Abstract
Although the clinical application of opioids for pain management is often hindered by undesired behavioral impairment, preclinical assays of antinociception typically do not provide information regarding the behaviorally disruptive effects of opioids that may accompany their antinociceptive effects. To address this, we modified a warm water tail withdrawal procedure to determine concurrently the effects of opioids on tail withdrawal latency (antinociception) and indices of food-maintained operant behavior (rates of responding and reinforcement density) in squirrel monkeys. Six opioid agonists were tested, and all produced dose-dependent antinociception and impairment of operant behavior. The ratio of ED50 values for both measures (behavioral impairment:antinociception) was used as a quantitative measure of therapeutic index. Nalbuphine had the highest ED50 ratio (4.88), reflecting antinociception with minimal behavioral disruption. Oxycodone, heroin, buprenorphine and methadone all produced similar ED50 ratios (0.82-1.14), whereas butorphanol yielded a significantly lower ED50 ratio (0.17) reflecting behavioral disruption at doses producing only minimal antinociception. The antinociceptive and behaviorally disruptive effects of oxycodone and buprenorphine were further characterized using Schild analysis to calculate apparent pA2 values for antagonism of the two drugs by naltrexone. These analyses suggest that μ-receptor mechanisms likely mediate both the antinociceptive and behaviorally disruptive effects of oxycodone (pA2 values: 8.13 and 8.57) and buprenorphine (pA2 values: 8.6 and 7.9).
Keywords: Opioid, antinociception, schedule-controlled behavior, oxycodone, squirrel monkey
Introduction
Opioid analgesics, which remain the primary treatment for moderate to severe pain, can exert other effects, such as sedation or reduced consciousness, that impair behavior and, depending on the circumstance, may thereby constrain their therapeutic utility5. In some instances, e.g., post-operative recovery, behavioral impairment may not be a problem and, indeed, opioid-induced sedation may be somewhat desirable. On the other hand, sedative effects may limit medication compliance in certain patient populations and, generally, are undesirable in the workplace and other non-hospital settings.44, 45
Both the sedative and antinociceptive effects of clinically useful opioid analgesics are mediated via μ-opioid mechanisms,18 and the presentation of such effects may depend on pharmacodynamic factors including receptor selectivity and efficacy that can vary among drugs. However, few studies have examined opioid analgesics with the intention of comparing the relative potency with which they produce antinociceptive and behaviorally disruptive effects,25, 38 and fewer still have concurrently evaluated these effects in the same subjects. This is somewhat surprising because effective analgesia without significant behavioral impairment remains a key challenge in pain management.9 Understanding the degree of behavioral impairment that may accompany the antinociceptive effects of opioid analgesics in preclinical studies should provide valuable information that can provide a rational basis for selecting among opioid medications depending on clinical setting and therapeutic goals.
While many preclinical assays of opioid antinociception are available (see Le Bars et al.24 for review), the warm water tail withdrawal assay remains the most commonly used in both rodent and primate species, and was first used to assess antinociception in monkeys by Dykstra and Woods.13 The present report describes a modification of this assay to allow concurrent assessment of both the antinociceptive and behaviorally disruptive effects of opioid analgesics. In this procedure, squirrel monkeys responded for a palatable food reinforcer in daily sessions during which the subject’s tail was immersed in hot water at selected times; the latency to withdraw the tail from the water was defined as a nociceptive response. Drugs studied included opioid full agonists (oxycodone, heroin, methadone) and partial or mixed-action agonists (buprenorphine, butorphanol, nalbuphine). Although each opioid produced significant antinociception (increase in tail withdrawal latencies) and, as well, significant behavioral impairment (decreases in response rate and reinforcement density), the magnitude of impairment and the ratio of ED50 values for antinociception and behavioral impairment varied considerably among drugs, supporting the utility of this approach as a measure of behavioral selectivity.
Methods
Subjects
Six adult male squirrel monkeys (Saimiri sciureus; 950-1050g; ranging from 11-16 years) were housed within a climate-controlled vivarium with a 12-hr light/dark cycle (lights on, 7 a.m.–7 p.m.) in the McLean Hospital Animal Care Facility (licensed by the U.S. Department of Agriculture and compliant with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, Commission on Life Sciences, National Research Council; 2011). Subjects were fed a high protein primate chow (Purina Monkey Chow, St. Louis, MO) supplemented with fruit and multivitamins and, except during testing, had unrestricted access to water in the home cage. Food intake was not restricted in the present studies; after daily weighing, diets were adjusted as needed to maintain stable body weights. Subjects had varying experimental histories involving treatment with drugs from different pharmacological classes, but had not received any drug injections or participated in any studies for at least 3 months prior to the present research. No subject previously had been involved in studies of nociception.
Throughout the present studies, the distal 10 cm of each subject’s tail was shaved regularly (2-3 × per week) to provide a cleared portion for tail-withdrawal experiments. The tail was inspected visually at the end of every session and there was no sign of injury or irritation throughout the duration of the study. Vaseline was applied post-session to ensure the area was moisturized, Behavioral experiments were conducted three to five days a week (Monday to Friday between 8:00 a.m. and 6:00 p.m.) under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at McLean Hospital. For each subject, drug test sessions were conducted no more frequently than twice per week and only following a training session in which response rate, reinforcement density and tail withdrawal latency were within the range of overall control values; single cycle 30-min training sessions were conducted on intervening days. Thus, a test session was conducted only following a training session in which the subject maintained its tail in 35°C water for ≥ 20 sec throughout all trials and gained at least 50 reinforcers in the 30-min session.
Apparatus
During experimental sessions, monkeys were seated in customized Plexiglas chairs that allowed their tails to hang freely behind the chair. While seated, subjects faced the chair’s front panel which was outfitted with two pairs of colored stimulus lights at eye level and a response lever below each set of lights. A receptacle was situated between the response levers; small volumes of sweetened condensed milk could be delivered into the receptacle via PE tubing connected to a pump outside the chamber. Responding on the left lever was reinforced under the schedule described below (“active” lever) whereas responding on the right lever had no scheduled consequences (“inactive” lever). During the session, each press of the active lever with a force of at least 0.2 N (lever press) produced an audible click of a relay and was recorded as a response.
Procedure
Experimental Sessions
Subjects were trained to respond under a fixed-ratio 10-response (FR10) schedule of food reinforcement (0.15ml of 30% sweetened condensed milk in water). Under this schedule, completion of 10 responses on the active lever during the illumination of red stimulus lights turned off the lights, triggered milk delivery and initiated a timeout (TO) period of 30-sec during which all stimulus lights remained off and responding had no scheduled consequences. After the 30-sec TO, the stimulus lights were re-illuminated and programmed schedule contingencies were again in effect. A 20-sec limited hold (LH) was imposed on the FR10 schedule requirement; that is, the elapse of 20-sec before the completion of 10 responses turned off stimulus lights and initiated the 30-sec TO but did not trigger milk delivery. Tail withdrawal latencies were measured, as described below, during each of the 30-sec TO periods.
Experimental sessions comprised four or five sequential cycles of the basic procedure described above to permit cumulative drug dosing during test sessions. During test sessions, each cycle began with a 10-min long timeout (LTO) during which no lights were on and responding had no programmed consequences. A cumulative dose of drug or injection of vehicle was administered shortly after the onset of the 10-min LTO. After the 10-min LTO elapsed, stimulus lights were illuminated, initiating a 5-min response component during which the FR10 schedule of food reinforcement was in effect. Upon the passage of the 5-min response component, stimulus lights were turned off, initiating the next cycle of the session.
Antinociception studies
Tap water first was heated on a hot plate to the desired temperature and then transferred to a plastic insulated thermos, which could be held by the experimenter. While the subject was seated in the customized chair with its tail hanging freely, the thermos was moved up and over the shaved distal portion of the subject’s tail to immerse approximately 8cm of the tail in either 35, 45, 50 or 55°C water in initial temperature-effect determinations, or 35 or 55°C water in antinociception studies. The latency for the subject to completely withdraw its tail from the thermos was measured with a stop watch and recorded by the experimenter. In the event the tail was not withdrawn from 35 or 45°C water within 20 seconds or from 50 or 55°C water within 10 seconds, the test was ended by removing the thermos and the tail withdrawal latency was recorded as maximum effect. The 10-sec maximum was imposed to eliminate any possibility of tissue damage following multiple tail dips. Different temperatures were tested in random order across monkeys, with the proviso that each temperature above 35°C was tested once every 15 minutes (once per cycle; see below) and followed by a test at 35°C. Following initial temperature-effect determinations, tails were submerged only in 35 or 55°C water in all subsequent studies of antinociception.
