Abstract
Purpose
We evaluated associations between personal and clinical social support and nonadherence to adjuvant endocrine therapy (AET) in a large, Northern California breast cancer (BC) cohort from an integrated healthcare network.
Methods
This study included 3,382 women from the Pathways Study diagnosed from 2005–2013 with stages I–III hormone receptor-positive BC and who responded to the Medical Outcomes Study Social Support and Interpersonal Processes of Care surveys, approximately two months post-diagnosis. We used logistic regression to evaluate associations between tertiles of social support and noninitiation (<2 consecutive prescription fills within a year after diagnosis). Among those who initiated treatment, we used proportional hazards regression to evaluate associations with discontinuation (≥90 day gap) and nonadherence (<80% medical possession ratio).
Results
Of those who initiated AET (79%), approximately one-fourth either discontinued AET or were nonadherent. AET noninitiation was more likely in women with moderate (adjusted OR=1.18, 95% CI: 0.96–1.46) or low (OR=1.30, 95% CI:1.05–1.62) vs. high personal social support (P-trend=.02). Women with moderate (HR=1.20, 95% CI:0.99–1.45) or low (HR=1.32, 95% CI:1.09–1.60) personal social support were also more likely to discontinue treatment (P-trend=.01). Furthermore, women with moderate (HR=1.25, 95% CI:1.02–1.53) or low (HR=1.38, 95% CI:1.12–1.70) personal social support had higher nonadherence (P-trend=.007). Associations with clinical social support and outcomes were similar. Notably, high clinical social support mitigated the risk of discontinuation when patients’ personal support was moderate or low (p-value=0.04).
Conclusions
Women with low personal or clinical social support had higher AET nonadherence. Clinician teams may need to fill support gaps that compromise treatment adherence.
Keywords: Social support, breast cancer, adjuvant endocrine therapy, nonadherence, women
Introduction
Randomized trials show that adjuvant endocrine therapy (AET) significantly improves long-term survival of breast cancer (BC) patients with hormone receptor-positive disease[1, 2]. Inadequate adherence and treatment interruptions may also compromise survival[3–5]. Despite this, only 40–60% of BC patients finish recommended courses of AET[6–9].
Supportive relationships have been related to better treatment adherence generally[10]. However, the influence of social support on adherence to AET in BC patients is unclear. A study of French women ages 18–40 years found that women reporting a small number of supportive persons in their social network had more tamoxifen interruptions[11] and a greater likelihood of tamoxifen discontinuation[12]. However, investigators reported no significant association between personal social support levels and AET adherence in the U.S. Breast Cancer Quality of Care Study (BQUAL) study[13]. Though one study showed that clinical social support, measured using Patient-Centered Care Items from National Initiative on Cancer Care Quality Breast Cancer Patient Survey, predicted better AET adherence[14], an education- and emotional support-based peer navigation intervention did not lead to improvements in adherence[15].
Social support may be critical to treatment management through its influence on treatment decisions[16], or diminution of emotional distress[17] and symptom severity[18, 19]. Given limited research, small previous studies, and a lack of research in diverse BC patients, we therefore evaluated associations between personal and clinical social support and AET nonadherence in a prospective cohort study of 3,382 ethnically and racially diverse women newly diagnosed with stages I–III hormone receptor-positive invasive BC.
METHODS
Study population
A total of 4,505 women with newly diagnosed invasive BC were recruited from Kaiser Permanente Northern California (KPNC), an integrated healthcare system, into the Pathways Study between January 2006 and May 2013. Details are previously reported[20]. The analytic population for this study included 3,382 women from the Pathways cohort who were diagnosed with stages I–III hormone receptor-positive BC. We excluded women missing information on hormone receptor status or stage (n=7); those with less than 11 months active KPNC membership in the year after diagnosis (n=111) (to ensure that analyses of noninitiation were not influenced by changes in KPNC enrollment); one woman who recurred within a year of diagnosis and prior to start of AET; and those missing data on social support (n=310). Analyses of discontinuation and nonadherence included 2,687 women who initiated treatment and filled at least two prescriptions. Written informed consent was obtained from all participants. The study was approved by the KPNC Institutional Review Board.
