Abstract
Purpose
HR+/HER2− aromatase inhibitor (AI)-resistant metastatic breast cancer can be treated with everolimus and a second AI until the cancer recurs. Targeting these everolimus-resistant patients with the latest standard of care, CDK4/6 inhibitors, has not been clearly addressed. Understanding the signaling transduction pathways, which everolimus resistance activates, will elucidate the mechanisms and offer treatment strategies of everolimus resistance.
Methods
To mimic the clinical setting, letrozole-resistant cells were used to generate an everolimus-resistant model (RAD-R). Reverse phase protein array (RPPA) was performed to reveal changes in the signaling transduction pathways, and expression levels of key proteins were analyzed. Inhibitors targeting the major signaling pathways, a CDK4/6 inhibitor palbociclib, and a mTORC1/2 inhibitor (MLN0128) were evaluated to establish resistance mechanisms of RAD-R.
Results
RPPA results from RAD-R indicated changes to significant regulatory pathways and up-regulation of p-AKT expression level associating with everolimus resistance. MLN0128, that inhibits the AKT phosphorylation, effectively suppressed the proliferation of RAD-R cells while treatment with palbociclib had no effect.
Conclusion
Among the many signaling transduction pathways, which are altered post everolimus-resistance, targeting dual mTORC1/2 is a possible option for patients who have recurrent disease from previous everolimus treatment.
Keywords: mTOR inhibitors, MLN0128, everolimus, AI resistance
Introduction
Majority of hormone responsive breast cancers respond to endocrine therapy while some develop resistance. Thus, it is clinically important to effectively prolong endocrine therapy sensitivity. Deregulation of phosphatidylinositol 3 kinase (PI3K)/akt murine thymoma viral oncogene (AKT)/mammalian target of rapamycin inhibitor (mTOR) signaling pathway, which is common in breast cancers, upregulates the mTOR pathway leading to endocrine therapy resistance [1], and targeting it can delay resistance to treatment [1, 2].
PI3K/AKT/mTOR pathway regulates cell proliferation, metabolism and migration [3]. mTOR is a serine/threonine kinase present in two complexes-mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) and cross-talks with AKT. Both complexes have intricate network of feedback loops and protein partners, substrates and specific regulators. mTORC1 regulates translation by phosphorylating components of the protein synthesis machinery, such as the ribosomal protein S6 kinase-1 (p70S6K1) and 4E-binding protein (4E-BP). mTORC2 promotes cell survival and actin cytoskeleton through activation of AKT via phosphorylation at Ser473 [4]. It has been observed that up-regulated mTOR pathway is associated with higher cell proliferation [5]. Moreover, clinical and laboratory studies show the cross-talk of PI3K/AKT/mTOR and estrogen receptor (ER). AKT can activate ER, irrespective of estrogen availability and this can lead to endocrine therapy resistance [6].
Premenopausal women [hormone receptor positive (HR+) / HER2−] in first- or second-line treatment receive single-agent chemotherapy and tamoxifen (antiestrogen). When recurrence occurs, fulvestrant (ICI 182780; Faslodex) is the next line of treatment. However, in postmenopausal women, non-steroidal aromatase inhibitors (AIs) (e.g. letrozole) or fulvestrant is used initially, but with recurrence to the endocrine therapy, everolimus (mTORC inhibitor; Afinitor) has been introduced with a second AI (e.g. exemestane), since 2012 [7].
Clinical trials with pan-PI3K inhibitors [8–10] and AKT inhibitors [11] have observed dose-limiting toxicities, and many preclinical studies point to mTOR as an operative target [11, 12]. In 2012, FDA approved everolimus to target the PI3K/AKT/mTOR pathway in AI-resistant metastatic breast cancer [7, 13]. Clinical trials such as BOLERO-2 observed HR+/HER2− advanced postmenopausal breast cancer patients who had progressed on prior non-steroidal AIs exhibited higher progression free survival (PFS) in the everolimus/steroidal AI (exemestane) group versus the exemestane/placebo group (7.8 months versus 3.2 months, respectively). However, everolimus clinical studies have shown unpredictable antitumor effects across various cancers due to lack of full inhibition of p-4E-BP1 sites, the existence of feedback loops and the inability to inhibit mTORC2 leading to everolimus resistance [4]. Many patients exhibit either de novo or acquired resistance to everolimus treatment; thus, it is imperative to treat this subset of patients whose regimen will next include chemotherapeutic agents [7, 11, 14]. Based on clinical observations of patient treatment regimen and development of resistance, we generated an everolimus-resistant cell line (RAD-R) for an in vitro mechanistic analysis of such clinically significant problem.
Even though CDK4/6 inhibitors, in combination with AIs, are recently being utilized as first and second line treatment for pre and postmenopausal women [15–17], some AI-resistant patients are still being treated with everolimus [7, 11, 14]. Thus, our RAD-R serves as an important model to check the response of current treatment options (CDK4/6 inhibitors) and other potential inhibitors in everolimus resistance. Recently, an ATP-competitive mTORC inhibitor MLN0128 (also known as TAK-228 or INK-128; Intellikine) was developed to target both mTORC1 and mTORC2, simultaneously [18], and was shown in this study to be effective in RAD-R.
