Abstract
Behavior does not differentiate ASD risk prior to 12 months of age, but biomarkers may inform risk before symptoms emerge. Click-evoked auditory brainstem responses (ABRs) may be worth consideration due to their measurement properties (non-invasiveness; reliability) and conceptual features (well-characterized neural generators), but participant characteristics and assessment protocols vary considerably across studies. Our goal is to perform a meta-analysis of the association between ABRs and ASD. Following an electronic database search (PubMed, Medline, PsycInfo, PsycArticles), we included papers that were written in English, included ASD and typically-developing (TD) groups, and reported the information needed to calculate standardized mean differences (Hedges’s g) for at least one ABR latency component (I, III, V, I–III, III–V, I–V). We weighted and averaged effect sizes across conditions and subsets of participants to yield one estimate per component per study. We then performed random-effects regressions to generate component-specific estimates. ASD was associated with longer ABR latencies for Waves III (g=0.5, 95%CI 0.1, 0.9), V (g=0.7, 95%CI 0.3, 1.1), I–III (g=0.7, 95%CI 0.2, 1.2), and I–V (g=0.6, 95%CI 0.2, 1.0). All components showed significant heterogeneity. Associations were strongest among participants ≤8 years of age and those without middle ear abnormalities or elevated auditory thresholds. In sum, associations between ABRs and ASD are medium-to-large in size, but exhibit heterogeneity. Identifying sources of heterogeneity is challenging, however, due to power limitations and co-occurrence of sample/design characteristics across studies. Research addressing the above limitations is crucial to determining the etiologic and/or prognostic value of ABRs for ASD.
Keywords: Evoked Potentials, Auditory, Brain Stem, Autism Spectrum Disorder
Introduction
Early interventions represent promising avenues for improving the functioning of children with autism spectrum disorder (ASD). While the impact of these interventions depends upon many factors (e.g., symptom severity), age at enrollment is a powerful predictor of their efficacy (Odom, Boyd et al. 2010, Wallace and Rogers 2010, Rogers, Vismara et al. 2014). Early diagnosis and/or reliable identification of ASD risk therefore represent pressing public health objectives.
To date, family history is the most clearly defined risk factor for ASD with a 10–20% recurrence rate within families and heritability estimates of 0.6–0.9 (Ronald, Happe et al. 2006, Constantino, Zhang et al. 2010, Tick, Bolton et al. 2016). This knowledge has motivated extensive work with infant siblings of children with ASD, which in turn, has informed the identification of early emerging, behavioral-level antecedents associated with diagnosis (Jones and Klin 2013, Constantino, Kennon-McGill et al. 2017). However, behavior does not reliably differentiate ASD risk prior to 12 months of age and diagnostic status is not considered reliable prior to age 2 (Lord, Risi et al. 2006, Zwaigenbaum, Thurm et al. 2007, Kleinman, Ventola et al. 2008, Rogers 2009). Thus, much effort has been devoted to identifying biomarkers (e.g., genetic, metabolic, immune) that may inform risk prior to the manifestation of behavioral-level symptoms (Newschaffer, Croen et al. 2007, Dawson 2008).
To this end, click-evoked auditory brainstem responses (hereafter, ABRs) may be a biomarker worth further consideration. ABRs are electrophysiological responses that reflect auditory pathway activation by broadband acoustic stimuli (i.e., clicks) from the cochlea through the rostral brainstem (Moore 1987a, Moore 1987b). ABRs consist of 5 waves (I–V) from which latencies and amplitudes can be derived, values that reflect the degree of dendritic branching, myelination, and synchrony of firing across populations of neurons in the central auditory pathway (Ponton, Moore et al. 1996). Well-characterized components include I, III, & V, which correspond to action potentials generated from the VIII cranial nerve, cochlear nucleus, and lateral lemniscus, respectively (Moore 1987a).
ABRs may advance our understanding of ASD for methodological and conceptual reasons. For example, ABRs are recorded using electrodes placed on the scalp and are thus a non-invasive assessment; this facilitates enrollment of participants without medical indications. ABRs also exhibit high signal-to-noise ratios. They are elicited by clicks that are presented in quick succession (e.g., 11/sec), which enables the administration of (and averaging across) thousands of trials within minutes. Because ABRs also demonstrate test-retest reliability (Yang, Stuart et al. 1993), components can be interpreted at an individual-level with clinical import (e.g., neonatal hearing screening programs) (Mason and Herrmann 1998). In addition, ABR neural generators are well-characterized, despite diverse efferent and afferent projections that converge on these generators (Winer 2005). Thus, waveform decomposition enables integration with other brain-based assessments to generate hypotheses and/or evaluate coherence of findings.
Additional features of the ABR are specifically relevant to ASD. Neuroanatomically, ASD is associated with: 1) smaller brainstem volume, driven primarily by grey matter reduction, and 2) a marked reduction in superior olivary neurons, projections from which contribute to the lateral lemniscus (i.e., Wave V) (Hashimoto, Tayama et al. 1995, Rodier 2002, Jou, Minshew et al. 2009, Jou, Frazier et al. 2013). In addition, ASD and ABRs exhibit sex differences. ASD affects 4–5 males per female (Centers for Disease Control 2014), and males produce longer ABR latencies for all major wave components across the lifespan (Jerger and Hall 1980, Li, Zhu et al. 2013). ASD and ABRs are also sensitive to perinatal health risks and exhibit family resemblance (Jiang 1998, Jerger, Chmiel et al. 1999, Maziade, Merette et al. 2000, Jiang, Brosi et al. 2005). Perhaps most importantly, given that prevailing etiologic hypotheses of ASD implicate alterations in perinatal brain development (Rodier, Ingram et al. 1996, Anderson, Jacobs-Stannard et al. 2007, Stoner, Chow et al. 2014), ABRs can be measured a time proximal to this proposed process.
Given these characteristics, it is not surprising that associations between ABRs and ASD have been explored for more than three decades. However, findings vary considerably from one study to the next – both in terms of the magnitude and the direction of associations. For example, effects range from null to large (Courchesne, Courchesne et al. 1985, Roth, Muchnik et al. 2012) and are not consistently linked to specific aspects of the ABR waveform. In addition, ASD has been associated with both slower and faster ABR wave latencies relative to non-ASD counterparts (Rumsey, Grimes et al. 1984, Kwon, Kim et al. 2007, Dabbous 2012). However, several factors currently impede a coherent synthesis of the literature. For example, studies vary in the age at ABR assessment, utilize different ABR collection methods, employ varying definitions of ASD, and do not consistently address the impact of potential confounders (e.g., sex). We therefore performed a meta-analysis of the association between ABRs and ASD to address these interpretational challenges.