Data collection
Pharmacy data and AET outcomes
We obtained information on filled prescriptions of tamoxifen and aromatase inhibitors (i.e., anastrozole, letrozole, and/or exemestane) from outpatient pharmacy records. We categorized patients as not having initiated AET (noninitiation) if they did not fill 2 or more consecutive prescriptions within the first year after diagnosis. Among women who initiated treatment, discontinuation was defined as a treatment refill gap ≥90 days following the last day medication was supplied. Time to discontinuation was assessed as the number of consecutive days from AET initiation to the start of the first medication gap that was 90 days or longer. We conducted sensitivity analyses using a gap ≥45 days. Finally, nonadherence to AET was defined as a medication possession ratio (MPR) <80% during the five-year period after treatment initiation. The MPR was calculated as the number of days covered by all AET prescriptions divided by the number of days since AET initiation[7, 21]. In sensitivity analyses, we also evaluated associations with time to first nonadherence (i.e. the first occurrence of MPR <80% after a prescription).
Clinical data
Data on number of positive lymph nodes, American Joint Committee on Cancer (AJCC) stage, human epidermal growth factor receptor 2 (HER2) status, breast surgery (lumpectomy, mastectomy), chemotherapy and radiation therapy were obtained from the KPNC Cancer Registry (KPNCCR)[22]. Breast surgery and radiation therapy data were also supplemented by other KPNC electronic data sources.
Personal and clinical social support
To measure personal social support, each woman was asked at study baseline (~2 months post-diagnosis), and at 6 month follow-up (~8 months post-diagnosis), to complete the 19-item Medical Outcomes Study Social Support (MOS-SS) survey, a multidimensional social support survey developed for patients with chronic conditions, which assesses emotional/informational support, tangible support, positive social interaction, and affectionate support[23]. Five-point Likert responses ranged from none to all of the time. Personal social support was computed as the sum of these values. The reliability for each of the sub-scales and overall index in our sample were previously reported (Cronbach’s alpha 0.89–0.96) and factor structure confirmed[19].
Clinical social support was assessed using the 18-item Interpersonal Processes of Care scale[24]. However, items pertaining to the originally reported dimensions of “lack of clarity”, “discrimination due to race/ethnicity”, and “disrespectful office staff”, confirmed in factor analysis (SAS PROC FACTOR; SAS Institute, Cary, NC), differed conceptually from the concept of social support. By contrast, the 10 question items used in the original domains of “compassion”, “elicited concerns”, “explained results”, and “treatment decided together” had conceptual overlap with informational, emotional, and appraisal types of social support[25], and loaded together in the factor analysis and were thus combined into a single measure of clinical social support. We further standardized scores. Personal and clinical social support scores were weakly correlated, r=0.33, p<0.001 with poor concordance (κ=−0.13), suggesting the two measures captured different phenomena.
Covariates
Covariates were assessed at study baseline except for menopausal symptoms and treatment side effects, which were assessed at the 6-month follow-up. Data on sociodemographic characteristics, lifestyle factors, and psychosocial factors were collected by trained staff who conducted in-home interviews.
Body mass index (BMI) was computed from height and weight from KPNC electronic sources at study enrollment; missing values were supplemented by self-reported data. Physical activity in MET (metabolic equivalent)-hours/week was assessed from the Arizona Activity Frequency Questionnaire (AAFQ)[26]. For pre-cancer comorbidity, we computed the Charlson-Deyo comorbidity index[27] using ICD-9 codes from electronic data sources and dichotomized scores as no vs. any comorbidity. Depressive symptoms were assessed as a score ≥16 on the Centers for Epidemiologic Studies Depression (CES-D) scale[28]. Alcohol was computed in grams/day and smoking was assessed as current, past, or never.
At the six-month follow-up, women were asked (yes/no) whether they had menopausal symptoms such as hot flashes or night sweats in the last six months. Women were also asked whether in the past 7 days they were bothered by side effects of treatment, with response categories ranging from not at all to very much.
Statistical analyses
Analyses of social support and initiation of AET
We evaluated differences in demographic, severity, and treatment factors for those with and without missing personal social support data. We examined associations of continuous covariates against tertiles of social support using analysis of variance, and examined covariate distributions by tertiles of social support for categorical variables.
We used logistic regression (SAS PROC LOGISTIC) to evaluate associations between tertiles of personal and clinical social support and AET noninitiation in the first year and computed tests for linear trend. Results minimally adjusted for age, days between diagnosis and baseline social assessment, stage, and race (Model 1) were compared with those adjusted additionally for multiple covariates (Model 2) hypothesized a priori to be potential confounding variables. We considered models adjusted for depressive symptoms and lifestyle factors including BMI, physical activity, alcohol consumption, and smoking, but these had little substantive effect on associations, and were dropped from final models (data not shown). Finally, we simultaneously adjusted models for both personal and clinical social support (Model 3) to evaluate their independent effects. Proportions of noninitiation, discontinuation, and nonadherence by categories of social support are included in Supplementary Figure 1 and parallel reported findings.
