Abstract
Once assumed only to be a waste product of anaerobe glycolytic activity, lactate is now recognized as an energy source in skeletal muscles. While lactate metabolism has been extensively studied in vivo, underlying cellular processes are poorly described. This study aimed to examine lactate metabolism in cultured human myotubes and to investigate effects of lactate exposure on metabolism of oleic acid and glucose. Lactic acid, fatty acid and glucose metabolism were studied in myotubes using [14C(U)]lactic acid, [14C]oleic acid and [14C(U)]glucose, respectively. Myotubes expressed both the MCT1, MCT2, MCT3 and MCT4 lactate transporters, and lactic acid was found to be a substrate for both glycogen synthesis and lipid storage. Pyruvate and palmitic acid inhibited lactic acid oxidation, whilst glucose and α-cyano-4-hydroxycinnamic acid inhibited lactic acid uptake. Acute addition of lactic acid inhibited glucose and oleic acid oxidation, whereas oleic acid uptake was increased. Pretreatment with lactic acid for 24 h did not affect glucose or oleic acid metabolism. By replacing glucose with lactic acid during the whole culturing period, glucose uptake and oxidation were increased by 2.8-fold and 3-fold, respectively, and oleic acid oxidation was increased 1.4-fold. Thus, lactic acid has an important role in energy metabolism of human myotubes.
Introduction
Lactate is produced from glucose through glycolysis and the conversion of pyruvate by lactate dehydrogenase (LDH). Once considered a waste product of anaerobic glycolysis, lactate is now recognized to be formed continuously also under fully aerobic conditions1. The main function of lactate is to serve as a precursor of hepatic gluconeogenesis, but also as an energy substrate for aerobic oxidation via the citric acid cycle (TCA cycle) in many peripheral tissues. In a recently published systematic analysis of the fluxes of circulating metabolites in mice, lactate was revealed as a primary source of carbon for the TCA cycle, demonstrating that the contribution of glucose to the TCA cycle in majority of tissues (except brain) occurs through circulating lactate2. The major organ of lactate production and utilization is skeletal muscle, especially during exercise. While substantial amount of research has been devoted to investigate lactate metabolism under various conditions in vivo3–6, underlying cellular processes are still not well described, and studies are mainly limited to cell lines7,8. Expanding knowledge on the cellular metabolism of lactate, as well as its impacts on fuel utilization of other energy substrates in skeletal muscle cells, is of importance, as these processes will affect whole-body energy homeostasis.
Depending on the level of contractile activity, energy consumption in skeletal muscles may vary considerably, with an estimated 1000-fold difference between rest and maximal contraction9. Energy required for cellular functions, including contraction, is provided by ATP hydrolysis to ADP and Pi. There are three main mechanisms to generate ATP in skeletal muscle; creatine kinase activity, glycolysis and mitochondrial oxidative phosphorylation. The different muscle fiber types differ in their metabolic preferences. Slow muscles (type I fibers) rely mainly on oxidative phosphorylation, whereas fast muscles (type II fibers) are primarily glycolytic. Plasma lactate concentrations increase rapidly during exercise, and the traditional idea has been that lactate is a waste product that is transported to the liver, kidneys or other organs for clearance1. However, it is now generally accepted that lactate is also taken up by the muscles and oxidized10. In fact, the contribution of muscles to total body lactate clearance is considerable during exercise10–12. Most lactate (75–80%) is disposed of immediately within the tissue or released for reuptake by working muscle13. Even in resting muscles, when partial pressure of oxygen is more than sufficient for oxidative phosphorylation to occur, concentrations of lactate and pyruvate are 1.0 mM and 0.1 mM, respectively14. After vigorous exercise, but still at oxygen pressure sufficient for maximal mitochondrial respiration, lactate to pyruvate concentration ratio may increase to 500 fold14. Thus, glycolysis proceeds to lactate production under both aerobic and anaerobic conditions, since equilibrium constant of LDH strongly favors lactate production14. Lower oxygen supply will inhibit oxidative phosphorylation, in which case production of lactate will exceed the rate of oxidative metabolism of pyruvate, resulting in higher lactate concentrations. A well-described effect of endurance exercise in skeletal muscles is increased mitochondrial content, which then serves as a larger sink for pyruvate. Increased mitochondrial oxidative activity requires lower levels of stimulators (ADP), and since some glycolytic enzymes are activated by the same stimulators, glycolysis will be reduced14. Thus, lactate is always formed in the process of glycolysis, but the amount produced and transported out of the cell is affected by several factors such as glycolysis rate, activity of oxidative enzymes, oxygen availability and activity of lactate transporters.
Lactate transport across the plasma membrane is mediated by proton-linked monocarboxylate transporters (MCTs), that belong to the SLC16 gene family15. The driving force for the lactate transport includes both trans-membrane concentration gradient and local proton availability. Of the 14 identified MCTs, MCTs 1–4 are the most characterized, playing important metabolic roles in most tissues, where MCT1 and MCT4 are the most important isoforms in skeletal muscles15. Detailed molecular mechanisms involved in the MCT regulation are still unclear, but probably include both transcriptional and post-transciptional regulations. Of importance is the upregulation of muscle MCT1 expression by exercise and MCT4 expression by hypoxia, implying important role of the latter transporter in cells relying on anaerobic glycolysis for energy production16. MCT1 has a higher affinity for lactate than MCT4, and is therefore thought to be more central in lactate uptake, whereas MCT4 probably is more suited for lactate extrusion17. MCT1 has been found predominantly in oxidative muscles, where it is required for lactate to enter the cells and be oxidized as an energy fuel15. Indeed, expression of MCT1 in skeletal muscles is strongly associated with oxidative capacity and mitochondrial content15. MCT4 is widely expressed, especially in tissues relying on glycolysis even when oxygen is available (aerobic glycolysis)15,18, where it mainly serves to export lactic acid. In skeletal muscle, MCT1 is upregulated by exercise and chronic stimulations, and well-trained subjects present higher MCT1 content than less trained subjects, which tend to express more MCT417. Furthermore, a 9-week exercise intervention on sedentary individuals increased mitochondrial MCT1 content of musculus vastus lateralis19. Protein contents of MCT4 have been reported to change only occasionally in response to exercise20–23. Hypoxia, on the other hand, is a potent stimulator of MCT4 (but not of MCT1 or MCT2), and this effect is mediated by hypoxia-inducible factor 1α (HIF-1α)16. Regulation of MCT4 expression under normoxic conditions is not clarified. MCT2 and MCT3 are less studied, but MCT2 is present in skeletal muscle and is primarily involved in import of lactate24. MCT3 is the least described of the four transporters, expression of which is confined to the retinal pigment epithelium and choroid plexus16. Higher abundance of MCT3 in fast-twitch glycolytic and fast-twitch oxidative than in slow-twitch muscle fibers has been reported25. As opposed to MCT2, MCT3 mainly facilitates efflux of lactate25.
