Abstract
As a new initiative, HPV self-sampling to non-attenders using the dry Evalyn self-sampling brush is offered in the Capital Region of Denmark. The use of a dry brush is largely uncharted territory in terms of analytical stability. In this study we aim to provide evidence on the analytical quality of dry HPV self-sampling brushes as a function of time and temperature.
We assessed the analytical stability of dry stored Evalyn brushes at three different temperatures, (4 °C, room temperature, 30 °C) and five different storage time points; T = 0 (baseline), 2, 4, 8, 16, and 32 weeks prior to HPV analysis using the BD Onclarity HPV assay.
Mean Ct value of the Onclarity internal control was used as comparator of cellularity across time and temperatures, with no or only borderline statistical differences observed. HPV detection was stable throughout the five time points. In addition, analytically amplifiable DNA copy numbers and DNA fragmentation was assessed using the Agena iPLEX Exome QC assay, with no or only borderline statistical differences observed.
In conclusion, the Evalyn brush is analytically stable with respect to human genomic material and HPV detection for up to 32 weeks at temperatures ranging from 4 °C to 30 °C.
Abbreviations: HPV, Human Papillomavirus; CSi, Copenhagen Self-sampling initiative; RT, Room temperature; HBB, Human Beta globin Control of the BD Onclarity HPV assay; UTM, Universal Transport Medium; CBD, BD CytoBrush in Diluent; MALDI-TOF MS, Matrix Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry; SAP, Shrimp Alkaline Phosphatase; Ct-value, Cycle Threshold value; ANOVA, Analysis of Variance statistical tests; Bp, Base pair; QA, Quality Assurance
Keywords: Self-sampling, Dry self-sampling brushes, Analytical stability, HPV, Cervical cancer screening
Highlights
-
•
The Capital Region of Denmark is implementing HPV self-sampling using a dry brush.
-
•
But how analytically stable is a dry collected self-sampling device?
-
•
Stability was established for both human DNA (all, N = 637) and HPV (positives, N = 41).
-
•
We found the Evalyn Self-sampling brush stable up to 6 month.
-
•
Neither 4 °C nor 30 °C changed the analytical stability of the dry brush.
1. Introduction
Human papillomavirus (HPV) based cervical cancer screening [1], [2], [3], [4], [5], [6], [7] in combination with self-collected samples is increasingly being investigated as an alternative to clinician collected samples aiming at increasing the coverage of cervical screening worldwide [8], [9], [10], [11], [12]. In organized screening programs, HPV self-sampling is considered as a potential alternative to screening non-attending women [13], [14], [15]. In Denmark, women aged 23–65 years are invited for screening for cervical cancer free of charge as a public cancer prevention program. In Denmark approximately 50% of all cervical cancers are diagnosed amongst the 25% women who do not participate in screening after being invited [16], [17], as also observed in similar North European countries with nationwide cervical cancer screening programs [18], [19]. In qualitative studies evaluating women´s preferences in screening, the main reasons for non-participation are reported as a combination between the discomfort/embarrassment of the associated gynecological examination and the general inconvenience of the doctor's visit [20], [21], [22]. To address the screening non-attendance, the Capital Region of Denmark launched a pilot implementation program in 2014, the Copenhagen Self-sampling initiative (CSi), offering HPV self-sampling brushes to approx. 24,000 screening non-attenders [9], [14], [23]. In CSi, we distributed the Evalyn self-sampling brush to invited women who actively opted in after invitation using a purpose designed and developed self-sampling kit [9]. After sampling in the privacy of the woman´s own home, the women returned the brush in a dry state to the laboratory for HPV analysis using a postage pre-paid envelope.
Multiple approaches to self-sampling have been described but two types of self-sampling devices have predominantly been used for larger self-sampling initiatives; a “wet” brush that requires the woman to re-suspend the brush in a supplied media immediately after sampling or a “dry” brush, shipped directly to the laboratory after sampling without further interaction by the woman. Comparing these two approaches, the use of a dry shipped self-sampling brush in our opinion holds a number of logistically and safety related advantages independently of the self-sampling device. Firstly, a dry brush can be transported by mail between the women and the laboratory without the potential for spillage and leakage during collection and transport. Secondly, any risk of potential skin irritation and harm by accidental consumption e.g. by a child in the household, is eliminated. Thirdly, shipment of liquid biological samples is more expensive and subject to more strict regulations than shipment of dry samples, at least in the EU. Fourthly, re-suspended, liquid samples represent a diagnostic quality assurance challenge as spillage by the woman or during transport reduce the analytical volume available for analysis after reception of sample at the laboratory [11]. Variable analytical volumes challenges the validity of the clinical cut off of the HPV assay used. Yet, despite these advantages, the use of dry collected and shipped brushes is largely uncharted territory in terms of analytical stability of the resulting human and viral material received for analysis in the laboratory. The objective of this study was therefore to provide data on the analytical quality of dry brushes for HPV self-sampling.
The central question asked was; what is the analytical stability of dry brushes under low, normal and extreme temperatures or during prolonged storage and transportation after sampling?
Little if any information is available in the literature concerning the stability of dry collected HPV self-sampling brushes even though HPV self-sampling is in the process of being implemented in several countries, including the Capital Region of Denmark.
2. Material and methods
2.1. Sample collection
Fully anonymized cervical swab samples from women undergoing HPV testing in the Danish cervical screening program were used. The swab samples were received in universal transport medium (UTM, Copan Diagnostics INC. Murriette, CA, USA) where the residual sample typically contains more than 1.0 ml after routine diagnostic testing. UTM (Hank's Balanced Salts Bovine Serum Albumin L-Cysteine Gelatin Sucrose L-Glutamic Acid HEPES Buffer Vancomycin Amphotericin B Colistin Phenol Red pH 7.3 +/− 0.2 @ 25 °C) contains no fixatives affecting genomic stability of the sample. Only samples of cervical origin were included. A total of 183 swab samples were used in the study.
