Abstract
Neurological disorders represent major health concerns in terms of comorbidity and mortality worldwide. Despite a tremendous increase in our understanding of the pathophysiological processes involved in disease progression and prevention, the accumulated knowledge so far resulted in relatively moderate translational benefits in terms of therapeutic interventions and enhanced clinical outcomes. Aiming at specific neural molecular pathways, different strategies have been geared to target the development and progression of such disorders. The kallikrein-kinin system (KKS) is among the most delineated candidate systems due to its ubiquitous roles mediating several of the pathophysiological features of these neurological disorders as well as being implicated in regulating various brain functions. Several experimental KKS models revealed that the inhibition or stimulation of the two receptors of the KKS system (B1R and B2R) can exhibit neuroprotective and/or adverse pathological outcomes. This updated review provides background details of the KKS components and their functions in different neurological disorders including temporal lobe epilepsy, traumatic brain injury, stroke, spinal cord injury, Alzheimer’s disease, multiple sclerosis and glioma. Finally, this work will highlight the putative roles of the KKS components as potential neurotherapeutic targets and provide future perspectives on the possibility of translating these findings into potential clinical biomarkers in neurological disease.
Keywords: Kallikrein-Kinin System, bradykinin, kinin, brain injury, neurological disorders, biomarkers
1. The kallikrein-kinin system and its role in the brain
The kallikrein-kinin system (KKS) constitutes an inflammatory response system that influences the biological systems with a number of pleiotropic functions that have critical roles in vascular permeability and inflammation as well as thrombosis and blood coagulation (Costa-Neto, Dillenburg-Pilla et al. 2008). The discovery of the KKS and the subsequent studies that revealed its components dramatically increased our understanding of disease processes (Costa-Neto, Dillenburg-Pilla et al. 2008). While the KKS plays a pivotal role in maintaining normal physiology such as vascular smooth muscle tone, its dysfunction yields detrimental outcomes to the organs involved (Marcondes and Antunes 2005). Indeed, the KKS has been linked to various disorders including: diabetic nephropathy and retinopathy (Jaffa, Kobeissy et al. 2012, Liu and Feener 2013), cardiovascular diseases (Delemasure, Blaes et al. 2013) and diseases of the kidney (Kakoki and Smithies 2009). In addition, the KKS components identified in the nervous system influence the integrity of the neurovascular unit and participate in the progression of neurological diseases (Perry 1991, Raidoo, Ramsaroop et al. 1996, Leeb-Lundberg, Marceau et al. 2005, Guevara-Lora 2012).
The major constituents of the KKS are the kallikrein enzymes, plasma kallikrein (Scarisbrick, Sabharwal et al.) and tissue kallikrein (TK or KLK1), which are serine proteases whose role is, in part, to liberate bradykinin (BK) and kallidin, respectively, from kininogens. Prekallikrein is synthesized by the liver and secreted into the blood for further processing. This enzyme is linked to the coagulation pathways at the level of factor XIIa which converts the prekallikrein into PK (Colman, Sartor et al. 1998, Schmaier 2016). The high-molecular-weight kininogen (HK) is processed by PK and releases the nonapeptide BK: Arg1-Pro2-Pro3-Gly4-Phe5-Ser6-Pro7-Phe8-Arg9, while the low-molecular-weight kininogen (LK) is processed by TK and produces a decapeptide: Lys1-Arg2-Pro3-Pro4-Gly5-Phe6-Ser7-Pro8-Phe9-Arg10 referred to as kallidin or Lys-BK. Moreover, both the LK and HK belong to the cystatin superfamily and have identical N-terminus but differ at their C-terminus (Brown and Dziegielewska 1997). The mature HK is a single chain glycoprotein composed of 626 amino acids with a molecular weight of 120 kDa, and can be divided into 6 domains (Pathak, Wong et al. 2013). The first 3 domains compose the heavy chain of the HK, while the last 2 domains make its light chain. Each domain has specific properties accounting for the versatile functions of the HK (Zhang, Claffey et al. 2000). The proposed role(s) of these domains include:
-
▪
Domain D2: allows interaction with the endothelial cell receptor C1q
-
▪
Domain D3: allows interaction with platelets
-
▪
Domain D4: anchors BK
-
▪
Domain D5 (Histidine-rich domain): permits binding to anionic surfaces
-
▪
Domain D6: facilitates binding of the PK and Hageman factor (coagulation factor XII)
In contrast, the LK is a splice variant composed of 409 amino acids with a molecular weight of 65 kDa. The heavy chain of the LK is composed of 3 domains identical to the ones composing the heavy chain of the HK, namely domains D1, D2 and D3. However, the light chain of the LK consists of only one domain, domain D5. Therefore, the main difference between the HK and LK is the lack of the D6 domain at the C-terminus of the LK illustrated in Figure 1 (Colman and Schmaier 1997). Although kininogens act as a kinin precursors, yet they have other roles in coagulation, fibrinolysis, protease inhibition and angiogenesis (Saito, Goldsmith et al. 1976, Schmaier, Zuckerberg et al. 1983, Hayashi, Amano et al. 2002).
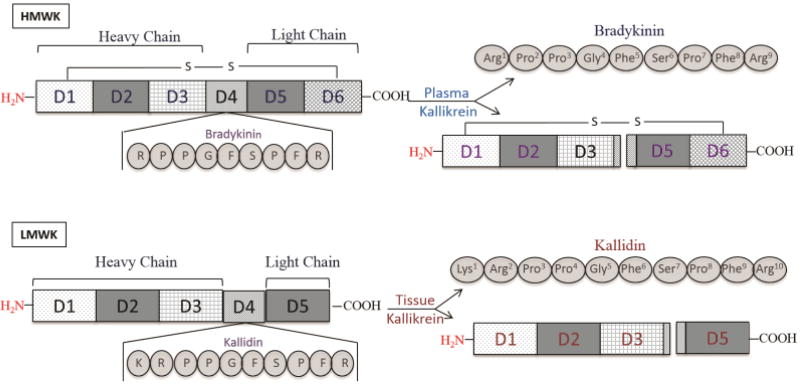
Figure 1. Schematic representation of the HK and LK domains.
The LK and HK have identical N-terminus but differ at their C-terminus. The mature HK is a single chain glycoprotein that can be divided into 6 domains. The first 3 domains (D1, D2 and D3) compose the heavy chain of the HK while the last 2 domains (D5 and D6) make its light chain. The heavy chain of the LK is composed of 3 domains identical to the ones composing the heavy chain of the HK; however, the light chain of the LK consists only of domain D5. The HK releases BK: RPPGFSPFR or Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg from domain 4 (D4) upon PK cleavage. The LK gives rise to Kallidin: KRPPGFSPFR or Lys-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg upon tissue kallikrein cleavage. LK: low-molecular-weight kininogen; HK: high-molecular-weight kininogen; PK: plasma kallikrein; BK: bradykinin.
As defined by Gilman and others, kinins are a group of oligopeptides with similar chemistry and pharmacological characteristics (Gilman 1990, Walker, Perkins et al. 1995). On the cerebrovascular level, these polypeptides belong to the family of autacoids that possess autocrine and/or paracrine functions due to their short-lived duration (Cyr, Lepage et al. 2001). They are involved in vasodilation, increasing vascular leakage and fluid extravasation, development of angioedema, blood pressure regulation, electrolyte homeostasis, renal hemodynamics, pain regulation, prostaglandin generation, and anti-thrombotic and anti-fibrotic activities (Jaffa, Rust et al. 1995, Nussberger, Cugno et al. 2002, Leeb-Lundberg, Marceau et al. 2005, Bergmann, Zheng et al. 2006, Tomita, Sanford et al. 2012). With a less than 30 seconds half-life and following their transient activity, kinins are subject to rapid degradation by peptidases known as kininases (Kayashima, Smithies et al. 2012). The kininase I-type, carboxypeptidase M (CPM), converts BK and kallidin into des-Arg9-BK and des-Arg10-kallidin, respectively (Zhang, Tan et al. 2011). Both kallidin and BK are digested by kininase II (dipeptidylcarboxypeptidase A, angiotensin converting enzyme (ACE)) which is the major BK proteolytic protease in plasma (Sheikh and Kaplan 1986, Erdos 1990, Jaspard, Wei et al. 1993). The same enzyme also converts angiotensin I into angiotensin II (Fuchs, Xiao et al. 2008). Together, kinins and their breakdown products encompass a diverse group of mediators that regulate several inflammatory pathways as described below (Gilman 1990).
1.1 Kinin receptors: kinin 1 and kinin 2 receptors
There are two receptors through which kinins act: kinin 1 receptor (B1R) and kinin 2 receptor (B2R). These two receptors belong to the family of G-protein coupled receptors (GPCRs) (Hess, Borkowski et al. 1992, Menke, Borkowski et al. 1994). In general, BK and kallidin bind to B2R which is ubiquitously expressed in healthy tissues whereas des-Arg9-BK and des-Arg10-kallidin bind to B1R which is expressed under stressful tissue insults (Marceau, Hess et al. 1998, Trabold, Eros et al. 2010). In the nervous system, B2R is localized in the cerebral cortex, thalamus, hypothalamus, brain stem, basal nuclei, ependyma of the lateral and third ventricles while B1R is present on neurons of the thalamus, spinal cord and hypothalamus (Raidoo and Bhoola 1997). Differential localization of KKS components in the CNS are summarized by Guevara-Lora I. (Guevara-Lora 2012). The sequence of events in the kinins activation process is illustrated in Figure 2.
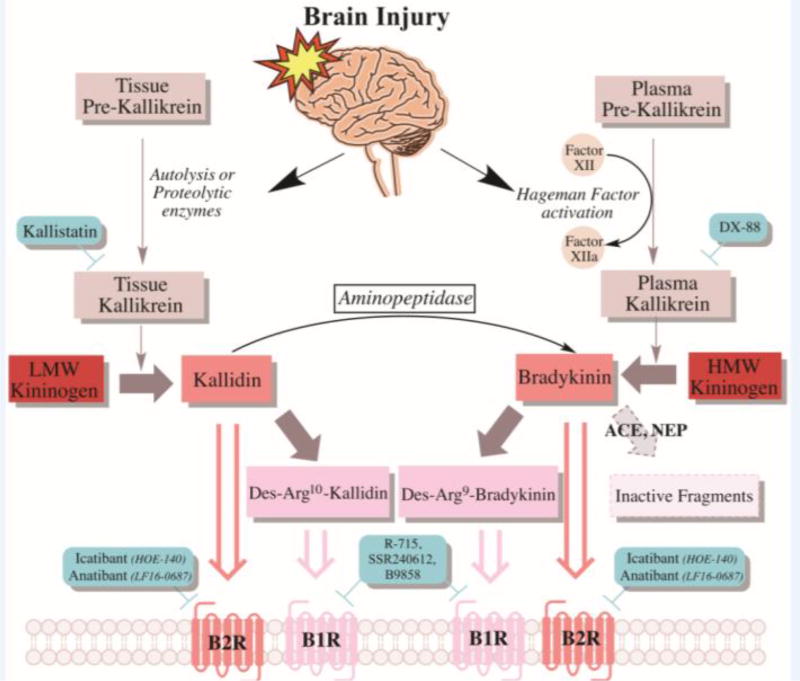
Figure 2. Tissue and plasma KKS activation pathway following brain injury.
Following brain injury, released proteolytic enzymes activate tissue kallikrein, which converts LK into Kallidin. An aminopeptidase can further convert Kallidin into BK. Another inflammatory protein released post-brain injury is PRCP, a plasma prekallikrein activator abundant in the brain. Hageman factor (Factor XII) activates PK, which converts HK into BK. Kallidin and BK are both agonists of the B2R, and can be further broken down into Des-Arg9-Kallidin and Des-Arg9-Bradykinin, which are B1R agonists. ACE and NEP can also degrade BK into inactive fragments. (The green rectangles represent major antagonists/inhibitors). LK: low-molecular-weight kininogen; HK: high-molecular-weight kininogen; PK: plasma kallikrein; BK: bradykinin; B1R: kinin 1 receptor; B2R: kinin 1 receptor; PRCP: prolylcarboxypeptidase; ACE: Angiotensin-converting enzyme; NEP: neutral endopeptidase.
Of interest, TK has been implicated in different brain insults; for example, during brain injury, released proteolytic enzymes activate TK that converts LK into Kallidin, which may be further converted into BK via an aminopeptidase. Prolylcarboxypeptidase (PRCP), a brain-rich prekallikrein activator, promotes activation of plasma pre-kallikrein (Shariat-Madar, Mahdi et al. 2002, Shariat-Madar, Mahdi et al. 2004, Wallingford, Perroud et al. 2009). Similarly, Hageman factor activates PK converting HK into BK. B2R is preferentially activated by BK or Kallidin, the breakdown products of which bind preferentially to B1R.
Furthermore, it has been shown that the expression of both receptors, B1R and B2R, is induced under pathological conditions of tissue injury due to oxidative stress, pro-inflammatory stimuli such as lipopolysaccharides, endotoxins, cytokines (IL-1β and TNF-α) exposure, and vasoactive peptide stimuli as observed in the renin-angiotensin system (Reza, Rasool et al.) (Sabourin, Morissette et al. 2002, Tschope, Schultheiss et al. 2002, Bossi, Fischetti et al. 2009, Jaffa, Kobeissy et al. 2012, Naffah-Mazzacoratti Mda, Gouveia et al. 2014). On the regulation of kinin receptors, post-translational modifications are common regulatory mechanisms in the KKS system. For instance, for B2R to sequester and get desensitized, its phosphorylation is required (Bachvarov, Houle et al. 2001, Blaukat, Pizard et al. 2001). The pharmacokinetic capacity of B2R to couple and uncouple to G-proteins has been found to be regulated by palmitoylation (Pizard, Blaukat et al. 2001). N-glycosylation is required for the efficient expression of B2R on the cell surface (Hess, Borkowski et al. 1992).
Although renin is found at low levels in the brain, yet the prorenin receptor (PRR) was recently identified to have high expression levels in the brain neurons and astrocytes (Shan, Cuadra et al. 2008, Takahashi, Hiraishi et al. 2010). This, in turn, can highlight on the critical roles of angiotensin receptors; the balance between the KKS and RAS is vital to maintain cerebrovascular homeostasis and blood-brain barrier (BBB) permeability in a multitude of neurological disorders including epilepsy, demyelinating diseases, stroke and dementias (Naffah-Mazzacoratti Mda, Gouveia et al. 2014). AbdAlla et al. showed that the B2R - Angiotensin II type 1 (AT1) receptor heterodimerization increased the responsiveness of the receptor to angiotensin II whereas B2R - B1R heterodimerization augments B1R signaling (AbdAlla, Lother et al. 2000, Kang, Ryberg et al. 2004). These findings have increased our knowledge of the KKS cross-talk between the neural involvement and other biological systems.
Different studies have discussed KKS involvement in both regular and pathophysiological brain functions indicating that regulation of the receptors of the KKS system (B1R and B2R), can exhibit neuroprotective and/or pathological outcomes. These conflicting evaluation generated more complications and confusion in the understanding of the KKS regulation as will be discussed in different sections (Albert-Weissenberger, Siren et al. 2013).
1.2 Kinin signaling: pathways cross talk in systems physiology
In the brain, TK has been identified in different brain regions including: the cerebral cortex, brain stem, cerebellum, hypothalamus, pituitary, pineal gland and its surrounding blood vessels, and the ependymal lining cells of the third ventricle (Scicli, Forbes et al. 1984, Simson, Dom et al. 1985, Chao, Chao et al. 1987, Kitagawa, Kizuki et al. 1991). The presence of TK in both endothelial cells and smooth muscle cells made it an essential factor in regulating vascular wall tone (Yayama, Shibata et al. 1998, Bergaya, Meneton et al. 2001). B2R activation raises intracellular calcium concentrations via activation of the phospholipase C (PLC) that subsequently produces diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) (Pinheiro, Paramos-de-Carvalho et al. 2013) (Leung, Cheng et al. 2006). This is followed by activation of protein kinase C (PKC), activation of phospholipase A2 (PLA2) and arachidonic acid production (Lal, Proulx et al. 1998, Li, Oehrlein et al. 1998). This elevation is followed by increased levels of inflammatory molecules such as prostaglandins and oxidative stress mediators, including reactive oxygen species (ROS) and nitric oxide (NO) (Raslan, Schwarz et al. 2010). Similarly, B1R also increases DAG and IP3 promoting the release of arachidonic acid (Tropea, Gummelt et al. 1993).
Upon binding of endothelium-derived relaxing factors (EDRFs) to their pertussis toxin-sensitive G(i) (serotonin and thrombin) or pertussis toxin-insensitive G(q) (Aguiar, Tuon et al.) (BK, histamine, ADP) receptors, downstream signaling responsible for NO generation is activated (Vanhoutte, Shimokawa et al. 2009). Specifically, nitric oxide synthase (endothelial or type III, eNOS) converts L-arginine to NO that diffuses through the endothelial cell membrane and reaches the underlying vascular smooth muscle cells (VSMCs) (Tousoulis, Kampoli et al. 2012). Although eNOS activation is involved in B1R signaling (Ignjatovic, Stanisavljevic et al. 2004), new data on B1R-dependent NO release suggest that it is mediated through the inducible isoform of NOS (iNOS), and not eNOS (Brovkovych, Zhang et al. 2011). Nevertheless, the released NO stimulates guanylyl cyclase (GC) in VSMCs, increases the production of cyclic guanosine monophosphate (cGMP) leading to the opening of potassium channels and hyperpolarization (Archer, Huang et al. 1994, Francis, Busch et al. 2010). Besides NO and prostacyclin, several endothelium-derived factors (EDHFs) such as arachidonic acid generated by the action of PLA2, may cause NO-independent hyperpolarizations of the VSMCs (Murota, Morita et al. 1987, Campbell, Gebremedhin et al. 1996, Bryan, You et al. 2005, Feletou and Vanhoutte 2006, Fleming and Busse 2006). Interestingly, more profound and prolonged NO production is noted with B1R compared to B2R, likely due to the rapid desensitization and internalization of B2R. In disease, when the ability of the endothelial cells to provoke hyperpolarizations- (whether NO-dependent or not)- is impaired, endothelial dysfunction appears to be one of the initial major steps resulting in atherosclerosis, stroke and coronary disease (Ross 1999, Vanhoutte 2003). In fact, besides its atheroprotective role, NO inhibits platelet aggregation, expression of adhesion molecules, infiltration of leukocytes, and the release of vasoconstrictors; hence, reducing the extent of inflammatory processes (Cooke 2004, Voetsch, Jin et al. 2004).
Taking into consideration that BK receptors belong to Class A Rhodopsin-like family of the seven transmembrane receptors, researchers have focused on downstream proteins and associated-pathways specific to this type of GPCRs (Roberts 1989, Farmer and Burch 1992, Hall 1997, Leeb-Lundberg, Marceau et al. 2005). Kinin signaling is essential for cell survival, proliferation, protection against oxidative stress as well as for maintaining cellular integrity and promoting differentiation as discussed later. For instance, Liu et al. demonstrated that TK upregulates the expression of the anti-apoptotic survival genes: brain-derived neurotrophic factor (BDNF) and Bcl-2 genes (Liu, Zhang et al. 2009). A parallel work emphasized the antioxidant features of TK and its ability to inhibit apoptosis, decrease ischemia-acidosis/reperfusion-induced injury, and promote in vitro cell survival via activation of the ERK1/2 signaling pathway (Zhang, Larner et al. 2009). BK was shown to activate the ERK/ElK-1/Ap-1 pathway in mesangial cells while its signaling process was mainly dependent on protein tyrosine phosphorylation (El-Dahr, Dipp et al. 1998). Tang et al. investigated the role and the mechanism of B2R in neuronal damage on a hypoxia/reperfusion (H/R) model of primary cultured neurons (Liu, Zhang et al. 2009). Following H/R, B2R expression was found to be increased as a physiologic response to neurological insult. Furthermore, it was discovered that BK could alleviate neuronal damage, increase ERK1/2 phosphorylation, reduce LDH release and decrease caspase-3 activity post-H/R (Liu, Zhang et al. 2009). In another study, B2R was shown to play a critical role in promoting calmodulin kinase II-mediated neuronal differentiation and maturation of major b-series gangliosides such as GT1b, GD1b and GD3 gangliosides. Indeed, exogenous gangliosides not only stimulate neuronal cells and induce calcium release from intracellular synaptic stores, but also activate calcium/calmodulin-dependent protein kinase II (CaMKII) and cdc42, and; thus, promoting the reorganization of cytoskeletal actin and dendritic differentiation (Kanatsu, Chen et al. 2012).
The neuroprotective role of BK was shown in a study by Martins et al., where BK showed marked neuroprotection of pyramidal neurons against N-methyl-D-aspartate (NMDA)-mediated excitotoxicity (Martins, Alves et al. 2012). This vital protective role involved the activation of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), which is responsible for Bad protein phosphorylation and consequent anti-apoptotic activity. Neuroprotection mediated by BK was independent of the MEK/MAPK activation cascade. In an attempt to study the role of BK on BR2, an immortalized murine microglial cell line, Ben-Shmuel et al. demonstrated that BK was capable of attenuating LPS-induced microglial cell death via B1R and B2R activation, and was also capable of reducing NO production by coupling to Gαi proteins and inhibiting the cAMP-PKA-CREB downstream signaling pathway (Ben-Shmuel, Danon et al. 2013). Thus, in this particular case, BK was shown to have a neuroprotective and an anti-inflammatory role. Also, downstream to BK, activating the ERK/NF-κB and JNK/c-Jun cascades by a Nox/ROS-dependent signal, which enhances c-Fos/AP-1 activity, in rat brain astrocytes (RBA-1) is essential for Heme Oxygenase-1 (HO-1) up-regulation and activation (Hsieh, Wang et al. 2010). Indeed, HO-1 is a stress-inducible protein that acts downstream of interleukin-10 and represents a potential therapeutic target for treating inflammatory diseases (Lee and Chau 2002). Also, activation of the ROS-dependent NF-E2-related factor 2 in astrocytes, was shown to contribute to HO-1 induction via BK (Hsieh, Wang et al. 2010).
Enzymatically-generated kinins are agonists of the B2R and must be processed by a carboxypeptidase to generate B1R agonists: des-Arg9-BDK or des-Arg10-kallidin (Zhang, Tan et al. 2008). In fact, B1R heterodimerizes with CPM producing des-Arg9-BK to generate signals stimulating pro-inflammatory processes (Zhang, Tan et al. 2008). In addition, the B1R- and CPM- dependent calcium signals require activation of the B2R by BK (Zhang, Tan et al. 2008). Previously, in neuro-2A cell model and in rat astrocytes exposed to BK, Ikeda et al. have shown that calcium increase was suppressed by a B2R antagonist, Hoe-140, but not by a B1R antagonist, des-Arg-Hoe-140, suggesting that the effect occurred specifically through B2R activation (Ikeda, Ueno et al. 2000). In a recent study, RT-qPCR confirmed the expression of B1R and B2R in U-138MG and U-251MG human glioma cell lines, and the activation of these receptors by des-Arg9-BK (B1R agonist) and BK (B2R agonist) highlighted on the role of these receptors in glioma cell expansion, growth and viability (Nicoletti, Erig et al. 2014). Nevertheless, treatment of these cell lines with SSR240612 (B1R antagonist) and HOE-140 lead to glioblastoma cell death in a concentration-dependent manner (Nicoletti, Erig et al. 2014). Moreover, the kinin-induced proliferative outcomes of these glioblastoma cell lineages were associated to the activation of PI3K/Akt and ERK 1/2 pathways (Nicoletti, Erig et al. 2014), confirming the previous findings of Zhang et al. (Zhang, Larner et al. 2009) and Liu et al. (Liu, Zhang et al. 2009) [discussed above].
1.3 Kinin signaling: pathways cross talk in neuroinflammation
Neuroinflammation is a complex orchestration of inflammatory infiltrates mainly neutrophils and microglia and their downstream consequences in terms of the released interleukins, cytokines and chemokines ultimately causing apoptosis, neurodegeneration, altered astrocytic function, vasodilation and increased permeability (Cherry, Olschowka et al. 2014). While a balanced neuroinflammatory response to an initial mechanical or chemical insult is necessary for healing and regeneration, uncontrolled inflammation may cause permanent neuronal loss, scarring and cytodestruction (Gao and Hong 2008, Abou-El-Hassan and Zaraket 2017).
Inflammation is the first line of defense of an organism following an injury or infection. It results in local vasodilation due to the release of mast cell and platelet constituents including: histamine, leukotrienes, serotonin and prostaglandins (Theoharides, Alysandratos et al. 2012). BK is another important vasodilator that has been shown to induce vasodilatation of resistance arteries in humans via nitric oxide (O'Kane, Webb et al. 1994) and endothelium-derived hyperpolarizing factor (EDHF) (Honing, Smits et al. 2000).