Method
Data Sources & Search Strategy
To identify candidate papers, we searched PubMed, Medline, PsycInfo, and PsycArticles using the following terms: (“auditory brain stem” or “auditory brainstem” or “audit$,”) and (“autism” or “autism spectrum disorder” or “PDD” or “disintigrative” or “asperger$”). This search, most recently implemented in May 2016, yielded 80 references that were evaluated for inclusion. First, we excluded references not written in English (N=4), along with reviews/commentaries (N=5), animal studies (N=9), case studies (N=1), and duplicate papers (N=4). We also excluded papers missing either: 1) click-evoked ABR or ASD data (N=30) or 2) a typically developing comparison (TD) group (N=7). To remain sensitive to secular changes in ASD conceptualization, following diagnoses were considered indicative of and are hereafter referred to as “ASD”: Infantile Amnesia, Autistic Disorder, Asperger’s Disorder, Pervasive Developmental Disorder, Pervasive Developmental Disorder-Not Otherwise Specified, or Childhood Disintegrative Disorder. In total, 60 papers were excluded in this first stage. Next, we examined citations within the remaining 20 papers to identify references missed by our database search. We identified an additional 5 papers using this manual search strategy, resulting in 25 papers eligible for further consideration. Finally, we obtained copies of these papers and evaluated whether standardized mean differences could be generated for at least one ABR latency component. We did not consider papers reporting only: 1) odds of ABR abnormality (N=2) (Cohen, Gardner et al. 2013, Demopoulos and Lewine 2015), because definitions for abnormality were non-comparable across studies, or 2) ABRs acquired via binaural stimulation, which are non-comparable to ABRs acquired monaurally (N=1) (Rosenblum, Arick et al. 1980). In total, we included 15 papers in this meta-analysis, all of which had a stated objective of assessing differences in ABR components between ASD and TD groups (Figure 1) (Taylor, Rosenblatt et al. 1982, Gillberg and Gillberg 1983, Rumsey, Grimes et al. 1984, Grillon, Courchesne et al. 1989, Sersen, Heaney et al. 1990, Wong and Wong 1991, Tharpe, Bess et al. 2006, Kwon, Kim et al. 2007, Tas, Yagiz et al. 2007, Russo, Nicol et al. 2009, Fujikawa-Brooks, Isenberg et al. 2010, Magliaro, Scheuer et al. 2010, Dabbous 2012, Roth, Muchnik et al. 2012, Miron, Roth et al. 2016). Because our analyses utilized published, aggregate-level data, our study is considered exempt by the Michigan State University Institutional Review Board.
Figure 1.
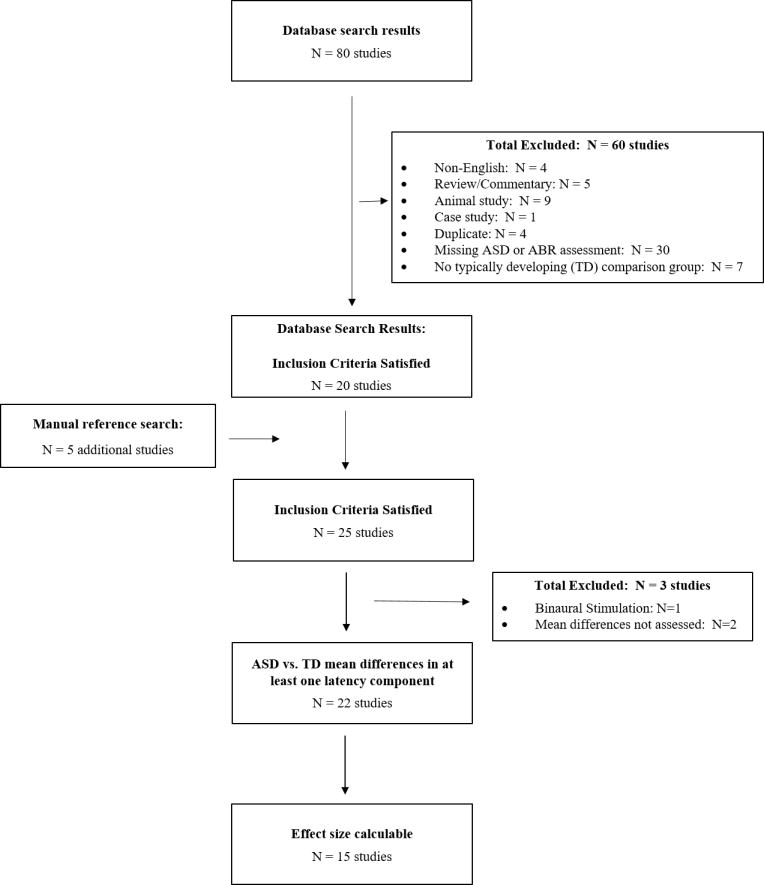
ABR components and effect size scoring
Well-characterized ABR components include waves I, III, & V, which can be reliably generated and measured across the lifespan (Jerger and Hall 1980, Skoe, Krizman et al. 2015). ABRs yield amplitudes and latencies that may reflect the processes of neuronal synchronization and myelination. However, only one study enabled effect size calculation for amplitudes (Grillon, Courchesne et al. 1989). Thus, ABR parameters of interest here include absolute (I, III, & V) and inter-peak latencies (IPLs: I–III, III–V, I–V). Absolute and inter-peak latencies differ in that the former is derived from the onset of the click (thus involving conduction and transduction) whereas the latter is derived from the onset of a particular wave.
We estimated effect sizes using Hedges’s g, a standardized mean difference score corrected for inflation due to small sample sizes. Hedges’ g is interpreted similarly to Cohen’s d, with estimates of 0.2, 0.5, and 0.8 corresponding to small, medium, and large effects, respectively. Study- and component-specific estimates of Hedges’s g were calculated to reflect latency differences between ASD and TD participants (g>0: ASD latency>TD latency; g<0: ASD latency<TD latency). To generate one estimate per parameter per study, effect sizes were weighted and averaged across all variable conditions (e.g., ear of stimulation) and subsets of participants (Card 2011). Exceptions included Fujikawa et al. (2010), from which we only utilized the 61/sec condition, and Miron et al. (2016), from which we only utilized infant data (see below). The first author abstracted the papers and calculated effect sizes (at the study- and component-level) on two separate occasions to identify and resolve any discrepancies. Disaggregated effect sizes by study and component are summarized in eTable 1.
Moderator Variables
We abstracted various study characteristics to characterize heterogeneity in effects across studies and address conceptual gaps in the literature. A summary of the study characteristics and coding decisions, generated by two independent abstractors, is reported in Table 1. We did not model preterm delivery as a moderator because perinatal health information was reported in only three of the studies included here (1 excluded preterm infants, 1 included preterm infants, and 1 excluded children with “infective prenatal conditions”) (Tas, Yagiz et al. 2007, Roth, Muchnik et al. 2012, Miron, Roth et al. 2016). A minimum of two studies was necessary to warrant interpretation of a specific moderator variable level.
Table 1.
