Abstract
N,N ′-diarylureas have recently emerged as a new antischistosomal chemotype. We now describe physicochemical profiling, in vitro ADME, plasma exposure, and ex vivo and in vivo activities against Schistosoma mansoni for twenty new N,N′-diarylureas designed primarily to increase aqueous solubility, but also to maximize structural diversity. Replacement of one of the 4-fluoro-3-trifluoromethylphenyl substructures of lead N,N′-diarylurea 1 with azaheterocycles and benzoic acids, benzamides, or benzonitriles decreased lipophilicity, and in most cases, increased aqueous solubility. There was no clear relationship between lipophilicity and metabolic stability, although all compounds with 3-trifluoromethyl-4-pyridyl substructures were metabolically stable. N,N′-diaryl ureas containing 4-fluoro-3-trifluoromethylphenyl, 3-trifluoromethyl-4-pyridyl, 2,2-difluorobenzodioxole, or 4-benzonitrile substructures had high activity against ex vivo S. mansoni and relatively low cytotoxicity. N,N-diaryl ureas with 3-trifluoromethyl-4-pyridyl and 2,2-difluorobenzodioxole substructures had the highest exposures whereas those with 4-fluoro-3-trifluoromethylphenyl substructures had the best in vivo antischistosomal activities. There was no direct correlation between compound exposure and in vivo activity.
Keywords: N,N′-diarylurea; antischistosomal; SAR
Graphical Abstract
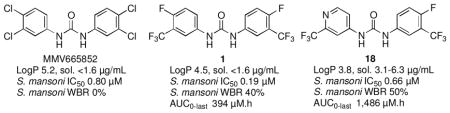
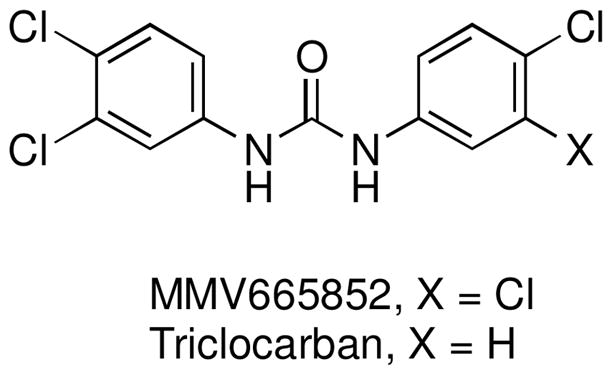
Schistosomiasis is a widespread tropical parasitic disease1; Schistosoma mansoni, S. haematobium and S. japonicum are the predominant pathogenic species.2–4 Praziquantel is the only drug used for treatment of schistosomiasis. Even so, praziquantel drug resistance is not yet widespread.4–7 We thus have a window of opportunity to identify a new antischistosomal drug. In this regard, several antischistosomal lead compounds have recently been identified by phenotypic screening of drug and chemical compound libraries.8–11 One of these was the symmetrical N,N′-diaryl urea MMV665582,9 a structural analog of triclocarban,12,13 an antibacterial agent used in detergents, cosmetics, and other products (Figure 1).
Figure 1.
With an IC50 of 0.8 μM, MMV665852 is only 4-fold less potent than praziquantel against ex vivo S. mansoni and has a 64-fold in vitro selectivity index. A single 400 mg/kg oral dose of MMV665852 administered to S. mansoni-infected mice reduced worm burden by 53%. In an initial pharmacokinetic investigation of MMV665852, this symmetrical N,N′-diarylurea was characterized by a half-life of 4.7 h and Cmax of 4.4 μM M at a 46.3 mg/kg oral dose.9 Thus, this very simple compound offers intriguing possibilities for further optimization, although this is tempered by its high Log P of 5.2, and the low aqueous solubility14 and potential pharmacological promiscuity12,13,15,16 of this compound class. Following the discovery of MMV665852, two subsequent studies17,18 established an initial SAR for this compound series: 1) substitution at positions 3 and 4 of the phenyl rings with H, F, Cl, CN, and CF3 groups was optimal; 2) substitution at positions 3 and 4 of the phenyl rings with OCH3, NH2 and other electron-donating groups diminished activity; 3) replacement of one of the phenyl rings with alkyl substituents diminished or abolished activity; 4) cyclization of the urea to imidazoline-2-ones abolished activity; and 5) replacement of the urea with carbamates, thioureas, sulfonamides, or oxalamides diminished or abolished activity. Concurrent with this work, we found that the symmetrical N,N′-diarylurea 1,19 a side-reaction product formed in the synthesis of aryl hydantoins,20 had promising antischistosomal activity, better than that of MMV665582. We now describe physicochemical profiling, in vitro ADME, plasma exposure, and ex vivo and in vivo activities against S. mansoni for a number of analogs of 1 (2–20, Table 1) designed primarily to increase aqueous solubility, but also to maximize structural diversity.