Kinins are well known inflammatory regulators outside the central nervous system (CNS). Despite a well-known distribution of the kinin system throughout the brain, the exact role of BK in the CNS is still under-investigated (Sarit, Lajos et al. 2012). Given the distribution of B1R and B2R in the CNS discussed above and their presence on neurons, astrocytes, microglia, oligodendrocytes, and endothelial cells (Jeftinija, Jeftinija et al. 1996, Simpson, Mehotra et al. 1997, Abbott 2000, Fleisher-Berkovich, Filipovich-Rimon et al. 2010), it has been proposed that, by acting on B1R and B2R, BK and substance P exert a significant pro-inflammatory role and can induce a neurogenic inflammation resulting in vasodilation, plasma extravasation and subsequent development of edema and neuronal degeneration (Raslan, Schwarz et al. 2010, Thornton, Ziebell et al. 2010). At the level of astrocytes, for instance, astrogliosis develops following an injury (Eng and Ghirnikar 1994). Subsequently, astrocytes then release calcium, ROS, glutamate, MMP-9, PLA2 and IL-6 in a B2R-activated ERK1/2 pathway and not via B1R (Jeftinija, Jeftinija et al. 1996, Hsieh, Yen et al. 2004, Hsieh, Wu et al. 2006, Kim, Cho et al. 2010). Microglia were also found to host B2R whose role was found to be neuroprotective in terms of lipopolysaccharide-induced microglial activation after the administration of BK whereas B1R stimulation induced microglial migration (Ifuku, Farber et al. 2007, Noda, Kariura et al. 2007). In addition, these mediators provoke BBB disruption; leading to edema, neuronal injury and eventually neuronal and glial cell death, further highlighting the prominent role of kinins in brain inflammation (Su, Cui et al. 2009, Raslan, Schwarz et al.). One of the findings related to the BK mechanism of action is its ability to regulate Claudin-5, a tight junction protein mainly found in the BBB (Zhou, Yang et al. 2014). In particular, Zhou et al. demonstrated that BK provokes intracellular calcium release in primary cultures of rat brain microvascular endothelial cells (BMECs) via the activation of IP3 and Ryanodine receptors found on the surface of the endoplasmic reticulum (ER). This calcium induced-calcium release provoked the internalization and downregulation of claudin-5, followed by a disruption of BBB permeability (Zhou, Yang et al. 2014). For instance, inhibition of calcium-dependent potassium channels resulted in significant inhibition of vasodilatation in cerebral arterioles exposed to hydrogen peroxide or BK-induced hydrogen peroxide (Gorlach and Wahl 1996, Sobey, Heistad et al. 1997).
Oxidative stress characterized by an excessive production of RAS byproducts, ROS and NADPH oxidase (Nox) along with matrix metalloproteinase-9 (MMP-9), cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2), iNOS induced by proinflammatory factors such as cytokines, interleukins (IL) and peroxidants is usually thought to be responsible for tissue injury associated with a range of brain injury, inflammation, and degenerative diseases (Theodoropoulos and Loumos 1991, Abbott 2000, Stegbauer, Lee et al. 2009, Uttara, Singh et al. 2009, Labandeira-Garcia, Rodriguez-Perez et al. 2017, Regoli and Gobeil 2017). A recent work by Yang et al. highlighted the contribution of BK in enhancing the production and release of different molecules mediating apoptotic cell death from astrocytes (Yang, Hsieh et al. 2013). More specifically, BK-treated RBA-1 cells were shown to release neurotoxic factors such as ROS, MMP-9 and HO-1/CO, inducing neuronal apoptosis via a caspase-3-dependent pathway. These multiple inflammatory effectors can be responsible for neurodegenerative disorders leading to neural injury, making BK a promising target in strategic pharmacological interventions (Yang, Hsieh et al. 2013). As shown in the previous section, BK has a protective effect in the health state, however; under stressful circumstances, BK worsens the initial insult.
Neuroglial cells are particularly susceptible to the injurious effects of oxidative stress. In brain astrocytes, Wu et al. showed that IL-1beta stimulated c-Src-dependent transactivation of platelet-derived growth factor receptor (PDGFR) acting downstream through the PDGFR/PI3K/Akt cascade to increase proMMP-9 expression (Wu, Hsieh et al. 2008). MMP-9 gene up-regulation and cell migration were found to be induced by BK via activation of a PKC-delta-dependent ERK/Elk-1 pathway resulting in tissue remodeling (Hsieh, Wu et al. 2008). Specifically, downstream to PKC-delta, ERK1/2 undergoes phosphorylation and translocation activating transcription factor Elk-1. Recently, Lin et al. showed that BK may also induce MMP-9 expression via the AP-1 (c-Fos/c-Jun) PKC-α-mediated NADPH oxidase 2 (Nox2) Nox2/ROS signaling pathway (Lin, Hsieh et al. 2012). From a different approach, BK activated PKC-delta, p42/p44 MAPK, and NF-κB sequence inducing cytosolic phospholipase A2 (cPLA2) expression as well as iNOS gene up-regulation (Hsieh, Wu et al. 2006, Wang, Hsieh et al. 2011). Produced NO enhanced astrocyte migration through tyrosine nitration of MMP-9 (Wang, Hsieh et al. 2011). IL-1 and TNF-α may also modulate cPLA2 activity and expression (Xu, Chalimoniuk et al. 2003). In the context of neurodegeneration, Stephenson et al. showed an increase in immunoreactivity to cPLA2 in astrocytes in the cortex of Alzheimer’s disease (AD) patients (Stephenson, Rash et al. 1999). The same pathway (PKC-delta, p42/p44, MAPK and NF-κB) resulted in upregulation of BK-induced cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) release (Hsieh, Wang et al. 2007). In the brain tissue, COX-2 leads to increased synthesis of prostanoids, potent inflammatory mediators that are upregulated in neuroinflammatory conditions (Ohtsuki, Kitagawa et al. 1996, Brambilla, Neary et al. 2002). At the level of enzymatic Kallidin formation, TK diminished glutamate-induced apoptosis by activating B2R that phosphorylated ERK1/2 (mainly ERK1), and in turn activated the transcription factor NF-κB (Liu, Zhang et al. 2009), which controls the expression of many inflammatory components and blocks cell apoptosis (Li and Stark 2002). Furthermore, tissue factor pathway inhibitor-2 (TFPI-2), a serine proteinase inhibitor of PK, promoted apoptosis via the Fas/FasL signaling pathway (Pan, Shi et al. 2011). Taken together, these findings underline a new set of mechanisms through which brain injury and inflammatory diseases can occur. All the above-mentioned downstream proteins/pathways, activated by the BK receptors, have been summarized and linked all together, and are illustrated in Figure 3.
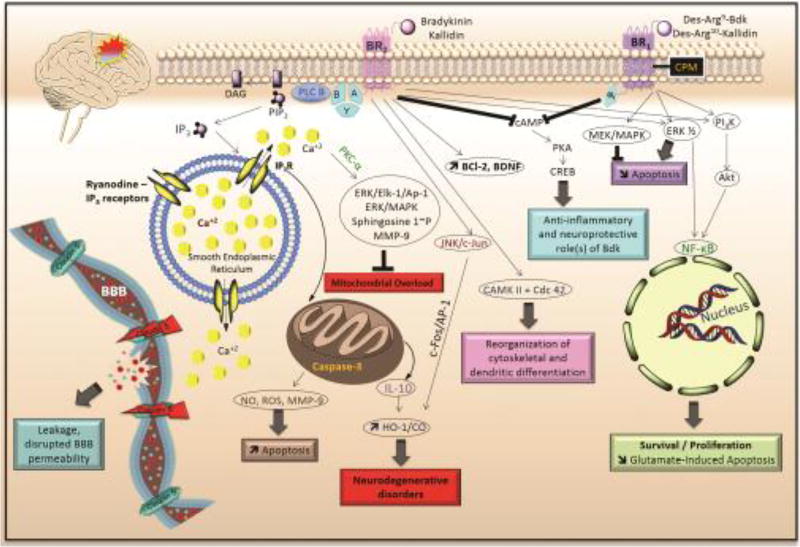
Figure 3. Signaling pathways of B1R and B2R leading to neuroinflammatory responses.
Following activation of BK receptors by their respective agonists, a cascade of pathways is activated, targeting the mitochondria, the smooth endoplasmic reticulum, the nucleus and the BBB leading to various responses of the cells, such as apoptosis, neurodegeneration, cytoskeletal reorganization, BBB leakage, survival and proliferation. Uncontrolled B2R GPCR-signaling disrupts the integrity of the BBB increasing BBB permeability as well as increased apoptosis and subsequent neurodegeneration post-mitochondrial overload, the outcome also observed via the JNK/c-Jun pathway. Cytoskeletal reorganization is mediated via CaMKII and cdc42. In contrast, inhibition of cAMP common to both B1R and B2R, promotes anti-inflammation and neuroprotection. B1R-induced decreased apoptosis is mediated via activation of the ERK1/2 (mainly ERK1) and PI3K-Akt pathways. BK: bradykinin; BBB: blood-brain barrier; B1R: kinin 1 receptor; B2R: kinin 1 receptor; GPCR: G protein-coupled receptor.
The distribution of the KKS system is quite complex as different components are present in the plasma, on the surface of blood cells and in various tissue types. The tight regulatory pathways add to its complexity as kinins are regulated by several metallopeptidases and serpins besides its multiple interactions with other biological systems such as the RAS (Motta and Tersariol 2017). Downstream KKS events are interlinked and may be activated by other mediators such as the RAS and complement pathways, not only those part of the KKS. However, as described, the multitude of pathways involved in disease progression is beneficial in terms of therapy. Combinatorial inhibition of at least two pro-inflammatory promoters may offer more protection by acting on several secondary sequelae including BBB disturbance, excitotoxicity, generation of ROS, edema, inflammation and scarring (Wu 2015, Soley Bda, Morais et al. 2016). Development of brain atrophy and subsequent dementia is a major debilitating consequence of severe injury to the nervous system caused, in part, by uncontrolled activation of the KKS pathways in disease (Bryant and Shariat-Madar 2009). It is therefore imperative to counteract the development and progression of long-term secondary consequences. For this, two approaches are possible: Up-down and down-up. In the up-down approach, a certain mediator (such as BK) that is inhibited would terminate all of its effector functions, whether beneficial or otherwise. In the down-up approach, the molecular effectors themselves (such as MMP) are inhibited, thus providing a more specific outcome. Post-intervention outcomes were also time-dependent, as KKS neuroinflammatory pathways display temporal alterations (Austinat, Braeuninger et al. 2009). Further research questions may focus on delineating the extent of pathway activation to best identify the optimal period of intervention at which KKS inhibitors are most beneficial. In parallel, dosing requirements and how long a therapy should be kept along with its associated side-effects are aspects that need to be established. Nevertheless, combining both approaches in the KKS pathways would be interesting to investigate.
2. The kinin system in neurological disorders: a novel contribution
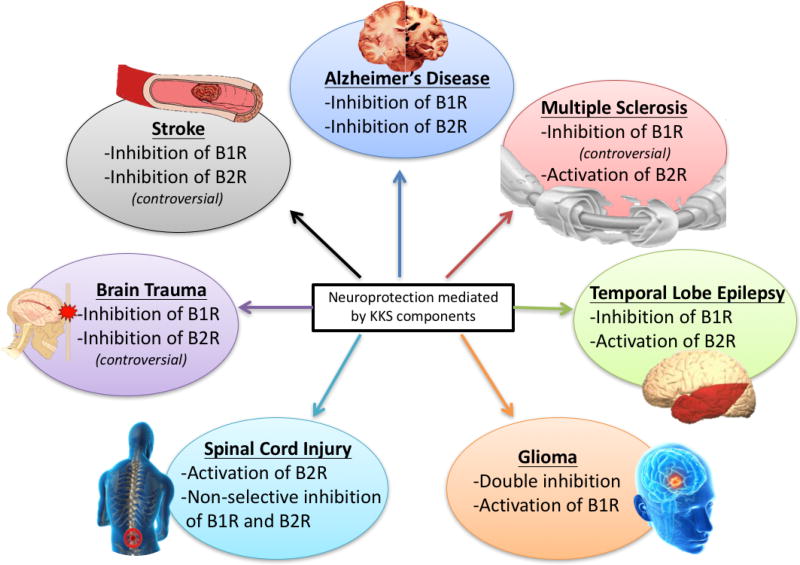
Neurological disorders encompass a number of different causalities including: genetic origins, congenital abnormalities, infections, lifestyle (diabetic neuropathy) or can be due to CNS insults such as brain/spinal cord injury. Diseases of the nervous system have distressing consequences and are extensively spread among the population, particularly among the elderly (Simonato, Bennett et al. 2013). Therefore, understanding mechanisms underlying these disorders is critical to identify effective neurotherapies and markers against these disorders. Of interest, kinins have recently emerged as “novel” potent stimulators of neural and neuroglial tissues inducing the synthesis and release of different pro-inflammatory mediators such as prostanoids and cytotoxins, which will lead to neural tissue damage and BBB disruption (Walker, Perkins et al. 1995). Nowadays, the participation of kinins in neurological disorders is under thorough investigation due to their key vital contribution in several neurological disorders. Having discussed the kinin global signaling pathways in the previous sections, in this part, we will review the physiological and pathological role of the KKS in the main neurological disorders, and present evidence of how this knowledge can be translated into new potential therapies.
2.1 Temporal lobe epilepsy
Temporal lobe epilepsy (TLE) is the most common and most studied adult epileptic syndrome (Zhang, Cui et al. 2002, Krook-Magnuson, Armstrong et al. 2013, Lee and Lee 2013, Pelliccia, Mai et al. 2013, Kandratavicius, Peixoto-Santos et al. 2015). The necessity to develop new drugs acting through different mechanisms resides in the fact that some patients are resistant to the currently available pharmacological therapies (Garga and Lowenstein 2006). In particular, one-third of epileptic patients develop resistance to modern anti-epileptic drugs (AEDs) such as phenytoin, carbamazepine, and valproate (Jain 2009). This resistance is thought to be nonspecific and involves the ATP-binding cassette sub-family B member 1 (ABCB1). Thus, novel treatment strategies would require the development of AEDs that are not ABCB1 substrates and that can inhibit or evade ABCB1 (Jain 2009). New AEDs have been designed to inhibit glutamatergic excitatory neurotransmission and despite their promising effects in terms of improved mood, these new AEDs were associated with anxiogenic effects (Mitchell, Seri et al. 2012). In this regard, some studies have been focusing on the KKS, which is activated in parallel to the glutamate excitatory pathway in the brain (Rodi, Couture et al. 2005). In 1999, Bregola et al. demonstrated that stimulation of B1R significantly increased glutamate outflow in cortical and hippocampal slices of two different models of TLE: kainate-treated (generalized seizures) and kindled (gradually intensifying seizures from local to generalized) rats; suggesting that the biological activity of B1R may involve epileptic hyper-excitability (Bregola, Varani et al. 1999).
Later on, Ongali et al. performed an autoradiographic analysis, a technique used to visualize radioactively labeled molecules or fragments using an X-ray film, and demonstrated a noteworthy drop of B2R binding sites, complemented by a marked rise in B1R labeling in the brain of rats submitted to the electric kindling model of epilepsy (Ongali, Campos et al. 2003). These discoveries provided evidence for the “pivotal” role of these two receptors in mediating epilepsy. It has also been shown that B1R contributes to the modulation of epileptic neuronal excitability (Mazzuferi, Binaschi et al. 2005).
Arganaraz et al. demonstrated enhanced kinin B1R and B2R mRNA levels in the hippocampi of pilocarpine model of epilepsy adult male Wistar rats 6 hours, 5 and 60 days after status epilepticus (SE) onset, further corroborating the involvement of B1R and B2R during acute, silent and chronic periods of TLE (Arganaraz, Silva et al. 2004). These findings were reiterated in human patients with TLE (Perosa, Arganaraz et al. 2007). Recently, thimet oligopeptidase (TOP), a metalloprotease whose substrates include BK, was found to be decreased in levels and activity in TLE patients compared to controls, thus potentially explaining the increase in KKS activation due to the low kininase TOP activity (Simoes, Visniauskas et al. 2014). More specifically, kinin B1R was considered to have a pro-convulsant role, as its absence reduced hippocampal cell death and mossy fiber sprouting, while the kinin B2R played an anticonvulsant role with an opposite effect on epileptogenesis (Adolfo Arganaraz, Regina Perosa et al. 2004).
Moreover, kinin receptors were found to be amplified in the pilocarpine-induced epilepsy animal model, with the preponderance of B2R in the acute disease state and B1R during the silent period (Silva, Goto et al. 2008). However, the fundamental role of B1R and B2R in TLE models is suggested to be unrelated to the inflammatory process, and kinin receptors in the CNS can be involved in seizure mechanisms by inducing an alteration in kininergic neurochemical exclusive pathways (Pereira, Gitai et al. 2008). In response to this last assumption, a recent study revealed an overexpression of the KKS and a neuron–glia interaction in the inflammatory process found in refractory TLE, associated with hippocampal sclerosis (TLE-HS) (Simoes, Perosa et al. 2011). This study focused on the co-localization of Kallikrein 1, a tissue enzyme responsible for kinin release in the inflammatory cascade, with B1R and B2R in the hippocampus of patients with TLE-HS (Simoes, Perosa et al. 2011).
In another study, Rodi et al. showed that B1R knockout (KO) mice were more vulnerable to kainate and kindling seizures compared to wild-type (WT) mice and this excitability was mainly due to the overexpression of B2R in the dorsal hippocampus and the piriform cortex of B1 KO mice (Rodi, Buzzi et al. 2013). As a proof of concept, kainic acid (KA) seizures ceased following pretreatment with the B2R antagonists HOE-140 (icatibant, 100nM) or LF 16-0687 (anatibant, 1lM) (Rodi, Buzzi et al. 2013).
All together, these studies indicate that the early molecular and cellular events leading to the alteration of a normal brain into an epileptic one involves B2R up-regulation when B1R is not present yet (Rodi, Buzzi et al. 2013), while later epileptic events (i.e. maintenance of hyper-excitability) depends on B1R expression (Bregola, Varani et al. 1999, Mazzuferi, Binaschi et al. 2005).
2.2 Traumatic brain injury
Traumatic brain injury (TBI) imposes a major public health concern worldwide and represents a leading cause of death, especially amongst young adults, (Maas, Stocchetti et al. 2008). Its incidence is rising sharply (Roozenbeek, Maas et al. 2013) and the current surge in military conflicts has resulted in a pronounced increase of military personnel returning from combat with mild TBI (Ling, Bandak et al. 2009). However, TBI is merely the tip of the iceberg; some of the TBI patients suffer multiple different long-term complications, including cognitive impairment, movement disorders, visual and/or auditory deficits, neuropsychiatric disorders, post-traumatic stress disorder (PTSD), chronic pain, and, may have a higher risk for the later development of post-traumatic epilepsy and dementia of the Alzheimer’s type (Risdall and Menon 2011, Shively, Scher et al. 2012).
The concept of TBI has changed and evolved over time, and currently, it is deemed a chronic disease process rather than an “event” (Masel and DeWitt 2010). Brain injury consists of two phases: primary and secondary injury (Table I, Figure 4). The original primary mechanical damage occurs at the time of the traumatic incident and is permanent and solely amenable to preventative actions (Abou-El-Hassan, Dia et al. 2017). The secondary phase, which occurs subsequent to the primary insult, is a multifactorial process started at the time of impact and evolving over the successive subacute phases to the chronic stages of recovery (Vink and Nimmo 2009). This secondary phase involves a variety of cellular responses and complex cascades of neurochemical and neurometabolic events aimed at restoring homeostasis (Abou-El-Hassan, Sukhon et al. 2017). Indeed, kininogen levels increase immediately following brain trauma and remain as such for 15 hours suggesting that the secondary post-injury events are related to endothelial and vascular repair mechanisms (Ellis, Chao et al. 1989).
Table I. Characteristics of primary and secondary insults following TBI.
The primary insult is mainly orchestrated by calpains, which cause necrotic cell death near the impact site. This occurs within minutes to hours, post-TBI, as depicted in Figure 4 (represented by one star on the timeline). The secondary insult is primarily mediated by caspases triggering apoptotic cell death, which occurs within days and again in the following weeks post-TBI (represented by two stars on the timeline of Figure 4).
| Primary Insult | Secondary Insult | |
|---|---|---|
| When |
|
|
| Consequence of |
|
|
| Effects |
|
|
| Cell Death |
|
|
| Brain Pathology |
|
|
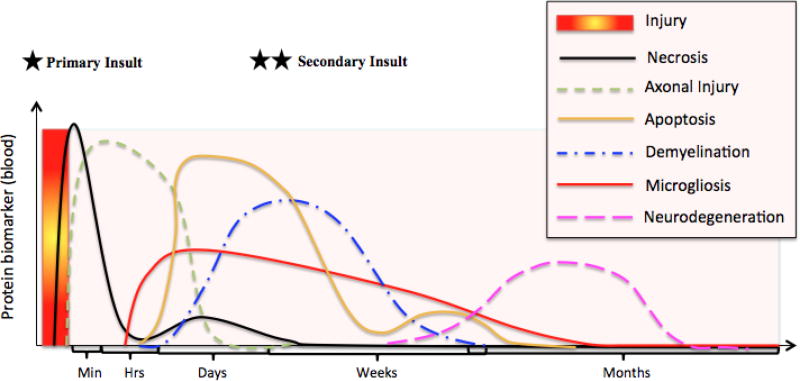
Figure 4. Maximal concentration of protein biomarkers in the blood over time, following brain injury.
The sequence of events taking place after a brain injury consists of: necrosis, axonal injury, gliosis/microgliosis, apoptosis, demyelination and neuroregeneration. Although these events occur in a consecutive manner, some of these events overlap over time.
Several published works have been conducted in order to understand the role of BK following brain trauma (Albert-Weissenberger, Siren et al. 2013, Hopp and Albert-Weissenberger 2015). The KKS was shown to be an essential mediator of secondary brain impairment, namely vasogenic brain edema (Stover, Dohse et al. 2000). Experimental models of CNS injury demonstrated that BK has a multifactorial involvement in the secondary phase of TBI. Indeed, BK triggers the release of glutamate and aspartate, and acts as a potent endothelium-dependent dilator of the brain vasculature (Rosenblum 1986, Jeftinija, Jeftinija et al. 1996, Thornton, Ziebell et al. 2010). Trabold et al. examined the role of the KKS in a mouse controlled cortical impact (CCI) model and demonstrated that BK levels were considerably increased 2 hours post-CCI, before decreasing over subsequent hours (Trabold, Eros et al. 2010). Indeed, in mongrel cats with vasogenic edema and disrupted BBB induced by cold injury and focal trauma, respectively, plasma kininogens were found to penetrate into necrotic and edematous brain tissue where activation of the KKS provoked additional cerebral ischemia (Maier-Hauff, Baethmann et al. 1984). In addition, cerebral edema was shown to be reduced in the presence of CP-0127, a specific BK antagonist, in a cold lesion model in rats (Narotam, Rodell et al. 1998). Consistently in a randomized, single-blind pilot study, 7-day infusion of CP-0127 (3.0 ug/kg/min) was compared to placebo in patients with focal cerebral contusions with an initial Glasgow Coma Score (GCS) of 9–14. CP-0127 seemed to block the secondary brain swelling by acting on the cerebral vasculature and limiting dys-autoregulation or by acting on the BBB to reduce cerebral edema (Narotam, Rodell et al. 1998). In this study, this BK antagonist proved to be extremely beneficial in preventing brain edema associated with cerebral contusion and obviating the need for neurosurgical intervention in the majority of treated patients. In one study, brain swelling due to perifocal cold injury edema in rabbits exposed to aprotinin and soybean trypsin inhibitor in order to inhibit the KKS, by interfering with plasma and TK, resulted in marked reduction of vasogenic edema, assessed by the decrease in weight of the traumatized hemisphere (Unterberg, Dautermann et al. 1986). In parallel, a recent study by Hopp et al. revealed a decrease in bradykinin-induced post-traumatic lesion size, brain edema and inflammation after inhibition of factor XIIa, a factor involved in the activation of PK (Hopp, Nolte et al. 2017).
In regards to the mRNA levels of B1R and B2R, there was a four-fold rise in the expression of the B1R mRNA at 6 hours after CCI (Trabold, Eros et al. 2010). In this same study, Trabold et al. further confirmed the role of BK and its receptors in TBI by using genetically engineered transgenic mice, which were deficient in either B1R or B2R. The authors found out that B2R−/− animals had a decrease of brain water content by 50% and did better on functional outcome tests following injury, in comparison to wild-type animals (Trabold, Eros et al. 2010). Albert-Weissenberger et al. showed an increase of kinin receptor mRNA levels on day 7, at later stages post-weight-drop brain injury, along with reduced axonal injury and astrogliosis post B1R blocking (Albert-Weissenberger, Stetter et al. 2012). Raslan et al.; however, reported that B1R inhibition rather than B2R reduced brain edema (Raslan, Schwarz et al. 2010).