Energy substrate preference in skeletal muscle is variable, and during the fed state, increased availability of plasma glucose stimulates glucose oxidation and fatty acid synthesis, whereas fatty acid oxidation increases both during fasting and sustained exercise26,27. However, when exercise intensity increases, the fuel preference shifts from fatty acid to glucose metabolism28,29. The ability to switch between energy substrates is thought to be a characteristic of healthy human myotubes. Adaptability refers to the cells ability to increase fatty acid oxidation with an increase in fatty acid availability. Suppressibility refers to the suppressive effect acutely added glucose has on the oxidation of fatty acids. Accordingly, metabolic inflexibility is defined as loss of ability to readily switch from fatty acid oxidation during fasting to glucose oxidation in the postprandial state30, and has also been associated with obesity and type 2 diabetes30,31. It has been shown that metabolic switching might be an intrinsic characteristic of human skeletal muscle cells32, and we have shown in other studies that metabolic switching of human myotubes could be changed by altering the extracellular milieu33–36. Role of lactate in metabolic switching of skeletal muscle cells has, to our knowledge, not been described.
The purpose of the present work was to study lactate metabolism in cultured human myotubes. The ability of human myotubes to use lactate as an energy source, but also as a substrate for storage as glycogen and intracellular lipids, was investigated. Additionally, effects of acute and chronic lactate exposure on the metabolism of the two other main energy substrates in human skeletal muscle cells, oleic acid and glucose, were studied.
Results
Lactate as an energy substrate for myotubes
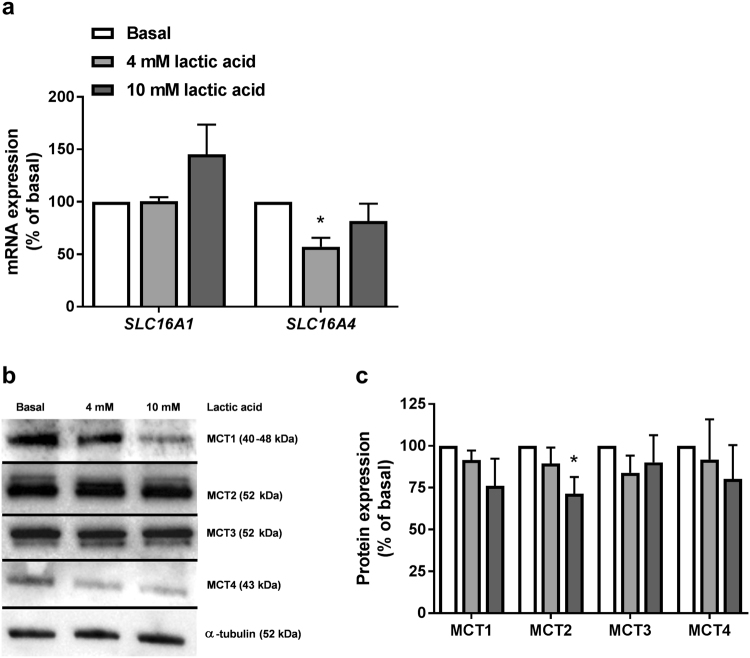
Extracellularly added lactate must be transported into muscle cells in order to function as an energy substrate. Expression levels of the two main lactic acid transporters in skeletal muscles, MCT1 (SLC16A1) and MCT4 (SLC16A4), were studied in fully differentiated myotubes grown in standard differentiation medium (5.5 mM glucose) at both mRNA (Fig. 1a) and protein level (Fig. 1b,c). Two other transporters, MCT2 and MCT3, were studied at protein level (Fig. 1b,c). mRNA expression level of MCT4 (SLC16A4) was reduced in cells treated with 4 mM lactic acid (Fig. 1a), while protein levels of other MCTs were unaffected after 24 h of lactic acid pretreatment, except for MCT2, which was reduced in cells treated with 10 mM lactic acid (Fig. 1b,c).
Figure 1.
mRNA and protein expression of lactate transporters. Satellite cells were cultured and differentiated to myotubes. They were kept in normal differentiation medium (basal, 5.5 mM glucose) or exposed to lactic acid (4 mM or 10 mM replacing glucose) for 24 h before total cell RNA and protein content were isolated. (a) mRNA expressions of SLC16A1 (MCT1) and SLC16A4 (MCT4). All values were corrected for the housekeeping gene acidic ribosomal phosphoprotein P0 (RPLP0), and presented as means ± SEM relative to basal (n = 5). (b,c) Protein expressions of MCT1–4. (b) One representative immunoblot. (c) Quantified immunoblots relative to basal. All values were corrected for the housekeeping control α-tubulin, and data are presented as means ± SEM relative to basal (n = 5). Dividing lines delineate blots from different gels. The samples derive from the same experiment, and all blots were processed in parallel. Full-length blots are presented in Supplementary Fig. 1. *Statistically significant vs. basal (p < 0.05, paired Student’s t-test).