2.2. Study design
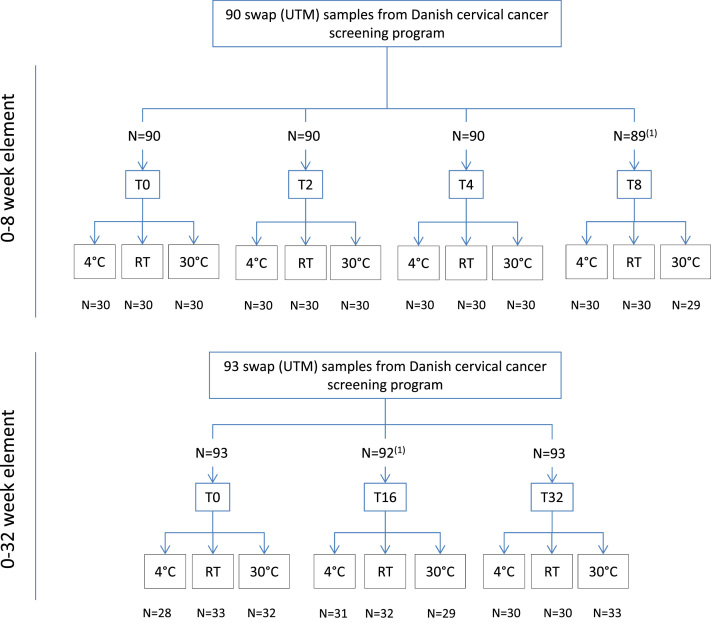
Biospecimens for an index (baseline, T0) and five different storage points were evaluated (2, 4, 8, 16 and 32 weeks) at three different temperatures: 4 °C, uncontrolled room temperature (RT, approximately 20–22 °C), and 30 °C (Fig. 1). In total, 639 individual Evalyn brushes were analyzed.
Fig. 1.
: Study Flowchart. 1 Two samples were excluded due to technical reasons during processing. 2 The sample number (n) for each of the temperature study periods is listed in Table 1.
To allow for direct comparison at different time points, up to four individual brushes were inoculated with each swab sample creating the biospecimens for analysis. Material limitations necessitated that not all time-points could be derived from the same swap samples, which resulted in the separation of the 8 (0, 2, 4, & 8 weeks) and 32 (0, 16, and 32 weeks) week time points into two separate study elements, now designated the 8 week and the 32 week study element. Both elements contained an index test at T = 0. For the 8 week time point (baseline plus 2, 4 & 8 weeks), 90 swab samples were used with four brushes per swab sample, resulting in a total 360 brushes, with approximately 30 brushes per evaluation point. For the 32 week time point (baseline plus 16 and 32 weeks), 93 swab samples were used with three brushes per swab sample, resulting in a total of 279 brushes or approximately 30 brushes per evaluation point.
2.3. Sample processing
The swab sample material was transferred to a 5 ml Eppendorf tube (Eppendorf, Hamburg, Germany). The Evalyn brushes were dipped briefly into the swab sample, swirled around three to four times and left to dry to mimic a home-taken cervical self-sample. The brushes were randomly allocated for the different temperatures and time points. Samples for baseline testing were stored overnight at the designated temperature and subsequently processed for HPV analysis to generate a T = 0 time point.
After incubation at the designated time and temperature points, the brush heads were removed and placed in an empty 5 ml Eppendorf tube. Three ml BD “CytoBrush in Diluent” medium (CBD, BD Diagnostics, Sparks, USA) were added and the samples were subsequently vortexed for 5 s and left for 15 min at room temperature. Afterwards, the brush heads were discarded and the samples were vortexed for an additional 5 s. Finally, 1.0 ml of re-suspended sample material was transferred to an empty BD sample tube for BD Onclarity HPV testing (Onclarity), and 0.2 ml was transferred to a 96 well plate for DNA extraction using Roche MagNA Pure 96 System.
2.4. BD Onclarity HPV assay
The CBD collected samples were tested using the Onclarity assay on the BD VIPER LT system [24], [25] which has previously been described in details [14]. The Onclarity assay report nine different genotypes groups (16, 18, 31, 45, 51, 52, 33/58, 35/39/68, 56/59/66) and harbors an internal human beta globin control (HBB). In summary, 1.0 ml aliquots of the re-suspended CBD material were transferred to an empty sample tube before being preheat treated for 30 min at 120 °C on the VIPER pre-warm station. The pre-warmed samples were subsequently transferred to the fully automated VIPER LT platform and tested with the Onclarity assay according to manufacturer´s recommendations. Two samples were excluded due to technical failure during processing.
2.5. Agena iPLEX Pro Exome QC
The Agena iPLEX Pro Exome QC assay (Agena Bioscience, Hamburg, Germany) is a relatively new quality assurance assay, not previously used on cervical screening samples. The Exome QC panel is a quantitative assay that evaluates the amount of available DNA in a sample and the number of amplifiable copies at five different target amplicon sizes (100, 200, 300, 400 and 500 base pair (bp.).). The assay harbors 21 SNP, 3 markers for gender identification and 25 copy number controls, and five markers per amplicon length in a single multiplexed assay. The assay uses the Matrix Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) technology. All 639 samples were tested with the Exome QC assay to evaluate the relative amount and fragmentation of the DNA in the samples.
DNA was purified from the residual re-suspended brush CDB diluent, 200 µl was transferred to a microtiter plate for DNA purification on the Roche MagNA Pure 96 platform, using the MagNA Pure LC Total Nucleic Acid Isolation kit (Roche Diagnostics). Elution volume was 100 µl.
An initial multiplex amplification PCR was set up with 2 µl DNA, followed by a Shrimp Alkaline Phosphatase (SAP) reaction (which removes excess Nucleotides). The iPLEX Pro single base extension PCR reaction was then performed, where a mix of oligonucleotide extension primers designed to anneal to the amplified DNA fragments were added together with extension enzyme and mass-modified dideoxynucleoside terminators. The extension products were subsequently de-salted with Clean Resin prior to being loaded into the MassARRAY Dx Nanodispenser RS1000 (Agena, Hamburg, Germany), which transfers the analyte to a spectroCHIP. Here the samples crystalizes with the matrix on the chip, which was analyzed on the MassARRAY Dx Analyzer 4 (MA4). The analyte crystals are irradiated by a laser, inducing desorption and ionization. The MA4 accelerates the samples to a detector that differentiates genetic variants by molecular mass.
2.6. Statistics
The Onclarity assay has a three well design with nine HPV genotype read-outs, the internal HBB control is included in each well, and the Ct-value of HBB in this study was calculated as an average of the three HBB individual Ct values of the three wells. The cut-off for all channels is uniformly Ct 34.2. The mean HBB Ct value was included in the analysis regardless of the validity status of the samples, hence HBB Ct-values above 34.2 were also included in the calculations. For genotype analysis only clinical positives (Ct values below the cut off of 34.2) were included in the calculations. For the Exome QC analysis, only samples with sufficient number of SNPs were included in the analysis. Mean Ct, mean available copy numbers, 95% confidence intervals, and standard deviations were calculated using IBM SPSS statistics ver. 22. One-way ANOVA test was used to calculate statistical difference between the different time and temperature points. Talking into account that some of the data points deviate by a large margin from the general distribution patterns, we also applied two non-parametric analysis to confirm the outcome of the one-way ANOVO (the Freidman and Kruskal-Wallis non-parametric analysis,SPSS).