Table 1.
Physicochemical and in vitro ADME properties for N,N′-diarylureas 1–20.
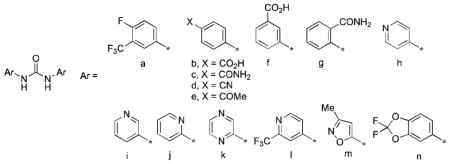
| |||||
|---|---|---|---|---|---|
| Compd | Ar | LogD7.4 a | PSA (Å2)b | Sol2.0/Sol6.5 (μg/mL)c | h/m CLint (μL/min/mg protein)d |
| 1 | a,a | 4.5 | 41.1 | <1.6/<1.6 | 10/7 |
| 2 | l,l | 3.0 | 66.9 | 12.5–25/12.5–25 | <7/<7 |
| 3 | h,h | 1.3 | 66.9 | >100/12.5–25 | <7/188 |
| 4 | k,k | 0.8 | 92.7 | 6.3–12.5/6.3–12.5 | 18/157 |
| 5 | n,n | 4.4 | 78.1 | <1.6/<1.6 | <7/12 |
| 6 | a,h | 3.2 | 54.0 | 6.3–12.5/6.3–12.5 | 12/37 |
| 7 | a,i | 3.0 | 54.0 | 50–100/12.5–25 | 13/154 |
| 8 | a,j | 3.7 | 54.0 | 1.6–3.1/<1.6 | 188/705 |
| 9 | a,k | 3.0 | 66.9 | 1.6–3.1/<1.6 | 210/160 |
| 10 | a,m | 3.2 | 67.2 | 12.5–25/6.3–12.5 | 73/108 |
| 11 | a,b | 1.5 | 81.3 | <1.6/12.5–25 | <7/<7 |
| 12 | a,f | 1.6 | 81.3 | 1.6–3.1/12.5–25 | <7/<7 |
| 13 | a,c | 2.7 | 84.2 | 6.3–12.5/6.3–12.5 | 28/22 |
| 14 | a,g | 3.3 | 84.2 | 1.6–3.1/1.6–3.1 | 81/69 |
| 15 | a,d | 3.8 | 64.9 | <1.6/<1.6 | <7/12 |
| 16 | a,e | 3.6 | 58.2 | <1.6/<1.6 | 16/14 |
| 17 | a,n | 4.5 | 59.6 | <1.6/<1.6 | <7/<7 |
| 18 | a,l | 3.8 | 54.0 | 3.1–6.3/3.1–6.3 | <7/<7 |
| 19 | d,l | 3.0 | 77.8 | 1.6–3.1/1.6–3.1 | <7/<7 |
| 20 | l,n | 3.7 | 72.5 | 3.1–6.3/3.1–6.3 | <7/16 |
LogD values were estimated by correlation of their chromatographic retention properties using a modified gradient HPLC method adapted from Lombardo et al.27
calculated using ChemAxon JChem for Excel
Compounds in DMSO were spiked into either pH 6.5 phosphate buffer or 0.01 M HCl (approx. pH 2.0) and analyzed by nephelometry28 to determine a concentration range.