Materials and methods
Cell lines
MCF-7aro and letrozole-resistant cell line (LET-R) were generated as previously described [19, 20]. Everolimus (RAD-001)-resistant cell line (RAD-R) was generated from LET-R cells by prolonged culture (8 months) in phenol-red free MEM1x and in the presence of the inhibitor and testosterone. MCF-7aro was cultured in phenol red MEM1x. Cell culture media was supplemented with 10% FBS or charcoal-dextran treated FBS for LET-R and RAD-R; 2 mM L-glutamine; 50mg/ml of G418; 1 mM sodium pyruvate; and 100 U/mL penicillin-streptomycin. Cell lines were authenticated at the Integrative Genomics Core of the City of Hope (City of Hope, Duarte, CA).
Reagents
Testosterone was purchased from Sigma (Sigma Chemical, St. Louis, MO); Fulvestrant (ICI 182780; Faslodex) and everolimus (RAD-001) from Tocris (Ellisville, MO); and palbociclib (PD0332991) and MLN0128 (also known as TAK-228 or INK-128) from Selleckchem (Houston, TX). Inhibitors Erlotinib, Lapatinib, MK-2206 and PPP (Picropodophyllotoxin) were purchased from Cayman Chemical (Ann Arbor, MI).
Determination of IC50
IC50 index was calculated by Calcusyn 2.1 software (Biosoft, Cambridge, UK) according to the Chou-Talalay method [21]. Cell viability was assayed after 6 days when cell growth reached the exponential phase using the MTT assay (Sigma, St. Louis).
Reverse phase protein array (RPPA)
Cells were treated with DMSO for 6 days. The cells were then lysed per the cell line lysate prep (6-well plate) protocol of the University of Texas, MD Anderson Cancer Center RPPA Core Facility-Functional Proteomics (Houston, Texas). Protein expression in these samples was then estimated through RPPA, as described by Kanaya et al.[22].
Western blot analysis
Forty-eight hours post inhibitor treatment, protein was extracted with Radioimmunoprecipitation assay (RIPA) buffer supplemented with 1mM phenylmethanesulfonyl fluoride (PMSF). Protein concentration was determined using the Bradford Protein Assay (BioRad, Hercules, CA). The following antibodies were used: p-AKT (Ser473)/AKT; p-4E-BP1 (Thr37/46)/4E-BP1; p-p70S6K (Thr389)/p70S6K; pRB/RB; p-HER2/ErbB2 (Y1248)/HER2/ErbB2 (D8F12);GAPDH (14C10) from Cell Signaling Technology (Beverly, MA); and β-actin (I-19R) from Santa Cruz Biotechnology (Dallas, Texas). Signal intensity was determined by ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA).
Results
Molecular signaling pathways are altered in RAD-R
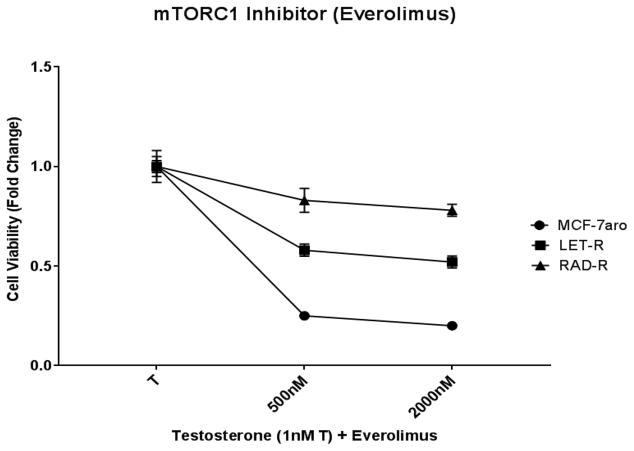
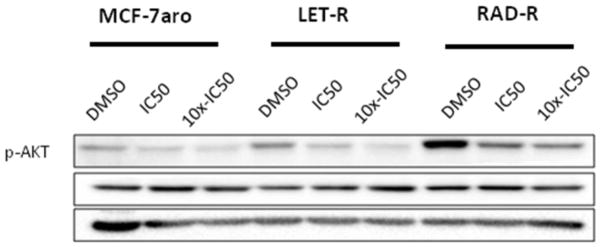
In order to characterize the signaling transduction pathways in everolimus resistance, we performed a RPPA analysis including HR+/endocrine responsive cell line (MCF-7aro), letrozole-resistant (LET-R) and everolimus-resistant (RAD-R) cell lines. MCF-7aro cells overexpress the aromatase enzyme [20] and testosterone (converted to estrogen) drives the proliferation of these cells. LET-R was generated from MCF-7aro by treatment with letrozole for an extended period of time [19]. In order to simulate the treatment regimen of patients in the clinic, RAD-R was generated from LET-R because everolimus is treated after patients fail AI treatments [7]. Fig. 1 indicates the response to everolimus in parental/everolimus sensitive cell line (MCF-7aro) while RAD-R exhibits reduced response. Also, reduced response to everolimus (approximately 40% cell death) is observed in LET-R. The lack of complete inhibition in LET-R can be attributed to the unpredictable antitumor effects of everolimus in AI-resistant breast cancer due to the existence of feedback loops which lead to AKT phosphorylation/activation because of the inability to inhibit the mTORC2 signaling pathway. With the use of the RPPA, we were able to identify the expression of several signaling transduction pathways which were altered in these cell lines, and we were able to compare the changes in the signaling pathways which occurred with the formation of resistance. With everolimus resistance, changes to the molecular signaling pathways included up-regulation of phospho-AKT (p-AKT), phospho-EGFR (p-EGFR), phospho-HER2 (p-HER2) and IGFR, and down-regulation of phospho-4E-BP1 (p-4E-BP1), phospho-p70S6K (p-p70S6K) and ER (Supplementary Fig. S1). Also, in comparison, these signaling pathways were either less-activated and/or exhibited no change in the endocrine therapy responsive (MCF-7aro) or AI-resistant (LET-R) cell line. The RPPA results show that with everolimus resistance, even though p-p70S6K and p-4E-BP1 are down-regulated, the up-regulation of p-AKT signaling pathways are imminent due to the inability of everolimus to inhibit mTORC2 activation of AKT.