2.7. Ethical approval
The study used completely anonymized residual clinical swab sample material which would otherwise have been discarded and the study did therefore neither need Medical Ethical Committee nor Data Protection Agency approval under current Danish Law.
3. Results
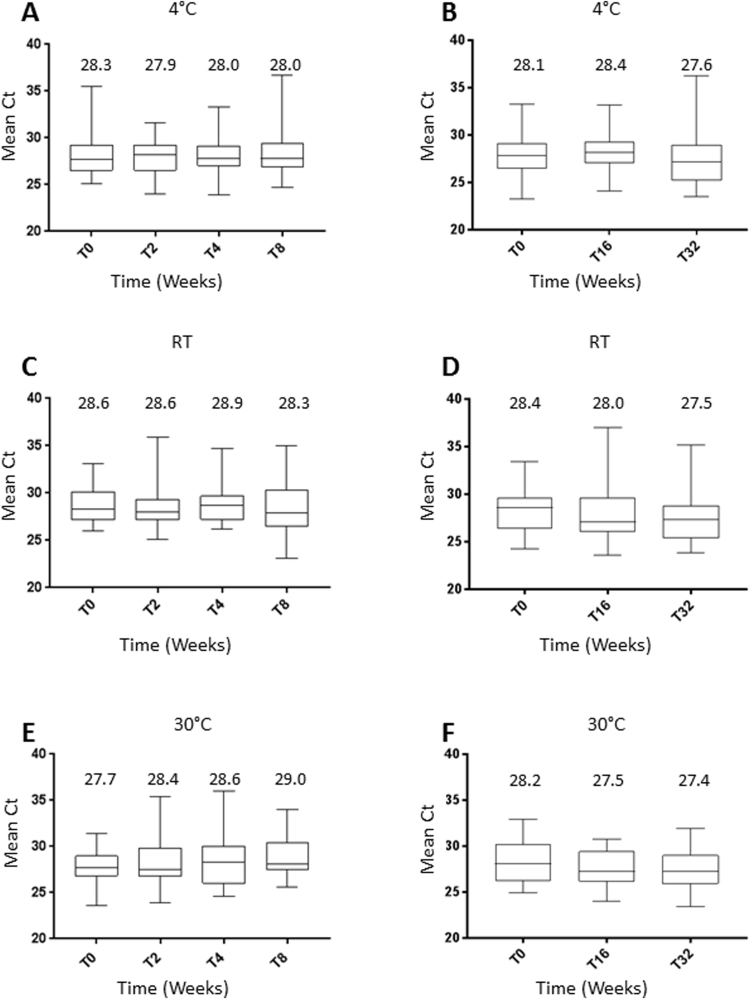
In total, three temperature conditions (4 °C, RT & 30 °C) and 6 time points were evaluated (Baseline and 2, 4, 8, 16 and 32 weeks, Fig. 1), representing 18 individual conditions. At 4 °C, the mean Ct-value of the internal HBB control, ranged from 27.9 to 28.3 (overall mean Ct 28.1, 95% CI 27.7–28.5) and 27.6–28.4 (28.0, 95% CI 27.5–28.6) for 0–8 and 0–32 weeks, respectively (Table 1 and Fig. 2A+B). No statistical difference within each group were observed (0–8 weeks; p-value .95, 0–32 weeks; p = 0.54, Table 1).
Table 1.
Stability of dry Self-sample brushes stored at 0–32 weeks, measured as relative cellularity using BD Onclarity human Beta-globin (HBB) internal control.
| Time points (weeks) | N Tested | N2 Invalids | HBB Ct Mean | St. dev. | 95% CI | P-value3 | |
|---|---|---|---|---|---|---|---|
| 4 °C 0–8 weeks | 0 | 30 | 1 | 28.3 | 2.4 | 27.4–29.2 | 0.95 |
| 2 | 30 | 0 | 27.9 | 1.9 | 27.2–28.6 | ||
| 4 | 30 | 0 | 28.0 | 1.8 | 27.3–28.7 | ||
| 8 | 30 | 2 | 28.0 | 2.6 | 27.1–29.0 | ||
| 4 °C 0–32 weeks | 01 | 28 | 0 | 28.1 | 2.6 | 27.1–29.1 | 0.54 |
| 16 | 31 | 0 | 28.4 | 2.3 | 27.5–29.2 | ||
| 32 | 30 | 1 | 27.6 | 3.1 | 26.5–28.8 | ||
| RT 0–8 weeks | 0 | 30 | 0 | 28.6 | 1.8 | 27.9–29.2 | 0.76 |
| 2 | 30 | 3 | 28.6 | 2.7 | 27.6–29.6 | ||
| 4 | 30 | 1 | 28.9 | 2.2 | 28.1–29.8 | ||
| 8 | 30 | 2 | 28.3 | 2.8 | 27.2–29.3 | ||
| RT 0–32 weeks | 0 | 33 | 0 | 28.4 | 2.3 | 27.6–29.2 | 0.39 |
| 16 | 32 | 2 | 28.0 | 3.3 | 26.8–29.2 | ||
| 32 | 30 | 1 | 27.5 | 2.6 | 26.5–28.4 | ||
| 30 °C 0–8 weeks | 0 | 30 | 0 | 27.7 | 1.9 | 26.9–28.4 | 0.20 |
| 2 | 30 | 1 | 28.4 | 2.4 | 27.5–29.3 | ||
| 4 | 30 | 2 | 28.6 | 2.9 | 27.5–29.6 | ||
| 8 | 29 | 0 | 29.0 | 2.2 | 28.2–29.8 | ||
| 30 °C 0–32 weeks | 0 | 32 | 0 | 28.2 | 2.2 | 27.4–29.0 | 0.25 |
| 16 | 29 | 0 | 27.5 | 1.8 | 26.8–28.3 | ||
| 32 | 33 | 0 | 27.4 | 2.1 | 26.7–28.2 |
Each of the two time studies, 0–8 weeks and 0–32 weeks, had individual baselines (T = 0).
Invalid outcomes were recorded when the Ct-value of the internal HBB control was above Ct 34.2.
P-value calculated using the One-way ANOVA test.