in vitro intrinsic clearance measured in human and mouse liver microsomes
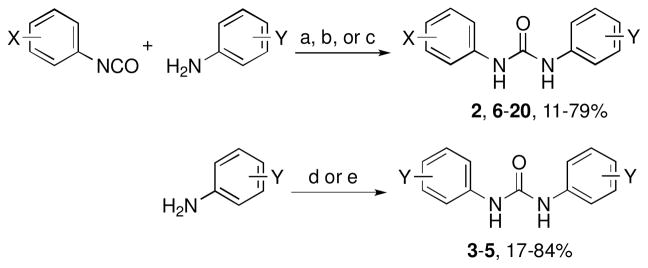
Target N,N′-diarylureas 2 and 6–20 were prepared (Supporting Information) by reactions of phenyl isocyanates (Table 1 Ar = a, d, l, n) with the requisite anilines under three slightly different reaction conditions (Scheme 1). Phenyl isocyanates were prepared in situ by treatment of the precursor anilines with triphosgene. Target N,N′-diarylureas 3,21 4,22 and 5 were prepared by treatment of the corresponding anilines with 1,1′-carbonyldiimidazole (CDI) (Scheme 1).
Scheme 1.
Reagents and conditions: (a) N,N-diisopropylethylamine, CH2Cl2, rt, 12 h (2, 17, 18); (b) THF, 25–80 °C; 12 h (6–9, 19, 20); (c) CH3CN, 80 °C, 12 h (10–16); (d) CDI, THF, rt, 48 h (3, 4); (e) CDI, 1,2-dimethoxyethane, reflux, 16 h (5).
We first consider the physicochemical and in vitro ADME properties of these analogs of 1 (Table 1). The calculated polar surface area (PSA) values of between 41 and 93 Å2 indicate that the polarity of these compounds is unlikely to be a rate-limiting factor for membrane permeability and oral bioavailability.23 Symmetrical N,N-diaryl urea 1 had a high LogD7.4 of 4.5, similar to that of 5.2 for MMV665852,9 and its kinetic solubility was very poor (<1.6 μg/mL). The symmetrical azaheterocycle N,N-diaryl ureas 2, 3, and 4 were considerably more polar and more soluble at both pH 2 and 6.5 compared to 1. In contrast, the symmetrical 2,2-difluorobenzodioxole N,N-diaryl urea 5 had a similar LogD7.4 to that of 1 and was not more soluble.
Substitution of one of the 4-fluoro-3-trifluromethylphenyl substructures of 1 with azaheterocycles 6–10 decreased lipophilicity, and with the exception of 8 and 9, increased solubility significantly. Replacing one of the 4-fluoro-3-trifluromethylphenyl substructures of 1 with benzoic acids (11, 12), benzamides (13, 14), a benzonitrile (15) or an acetophenone (16) decreased lipophilicity, and with the exception of 14, 15, and 16, all were more soluble than 1. Compounds where one of the 4-fluoro substituents were replaced with pyridine nitrogen atoms (18 vs. 1, 20 vs. 17, 19 vs. 15) were less lipophilic and marginally more soluble.
Twelve of twenty N,N-diaryl ureas (Table 1) had low intrinsic clearance values in human and mouse liver microsomes and three of these (1, 5, 17) were the most lipophilic of the series, possibly reflecting high protein binding in the microsomal test system. The eight N,N-diaryl ureas with intermediate to high intrinsic clearance contained either pyridine nitrogen atoms (4, 6–10) or primary carboxamide functional groups (13, 14). Notably, N,N-diaryl ureas with 3-trifluoromethyl-4-pyridyl substructures (2, 18–20) were metabolically stable.
As a gatekeeping assessment of antischistosomal activity, the N,N′-diaryl ureas were tested against newly transformed schistosomula (NTS)24 (Table 2). At 10 μM, 9 out of 20 of the compounds killed the NTS. A subsequent concentration titration revealed NTS IC50 values ranging from 0.15 to 5.6 μM. Further assessment indicated that these compounds killed adult S. mansoni at a similar IC50 range of 0.18 to 3.3 μM. The aryl substructures (Table 1) in the active N,N′-diaryl ureas were 4-fluoro-3-trifluoromethylphenyl = 3-trifluoromethyl-4-pyridyl > 2,2-difluorobenzodioxole > 4-benzonitrile. Notably, none of the new N,N′-diaryl ureas were more potent than 1 ex vivo.