To study antinociceptive effects of drugs, the latency to withdraw the tail from 55°C water was measured in each subject after treatment with vehicle or different doses of test drugs. Briefly, the subject’s tail was immersed in water (35 or 55°C) during each of the 30-sec TOs of the 5-min component. The latency to withdrawal from 55°C water was tested only once in each component and the cycle in which it was tested varied irregularly across consecutive components of the session and, for each drug, across monkeys; all other tail immersions during each component were at 35°C. Tail immersion in 55°C water occurred only when control values were obtained in the immediately preceding immersion in 35°C. This latter provision ensured that the subsequent withdrawal of the tail from 55°C water reflected a nociceptive response. The LH contingency insured that the number of determinations per component ranged from six to ten, depending on the number of reinforcement deliveries during the component. Each drug was studied by administering cumulative i.m. doses shortly after the onset of the 10-min LTO periods. Data from sessions in which sequential injections of saline vehicle were administered i.m. across components provided baseline control values. Training and test sessions typically comprised four or five cycles but were discontinued prior to completion of the 4th cycle if response rates during the preceding response component were below 0.2 responses per sec.
Two sets of experiments were conducted in the present study. First, the effects of drugs on both tail withdrawal latency and food-maintained performance were examined to compare the effects of opioids differing in selectivity and/or efficacy on both measures (n=4). Drugs included the μ-opioid full, or high-efficacy, agonists oxycodone (0.032-0.56 mg/kg), heroin (0.01-0.32 mg/kg) and methadone (0.032-1.0 mg/kg) and the mixed-action μ-opioid partial, or low-efficacy, agonists buprenorphine (0.0032-0.1 mg/kg), butorphanol (0.01-0.32 mg/kg) and nalbuphine (0.1-3.2 mg/kg). All drugs were administered using cumulative dosing to facilitate the collection of full dose-response data within single sessions. Based on preliminary observations and published data,7, 42 the 10-minute pretreatment time before each session cycle was sufficient to allow for onset of drug action; no significant drug degradation was expected during the four or five cumulative dosing cycles. Moreover, differences in time to onset were not expected to influence within-drug comparison of the effects of cumulative doses on the concurrently-determined behavioral measures. In a second set of experiments, antagonism of both the antinociceptive and behaviorally disruptive effects of oxycodone and buprenorphine by the opioid antagonist naltrexone (0.0032-0.1 mg/kg) was assessed to confirm common mechanisms of opioid action on both behavioral endpoints (n=5). Each dose of naltrexone was administered 10 minutes before the first dose of opioid agonist (i.e oxycodone or buprenorphine). Oxycodone was chosen because it is a widely prescribed opioid for analgesia and buprenorphine was studied due to its unique behavioral effects in squirrel monkeys (i.e. potent rate-decreasing effects not usually reported in other nonhuman primate studies33, 35, 36, 57).
Data Analysis
Overall rates of responding (resp/sec) were calculated for each cycle by dividing the number of lever-press responses emitted in the presence of stimulus lights by the time during which the stimulus lights were illuminated. Individual mean control values were calculated by averaging response rates obtained during the four components of the control sessions in which sequential injections of saline were administered. In order to assess tolerance that may have developed over the course of the study, the antinociceptive and behaviorally disruptive effects of oxycodone and buprenorphine were determined at the beginning and end of the experiments. There were no significant changes in the dose effect curves following repeated exposure to opioids, and the two determinations for each drug were averaged in individual subjects for subsequent analyses.
Statistical analysis was conducted with Prism version 5.02 (GraphPad Software Inc., San Diego, CA) with doses expressed as log-transformed values. ED50 values were calculated by linear interpolation for increases in tail withdrawal latency and decreases in response rate and reinforcement density (i.e., number of reinforcers per 5-minute response component) after agonists alone and after treatment with the μ-opioid antagonist naltrexone. The ED50 for decreases in response-rate for each drug was divided by the ED50 for its effects on tail withdrawal latency to provide an index of the behavioral selectivity of its antinociceptive effects. Similar ratios were also calculated using the ED50 for decreases in reinforcement density and the ED50 for increases in tail withdrawal latency. Group ED50 ratios for each behavioral measure were calculated from the average of individual ED50 values. Additionally, Schild analysis of data from naltrexone pretreatment studies was used to evaluate receptor mechanisms mediating the behavioral effects of oxycodone and buprenorphine in the present studies. Briefly, dose ratios were calculated by dividing the ED50 for the agonist studied in combination with naltrexone by the ED50 for the agonist alone. Schild analyses were conducted by plotting the log (dose ratio −1) as a function of the negative log of the molar dose of the antagonist; apparent pA2 values (with and without constraining slopes) were obtained from these plots2. Effects of drugs on response rates, reinforcement density and tail withdrawal latency were compared using one-way repeated measures analysis of variance, followed by Dunnett’s method of multiple comparison procedures; significance was set at p < 0.05.
Drugs
Oxycodone hydrochloride, nalbuphine hydrochloride and naltrexone hydrochloride were purchased from Sigma/RBI (Natick, MA). Heroin hydrochloride and buprenorphine hydrochloride were obtained from the National Institute on Drug Abuse (Rockville, MD). Methadone hydrochloride and butorphanol tartrate were obtained from, respectively, Eli Lilly (Indianapolos, IN) and Bristol Laboratories (Evansville, IN). Drugs were dissolved and diluted to desired concentrations in sterile saline. Drug doses are expressed in terms of their free-base weights. All drugs were administered by intramuscular (i.m.) injection into the calf or thigh muscle in volumes of 0.3 ml/kg body weight or less. The order of drugs tested varied among subjects. The acute effects of individual drugs and vehicles were studied using cumulative dosing procedures in which graded doses of each drug were administered in 0.25 or 0.5 log unit increments in sequential cycles of the test session.
Results
Baseline Nociception and Food-maintained Behavior
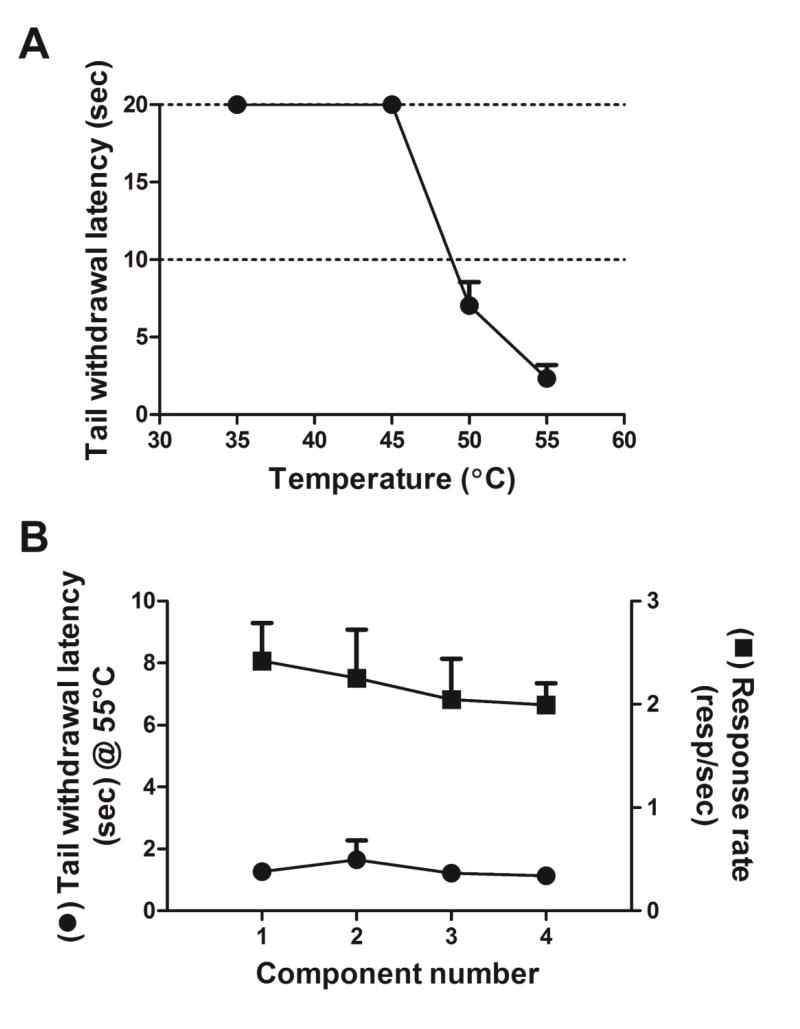
A reliable and orderly decrease in tail withdrawal latency occurred as a function of increased water temperature, with all subjects consistently keeping their tail immersed in 35°C and 45°C degree water for 20 seconds and all subjects consistently withdrawing their tail in less than 5 seconds when immersed in 55°C water (fig. 1a). Sequential i.m. injections of saline did not notably alter either tail withdrawal latency from 55°C water (1.6 ± 0.6 to 1.1 ± 0.1 sec) or food-maintained operant behavior across the four components of the test session (fig. 1b). Thus, mean rates of responding for the group of subjects ranged from 2.00 ± 0.21 to 2.42 ± 0.37 resp/s (fig. 1b) and reinforcement density was relatively invariant (ranging from 8.25 ± 0.75 to 8.75 ± 0.25 reinforcers per 5 min component), displaying no evidence of satiation throughout the test session.
