Abstract
Background
Methamphetamine (Meth) seeking progressively increases after withdrawal (incubation of Meth craving). We previously demonstrated an association between histone deacetylase 5 (HDAC5) gene expression in rat dorsal striatum and incubation of Meth craving. Here we used viral constructs to study the causal role of dorsal striatal HDAC5 in this incubation.
Methods
In Exp. 1 (over-expression), we injected adeno-associated virus (AAV) bilaterally into dorsal striatum to express either GFP (control) or a mutant form of HDAC5 (mHDAC5), which strongly localized to the nucleus. After training rats to self-administer Meth (10 days, 9 h/d), we tested the rats for relapse to Meth seeking on withdrawal days 2 and 30. In Exp. 2 (knockdown), we injected AAV bilaterally into dorsal striatum to express either a short hairpin RNA against luciferase (shLUC, control) or against HDAC5 (shHDAC5). After training rats to self-administer Meth, we tested the rats for relapse on withdrawal days 2 and 30. We also measured gene expression of other HDACs and potential HDAC5 downstream targets.
Results
We found that HDAC5 overexpression in dorsal striatum increased Meth seeking on withdrawal day 30 but not day 2. In contrast, HDAC5 knockdown in dorsal striatum decreased Meth seeking on withdrawal day 30 but not day 2; this manipulation also altered other HDACs (Hdac1 and Hdac4) and potential HDAC5 targets (Gnb4 and Suv39h1).
Conclusions
Results demonstrate a novel role of dorsal striatal HDAC5 in incubation of Meth craving. These findings also set up future work to identify HDAC5 targets that mediate this incubation.
Keywords: methamphetamine, incubation, HDAC5, dorsal striatum, epigenetics, relapse
Introduction
A key feature of drug addiction is high relapse rates during abstinence (1, 2). In rats, drug seeking progressively increases or incubates after withdrawal from drug self-administration (3–5). This incubation phenomenon was observed in rats across several drug classes (6–10), including methamphetamine (Meth) (11). Incubation of Meth craving was also observed in Meth-dependent patients (12). Recently, we and others have begun to explore mechanisms of incubation of Meth craving (13–17), extending previous studies that primarily focused on incubation of cocaine craving (4, 5, 18). In these studies, we found that reversible inactivation of either central amygdala (16) or dorsal striatum (DS) (15) decreased “incubated” Meth seeking after prolonged withdrawal. The Wolf group demonstrated a critical role of calcium-permeable AMPA receptors in nucleus accumbens (NAc) in this incubation (17). We also used a choice-based procedure to achieve voluntary abstinence and found that either systemic injections of a positive regulator of mGluR2 (AZD8529) (13) or inactivation of relapse-test activated Fos-neurons in dorsomedial striatum (14) decreased incubated Meth seeking after voluntary abstinence.
In the current study, we examined the role of the epigenetic enzyme, histone deacetylase 5 (HDAC5), in DS in incubation of Meth craving. HDAC5 is a member of class IIa HDACs that also includes HDAC4, 7 and 9 (19). Like other HDACs, HDAC5 generally suppresses gene expression by deacetylating histones (20). HDAC5 regulates gene expression and other signaling pathways in an activity-dependent manner by shuttling between the nucleus and cytoplasm, depending on its phosphorylation state (21, 22). Previous studies have shown that systemic injections of non-specific HDAC inhibitors decrease reinstatement of cocaine-induced conditioned place preference (CPP) in mice (23), and reinstatement of cocaine (24) and nicotine (25) seeking in rats, but enhance heroin seeking in rats (26). The Wood group demonstrated in mice that systemic injections of an HDAC3 (a class-I HDAC) inhibitor facilitate cocaine CPP extinction (27) while genetic deletion of HDAC3 in NAc enhances cocaine CPP acquisition (28). Kennedy et al. (29) found in mice that genetic deletion of HDAC1 (another class-I HDAC) in NAc or NAc injections of a class-I HDAC inhibitor decrease cocaine locomotor sensitization.
Here, we focused on HDAC5 in DS based on both earlier studies with cocaine and our recent study with Meth. For cocaine, previous studies showed a role of NAc HDAC5 in cocaine locomotor sensitization and CPP (30, 31); a recent study also showed a role of NAc HDAC5 in cocaine CPP and reinstatement induced by exposure to cocaine cues and cocaine priming injections (32). For Meth, we found that Hdac5 mRNA expression is increased in both DS homogenates and relapse-test activated DS neurons after prolonged withdrawal, when Meth seeking is high (15). Based on the latter evidence, we hypothesized that HDAC5 positively regulates incubation of Meth craving. There are no specific pharmacological compounds to manipulate HDAC5 function (33). Therefore, we used viral approaches to either overexpress or knockdown DS HDAC5 expression and determined whether these manipulations would increase or decrease, respectively, incubation of Meth craving. To overexpress HDAC5, we used an adeno-associated virus (AAV) expressing a mutant form of HDAC5 that increases its nuclear localization (32). To knockdown HDAC5 expression, we used an AAV expressing a short-hairpin against Hdac5 mRNA (34). After HDAC5 knockdown, we also measured mRNA expression of other DS HDACs (to determine whether HDAC5 knockdown leads to compensatory changes of other HDACs) and potential HDAC5 downstream targets identified in the previous study with HDAC5 knockout mice (30). Finally, we determined the effect of HDAC5 knockdown within either dorsomedial or dorsolateral striatum (DMS or DLS) on incubation of Meth craving to determine the role of HDAC5 in the two major DS anatomical sub-regions in this incubation.