Fig. 1. Cell proliferation response of MCF-7aro, LET-R and RAD-R cell lines to everolimus.
RAD-R cell line indicates acquired resistance while LET-R and MCF-7aro show response post 6 day treatment with everolimus. Cell proliferation was initiated upon the addition of testosterone (1nM T), and fold change of 1 was baseline at day 1 of MTT assay.
Estrogen receptor signaling pathways in RAD-R
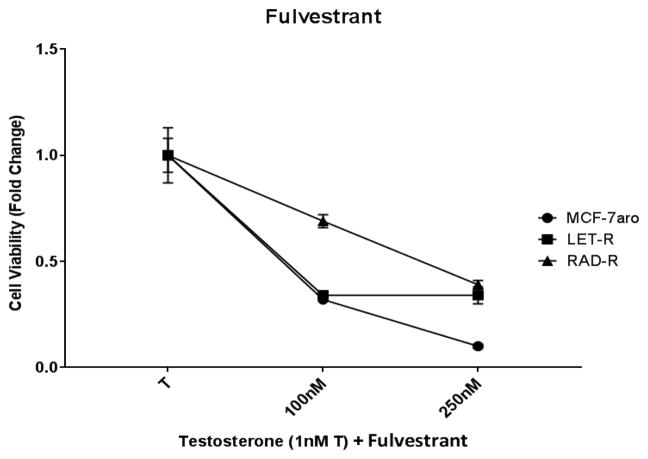
As previously reported, ER expression is constitutively active in AI resistance; thus, resistant cells do not rely on the ER signaling pathways for growth [23]. In RAD-R and LET-R, the expression of ER is down-regulated indicating reduced dependence of these cells from the ER signaling pathways (Supplementary Fig. S1). In a clinical setting, pre and postmenopausal HR+/HER2− women who have developed AI resistance, are given the ER antagonist fulvestrant as 2nd line treatment [7, 11]. In our cell line models, using increasing concentrations of fulvestrant, ER dependent MCF-7aro cells decreased cell viability while RAD-R and LET-R cells were less affected (Fig. 2), suggesting a decrease dependence of ER in driving cell proliferation in the two resistant cell lines.
Fig. 2. Cell proliferation response of MCF-7aro, LET-R and RAD-R cell lines to fulvestrant.
Six day cell viability shows inhibition of MCF-7aro cells post treatment with the estrogen receptor antagonist fulvestrant. LET-R and RAD-R were less responsive than MCF-7aro. Cell proliferation was initiated upon the addition of testosterone (1nM T), and fold change of 1 was baseline at day 1 of MTT assay.
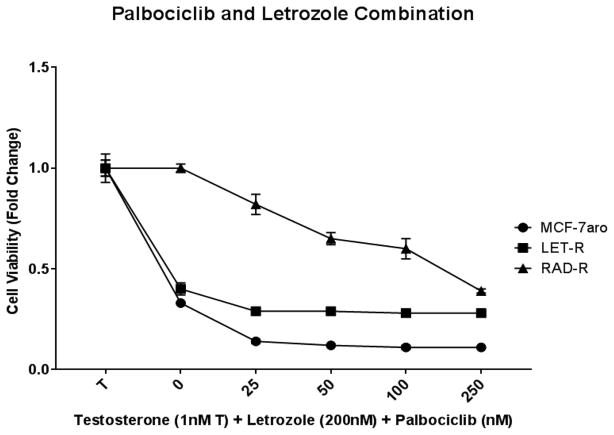
CDK4/6 inhibitors have been recently utilized for HR+/HER2− breast cancer, including AI-resistant diseases [11, 15–17]. In order to test the inhibition of RAD-R and mimic the clinical regimen, the cell lines were treated with a combination of palbociclib (CDK4/6 inhibitor) and letrozole. Proliferation suppression was observed in the MCF-7aro cell line, and LET-R displayed a decrease but to a lesser degree (Fig. 3). RAD-R had significantly reduced response; thus, suggesting CDK4/6 inhibitor treatment of everolimus-resistant patients would not be beneficial.
