Abstract
Background
Thrombocytopenia is frequently encountered in critically ill patients, often resulting in prophylactic transfusion of platelets for the prevention of bleeding complications. However, the efficacy of this practice remains unclear. The objective of this study was to determine the relationship between prophylactic platelet transfusion and bleeding complications in critically ill patients.
Methods
This is a retrospective cohort study of adults admitted to surgical, medical, or combined medical-surgical intensive care units (ICU) at a single academic institution between January 1, 2009 and December 31, 2013. Inclusion criteria included age ≥ 18 years and a platelet count measured during ICU admission. Propensity-matched analyses were used to evaluate associations between prophylactic platelet transfusions and the outcomes of interest with a primary outcome of red blood cell (RBC) transfusion in the ensuing 24 hours and secondary outcomes of ICU and hospital free days and changes in sequential organ failure assessment (SOFA) scores.
Results
A total of 40,693 patients were included in the investigation with 3,227 (7.9%) receiving a platelet transfusion and 1,065 (33.0%) for which platelet transfusion was prophylactic in nature. In propensity-matched analyses, 994 patients with prophylactic platelet transfusion were matched to those without a transfusion. Patients receiving prophylactic platelets had significantly higher RBC transfusion rates [OR 7.5 (5.9 – 9.5), p < 0.001], fewer ICU free days [mean (SD) 20.8 (9.1) vs 22.7 (8.3) days, p = 0.004], fewer hospital-free days [13.0 (9.7) vs 15.8 (9.4) days, p < 0.001], and less improvement in SOFA scores [mean decrease of 0.2 (3.6) vs. 1.8 (3.3), p < 0.001] in the subsequent 24 hours. These findings appeared robust, persisting in multiple predefined sensitivity analyses.
Conclusions
Prophylactic administration of platelets in the critically ill was not associated with improved clinical outcomes, though residual confounding may exist. Further investigation of platelet transfusion strategies in this population is warranted.
Keywords: platelets, intensive care, bleeding, transfusion, red blood cell, prophylaxis
Introduction
Derangements in the hematologic system are common in the critically ill, with thrombocytopenia occurring in approximately 50% of patients during the course of their intensive care unit (ICU) stay.1 The etiology of thrombocytopenia is often multifactorial and varies based upon underlying clinical diagnoses. It may be peripherally-mediated secondary to platelet consumption, sequestration, destruction, or dilution (e.g., disseminated intravascular coagulation, hypersplenism, active bleeding); centrally-mediated as the result of hypoproliferation (e.g., myelodysplasia, hematologic malignancy with myelosuppression); or mixed.2,3 Previous studies have shown that both absolute and relative (defined by percentage decrease from baseline) thrombocytopenia is associated with poor outcomes in the critically ill.1,4,5
Platelet transfusions are frequently utilized in critically ill patients with moderate-to-severe thrombocytopenia for the prevention of bleeding complications. However, the efficacy of such prophylactic transfusion episodes in the non-bleeding critically ill lacks robust clinical or experimental evidence and places strain on blood bank inventories. Unlike many other blood products, platelets must be stored at room temperature, resulting in limited shelf-life and logistical difficulties regarding the optimal and most cost-effective approach to platelet handling. While the American Association of Blood Banks (AABB) recommends prophylactic platelet transfusion in patients with decreased platelet production and platelet counts ≤ 10 × 109/L,6 thrombocytopenia in the critically ill is not always secondary to decreased production, and prophylactic transfusions are often provided at higher platelet count thresholds.7
The objective of this study was to assess the impact of prophylactic platelet transfusion on bleeding complications in critically ill patients with thrombocytopenia. We hypothesized that platelet transfusions would not be associated with improved clinical outcomes.
Materials and Methods
This is a retrospective cohort study conducted under the approval of the Mayo Clinic (Rochester, Minnesota) Institutional Review Board with a waived requirement for written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used in the design and conduct of the study.8
The study population included adult patients admitted to a medical, surgical, or combined medical-surgical ICU at an academic medical center. Basic ICU characteristics are provided in Supplemental Table 1. Inclusion criteria were age ≥ 18 years, ICU admission between January 1, 2009 and December 31, 2013, and the presence of a platelet count measured during ICU admission. For patients with multiple ICU admissions during the study period, only the first admission with a platelet count measurement was included. For patients with multiple platelet counts who did not receive platelet transfusion, the lowest platelet value was utilized as the qualifying platelet count. In patients with multiple platelet counts who did receive platelets during their first ICU admission, the platelet count immediately preceding platelet transfusion was utilized. Patients were excluded for lack of research authorization and prior inclusion in the study such that no patient was included twice. Patients receiving red blood cell (RBC) transfusion in the 24 hours before measurement of the qualifying platelet count were also excluded in order to minimize the risk of including those with active bleeding. Similarly, patients receiving RBC transfusion in the interval between platelet count measurement and platelet transfusion were also excluded.
The primary exposure variable was the presence or absence of prophylactic platelet transfusion in the 24 hours following measurement of the qualifying platelet count value. Prophylactic platelet transfusion was defined as the first platelet transfusion episode during the qualifying ICU admission, excluding those patients that had received RBC transfusion in the 24 hours before platelet count measurement or in the interval between platelet count measurement and platelet component administration. The presence and timing of all transfusion episodes were extracted from the electronic health record, with the timing of transfusion defined as the actual transfusion initiation time rather than the time of component issue from the blood bank. Of note, platelet transfusion orders at the study institution require selection of an indication from the following categories: platelet count ≤ 100 × 109/L with active bleeding; ≤ 10 × 109/L without active bleeding; ≤ 20 × 109/L with fever, disseminated intravascular coagulation, sepsis, or other conditions associated with platelet destruction; ≤ 20 × 109/L prior to central venous catheter insertion; ≤ 50 × 109/L requiring surgery; and active bleeding in the presence of antiplatelet therapies at any platelet count. Ordering providers may also select “other” with subsequent free text entry of the indication, and hence platelet transfusions are not strictly per protocol. Additionally, no indication is required for transfusion orders placed in surgical/procedural suites or during massive transfusion protocols.