Methods and Materials
Subjects, apparatus, intravenous surgery, meth self-administration, withdrawal phase, and relapse tests
AAV preparation & injections; immunohistochemistry, image acquisition & HDAC5 immunofluorescence quantification, RNA extraction, cDNA synthesis & qPCR, Immunoblotting
See SOM
Exp. 1: Effect of overexpressing nuclear HDAC5 in DS on incubation of Meth craving (Fig. 2A)
Figure 2. Overexpressing nuclear HDAC5 in dorsal striatum (DS) potentiated incubation of Meth craving.
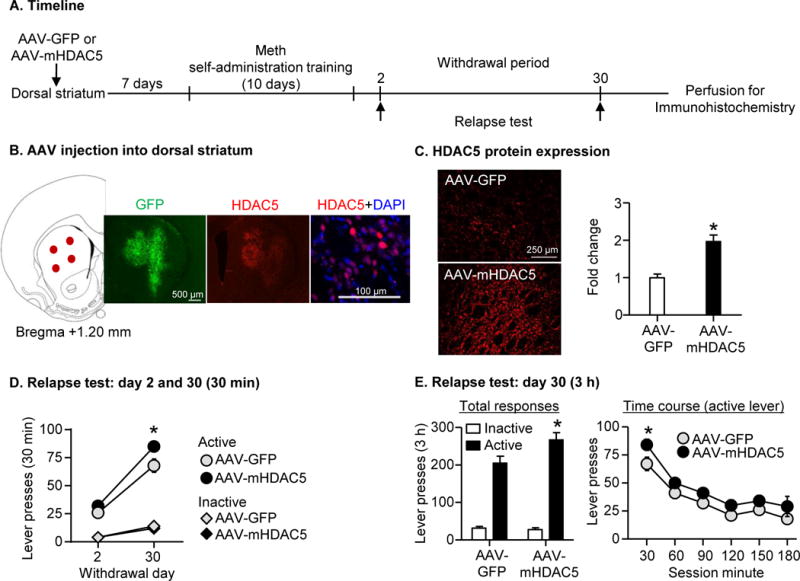
(A) Timeline of the experiment. (B) Left: representative anatomical locations of AAV injections (red dots) into DS [mm from Bregma (97)]; Right: representative images of GFP immunostaining from AAV-GFP group, HDAC5 immunostaining from AAV-mHDAC5 group, and HDAC5 (red) +DAPI (blue) double staining from AAV-mHDAC5 group (C) Left: representative images of HDAC5 immunostaining from DS of AAV-GFP and AAV-mHDAC5 groups; Right: relative HDAC5 fluorescent intensity in DS. Data are presented as fold change of mean values in the AAV-GFP group. * p<0.001; n=10-13 per group. Error bars indicate SEM. (D) Relapse test on withdrawal days 2 and 30: Data are mean±SEM of responses on the previously active lever and on the inactive lever during the 30-min relapse test on withdrawal day 2 and the first 30 min of the 3-h relapse test on withdrawal day 30. * Different from day 2, p<0.05, n=10-13 per group. (E) Relapse test on withdrawal day 30: Data are mean±SEM of responses on the previously active lever and on the inactive lever during the 3-h relapse test. During testing, lever-presses led to contingent presentations of the tone-light cue previously paired with Meth infusions during training, but not Meth. * Different from AAV-GFP, p<0.01, n=10-13 per group.
We performed intravenous surgery on two groups of rats (total n=23) and injected AAV2-GFP (n=10) or AAV2-mHDAC5 (n=13) bilaterally into the DS (see SOM and Fig. 2B). A week after surgery, we trained both groups of rats for Meth self-administration. On withdrawal day 2, we tested the rats for relapse to Meth seeking in a 30-min session. On withdrawal day 30, we tested the rats for relapse in a 3-h session. Active lever-presses during testing [the operational measure of drug seeking in incubation of craving studies (3, 4)] resulted in contingent presentations of the tone-light cue, previously paired with Meth infusions, but not the drug. After the final relapse test, we perfused the rats and processed their brains for immunohistochemistry (both GFP and HDAC5; see SOM). The duration of the test session on day 1 was 30 min to minimize carryover effect of extinction learning, which may subsequently decrease drug seeking on day 30 testing (35, 36).
We also validated HDAC5 knockdown at the protein levels in drug-naïve rats. We injected AAV-mHDAC5 into one DS hemisphere and AAV-mHDAC5 into the other hemisphere. Three weeks later, when AAV expression is maximal (37), we collected DS tissue for subsequent immunoblotting assays (see SOM and Fig. S1A).
Exp. 2: Effect of knocking down HDAC5 expression DS on incubation of Meth craving (Fig. 3A)
Figure 3. Knocking down HDAC5 expression in DS decreased incubation of Meth craving.