Fig. 3. Cell proliferation response of MCF-7aro, LET-R and RAD-R cell lines to palbociclib and letrozole combination.
Cell lines were incubated for 6 days with increasing concentrations of palbociclib and constant concentration of letrozole (LET) and testosterone (T). MCF-7aro shows the greatest response, and RAD-R only shows response at the highest concentrations. Cell proliferation was initiated upon the addition of testosterone (1nM T), and fold change of 1 was baseline at day 1 of MTT assay.
Signaling pathway analysis in RAD-R
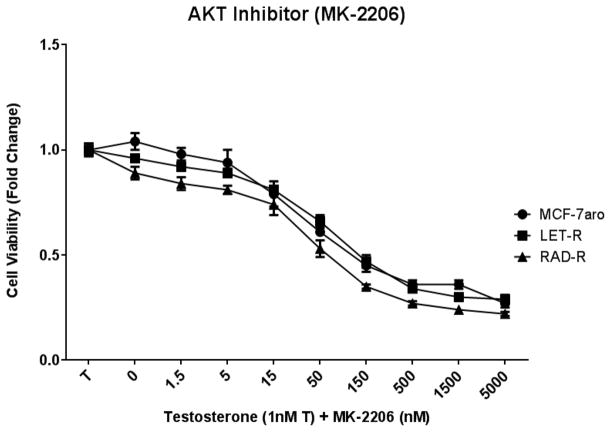
In order to analyze the signaling pathway activation in these cell lines, cell proliferation studies were conducted with inhibitors to AKT (MK-2206), HER2 (lapatinib), EGFR (erlotinib), and IGF1R (PPP). AKT inhibition affected the cell growth of all three cell lines (Fig. 4a) in a dose-dependent manner, and the p-AKT protein expression was reduced with increasing concentrations of the inhibitor (Fig. 4b). Moreover, p-AKT expression was higher in RAD-R versus MCF-7aro or LET-R. Concentrations of MK-2206 for three cell lines were based on the IC50 values, and 10x-IC50 concentrations were used to maximize AKT inhibition (Table 1).
Fig. 4. AKT inhibitor (MK-2206) treatment suppressed the proliferation of RAD-R and AKT phosphorylation.
IC50 concentrations were calculated for cell lines MCF-7aro (90nM), LET-R (80nM) and RAD-R (22nM). (a) Post 6 day treatment of testosterone (T) with increasing concentrations of MK-2206 shows growth inhibition of three cell lines. Cell proliferation was initiated upon the addition of testosterone (1nM T), and fold change of 1 was baseline at day 1 of MTT assay. (b) Western blot analysis shows dose-dependent inhibition of AKT phosphorylation with treatment at IC50 and 10x-IC50 concentrations
Table 1.
IC50 Concentration of Inhibitors
| Inhibitor | Cell Lines | ||
|---|---|---|---|
|
| |||
| MCF-7aro | LET-R | RAD-R | |
| Erlotinib (EGFR) | 1036nM | 9375nM | 2651nM |
| Everolimus (mTORC1) | 250nM | 2000nM | 8000nM |
| Lapatinib (HER2) | 1698nM | 2148nM | 389nM |
| MK-2206 (AKT) | 90nM | 80nM | 22nM |
| MLN0128 (mTORC1/2) | 5nM | 10nM | 50nM |
| Palbociclib (CDK4/6) | 50nM | 5000nM | 10000nM |
| PPP (IGF1R) | 69nM | 103nM | 84nM |
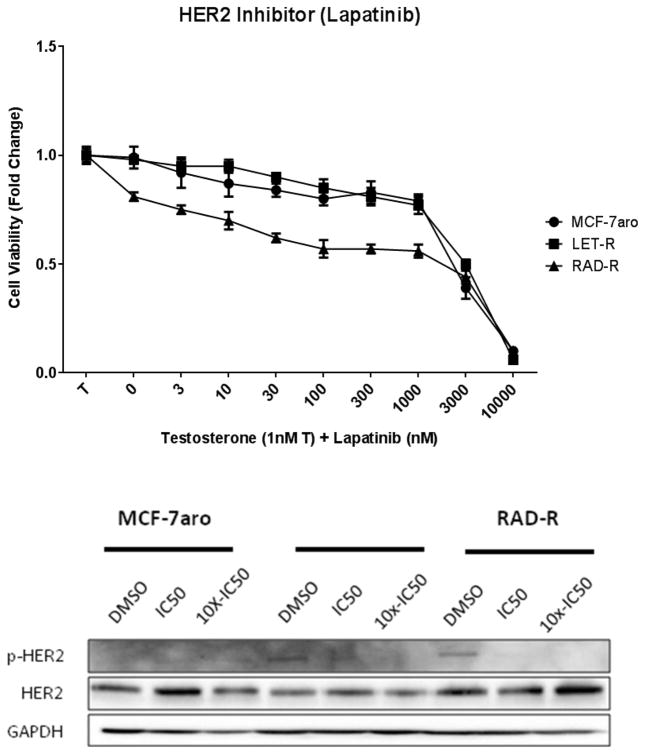
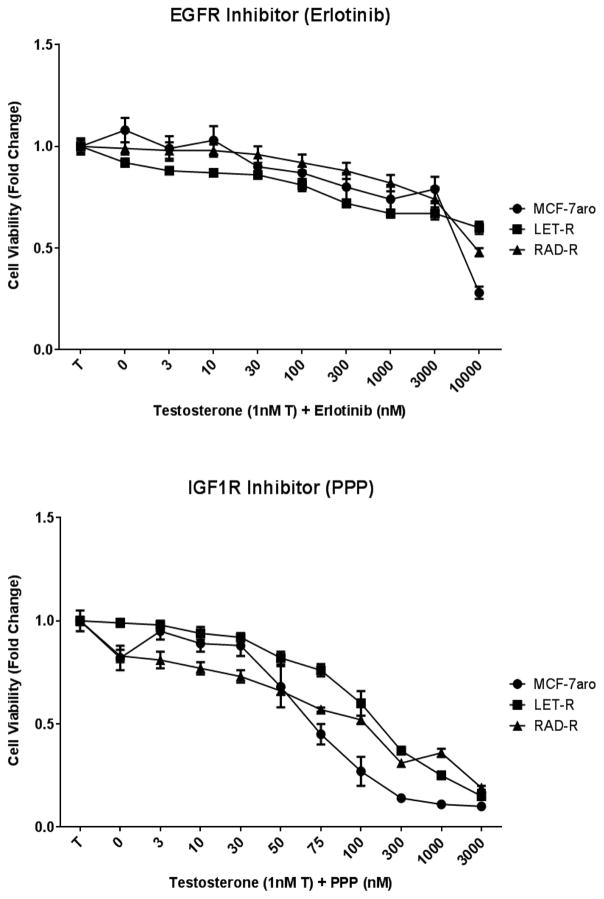