The primary outcome for this investigation was RBC transfusion within 24 hours of the qualifying platelet count value for non-platelet-transfused patients and within 24 hours of platelet transfusion for platelet-transfused patients. Secondary outcome measures included ICU and hospital-free days (defined as 28 minus the ICU or hospital length of stay in days, with patients dying prior to discharge and those with ICU or hospital durations greater than 28 days receiving a score of zero), ICU mortality, all-cause mortality within 30 days of ICU discharge, and changes in sequential organ failure assessment (SOFA) score 24 hours after the qualifying platelet count or platelet transfusion for non-transfused and transfused patients, respectively.
Relevant information for study participants was extracted using the Acute Care DataMart, an institutional resource that contains clinical, demographic, transfusion, and laboratory data for patients admitted to the ICU.9 Additional baseline characteristics were obtained from the Mayo Clinic Life Sciences System (MCLSS), a second institutional database.10 Extensive validation has been performed on these databases, and the accuracy of extracted data is superior to that collected by manual methods.11
Statistical Analysis
The binary end points of qualifying RBC transfusion (primary outcome), ICU mortality and 30-day mortality were modeled using logistic regression. ICU and hospital free day endpoints were modeled using negative binomial generalized linear models, while changes in SOFA scores from the time of platelet count measurement (controls) or platelet transfusion (cases) to 24 hours later were modeled using linear regression. To account for imbalances in measured covariates, individuals were matched with and without platelet transfusions using 1:1 propensity score matching without replacement. Missing data were imputed using recursive conditional mean imputation. The variable with the least missing was imputed first, then the variable with the next least missing, and so forth. Missing variables included hemoglobin (0.3%), INR (21.0%), and aPTT (35.0%). When comparing fully non-missing patients to patients with any missing value, patients with any missing were less likely to receive prophylactic platelet transfusion (1.1% to 4.7%), aspirin (45.0% to 56.8%), warfarin (10.5% to 26.8%), or be of male gender (53.7% to 62.5%; data not shown). The assumption of missing at random appeared reasonable for all other variables. The propensity score was constructed from a logistic regression model and matching using the SAS macro %gmatch (greedy-matching algorithm).12 The caliper width was set to 0.25 standard deviations of the logit propensity score.13 To assess pre-match and post-match imbalances, standardized differences were estimated for all baseline covariates.14 A standardized difference <10% was considered to be an adequate balance. It was decided a priori that any variables with inadequate balances would be adjusted for in the subsequent analyses. Following matching, statistical analyses were performed as previously described for unmatched analyses. The primary analysis was the primary (RBC transfusion) and secondary outcomes (ICU mortality, all-cause 30 day mortality, ICU free days, hospital free days, and change in SOFA score) in the propensity score matched dataset.
To further assess the robustness of study findings, multiple a priori sensitivity analyses were planned including restriction to 1) patients with platelet counts ≤ 50 × 109/L, which is commonly used as platelet transfusion threshold in surgical ICU patients and prior to invasive procedures; 2) patients with platelet counts ≤ 20 × 109/L, which is a commonly utilized platelet transfusion threshold; 3) medical ICU patients; 4) surgical ICU patients; and 5) exclusion of patients requiring surgical or interventional procedures outside of the ICU environment (e.g. interventional radiology procedures, complex endoscopy, coronary angiography) in the 24 hour period following platelet count measurement or platelet transfusion. This last sensitivity analysis was intended to mitigate the impact of the surgical procedure on subsequent RBC transfusions. Additionally, as a supplement to propensity-matched analyses, a multivariable regression model was run in the full cohort adjusting for all variables used in the propensity score matching.
It was decided a priori that an odds ratio of 1.5 for RBC transfusion in those who receive prophylactic transfusion compared to those who did not was clinically important. Assuming an RBC rate of 10% in those without prophylactic transfusion, we would have 80% power at 0.05 alpha significance level with 957 1:1 platelet transfusions to no platelet transfusion matched sets (alpha=0.05, beta=0.20). The prophylactic transfusion rate was estimated to be 4%, thus we would need to examine ~24,000 study participants to acquire 957 patients with prophylactic platelet transfusion. This number was inflated to 31,000 to facilitate a priori sensitivity analyses. All analyses were performed using SAS version 9.4.
Results
A total of 45,785 patients were admitted to an eligible ICU during the study period with 40,693 (88.9%) meeting inclusion criteria (Figure 1). Of the 37,466 patients not receiving platelets, 7,017 were excluded for having received an RBC transfusion in the 24 hours preceding platelet count measurement. Of the 3,227 patients receiving platelets, 2,162 patients were excluded due to receiving RBCs in the 24 hours preceding platelet count measurement or platelet transfusion, creating a total cohort of 31,514 patients including 14,171 surgical patients; 10,162 medical patients; and 7,181 patients in a mixed medical-surgical ICU. In total, 1,065 (3.4%) received a prophylactic platelet transfusion with a median (interquartile range) volume of 1 (1-1) units. The median time between platelet count measurement and platelet transfusion was 2.8 (1.4-6.2) hours.