Coding decisions for characteristics of studies included in the meta-analysis
| Age Group | Sex Matching | ASD group | ID | Middle Ear Abnormality | Elevated Auditory Threshold | Click Rate | Waves Available | |
|---|---|---|---|---|---|---|---|---|
| Taylor et al. (1982) | > 8 years | No | before DSM-IV1 | not reported | not reported | not excluded | < 27.5/s | I–III, III–V, I–V |
| Gillberg et al. (1983) | > 8 years | No | before DSM-IV2 | ASD only | not excluded | not excluded | not reported | V, I–V |
| Rumsey et al. (1984) | > 8 years | Yes | before DSM-IV | ASD only | not excluded | not excluded | < 27.5/s | III, I–III, I–V |
| Grillon et al. (1989) | > 8 years | Yes | before DSM-IV | none | not reported | excluded | < 27.5/s | I, III, V, I–III, III–V, I–V |
| Sersen et al. (1990) | > 8 years | Yes | before DSM-IV | ASD only | not reported | excluded | < 27.5/s | I, III, V |
| Wong and Wong (1991) | ≤ 8 years | No | before DSM-IV | ASD only | not reported | excluded | < 27.5/s | I, III, V, I–III, III–V, I–V |
| Tharpe et al. (2006) | > 8 years | Yes | DSM IV/IV-TR | ASD only | excluded | not excluded | < 27.5/s | I, III, V, I–III, III–V, I–V |
| Tas et al. (2007) | ≤ 8 years | No | DSM IV/IV-TR | not reported | excluded | not excluded | < 27.5/s | I, III, V, I–III, III–V, I–V |
| Kwon et al. (2007)3 | ≤ 8 years | Yes | DSM IV/IV-TR | not reported | not reported | not reported | < 27.5/s | I, III, V, III–V, I–V |
| Russo et al. (2009) | > 8 years | No | DSM IV/IV-TR4 | none | excluded | excluded | < 27.5/s | V |
| Magliaro et al. (2010) | > 8 years | No | DSM IV/IV-TR | not reported | excluded | excluded | < 27.5/s | I, III, V, I–III, III–V, I–V |
| Fujikawa et al. (2010) | > 8 years | No | DSM IV/IV-TR5 | ASD only | excluded | excluded | ≥ 27.5/s6 | I, III, V, I–III, III–V, I–V |
| Roth et al. (2012) | ≤ 8 years | Not Reported | DSM IV/IV-TR | not reported | excluded | excluded | ≥ 27.5/s | I, III, V, I–III, III–V, I–V |
| Dabbous et al. (2012)7 | ≤ 8 years | Yes 8 | DSM IV/IV-TR9 | not reported | excluded | excluded | ≥ 27.5/s | I, III, V, I–III, III–V, I–V |
| Miron et al. (2016) | ≤ 8 years10 | Yes | DSM IV/IV-TR | not reported | not reported | excluded | ≥ 27.5/s | I, III, V, I–III, III–V, I–V |
Note. ASD: Autism spectrum disorder; DSM: Diagnostic and Statistical Manual; ID: Intellectual disability; s: second; TD: typically developing
National Society for Autistic Children Criteria
Rutter (1978) criteria
ASD group only (n=71), because the AD group (n=20) was not described and appears to be a subset of this total
Parent-report substantiated by medical records
Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview-Revised (ADI-R)
Included only the ≥ 27.5/s group because click rate is a moderator variable in this analysis
All ASD cases exhibited “intolerance to noise” or hyperacusis
Derived from narrative text
Diagnosis by medical professional (criteria unspecified); child age within range defined by DSM-IV and article publication dates
Infancy condition only
Age at ABR assessment
Because neurodevelopmental processes impact ABR components up to 18 months of age (and perhaps again at preschool-age, adolescence, and middle to late adulthood) (Jerger and Hall 1980, Thivierge and Cote 1990, Skoe, Krizman et al. 2015, Spitzer, White-Schwoch et al. 2015), we grouped studies according to whether ABRs were assessed prior to 8 years of age. This corresponds to the age of peak prevalence for ASD (Yeargin-Allsopp, Rice et al. 2003) and occurs prior to the onset of salient pubertal events for most participants. When participant age ranges straddled this divide, the study was included in the ≥ 8 year old group. Miron et al. (2016) included separate toddler and infant samples. We only utilized the infant data from this study given that ABRs were likely assessed prior to the manifestation of behavioral-level ASD symptoms.
ASD case definition
We grouped studies according to whether ASD diagnoses were specified using criteria published prior to or following DSM-IV, the system that markedly broadened the conceptualization of the disorder (Volkmar, Reichow et al. 2014). For studies that did not report diagnostic criteria, this information was inferred by comparing the age range of the participants to the publication date for DSM-IV.
Intellectual Disability
Intellectual disability is a common comorbidity associated with ASD (Centers for Disease Control 2014). We evaluated whether studies characterized intellectual functioning, and if so, whether participants with ID were included in the ASD group, TD group, neither group, or both groups.
Sex matching
Males exhibit longer ABR latencies across the entire lifespan and are also more likely to have an ASD diagnosis compared to females. Thus, we classified studies according to whether the ASD and TD groups were matched on sex. Matching was inferred from the article’s text or if the calculated proportion of male participants was equivalent across the ASD and TD groups (i.e., ASD:TD ratio = 1.0).
Middle ear characterization
Middle ear abnormalities that impede conduction can lead to prolonged ABR latencies (particularly absolute latencies) (Gunnarson and Finitzo 1991, Hall and Grose 1993). Each study was evaluated to determine whether tympanometry and/or otoscopic examinations were performed, and if so, whether participants with abnormal findings were included or excluded from analysis.
Elevated auditory thresholds
Elevated auditory thresholds are associated with prolonged ABR latencies and are indicative of hearing loss (Jerger and Johnson 1988). Each study was evaluated to determine whether this information was reported and if so, whether participants with elevated thresholds were included or excluded from analysis.
Click rate
Click rates can be manipulated to exert varying levels of challenge to the auditory nerve, with faster click rates eliciting longer wave latencies across all ages and in the context of some demyelinating diseases (e.g., multiple sclerosis) (Jacobson, Murray et al. 1987, Jiang, Brosi et al. 2002). Studies were grouped according to whether they utilized rates above or below 27.5 clicks/second, because rates above this threshold have been associated with longer latencies in both neonates and adults (Jiang, Brosi et al. 1998). Although Fujikawa et al. (2010) utilized 2 different click rate conditions, we utilized data from the 61/second condition here to increase the sample size of the ≥ 27.5/second group.
Publication bias
We evaluated publication bias using Kendall’s tau and Eggert’s intercept, and interpreted significant findings on either test as indicative of bias (p<0.05, two-tailed). Because these tests may be underpowered (Card 2011), we also calculated the fail-safe N to estimate the minimum number of studies with an effect size of 0 needed to attenuate findings to non-significance.
Analytic Plan
We begin by providing an overview of the studies contributing to the meta-analysis. After generating one effect size per component per study, we performed random-effects regressions (one per component) to evaluate whether ABR latencies differed between ASD and TD participants. Next, we evaluated heterogeneity in these effects using the Q statistic (Lipsey and Wilson 2001). For components exhibiting significant heterogeneity, we used mixed-effects meta-regression to evaluate the contribution of each moderator to the variability in effects. Random effects variance was based upon methods of moments estimation. To adjust for multiple comparisons, we utilized a false discovery rate of 5% (corrected p=0.013, two-tailed) to minimize the impact of Type I error. Publication bias was evaluated only for parameters with significant effect sizes in the main (i.e., non-moderator) analysis.
Results
Of the 15 studies included in this meta-analysis, 14 employed cross-sectional designs and 1 employed a case-control design. The number of participants per study ranged from 16 to 167, and ages ranged between 3 months and 40 years (eAppendix). Six studies (40%) involved participants ≤ 8 years, nine studies (60%) utilized DSM-IV or DSM-IV-TR criteria to diagnose ASD, and seven studies (47%) matched the ASD and TD groups on sex (Table 1). Seven studies (47%) did not report any information on intellectual disability (ID), whereas six studies (40%) excluded ID only from the TD group. With respect to ABR acquisition protocols, seven studies (47%) excluded children with middle ear abnormalities and nine studies (60%) excluded children with hearing loss. A majority of studies (67%) employed click rates <27.5/second.
The number of studies contributing to component-specific effect size estimates varied from 11 (I; I–III; III–V) to 13 (V, I–V), with the number of participants ranging from 657 (I–III) to 862 (V) (Table 2). ASD was not associated with Wave I or III–V latencies. However, ASD was associated with longer latencies relative to TD counterparts for Waves III (g=0.5, 95%CI 0.1,0.9), V (g=0.7, 95%CI 0.3,1.1), I–III (g=0.7, 95%CI 0.2,1.2), and I–V (g=0.6, 95%CI 0.2,1.0). For all absolute and inter-peak latencies, we observed significant heterogeneity in these effects (all p< 0.001; eFigure 1A–1F).
Table 2.