In terms of B2R antagonism, a recent study by Ferreira et al. revealed that a subcutaneous injection of HOE-140 (10 nmol/kg) could protect against memory impairment in a moderate lateral fluid percussion injury (mLFPI) mouse model, where a plastic cannula was fixed into a 3 mm burr hole in the mice right parietal bone and connected to a fluid percussion device producing TBI (Ferreira, Rodrigues et al. 2014). The protective role of HOE-140 against memory deficits was explained by the reduction of inflammatory IL-1beta, TNF-α and oxidative-NOX in mLFPI-mediated impairments, which further prevented the increase in lesion volume. The reduction of such secondary brain damages resulted in a better cognitive performance, tested via object recognition task and elevated plus maze. Thus, this study demonstrated the protective involvement of B2R in memory deficits and brain damage post-mLFPI in mice (Ferreira, Rodrigues et al. 2014). Furthermore, recently, our lab has demonstrated that inhibition of B2R, using HOE-140, might play a protective role in PC12 cells subjected to the neurotoxic apoptotic specific drug staurosporine (STS) when compared to the inhibition of B1R and controls (Nokkari, Mouhieddine et al. 2015). STS was used to simulate cell activity following TBI (Zhang, Larner et al. 2009, Xu, Fan et al. 2014). Interestingly, we have shown a marked reduction in intracellular calcium release following B2R inhibition, exclusively. These effects were maintained following STS treatment, suggesting the potential therapeutic role of HOE-140. We also conducted a neuroproteomics study and identified a “survival” biological process developed by PC12 cell line post-HOE-140 treatment (Nokkari, Mouhieddine et al. 2015). The ability of PC12 cells to endure STS-mediated neurotoxicity, in the presence of a B2R antagonist, was shown to require activation of the transcription factor NF-κB, in addition to the presence of proteins such as Thioredoxin (TXN), Carbonyl reductase 1 (CBR1), aldehyde dehydrogenase 3 family member A1 (ALDH3A1), Histidine triad nucleotide-binding protein 1 (HINT1) and Microtubule-associated protein RP/EB family member 1 (MAPRE1); all of which are linked to cell death and inflammatory pathways following TBI (Kobeissy, Ottens et al. 2006, Kobeissy, Sadasivan et al. 2010, Boutte, Yao et al. 2012, Guingab-Cagmat, Newsom et al. 2012). The brain-specific creatine kinase B-type (CKB) was also discovered and was found to be upregulated in PC12 cells treated with STS and HOE-140 (Nokkari 2014). Remarkably, this protein was shown to lower oxidative stress and was also found to be involved in neuroprotection (Cunha, Martin-de-Saavedra et al. 2014). Stover et al. demonstrated that CCI-injured rats had 25% and 27% reduced vasogenic brain edema after administration of a low (3 mg/kg) and high dose (30 mg/kg) of the LF 16-0687Ms B2R antagonist (Pelliccia, Mai et al.). They also noted a decreased level of taurine, hypoxanthine and xanthine in the CSF of the brain-injured rats post-LF 16-0687Ms administration, suggesting a protective role of the B2R antagonist in the reduction of post-traumatic brain edema (Stover, Dohse et al. 2000). A similar outcome was reported when TBI was caused by either weight-drop closed head injury or a left parietal cortex focal cold injury (Pruneau, Chorny et al. 1999, Schulz, Plesnila et al. 2000). The therapeutic role of the B2R antagonist was further demonstrated in a study conducted by Hellal et al. on mice models of closed-head trauma (Hellal, Pruneau et al. 2003). In this study, B2R antagonist LF 16-0687Ms given 30 minutes post-injury lowered neurological deficit and cerebral edema when assessed 4 hours post-trauma-induced diffuse injury (Hellal, Pruneau et al. 2003). Similarly, the authors showed that neurological function was improved in B2R−/− mice when compared to B2R+/+ mice; thus, providing evidence that B2R plays a detrimental role in the development of neurological deficits, as well as in the inflammatory secondary damage resulting from diffuse TBI (Hellal, Pruneau et al. 2003). Comparable results were obtained by Ivashkova et al. after infusion of the B2R antagonist LF 18-1505T on CHT rat models; (Ivashkova, Svetnitsky et al. 2006). Another study emphasized the neuroprotective role of the B2R antagonist as compared to the B1R antagonist B9858. Specifically, HOE-140 was shown to reduce lesion volume and brain swelling induced by cold injury (−78°C) in the parietal cortex of rats and mice, a therapeutic effect that was not reproduced when using a B1R antagonist (Gorlach, Hortobagyi et al. 2001). The B2R antagonist reduced brain edema and improved neurological outcome after closed head trauma in rats without affecting physiologic values such as blood pressure and glucose concentration (Ivashkova, Svetnitsky et al. 2006). At the clinical level, there is no evidence on the effectivity of B2R antagonism in reducing mortality or disability after TBI (Ker and Blackhall 2008).
In a recent study by Hopp et al. aiming to study the neuroinflammotary outcomes post-factor XII inhibition, secondary brain damage was found to be alleviated in a cortical cryogenic lesion model in mice after inhibition of activated factor XII using recombinant human albumin-fused Infestin-4 (Hopp, Nolte et al. 2017). In the study, quantification of some interleukins, chemokines, cytokines, and adhesion molecules revealed a reduction in BBB disruption and brain edema as well as brain lesion size, thereby, promoting the therapeutic potential of pharmacological blocking of activated factor XII. In a CCI animal model, treatment with C1-INH at 10 min or 1-hour post-CCI revealed smaller lesions and better cognitive function (Longhi, Perego et al. 2008, Longhi, Perego et al. 2009).
On the translational level, Kunz et al. performed a comparative clinical study on 29 patients diagnosed with acute cerebral lesions (TBI, subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH) and ischemic stroke) (Kunz, Nussberger et al. 2013). Blood and CSF of patients were collected and processed to measure BK concentrations along with the extent of cerebral edema and ICP, which were compared to 7 patients with lumbar drainage as controls. The results confirmed the presence of an association between acute cerebral lesions and increased levels of BK in the CSF. Specifically, cerebral edema and ICP extent were associated with elevated CSF BK levels in TBI, SAH and ICH. As suggested in the study, BK-blocking agents could be potential novel therapeutic intervention in brain injuries (Kunz, Nussberger et al. 2013). Also, plasma and cerebrospinal fluid (CSF) levels of the stable BK metabolite, BK1–5, were considerably and distinctly increased in patients with acute trauma (34700 fmol/ml in patients vs. 34.9 fmol/ml in normal volunteers) (Marmarou, Guy et al. 2005).
Since different components of the KSS are present in the blood and CSF of brain trauma patients as well as in the biofluids of animal models of TBI strengthen the promising role of B2R antagonists in reducing brain edema, cytodestruction and neuroinflammation. However, data from clinical trials of B2R blockage did not provide successful translational benefits due to either adverse effects of B2R blocking agents or the low number of study participants (Marmarou, Nichols et al. 1999, Mahmoudian, Mehrpour et al. 2003, Marmarou, Guy et al. 2005, Shakur, Andrews et al. 2009).
Further well-designed and supported studies are needed to ascertain the prognosis of using B2R antagonists in terms of mortality and/or disability. Variation in the available TBI models (closed-head weight-drop, fluid percussion, CCI, cryogenic injury) besides inconsistent methodologies may justify some of the observed discrepancies.
2.3 Stroke
Stroke injury includes both ischemic and non-traumatic hemorrhage to the brain. Ischemic stroke is the most prevalent representing 80% or more of all strokes (Chao and Chao 2005, Hirtz, Thurman et al. 2007), and up till now, the middle cerebral artery occlusion (MCAO) model is the best candidate to study ischemic stroke (Chao and Chao 2005). In MCAO, mRNA and protein levels of B1R and B2R were significantly elevated as early as 4 hours post-insult and persisted through day 3 (Austinat, Braeuninger et al. 2009). In parallel, there is an increase in BK and kallidin levels, maximally at 12 hours post-MCAO (Groger, Lebesgue et al. 2005).
Liu et al. exposed human SH-SY5Y cells to oxygen and glucose deprivation (OGD) after which treatment with TK was found to enhance induction of autophagy by B2R upregulation (Liu, Lu et al. 2016). B2R antagonism in parallel to TK treatment resulted in a decrease in inflammation, oxidative stress, mortality rate and post-ischemic brain injury (Chao and Chao 2006). Pathways activated by TK included mitogen-activated protein kinase 1/2 (MEK1/2)/ ERK1/2 and AMP-activated protein kinase (AMPK)/tuberous sclerosis complex 2 (TSC2)/mammalian target of rapamycin (mTOR) (Liu, Zhang et al. 2009, Liu, Lu et al. 2016). Since diabetics are at an increased risk of developing ischemic stroke, TK administration was reported to be neuroprotective in terms of alleviating cerebral Ischemia-Reperfusion (I/R) Injury by activating the B2R-ERK1/2-CREB-Bcl-2 signaling cascade at the molecular level and reduced the infarct volume at the structural level (Shi, Yuan et al. 2016). In a different study, TK was shown to protect against I/R injury through the B2R Homer1b/c-ERK1/2 and Homer1b/c-PI3K-Akt signaling pathways (Su, Tang et al. 2012). Furthermore, in a rat stroke model, immediate rather than delayed TK treatment resulted in remarkably enhanced neurological deficits and decreased infarct size accomplished via suppressing TLR4/NF-κB levels and activating Nrf2 signaling pathway (Yang, Su et al. 2017). Thus, taken together, all of the above evidence supports TK as a potential therapeutic strategy for management of ischemic stroke.
Given the recent evidence that urinary TK is a stronger predictor of stroke recurrence compared to plasma TK (Zhang, Ding et al. 2011, Ran, Zhang et al. 2015), human urinary kallidinogenase (HUK), a TK extracted from urine, was recently reported to promote neuro-repair mechanisms; particularly angiogenesis in an MCAO animal stroke model via an ERK1/2 dependent-pathway (Han, Li et al. 2015). In a clinical trial to assess the clinical efficacy of HUK in treating acute cerebral infarction, 110 patients were randomly assigned two groups: one receiving HUK at a dose of 0.15 PNA unit in 100 mL saline infused intravenously once a day for 14 days and another control group (Li, Zha et al. 2015). Pre-treatment National Institutes of Health Stroke Scale (NIHSS) scores between the two groups were not significantly different, whereas NIHSS scores of both groups declined post-treatment and more so in the HUK group indicating the clinical efficacy of HUK in reducing stroke-induced impairment. Also, patients with level 3 hypertension who received HUK had a significantly lower Modified Rankin Scale (mRS) score and a higher recovery rate compared to the control group (Wu, Lyu et al. 2017). Unlike kallidin, BK concentrations were not significantly different between controls and stroke patients, yet, high PK levels may increase the risk of stroke in young women (Wagner, Kalb et al. 2002, Siegerink, Govers-Riemslag et al. 2010). This highlights the role of urinary TK as a predictor of stroke recurrence.
The role of PK in ischemic stroke has been investigated. As stated previously, PK cleaves HK into BK and activates coagulation factor XII. It can be inferred that PK is involved in thrombus formation and inflammation, which are the underlying causes of stroke. Interestingly, Gob et al. demonstrated that PK-deficient MCAO mice were less vulnerable to brain infarctions, infarct-associated hemorrhage and neurological deficits when compared to controls (Gob, Reymann et al. 2015). Furthermore, the maintenance of an intact BBB in prekallikrein deficient mice (klkb1−/−) highlighted the usefulness of PK inhibition in fighting thromboembolic consequences observed in stroke (Gob, Reymann et al. 2015). Mechanistically, Stavrou et al. recently showed that the reduced thrombosis observed in klkb1−/− mice was found to be due to increased Mas receptor, prostacyclin, aortic vasculoprotective transcription factors (Sirt1 and KLF4) and decreased tissue factor (Stavrou, Fang et al. 2015). Storini et al. demonstrated in their brain ischemia/reperfusion mouse model that DX-88 (a selective recombinant inhibitor of human PK), a drug approved for the treatment of hereditary angioedema which is a disease characterized by a deficiency of C1 inhibitor (C1-INH), induces a dose-dependent decline in the ischemic volume and swelling, lowers the amount of dying neurons, and drastically diminishes functional neurological damage for up to 7 days post-ischemia (Storini, Bergamaschini et al. 2006) (Caliezi, Wuillemin et al. 2000). C1-INH, an inhibitor of C1 and activated factor XII, is one of the major endogenous PK inhibitors (Caliezi, Wuillemin et al. 2000) and was found to reduce the infarct size post-MCAO in mice (De Simoni, Storini et al. 2003, Heydenreich, Nolte et al. 2012) via anti-inflammatory and anti-apoptotic mechanisms (Storini, Rossi et al. 2005). Aprotinin, another serine protease inhibitor, was found to reduce the risk of stroke in patients undergoing extracorporeal circulation when given at high doses along with improved neurologic deficits (Levy, Pifarre et al. 1995, Homi, Sheng et al. 2010). On the contrary, Mangano et al. reported an increased risk of stroke using aprotinin after which it was withdrawn from the market (Mangano, Tudor et al. 2006). A newer kallikrein inhibitor, CU-2010, had better myocardial outcomes but similar antifibrinolytic capacity compared to aprotinin (Dietrich, Nicklisch et al. 2009, Szabo, Veres et al. 2010).
A novel study by Langhauser et al. indicated that in a model of acute ischemic stroke in kininogen deficient Kgn1−/− mice, ischemic neurodegeneration and BBB permeability were prevented by a combination of antithrombotic and anti-inflammatory mechanisms but without increasing the risk of intracerebral hemorrhage (Langhauser, Gob et al. 2012). In MCAO-mice, tissue plasminogen activator (tPA) was found to promote PK activity via a factor XII-dependent mechanism exacerbating intracranial hemorrhage, edema and infarction (Simao, Ustunkaya et al. 2017). To decipher the role of factor XII in stroke, in factor XII-deficient mice as wells as in its inhibition using Infestin-4, significant protection against ischemia without an adverse increased risk of bleeding was noted (Kleinschnitz, Stoll et al. 2006, Hagedorn, Schmidbauer et al. 2010). Also, PK inhibition post-tPA treatment reduced MMP-9 levels resulting in reduced BBB permeability. Administration of both tPA and PK inhibitor lead to increased clot dissolution in vitro suggesting that the combined therapy of these two compounds may reduce cerebrovascular thrombotic disease without increasing the likelihood of bleeding. Clear evidence on the role of factor XII has been obtained in animal models; yet, in human, the data yielded contrasting findings. In young women, high levels of factor XIIa raised the odds ratio for stroke whereas in a study of 21 patients deficient for factor XII showed no thrombotic events over a 15-year follow-up period (Siegerink, Govers-Riemslag et al. 2010) (Girolami, Randi et al. 2005). Thus, further clinical research with well-controlled variables is warranted to accurately figure the protective outcomes of inhibiting factor XII.
The role of BK in neuronal damage has also been investigated in different experimental stroke models. After 8-minute long four-vessel occlusion of the forebrain in male Wistar rats, assessment at 3 days after reperfusion showed that BK administration attenuated the extensive neuronal degeneration via a decrease in cytoplasmic mitochondrial enzyme manganese superoxide dismutase (MnSOD) and caspase-3 as compared to placebo rats (Danielisova, Gottlieb et al. 2009). Interestingly, MnSOD is a mitochondrial anti-oxidant enzyme in the cytosol; hence a probable mechanism of protection might involve blockage of mitochondrial proteins release to the cytoplasm, in addition to blockage of the mitochondrial pathway of apoptosis in post-ischemic brain injury (Danielisova, Gottlieb et al. 2008). In another study by Bovenzi et al., BK treatment on primary porcine cerebral microvascular endothelial cells (pCMVEC) and brain micro-vessels in organo-typic explants revealed a major decline in the endothelial cell death elicited by hydrogen peroxide and lipopolysaccharides (Bovenzi, Savard et al. 2010). This phenomenon is thought to be triggered by reprogramming genes taking part in cell survival, such as Bcl-2, cyclooxygenase-2, and copper zinc-SOD (CuZnSOD). From a different approach, Danielisova et al. focused their study on the consequences of BK on endogenous SOD after transient forebrain ischemia in rats (Danielisova, Gottlieb et al. 2008). The authors also found a marked reduction in the activity of CuZnSOD 3 days post-ischemia, while the activity of MnSOD was found to be significantly increased. Given these mechanisms, Danielisova et al. confirmed that the administration of BK prior to the onset of permanent detrimental ischemic injury-related changes is neuroprotective (Danielisova, Gottlieb et al. 2014). Indeed, BK post-conditioning in a rat model reduced total infarct volumes, decreased the number of impaired neurons in the hippocampal CA1 region and inhibited the activity of the endogenous antioxidant enzymes, SOD and catalase enzymes. Importantly, BK treatment was shown to be effective after a strong MCAO attack (60 minutes) and also many hours post cerebral ischemia (Danielisova, Gottlieb et al. 2014). Furthermore, BK is thought to indirectly participate in the mechanism of action of ACE inhibitors as it was shown to confer microvascular endothelial protection and maintenance of vascular integrity in animal disease models and patients treated with ACE inhibitor and AT1 receptor antagonists (ARA) (Duncan, Kladis et al. 2000, Matsumoto, Manabe et al. 2003, Battegay, de Miguel et al. 2007, Bovenzi, Savard et al. 2010). BK conditioning was also found to show behavioral and memory improvements as assessed by a Barnes maze (Lalkovicova, Bonova et al. 2015). This finding provides a neuroprotective role of BK from transient global cerebral ischemia/reperfusion injury.
In the scope of endogenous regulation, urodilatin, a hormone that causes natriuresis, was reported to reduce brain edema in MCAO mice, highlighting the existence of endogenous BK antagonist and its potential to reverse the detrimental consequences of the KSS in stroke (Dobrivojevic, Spiranec et al. 2016). It was shown by Smeda et al. that Kyoto Wistar stroke-prone spontaneously hypertensive rats (SHRsp) with hypertensive encephalopathy (HE) or cerebral hemorrhage could not vasodilate to BK; however, protease-activated receptor 2 (PAR2) expression and vasodilation function were not affected in the MCA SHRsp after HE or cerebral hemorrhage. Therefore, PAR2 could be pharmacologically used to stimulate cerebrovascular dilation, ameliorating cerebrovascular vasospasm, which often occurs after cerebral hemorrhage (Smeda, McGuire et al. 2010).
Following the discovery of the beneficial effects of the specific non-peptide competitive B2R antagonist LF 16-0687Ms on brain edema evolution, and functional recovery post-TBI, the role of this same antagonist was investigated in rats with focal cerebral ischemia model (Zausinger, Lumenta et al. 2002). In this study, Sprague-Dawley rats were subjected to MCAO for 90 mins by an intraluminal filament and cerebral blood flow was recorded by flowmetry. B2R antagonism attenuated post-ischemic brain swelling, improved functional neurological recovery and limited ischemic tissue damage. Similarly, subarachnoid hemorrhage (SAH), mortality and secondary brain damage post-SAH in BK-knockout mice is mediated by B2R signaling (Scholler, Feiler et al. 2011). LF 16-0687Ms used by Klasner et al. and Ding-Zhou et al. and CP-0597 used by Relton et al. also reported similar findings in MCAO animal models, whereby B2R antagonism resulted in reduced infarct volume, penumbral damage, necrotic neurons, edema and functional outcomes (Relton, Beckey et al. 1997, Ding-Zhou, Margaill et al. 2003, Klasner, Lumenta et al. 2006). Similar protective findings were also reported using Bradyzide, a highly selective B2R antagonist (Su, Cui et al. 2009). The KKS promotes ischemic-stroke induced brain injuries via B2R (Groger, Lebesgue et al. 2005). In the study, tissue BK was maximally increased 12 hrs post-45 mins MCAO in mice, while B2R was upregulated 2 hrs after ischemia and remained so up to 24 hrs. Indeed, B2−/− mice had improved motor function, smaller infarcts, less brain edema and survived longer.
On the other hand, Xia et al. demonstrated that MCAO-induced ischemic/reperfusion damage was intensified in B2R knockout mice (Xia, Smith et al. 2006); while in another study they discovered that the presence of kallikrein leading to B2R activation, is necessary to prevent H/R-induced astrocytic apoptosis (Xia, Yin et al. 2004). Similar opposite results were reported by Austinat et al. when inhibiting B1R resulted in less brain infarction and edema formation in mice, whereas blocking B2R failed to give similar neuroprotective effect (Austinat, Braeuninger et al. 2009). In diabetic rats exposed to I/R injury, both B1R and B2R were upregulated relatively more in astrocytes and neurons, respectively (Sang, Liu et al. 2016). The study also showed the contrary to B2R antagonism, B1R inhibition yielded reduced infarct volume, neurological deficits, apoptosis, and degeneration at 24 hrs after reperfusion. In the context of diabetes, selective blockade or activation opens new avenues for ischemic stroke treatment. For instance, Desposito et al. recently showed a reduction in neurological deficit and infarct size in diabetic mice treated with B1R-agonist by 42–52% and IS by 66–71%, respectively (Desposito, Zadigue et al. 2017). Importantly, the beneficial effects of the KKS encompass the reduction of inflammatory cell infiltration, infarct size, neurological dysfunction, neuronal/glial cell apoptosis, as well as the promotion of angiogenesis and neurogenesis in ischemic brain (Ping, Chun et al. 2005). These effects are supplemented by a reduction of oxidative stress and activation of the apoptotic Akt-mediated signaling pathways (Ping, Chun et al. 2005).
2.4 Spinal cord injury
Spinal cord injury is a devastating condition that is generally caused by high-speed and impact injuries such as car accidents, falls, sport injuries and violence (Scicli, Forbes et al.). Damage to the spinal cord includes oligodendrocyte loss and consequent demyelination and impaired axonal function causing impairments/loss of motor, sensory, or autonomic function. In addition, edema and disruption of the blood spinal cord barrier (BSCB) are also observed (Kumar, Ropper et al. 2016). Controlling inflammatory process to prevent damage spread is emerging as an important component of the therapeutic strategy for SCI (Thornton, Ziebell et al. 2010).
Yan-feng et al. further confirmed the protective role of BK in the spinal cord ischemic injury in rats (Yan-Feng, Gang et al. 2008). They showed that BK pre-conditioning increased the expression of the basic fibroblast growth factor protein (bFGF) after ischemia. bFGF elevation activates or augments the signal transmission pathway in spinal cord parenchymal cells to protect neurons from injury (Xu, Kitada et al. 2006). However, this is not the case in post-conditioning where the tissue has already been ischemic (Yan-Feng, Gang et al. 2008). Following SCI, short-term outcomes post-BK treatment resulted in depolarization of interneurons and motoneurons in the ventral horn whereas long-term outcomes include spinal cord plasticity characterized by upregulation of glial-derived neurotrophic factor and an increase in microglia numbers via B2R (Mandadi, Leduc-Pessah et al. 2016). In fact, kininogen content in traumatized cord segments in rats increased temporally and kinin levels increased up to 40-fold in 2 hrs indicating the conversion of accumulated kininogen into kinin resulting in vasogenic brain edema and posttraumatic vascular injury (Xu, Hsu et al. 1991). Moreover, the modifications in the expression of aquaporin-4 (AQP4) - a protein highly expressed in the glial cells of the spinal cord - occur in parallel to water content changes in injured spinal cords. Knowing that BK pre-conditioning can help restore BSCB integrity, Xu et al., investigated whether pre-ischemic administration of BK could alter the expression of AQP4 (Xu, Gu et al. 2008). They found that BK was capable of reducing the expression level of AQP4 protein in the white matter, contributing to spinal cord edema fluid clearance, 72 hours post-reperfusion (Xu, Gu et al. 2008). These findings shed light on the molecular mechanism of spinal cord ischemic edema.
Secondary damage post-SCI is partly caused by inflammatory astrogliosis essentially regulated by PAR2 (Radulovic, Yoon et al. 2015). Greater motor coordination and strength were observed in SCI mice lacking PAR2 along with attenuated levels of glial fibrillary acidic protein (GFAP), vimentin and neurocan referred to as key hallmarks of astrogliosis, besides downregulation of TNF, IL-1β and IL-6. Mechanistically, the study revealed that neurosin (kallikrein 6) promotes IL-6 secretion in a PAR2 and STAT3-dependent manner. Therefore, designing antagonists designed against PAR2 represents a new approach in an attempt to downgrade the severity of secondary neurotrauma. To determine the role of a select number of kallikreins in the pathogenesis of SCI, Radulovic et al. quantified RNA of kallikrein 1, 5, 6, 7, 8 and 9 in postmortem human traumatic SCI cases (Radulovic, Yoon et al. 2013). The most marked elevation that remained so chronically is kallikrein 9 knowing that kallikreins 5, 6, 7 and 8 may activate pro-kallikrein 9 (Yoon, Blaber et al. 2009). Kallikreins 1, 5, 6, 7 and 9 are neurotoxic whose targeting should be considered in future neuroprotective strategies (Scarisbrick, Sabharwal et al. 2006, Scarisbrick, Linbo et al. 2008, Radulovic, Yoon et al. 2013, Yoon, Radulovic et al. 2013).
Administration of HOE-140 in an SCI rat model attenuated the extravasation of Evans blue and [131]I-sodium tracers indicating that disruption of BSCB permeability is also mediated via B2R (Sharma 2000). In overactive urinary bladder (OAB) rats subjected to SCI, B1R expression was elevated in the urinary bladder, dorsal root ganglion and spinal cord and that of B2R was elevated in the spinal cord (Forner, Andrade et al. 2012). More importantly, cystometry showed reduced amplitude and frequency of non-voiding contractions (NVCs) with both B1R and B2R antagonism. In terms of post-SCI neuropathic pain, transient receptor potential vanilloid subtype 1 (TRPV1) antagonist improved thermal hyperalgesia whereas B1R antagonist appeared to reverse thermal hyperalgesia (Rajpal, Gerovac et al. 2007). BK-induced hyperalgesia was inhibited by the selective TRPV4 antagonist HC-067047 in a PKC-dependent fashion, the outcome that was also inhibited using PKCε inhibitor (εV1–2) (Costa, Bicca et al. 2017). DomBourian et al. reported similar findings in rats subject to SCI (DomBourian, Turner et al. 2006). Successively, Pan et al. have established that BK participates in the early phase of BSCB disruption (Pan, Kastin et al. 2001). Importantly, B9430, a non-selective B1R and B2R antagonist, prevented BSCB breakdown without disturbing the selective transport system for TNF-α (required for neuro-regeneration). Thus, B9430 is considered an interesting potential therapeutic candidate to decrease tissue damage induced by SCI (Pan, Kastin et al. 2001). Thus, spinal cord ischemia protection mediated via BK pre-conditioning occurs via protection of vasculature, diminished permeability of the BSCB and amelioration of the ischemic neurons (Yan-Feng, Gang et al. 2008).
2.5 Alzheimer’s disease
Alzheimer’s disease (AD) is portrayed by the deposition of β-amyloid (Aβ) protein and neurofibrillary tangles of tau protein in the brain. This β-amyloid protein is a fragment of a larger protein, the amyloid precursor protein (APP) (Portt, Norman et al.). Particularly, the 1–11 region of Aβ is essential for activation of the contact-kinin system in AD (Bergamaschini, Donarini et al. 2001). In fact, HK has been massively cleaved in the CSF of AD patients potentially indicating an interaction of Aβ with factor XII and kallikrein production (Bergamaschini, Parnetti et al. 1998).