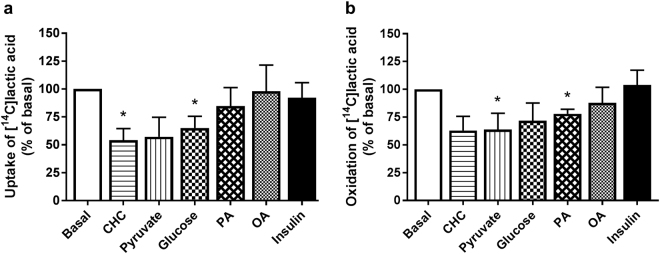
Further, lactate metabolism was studied by incubating myotubes with [14C]lactic acid (1 µCi/ml, 100 µM) for 4 h (Fig. 2). Lactic acid was clearly taken up by the cells (11.7 ± 5.3 nmol/mg protein) (Fig. 2a), and 45 ± 4% (5.3 ± 2.6 nmol/mg protein) of the amount taken up was completely oxidized to CO2 (Fig. 2b). Insulin (100 nM) did not stimulate lactic acid uptake or oxidation (Fig. 2a,b, respectively), and cytochalasin B (10 µM) did not inhibit lactic acid uptake (data not shown). However, uptake of lactic acid was reduced in the presence of 5 mM glucose (Fig. 2a), and oxidation was reduced by acute addition of 5 mM pyruvate (Fig. 2b). Palmitic acid (PA, 100 µM) also reduced oxidation of lactic acid (Fig. 2b), whereas oleic acid (OA, 100 µM) had no effect on either uptake or oxidation (Fig. 2a,b, respectively). The monocarboxylate inhibitor α-cyano-4-hydroxycinnamic acid (CHC, 1 µg/ml) inhibited lactic acid uptake to 54 ± 10% of basal uptake (Fig. 2a).
Figure 2.
Lactate metabolism in human myotubes and effects of insulin, other energy substrates and an inhibitor of monocarboxylate transporters. Lactic acid metabolism in human myotubes and the influence of acutely added insulin (100 nM), glucose (5 mM), pyruvate (5 mM), oleic acid (OA, 100 µM), palmitic acid (PA, 100 µM) or α-cyano-4-hydroxycinnamic acid (CHC, 1 µg/ml). Myotubes were incubated with [14C(U)]lactic acid (1 µCi/ml, 100 µM) for 4 h. (a) Uptake of lactic acid was assessed as the sum of oxidized [14C]lactic acid (CO2) and remaining cell-associated radioactivity (CA) radioactivity. Basal absolute value representing 100% (mean ± SEM): 11.7 ± 5.3 nmol/mg protein. (b) Oxidized [14C]lactic acid (CO2) was trapped in a filter and counted by liquid scintillation. Basal absolute value representing 100% (mean ± SEM): 5.3 ± 2.6 nmol/mg protein. All data are presented as means ± SEM, relative to basal (n = 4–6). *Statistically significant vs. basal (p < 0.05, paired Student’s t-test).
In addition to be taken up and oxidized, lactic acid was found to be a substrate for glycogen synthesis in human myotubes (Fig. 3), and insulin increased incorporation of lactic acid into glycogen in four out of six cell donors (Fig. 3).
Figure 3.
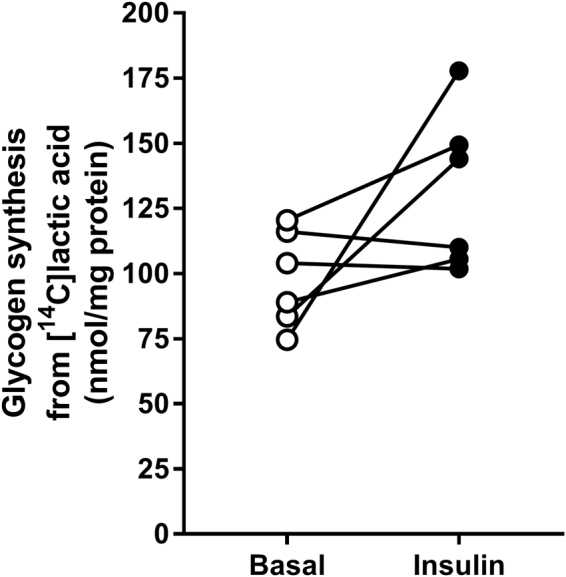
Glycogen synthesis from [14C]lactic acid. Myotubes were incubated with [14C(U)]lactic acid (1 µCi/ml, 20 mM) the last 24 h of the differentiation period. Glycogen synthesis was measured as incorporation of [14C(U)]lactic acid into glycogen in the presence or absence of 100 nM insulin for 3 h. All values are presented as means ± SEM (n = 6). *Statistically significant vs. basal (p < 0.05, paired Student’s t-test).
Lactic acid was also incorporated into cellular lipids (Fig. 4), with a total lipid content of 57.9 ± 29.5 nmol/mg protein. Incorporation into phospholipids (PL) constituted approximately 70% of total lipid content (Fig. 4). Diacylglycerol (DAG) and triacylglycerol (TAG) constituted approximately 10% and 20% of the total lipid content, respectively (Fig. 4), whereas both free fatty acids (FFA) and cholesteryl ester (CE) constituted approximately 2% of the total lipid content (Fig. 4). After hydrolysis of TAG and DAG, 90% of the incorporated lactic acid was recovered in the aqueous (glycerol) phase, whereas 10% was recovered in the organic (fatty acids) phase (data not shown).
Figure 4.
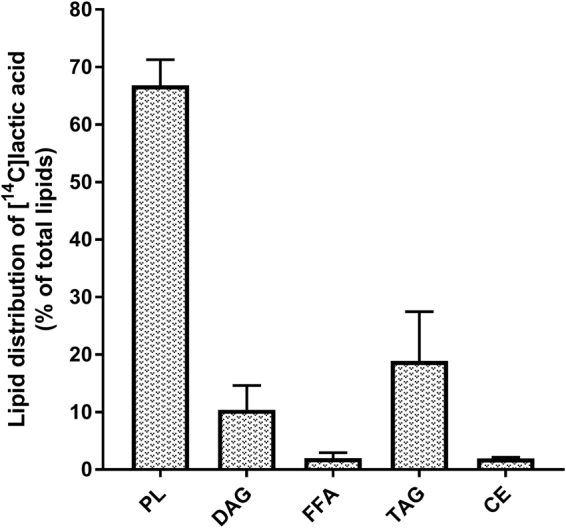
Incorporation of lactic acid into cellular lipids. Myotubes were incubated with [14C(U)]lactic acid (1 µCi/ml, 20 mM) for the last 24 h of the differentiation period before total cell lipids were extracted and separated by thin layer chromatography. Incorporation into cellular lipids calculated as % of total lipid content (57.9 ± 29.5 nmol/mg protein). Data are presented as means ± SEM (n = 3). PL, phospholipids; DAG, diacylglycerol; FFA, free fatty acids; TAG, triacylglycerol; CE, cholesteryl ester. *Statistically significant vs. basal (p < 0.05, paired Student’s t-test).