Figure 1.
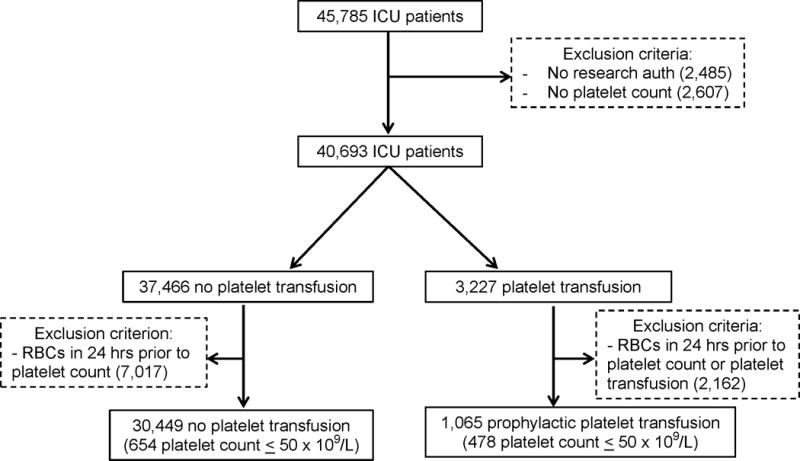
Study population flow diagram. ICU – intensive care unit, RBC – red blood cells.
Comparison of baseline clinical, demographic, and laboratory characteristics between platelet-transfused and non-platelet transfused patients are displayed in Table 1. Briefly, patients receiving platelets had a higher burden of comorbid disease, higher baseline SOFA scores, and more severe coagulation abnormalities. A total of 2,115 patients (6.7%) received a qualifying RBC transfusion, including 1,627 patients (5.3%) in the non-platelet group and 488 (45.8%) in the platelet-transfused group (p < 0.001). Results of univariate analyses in the non-matched cohort are displayed in Supplemental Table 2. Briefly, in unadjusted analyses those receiving platelets were more likely to have an RBC transfusion, increased mortality, fewer ICU and hospital free days, and smaller decreases in SOFA scores. The median RBC transfusion volume was 2 (1 – 2) units for both platelet-transfused and non-platelet transfused patients.
Table 1.
Standardized Differences In The Full Cohort and Propensity-Matched Cohort
| Full Cohort | Matched | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | No
Platelet Transfusion N=30449 |
Platelet Transfusion N=1065 |
Standardized Difference (%) |
p-value | No
Platelet Transfusion N=994 |
Platelet Transfusion N=994 |
Standardized Difference (%) |
p-value |
| Demographics | ||||||||
| Age | 62.4 (17.5) | 62.3 (15.2) | 0.5 | 0.888† | 62.8 (15.1) | 62.7 (15.2) | 0.4 | 0.935† |
| Male Sex | 17946 (58.9%) | 728 (68.4%) | 19.7 | <0.001‡ | 710 (72.1%) | 684 (69.4%) | 5.8 | 0.198‡ |
| White Race | 27419 (90.0%) | 962 (90.3%) | 0.9 | 0.764‡ | 896 (91.0%) | 890 (90.4%) | 2.1 | 0.642‡ |
| Laboratory Variables | ||||||||
| Albumin (g/dL) | 0.8 (1.6) | 1.6 (1.8) | 42.8 | <0.001† | 1.6 (1.8) | 1.5 (1.8) | 4.3 | 0.337† |
| Hemoglobin (g/dL) | 10.9 (1.9) | 10.1 (2.0) | 40.2 | <0.001† | 10.2 (1.9) | 10.2 (2.0) | 1.8 | 0.682† |
| INR | 1.3 (0.5) | 1.4 (0.6) | 30.2 | <0.001† | 1.4 (0.7) | 1.4 (0.6) | 2.3 | 0.608† |
| Platelet count (x 109/L) | 172.9 (81.8) | 72.8 (60.2) | 139.3 | <0.001† | 81.1 (50.1) | 77.5 (60.1) | 6.4 | 0.154† |
| Medications | ||||||||
| Aspirin | 16089 (52.8%) | 446 (41.9%) | 22.1 | <0.001‡ | 414 (42.0%) | 437 (44.4%) | 4.7 | 0.296‡ |
| Clopidogrel | 3568 (11.7%) | 77 (7.2%) | 15.4 | <0.001‡ | 83 (8.4%) | 76 (7.7%) | 2.6 | 0.563‡ |
| Factor Xa inhibitor | 53 (0.2%) | 3 (0.3%) | 2.3 | 0.439§ | 3 (0.3%) | 3 (0.3%) | 0.0 | 1.000§ |
| LMW heparin | 1885 (6.2%) | 43 (4.0%) | 9.8 | 0.004‡ | 39 (4.0%) | 39 (4.0%) | 0.0 | 1.000‡ |
| Thrombin inhibitor | 198 (0.7%) | 16 (1.5%) | 8.3 | 0.001‡ | 16 (1.6%) | 15 (1.5%) | 0.8 | 0.856‡ |
| Unfractionated heparin | 3074 (10.1%) | 72 (6.8%) | 12.0 | <0.001‡ | 71 (7.2%) | 70 (7.1%) | 0.4 | 0.930‡ |
| Warfarin | 6357 (20.9%) | 203 (19.1%) | 4.5 | 0.151‡ | 184 (18.7%) | 201 (20.4%) | 4.4 | 0.334‡ |
| Comorbidities | ||||||||
| Charlson score | 1.7 (2.1) | 2.2 (2.2) | 21.6 | <0.001† | 2.2 (2.4) | 2.2 (2.3) | 1.0 | 0.824† |
| SOFA score | 4.1 (3.3) | 8.7 (3.6) | 131.6 | <0.001† | 8.2 (3.9) | 8.4 (3.4) | 3.2 | 0.471† |
| AIDS | 27 (0.1%) | 0 (0.0%) | 4.2 | 1.000§ | 0 (0.0%) | 0 (0.0%) | 4.5 | 1.000§ |
| CHF | 4727 (15.5%) | 162 (15.2%) | 0.9 | 0.781‡ | 169 (17.2%) | 154 (15.6%) | 4.1 | 0.361‡ |