Table 2.
Ex vivo antischistosomal activity and cytotoxicity for selected N,N′-diarylureas.
| S. mansoni IC50 (μM) | Cytotoxicity IC50 (μM) | |||||
|---|---|---|---|---|---|---|
| Compd | NTS | adult | HFF | U-20S | HEK293 | HC-04 |
| 1 | 0.15 | 0.19 | 57 | >100 | >100 | >100 |
| 2 | 2.4 | 0.88 | >100 | >100 | >100 | >100 |
| 5 | 0.26 | 0.49 | 79 | >100 | >100 | >100 |
| 6 | 5.6 | >10 | ND | ND | ND | ND |
| 15 | 1.6 | 0.94 | 60 | >100 | >100 | >100 |
| 17 | 0.21 | 0.49 | 67 | >100 | >100 | >100 |
| 18 | 0.74 | 0.66 | 71 | >100 | >100 | >100 |
| 19 | 1.9 | 3.3 | >100 | >100 | >100 | >100 |
| 20 | 1.3 | 0.75 | 80 | >100 | >100 | >100 |
To assess host cell cytotoxicity, the active N,N′-diaryl ureas were tested for growth inhibition of four human cell lines: human foreskin fibroblast (HFF), kidney (HEK293), hepatocyte (HC04), and B lymphocyte (RAJI) (Table 2). The HFF cell line was inhibited by 6 out of 8 of the compounds with IC50 values ranging from 57 to 80 μM; the remaining cell lines were unaffected at compound concentrations up to 100 μM. Thus, these compounds appeared to have high antischistosomal selectivity, although the six compounds that inhibited the HFF cell line were also among the most potent against ex vivo S. mansoni with IC50 values < 1 μM.
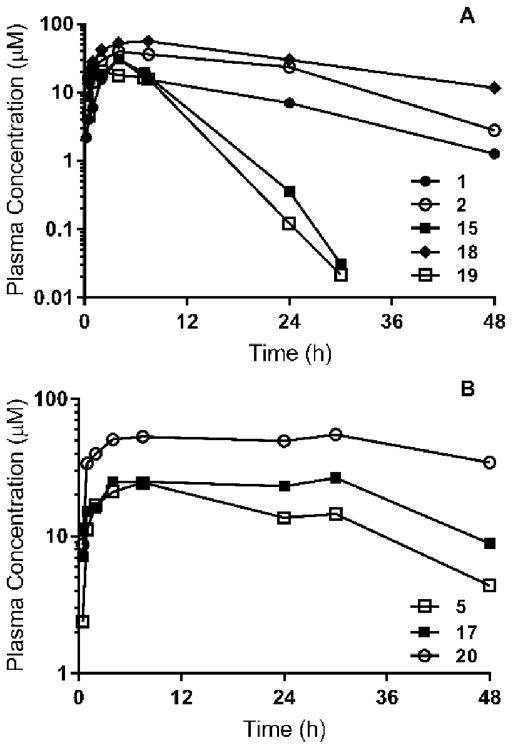
Single 100 mg/kg oral doses of the most active N,N′-diaryl ureas were administered to non-infected mice to assess exposure (Table 3). For logistical reasons, we made the assumption that exposure profiles generated in non-infected mice give a reasonable estimation of exposure in S. mansoni-infected mice. Plasma concentrations of 1 increased until 2 h post-dose after which they remained above 1 μM up to at least 48 h (Figure 2A). Similarly, plasma concentrations of 2 and 18 increased until about 4 h post-dose and then remained high for the duration of the 48 h post-dose sampling period. Based on the AUC values up to the last measured concentration, the overall systemic exposures of 2 and 18 were approximately 2–4-fold higher than that of 1. Absorption of 15 and 19 resulted in similar Tmax and Cmax values compared to 1, 2, and 18 (Table 1), however concentrations declined with a much shorter half-life than seen for either 1, 2, or 18 and were well below 1 μM by ~18 h post-dose. Based on in vitro studies with liver microsomes, both 15 and 19 were not highly susceptible to cytochrome P450-mediated metabolism, so alternative in vivo degradation/clearance pathways are likely for these benzonitriles. Unfortunately, the basis for these differences in half-life cannot be determined based only on the oral exposure data and additional studies with intravenous dosing would be needed to differentiate between absorption, distribution, and clearance-related differences.