Fig. 2.
Stability of dry Self-samples brushes stored at 0–32 weeks for three different temperatures (4 °C, RT, 30 °C), presented as box blot for Ct value of the internal control of BD Onclarity HPV assay.
At room temperature, the mean HBB Ct value ranged from 28.3 to 28.9 for 0–8 weeks (mean HBB Ct 28.6, 95% CI 28.2–29.0) and 27.5–28.4 for 0–32 weeks (28.0, 95% CI 27.4–28.5) (Table 1 and Fig. 2C+D). Again, no statistical difference within each group were observed (p-value=.76 for 0–8 weeks, p = 0.39 for 0–32 weeks, Table 1).
At 30 °C storage, we found the cellularity to be stable for up to 32 weeks (p = 0.20 for 0–8 weeks, p-value=.25 for 0–32 weeks, Table 1), with mean HBB Ct values ranging from 27.7 to 29.0 (mean HBB Ct: 28.4, 95% CI 28.0–28.8) and 27.4–28.2 (27.7, 95% CI 27.3–28.2) for 0–8 and 0–32 weeks respectively (Table 1 and Fig. 2E + F).
Finally, no statistical difference was observed comparing the three temperature groups using one-way ANOVA (p = 0.20 for 0–8 weeks, 0.72 for 0–32 weeks), nor when comparing the time points (p = 0792 for 0–8 weeks, p = 0.11 for 0–32 weeks (data not tabulated)).
In addition, direct comparison using one-way ANOVA and Kruskal-Wallis non-parametrical tests showed no difference between individual time-points across the three temperatures (data not shown).
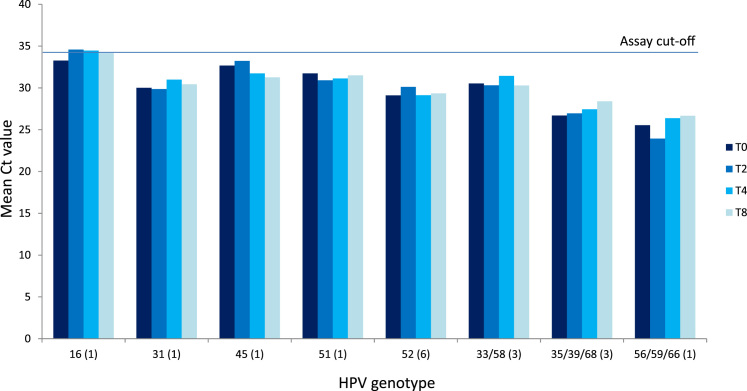
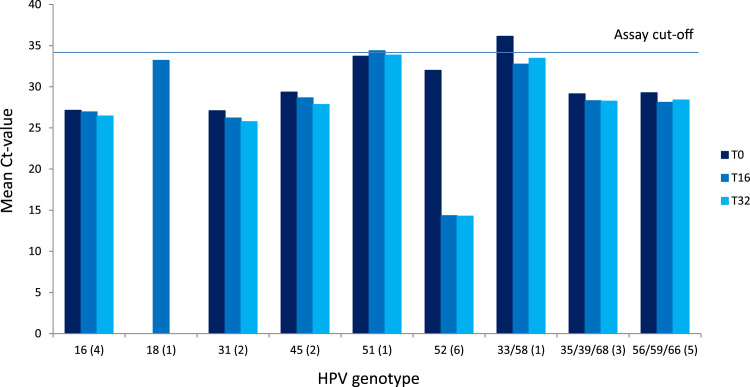
Stability of viral DNA on the dry self-sample brushes was evaluated over time and temperature by comparing HPV outcomes and resulting Ct values of those samples with known HPV content. The Onclarity assay reports Ct-value for nine different HPV genotype groups, and Fig. 3, Fig. 4 shows the mean Ct value of the nine genotype groups for the six time points (see Supplementary Tables 1+2). A total of 41 HPV positives samples were analyzed, with 16 HPV positive samples in the 8 week study element and 25 HPV positive samples in the 32 week study element. Between the two study elements, 14 of 16 and 17 of 25 samples, respectively, returned concordant results at all time points. In the 8 week study element, 2 samples had a discordant outcome between HPV positive and negative, with two of the four time points returned a Ct value just above assay cut off, indicating this to be “close to cutoff samples” irrespective of time and storage conditions (Fig. 3 and Supplementary Table 1). In the 32 week study element, of eight discordant samples, four were “close to cut-off”. The remaining four samples had 1 or 2 time points where the CT value for the HPV genotype was observed just below cut-off whereas the remaining time point the CT value for the HPV genotypes was 0.0 (Fig. 4 and Supplementary Table 2).
Fig. 3.
: Stability of HPV genotypes on dry self-sampling brushes at storage for 0–8 weeks. The horizontal line indicates the clinical cut-off of 34.2 Ct. Numbers in parentheses indicate number of samples with the stated genotype. One sample had a multiple infection of HPV45 and HPV52, which is why there are 17 infections and 16 samples.
Fig. 4.
: Stability of HPV genotypes on dry self-sampling brushes at storage for 0–32 weeks. The horizontal line indicates the clinical cut-point of 34.2 Ct. Numbers in parentheses indicate number of samples with the stated genotype.
The Exome QC assay report average number of amplifiable human DNA copies. In addition, this assay report a detailed composition describing the relative distribution between amplifiable copies at 100, 200, 300, 400, and 500 bp. length as a measure of the level of DNA fragmentation in the individual sample (Table 2). We observed a small, time dependent statistical difference in overall amplifiable DNA copies when comparing T0, T16 and T32 at 4 °C (P = 0.03), but this difference was only evident when comparing T0 and T16 (P = 0.045), whereas T0 and T32 showed borderline statistical difference (P = 0.07). No time dependent statistical difference was observed for the two other temperatures looking at average amplifiable DNA copies (Table 2). Here, DNA fragmentation at different base pair lengths showed some statistical difference for samples stored at 30 °C between the smallest fragments of 100, 200 and 300 bp. and at 4 °C for 300 and 400 bp. For the remaining time and temperature points, no or only borderline statistical difference between the measured compositions of fragments was observed (Table 2).
Table 2.