| Chronic pulmonary disease | 4584 (15.1%) | 117 (11.0%) | 12.1 | <0.001‡ | 111 (11.3%) | 114 (11.6%) | 1.0 | 0.832‡ |
| Connective tissue disease | 1217 (4.0%) | 27 (2.5%) | 8.2 | 0.016‡ | 21 (2.1%) | 25 (2.5%) | 2.7 | 0.551‡ |
| Dementia | 771 (2.5%) | 11 (1.0%) | 11.3 | 0.002‡ | 12 (1.2%) | 11 (1.1%) | 0.9 | 0.834‡ |
| DM w/o complications | 5033 (16.5%) | 169 (15.9%) | 1.8 | 0.568‡ | 142 (14.4%) | 164 (16.6%) | 6.2 | 0.171‡ |
| DM w/complications | 3347 (11.0%) | 115 (10.8%) | 0.6 | 0.842‡ | 101 (10.3%) | 108 (11.0%) | 2.3 | 0.609‡ |
| Hemiplegia | 245 (0.8%) | 13 (1.2%) | 4.2 | 0.139‡ | 10 (1.0%) | 13 (1.3%) | 2.8 | 0.529‡ |
| Kidney disease | 450 (1.5%) | 66 (6.2%) | 24.8 | <0.001‡ | 57 (5.8%) | 61 (6.2%) | 1.7 | 0.704‡ |
| Leukemia | 381 (1.3%) | 85 (8.0%) | 32.5 | <0.001‡ | 67 (6.8%) | 53 (5.4%) | 5.9 | 0.187‡ |
| Liver disease | 3261 (10.7%) | 191 (17.9%) | 20.7 | <0.001‡ | 176 (17.9%) | 182 (18.5%) | 1.6 | 0.726‡ |
| Lymphoma | 930 (3.1%) | 135 (12.7%) | 36.3 | <0.001‡ | 122 (12.4%) | 109 (11.1%) | 4.1 | 0.363‡ |
| Myocardial infarction | 5439 (17.9%) | 158 (14.8%) | 8.2 | 0.011‡ | 165 (16.8%) | 155 (15.7%) | 2.8 | 0.541‡ |
| Peptic ulcer disease | 1541 (5.1%) | 56 (5.3%) | 0.9 | 0.773‡ | 39 (4.0%) | 53 (5.4%) | 6.7 | 0.135‡ |
| PVD | 1714 (5.6%) | 62 (5.8%) | 0.8 | 0.789‡ | 59 (6.0%) | 59 (6.0%) | 0.0 | 1.000‡ |
| Solid tumor | 3720 (12.2%) | 136 (12.8%) | 1.7 | 0.588‡ | 129 (13.1%) | 125 (12.7%) | 1.2 | 0.788‡ |
| Solid tumor w/metastases | 923 (3.0%) | 23 (2.2%) | 5.5 | 0.101‡ | 29 (2.9%) | 21 (2.1%) | 5.2 | 0.252‡ |
Equal variance t-test
Chi-square test
Fisher exact test
Continuous variables presented as mean (standard deviation). Categorical variables presented as n (%).
AIDS – acquired immunodeficiency syndrome, CHF – congestive heart failure, DM – diabetes mellitus, INR – international normalized ratio, LMW – low molecular weight, PVD – peripheral vascular disease, SOFA – sequential organ failure assessment.
In total, 985 patients receiving prophylactic platelets were propensity-matched 1:1 with a patient not receiving platelets. The propensity-matching significantly reduced between group differences (Table 1, Figure 2). Of note, 80 patients receiving a platelet component were removed from propensity-matched analyses due to the lack of a suitable non-transfused subject. Propensity-matching largely removed between group differences resulting in similar covariate profiles (Table 1, Figure 2). The results of propensity-matched analyses are displayed in Table 2. Briefly, patients receiving platelets were more likely to receive an RBC transfusion [OR 7.5, 95% CI (5.9, 9.5), p < .001], fewer ICU-free days [mean (SD) 20.8 (9.1) vs 22.7 (8.1) days, mean rate ratio 0.91, 95% CI (0.85, 0.97), p = 0.005], and fewer hospital-free days [13.0 (9.7) vs 15.8 (9.4) days, mean rate ratio 0.8, 95% CI (0.7, 0.9), p < .001]. They also had less improvement in SOFA scores [mean decrease 0.2 (3.6) vs. 1.8 (3.3), mean difference 1.5, 95% CI (1.2, 1.8), p < .001] compared to their non-transfused counterparts. Changes in SOFA component scores are displayed in Supplemental Table 3, with platelet-transfused patients experiencing improvement only in the hematologic component with worsened respiratory, cardiovascular, and neurological component scores compared to non-transfused patients. There were no significant associations between platelet transfusion and mortality.
Figure 2.