Table 3.
Exposure parameters and in vivo antischistosomal activity of selected N,N′-diarylureas following administration of a single 100 mg/kg oral dose.
| Compd | Cmax (μM) | Tmax (h) | Half-Life (h) | AUC0-last (μM.h) | S. mansoni WBR (%) |
|---|---|---|---|---|---|
| 1 | 31 | 4 | 11 | 394 | 40 |
| 2 | 40 | 4.0 | 10 | 953 | 20 |
| 5 | 25 | c.n.d. | 13 | 679 | 0 |
| 15 | 31 | 4 | 2.7 | 216 | 37 |
| 17 | 27 | c.n.d. | c.n.d. | 989 | 0 |
| 18 | 56 | 7.5 | 18 | 1486 | 50 |
| 19 | 20 | 2.0 | 2.3 | 175 | 1.6 |
| 20 | 55 | c.n.d. | c.n.d. | 2273 | 2.1 |
c.n.d. = could not determine
Figure 2.
Plasma concentration versus time profiles following oral administration of 100 mg/kg to non-infected male Swiss outbred mice. Symbols represent the mean of n=2 mice at each time point. Panel A shows the profiles for 1, 2, 15, 18, and 19. Panel B shows the profiles for 5, 17, and 20.
Following oral administration, 5, 17, and 20 were each very slowly absorbed and exhibited relatively flat profiles precluding the assessment of Tmax (Figure 2B). The shape of these profiles likely reflects the high dose and very poor aqueous solubility leading to very prolonged absorption. For these three 2,2-difluorobenzodioxole-containing N,N-diaryl ureas, plasma concentrations remained above ~5 μM during the entire 48 h sampling period. Based on AUC0-last values ranging from 679 to 2273 μM.h, the exposure of 5, 17, and 20 was much higher than that of 1 (394 μM.h) but comparable to that for 2 and 18 (953 and 1486 μM.h). From these data, we see a trend that N,N-diaryl ureas containing 3-trifluoromethyl-4-pyridyl and 2,2-difluorobenzodioxole substructures had the highest plasma exposures of the compounds tested.
In vivo antischistosomal activity was determined by measuring worm burden reduction (WBR) values following administration of single 100 mg/kg oral doses to S. mansoni-infected mice (Table 3). None of the compounds tested showed high in vivo activity, but the three (1, 15, and 18) with moderate WBR values (37–50%) contained a 4-fluoro-3-trifluoromethylphenyl substructure. We also found that at this same 100 mg/kg dose, MMV665852 had no activity (0% WBR). There was no direct correlation between plasma exposure and in vivo activity. For example, even though all compounds had Cmax levels an order-of-magnitude greater than their S. mansoni IC50 values, and with the exception of 15 and 19, maintained high plasma concentrations for extended periods, their overall in vivo efficacy was weak to moderate with only 1 and 18 having WBR values of 40–50%. When 18 was administered as four consecutive 80 mg/kg oral doses, the WBR of 53% was no better than that of 50% obtained with a single 100 mg/kg dose. Given that most of the compounds in this series are quite lipophilic (Log D >3), the disappointing in vivo efficacy despite high plasma exposure could be a reflection of high plasma protein binding and low unbound concentrations with insufficient concentrations reaching the site of action within the worms to achieve the desired killing effect. Additional pharmacokinetic and pharmacodynamic studies are needed to better understand the relationship between plasma and/or tissue exposure and in vivo activity for this series of compounds. Even though none of the N,N-diaryl ureas had high in vivo activity, it is useful to note that at this same 100 mg/kg dose, praziquantel also has a low WBR value of only 15%;25 however, a higher 400 mg/kg dose of praziquantel reduces worm burden by 96%.26
In summary, replacement of one of the 4-fluoro-3-trifluoromethylphenyl substructures of 1 with azaheterocycles and benzoic acids, benzamides, or benzonitriles decreased lipophilicity, and in most cases, increased aqueous solubility. There was no clear relationship between lipophilicity and metabolic stability, although all N,N-diaryl ureas with 3-trifluoromethyl-4-pyridyl substructures were metabolically stable. N,N′-diaryl ureas containing 4-fluoro-3-trifluoromethylphenyl, 3-trifluoromethyl-4-pyridyl, 2,2-difluorobenzodioxole, or 4-benzonitrile substructures had high activity against ex vivo S. mansoni and relatively low cytotoxicity. N,N-diaryl ureas with 3-trifluoromethyl-4-pyridyl and 2,2-difluorobenzodioxole substructures had the highest exposures whereas those with 4-fluoro-3-trifluoromethylphenyl substructures had the best in vivo antischistosomal activities. Finally, there was no direct correlation between compound exposure and in vivo activity.