The role of the plasma KKS has been experimented by Ashby et al. on human brain tissues from post-mortem-confirmed cases of sporadic AD, vascular dementia (VaD) and controls (Ashby, Love et al. 2012). Enolase mRNA and protein levels were evaluated prior to PK measurements, to adjust for neuronal loss (Ashby, Love et al. 2012). It was demonstrated that adjusted PK mRNA and protein levels were particularly elevated in the frontal cortex in AD, and in both the frontal and temporal cortex in VaD (Ashby, Love et al. 2012). Moreover, PK activity was shown to be raised in both the frontal and temporal cortex in AD, which may be a consequence of Aβ accumulation (Ashby, Love et al. 2012). As PK is the enzyme that activates BK, it was also inferred that increased PK in AD correlates with increased BK release, whose activity was indeed found to induce the processing of APP via α-secretase (Nitsch, Kim et al. 1998, Ashby, Love et al. 2012). In addition, Iores-Marcal et al. showed that the KKS activation leads to an increase in CSF levels of BK in rats submitted to neurodegeneration (Iores-Marcal, Viel et al. 2006). Remarkably, only the degradation product of BK (Arg-Pro-Pro) was detected in the brain, implying the contribution of the kinin processing enzymes: prolyl oligopeptidase (POP) and/or neprilysin (Iores-Marcal, Viel et al. 2006). Given the fact that POP regulates the process of neuropeptides and inactivates BK, which is itself involved in senile Aβ plaque formation in AD, POP should be tightly controlled (Myohanen, Garcia-Horsman et al. 2009). However, BK is a double-edged sword and concerns should be raised about its dual role. In fact, although BK exerts inflammatory effects on neurons, it can also exert a neuroprotective effect on glial cells (Viel and Buck 2011).
Still, in the quest of early detectable AD features, Khan et al. presented ERK1 and ERK2 as peripheral molecular biomarkers, phosphorylated in response to BK-mediated activation of the inflammatory PKC pathway (Khan and Alkon 2006). Thus, differential ERK1 and ERK2 phosphorylation might be a clinically useful tool in the early diagnosis of AD (Khan and Alkon 2006). The secretory processing of APP can be quickened by the activation of different GPCRs, notably B2R (Racchi, Ianna et al. 1998). However, in PC12 cells, BK-induced increase in APP secretion is mediated by PKC; while in NRK-49F cells, vasopressin-mediated APP processing is PKC-independent (Nitsch, Kim et al. 1998). Modulation of neuropeptide receptors offers a conceivable novel strategy for the treatment of AD (Nitsch, Kim et al. 1998). Racchi et al. were among the first researchers to discover that APP secretion in human skin fibroblasts depends on the interaction of BK with B2R, but is independent of PKC activation (Racchi, Ianna et al. 1998). It is thought that the pathophysiological aspect of AD is due to a variation in signal transduction. In 1995, after demonstrating that human skin fibroblasts contain BK receptors that are intertwined with the phosphoinositide pathway (Huang, Toral-Barza et al. 1991), Huang et al. showed that the increase in BK-stimulated IP3 formation in AD fibroblasts was associated with an elevated BK receptor number, and that this scenario was occurring following PKC down-regulation (Huang, Lin et al. 1995, Huang and Gibson 1996). Furthermore, in another study performed on BK-treated human skin fibroblasts, the effect of BK on soluble APP secretion appeared to be B2R-dependent and PKC-independent (Racchi, Ianna et al. 1998). Moreover, Racchi et al. suggested that the alteration in PKC activity, adenylate cyclase activity and inositol turnover in familial and sporadic AD fibroblasts could present different phenotypical patterns linked to the presence or absence of specific mutations (Racchi, Ianna et al. 1998). In terms of calcium homeostasis, BK-induced greater calcium release primarily from intracellular stores in cultured fibroblasts isolated from AD patients compared to healthy controls (Peterson, Ratan et al. 1988, Ito, Oka et al. 1994, Gibson, Zhang et al. 1996). More specifically, B2R activation was shown to lead to Ser phosphorylation of the tau microtubule-associated protein in skin fibroblasts, in individuals who have or will develop AD because of Presenilin 1 mutations or Trisomy 21 (Jong, Ford et al. 2003). Also, CaM Kinase II and GSK-3 were elevated in accordance with tau hyperphosphorylation post-BK in rat brain (Sengupta, Grundke-Iqbal et al. 2006). Interestingly, injection of BK to rat hippocampus induced Alzheimer-like tau hyperphosphorylation in addition to a characteristic abnormal behavior as observed using an electronic attack jump platform (Wang and Wang 2002). This various signaling cascades in early features of AD represents an interesting molecular signature accessible in the brain but more easily in peripheral tissues (Hongpaisan, Sun et al. 2011). Furthermore, Khan et al. demonstrated that the ERK1/2 PKC-based biomarker (AD-biomarker) as assessed in skin fibroblasts has a high specificity and sensitivity at early stages of the disease, as confirmed by autopsy validation and clinical diagnosis (Khan and Alkon 2010). This peripheral biomarker has a remarkable pathophysiological relevance and diagnostic accuracy, and is more convenient for an early detection of AD as compared to direct measures of amyloid plaques, tau protein, Aβ1– 40 and/or Aβ1– 42 in the CSF of more advanced cases (Khan and Alkon 2010).
After the introduction of Aβ1–40, a significant increase in cGMP production was observed which was inhibited by a BR2 antagonist as well as by a selective PK inhibitor further indicating the activation of KSS in AD (Wirth, Fink et al. 1999). In a recent study, Prediger et al. reported that intracerebroventricular injection of Aβ1– 40 peptide results in the upregulation of the kinin B1R in the hippocampus and cortex of mice, and that the genetic deletion or pharmacological antagonism of kinin B1R and B2R is capable of ameliorating the cognitive deficits in Aβ1– 40-treated mice (Prediger, Medeiros et al. 2008). Noteworthy, both B1R and B2R antagonists, and principally the new orally active non-peptide antagonists, represent a putative therapeutic candidate for AD treatment (Prediger, Medeiros et al. 2008). In 2013, Lacoste et al. revealed the detrimental role of B1R on cognition and cerebral vessels in an AD transgenic mouse model, expressing the human APP gene (Tg-SwDI) (Lacoste, Tong et al. 2013). The study demonstrated a selective increase in astrocytic B1R protein expression, mainly concomitant with the presence of Aβ plaques in the hippocampus of the APP-transgenic mouse brain (Lacoste, Tong et al. 2013). Moreover, treatment of the APP cell line with the selective brain-penetrant B1R antagonist (SSR240612) reduced soluble brain Aβ1–42, diffuse and dense-core Aβ plaque levels, and led to an improvement in spatial learning and memory performance, better cerebrovascular function and reactivity, a reduction in microglial activation, along with amyloidosis repeal (Lacoste, Tong et al. 2013). Consistently, B1R proved to be involved in neurodegeneration and memory loss as knock-out B1R mice, and not knock-out B2R mice, had better memory retention measured by the frequency of conditioned avoidance responses (Lemos, Amaral et al. 2010). Hence, this work ascertains the deleterious effect of B1R in AD pathogenesis and proposes the use of SSR240612 as a potential neuroprotective therapy (Lacoste, Tong et al. 2013). A similar study was performed by Passos et al. with the same transgenic mouse model described above (Tg-SwDI); however, a different B1R antagonist was used: R-715 (Passos, Medeiros et al. 2013). Here, inhibition of B1R significantly increased the deposition of Aβ40 plaques in the brain, which was immediately followed by a marked reduction in the activation of glial cells and in the levels of pro-inflammatory molecules (Passos, Medeiros et al. 2013). Similarly, Asraf et al. recently showed that R-715 but not HOE 140 increased NO and TNF-α release besides microglial accumulation and amyloid deposition (Asraf, Torika et al. 2017). By regulating Aβ deposition and reducing neuroinflammation, the B1R represents a novel potential therapeutic approach for AD (Passos, Medeiros et al. 2013).
On one hand, these studies insinuate that B1R is involved in the pathogenesis of AD, as its inhibition abolishes amyloidosis, cerebrovascular and memory deficits. On the other hand, activation of B2R was linked to memory preservation (Caetano, Dong-Creste et al. 2015).
2.6 Multiple sclerosis
Multiple Sclerosis is a chronic, progressive immune-mediated disease in which myelin sheaths around axons of the brain and spinal cord deteriorate (Grigoriadis, van Pesch et al. 2015). These processes are closely related to diffuse neurodegeneration. Although the underlying mechanisms are still not fully elucidated, the inflammatory demyelinating disease process is believed to be driven by a T cell-mediated attack and failure of oligodendrocytes to counterbalance the demyelination (Nakahara, Maeda et al. 2012). Inflammation is an important hallmark of MS whereby myelin protein-specific T cells enter the brain, due to BBB disruption, and attack the myelin sheath (Compston and Coles 2008). A new method to treat MS consists of diminishing the infiltration of immune cells into the CNS via monoclonal antibodies (such as Natalizumab). Since BK has the ability to alter leukocyte recruitment by the production of chemo-attractant molecules, namely chemokines, the KKS has been investigated in the quest for potential therapeutic targets for MS (Souza, Lomez et al. 2004, Dutra 2017).
One study conducted on experimental autoimmune encephalomyelitis (EAE), which is an experimental animal model of human MS, demonstrated that B2R has two major effects on EAE severity: (i) regulating the expression of chemokines, including CCL2 and CCL5, and (Schuhmann, Mokhtarzadeh et al.) (ii) modulating leukocyte recruitment and inflammatory lesions in the CNS (Dos Santos, Roffe et al. 2008). Activation of B1R using B1R agonist R838, unlike B1R antagonist R715, limits encephalitogenic T lymphocyte recruitment to the CNS in a viral model of MS (Schulze-Topphoff, Prat et al. 2009). Furthermore, pharmacological modulation revealed that migration of human Th17 lymphocytes across the BBB endothelium is regulated by B1R indicating its crucial role in neuroinflammation and immune cell infiltration (Blaber, Ciric et al. 2004). Another study recognized B1R as a mediator of BBB disturbance and immune cell transport in EAE (Gobel, Pankratz et al. 2011). Dutra et al. established the central role of B1R in EAE and suggested that B1R exhibits a double role in the development of EAE via diverse mechanisms at every stage of the disease, mostly via modulation of Th1 and Th17- myelin-specific lymphocytes and glial cell-dependent pathways (Dutra, Leite et al. 2011).
It was also demonstrated that B1R-knock out EAE mice show less cognitive deficits and cholinergic dysfunction, along with blocked mRNA expression of IL-17 and INF-gamma, which are pro-inflammatory cytokines, in the lymph nodes, prefrontal cortex and spleen when compared to control mice (Dutra, Moreira et al. 2013). In addition, B1R was found to participate in the modulation of EAE neuropathic pain, by causing changes in astrocytic reactivity (Dutra, Bento et al. 2013). Therefore, MS patients suffering from neuropathic pain can benefit from treatment with B1R selective antagonists or drugs that reduce kinin release. Elevations in the levels of proinflammatory cytokines (Il-6, TNF-α, but not IL-1β), chemokines (MCP-1, MIP-3α, LIX), fractalkine and VEGF observed in EAE rats, were significantly downregulated post-B1R antagonism using DALBK (Podsiadlo, Sulkowski et al. 2017). However, a decrease in neuroprotective factors such as IL-10, IL-4, and CNTF was noticed. In a different study conducted on EAE rats, DALBK consistently prevented IFN-induced up-regulation of TNF-α and IL-6 release in astrocyte culture (Dutra, Bento et al. 2013). From a different perspective, Dutra et al. revealed that B1R gene deletion or antagonism significantly inhibited sensory hypersensitivity indicating a possible treatment of neuropathic pain in patients suffering from MS. In blood-derived mononuclear cells serially collected from 6 MS patients, Prat et al. quantified B1R mRNA levels and were found to correlate positively with the volume of magnetic resonance imaging (MRI) T2-weighted lesion (Prat, Biernacki et al. 2005). This finding promotes the use of this B1R as an index of disease activity in MS.
In a recent study, Enalapril, an ACE inhibitor, was administered to EAE mice at a dosage of 0.2 or 1.0 mg/kg/day (Uzawa, Mori et al. 2014). Interestingly, Enalapril alleviated the severity of EAE, attenuated CNS inflammation, demyelination and infiltration of IL-17-positive cells into the CNS, and enhanced serum BK levels (Uzawa, Mori et al. 2014). These favorable effects disappeared in the presence of R-715, a B1R antagonist (Uzawa, Mori et al. 2014). High levels of factor XII activity are present in the plasma of MS patients during relapse (Gobel, Pankratz et al. 2016). Targeting factor XII renders mice less susceptible to EAE along with a reduced number of IL-17-producing T cells indicating the promising role of combating factor XII in MS. ACE inhibitors were also reported to abolish apoptosis in growing control cultures (Weissgarten, Berman et al. 2007). Taken together, these results demonstrate the above-mentioned unfavorable role of B1R antagonists on EAE mice models. However, tight control of the KKS, through ACE inhibitors or B1R inhibitors, remains a valid therapeutic strategy for MS.
2.7 Glioma
Human tissue factor pathway inhibitor-2 (TFPI-2) protein that inhibits PK, inversely correlates with the progression of human gliomas; highest levels in the normal brain, lesser in low-grade gliomas and astrocytomas, and negligible levels in glioblastomas (Rao, Lakka et al. 2001). Further research by Konduri et al. revealed that TFPI-2 significantly promotes survival and importantly, an invasive phenotype of human gliomas (Koshikawa, Nakamura et al. 1996, Konduri, Rao et al. 2001, Tasiou, Konduri et al. 2001).
To elucidate the interactions between GBM cells and mesenchymal stem cells (MSC), Pillat et al. showed that BK reduced B1R and B2R in MSC, with concomitant elevation of these receptors in the GBM cell line, suggesting a BK-mediated GBM-MSC crosstalk essential for tumor progression and invasion (Pillat, Oliveira et al. 2016). Therapeutically and in an attempt to overcome the tight BBB, in the malignant F98 glioma rat model, treatment with B1R and B2R agonists enhanced delivery of the anticancer drug carboplatin to the brain as detected using inductively coupled plasma-mass spectrometry (Cote, Savard et al. 2013). Similar finding was previously reported by Cote et al. whereby a B2R analogue (R523) allowed the extravasation of hydrophilic macromolecular compounds towards the tumor (Cote, Savard et al. 2010). This approach increased survival and eventually, proves to be a novel way of improving neuro-bioavailability of chemotherapeutic agents.
To investigate the role of kinin receptors in brain malignancy, Nicoletti et al. injected GL-261 glioma cells into the right striatum of adult wild-type, B1R and B2R knockout (KOB1R and KOB2R) and B1R B2R double knockout mice (KOB1B2R) (Nicoletti, Senecal et al. 2016). In KOB1R or SSR240612-treated mice, tumor growth progressed uncontrollably which was blunted using HOE-140 whereas in KOB1B2R mice or upon combined administration of both B1R and B2R antagonists, elevation of B1R was normalized and a decrease in tumor size and mitotic index were noted. The same study revealed marked B1R immunopositivity in low-grade glioma patients indicating that either double receptor antagonism or B1R stimulation is of therapeutic value in the management of glioblastomas (Nicoletti, Senecal et al. 2016).
3. Kinin receptors at the borderline between therapeutic tools and biomarkers
3.1 Kinins as therapeutic targets
Over the past years, numerous studies have focused on B1R and B2R manipulation (inhibition and/or activation) as therapeutic targets in the treatment of different brain disorders such as temporal lobe epilepsy (Arganaraz, Silva et al. 2004), spinal cord injury (Xu, Kitada et al. 2006, Xu, Gu et al. 2008, Yan-Feng, Gang et al. 2008), Alzheimer’s disease (Huang, Lin et al. 1995, Nitsch, Kim et al. 1998, Jong, Ford et al. 2003, Prediger, Medeiros et al. 2008, Lacoste, Tong et al. 2013, Passos, Medeiros et al. 2013), ischemic stroke (Xia, Yin et al. 2004, Xia, Smith et al. 2006, Austinat, Braeuninger et al. 2009, Liu, Zhang et al. 2009, Scholler, Feiler et al. 2011, Woodfin, Hu et al. 2011, Waldner, Baethmann et al. 2012, Danielisova, Gottlieb et al. 2014) TBI (Narotam, Rodell et al. 1998, Gorlach, Hortobagyi et al. 2001, Zausinger, Lumenta et al. 2002, Hellal, Pruneau et al. 2003, Ivashkova, Svetnitsky et al. 2006, Liu, Zhang et al. 2009, Woodfin, Hu et al. 2011, Waldner, Baethmann et al. 2012, Albert-Weissenberger, Siren et al. 2013), and febrile illness (Soares, Santos et al. 2017). Nevertheless, the results obtained were controversial. Actually, some studies have emphasized the implication and protective role of B1R in brain injury while others have emphasized the critical role of B2R. The disparity of the experimental results is summarized in Table II. Although controversial, the results obtained offer encouraging therapeutic approaches for drug development as shown in Figure 5 (Rodell 1996, Heitsch 2002).
Table II. Controversial results regarding the protective effects of B1R versus B2R in different neurological disorders.
The table portrays the beneficial and harmful effects provoked by the activation (via an agonist) or inhibition (via an antagonist or by knock-out) of the B1R and B2R. The controversy can be seen when, in different studies, a different effect is obtained though the state of the receptor, the models, the type of injury performed and the neurological disorder tested are the same. Discordant results are also observed when the same effect is obtained after activating a receptor in one study, and after inactivating this same receptor in another study.
| Receptor | State of Receptor |
Neurological disorder tested |
Models | Type of injury performed |
Name of agonist or antagonist used |
Effects | Benefit (+) Harm (−) |
References |
|---|---|---|---|---|---|---|---|---|
| B1R | Activation | TLE | Rats | Kindling/Kainate | Lys-des-Arg9-BK |
|
− | (Bregola et al., 1999; Mazzuferi et al., 2005) |
| Glioma | Glioblastoma cells | BK |
|
− | (Pillat et al., 2016) | |||
| Glioma | Rats | B1R agonist (NG29) |
|
+ | (Cote et al., 2013) | |||
| MS | Mice | EAE | Des-Arg9-BK |
|
+ | (Dutra et al., 2011) | ||
| MS | Mice | EAE | R838 |
|
+ | (Schulze-Topphoff et al., 2009) | ||
| MS | Mice and MS patients | EAE | R838 |
|
− | (Gobel et al., 2011) | ||
| KO | TLE | Mice | Pilocarpine treated |
|
+ | (Arganaraz et al., 2004) | ||
| TBI | Mice | Cryogenic cortical injury |
|
+ | (Raslan et al., 2010) | |||
| Glioma | Mice | Injection of GL-261 glioma cells into the right striatum |
|
− | (Nicoletti et al., 2016) | |||
| MS | Mice | EAE |
|
+ | (Dutra et al., 2013) | |||
| Inhibition | Stroke | Mice | 60min transient MCAO surgery | R-715 | Diminished brain infarction and edema formation. | + | (Austinat et al., 2009) | |
| Stroke | Rats | MCAO | Lys-(des-Arg9-Leu8)-BK |
|
+ | (Sang et al., 2016) | ||
| SCI | Rats | Contusion | Lys-(des-Arg9-Leu8)-BK | Hyperalgesia | − | (Rajpal et al., 2007) | ||
| AD | Mice | Aβ1–40-treated | Des-Arg9 [Leu8]-BK | Improved cognitive deficits. | + | (Prediger et al., 2008) | ||
| AD | Mice | Transgenic mice overexpressing human APP (Tg-SwDI) | SSR240612 |
|
+ | (Lacoste et al., 2013) | ||
| AD | Mice | Tg-SwDI | R-715 | Increased deposition of Aβ plaques in the brain, directly followed by a marked reduction in the activation of glial cells and in the levels of pro-inflammatory molecules. | + | (Passos et al., 2013) | ||
| AD | Mice | Transgenic 5× familial AD mice | R-715 | Increased NO and TNF-α release besides microglial accumulation and amyloid deposition | − | (Asraf et al., 2017) | ||
| MS | Mice | EAE | Des-Arg9-[Leu8]-BK | Reduced neuropathic pain via changes in astrocytes reactivity. | + | (Dutra et al., 2013) | ||
| MS | Mice | EAE | R-715 | Increased CNS inflammation, demyelination and infiltration of IL-17 positive cells | − | (Uzawa et al., 2014) | ||
| MS | Mice | EAE | R715 | Increased encephalitogenic T lymphocyte recruitment to the CNS | − | (Schulze-Topphoff et al., 2009) | ||
| MS | Rats | EAE | DALBK |
|
+ | (Podsiadlo et al., 2017) | ||
| MS | Mice | EAE | DALBK |
|
+ | (Dutra et al., 2013) | ||
| TBI | Mice | Cryogenic cortical injury | R-715 |
|
+ | (Raslan et al., 2010) | ||
| TBI | Mice | Closed head model of focal TBI | R-715 |
|
+ | (Albert-Weissenberger et al., 2013) | ||
| B2R | Activation | Stroke | Rats | Cerebral I/R surgery | BK |
|
+ | (Xia et al., 2004) |
| Stroke | Rats (Primary cultured neurons) | H/R insult | BK |
|
+ | (Tang et al., 2009) | ||
| Stroke | Rats | Cerebral I/R surgery | BK |
|
− | (Woodfin et al., 2011) | ||
| Stroke | Mongolian gerbils | Cerebral I/R surgery | BK | Promoted secondary brain damage through an increase of vascular permeability and brain edema formation. | − | (Waldner et al., 2012) | ||
| Stroke | Rats | MCAO surgery | BK |
|
+ | (Danielisova et al., 2014) | ||
| SCI | Rats | Spinal cord ischemic injury | BK pre-conditioning | Reduced expression of AQP4 followed by clearance of spinal cord edema in the white matter 72hrs post-reperfusion. | + | (Xu et al., 2008) | ||
| SCI | Rats | Spinal cord ischemia injury model | BK pre-conditioning |
|
+ | (Yan-Feng et al., 2008) | ||
| SCI | Microglial cell culture | Spinal cord injury | BK |
|
+ | (Mandadi et al., 2016) | ||
| AD | Skin fibroblasts of AD donors | BK | Increased α-secretase APP processing in cell lines via PKC down-regulation. | − | (Huang et al., 1995; Nitsch et al., 1998) | |||
| AD | Non-immortalized human skin fibroblast of AD patients | BK | Ser phosphorylation of tau microtubule-associated protein in skin fibroblasts from persons who have or will develop AD due to Presenilin 1 mutations or Trisomy 21. | − | (Jong et al., 2003) | |||
| Glioma | Glioblastoma cells | BK | Increased B2R in glioblastoma cells | − | (Pillat et al., 2016) | |||
| Glioma | Rats | B2R agonist (R523) | Enhanced delivery of carboplatin to the brain | + | (Cote et al., 2013) | |||
| Glioma | Rats | R523 | Allowed extravasation of hydrophilic macromolecular agents towards the tumor | + | (Cote et al., 2010) | |||
| AD | Rats | BK | Abnormal behavior, disturbance in learning and memory | − | (Wang and Wang, 2002) | |||
| Stroke | Rats | NMDA-provoked excitotoxicity | BK | Marked neuroprotection of pyramidal neurons via activation of PI3 kinase, which is then responsible for Bad phosphorylation and subsequent anti-apoptotic activity. | + | (Martins et al., 2012) | ||
| KO | TLE | Mice | Pilocarpine treated |
|
− | (Arganaraz et al., 2004) | ||
| MS | Mice | EAE |
|
+ | (Dos Santos et al., 2008) | |||
| TBI | Mice | Closed head injury |
|
+ | (Hellal et al., 2003) | |||
| TBI | Mice | Closed head model of focal TBI |
|
− | (Albert-Weissenberger et al., 2013) | |||
| TBI | Mice | CCI | Reduced brain water content by half and better functional outcome tests in injured animals. | + | (Trabold et al., 2010) | |||
| Stroke | Mice | MCAO injury |
|
− | (Xia et al., 2006) | |||
| Stroke | Mice | SAH surgery |
|
+ | (Scholler et al., 2011) | |||
| Stroke | Mice (PK−/−) | MCAO injury |
|
+ | (Gob et al., 2015) | |||
| Inhibition | TLE | Mice | Kindle and kainate | HOE-140 (Icatibant) and LF16-0687 Ms (Anatibant) | Reduction of seizures. | + | (Rodi et al., 2013) | |
| TBI | Traumatic brain contusions patients | Focal closed cerebral contusion | 7 days infusion of CP-0127 | Reduced cerebral edema with no rise in ICP. | + | (Narotam et al., 1998) | ||
| TBI | Rats and mice | Parietal cortex cold injury | HOE-140 |
|
+ | (Gorlach et al., 2001) | ||
| TBI | Mice | Closed head injury | LF16-0687 Ms | Reduced neurological deficit and cerebral edema 4hrs post-injury. | + | (Hellal et al., 2003) | ||
| TBI | Rats | Closed head trauma | LF18-1505T |
|
+ | (Ivashkova et al., 2006) | ||
| TBI | Mice | mLFPI | HOE-140 |
|
+ | (Ferreira et al., 2014) | ||
| Stroke | Rats | Transient MCAO | LF16-0687 Ms |
|
+ | (Zausinger et al., 2002) | ||
| Stroke | Rats | MCAO | HOE-140 |
|
− | (Chao and Chao, 2006) | ||
| Stroke | Diabetic rats | I/R | Bradyzide |
|
− | (Sang et al., 2016) | ||
| Stroke | Rats (Primary cultured neurons) | H/R insult | HOE-140 |
|
− | (Tang et al., 2009) | ||
| Stroke | Rats | Cerebral I/R surgery | HOE-140 | Reduced BBB permeability. | + | (Woodfin et al., 2011) | ||
| Stroke | Mongolian gerbils | Cerebral I/R surgery | HOE-140 | Decreased number of rolling and adherent leukocytes as well as rolling platelets at the venular endothelium. | + | (Waldner et al., 2012) | ||
| AD | Mice | Aβ1– 40-treated | HOE-140 | Attenuation of short- and long-term cognitive deficits. | + | (Prediger et al., 2008) | ||
| SCI | Rats | T10–11 incision | HOE-140 |
|
+ | (Sharma, 2000) | ||
| Glioma | Glioma cell line SNB19 | Vector carrying TFPI-2 | TFPI-2 (indirect) |
|
− | (Konduri et al., 2001; Tasiou et al., 2001) | ||
| Brain Trauma | Severe TBI patients | LF16-0687 Ms (single subcutaneous injection) |
|
+ | (Marmarou et al., 2005) | |||
| Brain Trauma | Mice | CCI injury | LF16-0687 Ms |
|
+ | (Zweckberger and Plesnila, 2009) | ||
| Brain Trauma | Rats | Focal cold injury | LF16-0687 Ms | Reduced vasogenic brain edema | + | (Schulz et al., 2000) | ||
| Brain Trauma | Rats | Cryogenic cortical lesion | LF16-0687 Ms |
|
+ | (Pruneau et al., 1999) | ||
| Brain Trauma | Mice | Cryogenic cortical injury | HOE-140 |
|
− | (Raslan et al., 2010) |
Figure 5. Neuroprotection in neurological disorders is mediated by activation or inhibition of the KKS components: B1R and B2R.