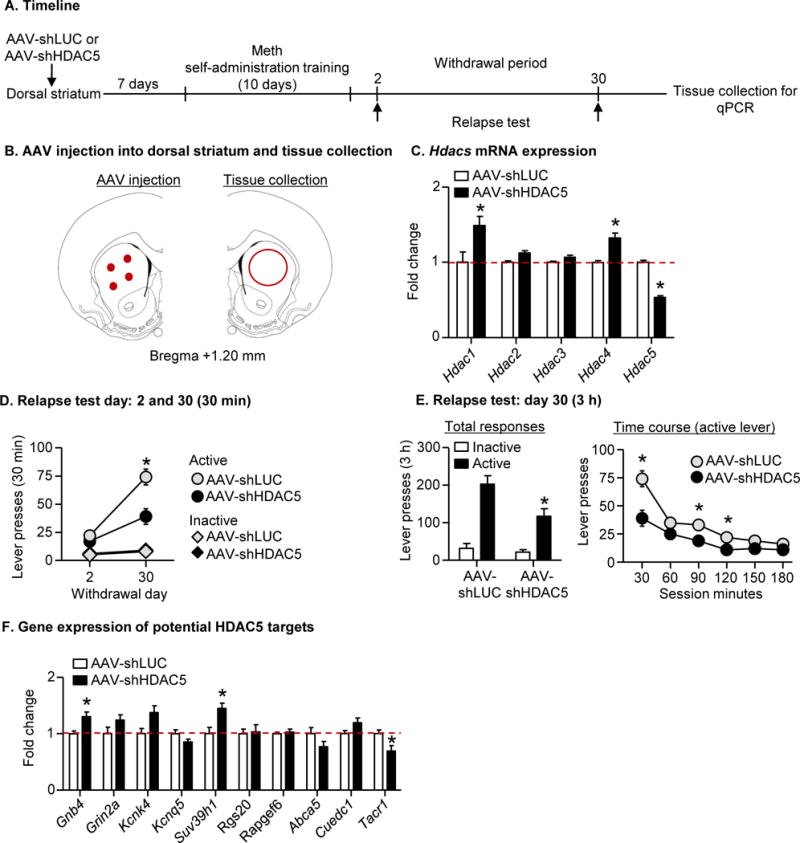
(A) Timeline of the experiment. (B) Left: representative anatomical location of AAV injections (red dots) DS [mm from Bregma (97)]; Right: a representative schematic of DS tissue collection (red circle). (C) Hdac mRNA expression. Data are presented as fold change of mean values in the AAV-shLUC group. Error bars indicate SEM. * p<0.05; n=9-11 per group (D) Relapse test on withdrawal days 2 and 30: Data are mean±SEM of responses on the previously active lever and on the inactive lever during the 30-min relapse test on withdrawal day 2 and the first 30 min of the 3-h relapse test on withdrawal day 30. * Different from day 2, p<0.05, n=11 per group. (E) Relapse test on withdrawal day 30: Data are mean±SEM of responses on the previously active lever and on the inactive lever during the 3-h relapse test. * Different from AAV-shHDAC5, p<0.01, n=11 per group. (F) Gene expression of potential HDAC5 targets. Data are presented as fold change of mean values in the AAV-shLUC group. Error bars indicate SEM. *p<0.05; n=10-11 per group.
We performed intravenous surgery on two groups of rats (total n=22) and injected AAV-shLUC (n=11) or AAV-shHDAC5 (n=11) bilaterally into the DS (see SOM). A week after surgery, we trained both groups of rats for Meth self-administration. On withdrawal day 2, we tested the rats for relapse in a 30-min session. On withdrawal day 30, we tested the rats for relapse in a 3-h session. After the final relapse tests, we collected DS tissue for subsequent qPCR analysis.
We also independently validated HDAC5 knockdown at both the mRNA (n=4) and protein (n=4) levels in drug-naïve rats. We injected AAV-shLUC into the DS of one hemisphere and AAV-shHDAC5 into the other hemisphere. We collected DS tissue three weeks for subsequent qPCR and immunoblotting assays (Fig. S1B).
Exp. 3: Effect of knocking down HDAC5 expression in DMS or DLS on incubation of Meth craving (Fig. S2 and Fig. S3)
We performed intravenous surgery on four groups of rats (total n=47). We injected AAV-shLUC or AAV-shHDAC5 into DMS (AAV-shLUC, n=11; AAV-shHDAC5, n=13) or DLS (AAV-shLUC, n=10; AAV-shHDAC5, n=13). A week after surgery, we trained the rats for Meth self-administration. On withdrawal day 2, we tested the rats for relapse in a 30-min session. On withdrawal day 30, we tested the rats for relapse in a 3-h session. After the final relapse tests, we collected DMS and DLS tissue for subsequent qPCR analysis.
Statistical analysis
See SOM
Results
Exp. 1: Effect of overexpressing nuclear HDAC5 in DS on incubation of Meth craving
The goal of Exp. 1 was to examine whether DS HDAC5 plays a sufficient role in incubation of Meth craving. For this purpose, we delivered AAV into the DS to overexpress a nuclear-localized HDAC5 (AAV-mHDAC5, Fig. 2B-C) and examined whether this overexpression would increase incubation on Meth craving.
Self-administration training (Fig. 1A)
Figure 1. Meth self-administration training.
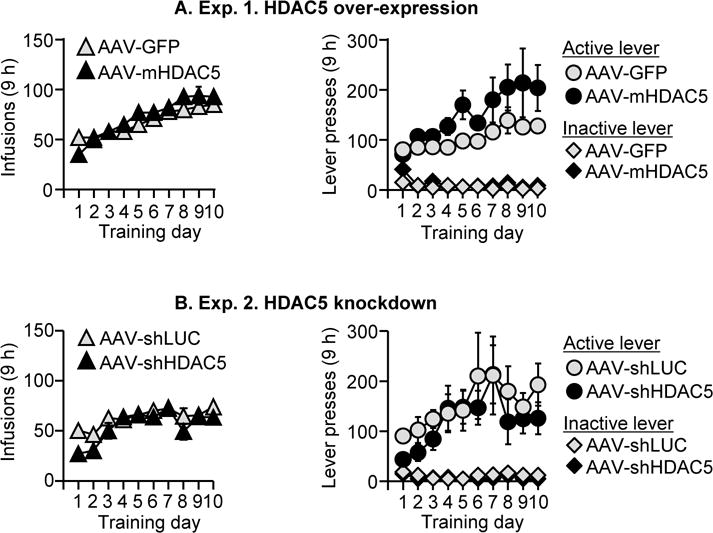
Data are mean ± SEM number of Meth infusions (0.1 mg/kg/infusion) and active and inactive lever-presses during the ten 9-h daily self-administration sessions for Exp. 1 (total n=23) and Exp. 2 (total n=22). During training, active lever presses were reinforced on an FR1 20-s timeout reinforcement schedule and Meth infusions were paired with a 5-s tone-light cue.