Supplementary Material
Highlights.
Expansion of antischistosomal N,N′-diaryl urea SAR.
3-Trifluoromethyl-4-pyridyl and 2,2-difluorobenzodioxole improve exposure.
4-Fluoro-3-trifluoromethylphenyl required for best antischistosomal activity.
Acknowledgments
We acknowledge the U.S. National Institutes of Health (GM103427-16, AI097802-02 and AI116723-01) and the European Research Council (ERC-2013-CoG 614739-A HERO) for financial support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: The great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gryseels B. Schistosomiasis. Infect Dis Clin North Am. 2012;26:383–397. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vale N, Gouveia MJ, Rinaldi G, Brindley PJ, Gärtner F, Correia da Costa JM. Praziquantel for schistosomiasis: Single-drug metabolism revisited, mode of action, and resistance. Antimicrob Agents Chemother. 2017;61:e02582–16. doi: 10.1128/AAC.02582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, Mutuku MW, Karanja DM, Colley DG, Black CL, Secor WE, Mkoji GM, Loker ES. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seto EY, Wong BK, Lu D, Zhong B. Human schistosomiasis resistance to praziquantel in China: Should we be worried? Am J Trop Med Hyg. 2011;85:74–82. doi: 10.4269/ajtmh.2011.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasinathan RS, Greenberg RM. Pharmacology and potential physiological significance of schistosome multidrug resistance transporters. Exp Parasitol. 2012;132:2–6. doi: 10.1016/j.exppara.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulla M-H, Ruelas DS, Wolff B, Snedecor J, Lim K-C, Xu F, Renslo AR, Williams J, McKerrow JH, Caffrey CR. Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl Trop Dis. 2009;3:e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingram-Sieber K, Cowan N, Panic G, Vargas M, Mansour NR, Bickle QD, Wells TN, Spangenberg T, Keiser J. Orally active antischistosomal early leads identified from the open access malaria box. PLoS Negl Trop Dis. 2014;8:e2610. doi: 10.1371/journal.pntd.0002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panic G, Vargas M, Keiser J, Scandale I. Activity profile of an FDA-approved compound library against Schistosoma mansoni. PLoS Negl Trop Dis. 2015;9:e0003962. doi: 10.1371/journal.pntd.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira LG, Andricopulo AD. Drug repositioning approaches to parasitic diseases: A medicinal chemistry perspective. Drug Discov Today. 2016;21:1699–1710. doi: 10.1016/j.drudis.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol. 2011;45:3109–3115. doi: 10.1021/es103650m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonoli D, Fürstenberger C, Boccard J, Hochstrasser D, Jeanneret F, Odermatt A, Rudaz S. Steroidomic footprinting based on ultra-high performance liquid chromatography coupled with qualitative and quantitative high-resolution mass spectrometry for the evaluation of endocrine disrupting chemicals in H295R cells. Chem Res Toxicol. 2015;28:955–966. doi: 10.1021/tx5005369. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Anderson M, Weisman JL, Lu M, Choy CJ, Boyd VA, Price J, Sigal M, Clark J, Connelly M, Zhu F, Guiguemde WA, Jeffries C, Yang L, Lemoff A, Liou AP, Webb TR, Derisi JL, Guy RK. Evaluation of diarylureas for activity against Plasmodium falciparum. ACS Med Chem Lett. 2010;1:460–465. doi: 10.1021/ml100083c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garuti L, Roberti M, Bottegoni G, Ferraro M. Diaryl urea: A privileged structure in anticancer agents. Curr Med Chem. 2016;23:1528–1548. doi: 10.2174/0929867323666160411142532. [DOI] [PubMed] [Google Scholar]