Global summary of the protection conferred by the BK receptors in different neurological disorders, including: Alzheimer’s disease, multiple sclerosis, temporal lobe epilepsy, spinal cord injury, traumatic brain injury, stroke. Some results are claimed by the majority of the studies, while others are still controversial. KKS: Kallikrein-Kinin system; B1R: kinin 1 receptor; B2R: kinin 1 receptor; TBI: traumatic brain injury.
Due to their demonstrated role in brain injury, several studies have evaluated the importance of the BK antagonist as a therapeutic target after brain trauma. In one study by Zweckberger, it has been demonstrated that Anatibant, a selective and potent antagonist of B2R, reduces the severity of brain edema by lowering intracranial pressure, and diminishes the “secondary loss of viable brain tissue” (Zweckberger and Plesnila 2009). Moreover, it has been verified that a single subcutaneous injection of Anatibant can treat secondary brain injuries with no adverse effects, in patients presenting with TBI (Marmarou, Guy et al. 2005). Furthermore, Raslan et al. showed that B1R−/− mice or the acute use of B1R antagonist R-715 attenuates cortical damage after focal brain injury in mice, but inhibition of B2R has no major protective effect. Mechanistically, the blockage of B1R was related to decreased CNS inflammation and BBB leakage after cryogenic cortical injury (Raslan, Schwarz et al. 2010).
In another study by Waldner et al, it has been shown that BK was implicated in the secondary brain damage observed following cerebral ischemia, by increasing vascular permeability and brain edema formation (Waldner, Baethmann et al. 2012). Intra-carotid injection of BK led to increased numbers of rolling and adherent leukocytes as well as rolling platelets at the venular endothelium. This was undone by adding a B2R antagonist and was not observed upon administration of a B1R antagonist. Thus, it was proposed that in order to reduce secondary brain damage, blockage of B2R but not B1R, might be of therapeutic benefit (Waldner, Baethmann et al. 2012). Similar results were demonstrated in the experimental model of stroke (cerebral reperfusion injury) where permeability was avoided by administering HOE-140.
Urodilatin, a hormone that causes natriuresis was found to reverse the BK-induced increase in ischemic lesion in MCAO mice highlighting the existence of endogenous BK antagonist (Dobrivojevic, Spiranec et al. 2016). In addition, it was shown that BK leads to IL-1β, contributing to BBB breakdown. The acute permeability response to BK relies on arachidonic acid formation via phospholipase A2 (PLA2) activation and subsequent ROS generation via cyclooxygenase and lipoxygenase (Woodfin, Hu et al. 2011). However, Albert-Weissenberger et al. demonstrated that inhibiting B1R, but not B2R reduces inflammation and edema formation in a closed head model of focal TBI (weight drop), thus exerting a protective effect against cortical cryolesions (Albert-Weissenberger, Siren et al. 2013). An amplified expression of B1R, and not B2R, was observed in the injured hemispheres of wild-type mice after 7 days of injury. Blocking of B1R showed reduced axonal injury and astroglial activation after injury induction (Albert-Weissenberger, Siren et al. 2013).
Interestingly, Cote et al. demonstrated that a B1R agonist could also be a valuable tool in the treatment of malignant brain gliomas (Cote, Bovenzi et al. 2012). The authors inoculated F98 glioma-implanted Fischer rats with natural and synthetic peptide B1R agonists, namely: LysdesArg9BK (LDBK) and SarLys[DPhe8]desArg9BK (NG29), and found out that NG29 induces selective blood-tumor barrier permeability via B1R activation and a COX-dependent pathway, and improves local distribution of diverse sized-therapeutics such as Magnevist, Gadomer, Carboplatin and endogenous albumin, at peri-tumoral sites, (Cote, Bovenzi et al. 2012). Therefore, it can be concluded that the transport of chemotherapeutic agents to treat brain tumors is highly enhanced when co-administered with synthetic biostable B1R agonists (Cote, Bovenzi et al. 2012).
3.2 Kinins as novel candidate biomarkers
Despite substantial advances, diagnosis and classification of many neurological disorders still rely on traditional unidimensional and insensitive approaches that ignore brain complexity and heterogeneity and the injury-specificity and inter-individual variability. These factors necessitate the urgent need for identifying sensitive and specific markers for such disorders. Brain injury, in concept, triggers cellular damage and neural disintegration, causing the release of proteins into biofluids (CSF and blood). In the urine of AD patients, level of acid-stable proteinase inhibitors such as trypsin inhibitors, and not kallikrein inhibitors, was elevated compared to controls (Sparro, Bonaiuto et al. 1996). This suggests that not only CSF and blood can be used for biomarker discovery, but also urine has its own fingerprint in the progression of AD. These proteins, upon identifications and assessment, can represent putative biomarkers to reveal the type and magnitude of the injury as well as insights into the underlying molecular pathophysiology (Mondello, Schmid et al. 2014). In addition, these molecules may be diagnostic factors themselves and therefore may be used to guide the treatment as targets of therapy, and monitor the biochemical effect of a drug (theragnostic markers).
Since 1984, a succession of studies provided evidence of the manifestation of the KKS in the brain and showed that this system has huge implications in the brain physiological or pathological conditions (Scicli, Forbes et al. 1984). This knowledge may have integral clinical implications; several studies have started exploring the potential of the KKS components as biomarkers of neurological disorders (Diamandis, Scorilas et al. 2004, Martinez-Morillo, Diamandis et al. 2012, Kunz, Nussberger et al. 2013). For instance, recent studies have reported elevated BK levels in human CSF after TBI, subarachnoid hemorrhage, intracerebral hemorrhage and ischemic stroke that interrelated with the intensity of edema formation (Kaplanski, Pruneau et al. 2002, Groger, Lebesgue et al. 2005, Tsuda 2009, Kunz, Nussberger et al. 2013, Dobrivojevic, Spiranec et al. 2015). These studies demonstrate a strong relationship between the KKS and brain degeneration suggesting that different components of the KKS could be useful in the diagnosis and improved discrimination of neurodegenerative disorders. However, given the lack of specificity of the components of the KKS, they should be coupled with simultaneous assessment of other brain-specific markers. This “multimarker-concept” approach is likely to provide independent and complementary prognostic information and improve the prognostic accuracy of a specific disease.
Altogether, these interpretations support the concept that the diverse components of the KKS can be utilized as markers of pathological processes underlying several neurological diseases; these can guide clinical diagnosis or monitor the progression of the disease as well as assess disease risk and prognosis. Importantly, they also appear to be risk factors themselves and therefore may be potential targets of therapy, providing opportunities to prevent the onset or progression of the disease.
4. Concluding remarks
To date, the major contribution of the KKS to the inflammatory pathways observed in several neurological disorders is no more questionable. The KKS influences normal growth and repair, the functions of which are disrupted in pathological conditions leading to uncontrolled inflammation and exacerbated neurodegeneration as discussed in several of the disorders (TBI, stroke, SCI etc.). For this reason, targeting the KKS in pathological settings is hypothesized to limit the extent of tissue loss and irreversible damage and regulate the hyperactivation of the inflammatory process. However, this theory when applied in reality, the resulting outcomes obtained with either inhibition or knockdown of BK receptor constitute the main controversy in the prognostic value of targeting components of the KSS in disease. This paradox between the varieties of effects observed post-KSS intervention has been well-demonstrated in different neurological disorders and even within the same condition which highlight a more complex “unidentified” roles of the KKS. Therefore, more thorough studies are warranted to decipher the exact roles of this ubiquities and multifunctional KKS which can be achieved via more advanced approaches such as proteomics and genomics. Future work using innovative therapeutic strategies will be critical to determine the importance of the KKS as a potential target for treating neurological disorders and preventing further downstream neurodegeneration.
The ultimate desired outcome is a reduction in the severity of injury, should it initially occur. This can be achieved at multiple levels, the best of which is the prevention of injury. At the bed-side clinical level, there is no skepticism on the role of general practitioners in providing holistic care to neurological patients in terms of ensuring personal safety, offering early screening services and providing pieces of advice on prevention of disease. The following level is bench-side; delineating detailed pathways, utilizing experimental models for drug development and uncovering accurate answers to the controversial outcomes observed from utilizing multiple approaches and levels of therapy. To alleviate inconsistency of research results, further diligent work is needed to standardize preclinical research protocols to account for discrepancies in animal species, injury model, drug administration and methods used to measure the observed outcomes. This prepares for a smooth transition to conducting clinical trials based on a solid preclinical evidence.
Highlights.
The vasodilatory kallikrein-kinin system (KKS), due to its ubiquitous roles, mediates several of the pathophysiological features of different neurological disorders as well as being implicated in regulating proper brain functions.
Recent studies have highlighted the different KKS involvement and functions in the brain and have identified that inhibition or stimulation its receptors (B1R and B2R), can exhibit neuroprotective and/or pathological outcomes.
The altered KKS dynamics can be utilized as putative biomarkers in different neurological disorders
Acknowledgments
This work is supported by a grant from the Lebanese National Council for Scientific Research (CNRS) [Grant title: Cell-Based Therapy Using Neural Stem Cell Coupled with BMP-4 as New treatment in traumatic brain injury; Lead PI Firas Kobeissy, In addition, this work was supported by the AUB-MPP grant “Dual Neurotherapeutic Effects of Docosahexaenoic Acid (DHA) and Arachidonic Acid (AA) with Neonatal Neural Stem Cell transplantation Post-Traumatic Brain Injury ”PI: FK.
Abbreviation List
- AD
Alzheimer’s type
- AEDs
anti-epileptic drugs
- BBB
blood-brain barrier
- Bdk
Bradykinin
- BDNF
brain-derived neurotrophic factor
- CPM
Carboxypeptidase M
- CNS
central nervous system
- CSF
cerebrospinal fluid
- GCS
Glasgow Coma Score
- GPCRs
G-protein coupled receptors
- HO-1
Heme Oxygenase-1
- HMWK
high molecular weight kininogen
- H/R
hypoxia/reperfusion
- ICH
intracerebral hemorrhage
- KA
kainic acid
- KKS
Kallikrein-Kinin System
- B1R
kinin 1 receptor
- B2R
kinin 2 receptor
- KO
knockout
- MCAO
middle cerebral artery occlusion
- mLFPI
moderate lateral fluid percussion injury
- NO
nitric oxide
- pCMVEC
porcine cerebral microvascular endothelial cells
- PTSD
post-traumatic stress disorder
- RBA-1
rat brain astrocytes
- ROS
reactive oxygen species
- RAS
renin angiotensin system
- SE
status epilepticus
- STS
Staurosporine
- SAH
subarachnoid hemorrhage
- TLE
Temporal lobe epilepsy
- TK
tissue kallikrein
- TBI
Traumatic brain injury
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407(6800):94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- Abou-El-Hassan H, Dia B, Choucair K, Eid SA, Najdi F, Baki L, Talih F, Eid AA, Kobeissy F. Traumatic brain injury, diabetic neuropathy and altered-psychiatric health: The fateful triangle. Med Hypotheses. 2017;108:69–80. doi: 10.1016/j.mehy.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Abou-El-Hassan H, Sukhon F, Assaf EJ, Bahmad H, Abou-Abbass H, Jourdi H, Kobeissy FH. Degradomics in Neurotrauma: Profiling Traumatic Brain Injury. Methods Mol Biol. 2017;1598:65–99. doi: 10.1007/978-1-4939-6952-4_4. [DOI] [PubMed] [Google Scholar]
- Abou-El-Hassan H, Zaraket H. Viral-derived complement inhibitors: current status and potential role in immunomodulation. Exp Biol Med (Maywood) 2017;242(4):397–410. doi: 10.1177/1535370216675772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfo Arganaraz G, Regina Perosa S, Cristina Lencioni E, Bader M, Abrao Cavalheiro E, da Graca Naffah-Mazzacoratti M, Pesquero JB, Antonio Silva J., Jr Role of kinin B1 and B2 receptors in the development of pilocarpine model of epilepsy. Brain Res. 2004;1013(1):30–39. doi: 10.1016/j.brainres.2004.03.046. [DOI] [PubMed] [Google Scholar]
- Albert-Weissenberger C, Siren AL, Kleinschnitz C. Ischemic stroke and traumatic brain injury: The role of the kallikrein-kinin system. Prog Neurobiol. 2013;101–102:65–82. doi: 10.1016/j.pneurobio.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Albert-Weissenberger C, Stetter C, Meuth SG, Gobel K, Bader M, Siren AL, Kleinschnitz C. Blocking of bradykinin receptor B1 protects from focal closed head injury in mice by reducing axonal damage and astroglia activation. J Cereb Blood Flow Metab. 2012;32(9):1747–1756. doi: 10.1038/jcbfm.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994;91(16):7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arganaraz GA, Silva JA, Jr, Perosa SR, Pessoa LG, Carvalho FF, Bascands JL, Bader M, da Silva Trindade E, Amado D, Cavalheiro EA, Pesquero JB, da Graca Naffah-Mazzacoratti M. The synthesis and distribution of the kinin B1 and B2 receptors are modified in the hippocampus of rats submitted to pilocarpine model of epilepsy. Brain Res. 2004;1006(1):114–125. doi: 10.1016/j.brainres.2003.12.050. [DOI] [PubMed] [Google Scholar]
- Ashby EL, Love S, Kehoe PG. Assessment of activation of the plasma kallikrein-kinin system in frontal and temporal cortex in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2012;33(7):1345–1355. doi: 10.1016/j.neurobiolaging.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Asraf K, Torika N, Danon A, Fleisher-Berkovich S. Involvement of the Bradykinin B1 Receptor in Microglial Activation: In Vitro and In Vivo Studies. Front Endocrinol (Lausanne) 2017;8:82. doi: 10.3389/fendo.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, Renne T, Kleinschnitz C. Blockade of bradykinin receptor B1 but not bradykinin receptor B2 provides protection from cerebral infarction and brain edema. Stroke. 2009;40(1):285–293. doi: 10.1161/STROKEAHA.108.526673. [DOI] [PubMed] [Google Scholar]
- Bachvarov DR, Houle S, Bachvarova M, Bouthillier J, Adam A, Marceau F. Bradykinin B(2) receptor endocytosis, recycling, and down-regulation assessed using green fluorescent protein conjugates. J Pharmacol Exp Ther. 2001;297(1):19–26. [PubMed] [Google Scholar]
- Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr Opin Pharmacol. 2007;7(2):151–157. doi: 10.1016/j.coph.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Ben-Shmuel S, Danon A, Fleisher-Berkovich S. Bradykinin decreases nitric oxide release from microglia via inhibition of cyclic adenosine monophosphate signaling. Peptides. 2013;40:133–140. doi: 10.1016/j.peptides.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Donarini C, Foddi C, Gobbo G, Parnetti L, Agostoni A. The region 1–11 of Alzheimer amyloid-beta is critical for activation of contact-kinin system. Neurobiol Aging. 2001;22(1):63–69. doi: 10.1016/s0197-4580(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Bergamaschini L, Parnetti L, Pareyson D, Canziani S, Cugno M, Agostoni A. Activation of the contact system in cerebrospinal fluid of patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12(2):102–108. doi: 10.1097/00002093-199806000-00008. [DOI] [PubMed] [Google Scholar]
- Bergaya S, Meneton P, Bloch-Faure M, Mathieu E, Alhenc-Gelas F, Levy BI, Boulanger CM. Decreased flow-dependent dilation in carotid arteries of tissue kallikrein-knockout mice. Circ Res. 2001;88(6):593–599. doi: 10.1161/01.res.88.6.593. [DOI] [PubMed] [Google Scholar]
- Bergmann S, Zheng D, Barredo J, Abboud MR, Jaffa AA. Renal kallikrein: a risk marker for nephropathy in children with sickle cell disease. J Pediatr Hematol Oncol. 2006;28(3):147–153. doi: 10.1097/01.mph.0000203722.91189.9d. [DOI] [PubMed] [Google Scholar]
- Blaber SI, Ciric B, Christophi GP, Bernett MJ, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6 proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;18(7):920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- Blaukat A, Pizard A, Breit A, Wernstedt C, Alhenc-Gelas F, Muller-Esterl W, Dikic I. Determination of bradykinin B2 receptor in vivo phosphorylation sites and their role in receptor function. J Biol Chem. 2001;276(44):40431–40440. doi: 10.1074/jbc.M107024200. [DOI] [PubMed] [Google Scholar]
- Bossi F, Fischetti F, Regoli D, Durigutto P, Frossi B, Gobeil F, Jr, Ghebrehiwet B, Peerschke EI, Cicardi M, Tedesco F. Novel pathogenic mechanism and therapeutic approaches to angioedema associated with C1 inhibitor deficiency. J Allergy Clin Immunol. 2009;124(6):1303–1310 e1304. doi: 10.1016/j.jaci.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutte AM, Yao C, Kobeissy F, May Lu XC, Zhang Z, Wang KK, Schmid K, Tortella FC, Dave JR. Proteomic analysis and brain-specific systems biology in a rodent model of penetrating ballistic-like brain injury. Electrophoresis. 2012;33(24):3693–3704. doi: 10.1002/elps.201200196. [DOI] [PubMed] [Google Scholar]
- Bovenzi V, Savard M, Morin J, Cuerrier CM, Grandbois M, Gobeil F., Jr Bradykinin protects against brain microvascular endothelial cell death induced by pathophysiological stimuli. J Cell Physiol. 2010;222(1):168–176. doi: 10.1002/jcp.21933. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Neary JT, Cattabeni F, Cottini L, D'Ippolito G, Schiller PC, Abbracchio MP. Induction of COX-2 and reactive gliosis by P2Y receptors in rat cortical astrocytes is dependent on ERK1/2 but independent of calcium signalling. J Neurochem. 2002;83(6):1285–1296. doi: 10.1046/j.1471-4159.2002.01239.x. [DOI] [PubMed] [Google Scholar]
- Bregola G, Varani K, Gessi S, Beani L, Bianchi C, Borea PA, Regoli D, Simonato M. Changes in hippocampal and cortical B1 bradykinin receptor biological activity in two experimental models of epilepsy. Neuroscience. 1999;92(3):1043–1049. doi: 10.1016/s0306-4522(99)00075-5. [DOI] [PubMed] [Google Scholar]
- Brovkovych V, Zhang Y, Brovkovych S, Minshall RD, Skidgel RA. A novel pathway for receptor-mediated post-translational activation of inducible nitric oxide synthase. J Cell Mol Med. 2011;15(2):258–269. doi: 10.1111/j.1582-4934.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WM, Dziegielewska KM. Friends and relations of the cystatin superfamily--new members and their evolution. Protein Sci. 1997;6(1):5–12. doi: 10.1002/pro.5560060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RM, Jr, You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology. 2005;102(6):1261–1277. doi: 10.1097/00000542-200506000-00028. [DOI] [PubMed] [Google Scholar]
- Bryant JW, Shariat-Madar Z. Human plasma kallikrein-kinin system: physiological and biochemical parameters. Cardiovasc Hematol Agents Med Chem. 2009;7(3):234–250. doi: 10.2174/187152509789105444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano AL, Dong-Creste KE, Amaral FA, Monteiro-Silva KC, Pesquero JB, Araujo MS, Montor WR, Viel TA, Buck HS. Kinin B2 receptor can play a neuroprotective role in Alzheimer's disease. Neuropeptides. 2015;53:51–62. doi: 10.1016/j.npep.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Caliezi C, Wuillemin WA, Zeerleder S, Redondo M, Eisele B, Hack CE. C1-Esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev. 2000;52(1):91–112. [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78(3):415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Chao J, Chao L. Kallikrein-kinin in stroke, cardiovascular and renal disease. Exp Physiol. 2005;90(3):291–298. doi: 10.1113/expphysiol.2004.028464. [DOI] [PubMed] [Google Scholar]
- Chao J, Chao L. Experimental therapy with tissue kallikrein against cerebral ischemia. Front Biosci. 2006;11:1323–1327. doi: 10.2741/1886. [DOI] [PubMed] [Google Scholar]
- Chao J, Chao L, Swain CC, Tsai J, Margolius HS. Tissue kallikrein in rat brain and pituitary: regional distribution and estrogen induction in the anterior pituitary. Endocrinology. 1987;120(2):475–482. doi: 10.1210/endo-120-2-475. [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RW, Sartor RB, Adam AA, DeLa Cadena RA, Stadnicki A. The plasma kallikrein-kinin system in sepsis, inflammatory arthritis, and enterocolitis. Clin Rev Allergy Immunol. 1998;16(4):365–384. doi: 10.1007/BF02737657. [DOI] [PubMed] [Google Scholar]
- Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90(10):3819–3843. [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Cooke JP. The pivotal role of nitric oxide for vascular health. Can J Cardiol. 2004;20(Suppl B):7B–15B. [PubMed] [Google Scholar]
- Costa R, Bicca MA, Manjavachi MN, Segat GC, Dias FC, Fernandes ES, Calixto JB. Kinin Receptors Sensitize TRPV4 Channel and Induce Mechanical Hyperalgesia: Relevance to Paclitaxel-Induced Peripheral Neuropathy in Mice. Mol Neurobiol. 2017 doi: 10.1007/s12035-017-0475-9. [DOI] [PubMed] [Google Scholar]
- Costa-Neto CM, Dillenburg-Pilla P, Heinrich TA, Parreiras-e-Silva LT, Pereira MG, Reis RI, Souza PP. Participation of kallikrein-kinin system in different pathologies. Int Immunopharmacol. 2008;8(2):135–142. doi: 10.1016/j.intimp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Cote J, Bovenzi V, Savard M, Dubuc C, Fortier A, Neugebauer W, Tremblay L, Muller-Esterl W, Tsanaclis AM, Lepage M, Fortin D, Gobeil F., Jr Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS One. 2012;7(5):e37485. doi: 10.1371/journal.pone.0037485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote J, Savard M, Bovenzi V, Dubuc C, Tremblay L, Tsanaclis AM, Fortin D, Lepage M, Gobeil F., Jr Selective tumor blood-brain barrier opening with the kinin B2 receptor agonist [Phe(8)psi(CH(2)NH)Arg(9)]-BK in a F98 glioma rat model: an MRI study. Neuropeptides. 2010;44(2):177–185. doi: 10.1016/j.npep.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Cote J, Savard M, Neugebauer W, Fortin D, Lepage M, Gobeil F. Dual kinin B1 and B2 receptor activation provides enhanced blood-brain barrier permeability and anticancer drug delivery into brain tumors. Cancer Biol Ther. 2013;14(9):806–811. doi: 10.4161/cbt.25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MP, Martin-de-Saavedra MD, Romero A, Egea J, Ludka FK, Tasca CI, Farina M, Rodrigues AL, Lopez MG. Both creatine and its product phosphocreatine reduce oxidative stress and afford neuroprotection in an in vitro Parkinson's model. ASN Neuro. 2014;6(6) doi: 10.1177/1759091414554945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Lepage Y, Blais C, Jr, Gervais N, Cugno M, Rouleau JL, Adam A. Bradykinin and des-Arg(9)-bradykinin metabolic pathways and kinetics of activation of human plasma. Am J Physiol Heart Circ Physiol. 2001;281(1):H275–283. doi: 10.1152/ajpheart.2001.281.1.H275. [DOI] [PubMed] [Google Scholar]
- Danielisova V, Gottlieb M, Bonova P, Nemethova M, Burda J. Bradykinin postconditioning ameliorates focal cerebral ischemia in the rat. Neurochem Int. 2014;72C:22–29. doi: 10.1016/j.neuint.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Danielisova V, Gottlieb M, Nemethova M, Burda J. Effects of bradykinin postconditioning on endogenous antioxidant enzyme activity after transient forebrain ischemia in rat. Neurochem Res. 2008;33(6):1057–1064. doi: 10.1007/s11064-007-9550-3. [DOI] [PubMed] [Google Scholar]