Effects of lactate on the metabolism of glucose and oleic acid
Acute effects of lactate exposure
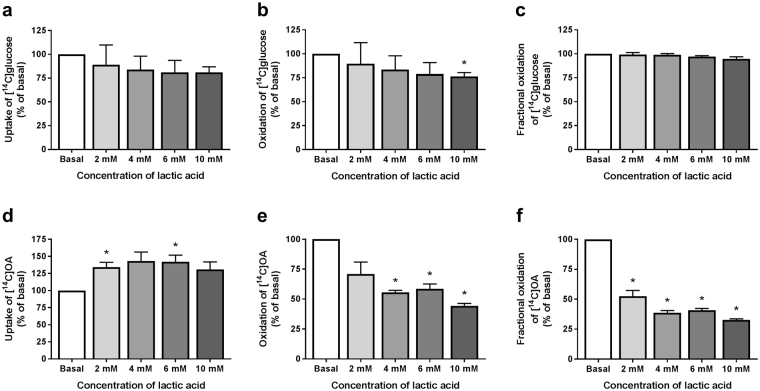
Acute addition of lactic acid (4 h) did not affect glucose uptake (Fig. 5a), whereas glucose oxidation was decreased when lactic acid was added at the highest concentration (10 mM, Fig. 5b). However, acutely added lactic acid had no effect on fractional glucose oxidation (Fig. 5c). Lactic acid increased oleic acid uptake (Fig. 5d), whereas oleic acid oxidation was markedly reduced (Fig. 5e), causing reduced fractional oleic acid oxidation at all concentrations (Fig. 5f).
Figure 5.
Glucose and oleic acid metabolism after acute addition of lactic acid at different concentrations. Glucose and oleic acid metabolism were studied using [14C(U)]glucose (1 µCi/ml, 200 µM) and [14C]oleic acid (OA, 1 µCi/ml, 100 µM) for 4 h, respectively, in presence or absence (basal) of lactic acid (2, 4, 6, or 10 mM). Uptake was assessed as the sum of oxidized lactic acid (trapped CO2) and remaining cell-associated (CA) radioactivity. Oxidation was measured as CO2 trapped in a filter and counted by liquid scintillation. Fractional oxidation was calculated as CO2/(CO2 + CA). (a) Uptake of glucose. Basal absolute value representing 100% (mean ± SEM): 120.9 ± 44.3 nmol/mg protein. (b) Oxidation of glucose. Basal absolute value representing 100% (mean ± SEM): 95.9 ± 39.5 nmol/mg protein. (c) Fractional oxidation of glucose. (d) Uptake of oleic acid (OA). Basal absolute value representing 100% (mean ± SEM): 164.7 ± 47.1 nmol/mg protein. (e) Oxidation of OA. Basal absolute value representing 100% (mean ± SEM): 38.3 ± 14.0 nmol/mg protein. All data are presented as means ± SEM relative to basal (n = 3). *Statistically significant vs. basal (p < 0.05, paired Student’s t-test).
Chronic effects of lactate exposure
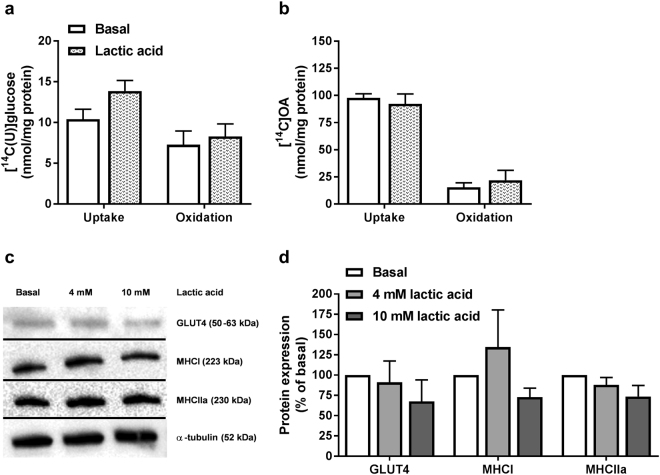
Chronic exposure to lactic acid (5 mM for 24 h), compared to chronic exposure to the same concentration of glucose, revealed a tendency towards enhanced glucose uptake (Fig. 6a), whereas oleic acid metabolism was unaffected (Fig. 6b). Furthermore, protein expressions of the glucose transporter GLUT4, as well as the fiber type markers MHCI (type I fibers) and MHCIIa (type II fibers) were unaffected by lactic acid (4 mM or 10 mM) exposure for 24 h (Fig. 6c,d).
Figure 6.
Glucose and oleic acid metabolism, protein expressions of GLUT4, MHCI and MHCIIa after incubation with lactic acid for 24 h. Myotubes were exposed to 5 mM lactic acid or standard glucose containing media (basal, 5.5 mM) for the last 24 h of the differentiation period. (a) Uptake and oxidation of [14C(U)]glucose (1 µCi/ml, 200 µM) over 4 h. (b) Uptake and oxidation of [14C]oleic acid (OA, 1 µCi/ml, 100 µM) over 4 h. Oxidation was measured as CO2 trapped in a filter and counted by liquid scintillation. Uptake was assessed as the sum of oxidized (trapped CO2) glucose or oleic acid and remaining cell-associated (CA) radioactivity. Data are presented as means ± SEM relative to basal (n = 3). (c,d) Protein expressions of GLUT4, MHCI and MHCIIa assessed by immunoblotting. (c) One representative immunoblot. (d) Quantified immunoblots relative to basal. All values were corrected for the housekeeping control α-tubulin, and data are presented as means ± SEM relative to basal (n = 5). Dividing lines delineate blots from different gels. The samples derive from the same experiment, and all blots were processed in parallell. Full-length blots are presented in Supplementary Fig. 6.