Fig. 1.
A. Temperature-response functions of group mean for tail withdrawal latency (secs) as a function of water temperature. Error bars represent ± SEM and, when not visible, are contained within the symbol. No injections were given when determining temperature-response functions. Ordinate: Latency to withdraw tail (secs) from water. Abscissa: Water temperature (°C). B. Tail withdrawal latency and response rates following i.m. injections of saline over four components. Left ordinate: Latency to withdraw tail (secs) from 55°C water. Right ordinate: Number of responses/sec. Abscissa: Component number.
Effects of Opioid Agonists
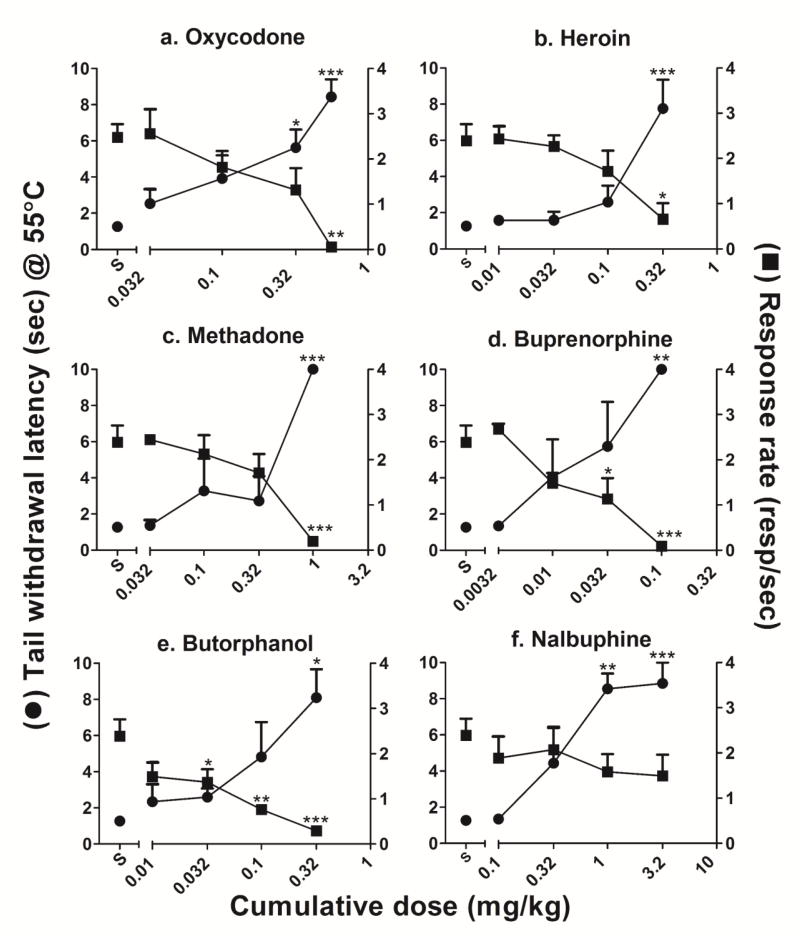
Fig. 2 presents the dose-response functions for tail withdrawal latency and rates of responding for each of the opioid agonists tested. Table 1 presents the corresponding numbers of reinforcers earned following each dose of opioid agonist. Oxycodone produced dose-dependent increases in tail withdrawal latency (F(4,15) = 7.94, P = 0.0012) and dose-dependent decreases in rates of responding (F(4,15) = 7.57, P = 0.0015). Decreases in rates of responding were accompanied by significant dose-dependent decreases in reinforcement density (F(4,12) = 133.3, P < 0.0001). The lowest dose of oxycodone (0.01 mg/kg) was without significant effect on tail withdrawal latency, rates of responding and reinforcement density, whereas the highest cumulative dose of oxycodone (0.56 mg/kg) produced highly significant increases in tail withdrawal latency and nearly abolished responding and reinforcement delivery. ED50 values for oxycodone’s effects on tail withdrawal latency, rates of responding and reinforcement density are presented in Table 2. The ratios of the ED50 values of behaviorally disruptive to antinociceptive effects for oxycodone (calculated by dividing either the ED50 for rates of responding or reinforcement density by the ED50 for tail withdrawal latency) were, respectively, 1.12 and 2.36.
Fig. 2.
Dose-response functions of group mean (±SEM) for the antinociceptive and response rate-disrupting effects following cumulative doses of a. oxycodone, b. heroin, c. methadone, d. buprenorphine, e. butorphanol and f. nalbuphine. Left ordinate: Latency to withdraw tail (secs) from 55°C water. Right ordinate: Number of responses/sec. Abscissa: cumulative dose of drug (mg/kg). * indicates P<0.05, ** indicates P<0.01, *** indicates P<0.001.
Table 1.
Number of reinforcers (± SEM) earned in a five-minute response component for each dose of agonist tested.
| Reinforcement density | ||||
|---|---|---|---|---|
| Oxycodone | 0.032 mg/kg | 0.1 mg/kg | 0.32 mg/kg | 0.56mg/kg |
| 9.25 ± 0.25 | 9.00 ± 0.00 | 8.67 ± 0.33 | 0.50 ± 0.50 | |
|
| ||||
| Heroin | 0.01 mg/kg | 0.032 mg/kg | 0.1 mg/kg | 0.32 mg/kg |
| 9.00 ± 0.00 | 9.00 ± 0.00 | 8.00 ± 0.71 | 3.75 ± 2.25 | |
|
| ||||
| Methadone | 0.032 mg/kg | 0.1 mg/kg | 0.32 mg/kg | 1.0 mg/kg |
| 9.00 ± 0.00 | 8.25 ± 0.75 | 8.00 ± 0.41 | 1.75 ± 1.03 | |
|
| ||||
| Buprenorphine | 0.0032 mg/kg | 0.01 mg/kg | 0.032 mg/kg | 0.1 mg/kg |
| 9.00 ± 0.00 | 8.25 ± 0.48 | 6.00 ± 2.12 | 1.00 ± 1.00 | |
|
| ||||
| Butorphanol | 0.01 mg/kg | 0.032 mg/kg | 0.1 mg/kg | 0.32 mg/kg |
| 8.00 ± 0.58 | 8.00 ± 0.58 | 6.00 ± 0.71 | 2.25 ± 1.03 | |
|
| ||||
| Nalbuphine | 0.1 mg/kg | 0.32 mg/kg | 1.0 mg/kg | 3.2 mg/kg |
| 8.00 ± 1.00 | 8.75 ± 0.48 | 8.00 ± 0.58 | 7.25 ± 1.03 | |
Table 2.