Autism spectrum disorder and its association with wave specific click-evoked auditory brainstem responses
| Wave Latency | No. of Studies | Sample Size | Random Effects | Q | p | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| g | 95%CI | p | |||||
| I | 11 | 768 | 0.1 | (−0.3, 0.5) | 0.60 | 69.9 | < 0.001 |
| III | 12 | 818 | 0.5 | (0.1, 0.9) | 0.03 | 89.6 | < 0.001 |
| V | 13 | 862 | 0.7 | (0.3, 1.1) | 0.001 | 96.5 | < 0.001 |
| I–III | 11 | 657 | 0.7 | (0.2, 1.2) | 0.007 | 82.6 | < 0.001 |
| III–V | 11 | 728 | 0.3 | (−0.1, 0.6) | 0.093 | 39.0 | < 0.001 |
| I–V | 13 | 833 | 0.6 | (0.2, 1.0) | 0.001 | 75.9 | < 0.001 |
Note. g (Hedges’ g); Q (test for heterogeneity); p (p value)
Tables 3a and 3b summarize moderator analyses for absolute and inter-peak latencies, respectively. None of the moderators were associated with Wave I latencies. For Waves III and V, age ≤ 8 years at ABR assessment, utilization of DSM-IV/IV-TR diagnostic criteria, exclusion of participants with middle ear abnormalities or hearing loss, and click rates ≥ 27.5/sec were associated with longer latencies for ASD versus TD participants (0.7<g<1.0, all p<0.013). Sex matching was not associated with Wave III, but was associated with Wave V; specifically, ASD was associated with longer latencies in both the matched and unmatched groups (0.4<g<0.7, all p<0.013). This general pattern of findings was replicated for inter-peak latencies I–III and I–V (0.6<g<1.0, all p<0.013), except that associations: 1) were not observed with ASD diagnostic criteria for wave I–III, and 2) extended to include participants for whom the presence of middle ear abnormalities was not reported (I–III: g=0.8, 95%CI 0.2,1.3; I–V g=0.9, 95%CI 0.4,1.4). In addition, associations between ASD and IPL I–V latencies were attenuated among studies that matched on sex (I–V: g=0.3, 95%CI −0.1, 0.8, p>0.013). Exclusion of participants with middle ear abnormalities was the only factor associated with IPL III–V (g=0.7, 95%CI 0.3,1.0).
Table 3a.
Moderator variables and effect sizes according to click evoked auditory brainstem response (ABR) absolute latencies1
| I | III | V | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| k | N | g | 95% CI | k | N | g | 95%CI | k | N | g | 95%CI | |
| Age Group | ||||||||||||
| ≤ 8 years | 6 | 541 | 0.0 | (−0.5, 0.6) | 6 | 541 | 0.8 | (0.2, 1.4) * | 6 | 297 | 1.0 | (0.4, 1.6) * |
| > 8 years | 5 | 227 | 0.2 | (−0.4, 0.9) | 6 | 277 | 0.2 | (−0.4, 1.4) | 7 | 565 | 0.5 | (−0.1, 1.1) |
| Sex Matching | ||||||||||||
| Yes | 5 | 341 | −0.1 | (−0.6, 0.3) | 7 | 427 | 0.2 | (−0.2, 0.5) | 6 | 377 | 0.4 | (0.1, 0.7) * |
| No | 5 | 326 | 0.0 | (−0.4, 0.5) | 4 | 290 | 0.6 | (0.1, 1.1) | 6 | 384 | 0.7 | (0.4, 1.0) * |
| Not Reported | 1 | 101 | 1.6 | (1.1, 2.1) * | 1 | 101 | 2.3 | (1.4, 3.1) * | 1 | 101 | 2.8 | (2.3, 3.3) * |
| ASD definition | ||||||||||||
| pre-DSM IV | 3 | 277 | 0.0 | (−0.8, 0.8) | 4 | 327 | 0.0 | (-0.7, 0.8) | 3 | 165 | 0.4 | (-0.5, 1.3) |
| DSM IV/IV-TR | 8 | 491 | 0.1 | (−0.4, 0.6) | 8 | 491 | 0.7 | (0.2, 1.2) * | 10 | 697 | 0.8 | (0.3, 1.3) * |
| Intellectual disability | ||||||||||||
| not reported | 6 | 415 | 0.2 | (−0.4, 0.7) | 6 | 415 | 0.8 | (0.2, 1.4) * | 6 | 415 | 0.9 | (0.3, 1.5) * |
| none | 1 | 16 | 0.2 | (−1.4, 1.8) | 1 | 16 | −0.8 | (−2.3, 0.8) | 2 | 55 | 0.1 | (−1.1, 1.3) |
| ASD only | 4 | 337 | 0.0 | (−0.7, 0.7) | 5 | 387 | 0.3 | (−0.4, 0.9) | 5 | 392 | 0.7 | (0.0, 1.4) |
| Middle Ear Abnormality | ||||||||||||
| not reported | 5 | 458 | 0.0 | (−0.6, 0.6) | 5 | 458 | 0.3 | (−0.4, 0.9) | 5 | 458 | 0.6 | (−0.1, 1.3) |
| excluded | 6 | 310 | 0.2 | (−0.4, 0.8) | 6 | 310 | 0.8 | (0.3, 1.4) * | 7 | 349 | 0.8 | (0.2, 1.4) * |
| not excluded | 0 | 0 | --- | ----- | 1 | 50 | −0.7 | (−2.0, 0.6) | 1 | 55 | 0.7 | (−0.9, 2.3) |
| Elevated Auditory Threshold | ||||||||||||
| not reported | 1 | 121 | 0.1 | (−1.0, 1.2) | 1 | 121 | 0.3 | (−1.1, 1.6) | 1 | 121 | 0.6 | (−1.0, 2.2) |
| excluded | 8 | 569 | 0.1 | (−1.4, 1.6) | 8 | 569 | 0.7 | (0.2, 1.2) * | 9 | 608 | 0.8 | (0.2, 1.3) * |
| not excluded | 2 | 78 | 0.1 | (−0.4, 0.7) | 3 | 128 | −0.1 | (−0.9, 0.8) | 3 | 133 | 0.6 | (−0.4, 1.5) |
| Click rate | ||||||||||||
| < 27.5/s | 7 | 517 | 0.1 | (−0.4, 0.6) | 8 | 567 | 0.2 | (−0.2, 0.7) | 8 | 556 | 0.6 | (0.0, 1.1) |
| ≥ 27.5/s | 4 | 251 | 0.1 | (−0.6, 0.8) | 4 | 251 | 1.0 | (0.3, 1.6) * | 4 | 251 | 1.0 | (0.2, 1.8) * |
| Not reported | 0 | 0 | --- | ----- | 0 | 0 | --- | ----- | 1 | 55 | 0.7 | (−0.9, 2.2) |
Note. df (degrees of freedom); g (Hedges’ g); k (number of studies); N (number of participants); Q (test for heterogeneity); p (p value)
A minimum of two studies was necessary to warrant interpretation of a specific moderator variable level.
corrected p < 0.013 (false discovery rate = 5%)
Table 3b.