Effects of prolonged lactate exposure
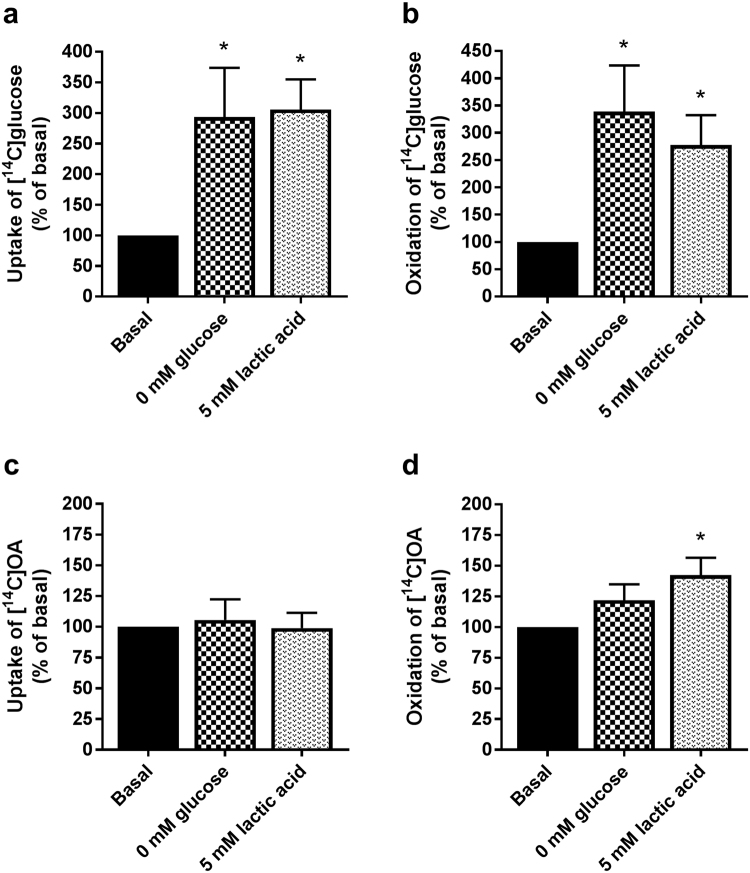
When increasing the exposure time by replacing glucose with lactic acid during the whole proliferation and differentiation period; both glucose uptake (Fig. 7a) and oxidation (Fig. 7b) were increased. Oleic acid uptake (Fig. 7c) was not affected, but oxidation was enhanced (Fig. 7d). The effect of lactic acid was comparable to removing glucose completely (0 mM glucose, Fig. 7). Replacing glucose with lactic acid only during the differentiation period (7 days) did not affect oleic acid metabolism (data not shown).
Figure 7.
Glucose and oleic acid metabolism after culturing myotubes in cell media where glucose was replaced by lactic acid or removed. Myotubes were cultured in regular cell media (basal, 5.5 mM glucose), cell media containing no glucose (0 mM glucose) or cell media where glucose were replaced with lactic acid (5 mM) for the whole proliferation and differentiation period. Oxidation was measured as CO2 trapped in a filter and counted by liquid scintillation. Uptake was assessed as the sum of oxidized (trapped CO2) lactic acid and remaining cell-associated (CA) radioactivity. (a,b) Uptake and oxidation of [14C(U)]glucose (1 µCi/ml, 200 µM). (c,d) Uptake and oxidation of [14C]oleic acid (OA, 1 µCi/ml, 100 µM). Absolute values (means ± SEM) representing 100%: glucose uptake; 16.7 ± 2.6 nmol/mg protein, glucose oxidation; 8.3 ± 0.8 nmol/mg protein, oleic acid (OA) uptake; 77.8 ± 12.9 nmol/mg protein and oleic acid (OA) oxidation; 7.1 ± 1.8 nmol/mg protein. Data are presented as means ± SEM relative to basal (n = 11). *Statistically significant vs. basal (p < 0.05, paired Student’s t-test).
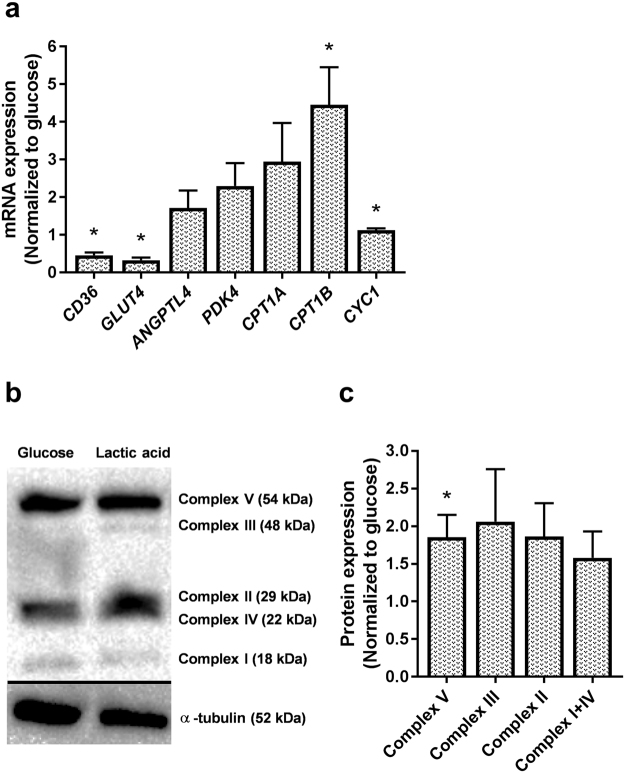
To further investigate underlying mechanisms in conditions where glucose was replaced by lactic acid for the entire culturing period, we studied mRNA and protein expressions of several factors involved in metabolic processes in skeletal muscles (Fig. 8). Compared to the cells grown in glucose-containing media, mRNA expressions of the transporters of glucose and fatty acids, solute carrier family 2 member 4, GLUT4 and CD36, respectively, were decreased in cells cultured in lactate-containing media (Fig. 8a). On the other hand, carnitine palmitoyl transferase 1B (CPT1B) and cytochrome c1 (CYC1), involved in the transport of fatty acids across the mitochondrial membrane and mitochondrial function, respectively, were increased in lactate-cultured cells (Fig. 8a). mRNA expression levels of ANGPTL4, a potent inhibitor of lipoprotein lipase activity induced in the fasting state by peroxisome proliferator activated receptors (PPARs)37, CPT1A and pyruvate dehydrogenase kinase 4 (PDK4), an inhibitor of the pyruvate dehydrogenase complex, were not changed in cells cultured in the presence of lactate (Fig. 8a). Protein expression of the electron transport chain’s complex V (ATP synthase subunit α) was also increased in the presence of lactic acid (Fig. 8b).