Analyses of social support, discontinuation, and nonadherence to AET
We employed Cox proportional hazard regression models (SAS PROC PHREG) for failure-time data to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for associations between tertiles of personal or clinical social support at study baseline, or at 6-month follow-up, and time to discontinuation or nonadherence. Person-years of follow-up were counted from the date of AET initiation until the date of the event, date of death, date of recurrence, date of disenrollment from KPNC, until five years elapsed from the date of the first prescription, or until end of study follow-up for this analysis (October 22, 2015), whichever came first. In addition to covariates included in models of noninitiation, we adjusted for menopausal symptoms and self-reported side effects of (any) treatment. Finally, we examined the cross-classification of personal social support and clinical social support to determine, in the presence of low or moderate levels of one type of social support, whether the other type of support may mitigate risk.
Stratified analyses
Finally, we conducted analyses stratified separately by stage at diagnosis (early [localized and regional with lymph nodes only] vs. late [regional, distant]), white vs. non-white race/ethnicity, age (< or ≥ median=60 years), education (<college, ≥college), presence of comorbidity, type of AET treatment (tamoxifen, aromatase inhibitors, both), indications of menopausal symptoms, and depressive symptoms. We assessed effect modification evaluating the cross-product of categorical social support terms and stratification variables and using likelihood ratio χ2 tests. Tests of statistical significance were two-sided. Statistically significant results denote p-values ≤0.05.
RESULTS
Of the 3,382 women eligible for AET, 2,687 (79%) initiated treatment with AET. Of these, 649 (24%) discontinued AET and 581 (22%) were nonadherent. Mean follow-up was 4.20 years (median=4.99, range 0.09–4.99 years). Women missing social support data were older and more likely to be African-American or Hispanic. They were slightly better represented among those with some college/vocational school and were less likely to have a post-graduate education and household income <$25,000. They had higher BMIs and were more likely to have a comorbidity or be a current smoker. However, missingness was not related to disease severity, treatment, physical activity, or alcohol intake (data not shown).
Baseline characteristics
Women with greater personal social support were younger and had higher incomes and generally healthier lifestyles with higher physical activity and a lower likelihood of current smoking (Table 1). They also had higher alcohol consumption. Hispanic women were more likely and Asian women less likely to have high personal social support. Women with high personal social support were less likely to be postmenopausal at diagnosis and were more likely to receive chemotherapy compared to those with low personal support; personal social support was unrelated to other treatment variables. Women with moderate support were least likely to be nulliparous. Women with low personal support were slightly more likely to have any comorbidity or stage I cancer. Clinical social support was similarly associated with covariates.
Table 1.
Baseline Characteristics of Hormone Receptor-Positive Breast Cancer Patients from the Pathways Study (N=3,382)
| Personal Social Support Categories
|
P* | |||
|---|---|---|---|---|
| High | Moderate | Low | ||
|
|
||||
| N (%) | 1,164 (34%) | 1,165 (34%) | 1,053 (31%) | |
| Range | 89.0–95.0 | 76.0–88.0 | 21.0–75.0 | |
| Personal social support (mean) | 92.9 | 82.0 | 64.3 | |
| Clinical social support (range) | 15.9–97.6 | 24.1–97.6 | 18.3–97.6 | |
| Clinical social support (mean) | 85.1 | 80.1 | 75.0 | |
| Time from diagnosis to post-diagnosis assessment (mean days) | 61.9 | 64.1 | 62.6 | .07 |
| Any comorbidity (%) | 8.2 | 11.8 | 11.4 | .01 |
| Treatment side effects, 6-month FU (%) | 48.4 | 49.6 | 51.6 | .49 |
| Menopausal symptoms, 6-month FU (%) | 38.9 | 39.9 | 35.3 | .01 |
