1097/PSY.0b013e3182571faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher-Kelly L, Calvanese P. Intensive momentary reporting of pain with an electronic diary: Reactivity, compliance, and patient satisfaction. Pain. 2003;104:343–351. doi: 10.1016/s0304-3959(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 129.Stone AA, Broderick JE, Shiffman SS, Schwartz JE. Understanding recall of weekly pain from a momentary assessment perspective: Absolute agreement, between- and within-person consistency, and judged change in weekly pain. Pain. 2004;107:61–69. doi: 10.1016/j.pain.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 130.Stone AA, Schwartz JE, Broderick JE, Shiffman S. Variability of momentary pain predicts recall of weekly pain: A consequence of the peak (or salience) memory heuristic. Pers Soc Psychol Bull. 2005;31:1340–1346. doi: 10.1177/0146167205275615. [DOI] [PubMed] [Google Scholar]
- 131.Stone AA, Shiffman S. Capturing momentary, self-report data: A proposal for reporting guidelines. Ann Behav Med. 2002;24:236–243. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- 132.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. Br Med J. 2002;324:1193–1194. doi: 10.1136/bmj.324.7347.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 134.Stull DE, Leidy NK, Parasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin. 2009;25:929–942. doi: 10.1185/03007990902774765. [DOI] [PubMed] [Google Scholar]
- 135.Tennen H, Affleck G, Zautra A. Depression history and coping with chronic pain: A daily process analysis. Health Psychol. 2006;25:370–379. doi: 10.1037/0278-6133.25.3.370. [DOI] [PubMed] [Google Scholar]
- 136.Turner JA, Mancl L, Aaron LA. Pain-related catastrophizing: a daily process study. Pain. 2004;110:103–111. doi: 10.1016/j.pain.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 137.Turner JA, Mancl L, Aaron LA. Brief cognitive-behavioral therapy for temporomandibular disorder pain: Effects on daily electronic outcome and process measures. Pain. 2005;117:377–387. doi: 10.1016/j.pain.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 138.Vendrig AA, Lousberg R. Within-person relationships among pain intensity, mood and physical activity in chronic pain: A naturalistic approach. Pain. 1997;73:71–76. doi: 10.1016/s0304-3959(97)00075-4. [DOI] [PubMed] [Google Scholar]
- 139.Viane I, Crombez G, Eccleston C, Devulder J, De Corte W. Acceptance of the unpleasant reality of chronic pain: effects upon attention to pain and engagement with daily activities. Pain. 2004;112:282–288. doi: 10.1016/j.pain.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 140.Weinland SR, Morris CB, Hu Y, Leserman J, Bangdiwala SI, Drossman DA. Characterization of episodes of irritable bowel syndrome using ecological momentary assessment. Am J Gastroenterol. 2011;106:1813–1820. doi: 10.1038/ajg.2011.170. [DOI] [PubMed] [Google Scholar]
- 141.Wen CKF, Schneider S, Stone AA, Spruijt-Metz D. Compliance with mobile ecological momentary assessment protocols in children and adolescents: A systematic review and metaanalysis. J Med Internet Res. 2017;19:e132. doi: 10.2196/jmir.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wig AD, Aaron LA, Turner JA, Huggins KH, Truelove E. Short-term clinical outcomes and patient compliance with temporomandibular disorder treatment recommendations. J Orofac Pain. 2004;18:203–213. [PubMed] [Google Scholar]
- 143.Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Williams DA, Gendreau M, Hufford MR, Groner K, Gracely RH, Clauw DJ. Pain assessment in patients with fibromyalgia syndrome. Clin J Pain. 2004;20:348–356. doi: 10.1097/00002508-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 145.Wolf LD, Davis MC, Yeung EW, Tennen HA. The within-day relation between lonely episodes and subsequent clinical pain in individuals with fibromyalgia: Mediating role of pain cognitions. J Psychosom Res. 2015;79:202–206. doi: 10.1016/j.jpsychores.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zautra A, Smith B, Affleck G, Tennen H. Examinations of chronic pain and affect relationships: Applications of a dynamic model of affect. J Consult Clin Psychol. 2001;69:786–795. doi: 10.1037//0022-006x.69.5.786. [DOI] [PubMed] [Google Scholar]
- 147.Zia J, Schroeder J, Munson S, Fogarty J, Nguyen L, Barney P, Heitkemper M, Ladabaum U. Feasibility and usability pilot study of a novel irritable bowel syndrome food and gastrointestinal symptom journal smartphone app. Clin Transl Gastroenterol. 2016;7:9. doi: 10.1038/ctg.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]