Moderator variables and effect sizes according to click evoked auditory brainstem response (ABR) inter-peak wave latencies1
| I–III | III–V | I–V | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| k | N | g | 95% CI | k | N | g | 95%CI | k | N | g | 95%CI | |
| Age Group | ||||||||||||
| ≤ 8 years | 5 | 420 | 1.0 | (0.3, 1.7) * | 6 | 541 | 0.3 | (−0.2, 0.7) | 6 | 541 | 0.9 | (0.4, 1.4) * |
| > 8 years | 6 | 237 | 0.4 | (−0.2, 1.0) | 5 | 187 | 0.2 | (−0.3, 0.7) | 7 | 292 | 0.4 | (−0.1, 0.9) |
| Sex Matching | ||||||||||||
| Yes | 5 | 212 | 0.5 | (−0.2, 1.1) | 5 | 283 | 0.1 | (−0.1, 0.6) | 6 | 333 | 0.3 | (−0.1, 0.8) |
| No | 5 | 344 | 0.6 | (−0.1, 1.2) | 5 | 344 | 0.5 | (0.0, 1.0) | 6 | 399 | 0.7 | (0.2, 1.1) * |
| Not calculable | 1 | 101 | 2.0 | (0.7, 3.3) * | 1 | 101 | 0.2 | (−1.0, 1.5) | 1 | 101 | 1.8 | (0.8, 2.8) * |
| ASD definition | ||||||||||||
| pre-DSM IV | 4 | 287 | 0.6 | (−0.3, 1.4) | 3 | 237 | 0.5 | (−0.1, 1.1) | 5 | 342 | 0.6 | (−0.1, 1.2) |
| DSM IV/IV-TR | 7 | 370 | 0.7 | (0.1, 1.4) | 8 | 491 | 0.2 | (−0.2, 0.5) | 8 | 491 | 0.7 | (0.2, 1.1) |
| Intellectual disability | ||||||||||||
| not reported | 6 | 293 | 0.9 | (0.3, 1.6) * | 7 | 469 | 0.2 | (−0.2, 0.6) | 7 | 469 | 0.7 | (0.2, 1.3) * |
| none | 1 | 16 | 1.0 | (−0.7, 2.7) | 1 | 16 | 0.0 | (−1.3, 1.3) | 1 | 16 | 0.6 | (−1.0, 2.2) |
| ASD only | 4 | 348 | 0.2 | (−0.6, 1.0) | 3 | 243 | 0.5 | (−0.1, 1.1) | 5 | 348 | 0.4 | (−0.2, 1.1) |
| Middle Ear Abnormality | ||||||||||||
| not reported | 4 | 297 | 0.8 | (0.2, 1.3) * | 5 | 444 | −0.1 | (−0.4, 0.3) | 5 | 418 | 0.9 | (0.4, 1.4) * |
| excluded | 6 | 310 | 0.9 | (0.2, 1.6) * | 6 | 284 | 0.7 | (0.3, 1.0) * | 7 | 365 | 0.6 | (0.2, 1.1) * |
| not excluded | 1 | 50 | −0.8 | (−2.2, 0.5) | 0 | 0 | --- | ---- | 1 | 50 | −0.7 | (−1.8, 0.4) |
| Elevated Auditory Threshold | ||||||||||||
| not reported | 0 | 0 | --- | ----- | 1 | 121 | 0.6 | (−0.5, 1.6) | 1 | 121 | 0.5 | (−0.7, 1.6) |
| excluded | 7 | 182 | 1.0 | (0.5, 1.5) * | 7 | 475 | 0.1 | (−0.3, 0.5) | 7 | 475 | 0.9 | (0.4, 1.4) * |
| not excluded | 4 | 475 | 0.1 | (−0.6, 0.8) | 3 | 132 | 0.6 | (−0.1, 1.3) | 5 | 237 | 0.2 | (−0.3, 0.8) |
| Click rate | ||||||||||||
| < 27.5/s | 7 | 406 | 0.5 | (−0.1, 1.1) | 7 | 477 | 0.4 | (0.0, 0.8) | 8 | 527 | 0.5 | (0.0, 1.0) |
| ≥ 27.5/s | 4 | 251 | 1.0 | (0.2, 1.8) * | 4 | 251 | 0.0 | (−0.5, 0.5) | 4 | 251 | 0.9 | (0.2, 1.6) * |
| Not reported | 0 | 0 | --- | ----- | 0 | 0 | --- | ----- | 1 | 55 | 0.4 | (−1.0, 1.7) |
Note. df (degrees of freedom); g (Hedges’ g); k (number of studies); N (number of participants); Q (test for heterogeneity); p (p value)
A minimum of two studies was necessary to warrant interpretation of a specific moderator variable level.
corrected p < 0.013 (false discovery rate = 5%)
We observed no evidence of publication bias across two indices assessing this effect (Kendall’s tau and Eggert’s test, all p>0.27; eTable 2). Approximately 119 (Wave III) to 290 (Wave V) studies with an effect size of zero would be required to attenuate main effects (Table 2) to non-significance.
Discussion
We performed a meta-analysis to assess the association between ASD and click-evoked ABRs and evaluated the impact of study characteristics that currently impede synthesis of the literature. We found that ASD was associated with longer ABR latencies relative to TD participants, particularly for waves III, V, I–III, and I–V. These associations were medium-to-large in size (0.5<g<0.7), but exhibited considerable heterogeneity. This variability was most consistently linked to participant age and ABR protocol characteristics.
For both absolute and inter-peak latencies, associations with ASD were limited to components involving neural transmission from the auditory nerve (wave I) to the cochlear nucleus (wave III). This raises the possibility that transmission involving wave I and wave III generators contribute to the findings observed here, given that no associations with wave I (click to auditory nerve) or III–V (cochlear nucleus to lateral lemniscus) were observed. Action potential velocity is determined primarily by degree of myelination, pathway length, and axonal diameter, but may also be influenced by the synchronization of neuronal firing or changes in synaptic efficacy (Eggermont 1988). To date, there is limited or equivocal evidence to suggest that these factors explain associations the findings observed here. For example, both hyper- and hypo-myelination of brainstem pathways have been linked to ASD (Hanaie, Mohri et al. 2016, Ouyang, Cheng et al. 2016), though we are unaware of studies that characterize these parameters for the central auditory pathway specifically. Furthermore, microscopic, imaging-based, and physiological findings that implicate brainstem-based anomalies in ASD do not necessarily mean that this brain region drives the complex neurological and behavioral features that accompany the disorder (e.g., weaker functional connectivity in frontal cortex; stronger cortical-subcortical connectivity; rapid sensory cortical expansion) (Minshew and Williams 2007, Hazlett, Gu et al. 2017). Indeed, longer ABR latencies associated with ASD may reflect activity of more distal brain regions that converge directly (e.g., corticofugal pathways) and/or indirectly (e.g., via the pons) on neural generators of the ABR. Disentangling how this diverse network of brain-based findings relate to one another is an important direction for future research.
Despite the medium-to-large effects observed at the aggregate-level, there was great variability in the magnitude and sometimes the direction of associations across individual studies. Moderator analyses suggested that effect size was related in part to ABR study characteristics – younger age at assessment, exclusion of participants with middle ear abnormalities or hearing loss, and faster click rates. With respect to age, cross-sectional findings suggest that ABR latencies decrease markedly during the first two years of life, decrease somewhat less steeply during preschool age, and then increase during middle childhood and adolescence to approach adult values, changes hypothesized to reflect brain-based developmental processes such as myelination, synaptogenesis, and pruning (Skoe, Krizman et al. 2015, Spitzer, White-Schwoch et al. 2015). Given these age-related changes, we repeated our analyses after classifying studies according to whether participants were assessed prior to 5 years; our results for waves III, V, I–III, and I–V were unchanged (0.9 < g < 1.3, all p < 0.013). Although it is unclear whether ABR assessment in early childhood is particularly sensitive to associations with ASD, age-related changes in ABR components underscore the importance of matching participants on this variable. One study did not match ASD and TD groups on age (Roth, Muchnik et al. 2012), and this might have contributed to the particularly large effects reported therein. However, when we excluded this study from the analysis, our main findings were altered by less than 0.2 across all components (data not shown). With respect to middle ear problems and elevated auditory thresholds, each are linked to ASD as well as longer ABR latencies (Stockard, Stockard et al. 1978, Gunnarson and Finitzo 1991, Moore, Hutchings et al. 1991, Hall and Grose 1993); however, estimates were larger following the exclusion of participants with these difficulties, suggesting that they do not account for the associations reported here. It is unclear why findings would strengthen when middle ear problems were excluded, particularly for inter-peak latencies, which do not incorporate conduction time. One possibility is that children with ASD are more likely to experience repeated occurrences of otitis media (OM) (Adams, Susi et al. 2016); repeated OM, in turn, has been linked with longer inter-peak latencies, even when middle ear problems are excluded at the time of the ABR assessment (Gunnarson and Finitzo 1991, Ferguson, Cook et al. 1998). Another possibility is that study characteristics such as exclusion of participants with middle ear problems are confounded by other factors that impact ABR latencies. For example, studies utilizing faster click rates often reported stronger associations between ABRs and ASD, but these studies were also likely to exclude participants with middle ear problems and hearing loss.