| Demographic characteristics | ||||
| Age at diagnosis (mean years) | 58.4 | 59.5 | 60.8 | <.001 |
| Ethnicity (%) | ||||
| Caucasian | 67.9 | 66.7 | 67.2 | .02 |
| African-American | 5.6 | 5.4 | 6.0 | |
| Asian | 10.4 | 13.7 | 14.9 | |
| Hispanic/Latino | 13.1 | 12.1 | 10.0 | |
| Other/unknown | 3.0 | 2.1 | 2.3 | |
| Education (%) | ||||
| High school or less | 17.1 | 14.9 | 14.1 | .35 |
| Some college/vocational school | 34.2 | 33.1 | 33.1 | |
| College graduate | 26.2 | 29.3 | 28.7 | |
| Post-graduate | 22.5 | 22.8 | 24.1 | |
| Household income (%) | ||||
| <$25,0000 | 6.8 | 7.7 | 13.2 | <.001 |
| $25,000–69,999 | 30.2 | 33.5 | 38.3 | |
| $70,000+ | 53.7 | 48.8 | 37.3 | |
| Unknown | 9.3 | 10.0 | 11.2 | |
| Employment (%) | ||||
| Full- or part-time | 48.9 | 48.7 | 42.8 | .02 |
| Not employed/retired | 48.6 | 49.5 | 54.7 | |
| Other/unknown | 2.5 | 1.8 | 2.5 | |
| Severity of disease (%) | ||||
| Stage | ||||
| I | 69.2 | 69.7 | 73.2 | .05 |
| II | 29.0 | 29.1 | 24.7 | |
| III | 1.9 | 1.2 | 2.1 | |
| Any lymph node involvement | 30.6 | 30.2 | 26.2 | .05 |
| HER-2-neu positive | 10.6 | 12.0 | 9.3 | .14 |
| Treatment in year after diagnosis (%) | ||||
| Chemotherapy | 44.4 | 40.8 | 35.7 | .004 |
| Lumpectomy | 61.9 | 61.0 | 64.6 | .21 |
| Radiation | 29.1 | 26.5 | 28.2 | .37 |
| Herceptin | 7.5 | 8.8 | 6.5 | .12 |
| Reproductive factors (%) | ||||
| Postmenopausal at diagnosis | 67.4 | 70.8 | 74.0 | .003 |
| Nulliparous | 20.1 | 16.9 | 23.6 | <.001 |
Pearson χ2 test or ANOVA/F-test
AET initiation
In minimally-adjusted models that included age, time between diagnosis and social assessment, race, and stage, women with moderate (OR=1.24, 95% CI: 1.00–1.52) or low (OR=1.40, 95% CI: 1.13–1.72) personal social support were more likely not to initiate AET treatment in the first year after diagnosis compared to women with high personal social support (Table 2). These associations remained significant after adjustment for multiple covariates (Table 2, Figure 1). Similarly, in multivariable-adjusted models, patients with moderate (OR=1.11, 95% CI: 0.90–1.38) or low (OR=1.25, 95% CI: 1.02–1.54) vs. high clinical social support were more likely not to initiate treatment (P =.03) (Table 2). When both personal and clinical social support were included in the same model, associations for each measure were further attenuated (Table 2).
Table 2.
Personal and Clinical Social Support and Relative Odds of Noninitiation of AET (N=3,382)
| % with outcome | Social Support Categories
|
P trend | |||
|---|---|---|---|---|---|
| High | Moderate | Low | |||
|
|
|||||
| Personal social support, N | 1,164 | 1,165 | 1,053 | ||
| Noninitiation | 21% | 206 | 243 | 246 | |
| OR, Model 1* | 1.00 | 1.24 | 1.40 | .002 | |
| 95% CI | (1.00–1.52) | (1.13–1.72) | |||
| OR, Model 2** | 1.00 | 1.18 | 1.30 | .02 | |
| 95% CI | (0.96–1.46) | (1.05–1.62) | |||
| OR, Model 3† | 1.00 | 1.15 | 1.23 | .08 | |
| 95% CI | (0.93–1.42) | (0.98–1.55) | |||
| Clinical social support, N | 1,307 | 1,001 | 1,069 | ||
| Noninitiation | 21% | 249 | 203 | 243 | |
| OR, Model 1* | 1.00 | 1.12 | 1.31 | .009 | |
| 95% CI | (0.91–1.38) | (1.07–1.60) | |||
| OR, Model 2** | 1.00 | 1.11 | 1.25 | .03 | |
| 95% CI | (0.90–1.38) | (1.02–1.54) | |||
| OR, Model 3† | 1.00 | 1.07 | 1.18 | .12 | |
| 95% CI | (0.86–1.33) | (1.00–1.50) | |||
Model 1: Adjusted for age (continuous), time between diagnosis and social support assessment (continuous), race (white (ref), black, Asian, Hispanic, other), and stage (I (ref), II, III)
Model 2: Adjusted additionally for chemotherapy (no (ref), yes), radiation (no (ref), yes), type of surgery (lumpectomy (ref), mastectomy, other), nodal status (positive, negative (ref), unknown), comorbidity at baseline (no (ref), yes, unknown), HER2 status (positive, negative (ref), unknown), educational status (high school or less, some college/vocational school (ref), college graduate, post-graduate) and income (<$25,000, $25,000–69,999, $70,000+ (ref), unknown). Further adjustment for alcohol use, physical activity, BMI, and smoking status did not substantively influence associations and these covariates were not included in models.