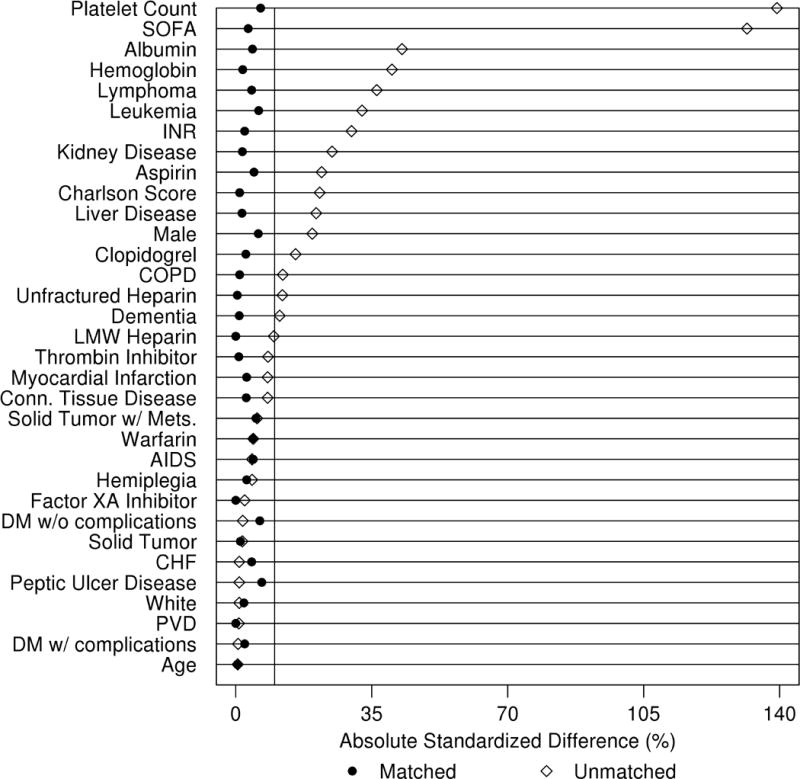
Standardized mean differences between unmatched and propensity-matched cohorts. Points to the right of the vertical reference line represent a standardized difference greater than 10.0 between those who received prophylactic platelet transfusion and those who did not. AIDS – acquired immunodeficiency syndrome; CHF – congestive heart failure; COPD – chronic obstructive pulmonary disease; DM – diabetes mellitus; INR – international normalized ratio; PVD – peripheral vascular disease; SOFA – sequential organ failure assessment
Table 2.
Primary and Secondary Outcomes in the Propensity-Matched Cohort
| No Platelet
Transfusion (N=985) |
Platelet
Transfusion (N=985) |
|||
|---|---|---|---|---|
| Outcome | Event (%) | Event (%) | OR (95% CI)† | p-value† |
| RBC transfusion within 24 hours | 102 (10.4%) | 456 (46.3%) | 7.5 (5.9, 9.5) | <0.001 |
| ICU mortality | 90 (9.1%) | 101 (10.3%) | 1.1 (0.8, 1.5) | 0.403 |
| All-cause 30-day mortality | 162 (16.4%) | 184 (18.7%) | 1.2 (0.9, 1.5) | 0.193 |
|
| ||||
| Outcome | Mean (SD) | Mean (SD) | Mean Rate Ratio (95% CI)‡ | p-value‡ |
| ICU-free days | 22.7 (8.3) | 20.8 (9.1) | 0.9 (0.9, 1.0) | 0.004 |
| Hospital-free days | 15.8 (9.4) | 13.0 (9.7) | 0.8 (0.7, 0.9) | <0.001 |
|
| ||||
| Outcome | Mean (SD) | Mean (SD) | Mean Difference (95% CI)§ | p-value§ |
| Change in SOFA score* | −1.8 (3.3) | −0.2 (3.6) | 1.5 (1.2, 1.8) | <0.001 |
CI – confidence interval, ICU – intensive care unit, RBC – red blood cell, SOFA – sequential organ failure assessment, SD – standard deviation.
Estimate and p-value comes from logistic regression. An odds ratio > 1 implies increased odds for the outcome in those receiving platelet transfusions.
Estimate and p-value comes from negative binomial regression. A rate ratio < 1 implies decreased mean for the outcome in those receiving platelet transfusions. Specifically, a rate ratio of 0.9 can be interpreted as a 10% decrease in the mean for those receiving platelet transfusions compared to those who did not.
Change in SOFA score defined as 24-hr SOFA score – Baseline SOFA score, with negative values reflecting improvement in SOFA scores in the ensuing 24 hours.
Estimate and p-value comes from linear regression. A mean difference > 0 implies less improvement in SOFA score in those receiving platelet transfusions.
As a sensitivity analysis to the propensity score matching, a multivariable model was run in the full cohort adjusting for all variables used in the propensity score matching (Supplemental Table 2). The results were consistent with the primary analysis (Table 2). Regarding those with marked thrombocytopenia, 1,132 patients (3.6%) had a qualifying platelet count ≤ 50 × 109/L, of which 478 (42%) received a platelet transfusion. There were 229 propensity matches where both the case and control had platelet counts ≤ 50 × 109/L. When restricting analyses to these patients, platelet administration remained associated with increased RBC transfusion rates but not with hospital or ICU free days or SOFA scores (Table 3). Similar results were seen at platelet counts ≤ 20 × 109/L (Supplemental Table 4). The results of propensity-matched analyses restricted to medical or surgical ICU patients are displayed in Supplemental Table 5. Briefly, platelet administration was associated with higher odds for RBC transfusion in both environments, deterioration of 24-hour SOFA scores in the medical ICU, and decreased improvement in SOFA scores in surgical patients. When excluding patients that required surgery or invasive procedures, outcomes remained consistent with the primary analyses (Supplemental Table 6). As a post-hoc analysis, we repeated the primary analysis adjusting for the effects of plasma transfusions administered in the 24 hours before or after platelet count measurement (non-platelet transfused group) or platelet transfusion (platelet transfused group), and the results were similar with the exception of hospital mortality, which was now significantly higher in patients with prophylactic platelet transfusions (Supplemental Table 7).