- Danielisova V, Gottlieb M, Nemethova M, Kravcukova P, Domorakova I, Mechirova E, Burda J. Bradykinin postconditioning protects pyramidal CA1 neurons against delayed neuronal death in rat hippocampus. Cell Mol Neurobiol. 2009;29(6–7):871–878. doi: 10.1007/s10571-009-9369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni MG, Storini C, Barba M, Catapano L, Arabia AM, Rossi E, Bergamaschini L. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischemia. J Cereb Blood Flow Metab. 2003;23(2):232–239. doi: 10.1097/01.WCB.0000046146.31247.A1. [DOI] [PubMed] [Google Scholar]
- Delemasure S, Blaes N, Richard C, Couture R, Bader M, Dutartre P, Girolami JP, Connat JL, Rochette L. Antioxidant/oxidant status and cardiac function in bradykinin B(1)- and B(2)-receptor null mice. Physiol Res. 2013;62(5):511–517. doi: 10.33549/physiolres.932496. [DOI] [PubMed] [Google Scholar]
- Desposito D, Zadigue G, Taveau C, Adam C, Alhenc-Gelas F, Bouby N, Roussel R. Neuroprotective effect of kinin B1 receptor activation in acute cerebral ischemia in diabetic mice. Sci Rep. 2017;7(1):9410. doi: 10.1038/s41598-017-09721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamandis EP, Scorilas A, Kishi T, Blennow K, Luo LY, Soosaipillai A, Rademaker AW, Sjogren M. Altered kallikrein 7 and 10 concentrations in cerebrospinal fluid of patients with Alzheimer's disease and frontotemporal dementia. Clin Biochem. 2004;37(3):230–237. doi: 10.1016/j.clinbiochem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Nicklisch S, Koster A, Spannagl M, Giersiefen H, van de Locht A. CU-2010--a novel small molecule protease inhibitor with antifibrinolytic and anticoagulant properties. Anesthesiology. 2009;110(1):123–130. doi: 10.1097/ALN.0b013e318191408c. [DOI] [PubMed] [Google Scholar]
- Ding-Zhou L, Margaill I, Palmier B, Pruneau D, Plotkine M, Marchand-Verrecchia C. LF 16-0687 Ms, a bradykinin B2 receptor antagonist, reduces ischemic brain injury in a murine model of transient focal cerebral ischemia. Br J Pharmacol. 2003;139(8):1539–1547. doi: 10.1038/sj.bjp.0705385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrivojevic M, Spiranec K, Gorup D, Erjavec I, Habek N, Radmilovic M, Unfirer S, Cosic A, Drenjancevic I, Gajovic S, Sindic A. Urodilatin reverses the detrimental influence of bradykinin in acute ischemic stroke. Exp Neurol. 2016;284(Pt A):1–10. doi: 10.1016/j.expneurol.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Dobrivojevic M, Spiranec K, Sindic A. Involvement of bradykinin in brain edema development after ischemic stroke. Pflugers Arch. 2015;467(2):201–212. doi: 10.1007/s00424-014-1519-x. [DOI] [PubMed] [Google Scholar]
- DomBourian MG, Turner NA, Gerovac TA, Vemuganti R, Miranpuri GS, Tureyen K, Satriotomo I, Miletic V, Resnick DK. B1 and TRPV-1 receptor genes and their relationship to hyperalgesia following spinal cord injury. Spine (Phila Pa 1976) 2006;31(24):2778–2782. doi: 10.1097/01.brs.0000245865.97424.b4. [DOI] [PubMed] [Google Scholar]
- Dos Santos AC, Roffe E, Arantes RM, Juliano L, Pesquero JL, Pesquero JB, Bader M, Teixeira MM, Carvalho-Tavares J. Kinin B2 receptor regulates chemokines CCL2 and CCL5 expression and modulates leukocyte recruitment and pathology in experimental autoimmune encephalomyelitis (EAE) in mice. J Neuroinflammation. 2008;5:49. doi: 10.1186/1742-2094-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AM, Kladis A, Jennings GL, Dart AM, Esler M, Campbell DJ. Kinins in humans. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R897–904. doi: 10.1152/ajpregu.2000.278.4.R897. [DOI] [PubMed] [Google Scholar]
- Dutra RC. Kinin receptors: Key regulators of autoimmunity. Autoimmun Rev. 2017;16(2):192–207. doi: 10.1016/j.autrev.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Dutra RC, Bento AF, Leite DF, Manjavachi MN, Marcon R, Bicca MA, Pesquero JB, Calixto JB. The role of kinin B1 and B2 receptors in the persistent pain induced by experimental autoimmune encephalomyelitis (EAE) in mice: evidence for the involvement of astrocytes. Neurobiol Dis. 2013;54:82–93. doi: 10.1016/j.nbd.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Dutra RC, Bento AF, Leite DF, Manjavachi MN, Marcon R, Bicca MA, Pesquero JB, Calixto JB. The role of kinin B and B receptors in the persistent pain induced by experimental autoimmune encephalomyelitis (EAE) in mice: Evidence for the involvement of astrocytes. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Dutra RC, Leite DF, Bento AF, Manjavachi MN, Patricio ES, Figueiredo CP, Pesquero JB, Calixto JB. The role of kinin receptors in preventing neuroinflammation and its clinical severity during experimental autoimmune encephalomyelitis in mice. PLoS One. 2011;6(11):e27875. doi: 10.1371/journal.pone.0027875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra RC, Moreira EL, Alberti TB, Marcon R, Prediger RD, Calixto JB. Spatial reference memory deficits precede motor dysfunction in an experimental autoimmune encephalomyelitis model: the role of kallikrein-kinin system. Brain Behav Immun. 2013;33:90–101. doi: 10.1016/j.bbi.2013.06.002. [DOI] [PubMed] [Google Scholar]
- El-Dahr SS, Dipp S, Baricos WH. Bradykinin stimulates the ERK-->Elk-1-->Fos/AP-1 pathway in mesangial cells. Am J Physiol. 1998;275(3 Pt 2):F343–352. doi: 10.1152/ajprenal.1998.275.3.F343. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Chao J, Heizer ML. Brain kininogen following experimental brain injury: evidence for a secondary event. J Neurosurg. 1989;71(3):437–442. doi: 10.3171/jns.1989.71.3.0437. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4(3):229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Erdos EG. Angiotensin I converting enzyme and the changes in our concepts through the years. Lewis K. Dahl memorial lecture. Hypertension. 1990;16(4):363–370. doi: 10.1161/01.hyp.16.4.363. [DOI] [PubMed] [Google Scholar]
- Farmer SG, Burch RM. Biochemical and molecular pharmacology of kinin receptors. Annu Rev Pharmacol Toxicol. 1992;32:511–536. doi: 10.1146/annurev.pa.32.040192.002455. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26(6):1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Ferreira AP, Rodrigues FS, Della-Pace ID, Mota BC, Oliveira SM, de Campos Velho Gewehr C, Bobinski F, de Oliveira CV, Brum JS, Oliveira MS, Furian AF, de Barros CS, dos Santos AR, Ferreira J, Fighera MR, Royes LF. HOE-140, an antagonist of B2 receptor, protects against memory deficits and brain damage induced by moderate lateral fluid percussion injury in mice. Psychopharmacology (Berl) 2014;231(9):1935–1948. doi: 10.1007/s00213-013-3336-x. [DOI] [PubMed] [Google Scholar]
- Fleisher-Berkovich S, Filipovich-Rimon T, Ben-Shmuel S, Hulsmann C, Kummer MP, Heneka MT. Distinct modulation of microglial amyloid beta phagocytosis and migration by neuropeptides (i) J Neuroinflammation. 2010;7:61. doi: 10.1186/1742-2094-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Busse R. Endothelium-derived epoxyeicosatrienoic acids and vascular function. Hypertension. 2006;47(4):629–633. doi: 10.1161/01.HYP.0000208597.87957.89. [DOI] [PubMed] [Google Scholar]
- Forner S, Andrade EL, Martini AC, Bento AF, Medeiros R, Koepp J, Calixto JB. Effects of kinin B(1) and B(2) receptor antagonists on overactive urinary bladder syndrome induced by spinal cord injury in rats. Br J Pharmacol. 2012;167(8):1737–1752. doi: 10.1111/j.1476-5381.2012.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Xiao HD, Hubert C, Michaud A, Campbell DJ, Adams JW, Capecchi MR, Corvol P, Bernstein KE. Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension. 2008;51(2):267–274. doi: 10.1161/HYPERTENSIONAHA.107.097865. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garga N, Lowenstein DH. Posttraumatic epilepsy: a major problem in desperate need of major advances. Epilepsy Curr. 2006;6(1):1–5. doi: 10.1111/j.1535-7511.2005.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Zhang H, Toral-Barza L, Szolosi S, Tofel-Grehl B. Calcium stores in cultured fibroblasts and their changes with Alzheimer's disease. Biochim Biophys Acta. 1996;1316(2):71–77. doi: 10.1016/0925-4439(96)00002-6. [DOI] [PubMed] [Google Scholar]
- Gilman AG. Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990. [Google Scholar]
- Girolami A, Randi ML, Ruzzon E, Zanon E, Girolami B. Myocardial infarction, other arterial thrombosis and invasive coronary procedures, in hemaophilia B: a critical evaluation of reported cases. J Thromb Thrombolysis. 2005;20(1):43–46. doi: 10.1007/s11239-005-2227-3. [DOI] [PubMed] [Google Scholar]
- Gob E, Reymann S, Langhauser F, Schuhmann MK, Kraft P, Thielmann I, Gobel K, Brede M, Homola G, Solymosi L, Stoll G, Geis C, Meuth SG, Nieswandt B, Kleinschnitz C. Blocking of plasma kallikrein ameliorates stroke by reducing thromboinflammation. Ann Neurol. 2015;77(5):784–803. doi: 10.1002/ana.24380. [DOI] [PubMed] [Google Scholar]
- Gobel K, Pankratz S, Asaridou CM, Herrmann AM, Bittner S, Merker M, Ruck T, Glumm S, Langhauser F, Kraft P, Krug TF, Breuer J, Herold M, Gross CC, Beckmann D, Korb-Pap A, Schuhmann MK, Kuerten S, Mitroulis I, Ruppert C, Nolte MW, Panousis C, Klotz L, Kehrel B, Korn T, Langer HF, Pap T, Nieswandt B, Wiendl H, Chavakis T, Kleinschnitz C, Meuth SG. Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nat Commun. 2016;7:11626. doi: 10.1038/ncomms11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel K, Pankratz S, Schneider-Hohendorf T, Bittner S, Schuhmann MK, Langer HF, Stoll G, Wiendl H, Kleinschnitz C, Meuth SG. Blockade of the kinin receptor B1 protects from autoimmune CNS disease by reducing leukocyte trafficking. J Autoimmun. 2011;36(2):106–114. doi: 10.1016/j.jaut.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Gorlach C, Hortobagyi T, Hortobagyi S, Benyo Z, Relton J, Whalley ET, Wahl M. Bradykinin B2, but not B1, receptor antagonism has a neuroprotective effect after brain injury. J Neurotrauma. 2001;18(8):833–838. doi: 10.1089/089771501316919193. [DOI] [PubMed] [Google Scholar]
- Gorlach, Wahl CM. Bradykinin dilates rat middle cerebral artery and its large branches via endothelial B2 receptors and release of nitric oxide. Peptides. 1996;17(8):1373–1378. doi: 10.1016/s0196-9781(96)00223-9. [DOI] [PubMed] [Google Scholar]
- Grigoriadis N, van Pesch V, Paradig MSG. A basic overview of multiple sclerosis immunopathology. Eur J Neurol. 2015;(22 Suppl 2):3–13. doi: 10.1111/ene.12798. [DOI] [PubMed] [Google Scholar]
- Groger M, Lebesgue D, Pruneau D, Relton J, Kim SW, Nussberger J, Plesnila N. Release of bradykinin and expression of kinin B2 receptors in the brain: role for cell death and brain edema formation after focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25(8):978–989. doi: 10.1038/sj.jcbfm.9600096. [DOI] [PubMed] [Google Scholar]
- Guevara-Lora I. Kinin-mediated inflammation in neurodegenerative disorders. Neurochem Int. 2012;61(1):72–78. doi: 10.1016/j.neuint.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Guingab-Cagmat JD, Newsom K, Vakulenko A, Cagmat EB, Kobeissy FH, Zoltewicz S, Wang KK, Anagli J. In vitro MS-based proteomic analysis and absolute quantification of neuronal-glial injury biomarkers in cell culture system. Electrophoresis. 2012;33(24):3786–3797. doi: 10.1002/elps.201200326. [DOI] [PubMed] [Google Scholar]
- Hagedorn I, Schmidbauer S, Pleines I, Kleinschnitz C, Kronthaler U, Stoll G, Dickneite G, Nieswandt B. Factor XIIa inhibitor recombinant human albumin Infestin-4 abolishes occlusive arterial thrombus formation without affecting bleeding. Circulation. 2010;121(13):1510–1517. doi: 10.1161/CIRCULATIONAHA.109.924761. [DOI] [PubMed] [Google Scholar]
- Hall JM. Bradykinin receptors. Gen Pharmacol. 1997;28(1):1–6. doi: 10.1016/s0306-3623(96)00174-7. [DOI] [PubMed] [Google Scholar]
- Han L, Li J, Chen Y, Zhang M, Qian L, Chen Y, Wu Z, Xu Y, Li J. Human Urinary Kallidinogenase Promotes Angiogenesis and Cerebral Perfusion in Experimental Stroke. PLoS One. 2015;10(7):e0134543. doi: 10.1371/journal.pone.0134543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi I, Amano H, Yoshida S, Kamata K, Kamata M, Inukai M, Fujita T, Kumagai Y, Furudate S, Majima M. Suppressed angiogenesis in kininogen-deficiencies. Lab Invest. 2002;82(7):871–880. doi: 10.1097/01.lab.0000018885.36823.d6. [DOI] [PubMed] [Google Scholar]
- Heitsch H. Non-peptide antagonists and agonists of the bradykinin B(2) receptor. Curr Med Chem. 2002;9(9):913–928. doi: 10.2174/0929867024606722. [DOI] [PubMed] [Google Scholar]
- Hellal F, Pruneau D, Palmier B, Faye P, Croci N, Plotkine M, Marchand-Verrecchia C. Detrimental role of bradykinin B2 receptor in a murine model of diffuse brain injury. J Neurotrauma. 2003;20(9):841–851. doi: 10.1089/089771503322385773. [DOI] [PubMed] [Google Scholar]
- Hess JF, Borkowski JA, Young GS, Strader CD, Ransom RW. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem Biophys Res Commun. 1992;184(1):260–268. doi: 10.1016/0006-291x(92)91187-u. [DOI] [PubMed] [Google Scholar]
- Heydenreich N, Nolte MW, Gob E, Langhauser F, Hofmeister M, Kraft P, Albert-Weissenberger C, Brede M, Varallyay C, Gobel K, Meuth SG, Nieswandt B, Dickneite G, Stoll G, Kleinschnitz C. C1-inhibitor protects from brain ischemia-reperfusion injury by combined antiinflammatory and antithrombotic mechanisms. Stroke. 2012;43(9):2457–2467. doi: 10.1161/STROKEAHA.112.660340. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Homi HM, Sheng H, Arepally GM, Mackensen GB, Grocott HP. Aprotinin improves functional outcome but not cerebral infarct size in an experimental model of stroke during cardiopulmonary bypass. Anesth Analg. 2010;111(1):38–45. doi: 10.1213/ANE.0b013e3181e0549f. [DOI] [PubMed] [Google Scholar]
- Hongpaisan J, Sun MK, Alkon DL. PKC epsilon activation prevents synaptic loss, Abeta elevation, and cognitive deficits in Alzheimer's disease transgenic mice. J Neurosci. 2011;31(2):630–643. doi: 10.1523/JNEUROSCI.5209-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing ML, Smits P, Morrison PJ, Rabelink TJ. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endotheliumdependent hyperpolarization. Hypertension. 2000;35(6):1314–1318. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- Hopp S, Albert-Weissenberger C. The kallikrein-kinin system: a promising therapeutic target for traumatic brain injury. Neural Regen Res. 2015;10(6):885–886. doi: 10.4103/1673-5374.158339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp S, Nolte MW, Stetter C, Kleinschnitz C, Siren AL, Albert-Weissenberger C. Alleviation of secondary brain injury, posttraumatic inflammation, and brain edema formation by inhibition of factor XIIa. J Neuroinflammation. 2017;14(1):39. doi: 10.1186/s12974-017-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HL, Wang HH, Wu CY, Jou MJ, Yen MH, Parker P, Yang CM. BK-induced COX-2 expression via PKC-delta-dependent activation of p42/p44 MAPK and NF-kappaB in astrocytes. Cell Signal. 2007;19(2):330–340. doi: 10.1016/j.cellsig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Wang HH, Wu CY, Yang CM. Reactive Oxygen Species-Dependent c-Fos/Activator Protein 1 Induction Upregulates Heme Oxygenase-1 Expression by Bradykinin in Brain Astrocytes. Antioxid Redox Signal. 2010;13(12):1829–1844. doi: 10.1089/ars.2009.2957. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Wu CY, Hwang TL, Yen MH, Parker P, Yang CM. BK-induced cytosolic phospholipase A2 expression via sequential PKC-delta, p42/p44 MAPK, and NF-kappaB activation in rat brain astrocytes. J Cell Physiol. 2006;206(1):246–254. doi: 10.1002/jcp.20457. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Wu CY, Yang CM. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes. Glia. 2008;56(6):619–632. doi: 10.1002/glia.20637. [DOI] [PubMed] [Google Scholar]
- Hsieh HL, Yen MH, Jou MJ, Yang CM. Intracellular signalings underlying bradykinin-induced matrix metalloproteinase-9 expression in rat brain astrocyte-1. Cell Signal. 2004;16(10):1163–1176. doi: 10.1016/j.cellsig.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Huang HM, Gibson G. Regulation of bradykinin-induced Ins(1,4,5)P3 formation by protein kinase C in human fibroblasts. Life Sci. 1996;59(18):1533–1543. doi: 10.1016/0024-3205(96)00484-5. [DOI] [PubMed] [Google Scholar]
- Huang HM, Lin TA, Sun GY, Gibson GE. Increased inositol 1,4,5-trisphosphate accumulation correlates with an up-regulation of bradykinin receptors in Alzheimer's disease. J Neurochem. 1995;64(2):761–766. doi: 10.1046/j.1471-4159.1995.64020761.x. [DOI] [PubMed] [Google Scholar]
- Huang HM, Toral-Barza L, Gibson GE. Interactions between inositol phosphates and cytosolic free calcium following bradykinin stimulation in cultured human skin fibroblasts. Biochim Biophys Acta. 1991;1091(3):409–416. doi: 10.1016/0167-4889(91)90208-f. [DOI] [PubMed] [Google Scholar]
- Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, Merrino VF, Kita S, Iwamoto T, Komuro I, Wang B, Cheung G, Ishikawa E, Ooboshi H, Bader M, Wada K, Kettenmann H, Noda M. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci. 2007;27(48):13065–13073. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic T, Stanisavljevic S, Brovkovych V, Skidgel RA, Erdos EG. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol Pharmacol. 2004;66(5):1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Ueno A, Naraba H, Matsuki N, Oh-Ishi S. Intracellular Ca2+ increase in neuro-2A cells and rat astrocytes following stimulation of bradykinin B2 receptor. Jpn J Pharmacol. 2000;84(2):140–145. doi: 10.1254/jjp.84.140. [DOI] [PubMed] [Google Scholar]
- Iores-Marcal LM, Viel TA, Buck HS, Nunes VA, Gozzo AJ, Cruz-Silva I, Miranda A, Shimamoto K, Ura N, Araujo MS. Bradykinin release and inactivation in brain of rats submitted to an experimental model of Alzheimer's disease. Peptides. 2006;27(12):3363–3369. doi: 10.1016/j.peptides.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91(2):534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkova Y, Svetnitsky A, Mayzler O, Pruneau D, Benifla M, Fuxman Y, Cohen A, Artru AA, Shapira Y. Bradykinin B2 receptor antagonism with LF 18-1505T reduces brain edema and improves neurological outcome after closed head trauma in rats. J Trauma. 2006;61(4):879–885. doi: 10.1097/01.ta.0000234722.98537.01. [DOI] [PubMed] [Google Scholar]
- Jaffa AA, Rust PF, Mayfield RK. Kinin, a mediator of diabetes-induced glomerular hyperfiltration. Diabetes. 1995;44(2):156–160. doi: 10.2337/diab.44.2.156. [DOI] [PubMed] [Google Scholar]
- Jaffa MA, Kobeissy F, Al Hariri M, Chalhoub H, Eid A, Ziyadeh FN, Jaffa AA. Global renal gene expression profiling analysis in B2-kinin receptor null mice: impact of diabetes. PLoS One. 2012;7(9):e44714. doi: 10.1371/journal.pone.0044714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain KK. Textbook of Personalized Medicine. Springer Science & Business Media; 2009. Drug Resistance in Epilepsy; pp. 262–263. [Google Scholar]
- Jaspard E, Wei L, Alhenc-Gelas F. Differences in the properties and enzymatic specificities of the two active sites of angiotensin I-converting enzyme (kininase II). Studies with bradykinin and other natural peptides. J Biol Chem. 1993;268(13):9496–9503. [PubMed] [Google Scholar]
- Jeftinija SD, Jeftinija KV, Stefanovic G, Liu F. Neuroligand-evoked calcium-dependent release of excitatory amino acids from cultured astrocytes. J Neurochem. 1996;66(2):676–684. doi: 10.1046/j.1471-4159.1996.66020676.x. [DOI] [PubMed] [Google Scholar]
- Jong YJ, Ford SR, Seehra K, Malave VB, Baenziger NL. Alzheimer's disease skin fibroblasts selectively express a bradykinin signaling pathway mediating tau protein Ser phosphorylation. FASEB J. 2003;17(15):2319–2321. doi: 10.1096/fj.02-1147fje. [DOI] [PubMed] [Google Scholar]
- Kakoki M, Smithies O. The kallikrein-kinin system in health and in diseases of the kidney. Kidney Int. 2009;75(10):1019–1030. doi: 10.1038/ki.2008.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu Y, Chen NH, Mitoma J, Nakagawa T, Hirabayashi Y, Higashi H. Gangliosides stimulate bradykinin B2 receptors to promote calmodulin kinase II-mediated neuronal differentiation. J Biochem. 2012;152(1):63–72. doi: 10.1093/jb/mvs055. [DOI] [PubMed] [Google Scholar]
- Kandratavicius L, Peixoto-Santos JE, Monteiro MR, Scandiuzzi RC, Carlotti CG, Jr, Assirati JA, Jr, Hallak JE, Leite JP. Mesial temporal lobe epilepsy with psychiatric comorbidities: a place for differential neuroinflammatory interplay. J Neuroinflammation. 2015;12:38. doi: 10.1186/s12974-015-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DS, Ryberg K, Morgelin M, Leeb-Lundberg LM. Spontaneous formation of a proteolytic B1 and B2 bradykinin receptor complex with enhanced signaling capacity. J Biol Chem. 2004;279(21):22102–22107. doi: 10.1074/jbc.M402572200. [DOI] [PubMed] [Google Scholar]
- Kaplanski J, Pruneau D, Asa I, Artru AA, Azez A, Ivashkova Y, Rudich Z, Shapira Y. LF 16-0687 Ms, a bradykinin B2 receptor antagonist, reduces brain edema and improves long-term neurological function recovery after closed head trauma in rats. J Neurotrauma. 2002;19(8):953–964. doi: 10.1089/089771502320317104. [DOI] [PubMed] [Google Scholar]
- Kayashima Y, Smithies O, Kakoki M. The kallikrein-kinin system and oxidative stress. Curr Opin Nephrol Hypertens. 2012;21(1):92–96. doi: 10.1097/MNH.0b013e32834d54b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ker K, Blackhall K. Beta-2 receptor antagonists for acute traumatic brain injury. Cochrane Database Syst Rev. 2008;(1):CD006686. doi: 10.1002/14651858.CD006686.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan TK, Alkon DL. An internally controlled peripheral biomarker for Alzheimer's disease: Erk1 and Erk2 responses to the inflammatory signal bradykinin. Proc Natl Acad Sci U S A. 2006;103(35):13203–13207. doi: 10.1073/pnas.0605411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan TK, Alkon DL. Early diagnostic accuracy and pathophysiologic relevance of an autopsy-confirmed Alzheimer's disease peripheral biomarker. Neurobiol Aging. 2010;31(6):889–900. doi: 10.1016/j.neurobiolaging.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Kim D, Cho SH, Kim JS, Jo SH, Lee SJ, Kim KT, Choi SY. Human astrocytic bradykinin B(2) receptor modulates zymosan-induced cytokine expression in 1321N1 cells. Peptides. 2010;31(1):101–107. doi: 10.1016/j.peptides.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Kitagawa A, Kizuki K, Moriya H, Kudo M, Noguchi T. Localization of kallikrein in rat pineal glands. Endocrinol Jpn. 1991;38(1):109–112. doi: 10.1507/endocrj1954.38.109. [DOI] [PubMed] [Google Scholar]
- Klasner B, Lumenta DB, Pruneau D, Zausinger S, Plesnila N. Therapeutic window of bradykinin B2 receptor inhibition after focal cerebral ischemia in rats. Neurochem Int. 2006;49(5):442–447. doi: 10.1016/j.neuint.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renne C, Gailani D, Nieswandt B, Renne T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203(3):513–518. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobeissy FH, Ottens AK, Zhang Z, Liu MC, Denslow ND, Dave JR, Tortella FC, Hayes RL, Wang KK. Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol Cell Proteomics. 2006;5(10):1887–1898. doi: 10.1074/mcp.M600157-MCP200. [DOI] [PubMed] [Google Scholar]
- Kobeissy FH, Sadasivan S, Buchanan M, Zhang Z, Gold MS, Wang KK. Methods in systems biology of experimental methamphetamine drug abuse. Methods Mol Biol. 2010;662:303–316. doi: 10.1007/978-1-60761-800-3_15. [DOI] [PubMed] [Google Scholar]
- Konduri SD, Rao CN, Chandrasekar N, Tasiou A, Mohanam S, Kin Y, Lakka SS, Dinh D, Olivero WC, Gujrati M, Foster DC, Kisiel W, Rao JS. A novel function of tissue factor pathway inhibitor-2 (TFPI-2) in human glioma invasion. Oncogene. 2001;20(47):6938–6945. doi: 10.1038/sj.onc.1204847. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Nakamura T, Tsuchiya N, Isaji M, Yasumitsu H, Umeda M, Miyazaki K. Purification and identification of a novel and four known serine proteinase inhibitors secreted by human glioblastoma cells. J Biochem. 1996;119(2):334–339. doi: 10.1093/oxfordjournals.jbchem.a021244. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Ropper AE, Lee SH, Han I. Propitious Therapeutic Modulators to Prevent Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9910-6. [DOI] [PubMed] [Google Scholar]