ED50 values for each behavioral measure for each agonist tested. ED50 ratios for antinociceptive to response rate disruptive effects and reinforcement density. ED50 values given are group means ± S.E.M in mg/kg and were determined from interpolation of individual (n=4) dose-response curves. * ED50 value (and therefore the ED50 ratio) was not determined due to the number of reinforcers remaining above 50% for 2 subjects despite significant disruption in response rates.
| Agonist | ED50 | Response rate disruption: tail withdrawal latency ratio | Reinforcement density: tail withdrawal latency ratio | ||
|---|---|---|---|---|---|
| Behavioral measure | |||||
| Oxycodone | |||||
| Tail withdrawal | 0.14 ± 0.09 | ||||
| Response rate disruption | 0.16 ± 0.04 | 1.12 | 2.36 | ||
| Reinforcement density | 0.34 ± 0.04 | ||||
|
| |||||
| Heroin | |||||
| Tail withdrawal | 0.18 ± 0.18 | ||||
| Response rate disruption | 0.15 ± 0.06 | 0.82 | 1.56 | ||
| Reinforcement density | 0.28 ± 0.20 | ||||
|
| |||||
| Methadone | |||||
| Tail withdrawal | 0.36 ± 0.10 | ||||
| Response rate disruption | 0.41 ± 0.07 | 1.14 | 1.22 | ||
| Reinforcement density | 0.44 ± 0.11 | ||||
|
| |||||
| Buprenorphine | |||||
| Tail withdrawal | 0.019 ± 0.01 | ||||
| Response rate disruption | 0.018 ± 0.004 | 0.98 | 1.93 | ||
| Reinforcement density | 0.036 ± 0.03 | ||||
|
| |||||
| Butorphanol | |||||
| Tail withdrawal | 0.13 ± 0.16 | ||||
| Response rate disruption | 0.02 ± 0.01 | 0.17 | 0.86 | ||
| Reinforcement density | 0.11 ± 0.04 | ||||
|
| |||||
| Nalbuphine | |||||
| Tail withdrawal | 0.39 ± 0.12 | ||||
| Response rate disruption | 1.91 ± 2.39 | 4.88 | ND* | ||
| Reinforcement density | ND* | ||||
Heroin produced dose-dependent increases in tail withdrawal latencies (F(4,15) = 9.9, P = 0.0004) and dose-dependent decreases in both rates of responding (F(4,15) = 4.57, P = 0.013) and reinforcement density (F(4,15) = 4.76, P = 0.011). The lowest dose of heroin (0.01 mg/kg) was without effect on tail withdrawal latency, rates of responding and reinforcement density, whereas the highest cumulative dose of heroin (0.32 mg/kg) caused a significant increase in the average tail withdrawal latency and significantly decreased both mean rates of responding and mean reinforcement density. The ED50 ratio values for heroin’s behaviorally disruptive and antinociceptive effects were 0.82 and 1.56 for, respectively, response rate and reinforcement density vs tail withdrawal latency (table 2).
Methadone also produced dose-dependent increases in tail withdrawal latency (F(4,15) = 12.49, P < 0.0001) and dose-dependent decreases in rates of responding (F(4,15) = 8.52, P = 0.0009) and reinforcement density (F(4,15) = 26.21, P < 0.0001). The lowest dose of methadone (0.032 mg/kg) was without antinociceptive effect whereas intermediate doses of methadone (0.1-0.32 mg/kg) resulted in an elevation of mean tail withdrawal latency that, due to individual variability, did not significantly differ from saline control values. The highest cumulative dose of methadone (1.0 mg/kg) resulted in maximum tail withdrawal latency (10 s) in all subjects, and significantly reduced both mean rates of responding and reinforcement density. Calculated ED50 ratios for methadone’s behaviorally disruptive and antinociceptive effects yielded values of 1.14 or 1.22 for, respectively, decreases in rates of responding or reinforcement density vs increases in tail withdrawal latency (table 2).
Like the full agonists described above, the mixed action μ-opioid partial agonist/κ-opioid antagonist buprenorphine produced dose-dependent increases in tail withdrawal latency (F(4,15) = 6.25, P = 0.0036) and dose-dependent decreases in both rates of responding (F(4,15) = 12.57, P = 0.0001) and reinforcement density (F(4,15) = 10.18, P= 0.0003). The lowest dose of buprenorphine (0.0032 mg/kg) was without significant effect on any measure compared to saline control, whereas effects of intermediate doses of buprenorphine (0.01 - 0.032 mg/kg) on tail withdrawal latency varied among subjects, yielding intermediate mean effects. For example, the cumulative dose of 0.01 mg/kg buprenorphine did not alter tail withdrawal latency in three of four subjects whereas the 0.5 log unit higher cumulative dose, 0.032 mg/kg, produced moderate increases in tail withdrawal latencies in two subjects and maximal antinociceptive effects (10 s) in the remaining two subjects. Less variability was observed in the behaviorally disruptive effects of buprenorphine. Thus, neither 0.0032 nor 0.01 mg/kg buprenorphine produced significant changes in rates of responding or reinforcement density whereas 0.032 mg/kg buprenorphine produced a significant decrease in mean response rates and a drop in reinforcement density compared to saline controls. The highest cumulative dose of buprenorphine (0.1 mg/kg) markedly increased tail withdrawal latency and nearly abolished responding in all four subjects. The ED50 ratios for buprenorphine’s behaviorally disruptive and antinociceptive effects yielded values of 0.98 or 1.93 for decreases in rates of responding or reinforcement density vs increases in tail withdrawal latency, respectively (table 2).
Butorphanol, a partial agonist at both μ- and κ-opioid receptors, produced dose-dependent increases in tail withdrawal latency (F(4,15) = 4.94, P = 0.0097) and significant behavioral disruption. Thus, decreases in response rates were evident even following cumulative doses that did not significantly increase tail withdrawal latencies (0.032 and 0.1 mg/kg). Reinforcement density was mildly decreased, compared to control values, following 0.032 mg/kg butorphanol and was significantly lowered by the cumulative dose of 0.1 mg/kg butorphanol. The highest cumulative dose of butorphanol (0.32 mg/kg) produced a significant increase in tail withdrawal latency and also markedly decreased rates of responding and reinforcement density in all subjects. The ED50 ratios for butorphanol’s behaviorally disruptive and antinociceptive effects were 0.17 and 0.86 for decreases in rates of responding or reinforcement density vs increases in tail withdrawal latency respectively (table 2).
Nalbuphine, a partial agonist at the μ-opioid receptor, produced dose-dependent increases in tail withdrawal latency in all four subjects (F(4,15) = 11.47, P = 0.0002). The lowest dose tested (0.1 mg/kg) had no effect on tail withdrawal latency in any subject whereas one subject reached the cutoff (10s) following an intermediate cumulative dose (0.32 mg/kg) of nalbuphine. There were significant increases in tail withdrawal latency in all four subjects following doses of 1.0 and 3.2 mg/kg nalbuphine. However, unlike the other opioid agonists, nalbuphine did not significantly alter rates of responding even following the highest cumulative dose of the μ-opioid partial agonist (3.2 mg/kg). Under these conditions, the ED50 ratio for nalbuphine’s behaviorally disruptive and antinociceptive effects was 4.88 for response rate disruption vs tail withdrawal latencies (table 2). Since the reinforcement density remained above 50% of saline control values in two subjects, the ED50 value (and ED50 ratio) was not calculated for reinforcement density.