We then examined whether effect size heterogeneity was associated with ASD symptoms or comorbid conditions. When ASD was diagnosed using either DSM-IV or –IV-TR criteria, which greatly broadened the symptoms linked to the disorder, stronger associations with several ABR latencies were reported. In addition, stronger associations were observed among studies that did not report the presence of intellectual disability in either the ASD or TD groups. The latter finding likely reflects its almost exclusive co-occurrence with the use of DSM-IV or –IV-TR criteria (Table 1), as it is unclear why lack of reporting would be related to strength in association. Indeed, two studies using DSM-IV/-IV-TR criteria but excluded ID from either the ASD group or both groups reported effect sizes comparable to aggregate-level analyses (Russo, Nicol et al. 2009, Fujikawa-Brooks, Isenberg et al. 2010). Furthermore, a comparison between mutually exclusive groups of ASD and ID participants suggested that ABR latencies were significantly longer in the ASD group (Wong and Wong 1991). Combined with the fact that earlier DSM versions identified the most severely affected children in the ASD spectrum, evidence to date suggests that associations between ASD and ABRs may not be driven by comorbid ID. Relatedly, links between ABR findings and ASD symptom dimensions (e.g., sensory hyper-/hypo-sensitivity; social communication deficits; restricted/ repetitive behaviors) are scarce. However, emerging evidence suggests that ASD with hyperacusis may be associated with faster ABR latencies relative to both TD counterparts and ASD children without hyperacusis (Dabbous 2012, Thabet and Zaghloul 2013). For language, studies employing more complex auditory brainstem processing protocols (e.g., speech probes; forward masking) have observed concurrent and prospective links with receptive language functioning (Russo, Nicol et al. 2009, Chonchaiya, Tardif et al. 2013), a process that is disturbed in a subset of children with ASD. Links to social communication deficits or restricted behaviors are currently unknown and under-investigated. In the end, marked improvements in the scope and depth of behavioral assessment are required to fully probe any association between ASD symptom dimensions and auditory brainstem processing findings. Indeed, most studies utilized medical record abstraction to define diagnostic status, with only one utilizing gold-standard ASD assessments (e.g., ADOS) (Fujikawa-Brooks, Isenberg et al. 2010).
There are limitations and alternative explanations that are important to consider when interpreting our results. First, as mentioned earlier, study characteristics often co-occurred. Thus, it is unclear the extent to which individual sample or study characteristics investigated here are related to ABR latencies. Separating these effects represents an important direction for future research. Second, studies to date are almost entirely cross-sectional in nature, with ABR assessment and case ascertainment for ASD taking place concurrently. Resolving the temporality of associations is critical to determining whether ABRs have etiologic and/or prognostic value in relation to ASD. Although two recent studies with infants suggest that it is possible for ABR findings to precede ASD diagnosis (Cohen, Gardner et al. 2013, Miron, Roth et al. 2016), additional evidence is needed. Third, with the exception of IPL I–V, associations between ASD and ABR latencies persisted when analyses were limited to studies that matched on sex. However, the absolute prevalence of female participants across studies was low, and this precluded a direct evaluation of effect modification by sex. Thus, it is unclear whether the findings presented here generalize to females, and this represents a very important area for future investigation. Fourth, our analyses involved many comparisons. Even though we used false discovery rates to reduce the impact of Type I error, the findings generated from our moderator analyses are in particular need of replication. Fifth, our moderator analyses do not represent the full complement of factors that may affect the association between ASD and ABRs. For example, preterm delivery is associated with longer ABR latencies relative to full-term counterparts who are matched according to either chronological or corrected age (Jiang and Li 2015, Stipdonk, Weisglas-Kuperus et al. 2016) and preterm delivery is a well-described risk factor for ASD. However, only three studies here reported specific information regarding perinatal health; one excluded preterm children (Roth, Muchnik et al. 2012), one included preterm children (Miron, Roth et al. 2016), and one excluded children with “infective prenatal conditions”(Tas, Yagiz et al. 2007). In addition, no study reported information regarding birth size or head circumference, the latter of which is positively associated with ABR latencies and has been extensively investigated in relation to ASD. Determining whether associations between ABRs and ASD differ across risk factors for the disorder (e.g., perinatal health; family history of ASD diagnosis) may provide important insights regarding the interpretation and potential application of ABRs in risk surveillance or elucidation of symptom profiles. Relatedly, it will be important to evaluate the specificity of findings to neurodevelopmental disorders beyond ASD. Indeed, longer ABR latencies have been linked with attention deficit/hyperactivity disorder and cerebral palsy (Sano, Kaga et al. 2005, Azzam and Hassan 2010). Currently, no direct comparisons between ASD and these disorders have been evaluated within the context of the same study. For these and other reasons (e.g., the lack of prospectively gathered data), the relevance of click-based ABRs in predicting risk for ASD or elucidating symptom profiles associated with the disorder is decidedly uncertain.
In sum, ASD may be associated with longer click-evoked ABR latencies. Findings vary greatly across studies, but effect sizes reported to date are substantial. Future work that utilizes prospective designs and addresses outstanding conceptual limitations are vital to informing the etiologic or prognostic value of ABRs for ASD.
Supplementary Material
Lay Summary.
Auditory brainstem responses (ABR) may be associated with ASD, but participant characteristics and assessment protocols vary considerably across individual studies. Our goal is to combine the results across these studies to facilitate clarity on the topic. Doing so represents a first step in evaluating whether ABRs yield potential for informing the etiology of ASD risk and/or ASD symptom profiles.