Stability of dry Self-sample brushes, measured as average amplifiable copy number of human genomic sample material.
| Time points (weeks) | 4 °C |
RT |
30 °C |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean1 | P-value3 | Mean | P-value | Mean | P-value | |||
| Average huDNA copy number | 0–8 weeks | 0 | 1034 | 0.41 | 1132 | 0.61 | 3923 | 0.37 |
| 2 | 1416 | 1012 | 3208 | |||||
| 4 | 3543 | 912 | 1078 | |||||
| 8 | 1013 | 2013 | 469 | |||||
| 0–32 weeks | 0 | 3489 | 0.03 | 1635 | 0.54 | 1216 | 0.54 | |
| 16 | 680 | 1184 | 1222 | |||||
| 32 | 1005 | 975 | 804 | |||||
| 2Relative number of 100 bp. fragments | 0–8 weeks | 0 | 678 | 0.44 | 609 | 0.91 | 1157 | 0.08 |
| 2 | 744 | 596 | 698 | |||||
| 4 | 669 | 629 | 715 | |||||
| 8 | 573 | 655 | 519 | |||||
| 0–32 weeks | 0 | 906 | 0.05 | 830 | 0.05 | 839 | 0.00 | |
| 16 | 618 | 606 | 595 | |||||
| 32 | 560 | 489 | 463 | |||||
| Relative number of 200 bp. fragments | 0–8 weeks | 0 | 252 | 0.79 | 190 | 0.678 | 748 | 0.03 |
| 2 | 250 | 246 | 265 | |||||
| 4 | 324 | 159 | 375 | |||||
| 8 | 193 | 284 | 88 | |||||
| 0–32 weeks | 0 | 1163 | 0.09 | 485 | 0.24 | 299 | 0.03 | |
| 16 | 111 | 181 | 209 | |||||
| 32 | 214 | 188 | 108 | |||||
| Relative number of 300 bp. fragments | 0–8 weeks | 0 | 1529 | 0.36 | 1387 | 0.616 | 3510 | 0.03 |
| 2 | 1511 | 1307 | 1662 | |||||
| 4 | 11125 | 1033 | 1604 | |||||
| 8 | 1211 | 1826 | 642 | |||||
| 0–32 weeks | 0 | 5018 | 0.04 | 2059 | 0.467 | 1811 | 0.69 | |
| 16 | 762 | 1379 | 2608 | |||||
| 32 | 1456 | 1280 | 1491 | |||||
| Relative number of 400 bp. Fragments | 0–8 weeks | 0 | 1253 | 0.35 | 1802 | 0.884 | 2990 | 0.44 |
| 2 | 1407 | 1660 | 11793 | |||||
| 4 | 2453 | 1577 | 1484 | |||||
| 8 | 1609 | 2193 | 683 | |||||
| 0–32 weeks | 0 | 4968 | 0.03 | 1905 | 0.90 | 1408 | 0.51 | |
| 16 | 1399 | 1730 | 1292 | |||||
| 32 | 1505 | 1594 | 1006 | |||||
| Relative number of 500 bp. fragments | 0–8 weeks | 0 | 1459 | 0.51 | 1673 | 0.554 | 11211 | 0.25 |
| 2 | 3168 | 1261 | 1623 | |||||
| 4 | 3158 | 1168 | 1267 | |||||
| 8 | 1480 | 5129 | 419 | |||||
| 0–32 weeks | 0 | 5389 | 0.05 | 2913 | 0.59 | 1735 | 0.50 | |
| 16 | 509 | 2025 | 1424 | |||||
| 32 | 1304 | 1337 | 956 | |||||
Average amplifiable copy numbers
Overall and relative composition of the sample evaluated as proportion of 100, 200, 300, 400 and 500 bp DNA fragments.
p=value calculated using the one-way ANOVA test
4. Discussion
HPV self-sampling is gaining acceptance in organized screening programs with implementation or large scale pilot implementation ongoing in, but not limited to, Denmark, Sweden, Norway and Holland. HPV self-sampling is an approach to improve screening coverage by offering screening non-attenders an alternative to the physician taken screening sample [9], [26], [27] and thereby overcoming some of the main reported reasons for non-attendance [20]. HPV self-sampling can also facilitate implementation of cervical screening in less favored regions where screening has not previously been a priority and/or where medical infrastructure is less developed [27], [28].
A key question of HPV based self-sampling is the clinical sensitivity for detection of disease [8], [29], but a recent, conclusive study by the Norwegian Cancer Registry convincingly showed that the right combination of self-sampling device and HPV test provides clinical sensitivity at par with regular physician taken liquid based cytology samples for cervical cancer screening [26]. However, a number of central questions for operationalization of HPV self-sampling remain unresolved; amongst them the analytical stability of self-collected samples for HPV diagnostics over time and different temperature conditions. In this study we investigated the analytical stability of dry Evalyn brushes for use in HPV self-sampling. Our aim was to show whether the quality of the DNA on a self-sampling brush will deteriorate over time as previously proposed [30], and/or if deterioration would be exacerbated in a dry state. In both instances, the validity of the resulting HPV analysis could be compromised.
The main finding of our study was that the dry Evalyn brushes using these biospecimens were analytically stable over a broad range of ambient temperatures and time up to 32 weeks. This observation was irrespective of whether the evaluation was conducted on human or viral DNA. The analytical stability was demonstrated for the overall human DNA content using the Onclarity HBB internal control, as well as longitudinally, comparing the ability to detect specific HPV genotypes across different storage conditions. A trend toward slightly lower amounts of analytical available material was seen at the 32 week time-point, though statistically insignificant.
The Exome QC assay was employed as a quality assurance (QA) assay evaluating the analytically available genomic material offering insight into the quality of the samples as a result of the storage conditions. This evaluation has relevance as heavy DNA fragmentation of a sample can challenge the validity of a diagnostic HPV test, given that majority of commercially available HPV assays typically rely on amplification of fragments above 200 bp. [31]. The results, however, showed that the overall amount of amplifiable DNA per sample and the level of DNA fragmentation did not significantly differ as a function of storage conditions. To our knowledge, this is the first report using an assay like the Exome QC as a QA measurement tool in cervical screening technology assessment.
The strength of the study is that the samples represent longitudinal storage of the same sample per data-point with three to four individual brushes per original sample, thereby eliminating the majority of sample-to-sample variation. The HPV analysis was performed using the automated Onclarity HPV assay which operates on clinically validated cervical screening cut offs for detection of HPV infections [24]. Had we chosen a more sensitive, less clinically specific HPV analysis, the HPV prevalence amongst the samples may have been higher, but the detection of HPV would not necessarily be relevant in a cervical cancer screening setting [24], [32]. Moreover, with 639 samples analyzed in total and almost 30 samples per time and temperature data-point, the dataset is statistically robust. Only two samples were invalid due to technical issues.