Figure 8.
mRNA and protein expressions of factors involved in regulation of metabolism in myotubes cultured in a lactic acid-containing medium. Myotubes were cultured in standard cell media (basal, 5.5 mM glucose) or cell media where glucose had been replaced with lactic acid (5 mM) for the whole proliferation and differentiation period. After ended differentiation, total cell RNA and protein contents were isolated. (a) mRNA expressions of CD36 molecule (CD36), solute carrier family 2 member 4 (GLUT4), angiopoietin like 4 (ANGPTL4), pyruvate dehydrogenase kinase 4 (PDK4), carnitine palmitoyl transferase 1 (CPT1A and B) and cytochrome c1 (CYC1). All values were corrected for the housekeeping control RPLP0, and presented as means ± SEM relative to basal (n = 5). (b,c) Expressions of proteins involved in mitochondrial phosphorylation, OXPHOS (complex I subunit NDUFB8, complex II subunit, complex III subunit core 2, complex IV subunit II and complex V (ATP synthase subunit α)). (b) One representative immunoblot. (c) Quantified immunoblots normalized to glucose. All values were corrected for the housekeeping control α-tubulin, normalized to glucose, and data are presented as means ± SEM relative to basal (n = 5). Dividing lines delineate blots from different gels. The samples derive from the same experiment, and all blots were processed in parallell. Full-length blots are presented in Supplementary Fig. 8. *Statistically significant vs. basal (p < 0.05, paired Student’s t-test).
Despite the observed changes in the metabolism of both glucose and fatty acids, the metabolic flexibility of myotubes grown and differentiated in lactic acid-containing media did not differ from cells grown in standard glucose-containing medium, assessed as glucose suppression of oleic acid oxidation (suppressibility) and the ability to increase oleic acid oxidation with increasing oleic acid concentration (adaptability) (data not shown).
Discussion
The aim of the present study was to explore the ability of cultured human myotubes to utilize lactate as a fuel source, but also to investigate effects of lactate on the metabolism of the two other main energy substrates, glucose and oleic acid. Lactate is no longer considered as only a waste product of glycolysis due to hypoxia, but also an important factor in cellular and whole body metabolism. Glucose and fatty acids are the two most important fuel sources of skeletal muscle. Moreover, skeletal muscle is the largest producer of lactate. Thus, shedding light on the impact of lactate on cellular metabolism of glucose and fatty acids in skeletal muscle cells is of particular interest for understanding whole-body energy balance, and has, to our knowledge, not been thoroughly explored. Our main finding was that isolated human skeletal muscle cells have the ability to metabolize lactate in terms of uptake, oxidation and storage not only in form of glycogen, but also as intracellular lipids. Furthermore, prolonged exposure to lactic acid increased glucose metabolism and oleic acid oxidation. These findings were accompanied by increased mRNA expression levels of CPT1B and CYC1, as well as higher protein expression of complex V (ATP synthase subunit α) of the mitochondrial respiratory chain, suggesting an overall improvement in mitochondrial function of myotubes exposed to lactic acid for an extended period.
The ability of the cells to take up extracellularly added lactate was first verified by showing expression of the monocarboxylate transporters responsible for lactate transport in skeletal muscles, MCT1 and MCT4, both at mRNA and protein level, and MCT2 and MCT3 at protein level. It has recently been shown in C2C12 cells that 16 mM lactate induced an increase in mRNA expression levels of both SLC16A1 (MCT1) and SLC16A4 (MCT4), but with different times of peak induction, suggesting different dynamics in the expression of the two transporters upon lactate stimulation38. We observed lower mRNA expression of SLC16A4 in cells pretreated with 4 mM lactic acid for 24 h, but not with 10 mM lactic acid. Lactate concentrations may reach 10–20 mM in the circulation39,40, or even 40 mM in the skeletal muscle after intense anaerobic exercise in humans, which has been shown to increase expression of both lactate transporters41. In another in vivo study, a progressive decrease in mRNA expression levels of both MCT1 and MCT4 over the period of 9–72 h after a single bout of exercise was reported42. Interestingly, in the same study, protein content of MCT1 was higher after 24–72 h compared to 9 h post-exercise, while MCT4 remained unchanged42, suggesting post-transcriptional modifications. Proteins and mRNA expressions may have distinct kinetics, which emphasizes importance of time points when taking biopsies post-exercise or cell-harvesting to interpret adaptive responses. Our finding of decreased mRNA level of MCT4 at 4 mM of lactic acid is supportive to in vivo results, and in the present study, both protein and RNA isolation was performed immediately after ended lactic acid treatment. The fact that the observed decrease in mRNA expression of SLC16A4 at 4 mM of lactic acid was not seen when concentration was increased to 10 mM is somewhat unexpected. However, it has been shown that MCT4 is upregulated in vitro by hypoxia-inducible factor-1α pathway, and hypoxia during exercise has been suggested to induce transient mRNA bursts of MCT4, followed by subsequent reduction in mRNA contents43. Thus, discrepancies between mRNA and protein levels, as well as inconsistencies regarding effects of exercise in vivo studies have been reported earlier, suggesting complex dynamics and post-transcriptional modifications that are yet to be described. The concentrations used in this study were in the range 2–10 mM, and at these conditions, no differences in mRNA expression of SLC16A1 or protein expressions of MCT1 or MCT4 were observed. As mentioned in the introduction, MCT1 has predominantly been found in oxidative fibers, whereas MCT4 is more abundant in glycolytic fibers19,44. Cultured human myotubes are generally limited with regards to their reflection of the fiber type of the muscle they are isolated from, and tend to mainly express fiber type markers of fast, glycolytic fibers45. It has also been shown that peroxisome proliferator activated receptor gamma coactivator −1α (PGC-1α) is a strong inducer of both MCT1 (but not MCT2 and MCT4) expression and uptake of lactic acid in muscles46. Expression of PGC-1α, which is a contraction-induced regulator of oxidative mitochondrial function, is reduced in cultured human myotubes47. Low expression of PGC-1α could