Effects of Naltrexone Pretreatment
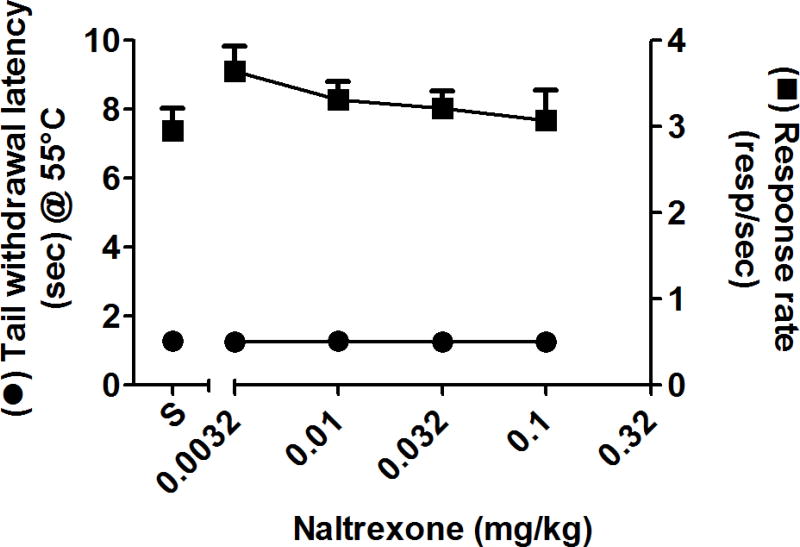
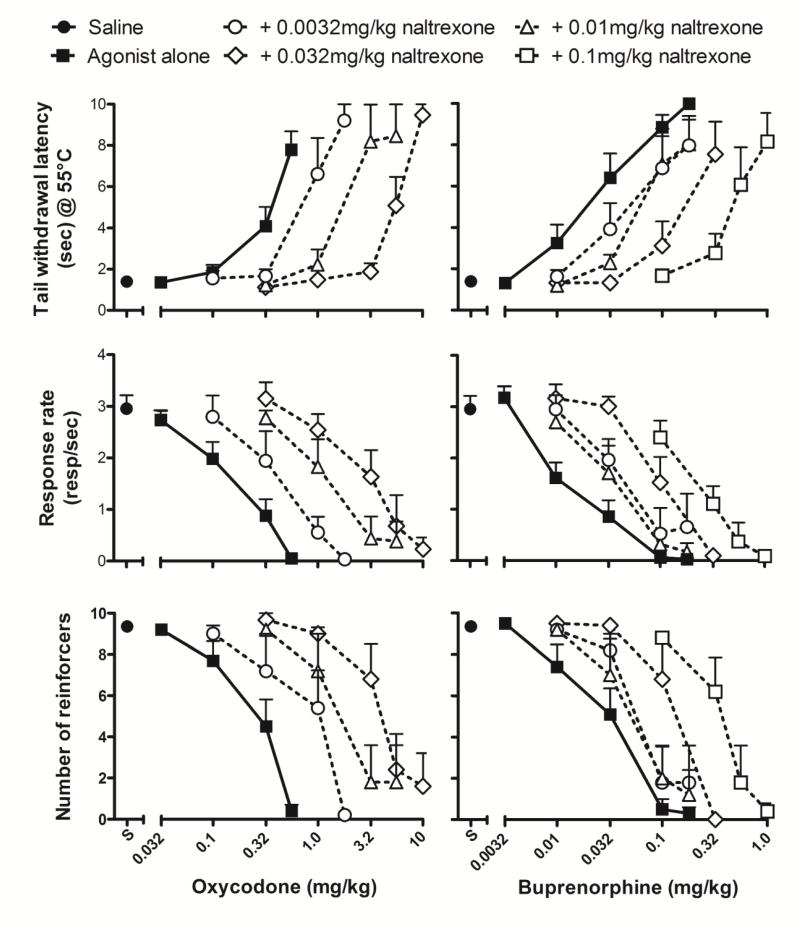
The effects of oxycodone and buprenorphine were redetermined in the presence of the opioid antagonist naltrexone. Tail withdrawal latency and food-maintained behavior were not altered by 0.0032-0.1 mg/kg naltrexone alone (fig. 3). Thus, mean rates of responding were within 85% of control values following treatment with 0.0032 and 0.1 mg/kg naltrexone, and mean tail-withdrawal latencies did not rise above 1.3 sec. However, doses of naltrexone without direct behavioral effects surmountably antagonized the antinociceptive and behaviorally disruptive effects of both opioid agonists, as indicated by increased ED50 values (table 3) and dose-dependent rightward shifts in the dose-response functions (fig. 4; oxycodone, left panels; buprenorphine, right panels). For example, in evaluating changes in tail withdrawal latency, 0.0032 - 0.032 mg/kg naltrexone produced approximately 2.3 - 17-fold rightward shifts in the oxycodone dose-response function and approximately 3.5 - 11-fold rightward shifts in the buprenorphine dose-response function. The highest pretreatment dose of naltrexone (0.1 mg/kg) shifted the dose-response function for buprenorphine’s effects on tail withdrawal latency slightly further to the right (approximately 15-fold). Naltrexone (0.0032 - 0.1 mg/kg) also surmountably antagonized the response rate decreasing effects of both opioids in a dose-related manner, producing approximately 4 - 28-fold and 1.5 - 6-fold rightward shifts in, respectively, oxycodone and buprenorphine dose-response functions. Orderly rightward shifts that similarly differed in magnitude for oxycodone and buprenorphine dose-response functions also were observed for reinforcement density (fig 4).
Fig. 3.
Dose-response functions of group mean (± SEM) for the antinociceptive effects and the response rates following cumulative doses of naltrexone. Left ordinate: Latency to withdraw tail (secs) from 55°C water. Right ordinate: Number of responses/sec. Abscissa: cumulative dose of naltrexone (mg/kg).
Table 3.
ED50 values for each behavioral measure of oxycodone and buprenorphine administered alone or after pretreatment with various doses of naltrexone. Values are given in mg/kg (±SEM).
| ED50 (mg/kg ± SEM) | |||||
|---|---|---|---|---|---|
|
| |||||
| Agonist alone |
+ naltrexone 0.0032 mg/kg |
+ naltrexone 0.01 mg/kg |
+ naltrexone 0.032 mg/kg |
+ naltrexone 0.1 mg/kg |
|
| Oxycodone | |||||
| Tail withdrawal | 0.29 ± 0.07 | 0.67 ± 0.19 | 1.74 ± 0.23 | 5.02 ± 0.87 | – |
| Response rate disruption | 0.12 ± 0.02 | 0.48 ± 0.14 | 1.57 ± 1.03 | 3.33 ± 1.49 | – |
| Reinforcement density | 0.22 ± 0.05 | 0.65 ± 0.19 | 1.26 ± 0.29* | 2.78 ± 0.64* | – |
|
| |||||
| Buprenorphine | |||||
| Tail withdrawal | 0.02 ± 0.01 | 0.07 ± 0.05 | 0.09 ± 0.17 | 0.22 ± 0.60 | 0.31 ± 0.85 |
| Response rate disruption | 0.02 ± 0.01 | 0.03 ± 0.03 | 0.04 ± 0.01 | 0.09 ± 0.02 | 0.11 ± 0.10 |
| Reinforcement density | 0.02 ± 0.01 | 0.05 ± 0.01* | 0.07 ± 0.10 | 0.12 ± 0.02 | 0.14 ± 0.10 |
n=4 (ED50 values could not be calculated for reinforcement density in one subject because despite a significant increase in tail withdrawal latency and a significant decrease in rates of responding, the reinforcement density remained above 50% of max reinforcers available).
Fig. 4.
Naltrexone antagonism of the antinociceptive (top), response rate-disrupting (middle) and reinforcement density-altering (bottom) effects of oxycodone (left) and buprenorphine (right). Top ordinate: Latency to withdraw tail (secs) from 55°C water. Middle ordinate: Number of responses/sec. Bottom ordinate: Number of reinforcers gained over 5 min response component. Abscissa: Cumulative dose of drug (mg/kg; oxycodone, left and buprenorphine, right).
Apparent pA2 Analysis
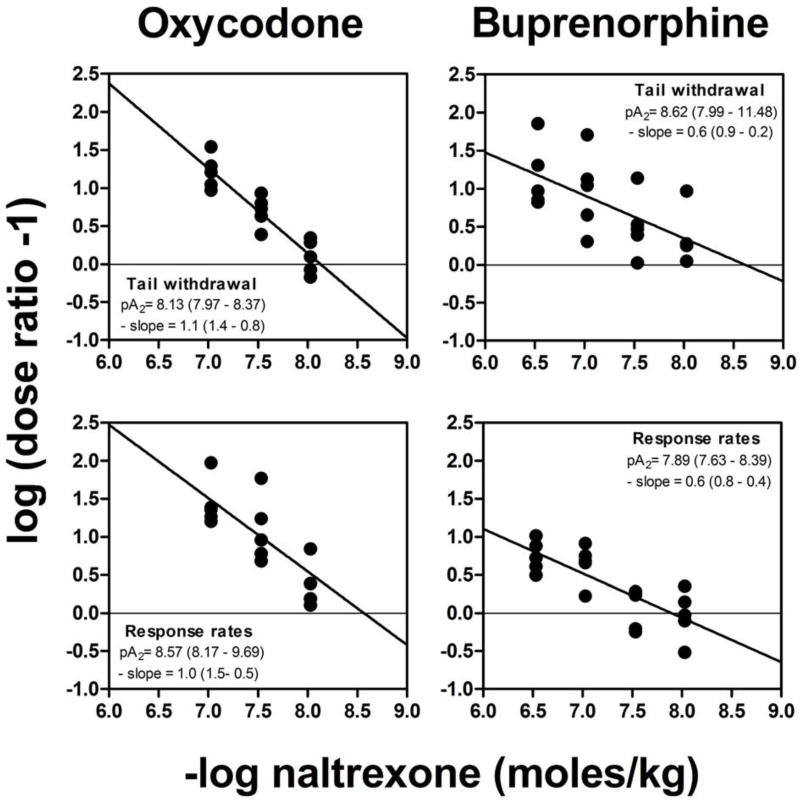
Naltrexone pretreatment did not significantly alter the slopes of the oxycodone or buprenorphine dose-response curves, indicating that equi-effective doses of the agonists were independent of level of response, and permitting apparent pA2 analysis. Fig. 5 shows the Schild plots from which the pA2 values were determined, using the dose ratio values obtained from data shown in the top four panels of fig. 4. The Schild plots shown on the left of fig. 5 indicate that the apparent pA2 values for oxycodone/naltrexone combinations are similar for antinociceptive and response rate-disruptive measures [respectively, 8.13 (slope = −1.1) and 8.57 (slope = −1.0)]. The Schild plots shown on the right of fig. 5 indicate that the pA2 values for buprenorphine/naltrexone combinations under both behavioral measures are also similar (8.62 and 7.89 for tail withdrawal latency and response rate disruption, respectively); however, the slope of the regression line in both buprenorphine Schild plots is significantly less than unity (slope = −0.6). Table 4 compares the pA2 values obtained for oxycodone and buprenorphine calculated with unconstrained slopes and slopes of regression lines constrained to −1.0. The pA2 values calculated for the effects of oxycodone and buprenorphine on both tail withdrawal latency and rates of responding are not significantly altered by constraining the slope of the regression line to −1.0.