We chose three temperatures representing both ends of the temperature scale, from 4 °C to 30 °C. Whereas 4 °C is the common temperature setting of refrigerators, 30 °C represents an ambient temperature rarely experienced for prolonged periods in northern European countries like Denmark. Nonetheless, from a logistics point of view, a self-collected sample left on a night stand, in a bath room cupboard, or stranded in a mail sorting central may be exposed to higher than geographical average temperatures and as such, the 30 °C setting is of relevance even for Northern Europe. Furthermore, analytical stability data at this temperature is important for HPV self-sampling outside the cooler northern European countries. We evaluated analytical stability over a full 6 month (32 week) period interspaced with intermediate time points. The CSi study showed that 50% of the distributed self-samples were returned to the laboratory within 2 weeks, 75% within 45 days, and 90% within 50 days. At 180 days (6 months) 99% of all samples were received [6], which is also consistent with other study observations [9], [25], [26], [27], [28], [29], [30]. Moreover, the 6 months stability time point also reflect the fact that as a screening service provider we do not control the return date of the brushes, but can only encourage participants to return the brushes using a reminder strategy [6]. Therefore, it is of high clinical value to know if the analytical stability is time sensitive, allowing one to define a relevant expiration date for diagnostic use of a returned self-sampling brush.
The limitation of the study is first and foremost the proxy nature of the biospecimens used and the use of cervical swabs for in situ creation of biospecimens has its pros and cons. Though the biospecimens generated by dipping Evalyn brushes into clinical swap samples represent relevant cervico-vaginal material, the concentration of material settling on the brush as evaluated by HBB Ct values was far less than in our previously published HPV self-sampling implementation (The Copenhagen Self-sampling Initiative, CSi [9], [23], [33]). In the CSi the average HBB Ct on 4620 evaluated, individual real-life Evalyn HPV self-samples was Ct21.8 (data not shown) which is substantially less than the HBB Ct26–29 observed here. Paradoxically, this is at the same time a strength of this study as “thin samples” in theory could deteriorate into discordance between time and temperature point faster than more full, robust samples. On the pro side also is the lab controlled “dipping procedure” ensuring a uniform expose of the brush heads to sample material prior to drying which is a prerequisite for a longitudinal comparison between subsamples. On the other hand, this is still an artificial approximation of self-collected samples, but to transfer this study to a real life setting would entail asking women to return 4 or more self-collected samples and subsequently analyze them over time. From a practicality and logistic point of view, this was beyond the scope of this study, and even in such a setting variations between serial samples could confound the results. Furthermore, given that no statistically differences in analytical stability was observed at 32 weeks, a longer time point could have been included to show when significant deterioration of the analytical material would set in.
Finally, the numbers of HPV positive samples is relatively small compared to the overall number of samples included. In retrospect, this study could have benefitted from a pre-screening of samples in order to enrich for HPV positive samples.
Dry or a wet brush: Which is the optimal choice of utensil for HPV self-sampling? From an operationalization point of view, we think that the use of dry self-sampling brushes is more advantageous than using a kit including a resuspension/shipping liquid based. It is arguably safer as there is no risk of accidental ingestion of the distributed liquid, no potential skin irritation upon use, and no risk of spillage or leakage during transport or collection. Importantly from the laboratory point of view, the assay input volume for the end-point analysis is always the same when the resuspension of the brush is conducted in the laboratory.
Several studies have compared dry and wet sample collection and in all cases found the two self-sample modes compatible [4], [30], [34], [35], [36], [37]. However, to the best of our knowledge no one has looked specifically at the storage and temperature limitations of dry HPV self-sampling brushes. Two studies have compared refrigerated stored dry and wet samples, shipped at room temperature [30] or storage and shipment at 4 °C [35]. Wolfrum et al., [37] observed a tendency for loss of HPV genotypes when a dry Dacron self-sampling brush was stored for more than one week. They also observed a decrease in DNA amounts over time [37]. We did not observe this, but the choice of HPV DNA detection assays and processing protocols differed between the studies. Feng et al. [30] stored Dacron self-sampling brushes refrigerated for 6 month prior to shipment at ambient temperature and observed good agreement between wet and dry samples. Eperon et al., [38] stored the self-samples at ambient temperatures from 5 to 15 days. A study by Lin et al. [39] using cobas HPV test on physician taken dry samples and LBC samples stored for 2–28 days at room temperature found no differences [39]. However the study did not look at Ct values, so a direct comparison with our study is not possible. Compared to various Dacron swabs, the Evalyn brush is a different sampling device based upon electrostatically charged polypropylene filaments catching and holding on to the sample material. The Evalyn collection device is currently being implemented as the self-sampling device of choice in a number of screening programs, e.g. in the Dutch and the Danish programs. Whereas three studies have looked at the performance of the Evalyn brush in comparison with other self-sampling methods [26], [36], [40], this is the first report on the analytical stability of Evalyn collected self-samples.
In conclusion, a dry Evalyn self-sampling brush represents an analytically stable collection device with respect to human and HPV material from cervico-vaginal self-collected samples. Analytical stability in this study using biospecimens were observed for up to 32 weeks, and under various environmental conditions ranging from 4° to 30°C. Together, this is encouraging and has important implications for the implementation of HPV based cervical cancer screening using self-collected samples, as logistic and time sensitivity issues do not appear to be of an immediate concern within a reasonable time period after sample collection.
Acknowledgements
We thank BD diagnostics for supplying the reagents for the Onclarity HPV testing. We thank Axlab A/S for supplying the Rover Evalyn brushes for the study. We thank Janni Uyen Hoa Lam for technical support during the study.
J.B. and D.M.E. designed the study. D.M.E., G.P.A., C.P., and H.P. performed the laboratory work. D.M.E and J.B analyzed the data. D.M.E and J.B drafted the manuscript. All authors contributed to revisions of the manuscript, participated in the decision to submit, and had full access to all of the data in the study.
The funders of the study (see above) had the right to read the manuscript and comment upon it, but had no editorial right, nor had they any role in the final interpretation of the data.
Acknowledgments
Funding
BD diagnostics supplied the reagents for running the Onclarity HPV tests. Axlab A/S supplied the Rover Evalyn brushes for the study. No other funding was received. The manufacturer of the Evalyn brush, Rovers, Oss, The Netherlands, had no part in the study.
Declaring of competing interest
Ditte Møller Ejegod attended meetings with various HPV device manufacturers.