See SOM
HDAC5 protein expression (Fig. 2C)
AAV-mHDAC5 group showed ~2-fold increase of HDAC5 immunofluorescence intensity in DS compared with the AAV-GFP group (t21=4.5, p<0.001). We also validated AAV-mHDAC5 using immunoblotting in drug-naïve rats and obtained similar results (Fig. S1A).
Relapse tests (Fig. 2D-E)
DS HDAC5 overexpression increased Meth seeking on withdrawal day 30, but not day 2. As we tested the rats for relapse in a 30-min session on day 2 and in a 3-h session on day 30, we performed two analyses. The first analysis of the relapse tests on day 2 and 30 included the between-subjects factor of Virus Condition (AAV-GFP, AAV-mHDAC5), the within-subjects factor of Withdrawal Day (2, 30), and inactive lever as a covariate; for this analysis, we used the data from the 30-min relapse test on day 2 and the first 30 min of the relapse test on day 30. The analysis showed significant main effects of Withdrawal Day (F1,19=42.9, p<0.001) and Virus Condition (F1,19=5.6, p=0.029) and an approaching significant interaction between the two factors (F1,19=3.5, p=0.075). The second analysis of day 30 only included the between-subjects factor of Virus Condition, the within-subjects factor of Session Minute (30 min intervals), and inactive lever as a covariate; for this analysis, we used the data from the 3-h relapse test. The analysis showed main effects of Virus Condition (F1,21=5.4, p=0.030) and Session Minutes (F5,105=34.6, p<0.001), but no interaction between the two factors (p>0.1); the lack of interaction suggests that HDAC5 over-expression had no effect on within-session extinction learning.
In summary, the data in Exp. 1 demonstrated that DS HDAC5 overexpression modestly potentiated incubated Meth seeking, and establish that HDAC5 in this region plays a sufficient role in this incubation.
Exp. 2: Effect of knocking down HDAC5 expression in DS on incubation of Meth craving
The goal of Exp. 2 was to examine whether HDAC5 also plays a necessary role in incubation of Meth craving. For this purpose, we delivered AAV into the DS to express a short-hairpin RNA against HDAC5 (AAV-shHDAC5), which decreased Hdac5 expression at both the mRNA and protein levels (Fig. S1). We examined whether this downregulation would decrease incubation of Meth craving. We used AAV expressing a short hairpin RNA against nanoluciferase, not expressed in mammals, as the control AAV (AAV-shLUC).
Self-administration training (Fig. 1B)
See SOM
Gene expression of HDACs (Fig. 3C)
We first validated HDAC5 knockdown in DS of drug-naïve rats (Fig. S1). HDAC5 of the AAV-shHDAC5 injected hemisphere decreased to ~50% of AAV-shLUC injected hemispheres at both the mRNA (t3=6.8, p=0.006) and protein (t3=4.3, p=0.023) levels. HDAC5 knockdown (Fig. 3C; t19=12.7, p<0.001) also led to a compensatory increase in expression of Hdac1 and Hdac4 mRNA levels (Hdac1: t19=2.7, p=0.015; Hdac4: t19=2.6, p=0.016).
Relapse tests (Fig. 3D-E)
HDAC5 knockdown decreased Meth seeking on withdrawal day 30, but not day 2. We performed two analyses identical to those described in Exp. 1. The first analysis of days 2 and 30 included the between-subjects factor of Virus Condition (AAV-shLUC, AAV-shHDAC5), the within-subjects factor of Withdrawal Day, and inactive lever as a covariate; for this analysis, we used the data from the 30-min relapse test on day 2 and the first 30 min of the relapse test on day 30. The analysis showed a significant interaction between Withdrawal Day and Virus Condition (F1,18=12.0, p=0.003). The second analysis of day 30 included the between-subjects factor of Virus Condition, the within-subjects factor of Session Minutes, and inactive lever as a covariate; for this analysis, we used the data from the 3-h relapse test. This analysis showed a significant interaction between Virus Condition and Session Minutes (F5,100=5.1, p<0.001).
Gene expression of potential HDAC5 targets (Fig. 3F)
Based on previous study in HDAC5 knockout mice (30), we measured mRNA expression of ten HDAC5 targets. Consistent with previous findings, Gnb4 and Suv39h1 in the DS of the AAV-shHDAC5 group increased compared with AAV-shLUC controls (Gnb4: t19=3.0, p=0.007; Suv39h1: t19=3.1, p=0.006). In contrast, other genes either did not change (Grin2a, Kcnk4, Kcnq5, Rgs20, Rapgef6, Abca5, Cuedc1, p>0.05) or decreased (Tacr1: t19=2.7, p=0.015).
In summary, the data in Exp. 2 demonstrated that dorsal striatal HDAC5 knockdown decreased incubated Meth seeking, indicating that HDAC5 in this brain region plays an important role in incubation of Meth craving. Additionally, downregulation of HDAC5 led to increased expression of other HDACs and some HDAC5 targets.
Exp. 3: Effect of knocking down HDAC5 expression in DMS or DLS on incubation of Meth craving
The goal of Exp. 3 was to examine whether the role of DS HDAC5 in incubation of Meth craving is sub-region specific. For this purpose, we delivered AAV-shHDAC5 into either DMS or DLS and examined whether downregulation of HDAC5 in either region would decrease incubation of Meth craving.
Self-administration training (Fig. S2)
See SOM.
Gene expression of HDAC5 (Fig. S3C)
We validated HDAC5 knockdown in DMS and DLS. HDAC5 expression in DMS or DLS of the AAV-shHDAC5 group decreased to ~50% of its respective AAV-shLUC group (DMS: t20=7.8, p<0.001; DLS: t20=8.0, p<0.001).