partially explain lack of increase in protein expression of MCT1 in our study. We further found that both MCT2 and MCT3 were expressed in cultured human myotubes. Interestingly, protein level of MCT2 was reduced in myotubes exposed to the highest concentration of lactic acid (10 mM). Whether MCT2 adapts to chronically increased muscle activity is still not defined, although it was reported in one study that it is not regulated by PGC-1α, nor does it change in response to contractions46. MCT2 is expressed in skeletal muscles and it is primarily involved in import of lactic acid, with a higher affinity for both lactic acid and pyruvate than MCT146. This makes it a suitable transporter in tissues taking up large amounts of lactic acid as a fuel. In spite of that, expression of MCT2 does not appear to correlate to oxidative capacity of different muscles, nor is it more abundant in oxidative muscles, in contrast to MCT146. Mechanisms of regulation of MCT2 are also not clarified. MCT3, which is even less described, was also expressed in myotubes in the present study, but protein expression of this transport was unaffected by different concentrations of lactic acid. Higher abundance of MCT3 in fast-twitch glycolytic and fast-twitch oxidative than in slow-twitch muscle fibers has been reported, and it appears to be involved in the efflux of lactic acid25. It is important to keep in mind that in vivo, most muscles contain a mixture of both primarily glycolytic and primarily oxidative muscle fibers, and the proportion of each fiber type will depend on many factors from genetics to whether that particular muscle uses high-intensity exercise (glycolytic) or endurance exercise (oxidative). Thus, both expression and regulation of the MCTs is probably tightly associated with the particular muscle and its metabolic activity.
The fact that all four transporters were expressed in the cells suggests that cultured myotubes should be able to transport lactate across membranes. This was confirmed by flux studies of labelled lactate, which was shown to be taken up by the cells, and approximately 45% was further oxidized to CO2. This is in accordance with the in vivo findings, where it has been shown that muscle fibers can take up lactate for subsequent metabolic use of intramuscular lactate48,49. Oxidative muscle fibers predominantly oxidize lactate, whereas glycolytic fibers primarily convert lactate to glycogen50–53. A probable pathway of intracellular lactate disposal involves conversion to pyruvate followed by entry into the Krebs cycle54. We further show that, in addition to being oxidized, lactate can be incorporated and stored as glycogen as well. This is in accordance with in vivo studies; glyconeogenesis from lactate has been observed in human muscles55. Studies on rat muscle showed both glyceroneogenesis and glyconeogenesis from lactate, and reverse flux of pyruvate to phosphoenolpyruvate was found to be the common route56. Indeed, during exercise, lactate is by far the most important gluconeogenic precursor in humans, as it is during fasting57.
Further, we showed that lactate can be incorporated in complex intracellular lipids. Chen et al. showed that lactate was metabolized to lipids in cultured HeLa cells and H460 human lung cancer cells58, and Jin et al. showed in studies on rat muscle that lactic acid could be converted to glycerol56. It has also been reported that lactate, at physiological concentrations, can induce triglyceride accumulation in adipocytes of both humans, mice and rats59, and recently, a dose-dependent lactate-induced induction of triglyceride content was demonstrated in C2C12 cells at concentrations of 16 mM and 20 mM38. As far as we know, incorporation of lactate to lipids has never before been observed in human skeletal muscle cells. While the majority of lactic acid was incorporated into phospholipids in the present study, partition to TAG was also substantial. Of the lactic acid incorporated in DAG and TAG, 90% was recovered in the aqueous (glycerol) phase, whereas 10% was recovered in the organic (fatty acid) phase.
We further found that pyruvate decreased both uptake and oxidation of lactic acid, as would be expected of a pyruvate metabolite. Glucose was also found to decrease both uptake and oxidation of lactate, which is in line with reports of human myotubes being highly glycolytic when grown in presence of glucose60, in addition to having low mitochondrial oxidative capacity resembling fast (glycolytic) muscle fibers61. Cultured human myotubes’ reliance on glucose as favorable fuel is also demonstrated by our observations that higher concentrations of acutely added lactate were necessary to suppress glucose oxidation than oleic acid oxidation (10 mM vs. 4 mM, respectively). Palmitic acid also caused a decrease in lactate oxidation. The monocarboxylic acid transport inhibitor CHC caused, as expected, a decrease in lactate uptake. Insulin had no effect on lactate metabolism and cytochalasin B did not inhibit lactate uptake, implying an uptake mechanism independent of translocation of transporters.
Acutely added lactate (4 h) suppressed both glucose and oleic acid metabolism, which is in agreement with a previous study where increased plasma lactate was associated with a decline in plasma FFAs, an anti-lipolytic effect on adipose tissue, and an inhibitory effect on muscle fat oxidation54. Chronic treatment (24 h) with 5 mM lactic acid, on the other hand, had no effect on either glucose or oleic acid metabolism; neither did it affect expression of the glucose transporter GLUT4. In these cells, glucose in the culturing media was replaced with the same concentration of lactate (5 mM) for a period of 24 h prior to harvesting of the cells, but not during the experiment. Therefore, possible explanations for the lack of effects may be too short pretreatment and absence of lactate during the 4 h course of experiment.
To increase exposure time to lactate, cells were exposed to either lactate or glucose for the entire culturing period. At these conditions, both glucose uptake and oxidation were increased, whereas oleic acid oxidation was increased without a corresponding effect on uptake. Thus, by replacing glucose with lactate for the entire culturing period, we induced an increase in the oxidative metabolism of the cells, which was mainly reflected as an increase in glucose metabolism. Based on these results, one may suggest that lactate forces the cells towards a state of energy deprivation and thereby increases the oxidative capacity of the cells, as metabolism levels were fairly similar as after culturing in media containing no glucose.