Helle Pedersen attended meetings with various HPV device manufacturers
Garazi Pena Alzua has no competing interest to declare
Camilla Pedersen has no competing interest to declare
Jesper Bonde Hansen attended meetings with various HPV device manufacturers. JB used to serve as a paid advisor to Roche and Genomica, and has received honoraria from Hologic/Gen-probe, Roche, Qiagen, Genomica, and BD Diagnostics for lectures.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2018.04.005.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Bulkmans N.W., Berkhof J., Rozendaal L., van Kemenade F.J., Boeke A.J., Bulk S., Voorhorst F.J., Verheijen R.H., van Groningen K., Boon M.E. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 2.Kitchener H.C., Almonte M., Gilham C., Dowie R., Stoykova B., Sargent A., Roberts C., Desai M., Peto J., Group A.T.S. ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening. Health Technol. Assess. 2009;13(51):1–150. doi: 10.3310/hta13510. (iii-iv) [DOI] [PubMed] [Google Scholar]
- 3.Leinonen M., Nieminen P., Kotaniemi-Talonen L., Malila N., Tarkkanen J., Laurila P., Anttila A. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J. Natl. Cancer Inst. 2009;101(23):1612–1623. doi: 10.1093/jnci/djp367. [DOI] [PubMed] [Google Scholar]
- 4.Mayrand M.H., Duarte-Franco E., Rodrigues I., Walter S.D., Hanley J., Ferenczy A., Ratnam S., Coutlee F., Franco E.L. Canadian Cervical Cancer Screening Trial Study G: human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N. Engl. J. Med. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 5.Naucler P., Ryd W., Tornberg S., Strand A., Wadell G., Elfgren K., Radberg T., Strander B., Johansson B., Forslund O. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N. Engl. J. Med. 2007;357(16):1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 6.Ronco G., Dillner J., Elfstrom K.M., Tunesi S., Snijders P.J., Arbyn M., Kitchener H., Segnan N., Gilham C., Giorgi-Rossi P. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383(9916):524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 7.Ronco G., Giubilato P., Naldoni C., Zorzi M., Anghinoni E., Scalisi A., Dalla Palma P., Zanier L., Barca A., Angeloni C. Extension of organised cervical cancer screening programmes in Italy and their process indicators: 2008 activity. Epidemiol. Prev. 2010;34(5–6Suppl 4):35–51. [PubMed] [Google Scholar]
- 8.Arbyn M., Verdoodt F., Snijders P.J., Verhoef V.M., Suonio E., Dillner L., Minozzi S., Bellisario C., Banzi R., Zhao F.H. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 9.Lam J.U., Rebolj M., Moller Ejegod D., Pedersen H., Rygaard C., Lynge E., Thirstrup Thomsen L., Kruger Kjaer S., Bonde J. Human papillomavirus self-sampling for screening nonattenders: opt-in pilot implementation with electronic communication platforms. Int. J. Cancer. 2017;140(10):2212–2219. doi: 10.1002/ijc.30647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snijders P.J., Verhoef V.M., Arbyn M., Ogilvie G., Minozzi S., Banzi R., van Kemenade F.J., Heideman D.A., Meijer C.J. High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int. J. Cancer. 2013;132(10):2223–2236. doi: 10.1002/ijc.27790. [DOI] [PubMed] [Google Scholar]
- 11.Enerly E., Bonde J., Schee K., Pedersen H., Lonnberg S., Nygard M. Self-sampling for human papillomavirus testing among non-attenders increases attendance to the norwegian cervical cancer screening programme. PLoS One. 2016;11(4):e0151978. doi: 10.1371/journal.pone.0151978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tranberg M., Bech B.H., Blaakaer J., Jensen J.S., Svanholm H., Andersen B. Preventing cervical cancer using HPV self-sampling: direct mailing of test-kits increases screening participation more than timely opt-in procedures - a randomized controlled trial. BMC Cancer. 2018;18(1):273. doi: 10.1186/s12885-018-4165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrossi S., Thouyaret L., Herrero R., Campanera A., Magdaleno A., Cuberli M., Barletta P., Laudi R., Orellana L. team EMAS: effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): a population-based cluster-randomised trial. Lancet Glob. Health. 2015;3(2):e85–e94. doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- 14.Lam J.U.H., Rebolj M., Ejegod D.M., Pedersen H., Rygaard C., Lynge E., Harder E., Thomsen L.T., Kjaer S.K., Bonde J. Prevalence of human papillomavirus in self-taken samples from screening non-attenders. J. Clin. Microbiol. 2017 doi: 10.1128/JCM.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao F.H., Lewkowitz A.K., Chen F., Lin M.J., Hu S.Y., Zhang X., Pan Q.J., Ma J.F., Niyazi M., Li C.Q. Pooled analysis of a self-sampling HPV DNA Test as a cervical cancer primary screening method. J. Natl. Cancer Inst. 2012;104(3):178–188. doi: 10.1093/jnci/djr532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Program) DKfLDQADfcCS: dansk Kvalitetsdatabase for Livmoderhalskræft. Dansk Kvalitetsdatabase for Livmoderhalskræft Årsrapport. 2014;2015(2015):45. [Google Scholar]
- 17.Dugue P.A., Lynge E., Bjerregaard B., Rebolj M. Non-participation in screening: the case of cervical cancer in Denmark. Prev. Med. 2012;54(3–4):266–269. doi: 10.1016/j.ypmed.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Nygard J.F., Nygard M., Skare G.B., Thoresen S.O. Screening histories of women with CIN 2/3 compared with women diagnosed with invasive cervical cancer: a retrospective analysis of the Norwegian Coordinated Cervical Cancer Screening Program. Cancer Causes Control. 2005;16(4):463–474. doi: 10.1007/s10552-004-6295-z. [DOI] [PubMed] [Google Scholar]
- 19.Andrae B., Kemetli L., Sparen P., Silfverdal L., Strander B., Ryd W., Dillner J., Tornberg S. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J. Natl. Cancer Inst. 2008;100(9):622–629. doi: 10.1093/jnci/djn099. [DOI] [PubMed] [Google Scholar]
- 20.Nelson E.J., Maynard B.R., Loux T., Fatla J., Gordon R., Arnold L.D. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex. Transm. Infect. 2017;93(1):56–61. doi: 10.1136/sextrans-2016-052609. [DOI] [PubMed] [Google Scholar]