Fig. 5.
Schild plots for naltrexone as an antagonist of the antinociceptive (top) and response rate-disrupting (bottom) effects of oxycodone (left) and buprenorphine (right). Ordinate: logarithm of the dose ratio (ED50 of the agonist in the presence of naltrexone divided by the ED50 of the agonist alone), −1. The data for ED50 values are from fig. 4 (see text for further details). Abscissa: negative logarithm of molar doses of naltrexone.
Table 4.
Apparent pA2 values for naltrexone as an antagonist of antinociceptive and response rate-disrupting effects of oxycodone and buprenorphine. Values are moles per kilogram and slope of the Schild plot
| Agonist | Slope unconstrained
|
Slope constrained
|
|
|---|---|---|---|
| pA2 (95% CI) | -Slope (95% CI) | pA2 (95% CI) | |
| Oxycodone | |||
| Antinociceptive effects | 8.13 (7.94 – 8.37) | 1.1 (1.4 – 0.8) | 8.20 (8.08 – 8.31) |
| Response rate effects | 8.57 (8.17 – 9.69) | 1.0 (1.5 – 0.5) | 8.53 (8.34 – 8.72) |
|
| |||
| Buprenorphine | |||
| Antinociceptive effects | 8.62 (7.99 – 11.48) | 0.6 (0.9 – 0.2) | 8.02 (7.79 – 8.25) |
| Response rate effects | 7.89 (7.63 – 8.39) | 0.6 (0.8 – 0.4) | 7.64 (7.47 – 7.81) |
Discussion
The warm water tail withdrawal assay has been particularly valuable in studying the role of efficacy and subtype selectivity in opioid antinociception in both rodent and primate species.22, 54, 56 One limitation of this assay, however, is that the antinociceptive effects of opioids are typically measured without obtaining companion data regarding their behaviorally disruptive effects. Thus, determining the relative potency with which drugs may concurrently produce antinociception and undesirable behavioral disruption requires measurement of the latter effects in separate experiments, complicating the comparison of antinociceptive selectivity across established and novel drugs. This consideration is especially relevant to research in nonhuman primates in which, due to cost and ethical concerns, it is desirable to efficiently obtain information regarding the behavioral selectivity with which opioids produce antinociception. To address this, a conventional warm water tail withdrawal assay was modified to concurrently measure opioid-induced changes in tail withdrawal latency and disruptions in operant performance in the same subject. Using this approach, the present studies show that relative potency of opioids with respect to these measures can vary greatly among drugs. The difference between these opioids with respect to undesirable behavioral effects of antinociceptive doses (discussed in detail below), comports well with clinical observations,53, 58 illustrating the ability of this approach to predict clinically meaningful differences among opioid analgesics. In view of the increased awareness of the need for improved preclinical assays to evaluate analgesic drugs in a more clinically relevant manner,20, 31 the present approach may provide an efficient means for achieving this goal.
The μ-opioid agonists oxycodone, heroin and methadone produced dose-related increases in tail withdrawal latency and disruptions in operant performance characterized by decreases in both response rate and reinforcement density. These findings were not unexpected and agree with previous results demonstrating the antinociceptive effects of these agonists in tail withdrawal procedures.3, 4, 6, 19, 21, 23, 29, 40, 43 They also agree with results of previous studies in rodents and/or monkeys showing that these opioids can impair operant performance by reducing rates of food-maintained responding.4, 11, 41, 51 In previous studies, however, the antinociceptive and behaviorally disruptive effects of opioids were not concurrently evaluated, precluding a quantitative determination of the behavioral selectivity of opioid antinociception in the same individuals. Based upon the present data, it seems clear that oxycodone, heroin and methadone, notwithstanding some reported differences in agonist efficacy,39 are not selective with respect to antinociceptive versus behaviorally disruptive effects, i.e., the ratio of ED50 values for decreases in response rate and increases in tail withdrawal latency approximates unity and does not greatly differ among the three drugs (0.78-1.28).
In contrast to the similarity among full agonists, the ratio of ED50 values varied among lower-efficacy mixed-action opioids. It was lowest for butorphanol (0.17), suggesting disruption of operant behavior at doses lower than those producing antinociception, and highest for nalbuphine (4.88), suggesting its antinociceptive effects were not accompanied by prominent behaviorally-disruptive effects. Both butorphanol and nalbuphine have been characterized as mixed-action μ/κ opioid agonists.15, 26, 49 However, nalbuphine generally has displayed lower μ and κ in vivo efficacy than butorphanol.27, 52 In squirrel monkeys, butorphanol but not nalbuphine, has been shown to produce cyclazocine-like discriminative stimulus effects at doses comparable to those given in the present studies whereas, in squirrel monkeys that discriminated the κ-opioid full agonist enadoline, nalbuphine produced predominantly κ-antagonist effects.7, 47, 48 In conjunction, these previous findings suggest that butorphanol, but not nalbuphine, produced κ-mediated agonist actions in the present experiments that markedly disrupted food-maintained behavior at doses lower than those that increased tail withdrawal latency. Previous Schild analysis has demonstrated that butorphanol’s antinociceptive effects are likely μ-mediated14 and, thus, the divergence between butorphanol’s behaviorally disruptive and antinociceptive effects may be consequent to its 2-fold higher binding affinity for κ- than μ-opioid receptors.59
Nalbuphine appears to have sufficient μ-opioid efficacy to markedly increase tail withdrawal latency under the present conditions but, unlike butorphanol, insufficient μ-related or κ-related efficacy to comparably disrupt food-maintained behavior. Previous studies have shown that nalbuphine can produce dose-related decreases in operant performance; however, its potency may differ across primate species. For example, doses of nalbuphine as low as 1.0 mg/kg have been reported to markedly reduce or eliminate schedule-controlled responding in rhesus monkeys3, 51 whereas doses up to 10-100 mg/kg were required to produce comparable effects in squirrel monkeys.7, 41 Notwithstanding such differences, the ability of nalbuphine, but not other opioids, to increase tail withdrawal latency at doses that did not disrupt operant performance suggests that the μ-related efficacy requirement for disrupting operant behavior was greater than that for producing antinociception under the present experimental conditions. These results also would predict that, under some conditions, nalbuphine may provide clinical analgesia comparable to that produced by higher-efficacy opioids such as morphine, yet produce lesser behavioral disruption. This prediction appears to agree well with reported findings.1, 50, 58 Nonetheless, as discussed below, the antinociceptive effects of nalbuphine can vary with the intensity of the nociceptive stimulus and, similarly, the equivalent analgesic efficacy of nalbuphine and morphine may greatly depend on the clinical situation. Thus, in the study by Akshat et al.1 notwithstanding their equivalent effects post-operatively, morphine provided superior analgesia for intraoperative pain management.