Relapse tests (Fig. S3D-F)
HDAC5 knockdown in DMS or DLS had no significant effect on Meth seeking on either withdrawal day 2 or 30. We analyzed the DMS and DLS HDAC5 knockdown experiments separately. We performed two analyses in each experiment like those described in Exp. 1, except that we did not use inactive lever presses as a covariate (see SOM). The first analysis included the between-subjects factor of Virus Condition and the within-subjects factor of Withdrawal Day; for this analysis, we used the data from the 30-min relapse test on day 2 and the first 30 min of the relapse test on day 30. The analysis showed significant main effects of Withdrawal Day (DMS: F1,22=20.2, p<0.001; DLS: F1,21=62.4, p<0.001), but not Virus Condition (DMS: F1,22=0.6, p=0.811; DLS: F1,21=3.2, p=0.087). For the DMS, the interaction between the two factors was not significant (p>0.05), whereas for the DLS the interaction was approaching statistical significance (F1,21=3.5, p=0.074). The second analysis of day 30 included the between-subjects factor of Virus Condition and the within-subjects factor of Session Minutes; for this analysis, we used the data from the 3-h relapse test. This analysis showed a main effect of Session Minute (DMS: F5,110=18.3, p<0.001; DLS: F5,105=29.5, p<0.001), but again no interaction between Virus Condition and Session Minutes (p values>0.05).
In summary, the data in Exp. 3 demonstrated that HDAC5 knockdown in DMS or DLS alone had no significant effect on incubated Meth seeking.
Discussion
We used AAVs to study the role of HDAC5 in DS in incubation of Meth craving. The main finding of our study is that HDAC5 overexpression or knockdown in DS increased or decreased, respectively, ‘incubated’ Meth seeking during late withdrawal. In contrast, neither HDAC5 overexpression nor knockdown in DS influenced Meth seeking during early withdrawal. Additionally, HDAC5 knockdown in DS changed the transcriptional profiles of some of its putative downstream targets and increased the expression of other Hdacs (Hdac1 and 4). Finally, HDAC5 knockdown in either DMS or DLS alone had no effect on incubation of Meth craving. Together, our results demonstrated that HDAC5 function in the entire DS, but not a specific sub-region, plays an important role in incubation of Meth craving.
Role of epigenetic mechanisms in DS in animal models of addiction
Previous studies on epigenetic mechanisms assessed cocaine-induced psychomotor stimulation and CPP (28–31, 38–44), cocaine self-administration (40, 45–50), extinction of cocaine or morphine CPP (23, 27, 51–53), and cocaine cue- and cocaine priming-induced reinstatement (24, 44, 54). Recent epigenetic studies also examined extinction and reinstatement of nicotine seeking (25), heroin-priming induced reinstatement (26), and alcohol consumption (55–59). However, only one published study has examined the causal role of persistent epigenetic adaptations in drug seeking after prolonged withdrawal from extended access drug self-administration. Massart et al. (60) reported that incubation of cocaine craving is associated with time-dependent changes of DNA methylation in NAc and that DNA methylation positively regulates this incubation. Our current study extends this previous work and provides new evidence that histone modification plays a causal role in persistent drug seeking after prolonged withdrawal.
Our results on the role of epigenetic mechanisms in DS in relapse extend previous studies on the role of epigenetic changes in the NAc (41, 42), mPFC (46, 48, 57), amygdala (56), and VTA (51, 61) in the behavioral effects of addictive drugs. Our results are also in agreement with previous studies on the role of DS (either DMS or DLS, or both) in cue- or context-induced cocaine (62–65), Meth (15, 66), and heroin (67) seeking. Recent evidence also indicates a role of DS in alcohol taking and seeking (68–74).
Regarding epigenetic mechanisms in DS, the only evidence available comes from the Kenny group who demonstrated critical roles of local microRNA-212 (75) and methyl CpG binding protein 2 (MeCP2, a transcriptional repressor) (50) in escalation of cocaine self-administration. Taken together with our data, these findings highlight the role of epigenetic mechanisms in DS in drug reward and relapse.
Role of striatal HDAC5 in the behavioral effects of psychostimulant drugs
Our focus on HDAC5 was initially inspired by two publications on the role of HDAC5 in cocaine’s effects. At the molecular level, Renthal et al. (30) and Taniguchi et al. (31) demonstrated that repeated cocaine exposure induces transient changes in striatal HDAC5 activity. At the behavioral level, Renthal et al. (30) reported that HDAC5 knockout mice show increased cocaine CPP and that viral overexpression of HDAC5 in NAc decreases drug CPP. Taniguchi et al. (31) reported that viral overexpression of a nuclear-localized HDAC5 in NAc decreases cocaine CPP. Very recently, Taniguchi et al (32) extended their previous findings and reported that viral overexpression of the nuclear-localized HDAC5 decreases cue-induced and cocaine priming-induced reinstatement of cocaine seeking. Overall, these data indicate that cocaine alters HDAC5 activity in NAc and that HDAC5 in NAc negatively regulates cocaine reward and relapse.
In contrast, we found a time-dependent increase of Hdac5 mRNA expression in rat DS (but not NAc, unpublished data) when Meth seeking is high (incubated) (15). Together with our current study, these data indicate that HDAC5 in DS positively regulates incubation of Meth craving. What might account for the different roles of striatal HDAC5?
One factor is the duration and amount of drug exposure, which are significantly lower in CPP or short-access self-administration than in extended-access self-administration studies. Indeed, it is well-established that extended drug self-administration causes different physiological and behavioral effects from cocaine CPP or short-access cocaine self-administration (5, 76–78). Another factor is the drug itself: cocaine versus Meth. Recent evidence suggests that the mechanisms of relapse to cocaine and Meth seeking are at least partially dissociable (79). For example, we found that inactivation of ventral mPFC, which inhibits incubation of cocaine craving (80), has no effect on incubation of Meth craving (16). Additionally, Fos expression in NAc only increased after context-induced reinstatement of cocaine but not Meth seeking (66, 81).