HER2 inhibition with lapatinib decreased the cell growth of all three cell lines but was effective only at micromolar concentrations (Fig. 5a); thus, rendering this inhibitor not feasible for everolimus resistance. Protein expression analysis revealed that the inhibitor was effective in targeting HER2 as seen in the reduction of p-HER2 protein expression in RAD-R (Fig. 5b). These results suggest that HER2 is not a key driver of everolimus resistance. Moreover, inhibition of EGFR with erlotinib affected the cell proliferation of the cell lines only at 10 μM concentration (Fig. 6a). Inhibition of IGF1R with PPP exhibited dose-dependent response, in all three cell lines, with greater inhibition of the MCF-7aro cells (Fig. 6b).
Fig. 5. HER2 inhibitor (Lapatinib) treatment suppresses cell proliferation at micromolar concentrations.
IC50 concentrations were calculated for cell lines MCF-7aro (1698nM), LET-R (2148nM) and RAD-R (389nM). (a) Post 6 day treatment of testosterone (T) with increasing concentrations of lapatinib shows growth inhibition of the three cell lines at micromolar concentrations. Cell proliferation was initiated upon the addition of testosterone (1nM T), and fold change of 1 was baseline at day 1 of MTT assay. (b) Western blot analysis shows dose-dependent reduction of p-HER2 expression in RAD-R with treatment at IC50 and 10x-IC50 concentrations
Fig. 6. EGFR (Erlotinib) and IGF1R (PPP) treatment of three cell lines.
(a) IC50 concentrations of EGFR (erlotinib) were calculated for cell lines MCF-7aro (1036nM), LET-R (9375nM) and RAD-R (2651nM). Post 6 day treatment of testosterone (T) with increasing concentrations of erlotinib shows no difference of growth suppression between the three cell lines. (b) IGF1R (PPP) IC50 concentrations were calculated for cell lines MCF-7aro (69nM), LET-R (103nM) and RAD-R (84nM). Six day treatment of testosterone (T) with increasing concentrations of PPP shows growth inhibition of the cell lines at increasing concentrations with greater effect on the MCF-7aro cells. Cell proliferation for (a) and (b) was initiated with the addition of testosterone (1nM T); fold change of 1 was baseline at day 1 of MTT assay
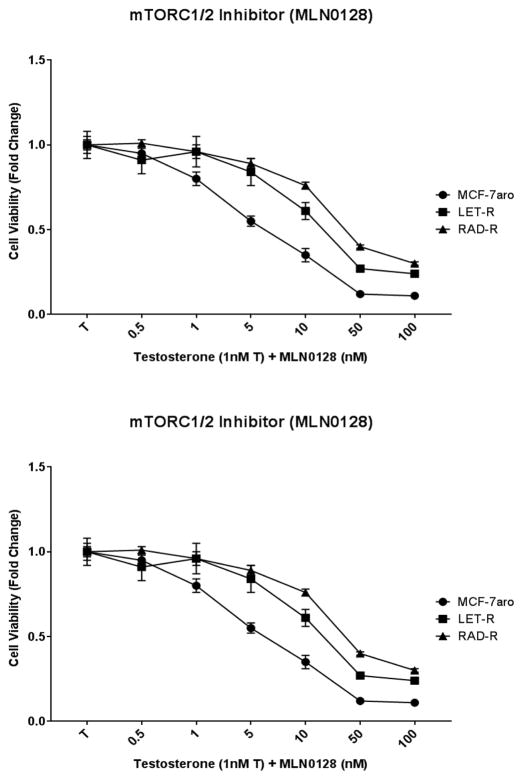
MCF-7aro, LET-R and RAD-R cell lines were also subjected to MLN0128 (dual mTORC inhibitor). Treatment with MLN0128 exhibited dose-dependent inhibition of cell proliferation, at low nanomolar concentrations (Fig. 7a). For all three cell lines, MLN0128 was a potent inhibitor. Therefore, MLN0128 is thought to be a suitable inhibitor of RAD-R, with a higher potency than other inhibitors.