- 16.Siddique MUM, McCann GJ, Sonawane V, Horley N, Williams IS, Joshi P, Bharate SB, Jayaprakash V, Sinha BN, Chaudhuri B. Biphenyl urea derivatives as selective CYP1B1 inhibitors. Org Biomol Chem. 2016;14:8931–8936. doi: 10.1039/c6ob01506a. [DOI] [PubMed] [Google Scholar]
- 17.Cowan N, Dätwyler P, Ernst B, Wang C, Vennerstrom JL, Spangenberg T, Keiser J. Activities of N,N′-Diarylurea MMV665852 analogs against Schistosoma mansoni. Antimicrob Agents Chemother. 2015;59:1935–1941. doi: 10.1128/AAC.04463-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao H, Liu F, Chen J, Li Y, Cui J, Qiao C. Antischistosomal activity of N,N′-arylurea analogs against Schistosoma japonicum. Bioorg Med Chem Lett. 2016;26:1386–1390. doi: 10.1016/j.bmcl.2016.01.075. [DOI] [PubMed] [Google Scholar]
- 19.Finger GC, Dickerson DR, Orlopp DE, Ehrmantraut JW. Aromatic fluorine compounds. XII. N-(fluorophenyl) carbamates. J Med Chem. 1964;7:572–573. doi: 10.1021/jm00334a041. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Zhao Q, Vargas M, Jones JO, White KL, Shackleford DM, Chen G, Saunders J, Ng ACF, Chiu FCK, Dong Y, Charman SA, Keiser J, Vennerstrom JL. Revisiting the SAR of the antischistosomal aryl hydantoin (Ro 13-3978) J Med Chem. 2016;59:10705–10718. doi: 10.1021/acs.jmedchem.6b01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weilandt T, Troff RW, Saxell H, Rissanen K, Schalley CA. Metallo-supramolecular self-assembly: the case of triangle-square equilibria. Inorg Chem. 2008;47:7588–7598. doi: 10.1021/ic800334k. [DOI] [PubMed] [Google Scholar]
- 22.Stanovnik B, Tisler M, Golob V, Hvala I, Nikolic O. Heterocycles. CXCVIII. Heteroacyl azides as acylating agents for aromatic or heteroaromatic amines. J Heterocyclic Chem. 1980;17:733–736. [Google Scholar]
- 23.Palm K, Stenberg P, Luthman K, Artursson P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm Res. 1997;14:568–571. doi: 10.1023/a:1012188625088. [DOI] [PubMed] [Google Scholar]
- 24.Keiser J. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology. 2010;137:589–603. doi: 10.1017/S0031182009991739. [DOI] [PubMed] [Google Scholar]
- 25.Keiser J, Manneck T, Vargas M. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J Antimicrob Chemother. 2011;66:1791–1797. doi: 10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
- 26.Dong Y, Chollet J, Vargas M, Mansour NR, Bickle Q, Alnouti Y, Huang J, Keiser J, Vennerstrom JL. Praziquantel analogs with activity against juvenile Schistosoma mansoni. Bioorg Med Chem Lett. 2010;20:2481–2484. doi: 10.1016/j.bmcl.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Lombardo F, Shalaeva MY, Tupper KA, Gao F. ElogDoct: A tool for lipophilicity determination in drug discovery. 2. basic and neutral compounds. J Med Chem. 2001;44:2490–2497. doi: 10.1021/jm0100990. [DOI] [PubMed] [Google Scholar]
- 28.Bevan CD, Lloyd RS. A high-throughput screening method for the determination of aqueous drug solubility using laser nephelometry in microtiter plates. Anal Chem. 2000;72:1781–1787. doi: 10.1021/ac9912247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.