As an epigenetic enzyme, HDAC5 can affect many genes (30), which leads to two final questions: 1) What are HDAC5’s targets? and 2) Does HDAC5 exerts its role in incubation of Meth craving through these targets? Comprehensive answers to these questions are beyond the scope of our current paper, but we did an initial exploration. Based on previous microarray data in HDAC5 knockout mouse NAc after cocaine exposure (30), we probed gene expression of ten potential HDAC5 targets after HDAC5 knockdown in DS (Exp. 2). We found four genes that showed a consistent expression pattern with the previous study in mice: increased mRNA expression for Gnb4 and Suv39h1, but no change for Grin2a and Kcnk4, while several other genes either did not change (Kcnq5, Rgs20, Rapgef6, Abca5, Cuedc1) or exhibited decreased mRNA expression (Tacr1). We also probed Npas4, an HDAC5’s target recently identified by Taniguchi et al (32). However, we found that Npas4 mRNA expression decreased after HDAC5 knockdown in DS (data not shown). These inconsistencies can be due to the complexity of epigenetic regulation where multiple epigenetic factors modulate gene expression simultaneously (82).
Another potential factor is the compensatory increase of HDAC1 and HDAC4 after HDAC5 knockdown in DS. Based on previous evidence implicating a role of striatal HDAC1 in cocaine locomotor sensitization (29) and a role of striatal HDAC4 in cocaine CPP (83), it is possible that increased HDAC1 or HDAC4 expression (or both) contributes to the decreased incubated Meth seeking after HDAC5 knockdown in DS.
Finally, it should be noted that probing a transcriptional profile after reducing HDAC5 expression can only indirectly identify HDAC5’s targets. Direct evidence comes from chromatin immunoprecipitation (ChIP) against HDAC5 followed by qPCR or genome-wide sequencing. Indeed, HDAC5’s role in regulating gene expression also depends on its deacetylase activity (84–87) and subcellular localization (21, 22). However, elucidating genomic binding, enzymatic activity, or subcellular localization of HDAC5 is beyond the scope of our current paper and will be explored in the future.
Overall, our data suggest that HDAC5 modulates incubation of Meth craving by altering the transcriptional regulation of its direct or indirect gene targets. For example, upregulation of Suv39h1 [a histone methyltransferase acting as a transcription repressor (88, 89)] may contribute to this incubation by remodeling connectivity of activated neurons (90). Additionally, upregulation of Gnb4 [a G-protein that modulates calcium and potassium ion channels (91)] may contribute to incubation of Meth craving by activating G-protein-gated inwardly rectifying potassium (GIRK) currents (92, 93).
Methodological and interpretation issues
From an anatomical and functional perspective, a main question is whether the role of DS HDAC5 in incubation of Meth craving is sub-region specific. This is a relevant question, because there is evidence showing that DMS and DLS play different roles in cue- and context-induced drug seeking in rat relapse models (79). To answer this question, we performed sub-region specific HDAC5 knockdown in DMS or DLS. Our data showed that knocking down HDAC5 expression in DMS or DLS alone had no significant effect on incubation of Meth craving (Fig. S3), suggesting that HDAC5 function in the entire DS, but not a specific sub-region, must be manipulated to appreciably control incubation of Meth craving.
Another main question is whether the role of DS HDAC5 in incubation of Meth craving is cell-type specific. Endogenous HDAC5 is enriched in neurons (94). For Exp. 1, we used AAV2 serotype (95) to increase the neurotropism of HDAC5 expression. For Exp. 2, we used AAV1, which expresses in both neurons and glial cells (95). Therefore, we cannot exclude the possibility that the behavioral effects in our study are mediated by non-neuronal HDAC5 such as glia cells. We believe that this possibility is unlikely, because we previously found that blockade of toll-like receptor 4, an innate immune receptor expressed in primarily in microglia, contributes to incubation of heroin but not Meth craving (36).
Another unresolved issue is whether HDAC5 in D1-expressing, D2-expressing, or both cell types in DS is critical for incubation of Meth craving. Based on our recent studies showing that incubation of Meth craving is associated with increased activity [assessed by the neuronal activity marker, Fos (96)] in both DS cell types (14, 15), we speculate that HDAC5 activity in both D1- and D2-expressing DS neurons is important for this incubation. In future studies, we hope to develop cell-type specific approaches to test whether perturbing HDAC5 expression in different DS cell types would have a similar effect on incubation of Meth craving.
Finally, our data suggest that HDAC5 plays a selective role in incubated Meth seeking during late withdrawal, but not “non-incubated” Meth seeking during early withdrawal. However, this conclusion should be made with caution for two reasons. First, under our experimental conditions, viral expression has been at its maximal expression for a longer duration during late versus early withdrawal. Second, as drug seeking is substantially lower during the early versus late withdrawal relapse tests, negative findings during the early withdrawal relapse tests after DS HDAC5 overexpression or knockdown can be due to a floor effect of low operant responding during early withdrawal.