- 21.Leinonen M.K., Campbell S., Klungsoyr O., Lonnberg S., Hansen B.T., Nygard M. Personal and provider level factors influence participation to cervical cancer screening: a retrospective register-based study of 1.3 million women in Norway. Prev. Med. 2017;94:31–39. doi: 10.1016/j.ypmed.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Waller J., Jackowska M., Marlow L., Wardle J. Exploring age differences in reasons for nonattendance for cervical screening: a qualitative study. BJOG. 2012;119(1):26–32. doi: 10.1111/j.1471-0528.2011.03030.x. [DOI] [PubMed] [Google Scholar]
- 23.Lam J.U.H., Elfstrom K.M., Ejegod D.M., Pedersen H., Rygaard C., Rebolj M., Lynge E., Juul K.E., Kjaer S.K., Dillner J. High-grade cervical intraepithelial neoplasia in human papillomavirus self-sampling of screening non-attenders. Br. J. Cancer. 2017 doi: 10.1038/bjc.2017.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ejegod D., Bottari F., Pedersen H., Sandri M.T., Bonde J. TheBD Onclarity HPV Assay on Samples Collected in SurePath Medium Meets the International Guidelines for Human Papillomavirus Test Requirements for Cervical Screening. J. Clin. Microbiol. 2016;54(9):2267–2272. doi: 10.1128/JCM.00508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejegod D., Junge J., Franzmann M., Kirschner B., Bottari F., Sideri M., Sandri M.T., Bonde J. Clinical and analytical performance of the BD Onclarity HPV assay for detection of CIN2+ lesions on SurePath samples. Papillomavirus Res. 2016;2:31–37. doi: 10.1016/j.pvr.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leinonen M.K., Schee K., Jonassen C.M., Lie A.K., Nystrand C.F., Rangberg A., Furre I.E., Johansson M.J., Trope A., Sjoborg K.D. Safety and acceptability of human papillomavirus testing of self-collected specimens: a methodologic study of the impact of collection devices and HPV assays on sensitivity for cervical cancer and high-grade lesions. J. Clin. Virol. 2017;99–100:22–30. doi: 10.1016/j.jcv.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Verdoodt F., Jentschke M., Hillemanns P., Racey C.S., Snijders P.J., Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur. J. Cancer. 2015;51(16):2375–2385. doi: 10.1016/j.ejca.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Allen-Leigh B., Uribe-Zuniga P., Leon-Maldonado L., Brown B.J., Lorincz A., Salmeron J., Lazcano-Ponce E. Barriers to HPV self-sampling and cytology among low-income indigenous women in rural areas of a middle-income setting: a qualitative study. BMC Cancer. 2017;17(1):734. doi: 10.1186/s12885-017-3723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbyn M., Castle P.E. Offering self-sampling kits for HPV testing to reach women who do not attend in the regular cervical cancer screening program. Cancer Epidemiol. Biomark. Prev. 2015;24(5):769–772. doi: 10.1158/1055-9965.EPI-14-1417. [DOI] [PubMed] [Google Scholar]
- 30.Feng Q., Cherne S., Winer R.L., Popov V., Zambrano H., Yerovi C., Hawes S.E., Koutsky L.A., Kiviat N.B. Evaluation of transported dry and wet cervical exfoliated samples for detection of human papillomavirus infection. J. Clin. Microbiol. 2010;48(9):3068–3072. doi: 10.1128/JCM.00736-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Thurah L., Bonde J., Lam J.U.H., Rebolj M. Concordant testing results between various human papillomavirus assays in primary cervical cancer screening: systematic review. Clin. Microbiol. Infect. 2018;24(1):29–36. doi: 10.1016/j.cmi.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Meijer C.J., Berkhof J., Castle P.E., Hesselink A.T., Franco E.L., Ronco G., Arbyn M., Bosch F.X., Cuzick J., Dillner J. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer. 2009;124(3):516–520. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam J.U.H., Rebolj M., Ejegod D.M., Pedersen H., Rygaard C., Lynge E., Harder E., Thomsen L.T., Kjaer S.K., Bonde J. Prevalence of human papillomavirus in self-taken samples from screening nonattenders. J. Clin. Microbiol. 2017;55(10):2913–2923. doi: 10.1128/JCM.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catarino R., Vassilakos P., Bilancioni A., Vanden Eynde M., Meyer-Hamme U., Menoud P.A., Guerry F., Petignat P. Randomized comparison of two vaginal self-sampling methods for human papillomavirus detection: dry swab versus FTA cartridge. PLoS One. 2015;10(12):e0143644. doi: 10.1371/journal.pone.0143644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerigo H., Coutlee F., Franco E.L., Brassard P. Dry self-sampling versus provider-sampling of cervicovaginal specimens for human papillomavirus detection in the Inuit population of Nunavik, Quebec. J. Med. Screen. 2012;19(1):42–48. doi: 10.1258/jms.2012.012011. [DOI] [PubMed] [Google Scholar]
- 36.Jentschke M., Chen K., Arbyn M., Hertel B., Noskowicz M., Soergel P., Hillemanns P. Direct comparison of two vaginal self-sampling devices for the detection of human papillomavirus infections. J. Clin. Virol. 2016;82:46–50. doi: 10.1016/j.jcv.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Wolfrum S.G., Koutsky L.A., Hughes J.P., Feng Q., Xi L.F., Shen Z., Winer R.L. Evaluation of dry and wet transport of at-home self-collected vaginal swabs for human papillomavirus testing. J. Med. Microbiol. 2012;61(Pt 11):1538–1545. doi: 10.1099/jmm.0.046110-0. [DOI] [PubMed] [Google Scholar]
- 38.Eperon I., Vassilakos P., Navarria I., Menoud P.A., Gauthier A., Pache J.C., Boulvain M., Untiet S., Petignat P. Randomized comparison of vaginal self-sampling by standard vs. dry swabs for human papillomavirus testing. BMC Cancer. 2013;13:353. doi: 10.1186/1471-2407-13-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin C.Q., Zeng X., Cui J.F., Liao G.D., Wu Z.N., Gao Q.Q., Zhang X., Yu X.Z., Chen W., Xi M.R. Stability study of cervical specimens collected by swab and stored dry followed by human papillomavirus DNA detection using the cobas 4800 test. J. Clin. Microbiol. 2017;55(2):568–573. doi: 10.1128/JCM.02025-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahomed K., Evans D., Sauls C., Richter K., Smith J., Firnhaber C. Human papillomavirus (HPV) testing on self-collected specimens: perceptions among HIV positive women attending rural and urban clinics in South Africa. Pan Afr. Med J. 2014;17:189. doi: 10.11604/pamj.2014.17.189.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material