Model 3: Simultaneously adjusted for personal and clinical social support.
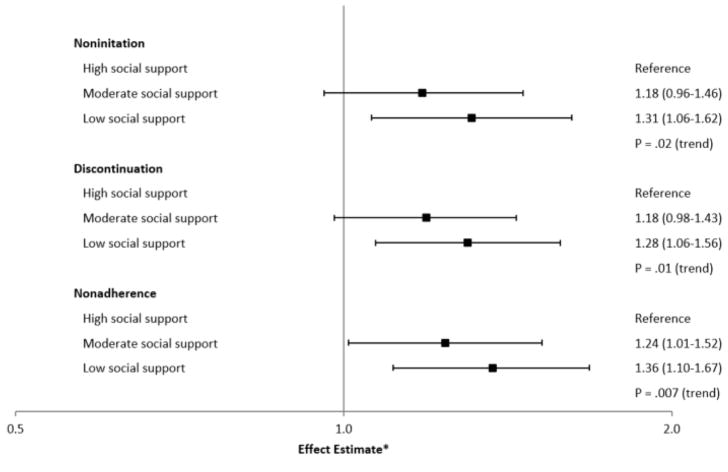
Figure 1.
Levels of personal social support and noninitiation (N=3,382), discontinuation and nonadherence (N=2,687) to AHT in hormone receptor-positive breast cancer patients from the Pathways Study
* The effect estimates are odds ratios (noninitiation) and hazard ratios (discontinuation and nonadherence); the horizontal axis is presented on a log scale. Associations of clinical social support and outcomes were similar in main analyses.
AET discontinuation and nonadherence
In multivariable-adjusted analyses, women with moderate (HR=1.18, 95% CI: 0.98–1.43) or low (HR=1.28, 95% CI: 1.06–1.56) personal social support had a higher risk of treatment discontinuation (P =.01) (Table 3, Figure 1). Furthermore, women with moderate (HR=1.24, 95% CI: 1.01–1.52) or low (HR=1.36, 95% CI: 1.10–1.67) personal social support also had greater nonadherence (P =.007) (Table 3, Figure 1) Results were similar in sensitivity analyses (data not shown). Results for personal social support were stronger when we examined associations of social support at 6-month follow-up and subsequent outcomes (Table 3).
Table 3.
Hazard Ratios of Personal and Clinical Social Support and AET Discontinuation or Nonadherence (N=2,682)
| % with outcome | Social Support Categories
|
P trend | |||
|---|---|---|---|---|---|
| High | Moderate | Low | |||
|
|
|||||
| Personal social support | |||||
| Baseline, N | 958 | 922 | 807 | ||
| Discontinuation | 24% | 201 | 226 | 222 | |
| HR, Model 1* | 1.00 | 1.21 | 1.37 | .001 | |
| 95% CI | (1.00–1.46) | (1.13–1.67) | |||
| HR, Model 2** | 1.00 | 1.18 | 1.28 | .01 | |
| 95% CI | (0.98–1.43) | (1.06–1.56) | |||
| HR, Model 3† | 1.00 | 1.13 | 1.18 | .13 | |
| 95% CI | (0.93–1.38) | (1.00–1.48) | |||
| Nonadherence | 22% | 174 | 206 | 201 | |
| HR, Model 1* | 1.00 | 1.27 | 1.48 | <.001 | |
| 95% CI | (1.04–1.55) | (1.20–1.81) | |||
| HR, Model 2** | 1.00 | 1.24 | 1.36 | .007 | |
| 95% CI | (1.01–1.52) | (1.10–1.67) | |||
| HR, Model 3† | 1.00 | 1.20 | 1.28 | .03 | |
| 95% CI | (0.97–1.47) | (1.03–1.59) | |||
|
| |||||
| Clinical social support | |||||
| Baseline, N | 1,058 | 798 | 826 | ||
| Discontinuation | 24% | 225 | 194 | 229 | |
| HR, Model 1* | 1.00 | 1.21 | 1.45 | <.001 | |
| 95% CI | (1.00–1.47) | (1.21–1.75) | |||
| HR, Model 2** | 1.00 | 1.20 | 1.37 | <.001 | |
| 95% CI | (0.99–1.46) | (1.14–1.65) | |||
| HR, Model 3† | 1.00 | 1.31 | 1.31 | .01 | |
| 95% CI | (0.96–1.42) | (1.07–1.59) | |||
| Nonadherence | 22% | 201 | 177 | 202 | |
| HR, Model 1* | 1.00 | 1.23 | 1.38 | .001 | |
| 95% CI | (1.00–1.50) | (1.13–1.68) | |||
| HR, Model 2** | 1.00 | 1.21 | 1.28 | .004 | |
| 95% CI | (0.99–1.48) | (1.05–1.57) | |||
| HR, Model 3† | 1.00 | 1.16 | 1.20 | .07 | |
| 95% CI | (0.94–1.42) | (0.97–1.48) | |||
Models adjusted for covariates listed in Table 2.