Conclusions
We demonstrated that DS HDAC5 plays an important role in incubation of Meth craving. This finding is consistent with our previous study showing time-dependent increases of DS HDAC5 mRNA expression during this incubation (15). In contrast, this novel role of HDAC5 in Meth seeking after extended-access drug self-administration contrasts with previous findings on the enzyme’s role in cocaine’s behavioral effects (assessed by CPP, locomotor sensitization, and short-access self-administration) (30–32), indicating that there are dissociated epigenetic mechanisms across drug classes, striatal regions, and behavioral procedures. Finally, HDAC5 likely exerts its role in incubation of Meth craving through its downstream gene targets, which can be further characterized by combining RNA-seq and ChIP-seq in future studies. Finally, from a clinical perspective, our study suggests that selective HDAC5 inhibitors could be a potential therapeutic target for decreasing Meth craving and relapse after prolonged withdrawal.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Drug Abuse, Intramural Research Program (YS) and NIDA extramural funds (P01 DA008227 to EJN, R01 DA032708 to CWC). MBC received support from a NIH predoctoral fellowship (F31 DA035073). JZ received support from China Scholarship Council (No. 201604910135). HS was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST), Republic of Korea (No. 2016R1A2B2006474). AAV particles expressing shRNA (HS provided the plasmid) were constructed and packaged by the NIDA Intramural Genetic Engineering and Viral Vector Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: XL, CWC, EJN, and YS conceived the project, provided intellectual input, and wrote the paper. XL, KW, TZ, OML, JMB, JZ and FS carried out experiments. MBC, CTR and BKH developed and validated the AAV constructs. HS provided the plasmids.
Financial Disclosure
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Jaffe JH. Drug addiction and drug abuse. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 8th. New York: Pergamon Press; 1990. pp. 522–573. [Google Scholar]
- 2.Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Pickens CL, Airavaara M, Theberge FR, Fanous S, Hope B, Shaham Y. Neurobiology of incubation of cocaine craving. Trends in Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 2016;17:351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 7.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 9.Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, et al. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, et al. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, et al. Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. J Neurosci. 2017;37:1014–1027. doi: 10.1523/JNEUROSCI.3091-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Rubio FJ, Zeric T, Bossert JM, Kambhampati S, Cates HM, et al. Incubation of methamphetamine craving is associated with selective increases in expression of Bdnf and trkb, glutamate receptors, and epigenetic enzymes in cue-activated fos-expressing dorsal striatal neurons. J Neurosci. 2015b;35:8232–8244. doi: 10.1523/JNEUROSCI.1022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2015a;40:1297–1306. doi: 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, et al. AMPA receptor plasticity in accumbens core contributes to incubation of methamphetamine craving. Biol Psychiatry. 2016;80:661–670. doi: 10.1016/j.biopsych.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 2014;76(Pt B):287–300. doi: 10.1016/j.neuropharm.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra M, Verdin E. Regulatory signal transduction pathways for class IIa histone deacetylases. Curr Opin Pharmacol. 2010;10:454–460. doi: 10.1016/j.coph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romieu P, Deschatrettes E, Host L, Gobaille S, Sandner G, Zwiller J. The inhibition of histone deacetylases reduces the reinstatement of cocaine-seeking behavior in rats. Curr Neuropharmacol. 2011;9:21–25. doi: 10.2174/157015911795017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castino MR, Cornish JL, Clemens KJ. Inhibition of histone deacetylases facilitates extinction and attenuates reinstatement of nicotine self-administration in rats. PLoS One. 2015;10:e0124796. doi: 10.1371/journal.pone.0124796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen WS, Xu WJ, Zhu HQ, Gao L, Lai MJ, Zhang FQ, et al. Effects of histone deacetylase inhibitor sodium butyrate on heroin seeking behavior in the nucleus accumbens in rats. Brain Res. 2016;1652:151–157. doi: 10.1016/j.brainres.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A. 2013;110:2647–2652. doi: 10.1073/pnas.1213364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogge GA, Singh H, Dang R, Wood MA. HDAC3 is a negative regulator of cocaine-context-associated memory formation. J Neurosci. 2013;33:6623–6632. doi: 10.1523/JNEUROSCI.4472-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy PJ, Feng J, Robison AJ, Maze I, Badimon A, Mouzon E, et al. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci. 2013;16:434–440. doi: 10.1038/nn.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi M, Carreira MB, Smith LN, Zirlin BC, Neve RL, Cowan CW. Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron. 2012;73:108–120. doi: 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniguchi M, Carreira MB, Cooper YA, Bobadilla AC, Heinsbroek JA, Koike N, et al. HDAC5 and its target gene, Npas4, function in the nucleus accumbens to regulate cocaine-conditioned behaviors. Neuron. 2017;96:130–144 e136. doi: 10.1016/j.neuron.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics. 2012;4:5. doi: 10.1186/1868-7083-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi M, Lee SH, Wang SE, Ko SY, Song M, Choi JS, et al. Ketamine produces antidepressant-like effects through phosphorylation-dependent nuclear export of histone deacetylase 5 (HDAC5) in rats. Proc Natl Acad Sci U S A. 2015;112:15755–15760. doi: 10.1073/pnas.1513913112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venniro M, Zhang M, Shaham Y, Caprioli D. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female Rats. Neuropsychopharmacology. 2017;42:1126–1135. doi: 10.1038/npp.2016.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 2013;73:729–737. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malvaez M, Mhillaj E, Matheos DP, Palmery M, Wood MA. CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviors. J Neurosci. 2011;31:16941–16948. doi: 10.1523/JNEUROSCI.2747-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogge GA, Wood MA. The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology. 2013;38:94–110. doi: 10.1038/npp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, et al. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci. 2015;35:8948–8958. doi: 10.1523/JNEUROSCI.5227-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, et al. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2010;35:913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Host L, Dietrich JB, Carouge D, Aunis D, Zwiller J. Cocaine self-administration alters the expression of chromatin-remodelling proteins; modulation by histone deacetylase inhibition. J Psychopharmacol. 2011;25:222–229. doi: 10.1177/0269881109348173. [DOI] [PubMed] [Google Scholar]