The antinociceptive and rate-decreasing effects of buprenorphine occurred with similar potency in the present studies, yielding a ratio of ED50 values that were not markedly different from those of the full agonists. These findings are notable because buprenorphine has been characterized as a partial, or low-efficacy μ-agonist with κ-antagonist and, with much lesser potency, NOP-agonist activity.28, 32, 46 Although the antinociceptive and behaviorally disruptive effects of buprenorphine are most likely due to its μ-agonist activity, its slow dissociation from the μ-receptor has been shown to produce a complex and time-dependent profile of agonist and antagonist effects.42 In behavioral studies in rhesus monkeys, acutely-administered buprenorphine has been shown to behave as a low-efficacy μ-agonist, i.e., its antinociceptive effects are evident when the nociceptive stimulus intensity is low but not high, and it serves to antagonize the disruptive effects of higher-efficacy μ-agonists on food-maintained behavior.42, 55 However, the direct effects of acutely-administered buprenorphine on operant performance in rhesus monkeys have been less consistent: significant (>50%) decreases in food-maintained responding have been produced by a wide range of buprenorphine doses, but have not been observed in other studies.33, 35, 36, 57 In contrast, acutely administered buprenorphine consistently has been shown to decrease food-maintained behavior in squirrel monkeys (present results;10, 12). It is unclear what may account for the difference in buprenorphine’s effects in these primate species. It is unlikely that squirrel monkeys are more sensitive to the effects of μ-opioids on food-maintained behavior, as the behaviorally disruptive doses of other opioids are comparable in rhesus and squirrel monkeys. Possibly, buprenorphine’s rate-decreasing effects in squirrel monkeys involve non-μ receptor-mediated actions. However, notwithstanding considerable variability in antagonism by naltrexone among individuals (see Walker et al.54) and despite regression line slopes that are significantly less than unity, the present Schild analyses strongly suggest that, like oxycodone, buprenorphine’s effects on both tail withdrawal latency and behavioral disruption can be attributed to its μ-mediated actions. Alternatively, it may be that buprenorphine has higher μ-efficacy in squirrel monkeys than rhesus monkeys. In this regard, previous studies have attempted but not been able to demonstrate antagonism of morphine’s effects by buprenorphine in squirrel monkeys,12 suggesting that, in contrast to its effects in rhesus monkeys, buprenorphine does not serve as a partial agonist/antagonist. It is well known that there are marked differences in opioid receptor subtype distribution and densities in different tissue preparations, and across species.17, 30, 34 Thus, it perhaps is not surprising that the behavioral effects of mixed-action ligands like buprenorphine also may differ across species.
It is well understood that the effects of opioids on antinociception and other behavioral endpoints may depend greatly on the parameters of the study. High-efficacy agonists may have antinociceptive effects regardless of differences in control values produced by nociceptive stimuli differing in intensity, whereas low-efficacy agonists may display antinociceptive effects only when stimulus intensity is low.8, 37 In the present experiments, we demonstrated that the latency to tail withdrawal varied directly in relation to intensity, i.e., water temperature, but that all drugs produced a near maximal increase in tail withdrawal latency from water heated to 55°C. This temperature previously has been used to distinguish between low- and high-efficacy μ-opioids in similar studies in rhesus monkeys, and produced consistent and relatively short latency to tail withdrawal in the present experiments. However, the ‘sensor’ (i.e., tail) is very different between primate species, and it is likely that similar distinctions between low- and high-efficacy μ-opioids would emerge in squirrel monkeys at a higher water temperature. Along analogous lines, the rate-decreasing effects of opioids also may vary depending on response requirement or the schedule of reinforcement.16 However, although the ED50 values for rate-decreasing effects of opioids might change under differing schedule requirements, it is likely that the order of behavioral selectivity across drugs will remain relatively constant. In the same regard, although the ratio of ED50 values increases when a decrease in reinforcement density rather than response rate is used as the measure of behavioral disruption, the order of behavioral selectivity across the opioids is similar across the two measures of behavioral disruption. Thus, in contrast to the degree of behavioral selectivity, the μ-related activity of the opioids does not depend on the behavioral endpoint.
Highlights.
Opioid antinociceptive and behaviorally disruptive effects are measured concurrently.
The ratio of ED50 values for both measures are calculated, and differ among opioids.
These ratios indicate the behavioral selectivity of opioid antinociception.
ED50 ratios serve as preclinical estimates of therapeutic index.
Opioids with differing ED50 ratios may have differing scopes of clinical utility.
Perspective.
This article presents an assay that allows for the concurrent assessment of the antinociceptive and behaviorally disruptive effects of opioids. Our results demonstrate that the tail withdrawal assay in squirrel monkeys can provide a useful index of the behavioral selectivity with which opioids produce antinociception.
Acknowledgments
The authors thank Dr R.D. Spealman for his comments on an earlier version of this manuscript.
This work was supported by National Institutes of Health [RO1-DA035857].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no conflict of interest.
AUTHORSHIP CONTRIBUTIONS
Participated in research design: Withey, Paronis, Bergman.
Conducted experiments: Withey
Performed data analysis: Withey
Wrote or contributed to the writing of the manuscript: Withey, Paronis, Bergman
References
- 1.Akshat S, Ramachandran R, Rewari V, Chandralekha, Trikha A, Sinha R. Morphine versus Nalbuphine for Open Gynaecological Surgery: A Randomized Controlled Double Blinded Trial. Pain research and treatment. 2014;2014:727952. doi: 10.1155/2014/727952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. British journal of pharmacology and chemotherapy. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks ML, Rice KC, Negus SS. Antinociceptive interactions between Mu-opioid receptor agonists and the serotonin uptake inhibitor clomipramine in rhesus monkeys: role of Mu agonist efficacy. J Pharmacol Exp Ther. 2010;335:497–505. doi: 10.1124/jpet.110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Experimental and clinical psychopharmacology. 2004;12:163–172. doi: 10.1037/1064-1297.12.3.163. [DOI] [PubMed] [Google Scholar]
- 5.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain physician. 2008;11:S105–120. [PubMed] [Google Scholar]
- 6.Bowen CA, Fischer BD, Mello NK, Negus SS. Antagonism of the antinociceptive and discriminative stimulus effects of heroin and morphine by 3-methoxynaltrexone and naltrexone in rhesus monkeys. J Pharmacol Exp Ther. 2002;302:264–273. doi: 10.1124/jpet.302.1.264. [DOI] [PubMed] [Google Scholar]
- 7.Carey GJ, Bergman J. Enadoline discrimination in squirrel monkeys: effects of opioid agonists and antagonists. J Pharmacol Exp Ther. 2001;297:215–223. [PubMed] [Google Scholar]
- 8.Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology. 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- 9.Corbett AD, Henderson G, McKnight AT, Paterson SJ. 75 years of opioid research: the exciting but vain quest for the Holy Grail. British journal of pharmacology. 2006;147(Suppl 1):S153–162. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeRossett SE, Holtzman SG. Effects of naloxone, diprenorphine, buprenorphine and etorphine on unpunished and punished food-reinforced responding in the squirrel monkey. J Pharmacol Exp Ther. 1984;228:669–675. [PubMed] [Google Scholar]
- 11.Doty P, Picker MJ, Dykstra LA. Differential cross-tolerance to opioid agonists in morphine-tolerant squirrel monkeys responding under a schedule of food presentation. European journal of pharmacology. 1989;174:171–180. doi: 10.1016/0014-2999(89)90309-9. [DOI] [PubMed] [Google Scholar]
- 12.Dykstra LA. Behavioral effects of buprenorphine and diprenorphine under a multiple schedule of food presentation in squirrel monkeys. J Pharmacol Exp Ther. 1983;226:317–323. [PubMed] [Google Scholar]
- 13.Dykstra LA, Woods JH. A tail withdrawal procedure for assessing analgesic activity in rhesus monkeys. Journal of pharmacological methods. 1986;15:263–269. doi: 10.1016/0160-5402(86)90056-2. [DOI] [PubMed] [Google Scholar]
- 14.Garner HR, Burke TF, Lawhorn CD, Stoner JM, Wessinger WD. Butorphanol-mediated antinociception in mice: partial agonist effects and mu receptor involvement. J Pharmacol Exp Ther. 1997;282:1253–1261. [PubMed] [Google Scholar]