In multivariable-adjusted models, women with moderate (HR=1.20, 95% CI: 0.99–1.46) or low (HR=1.37, 95% CI: 1.14–1.59) clinical social support had higher risks of discontinuation (P <.001) (Table 3). Furthermore, women with moderate (HR=1.21, 95% CI: 0.99–1.48) or low (HR=1.28, 95% CI: 1.05–1.57) clinical social support had higher risks of nonadherence (P =.004) (Table 3).
Baseline levels of both personal and clinical social support were significantly related to discontinuation with and without simultaneous adjustment for both types of social support (Table 3). However, upon simultaneous adjustment, personal social support remained related, but clinical social support was no longer related, to nonadherence (Table 3). Evaluating the 6-month measures, personal social support was more strongly related to discontinuation and nonadherence than was clinical social support (data not shown).
In analyses examining the combined effect of clinical and personal social support, high levels of clinical support mitigated the risk of discontinuation among those with moderate or low personal support (Table 4). By contrast, high levels of personal social support appeared to mitigate the risk of nonadherence among those reporting moderate or low clinical social support (Table 4).
Table 4.
Hazard Ratios of AET Outcome by Level of Personal and Clinical Social Support* (N=2,682)
| N | Discontinuation | HR | 95% CI | Nonadherence | HR | 95% CI | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Category | |||||||
| Low clinical support and | |||||||
| Low personal social support**†‡§ | 384 | 109 | 1.54 | (1.17–2.03) | 98 | 1.48 | (1.11–1.98) |
| Moderate personal social support** | 278 | 78 | 1.59 | (1.18–2.15) | 68 | 1.44 | (1.05–1.97) |
| High personal social support†‡ | 164 | 42 | 1.41 | (0.98–2.03) | 36 | 1.22 | (0.83–1.81) |
| Moderate clinical support and | |||||||
| Low personal social support † | 245 | 68 | 1.50 | (1.10–2.04) | 61 | 1.48 | (1.07–2.05) |
| Moderate personal social support | 306 | 71 | 1.28 | (0.95–1.74) | 73 | 1.40 | (1.03–1.91) |
| High personal social support † | 247 | 55 | 1.23 | (0.88–1.71) | 43 | 1.03 | (0.71–1.48) |
| High clinical support and | |||||||
| Low personal social support**§ | 176 | 45 | 1.25 | (0.88–1.78) | 42 | 1.28 | (0.89–1.85) |
| Moderate personal social support** | 338 | 77 | 1.20 | (0.89–1.61) | 65 | 1.10 | (0.80–1.51) |
| High personal social support (ref) | 544 | 103 | 1.00 | 94 | 1.00 | ||
Model adjusted for Model 2 covariates in Table 2.
Discontinuation
** Among those with low or moderate personal social support, comparison of those with low vs. high clinical social support, p-value=0.04
† Among those with low or moderate clinical social support, comparison of those with low vs. high personal social support, p-value=0.29
Nonadherence
** Among those with low or moderate personal social support, comparison of those with low vs. high clinical social support, p-value=0.11
† Among those with low or moderate clinical social support, comparison of those with low vs. high personal social support, p-value=0.05
Stratified analyses
Associations of personal or clinical social support with AET noninitiation, discontinuation or nonadherence of AET did not differ markedly by age, race/ethnicity, education, stage, comorbidity or depressive symptomatology (data not shown). Furthermore, associations were qualitatively similar across type of hormonal therapy and self-reported menopausal symptoms for discontinuation (Supplementary Table 1) and nonadherence (data not shown).
DISCUSSION
In this study, those with low personal or clinical social support were more likely not to initiate treatment with AET. Furthermore, among women who initiated treatment, those with low personal or clinical social support were more likely to discontinue or not fully adhere to treatment. High clinical social support appeared to mitigate the risk of discontinuation among women with moderate or low personal social support; high levels of personal social support appeared to mitigate the risk of nonadherence when clinical social support was moderate or low. Findings were similar for specific types of AET or by level of menopausal symptoms. Associations were independent of socioeconomic status, disease severity, reproductive and lifestyle factors, self-reported side effects of treatment and menopausal symptoms, and depressive symptomatology. These findings are novel and emphasize the importance of both personal and clinical social support in adherence to AET in women with BC.