Table 3.
Primary and Secondary Outcomes in the Propensity-Matched Cohort With Platelet Counts ≤ 50 × 109/L
| No Platelet
Transfusion (N=229) |
Platelet
Transfusion (N=229) |
|||
|---|---|---|---|---|
| Outcome | Event (%) | Event (%) | OR (95% CI)† | p-value† |
| RBC transfusion within 24 hours | 29 (12.7%) | 106 (46.3%) | 5.9 (3.7, 9.5) | <0.001 |
| ICU mortality | 40 (17.5%) | 41 (17.9%) | 1.0 (0.6, 1.7) | 0.903 |
| All-cause 30-day mortality | 75 (32.8%) | 81 (35.4%) | 1.1 (0.8, 1.7) | 0.554 |
|
| ||||
| Outcome | Mean (SD) | Mean (SD) | Mean Rate Ratio (95% CI)‡ | p-value‡ |
| ICU-free days | 19.9 (10.5) | 18.3 (10.6) | 0.9 (0.8, 1.1) | 0.399 |
| Hospital-free days | 10.2 (10.0) | 7.8 (9.4) | 0.8 (0.5, 1.1) | 0.144 |
|
| ||||
| Outcome | Mean (SD) | Mean (SD) | Mean Difference (95% CI)§ | p-value§ |
| Change in SOFA score* | −0.3 (2.9) | −0.5 (3.5) | −0.2 (−0.8, 0.4) | 0.553 |
CI – confidence interval, ICU – intensive care unit, RBC – red blood cell, SOFA – sequential organ failure assessment, SD – standard deviation.
Estimate and p-value comes from logistic regression. An odds ratio > 1 implies increased odds for the outcome in those receiving platelet transfusions.
Estimate and p-value comes from negative binomial regression. A rate ratio < 1 implies decreased mean for the outcome in those receiving platelet transfusions. Specifically, a rate ratio of 0.9 can be interpreted as a 10% decrease in the mean for those receiving platelet transfusions compared to those who did not.
Change in SOFA score defined as 24-hr SOFA score – Baseline SOFA score, with negative values reflecting improvement in SOFA scores in the ensuing 24 hours.
Estimate and p-value comes from linear regression. A mean difference > 0 implies less improvement in SOFA score in those receiving platelet transfusions
Discussion
Thrombocytopenia is frequently encountered in the critically ill; however, the utility of platelet transfusions in the absence of bleeding remains unclear. In this investigation, prophylactic platelet transfusions were not associated with improved outcomes in a diverse cohort of critically ill patients. To the contrary, patients receiving platelets had higher rates of RBC transfusion in the subsequent 24 hours, fewer ICU- and hospital-free days, and less improvement in SOFA scores. These relationships persisted in multiple predefined sensitivity analyses including limitation to medical or surgical ICU patients. In addition, similar results were seen with varying thresholds of thrombocytopenia.
Platelets, as they exist innately in the human body, have increasingly been recognized for their beneficial effects on a variety of physiologic processes. Besides their impact on thrombosis and hemostasis, platelets also participate in inflammatory responses, enhance endothelial barrier function, and promote wound healing, tissue regeneration, and angiogenesis.15,16 It is therefore not surprising that thrombocytopenia has been identified as a poor prognostic marker for critically ill patients and has been utilized as a marker for organ impairment.1,4,5,16–19 In the critically ill, the mechanisms driving thrombocytopenia are often multifactorial with platelet declines typically occurring as a consequence of underlying illness or in response to therapeutic interventions. Although platelet transfusions are frequently utilized for the quantitative restoration of platelet levels, the ability of platelet transfusions to restore normal platelet function and physiology remains unclear.