Acknowledgments
This project was supported in part by a grant from the National Institutes of Health (R21 DC01550) to Nicole Talge. Dr. Talge also acknowledges Julie Markant, Ph.D. (Tulane University) for her insights while reviewing drafts of the manuscript and Isabella Frownfelter (MSU) for her reliability coding efforts.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Adams DJ, Susi A, Erdie-Lalena CR, Gorman G, Hisle-Gorman E, Rajnik M, Elrod M, Nylund CM. Otitis Media and Related Complications Among Children with Autism Spectrum Disorders. J Autism Dev Disord. 2016;46(5):1636–1642. doi: 10.1007/s10803-015-2689-x. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Jacobs-Stannard A, Chawarska K, Volkmar FR, Kliman HJ. Placental trophoblast inclusions in autism spectrum disorder. Biol Psychiatry. 2007;61(4):487–491. doi: 10.1016/j.biopsych.2006.03.068. [DOI] [PubMed] [Google Scholar]
- Azzam H, Hassan D. Auditory brainstem timing and cortical processing in attention deficit hyperactivity disorder. Curr Psychiatr. 2010;17(1):15–20. [Google Scholar]
- Card NA. Applied Meta-Analysis for Social Science Research. New York, NY: Guilford Press; 2011. [Google Scholar]
- Centers for Disease Control. Prevalence of autism spectrum disorder among children aged 8 years - Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(2):1–22. [PubMed] [Google Scholar]
- Chonchaiya W, Tardif T, Mai X, Xu L, Li M, Kaciroti N, Kileny PR, Shao J, Lozoff B. Developmental trends in auditory processing can provide early predictions of language acquisition in young infants. Dev Sci. 2013;16(2):159–172. doi: 10.1111/desc.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Gardner JM, Karmel BZ, Phan HT, Kittler P, Gomez TR, Gonzalez MG, Lennon EM, Parab S, Barone A. Neonatal brainstem function and 4-month arousal-modulated attention are jointly associated with autism. Autism Res. 2013;6(1):11–22. doi: 10.1002/aur.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Kennon-McGill S, Weichselbaum C, Marrus N, Haider A, Glowinski AL, Gillespie S, Klaiman C, Klin A, Jones W. Infant viewing of social scenes is under genetic control and is atypical in autism. Nature. 2017;547(7663):340–344. doi: 10.1038/nature22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167(11):1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Courchesne RY, Hicks G, Lincoln AJ. Functioning of the brain-stem auditory pathway in non-retarded autistic individuals. Electroencephalogr Clin Neurophysiol. 1985;61(6):491–501. doi: 10.1016/0013-4694(85)90967-8. [DOI] [PubMed] [Google Scholar]
- Dabbous AO. Characteristics of auditory brainstem response latencies in children with autism spectrum disorders. Audiol Med. 2012;10:122–131. [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20(3):775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Demopoulos C, Lewine JD. Audiometric Profiles in Autism Spectrum Disorders: Does Subclinical Hearing Loss Impact Communication? Autism Res. 2015 doi: 10.1002/aur.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. On the rate of maturation of sensory evoked potentials. Electroencephalogr Clin Neurophysiol. 1988;70(4):293–305. doi: 10.1016/0013-4694(88)90048-x. [DOI] [PubMed] [Google Scholar]
- Ferguson MO, Cook RD, Hall JW, 3rd, Grose JH, Pillsbury HC., 3rd Chronic conductive hearing loss in adults: effects on the auditory brainstem response and masking-level difference. Arch Otolaryngol Head Neck Surg. 1998;124(6):678–685. doi: 10.1001/archotol.124.6.678. [DOI] [PubMed] [Google Scholar]
- Fujikawa-Brooks S, Isenberg AL, Osann K, Spence MA, Gage NM. The effect of rate stress on the auditory brainstem response in autism: a preliminary report. Int J Audiol. 2010;49(2):129–140. doi: 10.3109/14992020903289790. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Gillberg IC. Infantile autism: a total population study of reduced optimality in the pre-, peri-, and neonatal period. J Autism Dev Disord. 1983;13(2):153–166. doi: 10.1007/BF01531816. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Akshoomoff N. Brainstem and middle latency auditory evoked potentials in autism and developmental language disorder. J Autism Dev Disord. 1989;19(2):255–269. doi: 10.1007/BF02211845. [DOI] [PubMed] [Google Scholar]
- Gunnarson AD, Finitzo T. Conductive hearing loss during infancy: effects on later auditory brain stem electrophysiology. J Speech Hear Res. 1991;34(5):1207–1215. doi: 10.1044/jshr.3405.1207. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH. The effect of otitis media with effusion on the masking-level difference and the auditory brainstem response. J Speech Hear Res. 1993;36(1):210–217. doi: 10.1044/jshr.3601.210. [DOI] [PubMed] [Google Scholar]
- Hanaie R, Mohri I, Kagitani-Shimono K, Tachibana M, Matsuzaki J, Hirata I, Nagatani F, Watanabe Y, Fujita N, Taniike M. White matter volume in the brainstem and inferior parietal lobule is related to motor performance in children with autism spectrum disorder: A voxel-based morphometry study. Autism Res. 2016;9(9):981–992. doi: 10.1002/aur.1605. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, Kuroda Y. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord. 1995;25(1):1–18. doi: 10.1007/BF02178163. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL, Constantino JN, Dager SR, Estes AM, Evans AC, Fonov VS, Gerig G, Kostopoulos P, McKinstry RC, Pandey J, Paterson S, Pruett JR, Schultz RT, Shaw DW, Zwaigenbaum L, Piven J Network I, Clinical S, Data Coordinating C, Image Processing C, Statistical A. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JT, Murray TJ, Deppe U. The effects of ABR stimulus repetition rate in multiple sclerosis. Ear Hear. 1987;8(2):115–120. doi: 10.1097/00003446-198704000-00009. [DOI] [PubMed] [Google Scholar]
- Jerger J, Chmiel R, Tonini R, Murphy E, Kent M. Twin study of central auditory processing disorder. J Am Acad Audiol. 1999;10(10):521–528. [PubMed] [Google Scholar]
- Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106(7):387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Jerger J, Johnson K. Interactions of age, gender, and sensorineural hearing loss on ABR Latency. Ear & Hearing. 1988;9:168–176. doi: 10.1097/00003446-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Jiang ZD. Maturation of peripheral and brainstem auditory function in the first year following perinatal asphyxia: a longitudinal study. J Speech Lang Hear Res. 1998;41(1):83–93. doi: 10.1044/jslhr.4101.83. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Brosi DM, Li ZH, Chen C, Wilkinson AR. Brainstem auditory function at term in preterm babies with and without perinatal complications. Pediatr Res. 2005;58(6):1164–1169. doi: 10.1203/01.pdr.0000183783.99717.2b. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Brosi DM, Wilkinson AR. Immaturity of electrophysiological response of the neonatal auditory brainstem to high repetition rates of click stimulation. Early Hum Dev. 1998;52(2):133–143. doi: 10.1016/s0378-3782(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Brosi DM, Wilkinson AR. Auditory neural responses to click stimuli of different rates in the brainstem of very preterm babies at term. Pediatr Res. 2002;51(4):454–459. doi: 10.1203/00006450-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Li ZH. Mild maturational delay of the brainstem at term in late preterm small-for-gestation age babies. Early Hum Dev. 2015;91(4):265–269. doi: 10.1016/j.earlhumdev.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Frazier TW, Keshavan MS, Minshew NJ, Hardan AY. A two-year longitudinal pilot MRI study of the brainstem in autism. Behav Brain Res. 2013;251:163–167. doi: 10.1016/j.bbr.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou RJ, Minshew NJ, Melhem NM, Keshavan MS, Hardan AY. Brainstem volumetric alterations in children with autism. Psychol Med. 2009;39(8):1347–1354. doi: 10.1017/S0033291708004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JM, Ventola PE, Pandey J, Verbalis AD, Barton M, Hodgson S, Green J, Dumont-Mathieu T, Robins DL, Fein D. Diagnostic stability in very young children with autism spectrum disorders. J Autism Dev Disord. 2008;38(4):606–615. doi: 10.1007/s10803-007-0427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Kim J, Choe BH, Ko C, Park S. Electrophysiologic assessment of central auditory processing by auditory brainstem responses in children with autism spectrum disorders. J Korean Med Sci. 2007;22(4):656–659. doi: 10.3346/jkms.2007.22.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhu L, Mai X, Shao J, Lozoff B, Zhao Z. Sex and gestational age effects on auditory brainstem responses in preterm and term infants. Early Hum Dev. 2013;89(1):43–48. doi: 10.1016/j.earlhumdev.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Magliaro FC, Scheuer CI, Assumpcao FB, Junior, Matas CG. Study of auditory evoked potentials in autism. Pro Fono. 2010;22(1):31–36. doi: 10.1590/s0104-56872010000100007. [DOI] [PubMed] [Google Scholar]