Fig. 7. RAD-R cells respond to the dual mTORC inhibitor MLN0128 by displaying growth inhibition and down-regulation of p-AKT expression.
(a) Six day cell viability of MLN0128 treatment shows dose-dependent growth inhibition of the cell lines. Cell proliferation was initiated upon the addition of testosterone (1nM T), and fold change of 1 was baseline at day 1 of MTT assay. (b) Western blot analysis shows signaling pathways affected after 48 hour treatment. IC50 concentrations of MLN0128, palbociclib and everolimus were 5, 50, 250nM for MCF-7aro; 10, 5000, 2000nM for LET-R; and 50, 10000 and 8000nM for RAD-R, respectively
Dual mTORC inhibitor targets RAD-R
Utilizing the IC50 concentrations (Table 1), from the dose response experiments, we performed protein expression analysis on the molecular signaling pathways in the three cell lines. p-AKT protein expression was down-regulated in MCF-7aro cell line with MLN0128, palbociclib, and everolimus treatment (Fig. 7b). In LET-R and RAD-R cell lines, MLN0128 down-regulated p-AKT expression and everolimus treatment definitively up-regulated p-AKT expression in RAD-R. In all three cell lines, MLN0128 showed greater p-AKT protein inhibition than everolimus but both inhibitors reduced p-p70S6 expression which is downstream of both the mTORC pathways. Also, MLN0128 down-regulated expression levels of p-4E-BP1 and 4E-BP1 in LET-R and RAD-R but not in the MCF-7aro cells. As expected, palbociclib reduced the p-RB/RB protein expression levels and interestingly also reduced p-p70S6K expression in MCF-7aro and LET-R. Furthermore, p-AKT was still detected after treatment of RAD-R with palbociclib (Fig. 7b).
Discussion
Everolimus resistance has been thought to be due to the lack of p-4E-BP1 inhibition, the existence of feedback loops and the inability to inhibit mTORC2-mediated AKT activation/phosphorylation [4, 18]. Based on clinical observations and lack of well-established inhibitors for AKT and PI3K, and the rise of everolimus resistance, we generated everolimus-resistant cell line for a mechanistic analysis and for seeking a better drug against such resistance.
Following the treatment regime for HR+/HER2− advanced breast cancer, RAD-R cell line was derived from letrozole-resistant cell line (LET-R) [19] by long-term treatment with everolimus. In comparison to the MCF-7aro and LET-R cell lines, the RAD-R cell line shows resistance to everolimus but exhibits a dose-dependent response to the dual mTORC inhibitor (MLN0128) (Fig.1 vs. Fig. 7a). MCF-7aro cells, which are HR+/HER2− and use the overexpressed aromatase enzyme to convert testosterone to estrogen and drive cell proliferation, respond both to everolimus and MLN0128. MCF-7aro (parental/everolimus sensitive) cell line was more sensitive than LET-R and RAD-R. Both mTORC1 and mTORC2 are activated and cross-talk with ER-mediated regulation in hormone-responsive cells like MCF-7aro. In the clinical setting, when women develop letrozole resistance, they are then treated with a second AI (exemestane) and everolimus [7, 11]. As expected, the LET-R responds to both everolimus and MLN0128 by reducing cell proliferation (Fig. 1, 7a). Thus, MLN0128 is effective in both resistant cell lines (LET-R and RAD-R).
Recently, high PFS is observed in HR+/HER2− metastatic breast cancer patients treated with CDK4/6 inhibitor (e.g. palbociclib) in combination with endocrine therapy [15, 16, 24]. Therefore, we tested the possibility that inhibition of the cell cycle progression would also inhibit proliferation of RAD-R. With palbociclib treatment, RAD-R cells exhibited response only at much higher concentrations than that of MCF-7aro or LET-R cells (Fig. 3). Our results from this preclinical study are significant to support the clinical observation that palbociclib is not effective to overcome everolimus resistance [25–27]. Moreover, it was reported in the 2017 Annual Meeting of the American Society of Clinical Oncology (ASCO) that treatment with palbociclib (after everolimus) leads to a low drug response/clinical benefit [28].