- 15.Gerak LR, Butelman ER, Woods JH, France CP. Antinociceptive and respiratory effects of nalbuphine in rhesus monkeys. J Pharmacol Exp Ther. 1994;271:993–999. [PubMed] [Google Scholar]
- 16.Goldberg SR, Morse WH, Goldberg DM. Acute and chronic effects of naltrexone and naloxone on schedule-controlled behavior of squirrel monkeys and pigeons. J Pharmacol Exp Ther. 1981;216:500–509. [PubMed] [Google Scholar]
- 17.Gyand EA, Kosterlitz HW. Agonist and antagonist actions of morphine-like drugs on the guinea-pig isolated ileum. British journal of pharmacology and chemotherapy. 1966;27:514–527. doi: 10.1111/j.1476-5381.1966.tb01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes AG, Tyers MB. Determination of receptors that mediate opiate side effects in the mouse. British journal of pharmacology. 1983;79:731–736. doi: 10.1111/j.1476-5381.1983.tb10011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob JC, Poklis JL, Akbarali HI, Henderson G, Dewey WL. Ethanol Reversal of Tolerance to the Antinociceptive Effects of Oxycodone and Hydrocodone. J Pharmacol Exp Ther. 2017;362:45–52. doi: 10.1124/jpet.117.241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kangas BD, Bergman J. Operant nociception in nonhuman primates. Pain. 2014;155:1821–1828. doi: 10.1016/j.pain.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishioka S, Ko MC, Woods JH. Diltiazem enhances the analgesic but not the respiratory depressant effects of morphine in rhesus monkeys. European journal of pharmacology. 2000;397:85–92. doi: 10.1016/s0014-2999(00)00248-x. [DOI] [PubMed] [Google Scholar]
- 22.Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo A, Wyse BD, Meutermans W, Smith MT. In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two opioids have the same profile. British journal of pharmacology. 2015;172:532–548. doi: 10.1111/bph.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 25.Leander JD. Comparison of morphine, meperidine, anileridine, and alphaprodine on schedule-controlled responding and analgesia. Pharmacol Biochem Behav. 1980;12:797–801. doi: 10.1016/0091-3057(80)90168-9. [DOI] [PubMed] [Google Scholar]
- 26.Leander JD. Evidence that nalorphine, butorphanol and oxilorphan are partial agonists at a kappa-opioid receptor. European journal of pharmacology. 1983;86:467–470. doi: 10.1016/0014-2999(83)90198-x. [DOI] [PubMed] [Google Scholar]
- 27.Liguori A, Morse WH, Bergman J. Respiratory effects of opioid full and partial agonists in rhesus monkeys. J Pharmacol Exp Ther. 1996;277:462–472. [PubMed] [Google Scholar]
- 28.Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Current neuropharmacology. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maguire DR, Yang W, France CP. Interactions between mu-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J Pharmacol Exp Ther. 2013;345:354–362. doi: 10.1124/jpet.113.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends in neurosciences. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 31.Mao J. Current challenges in translational pain research. Trends in pharmacological sciences. 2012;33:568–573. doi: 10.1016/j.tips.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 33.Mello NK, Bree MP, Lukas SE, Mendelson JH. Buprenorphine effects on food-maintained responding in Macaque monkeys. Pharmacol Biochem Behav. 1985;23:1037–1044. doi: 10.1016/0091-3057(85)90111-x. [DOI] [PubMed] [Google Scholar]
- 34.Miller L, Shaw JS, Whiting EM. The contribution of intrinsic activity to the action of opioids in vitro. British journal of pharmacology. 1986;87:595–601. doi: 10.1111/j.1476-5381.1986.tb10202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moerschbaecher JM, Devia C, Brocklehurst C. Effects of mixed agonist-antagonist opioids on the acquisition of conditional discriminations in monkeys. J Pharmacol Exp Ther. 1987;240:74–81. [PubMed] [Google Scholar]
- 36.Moerschbaecher JM, Mastropaolo J, Winsauer PJ, Thompson DM. Effects of opioids on accuracy of a fixed-ratio discrimination in monkeys and rats. J Pharmacol Exp Ther. 1984;230:541–549. [PubMed] [Google Scholar]
- 37.Morgan D, Cook CD, Smith MA, Picker MJ. An examination of the interactions between the antinociceptive effects of morphine and various mu-opioids: the role of intrinsic efficacy and stimulus intensity. Anesthesia and analgesia. 1999;88:407–413. doi: 10.1097/00000539-199902000-00035. [DOI] [PubMed] [Google Scholar]
- 38.Morgan D, Picker MJ. Contribution of individual differences to discriminative stimulus, antinociceptive and rate-decreasing effects of opioids: importance of the drug’s relative intrinsic efficacy at the mu receptor. Behavioural pharmacology. 1996;7:261–284. [PubMed] [Google Scholar]
- 39.Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. British journal of pharmacology. 2011;164:1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan RW, Nicholson KL. Characterization of the antinociceptive effects of the individual isomers of methadone after acute and chronic administrations. Behavioural pharmacology. 2011;22:548–557. doi: 10.1097/FBP.0b013e328349ab0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveto AH, Picker MJ, Dykstra LA. Acute and chronic morphine administration: effects of mixed-action opioids in rats and squirrel monkeys responding under a schedule of food presentation. J Pharmacol Exp Ther. 1991;257:8–18. [PubMed] [Google Scholar]
- 42.Paronis CA, Bergman J. Buprenorphine and opioid antagonism, tolerance, and naltrexone-precipitated withdrawal. J Pharmacol Exp Ther. 2011;336:488–495. doi: 10.1124/jpet.110.173823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rady JJ, Portoghese PS, Fujimo JM. Methadone and heroin antinociception: predominant delta-opioid-receptor responses in methadone-tolerant mice. Japanese journal of pharmacology. 2002;88:319–331. doi: 10.1254/jjp.88.319. [DOI] [PubMed] [Google Scholar]
- 44.Reid MC, Henderson CR, Jr, Papaleontiou M, Amanfo L, Olkhovskaya Y, Moore AA, Parikh SS, Turner BJ. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain medicine (Malden, Mass) 2010;11:1063–1071. doi: 10.1111/j.1526-4637.2010.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson JP. United States worker’s compensation and disability. In: Ballantyne J, Tauben D, editors. Expert decision making on opioid treatment. Oxford University Press; New York: 2013. pp. 103–114. [Google Scholar]
- 46.Sadee W, Rosenbaum JS, Herz A. Buprenorphine: differential interaction with opiate receptor subtypes in vivo. J Pharmacol Exp Ther. 1982;223:157–162. [PubMed] [Google Scholar]
- 47.Schaefer GJ, Holtzman SG. Discriminative effects of cyclazocine in the squirrel monkey. J Pharmacol Exp Ther. 1978;205:291–301. [PubMed] [Google Scholar]
- 48.Schaefer GJ, Holtzman SG. Morphine-like stimulus effects in the monkey: opioids with antagonist properties. Pharmacol Biochem Behav. 1981;14:241–245. doi: 10.1016/0091-3057(81)90250-1. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt WK, Tam SW, Shotzberger GS, Smith DH, Jr, Clark R, Vernier VG. Nalbuphine. Drug and alcohol dependence. 1985;14:339–362. doi: 10.1016/0376-8716(85)90066-3. [DOI] [PubMed] [Google Scholar]
- 50.Shokri H, Ali I. Nalbuphine vs morphine as part of intravenous post cardiac surgery. Journal of Anesthesia and Clinical Research. 2014;5 [Google Scholar]
- 51.Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS. Opioid interactions in rhesus monkeys: effects of delta + mu and delta + kappa agonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther. 2003;307:1054–1064. doi: 10.1124/jpet.103.056515. [DOI] [PubMed] [Google Scholar]
- 52.Vivian JA, DeYoung MB, Sumpter TL, Traynor JR, Lewis JW, Woods JH. kappa-Opioid receptor effects of butorphanol in rhesus monkeys. J Pharmacol Exp Ther. 1999;290:259–265. [PubMed] [Google Scholar]
- 53.Walker DJ, Zacny JP, Galva KE, Lichtor JL. Subjective, psychomotor, and physiological effects of cumulative doses of mixed-action opioids in healthy volunteers. Psychopharmacology. 2001;155:362–371. doi: 10.1007/s002130100723. [DOI] [PubMed] [Google Scholar]
- 54.Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- 55.Walker EA, Zernig G, Woods JH. Buprenorphine antagonism of mu opioids in the rhesus monkey tail-withdrawal procedure. J Pharmacol Exp Ther. 1995;273:1345–1352. [PubMed] [Google Scholar]
- 56.Woods JH, Winger G. Behavioral characterization of opioid mixed agonist-antagonists. Drug and alcohol dependence. 1987;20:303–315. doi: 10.1016/0376-8716(87)90004-4. [DOI] [PubMed] [Google Scholar]
- 57.Young AM, Stephens KR, Hein DW, Woods JH. Reinforcing and discriminative stimulus properties of mixed agonist-antagonist opioids. J Pharmacol Exp Ther. 1984;229:118–126. [PubMed] [Google Scholar]
- 58.Zeng Z, Lu J, Shu C, Chen Y, Guo T, Wu QP, Yao SL, Yin P. A comparision of nalbuphine with morphine for analgesic effects and safety : meta-analysis of randomized controlled trials. Sci Rep. 2015;5:10927. doi: 10.1038/srep10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang B, Zhang T, Sromek AW, Scrimale T, Bidlack JM, Neumeyer JL. Synthesis and binding affinity of novel mono- and bivalent morphinan ligands for kappa, mu, and delta opioid receptors. Bioorganic & medicinal chemistry. 2011;19:2808–2816. doi: 10.1016/j.bmc.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]