Data on social support and AET adherence are both sparse and mixed. In hormone-receptor positive BC patients from the ELIPPSE40 study of French women ages 18–40 years, investigators reported that those with low levels of personal social support, measured as the number of persons in one’s network providing support, a structural measure assessed 10 months post-diagnosis, had greater tamoxifen interruptions[11] (n=196) and a greater likelihood of tamoxifen discontinuation[12] (n=288). However, in 523 women from the BQUAL study who were also from the Pathways Study, investigators reported no significant association between personal social support, measured using the MOS-SS survey, a functional social support measure and one of numerous psychosocial measures examined, and AET (tamoxifen or aromatase inhibitors) nonadherence[13], though findings were suggestive. Both studies were small and predominantly included white women; nonsignificant findings appeared due to limited statistical power. Findings regarding clinical social support and nonadherence have also been mixed[14, 15].
Our findings, in a considerably larger and more diverse population, confirm the roles of both personal and clinical social support in AET adherence. The magnitudes of effects were moderate, monotonic, and robust to multiple adjustment. Associations with outcomes were furthermore consistent across levels of self-reported symptoms and different types of treatment, even though treatment side effects are an important predictor of adherence[6] and tamoxifen and aromatase inhibitors have different side effect profiles[29].
Associations for noninitiation were attenuated with simultaneous adjustment for personal and clinical social support suggesting that self-reports of social support could be, in part, related to a single underlying factor such as anxiety, hostility, difficulty in managing relationships, or general ability to elicit support. However, the fact that personal social support measured approximately 8 months after diagnosis, was more strongly predictive of outcomes than was clinical social support, suggests that personal social support may become increasingly relevant to persistence with treatment the further from diagnosis. This is consistent with traditional post-diagnosis patterns of care in which clinician support declines after primary treatment. Nonetheless, results of the combined effect of both types of support provided evidence that each type of support contributed to more optimal outcomes. Future work might involve interventions designed to improve relationships between patients and the clinical team or help patients connect to supportive others.
This is the largest prospective study to examine associations of both personal and clinical social support and adherence to AET, conducted in a large, diverse population of 3,382 women with hormone-receptor-positive BC. A strength of the study was the ability to evaluate associations of social support measured at two timepoints including a baseline measure taken close in time to BC diagnosis and prior to treatment. Other strengths included well-established measures of social support, linkage to pharmacy records ensuring objective information on AET prescription fills, and the ability to examine associations with noninitiation, discontinuation, and nonadherence. This study included a racially and ethnically diverse population, thus improving generalizability compared with previous studies. Other important strengths included the ability to examine associations for different types of treatment and ability to adjust for many covariates including education and income.
Limitations included the lack of verification that women consumed medications and missing information on social support, the latter potentially leading to an underestimate in associations. Though findings appeared consistent across sociodemographic groups, findings need to be replicated in other populations and should include greater representation of women from racial/ethnic minority groups. Further work is needed to explore mechanisms linking social relationships to AET adherence to inform the design of effective interventions in women with low social support.
In summary, among women with hormone receptor-positive BC, those with low personal or clinical social support had greater risks of AET noninitiation, discontinuation, and nonadherence. However, each type of support helped mitigate the risk of nonadherence. Clinicians should be cognizant of the context of patients’ social relationships which may influence management of treatment. Clinical teams may also need to fill support gaps that may compromise treatment adherence.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health, National Cancer Institute Grant K07 CA187403 (PI: C. Kroenke), R01 CA105274 (PI: L Kushi) and U01 CA195565 (mPI: L Kushi, C. Ambrosone).
Footnotes
The authors report no conflicts of interest.
Author contributions: CH Kroenke contributed to conceptualization and design of the study, analysis, writing and interpretation. DL Hershman contributed to interpretation and editing. SL Gomez contributed to data collection, interpretation, and editing. SR Adams and EH Eldridge contributed to analysis, writing, and editing. ML Kwan contributed to data collection and editing. IJ Ergas contributed to analysis. A Kubo contributed to editing. LH Kushi contributed to data collection and editing. All authors of this research paper have approved the final version submitted.