- 49.Fonteneau M, Filliol D, Anglard P, Befort K, Romieu P, Zwiller J. Inhibition of DNA methyltransferases regulates cocaine self-administration by rats: a genome-wide DNA methylation study. Genes Brain Behav. 2017;16:313–327. doi: 10.1111/gbb.12354. [DOI] [PubMed] [Google Scholar]
- 50.Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN, et al. Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat Neurosci. 2015;18:415–422. doi: 10.1038/nn.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, et al. Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang WS, Kang S, Liu WT, Li M, Liu Y, Yu C, et al. Extinction of aversive memories associated with morphine withdrawal requires ERK-mediated epigenetic regulation of brain-derived neurotrophic factor transcription in the rat ventromedial prefrontal cortex. J Neurosci. 2012;32:13763–13775. doi: 10.1523/JNEUROSCI.1991-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang ZJ, Martin JA, Mueller LE, Caccamise A, Werner CT, Neve RL, et al. BRG1 in the nucleus accumbens regulates cocaine-seeking behavior. Biol Psychiatry. 2016;80:652–660. doi: 10.1016/j.biopsych.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warnault V, Darcq E, Levine A, Barak S, Ron D. Chromatin remodeling–a novel strategy to control excessive alcohol drinking. Transl Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darcq E, Warnault V, Phamluong K, Besserer GM, Liu F, Ron D. MicroRNA-30a-5p in the prefrontal cortex controls the transition from moderate to excessive alcohol consumption. Mol Psychiatry. 2015;20:1261. doi: 10.1038/mp.2014.155. [DOI] [PubMed] [Google Scholar]
- 58.Barbier E, Tapocik JD, Juergens N, Pitcairn C, Borich A, Schank JR, et al. DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavior and plasticity. J Neurosci. 2015;35:6153–6164. doi: 10.1523/JNEUROSCI.4571-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon-O’Brien E, Alaux-Cantin S, Warnault V, Buttolo R, Naassila M, Vilpoux C. The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals. Addict Biol. 2015;20:676–689. doi: 10.1111/adb.12161. [DOI] [PubMed] [Google Scholar]
- 60.Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, et al. Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci. 2015;35:8042–8058. doi: 10.1523/JNEUROSCI.3053-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120:202–209. doi: 10.1111/j.1471-4159.2011.07571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 64.Pacchioni AM, Gabriele A, See RE. Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res. 2011;218:296–300. doi: 10.1016/j.bbr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murray JE, Belin D, Everitt BJ. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 2012;37:2456–2466. doi: 10.1038/npp.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rubio FJ, Liu QR, Li X, Cruz FC, Leao RM, Warren BL, et al. Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. J Neurosci. 2015;35:5625–5639. doi: 10.1523/JNEUROSCI.4997-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–591. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darcq E, Morisot N, Phamluong K, Warnault V, Jeanblanc J, Longo FM, et al. The neurotrophic factor receptor p75 in the rat dorsolateral striatum drives excessive alcohol drinking. J Neurosci. 2016;36:10116–10127. doi: 10.1523/JNEUROSCI.4597-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, et al. Alcohol elicits functional and structural plasticity selectively in dopamine D1 receptor-expressing neurons of the dorsomedial striatum. J Neurosci. 2015;35:11634–11643. doi: 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeanblanc J, He DY, Carnicella S, Kharazia V, Janak PH, Ron D. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. J Neurosci. 2009;29:13494–13502. doi: 10.1523/JNEUROSCI.2243-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeanblanc J, Logrip ML, Janak PH, Ron D. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci. 2013;37:607–612. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- 78.Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 56 Suppl. 2009;1:177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, et al. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J Neurosci. 2014;34:7437–7446. doi: 10.1523/JNEUROSCI.0238-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 83.Penrod RD, Carreira MB, Taniguchi M, Kumar J, Maddox SA, Cowan CW. Novel role and regulation of HDAC4 in cocaine-related behaviors. Addict Biol. 2017 doi: 10.1111/adb.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- 85.Fischle W, Dequiedt F, Fillion M, Hendzel MJ, Voelter W, Verdin E. Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo. J Biol Chem. 2001;276:35826–35835. doi: 10.1074/jbc.M104935200. [DOI] [PubMed] [Google Scholar]
- 86.Yang WM, Tsai SC, Wen YD, Fejer G, Seto E. Functional domains of histone deacetylase-3. J Biol Chem. 2002;277:9447–9454. doi: 10.1074/jbc.M105993200. [DOI] [PubMed] [Google Scholar]
- 87.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- 89.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- 90.Ding X, Liu S, Tian M, Zhang W, Zhu T, Li D, et al. Activity-induced histone modifications govern Neurexin-1 mRNA splicing and memory preservation. Nat Neurosci. 2017;20:690–699. doi: 10.1038/nn.4536. [DOI] [PubMed] [Google Scholar]
- 91.Ruiz-Velasco V, Ikeda SR, Puhl HL. Cloning, tissue distribution, and functional expression of the human G protein beta 4-subunit. Physiol Genomics. 2002;8:41–50. doi: 10.1152/physiolgenomics.00085.2001. [DOI] [PubMed] [Google Scholar]
- 92.Rifkin RA, Moss SJ, Slesinger PA. G protein-gated potassium channels: a link to drug addiction. Trends Pharmacol Sci. 2017;38:378–392. doi: 10.1016/j.tips.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mayfield J, Blednov YA, Harris RA. Behavioral and genetic evidence for GIRK channels in the CNS: role in physiology, pathophysiology, and drug addiction. Int Rev Neurobiol. 2015;123:279–313. doi: 10.1016/bs.irn.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ. Distribution of histone deacetylases 1-11 in the rat brain. J Mol Neurosci. 2007;31:47–58. doi: 10.1007/BF02686117. [DOI] [PubMed] [Google Scholar]
- 95.Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res. 2015;93:144–157. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 96.Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, et al. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14:743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