- Kunz M, Nussberger J, Holtmannspotter M, Bitterling H, Plesnila N, Zausinger S. Bradykinin in blood and cerebrospinal fluid after acute cerebral lesions: correlations with cerebral edema and intracranial pressure. J Neurotrauma. 2013;30(19):1638–1644. doi: 10.1089/neu.2012.2774. [DOI] [PubMed] [Google Scholar]
- Labandeira-Garcia JL, Rodriguez-Perez AI, Garrido-Gil P, Rodriguez-Pallares J, Lanciego JL, Guerra MJ. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front Aging Neurosci. 2017;9:129. doi: 10.3389/fnagi.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Tong XK, Lahjouji K, Couture R, Hamel E. Cognitive and cerebrovascular improvements following kinin B1 receptor blockade in Alzheimer's disease mice. J Neuroinflammation. 2013;10:57. doi: 10.1186/1742-2094-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal MA, Proulx PR, Hebert RL. A role for PKC epsilon and MAP kinase in bradykinin-induced arachidonic acid release in rabbit CCD cells. Am J Physiol. 1998;274(4 Pt 2):F728–735. doi: 10.1152/ajprenal.1998.274.4.F728. [DOI] [PubMed] [Google Scholar]
- Lalkovicova M, Bonova P, Burda J, Danielisova V. Effect of Bradykinin Postconditioning on Ischemic and Toxic Brain Damage. Neurochem Res. 2015;40(8):1728–1738. doi: 10.1007/s11064-015-1675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhauser F, Gob E, Kraft P, Geis C, Schmitt J, Brede M, Gobel K, Helluy X, Pham M, Bendszus M, Jakob P, Stoll G, Meuth SG, Nieswandt B, McCrae KR, Kleinschnitz C. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood. 2012;120(19):4082–4092. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee JS. Temporal lobe epilepsy surgery in children versus adults: from etiologies to outcomes. Korean J Pediatr. 2013;56(7):275–281. doi: 10.3345/kjp.2013.56.7.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57(1):27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Lemos MT, Amaral FA, Dong KE, Bittencourt MF, Caetano AL, Pesquero JB, Viel TA, Buck HS. Role of kinin B1 and B2 receptors in memory consolidation during the aging process of mice. Neuropeptides. 2010;44(2):163–168. doi: 10.1016/j.npep.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Leung PC, Cheng KT, Liu C, Cheung WT, Kwan HY, Lau KL, Huang Y, Yao X. Mechanism of non-capacitative Ca2+ influx in response to bradykinin in vascular endothelial cells. J Vasc Res. 2006;43(4):367–376. doi: 10.1159/000094096. [DOI] [PubMed] [Google Scholar]
- Levy JH, Pifarre R, Schaff HV, Horrow JC, Albus R, Spiess B, Rosengart TK, Murray J, Clark RE, Smith P. A multicenter, double-blind, placebo-controlled trial of aprotinin for reducing blood loss and the requirement for donor-blood transfusion in patients undergoing repeat coronary artery bypass grafting. Circulation. 1995;92(8):2236–2244. doi: 10.1161/01.cir.92.8.2236. [DOI] [PubMed] [Google Scholar]
- Li C, Zha OG, He QY, Wu YZ, Wang TS, Teng JF. Study on the clinical efficacy of Human Urinary Kalllikrein in the treatment of acute cerebral infarction according to TOAST classification. Pak J Pharm Sci. 2015;28(4 Suppl):1505–1510. [PubMed] [Google Scholar]
- Li H, Oehrlein SA, Wallerath T, Ihrig-Biedert I, Wohlfart P, Ulshofer T, Jessen T, Herget T, Forstermann U, Kleinert H. Activation of protein kinase C alpha and/or epsilon enhances transcription of the human endothelial nitric oxide synthase gene. Mol Pharmacol. 1998;53(4):630–637. doi: 10.1124/mol.53.4.630. [DOI] [PubMed] [Google Scholar]
- Li X, Stark GR. NFkappaB-dependent signaling pathways. Exp Hematol. 2002;30(4):285–296. doi: 10.1016/s0301-472x(02)00777-4. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hsieh HL, Shih RH, Chi PL, Cheng SE, Chen JC, Yang CM. NADPH oxidase 2-derived reactive oxygen species signal contributes to bradykinin-induced matrix metalloproteinase-9 expression and cell migration in brain astrocytes. Cell Commun Signal. 2012;10(1):35. doi: 10.1186/1478-811X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, Ecklund J. Explosive Blast Neurotrauma. Journal of Neurotrauma. 2009;26(6):815–825. doi: 10.1089/neu.2007.0484. [DOI] [PubMed] [Google Scholar]
- Liu J, Feener EP. Plasma kallikrein-kinin system and diabetic retinopathy. Biol Chem. 2013;394(3):319–328. doi: 10.1515/hsz-2012-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang R, Liu K, Zhou H, Tang Y, Su J, Yu X, Yang X, Tang M, Dong Q. Tissue kallikrein alleviates glutamate-induced neurotoxicity by activating ERK1. J Neurosci Res. 2009;87(16):3576–3590. doi: 10.1002/jnr.22151. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang R, Liu K, Zhou H, Yang X, Liu X, Tang M, Su J, Dong Q. Tissue kallikrein protects cortical neurons against in vitro ischemia-acidosis/reperfusion-induced injury through the ERK1/2 pathway. Exp Neurol. 2009;219(2):453–465. doi: 10.1016/j.expneurol.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lu Z, Cui M, Yang Q, Tang Y, Dong Q. Tissue kallikrein protects SH-SY5Y neuronal cells against oxygen and glucose deprivation-induced injury through bradykinin B2 receptor-dependent regulation of autophagy induction. J Neurochem. 2016;139(2):208–220. doi: 10.1111/jnc.13690. [DOI] [PubMed] [Google Scholar]
- Longhi L, Perego C, Ortolano F, Zanier ER, Bianchi P, Stocchetti N, McIntosh TK, De Simoni MG. C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit Care Med. 2009;37(2):659–665. doi: 10.1097/CCM.0b013e318195998a. [DOI] [PubMed] [Google Scholar]
- Longhi L, Perego C, Zanier ER, Ortolano F, Bianchi P, Stocchetti N, De Simoni MG. Neuroprotective effect of C1-inhibitor following traumatic brain injury in mice. Acta Neurochir Suppl. 2008;102:381–384. doi: 10.1007/978-3-211-85578-2_73. [DOI] [PubMed] [Google Scholar]
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- Mahmoudian M, Mehrpour M, Benaissa F, Siadatpour Z. A preliminary report on the application of noscapine in the treatment of stroke. Eur J Clin Pharmacol. 2003;59(8–9):579–581. doi: 10.1007/s00228-003-0676-1. [DOI] [PubMed] [Google Scholar]
- Maier-Hauff K, Baethmann AJ, Lange M, Schurer L, Unterberg A. The kallikrein-kinin system as mediator in vasogenic brain edema. Part 2: Studies on kinin formation in focal and perifocal brain tissue. J Neurosurg. 1984;61(1):97–106. doi: 10.3171/jns.1984.61.1.0097. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Leduc-Pessah H, Hong P, Ejdrygiewicz J, Sharples SA, Trang T, Whelan PJ. Modulatory and plastic effects of kinins on spinal cord networks. J Physiol. 2016;594(4):1017–1036. doi: 10.1113/JP271152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano DT, Tudor IC, Dietzel C, G. Multicenter Study of Perioperative Ischemia Research. Ischemia R, Education F. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354(4):353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50(3):357–386. [PubMed] [Google Scholar]
- Marcondes S, Antunes E. The plasma and tissue kininogen-kallikrein-kinin system: role in the cardiovascular system. Curr Med Chem Cardiovasc Hematol Agents. 2005;3(1):33–44. doi: 10.2174/1568016052773351. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Guy M, Murphey L, Roy F, Layani L, Combal JP, Marquer C, C. American Brain Injury A single dose, three-arm, placebo-controlled, phase I study of the bradykinin B2 receptor antagonist Anatibant (LF16-0687Ms) in patients with severe traumatic brain injury. J Neurotrauma. 2005;22(12):1444–1455. doi: 10.1089/neu.2005.22.1444. [DOI] [PubMed] [Google Scholar]
- Marmarou A, Nichols J, Burgess J, Newell D, Troha J, Burnham D, Pitts L. Effects of the bradykinin antagonist Bradycor (deltibant, CP-1027) in severe traumatic brain injury: results of a multi-center, randomized, placebo-controlled trial. American Brain Injury Consortium Study Group. J Neurotrauma. 1999;16(6):431–444. doi: 10.1089/neu.1999.16.431. [DOI] [PubMed] [Google Scholar]
- Martinez-Morillo E, Diamandis A, Romaschin AD, Diamandis EP. Kallikrein 6 as a serum prognostic marker in patients with aneurysmal subarachnoid hemorrhage. Plos One. 2012;7(9):e45676. doi: 10.1371/journal.pone.0045676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins AH, Alves JM, Perez D, Carrasco M, Torres-Rivera W, Eterovic VA, Ferchmin PA, Ulrich H. Kinin-B2 receptor mediated neuroprotection after NMDA excitotoxicity is reversed in the presence of kinin-B1 receptor agonists. PLoS One. 2012;7(2):e30755. doi: 10.1371/journal.pone.0030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Manabe H, Ochiai J, Fujita N, Takagi T, Uemura M, Naito Y, Yoshida N, Oka S, Yoshikawa T. An AT1-receptor antagonist and an angiotensin-converting enzyme inhibitor protect against hypoxia-induced apoptosis in human aortic endothelial cells through upregulation of endothelial cell nitric oxide synthase activity. Shock. 2003;19(6):547–552. doi: 10.1097/01.shk.0000070734.34700.80. [DOI] [PubMed] [Google Scholar]
- Mazzuferi M, Binaschi A, Rodi D, Mantovani S, Simonato M. Induction of B1 bradykinin receptors in the kindled hippocampus increases extracellular glutamate levels: a microdialysis study. Neuroscience. 2005;135(3):979–986. doi: 10.1016/j.neuroscience.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Menke JG, Borkowski JA, Bierilo KK, MacNeil T, Derrick AW, Schneck KA, Ransom RW, Strader CD, Linemeyer DL, Hess JF. Expression cloning of a human B1 bradykinin receptor. J Biol Chem. 1994;269(34):21583–21586. [PubMed] [Google Scholar]
- Mitchell JW, Seri S, Cavanna AE. Pharmacotherapeutic and Non-Pharmacological Options for Refractory and Difficult-to-Treat Seizures. J Cent Nerv Syst Dis. 2012;4:105–115. doi: 10.4137/JCNSD.S8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello S, Schmid K, Berger RP, Kobeissy F, Italiano D, Jeromin A, Hayes RL, Tortella FC, Buki A. The challenge of mild traumatic brain injury: role of biochemical markers in diagnosis of brain damage. Med Res Rev. 2014;34(3):503–531. doi: 10.1002/med.21295. [DOI] [PubMed] [Google Scholar]
- Motta G, Tersariol ILS. Modulation of the Plasma Kallikrein-Kinin System Proteins Performed by Heparan Sulfate Proteoglycans. Front Physiol. 2017;8:481. doi: 10.3389/fphys.2017.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murota S, Morita I, Kanayasu T. Kallikrein produces a new peptide that specifically stimulates prostacyclin formation in vascular endothelial cells by activating phospholipase A2. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:569–572. [PubMed] [Google Scholar]
- Myohanen TT, Garcia-Horsman JA, Tenorio-Laranga J, Mannisto PT. Issues about the physiological functions of prolyl oligopeptidase based on its discordant spatial association with substrates and inconsistencies among mRNA, protein levels, and enzymatic activity. J Histochem Cytochem. 2009;57(9):831–848. doi: 10.1369/jhc.2009.953711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naffah-Mazzacoratti Mda G, Gouveia TL, Simoes PS, Perosa SR. What have we learned about the kallikrein-kinin and renin-angiotensin systems in neurological disorders? World J Biol Chem. 2014;5(2):130–140. doi: 10.4331/wjbc.v5.i2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara J, Maeda M, Aiso S, Suzuki N. Current concepts in multiple sclerosis: autoimmunity versus oligodendrogliopathy. Clin Rev Allergy Immunol. 2012;42(1):26–34. doi: 10.1007/s12016-011-8287-6. [DOI] [PubMed] [Google Scholar]
- Narotam PK, Rodell TC, Nadvi SS, Bhoola KD, Troha JM, Parbhoosingh R, van Dellen JR. Traumatic brain contusions: a clinical role for the kinin antagonist CP-0127. Acta Neurochir (Wien) 1998;140(8):793–802. doi: 10.1007/s007010050181. discussion 802-793. [DOI] [PubMed] [Google Scholar]
- Nicoletti NF, Erig TC, Zanin RF, Pereira TC, Bogo MR, Campos MM, Morrone FB. Mechanisms involved in kinin-induced glioma cells proliferation: the role of ERK1/2 and PI3K/Akt pathways. J Neurooncol. 2014;120(2):235–244. doi: 10.1007/s11060-014-1549-4. [DOI] [PubMed] [Google Scholar]
- Nicoletti NF, Senecal J, da Silva VD, Roxo MR, Ferreira NP, de Morais RL, Pesquero JB, Campos MM, Couture R, Morrone FB. Primary Role for Kinin B1 and B2 Receptors in Glioma Proliferation. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0265-9. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Kim C, Growdon JH. Vasopressin and bradykinin regulate secretory processing of the amyloid protein precursor of Alzheimer's disease. Neurochem Res. 1998;23(5):807–814. doi: 10.1023/a:1022423813362. [DOI] [PubMed] [Google Scholar]
- Noda M, Kariura Y, Pannasch U, Nishikawa K, Wang L, Seike T, Ifuku M, Kosai Y, Wang B, Nolte C, Aoki S, Kettenmann H, Wada K. Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia. J Neurochem. 2007;101(2):397–410. doi: 10.1111/j.1471-4159.2006.04339.x. [DOI] [PubMed] [Google Scholar]
- Nokkari A. Characterization of The Kallikrein-Kinin System Post Chemical Neuronal Injury: An in vitro Biochemical and Neuroproteomics Assessment. 2014 doi: 10.1371/journal.pone.0128601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokkari A, Mouhieddine TH, Itani MM, Abou-Kheir W, Daoud G, Zhu R, Meshref Y, Soueid J, Al Hariri M, Mondello S, Jaffa AA, Kobeissy F. Characterization of the Kallikrein-Kinin System Post Chemical Neuronal Injury: An In Vitro Biochemical and Neuroproteomics Assessment. PLoS One. 2015;10(6):e0128601. doi: 10.1371/journal.pone.0128601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med. 2002;347(8):621–622. doi: 10.1056/NEJM200208223470820. [DOI] [PubMed] [Google Scholar]
- O'Kane KP, Webb DJ, Collier JG, Vallance PJ. Local L-NG-monomethylarginine attenuates the vasodilator action of bradykinin in the human forearm. Br J Clin Pharmacol. 1994;38(4):311–315. doi: 10.1111/j.1365-2125.1994.tb04359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki T, Kitagawa K, Yamagata K, Mandai K, Mabuchi T, Matsushita K, Yanagihara T, Matsumoto M. Induction of cyclooxygenase-2 mRNA in gerbil hippocampal neurons after transient forebrain ischemia. Brain Res. 1996;736(1–2):353–356. doi: 10.1016/0006-8993(96)00948-1. [DOI] [PubMed] [Google Scholar]
- Ongali B, Campos MM, Bregola G, Rodi D, Regoli D, Thibault G, Simonato M, Couture R. Autoradiographic analysis of rat brain kinin B1 and B2 receptors: normal distribution and alterations induced by epilepsy. J Comp Neurol. 2003;461(4):506–519. doi: 10.1002/cne.10706. [DOI] [PubMed] [Google Scholar]
- Pan JJ, Shi HM, Luo XP, Ma D, Li Y, Zhu J, Liang W, Mu JG, Li J. Recombinant TFPI-2 enhances macrophage apoptosis through upregulation of Fas/FasL. Eur J Pharmacol. 2011;654(2):135–141. doi: 10.1016/j.ejphar.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Gera L, Stewart JM. Bradykinin antagonist decreases early disruption of the blood-spinal cord barrier after spinal cord injury in mice. Neurosci Lett. 2001;307(1):25–28. doi: 10.1016/s0304-3940(01)01904-8. [DOI] [PubMed] [Google Scholar]
- Passos GF, Medeiros R, Cheng D, Vasilevko V, Laferla FM, Cribbs DH. The bradykinin B1 receptor regulates Abeta deposition and neuroinflammation in Tg-SwDI mice. Am J Pathol. 2013;182(5):1740–1749. doi: 10.1016/j.ajpath.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak M, Wong SS, Dreveny I, Emsley J. Structure of plasma and tissue kallikreins. Thromb Haemost. 2013;109(6) doi: 10.1160/TH12-11-0840. [DOI] [PubMed] [Google Scholar]
- Pelliccia V, Mai R, Francione S, Gozzo F, Sartori I, Nobili L, Lo Russo G, Pizzanelli C, Tassi L. Ictal EEG modifications in temporal lobe epilepsy. Epileptic Disord. 2013;15(4):392–399. doi: 10.1684/epd.2013.0615. [DOI] [PubMed] [Google Scholar]
- Pereira MG, Gitai DL, Paco-Larson ML, Pesquero JB, Garcia-Cairasco N, Costa-Neto CM. Modulation of B1 and B2 kinin receptors expression levels in the hippocampus of rats after audiogenic kindling and with limbic recruitment, a model of temporal lobe epilepsy. Int Immunopharmacol. 2008;8(2):200–205. doi: 10.1016/j.intimp.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Perosa SR, Arganaraz GA, Goto EM, Costa LG, Konno AC, Varella PP, Santiago JF, Pesquero JB, Canzian M, Amado D, Yacubian EM, Carrete H, Jr, Centeno RS, Cavalheiro EA, Silva JA, Jr, Mazzacoratti Mda G. Kinin B1 and B2 receptors are overexpressed in the hippocampus of humans with temporal lobe epilepsy. Hippocampus. 2007;17(1):26–33. doi: 10.1002/hipo.20239. [DOI] [PubMed] [Google Scholar]
- Perry EK. Neurotransmitters and diseases of the brain. Br J Hosp Med. 1991;45(2):73–83. [PubMed] [Google Scholar]
- Peterson C, Ratan RR, Shelanski ML, Goldman JE. Altered response of fibroblasts from aged and Alzheimer donors to drugs that elevate cytosolic free calcium. Neurobiol Aging. 1988;9(3):261–266. doi: 10.1016/s0197-4580(88)80063-0. [DOI] [PubMed] [Google Scholar]
- Pillat MM, Oliveira MN, Motaln H, Breznik B, Glaser T, Lah TT, Ulrich H. Glioblastoma-mesenchymal stem cell communication modulates expression patterns of kinin receptors: Possible involvement of bradykinin in information flow. Cytometry A. 2016;89(4):365–375. doi: 10.1002/cyto.a.22800. [DOI] [PubMed] [Google Scholar]
- Ping A, Chun ZX, Xue XY. Bradykinin preconditioning induces protective effects against focal cerebral ischemia in rats. Brain Res. 2005;1059(2):105–112. doi: 10.1016/j.brainres.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Pinheiro AR, Paramos-de-Carvalho D, Certal M, Costa C, Magalhaes-Cardoso MT, Ferreirinha F, Costa MA, Correia-de-Sa P. Bradykinin-induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell Commun Signal. 2013;11:70. doi: 10.1186/1478-811X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizard A, Blaukat A, Michineau S, Dikic I, Muller-Esterl W, Alhenc-Gelas F, Rajerison RM. Palmitoylation of the human bradykinin B2 receptor influences ligand efficacy. Biochemistry. 2001;40(51):15743–15751. doi: 10.1021/bi011600t. [DOI] [PubMed] [Google Scholar]
- Podsiadlo K, Sulkowski G, Dabrowska-Bouta B, Struzynska L. Blockade of the kinin B1 receptor affects the cytokine/chemokine profile in rat brain subjected to autoimmune encephalomyelitis. Inflammopharmacology. 2017 doi: 10.1007/s10787-017-0312-9. [DOI] [PubMed] [Google Scholar]
- Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochim Biophys Acta. 2011;1813(1):238–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Prat A, Biernacki K, Saroli T, Orav JE, Guttmann CR, Weiner HL, Khoury SJ, Antel JP. Kinin B1 receptor expression on multiple sclerosis mononuclear cells: correlation with magnetic resonance imaging T2-weighted lesion volume and clinical disability. Arch Neurol. 2005;62(5):795–800. doi: 10.1001/archneur.62.5.795. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Medeiros R, Pandolfo P, Duarte FS, Passos GF, Pesquero JB, Campos MM, Calixto JB, Takahashi RN. Genetic deletion or antagonism of kinin B(1) and B(2) receptors improves cognitive deficits in a mouse model of Alzheimer's disease. Neuroscience. 2008;151(3):631–643. doi: 10.1016/j.neuroscience.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Pruneau D, Chorny I, Benkovitz V, Artru A, Roitblat L, Shapira Y. Effect of LF 16-0687MS, a new nonpeptide bradykinin B2 receptor antagonist, in a rat model of closed head trauma. J Neurotrauma. 1999;16(11):1057–1065. doi: 10.1089/neu.1999.16.1057. [DOI] [PubMed] [Google Scholar]
- Racchi M, Ianna P, Binetti G, Trabucchi M, Govoni S. Bradykinin-induced amyloid precursor protein secretion: a protein kinase C-independent mechanism that is not altered in fibroblasts from patients with sporadic Alzheimer's disease. Biochem J. 1998;330(Pt 3):1271–1275. doi: 10.1042/bj3301271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Larson N, Wu J, Linbo R, Burda JE, Diamandis EP, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein cascades in traumatic spinal cord injury: in vitro evidence for roles in axonopathy and neuron degeneration. J Neuropathol Exp Neurol. 2013;72(11):1072–1089. doi: 10.1097/NEN.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic M, Yoon H, Wu J, Mustafa K, Fehlings MG, Scarisbrick IA. Genetic targeting of protease activated receptor 2 reduces inflammatory astrogliosis and improves recovery of function after spinal cord injury. Neurobiol Dis. 2015;83:75–89. doi: 10.1016/j.nbd.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raidoo DM, Bhoola KD. Kinin receptors on human neurones. J Neuroimmunol. 1997;77(1):39–44. doi: 10.1016/s0165-5728(97)00048-9. [DOI] [PubMed] [Google Scholar]
- Raidoo DM, Ramsaroop R, Naidoo S, Bhoola KD. Regional distribution of tissue kallikrein in the human brain. Immunopharmacology. 1996;32(1–3):39–47. doi: 10.1016/0162-3109(96)00007-0. [DOI] [PubMed] [Google Scholar]
- Rajpal S, Gerovac TA, Turner NA, Tilghman JI, Allcock BK, McChesney SL, Miranpuri GS, Park SW, Resnick DK. Antihyperalgesic effects of vanilloid-1 and bradykinin-1 receptor antagonists following spinal cord injury in rats. J Neurosurg Spine. 2007;6(5):420–424. doi: 10.3171/spi.2007.6.5.420. [DOI] [PubMed] [Google Scholar]
- Ran X, Zhang Q, Wang DW. Tissue Kallikrein Activity, Detected by a Novel Method, May Be a Predictor of Recurrent Stroke: A Case-Control Study. Dis Markers. 2015:159750. doi: 10.1155/2015/159750. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CN, Lakka SS, Kin Y, Konduri SD, Fuller GN, Mohanam S, Rao JS. Expression of tissue factor pathway inhibitor 2 inversely correlates during the progression of human gliomas. Clin Cancer Res. 2001;7(3):570–576. [PubMed] [Google Scholar]
- Raslan F, Schwarz T, Meuth SG, Austinat M, Bader M, Renne T, Roosen K, Stoll G, Siren AL, Kleinschnitz C. Inhibition of bradykinin receptor B1 protects mice from focal brain injury by reducing blood-brain barrier leakage and inflammation. J Cereb Blood Flow Metab. 2010;30(8):1477–1486. doi: 10.1038/jcbfm.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D, Gobeil F. Kallikrein-kinin system as the dominant mechanism to counteract hyperactive renin-angiotensin system. Can J Physiol Pharmacol. 2017;95(10):1117–1124. doi: 10.1139/cjpp-2016-0619. [DOI] [PubMed] [Google Scholar]
- Relton JK, Beckey VE, Hanson WL, Whalley ET. CP-0597, a selective bradykinin B2 receptor antagonist, inhibits brain injury in a rat model of reversible middle cerebral artery occlusion. Stroke. 1997;28(7):1430–1436. doi: 10.1161/01.str.28.7.1430. [DOI] [PubMed] [Google Scholar]
- Risdall JE, Menon DK. Traumatic brain injury. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):241–250. doi: 10.1098/rstb.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RA. Bradykinin receptors: characterization, distribution and mechanisms of signal transduction. Prog Growth Factor Res. 1989;1(4):237–252. doi: 10.1016/0955-2235(89)90013-6. [DOI] [PubMed] [Google Scholar]
- Rodell TC. The kallikrein/kinin system and kinin antagonists in trauma. Immunopharmacology. 1996;33(1–3):279–283. doi: 10.1016/0162-3109(96)00071-9. [DOI] [PubMed] [Google Scholar]
- Rodi D, Buzzi A, Barbieri M, Zucchini S, Verlengia G, Binaschi A, Regoli D, Boschi A, Ongali B, Couture R, Simonato M. Bradykinin B(2) receptors increase hippocampal excitability and susceptibility to seizures in mice. Neuroscience. 2013;248:392–402. doi: 10.1016/j.neuroscience.2013.06.038. [DOI] [PubMed] [Google Scholar]
- Rodi D, Couture R, Ongali B, Simonato M. Targeting kinin receptors for the treatment of neurological diseases. Curr Pharm Des. 2005;11(10):1313–1326. doi: 10.2174/1381612053507422. [DOI] [PubMed] [Google Scholar]
- Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9(4):231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- Rosenblum WI. Endothelial dependent relaxation demonstrated in vivo in cerebral arterioles. Stroke. 1986;17(3):494–497. doi: 10.1161/01.str.17.3.494. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sabourin T, Morissette G, Bouthillier J, Levesque L, Marceau F. Expression of kinin B(1) receptor in fresh or cultured rabbit aortic smooth muscle: role of NF-kappa B. Am J Physiol Heart Circ Physiol. 2002;283(1):H227–237. doi: 10.1152/ajpheart.00978.2001. [DOI] [PubMed] [Google Scholar]