Bleeding is the most feared consequence of thrombocytopenia. While the thrombocytopenic patient with active bleeding is likely to benefit from platelet transfusion (though precise thresholds for transfusion remain poorly defined), there exists remarkable equipoise over the utility of platelet transfusions for non-bleeding patients with thrombocytopenia. From studies of patients with bone marrow hypoproliferation, spontaneous bleeding events are rare with platelet counts greater than 20 × 109/L.20 However, many patients receive prophylactic platelet transfusions at higher platelet counts. Indeed, clinically relevant platelet dysfunction may exist and potentially increase the risk of bleeding at higher platelet count levels in commonly encountered clinical scenarios (e.g., trauma, septic shock, antiplatelet therapy). Conversely, severe thrombocytopenia is not always associated with increased rates of bleeding. Perhaps most notably, endothelial activation with associated release of von Willebrand Factor may compensate for low platelet numbers in those with severe liver disease.21 It is therefore not particularly surprising that the thresholds utilized for platelet transfusion vary greatly amongst providers, with differing guidelines for platelet transfusion largely based upon expert opinion.6,22,23
In non-surgical patients, the most widely identified platelet count threshold for transfusion is 10 × 109/L; however, this is often increased to 20-30 × 109/L for patients with additional risk factors for bleeding including severe hepatic or renal impairment or ongoing coagulopathy (i.e., disseminated intravascular coagulation).24,25 In patients undergoing surgical or interventional procedures, even higher platelet counts are typically maintained (e.g., 50-100 × 109/L) in the immediate preoperative and postoperative periods despite the lack of compelling evidence to either support or refute this practice.23,26,27 In the current investigation, which included a large and heterogeneous cohort of surgical and medical ICU patients, prophylactic platelet therapy was not associated with fewer bleeding complications in propensity-matched analyses. Conversely, patients who received prophylactic platelet therapy were more likely to receive an RBC transfusion in the ensuing 24 hours, with odds for this outcome seven times greater than their non-transfused counterparts. This relationship remained after the exclusion of patients requiring surgical or interventional procedures in the ensuring 24 hours, and also after restricting the analyses to those patients with platelet counts less than or equal to 50 × 109/L and 20 × 109/L. While the driving factor behind the higher incidence of RBC transfusion seen in patients transfused with platelets remains unclear, there are several possible explanations. The first relates to provider-specific transfusion practices, such that providers with greater tendency to administer platelets prophylactically were also perhaps more inclined to transfuse erythrocytes. While plausible, this relationship remains conjectural. It may also be that patients receiving platelet transfusion were more critically ill than their non-transfused counterparts, despite successful propensity-matching including near identical pre-transfusion SOFA scores, comorbidity profiles, and laboratory characteristics including hemoglobin concentrations. As an alternative explanation, it is possible that platelet transfusion contributed to hemodilution, which in some patients may have dropped hemoglobin values below thresholds for RBC transfusion; however, it must also be recognized that platelet components are not a particularly high-volume transfusion product.
In addition to increased RBC transfusion rates, platelet-transfused patients had fewer ICU and hospital free days and less improvement in their SOFA scores in the ensuing 24 hours, suggesting that platelet transfusion did not result in improved clinical outcomes. This was seen in both medical and surgical ICU populations. Interestingly, platelet transfused patients had inferior SOFA component scores following transfusion in every organ system except for the renal system, with equivalent scores between groups, and the hematologic system, in which platelet transfused patients experienced improved scores directly attributed to higher platelet counts following transfusion.
There are several notable limitations of this investigation. The first relates to the retrospective design which does not allow for the precise determination of the indications for transfusion therapies. As such, some platelet transfusions may have been given in response to active hemorrhage rather than for the prevention of future bleeding complications. In an attempt to limit this possibility, we excluded any patient that received RBC transfusion in the 24-hour period preceding platelet count measurement or platelet transfusion, assuming that most patients with active bleeding secondary to thrombocytopenia would receive RBCs in addition to platelets. Of note, this may have also inadvertently excluded some non-bleeding patients from further analyses. Additionally, the primary outcome measure of RBC transfusion was utilized as a surrogate for bleeding, but patients may have been transfused for other reasons (e.g., anemia of chronic disease, dilutional anemia). However, given that both groups had similar baseline hemoglobin values and comorbidity profiles in matched analyses, differences in RBC transfusion rates are unlikely to be explained entirely by the heterogeneity of provider-responses to stable, non-bleeding anemia. Other surrogates for bleeding complications such as hemoglobin and hematocrit levels were not utilized given concerns for variation based upon the presence of other common clinical factors (e.g. crystalloid and non-blood colloid administration, phlebotomy, diuresis). Documented bleeding episodes (e.g. gastrointestinal hemorrhage) were not included given lack of standardization for reporting in our electronic health record environment, resulting in an inability to objectively identify events. Another limitation related to the retrospective nature of this study is the potential for residual confounding. Despite careful propensity-matching on observed variables, the possibility for residual confounding persists such that the platelet-transfused group could have been representative of a sicker patient population. Additionally, INR and aPTT values were missing for 20% and 35% of patients, respectively, and these missing variables were imputed. Significant differences were noted when patients with any missing were compared to non-missing, including prophylactic platelet transfusion, aspirin use, warfarin use, and gender. The data were assumed to be missing at random, but this assumption could not be tested, and may have biased our results. Moreover, due to the paucity of patients with platelet counts less than 10 × 109/L that did not receive prophylactic platelets (n = 2), we could not assess the utility of platelet transfusion at such severe levels of thrombocytopenia. Hence, we were only able to assess the impact of prophylactic platelet transfusions utilizing a minimum platelet count threshold of 20 × 109/L, and are unable to comment on the utility of platelet transfusion for platelet counts below 10 × 109/L. Other limitations include some of the sensitivity analyses being underpowered, and the power of the propensity score matched samples being somewhat limited. The observed odds ratios of 1.2 for all-cause 30 day mortality and 1.1 for ICU mortality may be clinically important, but the study was not powered to detect differences that small. Notably, the same estimates were significant when analyzing the full cohort in multivariable models that adjusted for variables. Finally, it must be acknowledged that while clinical trajectories did not improve for the cohort as a whole following platelet transfusion, it is possible that certain subpopulations may indeed benefit from the intervention, though these subgroups have yet to be identified.
Conclusions
Prophylactic platelet transfusion in a large cohort of medical and surgical ICU patients was not associated with fewer bleeding complications or improved clinical outcomes. Conversely, patients receiving platelet therapy were more likely to receive RBCs in the subsequent 24 hours, experience less improvement in organ function, and spend more time in the ICU and hospital. In lights of these findings, more conservative management of thrombocytopenia may be warranted in non-bleeding critically ill patients. Clinical trials specifically addressing this important knowledge gap are critically needed.
Supplementary Material
Key Points.