References
- 1.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, Gelber RD, Goldhirsch A, Smith I, Rabaglio M, et al. Treatment Adherence and Its Impact on Disease-Free Survival in the Breast International Group 1–98 Trial of Tamoxifen and Letrozole, Alone and in Sequence. J Clin Oncol. 2016;34(21):2452–2459. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh KP, Chen LC, Cheung KL, Chang CS, Yang YH. Interruption and non-adherence to long-term adjuvant hormone therapy is associated with adverse survival outcome of breast cancer women--an Asian population-based study. PLoS One. 2014;9(2):e87027. doi: 10.1371/journal.pone.0087027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013;108(7):1515–1524. doi: 10.1038/bjc.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 9.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 10.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23(2):207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 11.Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, Carrieri MP, Giorgi R. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23(4):882–890. doi: 10.1093/annonc/mdr330. [DOI] [PubMed] [Google Scholar]
- 12.Huiart L, Bouhnik AD, Rey D, Tarpin C, Cluze C, Bendiane MK, Viens P, Giorgi R. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer. 2012;48(13):1939–1946. doi: 10.1016/j.ejca.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Hershman DL, Kushi LH, Hillyer GC, Coromilas E, Buono D, Lamerato L, Bovbjerg DH, Mandelblatt JS, Tsai WY, Zhong X, et al. Psychosocial factors related to non-persistence with adjuvant endocrine therapy among women with breast cancer: the Breast Cancer Quality of Care Study (BQUAL) Breast Cancer Res Treat. 2016;157(1):133–143. doi: 10.1007/s10549-016-3788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45(5):431–439. doi: 10.1097/01.mlr.0000257193.10760.7f. [DOI] [PubMed] [Google Scholar]
- 15.Ell K, Vourlekis B, Xie B, Nedjat-Haiem FR, Lee PJ, Muderspach L, Russell C, Palinkas LA. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115(19):4606–4615. doi: 10.1002/cncr.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shelton RC, Clarke Hillyer G, Hershman DL, Leoce N, Bovbjerg DH, Mandelblatt JS, Kushi LH, Lamerato L, Nathanson SD, Ambrosone CB, et al. Interpersonal influences and attitudes about adjuvant therapy treatment decisions among non-metastatic breast cancer patients: an examination of differences by age and race/ethnicity in the BQUAL study. Breast Cancer Res Treat. 2013;137(3):817–828. doi: 10.1007/s10549-012-2370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ates O, Soylu C, Babacan T, Sarici F, Kertmen N, Allen D, Sever AR, Altundag K. Assessment of psychosocial factors and distress in women having adjuvant endocrine therapy for breast cancer: the relationship among emotional distress and patient and treatment-related factors. Springerplus. 2016;5:486. doi: 10.1186/s40064-016-2136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochayon L, Tunin R, Yoselis A, Kadmon I. Symptoms of hormonal therapy and social support: Is there a connection? Comparison of symptom severity, symptom interference and social support among breast cancer patients receiving and not receiving adjuvant hormonal treatment. Eur J Oncol Nurs. 2015;19(3):260–267. doi: 10.1016/j.ejon.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke CH, Kwan ML, Neugut AI, Ergas IJ, Wright JD, Caan BJ, Hershman D, Kushi LH. Social networks, social support mechanisms, and quality of life after breast cancer diagnosis. Breast Cancer Res Treat. 2013;139(2):515–527. doi: 10.1007/s10549-013-2477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwan ML, Ambrosone CB, Lee MM, Barlow J, Krathwohl SE, Ergas IJ, Ashley CH, Bittner JR, Darbinian J, Stronach K, et al. The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer Causes Control. 2008;19(10):1065–1076. doi: 10.1007/s10552-008-9170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oehrli MD, Quesenberry CP, Leyden W. Kaiser Permanente Northern California Cancer Registry. Nov, 2006. Annual Report on Trends, Incidence, and Outcomes. [Google Scholar]
- 23.Sherbourne CD, Stewart AL. The MOS social support survey. Social science & medicine (1982) 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AL, Napoles-Springer AM, Gregorich SE, Santoyo-Olsson J. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health Serv Res. 2007;42(3 Pt 1):1235–1256. doi: 10.1111/j.1475-6773.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.House JS. Work stress and social support. Reading, MA: 1981. [Google Scholar]
- 26.Staten LK, Taren DL, Howell WH, Tobar M, Poehlman ET, Hill A, Reid PM, Ritenbaugh C. Validation of the Arizona Activity Frequency Questionnaire using doubly labeled water. Med Sci Sports Exerc. 2001;33(11):1959–1967. doi: 10.1097/00005768-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 29.Garreau JR, Delamelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg. 2006;192(4):496–498. doi: 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.