- Mason JA, Herrmann KR. Universal infant hearing screening by automated auditory brainstem response measurement. Pediatrics. 1998;101(2):221–228. doi: 10.1542/peds.101.2.221. [DOI] [PubMed] [Google Scholar]
- Maziade M, Merette C, Cayer M, Roy MA, Szatmari P, Cote R, Thivierge J. Prolongation of brainstem auditory-evoked responses in autistic probands and their unaffected relatives. Arch Gen Psychiatry. 2000;57(11):1077–1083. doi: 10.1001/archpsyc.57.11.1077. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron O, Roth D, Gabis LV, Henkin Y, Shefer S, Dinstein I, Geva R. Prolonged auditory brainstem responses in infants with autism. Autism Res. 2016;9(6):689–695. doi: 10.1002/aur.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Hutchings ME, Meyer SE. Binaural masking level differences in children with a history of otitis media. Audiology. 1991;30(2):91–101. doi: 10.3109/00206099109072874. [DOI] [PubMed] [Google Scholar]
- Moore JK. The human auditory brain stem as a generator of auditory evoked potentials. Hear Res. 1987a;29(1):33–43. doi: 10.1016/0378-5955(87)90203-6. [DOI] [PubMed] [Google Scholar]
- Moore JK. The human auditory brain stem: a comparative view. Hear Res. 1987b;29(1):1–32. doi: 10.1016/0378-5955(87)90202-4. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, Mandell DS, Miller LA, Pinto-Martin J, Reaven J, Reynolds AM, Rice CE, Schendel D, Windham GC. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Odom SL, Boyd BA, Hall LJ, Hume K. Evaluation of comprehensive treatment models for individuals with autism spectrum disorders. J Autism Dev Disord. 2010;40(4):425–436. doi: 10.1007/s10803-009-0825-1. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Cheng H, Mishra V, Gong G, Mosconi MW, Sweeney J, Peng Y, Huang H. Atypical age-dependent effects of autism on white matter microstructure in children of 2–7 years. Hum Brain Mapp. 2016;37(2):819–832. doi: 10.1002/hbm.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton CW, Moore JK, Eggermont JJ. Auditory brain stem response generation by parallel pathways: differential maturation of axonal conduction time and synaptic transmission. Ear Hear. 1996;17(5):402–410. doi: 10.1097/00003446-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Rodier PM. Converging evidence for brain stem injury in autism. Dev Psychopathol. 2002;14(3):537–557. doi: 10.1017/s0954579402003085. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370(2):247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord. 2014;44(12):2981–2995. doi: 10.1007/s10803-014-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Happe F, Price TS, Baron-Cohen S, Plomin R. Phenotypic and genetic overlap between autistic traits at the extremes of the general population. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1206–1214. doi: 10.1097/01.chi.0000230165.54117.41. [DOI] [PubMed] [Google Scholar]
- Rosenblum SM, Arick JR, Krug DA, Stubbs EG, Young NB, Pelson RO. Auditory brainstem evoked responses in autistic children. J Autism Dev Disord. 1980;10(2):215–225. doi: 10.1007/BF02408472. [DOI] [PubMed] [Google Scholar]
- Roth DA, Muchnik C, Shabtai E, Hildesheimer M, Henkin Y. Evidence for atypical auditory brainstem responses in young children with suspected autism spectrum disorders. Dev Med Child Neurol. 2012;54(1):23–29. doi: 10.1111/j.1469-8749.2011.04149.x. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Grimes AM, Pikus AM, Duara R, Ismond DR. Auditory brainstem responses in pervasive developmental disorders. Biol Psychiatry. 1984;19(10):1403–1418. [PubMed] [Google Scholar]
- Russo N, Nicol T, Trommer B, Zecker S, Kraus N. Brainstem transcription of speech is disrupted in children with autism spectrum disorders. Dev Sci. 2009;12(4):557–567. doi: 10.1111/j.1467-7687.2008.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Kaga K, Kitazumi E, Kodama K. Sensorineural hearing loss in patients with cerebral palsy after asphyxia and hyperbilirubinemia. Int J Pediatr Otorhinolaryngol. 2005;69(9):1211–1217. doi: 10.1016/j.ijporl.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Sersen EA, Heaney G, Clausen J, Belser R, Rainbow S. Brainstem auditory-evoked responses with and without sedation in autism and Down’s syndrome. Biol Psychiatry. 1990;27(8):834–840. doi: 10.1016/0006-3223(90)90464-d. [DOI] [PubMed] [Google Scholar]
- Skoe E, Krizman J, Anderson S, Kraus N. Stability and plasticity of auditory brainstem function across the lifespan. Cereb Cortex. 2015;25(6):1415–1426. doi: 10.1093/cercor/bht311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer E, White-Schwoch T, Carr KW, Skoe E, Kraus N. Continued maturation of the click-evoked auditory brainstem response in preschoolers. J Am Acad Audiol. 2015;26(1):30–35. doi: 10.3766/jaaa.26.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipdonk L, Weisglas-Kuperus N, Franken M, Nasserine K, Dudink J, Goedegebure A. Auditory brainstem maturation in normal hearing infants born preterm: a meta-analysis. Dev Med Child Neurol. 2016;58:1009–1015. doi: 10.1111/dmcn.13151. [DOI] [PubMed] [Google Scholar]
- Stockard JJ, Stockard JE, Sharbrough FW. Nonpathologic factors influencing brainstem auditory evoked potentials. Am J EEG Technol. 1978;18:177–209. [Google Scholar]
- Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA, Lein ES, Courchesne E. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370(13):1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas A, Yagiz R, Tas M, Esme M, Uzun C, Karasalihoglu AR. Evaluation of hearing in children with autism by using TEOAE and ABR. Autism. 2007;11(1):73–79. doi: 10.1177/1362361307070908. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Rosenblatt B, Linschoten L. Auditory brainstem response abnormalities in autistic children. Can J Neurol Sci. 1982;9:429–433. doi: 10.1017/s0317167100044346. [DOI] [PubMed] [Google Scholar]
- Thabet EM, Zaghloul HS. Auditory profile and high resolution CT scan in autism spectrum disorders children with auditory hypersensitivity. Eur Arch Otorhinolaryngol. 2013;270(8):2353–2358. doi: 10.1007/s00405-013-2482-4. [DOI] [PubMed] [Google Scholar]
- Tharpe AM, Bess FH, Sladen DP, Schissel H, Couch S, Schery T. Auditory characteristics of children with autism. Ear Hear. 2006;27(4):430–441. doi: 10.1097/01.aud.0000224981.60575.d8. [DOI] [PubMed] [Google Scholar]
- Thivierge J, Cote R. Brain-stem auditory evoked response: normative values in children. Electroencephalogr Clin Neurophysiol. 1990;77:309–313. doi: 10.1016/0168-5597(90)90069-p. [DOI] [PubMed] [Google Scholar]
- Tick B, Bolton P, Happe F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57(5):585–595. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Reichow B, Westphal A, Mandell DS, editors. Handbook of Autism and Pervasive Developmental Disorders. 4. Hoboken, NJ: Wiley; 2014. Autism and the autism spectrum: Diagnostic concepts. [Google Scholar]
- Wallace KS, Rogers SJ. Intervening in infancy: implications for autism spectrum disorders. J Child Psychol Psychiatry. 2010;51(12):1300–1320. doi: 10.1111/j.1469-7610.2010.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hear Res. 2005;207(1–2):1–9. doi: 10.1016/j.heares.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Wong V, Wong SN. Brainstem auditory evoked potential study in children with autistic disorder. J Autism Dev Disord. 1991;21(3):329–340. doi: 10.1007/BF02207329. [DOI] [PubMed] [Google Scholar]
- Yang EY, Stuart A, Stenstrom R, Green WB. Test-retest variability of the auditory brainstem response to bone-conducted clicks in newborn infants. Audiology. 1993;32(2):89–94. doi: 10.3109/00206099309071859. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, Kau A, Klin A, Lord C, Landa R, Rogers S, Sigman M. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. J Autism Dev Disord. 2007;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