The mechanism of CDK4/6 inhibition resistance is still under investigation. CDK4/6 inhibitors target the retinoblastoma (RB) tumor suppressor protein leading to G1/S-phase cell cycle arrest [17, 29–31]. It has been shown that hyper-phosphorylated RB interacts with mTORC2 to suppress mTORC2 activation of AKT. But inhibition of RB phosphorylation by CDK4/6 inhibitors attenuates the suppression of mTORC2; thus, resulting in upregulated AKT phosphorylation/activation. This is specifically seen through elevated expression level of p-AKT [25, 27] (Fig. 7b). Furthermore, studies from our laboratory have revealed that a functional ER-mediated cell cycle progression is important for the response of CDK4/6 inhibitors (unpublished data). Thus, a decrease of fulvestrant response of RAD-R (Fig. 2) suggests a reduced involvement of ER-mediated cell proliferation and could contribute partially to its weak response to palbociclib (Fig. 3).
Moreover, many clinical and laboratory studies have shown the cross-talk of PI3K/mTOR and the ER [6]. However, because only mTORC1 is inhibited by everolimus, this treatment leads to everolimus resistance through a negative feedback loop between S6K, PI3K and mTORC2 which upregulates p-AKT [32, 33]. This was evident in RAD-R which exhibited an increase of p-AKT expression (Fig. 4b, 7b). With the formation of RAD-R, RPPA results indicated up-regulation of molecular signaling pathways, such as p-AKT, p-EFGR, p-HER2 and IGFR (Supplementary Fig. S1). Moreover, the signaling transduction pathways, which are activated through these growth factor receptors, converge on the AKT/mTOR signaling pathway [13]; thus, it was rational to analyze these pathways using inhibitors available to us.
Inhibitors to HER2 (lapatinib) and EGFR (erlotinib) were only effective at higher concentrations, suggesting that they are not key regulatory molecules (Fig. 5a, 6a). PPP inhibited the proliferation of RAD-R, but this is not a clinically relevant inhibitor for IGF1R (Fig. 6b). By using a selective inhibitor to target AKT (MK-2206), it was evident that inhibition of resistant cells was possible (Fig. 4a). Activation of these signaling pathways up-regulates the PI3K/AKT/mTOR network. However, effective PI3K [8–10] or AKT inhibitors [11] are not yet available for clinical use. Therefore, targeting both mTORC1 and mTORC2 to prevent AKT phosphorylation, would be an option [11, 12].
One recent study from the University of Texas MD Anderson Cancer Center reported rapid disease progression of CDK4/6 inhibitor plus everolimus resistance. But stable disease, over 20 months, was observed with MLN0128 plus a VEGF inhibitor (ziv-Aflibercept) [34]. Thus, even with CDK4/6 inhibitor resistance and/or everolimus resistance, as a model mTORC1/2 inhibitor, MLN0128 can serve to increase PFS. Currently, there are clinical trials which combine MLN0128 with AI inhibitors (exemestane) or estrogen antagonists (tamoxifen or fulvestrant) in advanced breast cancer [35]. Therefore, the results presented in this manuscript show that the mTORC1/2 signal transduction pathway is a critical target following everolimus resistance, and the dual mTORC inhibitor MLN0128 can benefit these patients the best.
Conclusion
Since 2012, AI-resistant metastatic breast cancer has been treated with everolimus which leads to resistance in majority of patients. Mechanistic studies have been carried out using an informative everolimus-resistant cell line which is derived from a letrozole-resistant line. Among the many signaling transduction pathways, which are altered post everolimus resistance, targeting dual mTORC1/2, but not CDK4/6, may be effective in patients whose next therapeutic option is chemotherapy.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Panda Charitable Foundation and the National Cancer Institute (P30CA033572).