Question
Is prophylactic transfusion of platelets associated with fewer bleeding complications and improved outcomes in critically ill patients?
Findings
In this propensity matched study, those receiving a prophylactic platelet transfusion were more likely to receive red blood cell transfusions, experience fewer ICU and hospital free days, and have less improvement in sequential organ failure assessment (SOFA) scores than their non-transfused counterparts.
Meaning
Prophylactic transfusion of platelets in critically ill patients may not be associated with improved clinical outcomes, though further investigation is clearly warranted.
Acknowledgments
The authors would like to thank Timothy J. Weister, MSN, RN (study coordinator, Anesthesia Clinical Research Unit, Mayo Clinic, Rochester, MN) for his assistance with data extraction.
Sources of Funding: The study was made possible by funding from the Mayo Clinic Department of Anesthesiology and Perioperative Medicine and the Critical Care Integrated Multidisciplinary Practice, Rochester, Minnesota. In addition, this study was supported by an NIH R01 grant (HL121232) to Dr. Kor.
Footnotes
Conflicts of Interest: No conflicts of interests exist for the submitted materials.
Author Contributions:
M.A. Warner – This author helped design the study, conduct the study, analyze the data, and write the manuscript
A. Chandran – This author helped design the study, conduct the study, and write the manuscript.
R.D. Frank – This author helped analyze the data and write the manuscript.
D.J. Kor – This author helped design the study, conduct the study, and the write the manuscript.
References
- 1.Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139:271–8. doi: 10.1378/chest.10-2243. [DOI] [PubMed] [Google Scholar]
- 2.Antier N, Quenot JP, Doise JM, Noel R, Demaistre E, Devilliers H. Mechanisms and etiologies of thrombocytopenia in the intensive care unit: impact of extensive investigations. Ann Intensive Care. 2014;4:24. doi: 10.1186/s13613-014-0024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiolliere F, Serre-Sapin AF, Reignier J, et al. Epidemiology and outcome of thrombocytopenic patients in the intensive care unit: results of a prospective multicenter study. Intensive Care Med. 2013;39:1460–8. doi: 10.1007/s00134-013-2963-3. [DOI] [PubMed] [Google Scholar]
- 4.Moreau D, Timsit JF, Vesin A, et al. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007;131:1735–41. doi: 10.1378/chest.06-2233. [DOI] [PubMed] [Google Scholar]
- 5.Strauss R, Wehler M, Mehler K, Kreutzer D, Koebnick C, Hahn EG. Thrombocytopenia in patients in the medical intensive care unit: bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002;30:1765–71. doi: 10.1097/00003246-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 7.Arnold DM, Crowther MA, Cook RJ, et al. Utilization of platelet transfusions in the intensive care unit: indications, transfusion triggers, and platelet count responses. Transfusion. 2006;46:1286–91. doi: 10.1111/j.1537-2995.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 9.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28:42. 4–5. [PubMed] [Google Scholar]
- 10.Chute CG, Beck SA, Fisk TB, Mohr DN. The Enterprise Data Trust at Mayo Clinic: a semantically integrated warehouse of biomedical data. J Am Med Inform Assoc. 2010;17:131–5. doi: 10.1136/jamia.2009.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh B, Singh A, Ahmed A, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87:817–24. doi: 10.1016/j.mayocp.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekhon JS. Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching package for R. Journal of Statistical Software. 2011;42:1–52. [Google Scholar]
- 13.Bergstralh EJ, Kosanke JL. Computerized matching of cases to controls. Technical Report 56 Mayo Foundation. 1995 [Google Scholar]
- 14.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 15.Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 16.Thachil J. Platelets in Inflammatory Disorders: A Pathophysiological and Clinical Perspective. Semin Thromb Hemost. 2015;41:572–81. doi: 10.1055/s-0035-1556589. [DOI] [PubMed] [Google Scholar]
- 17.Baughman RP, Lower EE, Flessa HC, Tollerud DJ. Thrombocytopenia in the intensive care unit. Chest. 1993;104:1243–7. doi: 10.1378/chest.104.4.1243. [DOI] [PubMed] [Google Scholar]
- 18.Crowther MA, Cook DJ, Meade MO, et al. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005;20:348–53. doi: 10.1016/j.jcrc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28:1871–6. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 20.Tosetto A, Balduini CL, Cattaneo M, et al. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2009;124:e13–8. doi: 10.1016/j.thromres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Hugenholtz GG, Porte RJ, Lisman T. The platelet and platelet function testing in liver disease. Clin Liver Dis. 2009;13:11–20. doi: 10.1016/j.cld.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman L, Bercovitz RS, Sholapur NS, Heddle NM, Stanworth SJ, Arnold DM. Platelet transfusions for critically ill patients with thrombocytopenia. Blood. 2014;123:1146–51. doi: 10.1182/blood-2013-02-435693. quiz 280. [DOI] [PubMed] [Google Scholar]
- 23.Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–36. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation. An international consensus. Thromb Haemost. 2016;115:896–904. doi: 10.1160/TH15-09-0740. [DOI] [PubMed] [Google Scholar]
- 25.Thachil J, Warkentin TE. How do we approach thrombocytopenia in criticall ill patients? Br J Haematol. 2016 doi: 10.1111/bjh.14482. [DOI] [PubMed] [Google Scholar]
- 26.Samama CM, Djoudi R, Lecompte T, Nathan N, Schved JF. Perioperative platelet transfusion. Recommendations of the French Health Products Safety Agency (AFSSAPS) 2003. Minerva Anestesiol. 2006;72:447–52. [PubMed] [Google Scholar]
- 27.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