- Saito H, Goldsmith G, Waldmann R. Fitzgerald factor (high molecular weight kininogen) clotting activity in human plasma in health and disease in various animal plasmas. Blood. 1976;48(6):941–947. [PubMed] [Google Scholar]
- Sang H, Liu L, Wang L, Qiu Z, Li M, Yu L, Zhang H, Shi R, Yu S, Guo R, Ye R, Liu X, Zhang R. Opposite roles of bradykinin B1 and B2 receptors during cerebral ischaemia-reperfusion injury in experimental diabetic rats. Eur J Neurosci. 2016;43(1):53–65. doi: 10.1111/ejn.13133. [DOI] [PubMed] [Google Scholar]
- Sarit BS, Lajos G, Abraham D, Ron A, Sigal FB. Inhibitory role of kinins on microglial nitric oxide and tumor necrosis factor-alpha production. Peptides. 2012;35(2):172–181. doi: 10.1016/j.peptides.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Linbo R, Vandell AG, Keegan M, Blaber SI, Blaber M, Sneve D, Lucchinetti CF, Rodriguez M, Diamandis EP. Kallikreins are associated with secondary progressive multiple sclerosis and promote neurodegeneration. Biol Chem. 2008;389(6):739–745. doi: 10.1515/BC.2008.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Sabharwal P, Cruz H, Larsen N, Vandell AG, Blaber SI, Ameenuddin S, Papke LM, Fehlings MG, Reeves RK, Blaber M, Windebank AJ, Rodriguez M. Dynamic role of kallikrein 6 in traumatic spinal cord injury. Eur J Neurosci. 2006;24(5):1457–1469. doi: 10.1111/j.1460-9568.2006.05021.x. [DOI] [PubMed] [Google Scholar]
- Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- Schmaier AH, Zuckerberg A, Silverman C, Kuchibhotla J, Tuszynski GP, Colman RW. High-molecular weight kininogen. A secreted platelet protein. J Clin Invest. 1983;71(5):1477–1489. doi: 10.1172/JCI110901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler K, Feiler S, Anetsberger S, Kim SW, Plesnila N. Contribution of bradykinin receptors to the development of secondary brain damage after experimental subarachnoid hemorrhage. Neurosurgery. 2011;68(4):1118–1123. doi: 10.1227/NEU.0b013e31820a0024. [DOI] [PubMed] [Google Scholar]
- Schuhmann MU, Mokhtarzadeh M, Stichtenoth DO, Skardelly M, Klinge PM, Gutzki FM, Samii M, Brinker T. Temporal profiles of cerebrospinal fluid leukotrienes, brain edema and inflammatory response following experimental brain injury. Neurol Res. 2003;25(5):481–491. doi: 10.1179/016164103101201896. [DOI] [PubMed] [Google Scholar]
- Schulz J, Plesnila N, Eriskat J, Stoffel M, Pruneau D, Baethmann A. LF16-0687 a novel non-peptide bradykinin B2 receptor antagonist reduces vasogenic brain edema from a focal lesion in rats. Acta Neurochir Suppl. 2000;76:137–139. doi: 10.1007/978-3-7091-6346-7_28. [DOI] [PubMed] [Google Scholar]
- Schulze-Topphoff U, Prat A, Prozorovski T, Siffrin V, Paterka M, Herz J, Bendix I, Ifergan I, Schadock I, Mori MA, Van Horssen J, Schroter F, Smorodchenko A, Han MH, Bader M, Steinman L, Aktas O, Zipp F. Activation of kinin receptor B1 limits encephalitogenic T lymphocyte recruitment to the central nervous system. Nat Med. 2009;15(7):788–793. doi: 10.1038/nm.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicli AG, Forbes G, Nolly H, Dujovny M, Carretero OA. Kallikrein-kinins in the central nervous system. Clin Exp Hypertens A. 1984;6(10–11):1731–1738. doi: 10.3109/10641968409046068. [DOI] [PubMed] [Google Scholar]
- Sengupta A, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of tau by protein kinases in rat brain. Neurochem Res. 2006;31(12):1473–1480. doi: 10.1007/s11064-006-9205-9. [DOI] [PubMed] [Google Scholar]
- Shakur H, Andrews P, Asser T, Balica L, Boeriu C, Quintero JD, Dewan Y, Druwe P, Fletcher O, Frost C, Hartzenberg B, Mantilla JM, Murillo-Cabezas F, Pachl J, Ravi RR, Ratsep I, Sampaio C, Singh M, Svoboda P, Roberts I. The BRAIN TRIAL: a randomised, placebo controlled trial of a Bradykinin B2 receptor antagonist (Anatibant) in patients with traumatic brain injury. Trials. 2009;10:109. doi: 10.1186/1745-6215-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol. 2008;93(5):701–708. doi: 10.1113/expphysiol.2008.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem. 2002;277(20):17962–17969. doi: 10.1074/jbc.M106101200. [DOI] [PubMed] [Google Scholar]
- Shariat-Madar Z, Mahdi F, Schmaier AH. Recombinant prolylcarboxypeptidase activates plasma prekallikrein. Blood. 2004;103(12):4554–4561. doi: 10.1182/blood-2003-07-2510. [DOI] [PubMed] [Google Scholar]
- Sharma HS. A bradykinin BK2 receptor antagonist HOE-140 attenuates blood-spinal cord barrier permeability following a focal trauma to the rat spinal cord. An experimental study using Evans blue, [131]I-sodium and lanthanum tracers. Acta Neurochir Suppl. 2000;76:159–163. doi: 10.1007/978-3-7091-6346-7_32. [DOI] [PubMed] [Google Scholar]
- Sheikh IA, Kaplan AP. Studies of the digestion of bradykinin, lysyl bradykinin, and kinin-degradation products by carboxypeptidases A, B, and N. Biochem Pharmacol. 1986;35(12):1957–1963. doi: 10.1016/0006-2952(86)90727-6. [DOI] [PubMed] [Google Scholar]
- Shi R, Yuan K, Hu B, Sang H, Zhou L, Xie Y, Xu L, Cao Q, Chen X, Zhao L, Xiong Y, Xu G, Liu X, Liu L, Zhang R. Tissue Kallikrein Alleviates Cerebral Ischemia-Reperfusion Injury by Activating the B2R-ERK1/2-CREB-Bcl-2 Signaling Pathway in Diabetic Rats. Oxid Med Cell Longev. 2016:1843201. doi: 10.1155/2016/1843201. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol. 2012;69(10):1245–1251. doi: 10.1001/archneurol.2011.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegerink B, Govers-Riemslag JW, Rosendaal FR, Ten Cate H, Algra A. Intrinsic coagulation activation and the risk of arterial thrombosis in young women: results from the Risk of Arterial Thrombosis in relation to Oral contraceptives (RATIO) case-control study. Circulation. 2010;122(18):1854–1861. doi: 10.1161/CIRCULATIONAHA.110.943738. [DOI] [PubMed] [Google Scholar]
- Silva JA, Jr, Goto EM, Perosa SR, Arganaraz GA, Cavalheiro EA, Naffah-Mazzacoratti MG, Pesquero JB. Kinin B1 receptors facilitate the development of temporal lobe epilepsy in mice. Int Immunopharmacol. 2008;8(2):197–199. doi: 10.1016/j.intimp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Simao F, Ustunkaya T, Clermont AC, Feener EP. Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke. Blood. 2017 doi: 10.1182/blood-2016-09-740670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes PS, Perosa SR, Arganaraz GA, Yacubian EM, Carrete H, Jr, Centeno RS, Varella PP, Santiago JF, Canzian M, Silva JA, Jr, Mortara RA, Amado D, Cavalheiro EA, Mazzacoratti Mda G. Kallikrein 1 is overexpressed by astrocytes in the hippocampus of patients with refractory temporal lobe epilepsy, associated with hippocampal sclerosis. Neurochem Int. 2011;58(4):477–482. doi: 10.1016/j.neuint.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Simoes PS, Visniauskas B, Perosa SR, Yacubian EM, Centeno R, Canzian M, Lopes-Cendes I, Maurer Morelli CV, Carrete H, Jr, Cavalheiro EA, Tufik S, Chagas JR, Mazzacoratti Mda G. Expression and activity of thimet oligopeptidase (TOP) are modified in the hippocampus of subjects with temporal lobe epilepsy (TLE) Epilepsia. 2014;55(5):754–762. doi: 10.1111/epi.12606. [DOI] [PubMed] [Google Scholar]
- Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gray SJ, Lowenstein PR, Vandenberghe LH, Wilson TJ, Wolfe JH, Glorioso JC. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9(6):298. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PB, Mehotra S, Lange GD, Russell JT. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J Biol Chem. 1997;272(36):22654–22661. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- Simson JA, Dom R, Chao J, Woodley C, Chao L, Margolius HS. Immunocytochemical localization of tissue kallikrein in brain ventricular epithelium and hypothalamic cell bodies. J Histochem Cytochem. 1985;33(9):951–953. doi: 10.1177/33.9.3894505. [DOI] [PubMed] [Google Scholar]
- Smeda JS, McGuire JJ, Daneshtalab N. Protease-activated receptor 2 and bradykinin-mediated vasodilation in the cerebral arteries of stroke-prone rats. Peptides. 2010;31(2):227–237. doi: 10.1016/j.peptides.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Soares DM, Santos DR, Rummel C, Ott D, Melo MCC, Roth J, Calixto JB, Souza GEP. The relevance of kalikrein-kinin system via activation of B2 receptor in LPS-induced fever in rats. Neuropharmacology. 2017;126:84–96. doi: 10.1016/j.neuropharm.2017.08.019. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Heistad DD, Faraci FM. Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke. 1997;28(11):2290–2294. doi: 10.1161/01.str.28.11.2290. discussion 2295. [DOI] [PubMed] [Google Scholar]
- Soley Bda S, Morais RL, Pesquero JB, Bader M, Otuki MF, Cabrini DA. Kinin receptors in skin wound healing. J Dermatol Sci. 2016;82(2):95–105. doi: 10.1016/j.jdermsci.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Souza DG, Lomez ES, Pinho V, Pesquero JB, Bader M, Pesquero JL, Teixeira MM. Role of bradykinin B2 and B1 receptors in the local, remote, and systemic inflammatory responses that follow intestinal ischemia and reperfusion injury. J Immunol. 2004;172(4):2542–2548. doi: 10.4049/jimmunol.172.4.2542. [DOI] [PubMed] [Google Scholar]
- Sparro G, Bonaiuto S, Galdenzi G, Eleuteri AM, Angeletti M, Lupidi G, Tacconi R, Giannandrea E, Vesprini A, Fioretti E. Acid-stable serine proteinase inhibitors in the urine of Alzheimer disease subjects. Dis Markers. 1996;13(1):31–41. doi: 10.1155/1996/193092. [DOI] [PubMed] [Google Scholar]
- Stavrou EX, Fang C, Merkulova A, Alhalabi O, Grobe N, Antoniak S, Mackman N, Schmaier AH. Reduced thrombosis in Klkb1−/− mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125(4):710–719. doi: 10.1182/blood-2014-01-550285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegbauer J, Lee DH, Seubert S, Ellrichmann G, Manzel A, Kvakan H, Muller DN, Gaupp S, Rump LC, Gold R, Linker RA. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci U S A. 2009;106(35):14942–14947. doi: 10.1073/pnas.0903602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J, Little S, Kramer R, Clemens J. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27(2):110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Storini C, Bergamaschini L, Gesuete R, Rossi E, Maiocchi D, De Simoni MG. Selective inhibition of plasma kallikrein protects brain from reperfusion injury. J Pharmacol Exp Ther. 2006;318(2):849–854. doi: 10.1124/jpet.106.105064. [DOI] [PubMed] [Google Scholar]
- Storini C, Rossi E, Marrella V, Distaso M, Veerhuis R, Vergani C, Bergamaschini L, De Simoni MG. C1-inhibitor protects against brain ischemia-reperfusion injury via inhibition of cell recruitment and inflammation. Neurobiol Dis. 2005;19(1–2):10–17. doi: 10.1016/j.nbd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Stover JF, Dohse NK, Unterberg AW. Significant reduction in brain swelling by administration of nonpeptide kinin B2 receptor antagonist LF 16-0687Ms after controlled cortical impact injury in rats. J Neurosurg. 2000;92(5):853–859. doi: 10.3171/jns.2000.92.5.0853. [DOI] [PubMed] [Google Scholar]
- Su J, Cui M, Tang Y, Zhou H, Liu L, Dong Q. Blockade of bradykinin B2 receptor more effectively reduces postischemic blood-brain barrier disruption and cytokines release than B1 receptor inhibition. Biochem Biophys Res Commun. 2009;388(2):205–211. doi: 10.1016/j.bbrc.2009.07.135. [DOI] [PubMed] [Google Scholar]
- Su J, Tang Y, Zhou H, Liu L, Dong Q. Tissue kallikrein protects neurons from hypoxia/reoxygenation-induced cell injury through Homer1b/c. Cell Signal. 2012;24(11):2205–2215. doi: 10.1016/j.cellsig.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Szabo G, Veres G, Radovits T, Haider H, Krieger N, Bahrle S, Niklisch S, Miesel-Groschel C, van de Locht A, Karck M. The novel synthetic serine protease inhibitor CU-2010 dose-dependently reduces postoperative blood loss and improves postischemic recovery after cardiac surgery in a canine model. J Thorac Cardiovasc Surg. 2010;139(3):732–740. doi: 10.1016/j.jtcvs.2009.10.059. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hiraishi K, Hirose T, Kato I, Yamamoto H, Shoji I, Shibasaki A, Kaneko K, Satoh F, Totsune K. Expression of (pro)renin receptor in the human brain and pituitary, and co-localisation with arginine vasopressin and oxytocin in the hypothalamus. J Neuroendocrinol. 2010;22(5):453–459. doi: 10.1111/j.1365-2826.2010.01980.x. [DOI] [PubMed] [Google Scholar]
- Tasiou A, Konduri SD, Yanamandra N, Dinh DH, Olivero WC, Gujrati M, Obeyesekere M, Rao JS. A novel role of tissue factor pathway inhibitor-2 in apoptosis of malignant human gliomas. Int J Oncol. 2001;19(3):591–597. doi: 10.3892/ijo.19.3.591. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos G, Loumos V. A compiled computer program for assisting the diagnosis of gastrointestinal helminths of humans and animals. Comput Methods Programs Biomed. 1991;36(4):237–238. doi: 10.1016/0169-2607(91)90092-8. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton E, Ziebell JM, Leonard AV, Vink R. Kinin receptor antagonists as potential neuroprotective agents in central nervous system injury. Molecules. 2010;15(9):6598–6618. doi: 10.3390/molecules15096598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Sanford RB, Smithies O, Kakoki M. The kallikrein-kinin system in diabetic nephropathy. Kidney Int. 2012;81(8):733–744. doi: 10.1038/ki.2011.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10(1):4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- Trabold R, Eros C, Zweckberger K, Relton J, Beck H, Nussberger J, Muller-Esterl W, Bader M, Whalley E, Plesnila N. The role of bradykinin B(1) and B(2) receptors for secondary brain damage after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2010;30(1):130–139. doi: 10.1038/jcbfm.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea MM, Gummelt D, Herzig MS, Leeb-Lundberg LM. B1 and B2 kinin receptors on cultured rabbit superior mesenteric artery smooth muscle cells: receptor-specific stimulation of inositol phosphate formation and arachidonic acid release by des-Arg9-bradykinin and bradykinin. J Pharmacol Exp Ther. 1993;264(2):930–937. [PubMed] [Google Scholar]
- Tschope C, Schultheiss HP, Walther T. Multiple interactions between the renin-angiotensin and the kallikrein-kinin systems: role of ACE inhibition and AT1 receptor blockade. J Cardiovasc Pharmacol. 2002;39(4):478–487. doi: 10.1097/00005344-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Tsuda K. Role of bradykinin and catecholamines in cerebral infarction and brain edema. Stroke. 2009;40(4):e103. doi: 10.1161/STROKEAHA.109.547752. author reply e104. [DOI] [PubMed] [Google Scholar]
- Unterberg A, Dautermann C, Baethmann A, Muller-Esterl W. The kallikrein-kinin system as mediator in vasogenic brain edema. Part 3: Inhibition of the kallikrein-kinin system in traumatic brain swelling. J Neurosurg. 1986;64(2):269–276. doi: 10.3171/jns.1986.64.2.0269. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzawa A, Mori M, Taniguchi J, Kuwabara S. Modulation of the kallikrein/kinin system by the angiotensin-converting enzyme inhibitor alleviates experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2014;178(2):245–252. doi: 10.1111/cei.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelial control of vasomotor function: from health to coronary disease. Circ J. 2003;67(7):572–575. doi: 10.1253/circj.67.572. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196(2):193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Viel TA, Buck HS. Kallikrein-kinin system mediated inflammation in Alzheimer's disease in vivo. Curr Alzheimer Res. 2011;8(1):59–66. doi: 10.2174/156720511794604570. [DOI] [PubMed] [Google Scholar]
- Vink R, Nimmo AJ. Multifunctional drugs for head injury. Neurotherapeutics. 2009;6(1):28–42. doi: 10.1016/j.nurt.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voetsch B, Jin RC, Loscalzo J. Nitric oxide insufficiency and atherothrombosis. Histochem Cell Biol. 2004;122(4):353–367. doi: 10.1007/s00418-004-0675-z. [DOI] [PubMed] [Google Scholar]
- Wagner S, Kalb P, Lukosava M, Hilgenfeldt U, Schwaninger M. Activation of the tissue kallikrein-kinin system in stroke. J Neurol Sci. 2002;202(1–2):75–76. doi: 10.1016/s0022-510x(02)00208-3. [DOI] [PubMed] [Google Scholar]
- Waldner MJ, Baethmann A, Uhl E, Lehmberg J. Bradykinin-induced leukocyte- and platelet-endothelium interactions in the cerebral microcirculation. Brain Res. 2012;1448:163–169. doi: 10.1016/j.brainres.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Walker K, Perkins M, Dray A. Kinins and kinin receptors in the nervous system. Neurochem Int. 1995;26(1):1–16. doi: 10.1016/0197-0186(94)00114-a. discussion 17–26. [DOI] [PubMed] [Google Scholar]
- Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest. 2009;119(8):2291–2303. doi: 10.1172/JCI37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. J Cell Physiol. 2011;226(9):2244–2256. doi: 10.1002/jcp.22560. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang J. Injection of bradykinin or cyclosporine A to hippocampus induces Alzheimer-like phosphorylation of Tau and abnormal behavior in rats. Chin Med J (Engl) 2002;115(6):884–887. [PubMed] [Google Scholar]
- Weissgarten J, Berman S, Efrati S, Rapoport M, Cohn M, Modai D, Averbukh Z. Apoptosis and proliferation of mesangial cells isolated from kidneys undergoing compensatory growth following contralateral nephrectomy: role of the renin-angiotensin system. Med Sci Monit. 2007;13(1):BR16–23. [PubMed] [Google Scholar]
- Wirth KJ, Fink E, Rudolphi K, Heitsch H, Deutschlander N, Wiemer G. Amyloid beta-(1–40) stimulates cyclic GMP production via release of kinins in primary cultured endothelial cells. Eur J Pharmacol. 1999;382(1):27–33. doi: 10.1016/s0014-2999(99)00576-2. [DOI] [PubMed] [Google Scholar]
- Woodfin A, Hu DE, Sarker M, Kurokawa T, Fraser P. Acute NADPH oxidase activation potentiates cerebrovascular permeability response to bradykinin in ischemia-reperfusion. Free Radic Biol Med. 2011;50(4):518–524. doi: 10.1016/j.freeradbiomed.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Hsieh HL, Sun CC, Tseng CP, Yang CM. IL-1 beta induces proMMP-9 expression via c-Src-dependent PDGFR/PI3K/Akt/p300 cascade in rat brain astrocytes. J Neurochem. 2008;105(4):1499–1512. doi: 10.1111/j.1471-4159.2008.05318.x. [DOI] [PubMed] [Google Scholar]
- Wu D, Lyu Y, Zhong P, Liu F, Liu X. Human Urinary kallidinogenase promotes good recovery in ischemic stroke patients with level 3 hypertension. Brain Behav. 2017;7(8):e00752. doi: 10.1002/brb3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Contact pathway of coagulation and inflammation. Thromb J. 2015;13:17. doi: 10.1186/s12959-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Smith RS, Jr, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension. 2006;47(4):752–761. doi: 10.1161/01.HYP.0000214867.35632.0e. [DOI] [PubMed] [Google Scholar]
- Xia CF, Yin H, Borlongan CV, Chao L, Chao J. Kallikrein gene transfer protects against ischemic stroke by promoting glial cell migration and inhibiting apoptosis. Hypertension. 2004;43(2):452–459. doi: 10.1161/01.HYP.0000110905.29389.e5. [DOI] [PubMed] [Google Scholar]
- Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukot Essent Fatty Acids. 2003;69(6):437–448. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Xu J, Hsu CY, Junker H, Chao S, Hogan EL, Chao J. Kininogen and kinin in experimental spinal cord injury. J Neurochem. 1991;57(3):975–980. doi: 10.1111/j.1471-4159.1991.tb08246.x. [DOI] [PubMed] [Google Scholar]
- Xu T, Fan X, Tan Y, Yue Y, Chen W, Gu X. Expression of PHB2 in rat brain cortex following traumatic brain injury. Int J Mol Sci. 2014;15(2):3299–3318. doi: 10.3390/ijms15023299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WB, Gu YT, Wang YF, Lu XH, Jia LS, Lv G. Bradykinin preconditioning modulates aquaporin-4 expression after spinal cord ischemic injury in rats. Brain Res. 2008;1246:11–18. doi: 10.1016/j.brainres.2008.09.087. [DOI] [PubMed] [Google Scholar]
- Xu Y, Kitada M, Yamaguchi M, Dezawa M, Ide C. Increase in bFGF-responsive neural progenitor population following contusion injury of the adult rodent spinal cord. Neurosci Lett. 2006;397(3):174–179. doi: 10.1016/j.neulet.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Yan-Feng W, Gang L, Yan-Ting G. Bradykinin preconditioning induces protective effects on the spinal cord ischemic injury of rats. Neurosci Lett. 2008;433(2):114–118. doi: 10.1016/j.neulet.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Yang CM, Hsieh HL, Lin CC, Shih RH, Chi PL, Cheng SE, Hsiao LD. Multiple factors from bradykinin-challenged astrocytes contribute to the neuronal apoptosis: involvement of astroglial ROS, MMP-9, and HO-1/CO system. Mol Neurobiol. 2013;47(3):1020–1033. doi: 10.1007/s12035-013-8402-1. [DOI] [PubMed] [Google Scholar]
- Yang J, Su J, Wan F, Yang N, Jiang H, Fang M, Xiao H, Wang J, Tang J. Tissue kallikrein protects against ischemic stroke by suppressing TLR4/NF-kappaB and activating Nrf2 signaling pathway in rats. Exp Ther Med. 2017;14(2):1163–1170. doi: 10.3892/etm.2017.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayama K, Shibata H, Takano M, Okamoto H. Expression of low-molecularweight kininogen in mouse vascular smooth muscle cells. Biol Pharm Bull. 1998;21(7):772–774. doi: 10.1248/bpb.21.772. [DOI] [PubMed] [Google Scholar]
- Yoon H, Blaber SI, Debela M, Goettig P, Scarisbrick IA, Blaber M. A completed KLK activome profile: investigation of activation profiles of KLK9, 10, and 15. Biol Chem. 2009;390(4):373–377. doi: 10.1515/BC.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Radulovic M, Wu J, Blaber SI, Blaber M, Fehlings MG, Scarisbrick IA. Kallikrein 6 signals through PAR1 and PAR2 to promote neuron injury and exacerbate glutamate neurotoxicity. J Neurochem. 2013;127(2):283–298. doi: 10.1111/jnc.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zausinger S, Lumenta DB, Pruneau D, Schmid-Elsaesser R, Plesnila N, Baethmann A. Effects of LF 16-0687 Ms, a bradykinin B(2) receptor antagonist, on brain edema formation and tissue damage in a rat model of temporary focal cerebral ischemia. Brain Res. 2002;950(1–2):268–278. doi: 10.1016/s0006-8993(02)03053-6. [DOI] [PubMed] [Google Scholar]
- Zhang JC, Claffey K, Sakthivel R, Darzynkiewicz Z, Shaw DE, Leal J, Wang YC, Lu FM, McCrae KR. Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: partial activity within domain 5. FASEB J. 2000;14(15):2589–2600. doi: 10.1096/fj.99-1025com. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ding H, Yan J, Wang W, Ma A, Zhu Z, Cianflone K, Hu FB, Hui R, Wang DW. Plasma tissue kallikrein level is negatively associated with incident and recurrent stroke: a multicenter case-control study in China. Ann Neurol. 2011;70(2):265–273. doi: 10.1002/ana.22404. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, Honer WG, Corcoran ME. Relations between brain pathology and temporal lobe epilepsy. J Neurosci. 2002;22(14):6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem. 2011;286(21):18547–18561. doi: 10.1074/jbc.M110.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tan F, Zhang Y, Skidgel RA. Carboxypeptidase M and kinin B1 receptors interact to facilitate efficient b1 signaling from B2 agonists. J Biol Chem. 2008;283(12):7994–8004. doi: 10.1074/jbc.M709837200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Larner SF, Liu MC, Zheng W, Hayes RL, Wang KK. Multiple alphaII-spectrin breakdown products distinguish calpain and caspase dominated necrotic and apoptotic cell death pathways. Apoptosis. 2009;14(11):1289–1298. doi: 10.1007/s10495-009-0405-z. [DOI] [PubMed] [Google Scholar]
- Zhou L, Yang B, Wang Y, Zhang HL, Chen RW, Wang YB. Bradykinin regulates the expression of claudin-5 in brain microvascular endothelial cells via calcium-induced calcium release. J Neurosci Res. 2014;92(5):597–606. doi: 10.1002/jnr.23350. [DOI] [PubMed] [Google Scholar]
- Zweckberger K, Plesnila N. Anatibant, a selective non-peptide bradykinin B2 receptor antagonist, reduces intracranial hypertension and histopathological damage after experimental traumatic brain injury. Neurosci Lett. 2009;454(2):115–117. doi: 10.1016/j.neulet.2009.02.014. [DOI] [PubMed] [Google Scholar]