Abbreviations
- AI
Aromatase inhibitor
- HR
Hormone receptor
- LET-R
Letrozole-resistant cell line
- PFS
Progression free survival
- RAD-R
Everolimus-resistant cell line
- RPPA
Reverse phase protein array
Footnotes
Compliance with ethical standards
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Paplomata E, O'Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6(4):154–66. doi: 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Margariti N, et al. “Overcoming breast cancer drug resistance with mTOR inhibitors”. Could it be a myth or a real possibility in the short-term future? Breast Cancer Res Treat. 2011;128(3):599–606. doi: 10.1007/s10549-010-0986-9. [DOI] [PubMed] [Google Scholar]
- 3.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169(2):361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Vilar E, Perez-Garcia J, Tabernero J. Pushing the envelope in the mTOR pathway: the second generation of inhibitors. Mol Cancer Ther. 2011;10(3):395–403. doi: 10.1158/1535-7163.MCT-10-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Missiaglia E, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28(2):245–55. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy LC, Seekallu SV, Watson PH. Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer. 2011;18(1):R1–14. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]
- 7.Mestres JA, et al. Defining the optimal sequence for the systemic treatment of metastatic breast cancer. Clin Transl Oncol. 2017;19(2):149–161. doi: 10.1007/s12094-016-1520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma CX, et al. A Phase I Trial of BKM120 (Buparlisib) in Combination with Fulvestrant in Postmenopausal Women with Estrogen Receptor-Positive Metastatic Breast Cancer. Clin Cancer Res. 2016;22(7):1583–91. doi: 10.1158/1078-0432.CCR-15-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma CX, et al. A Phase I Study of the AKT Inhibitor MK-2206 in Combination with Hormonal Therapy in Postmenopausal Women with Estrogen Receptor-Positive Metastatic Breast Cancer. Clin Cancer Res. 2016;22(11):2650–8. doi: 10.1158/1078-0432.CCR-15-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer IA, et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2014;32(12):1202–9. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brufsky AM, Dickler MN. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist. 2018 doi: 10.1634/theoncologist.2017-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkabets M, et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med. 2013;5(196):196ra99. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo HS, Vidula N, Ma C. Improving Response to Hormone Therapy in Breast Cancer: New Targets, New Therapeutic Options. Am Soc Clin Oncol Educ Book. 2016;35:e40–54. doi: 10.1200/EDBK_159198. [DOI] [PubMed] [Google Scholar]
- 14.Yi Z, Ma F. Biomarkers of Everolimus Sensitivity in Hormone Receptor-Positive Breast Cancer. J Breast Cancer. 2017;20(4):321–326. doi: 10.4048/jbc.2017.20.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 17.Fry DW, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–38. [PubMed] [Google Scholar]
- 18.Ingels A, et al. Preclinical trial of a new dual mTOR inhibitor, MLN0128, using renal cell carcinoma tumorgrafts. Int J Cancer. 2014;134(10):2322–9. doi: 10.1002/ijc.28579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, et al. New experimental models for aromatase inhibitor resistance. J Steroid Biochem Mol Biol. 2007;106(1–5):8–15. doi: 10.1016/j.jsbmb.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun XZ, Zhou D, Chen S. Autocrine and paracrine actions of breast tumor aromatase. A three-dimensional cell culture study involving aromatase transfected MCF-7 and T-47D cells. J Steroid Biochem Mol Biol. 1997;63(1–3):29–36. doi: 10.1016/s0960-0760(97)00068-x. [DOI] [PubMed] [Google Scholar]
- 21.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Kanaya N, et al. Characterization of patient-derived tumor xenografts (PDXs) as models for estrogen receptor positive (ER+HER2− and ER+HER2+) breast cancers. J Steroid Biochem Mol Biol. 2017;170:65–74. doi: 10.1016/j.jsbmb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masri S, et al. Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines. J Steroid Biochem Mol Biol. 2010;118(4–5):277–82. doi: 10.1016/j.jsbmb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristofanilli M, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 25.Bonelli MA, et al. Combined Inhibition of CDK4/6 and PI3K/AKT/mTOR Pathways Induces a Synergistic Anti-Tumor Effect in Malignant Pleural Mesothelioma Cells. Neoplasia. 2017;19(8):637–648. doi: 10.1016/j.neo.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes J, et al. The next era of treatment for hormone receptor-positive, HER2-negative advanced breast cancer: Triplet combination-based endocrine therapies. Cancer Treat Rev. 2017;61:53–60. doi: 10.1016/j.ctrv.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, et al. Inhibition of Rb Phosphorylation Leads to mTORC2-Mediated Activation of Akt. Mol Cell. 2016;62(6):929–942. doi: 10.1016/j.molcel.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opyrcha M. Palbociclib Given After Everolimus Is Associated With Low Response and Clinical Benefit Rates in HR+ Metastatic Breast Cancer. 2017 Annual Meeting of the American Society of Clinical Oncology (ASCO); May 23, 2017; Chicago, Illinois: Oncology and Metastatic Breast Cancer; [Google Scholar]
- 29.Curigliano G, et al. Pharmacokinetic drug evaluation of ribociclib for the treatment of metastatic, hormone-positive breast cancer. Expert Opin Drug Metab Toxicol. 2017;13(5):575–581. doi: 10.1080/17425255.2017.1318848. [DOI] [PubMed] [Google Scholar]
- 30.Witkiewicz AK, Knudsen ES. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16(3):207. doi: 10.1186/bcr3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang C, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36(16):2255–2264. doi: 10.1038/onc.2016.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan X, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 34.Bashour SI, et al. Rapid Breast Cancer Disease Progression Following Cyclin Dependent Kinase 4 and 6 Inhibitor Discontinuation. J Cancer. 2017;8(11):2004–2009. doi: 10.7150/jca.18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trials, C. MLN0128 | Breast Cancer. 2018 [cited 2018 February]; Available from: https://clinicaltrials.gov/ct2/results?term=MLN0128&cond=Breast+Cancer.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.