To shed light on the underlying mechanisms of the observed effects, we studied protein and mRNA expression levels of several metabolically important factors. Compared to the cells grown in glucose-containing media, mRNA expressions of the transporters of glucose and fatty acids, GLUT4 and CD36, respectively, were decreased in cells cultured in presence of lactic acid. On the other hand, expressions of two important markers of mitochondrial function and fatty acid oxidation, CYC1 and CPT1B, respectively, were both increased after prolonged exposure to lactate, as was protein expression of complex V (ATP synthase subunit α) of the mitochondrial respiratory chain. These data generally support functional results and suggest an increased transport of the fatty acids taken up across the inner mitochondrial membrane, an improved mitochondrial function and establishment of a more oxidative cell model in general. However, decreased level of GLUT4 was in contrast to the observed increase in glucose uptake, but this observation can probably be explained by generally low GLUT4 expression in skeletal muscle cells in vitro62, and the fact that basal glucose uptake can mainly be mediated by other glucose transporters (GLUT1 and 3) in primary human myotubes63,64. Discrepancies between GLUT4 levels and glucose uptake have also been reported previously47.
We have previously observed a remodeling of oxidative energy metabolism by replacing glucose with galactose as carbohydrate source during growth and differentiation65. In general, myotubes became more oxidative and seemed to utilize glucose better than oleic acid, which we suggested implied an improved metabolic switching65. In the present study, however, none of the parameters of metabolic switching were affected in cells that had been cultured under conditions where glucose was replaced by lactate. Thus, although replacing glucose with lactate had a positive effect on both oleic acid and glucose oxidation, the metabolic flexibility of the cells was unaffected.
In conclusion, cultured human skeletal muscle cells are able to take up, oxidize and store lactate in complex lipids and glycogen. Further, we have found that lactate exposure affects the metabolism of glucose and oleic acid in cultured human myotubes, and this impact appears to be related to the duration of exposure to lactate. These findings suggest an important role of lactate in energy metabolism of human skeletal muscle cells.
Materials and methods
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM-Glutamax™) low glucose, DMEM without glucose, fetal bovine serum (FBS), sodium pyruvate, Dulbecco’s Phosphate Buffered Saline (DPBS) without Mg2+ and Ca2+, penicillin/streptomycin (10000 IE/ml), amphotericin B, and trypsin-EDTA were from Gibco Invitrogen (Gibco, Life Technologies, Paisley, UK). Ultroser G was from Pall (Cergy-Saint-Christophe, France) and insulin (Actrapid® Penfill® 100 IE/ml) was from Novo Nordisk (Bagsvaerd, Denmark). SkBM-kit (SkGM) and BioWhittaker® Phosphate Buffered Saline (PBS) were from Lonza (Wakersville, MD, US). VWR® Grade 703 Blotting Paper was from VWR (Poole, UK). Bovine serum albumin (BSA, essentially fatty acid free), L-carnitine, D-glucose, oleic acid (OA, 18:1, n-9), palmitic acid (PA, 16:0), sodium lactic acid, trypan blue 0.4% solution, HEPES, DMSO, L-glutamine, gentamicin, cytochalasin B, α-cyano-4-hydroxycinnamate (CHC), protease inhibitor, phosphatase II inhibitor, β-mercaptoethanol, and Ponceau S solution were from Sigma-Aldrich (St.Louis, MO, US). D-[14C(U)]glucose (2.9 mCi/mmol), [1-14C]oleic acid (53 mCi/mmol and 56.3 mCi/mmol) and L-[14C(U)]lactic acid (118.4 mCi/mmol and 150.6 mCi/mmol) were provided by PerkinElmer® (Boston, MA, US). [14C(U)]glucose (10 mCi/mmol) was from American Radiolabeled Chemicals, Inc. (St. Louis, MO, US). 96-well Corning® CellBIND® tissue culture plates were from (Schiphol-Rijk, the Netherlands). Unifilter®−96 GF/B, 96-well Isoplate®, OptiPhase Supermix, and TopSeal®-A transparent film were from PerkinElmer (Shelton, CT, US). Nunc™ Cell Culture Treated Flasks with Filter Caps, Nunc™ 96-MicroWell™ plates, TaqMan reverse transcription kit reagents, High-Capacity cDNA Reverse Transcription Kit, primers for TaqMan PCR, MicroAmp® Optical 96-well Reaction Plate, MicroAmp® Optical Adhesive Film, and Power SYBR® Green PCR Master Mix were obtained from ThermoFisher Scientific (Roskilde, Denmark). Glycerol, Tris-HCl and thin layer chromatography plates were from Merck (Darmstadt, Germany). Amersham™ Protran™ Premium 0.45 µm NC Nitrocellulose Blotting Membrane was from Amersham™ (GE Healthcare, Esbjerg, Denmark). Bio Rad Protein Assay Dye Reagent Concentrate, Clarity™ Western ECL Substrate, Tris/glycine buffer, Tris/glycine/SDS buffer, SDS, Tween 20, brom-phenyl blue, Goat Anti-Rabbit IgG (H + L)-HRP Conjugate (170–6515) secondary antibody, Goat Anti-Mouse IgG (H + L)-HRP Conjugate (170–6516) secondary antibody, and Mini-Protean® TGX™ gels (4–20%) were from Bio-Rad (Copenhagen, Denmark). The antibodies against GLUT4 (sc-53566)66, MCT1 (H-1) (sc-365501)67 and MCT4 (G-7)68 (sc-376465) were from Santa Cruz Biotechnology (Santa Cruz, CA, US). The antibody against MHCI (MAB1628)69 was from Millipore (Temecula, CA, US). The human total OXPHOS antibody (ab110411)65 and antibodies against MCT2 (ab129290)70 and SLC16A8 (ab60333)70 were from Abcam (Cambridge, UK). The antibodies against MHCIIa (3403)71 and α-tubulin (2144)72 were from Cell Signaling Technology Inc. (Beverly, MA, US). QIAshredder and RNeasy Plus Mini Kit were from QIAGEN (Venlo, the Netherlands).
Ethics statement
The biopsies were obtained with informed written consent and approval by the Regional Committee for Medical and Health Research Ethics South East, Oslo, Norway (reference number: S-04133). The study adhered to the Declaration of Helsinki.
Human skeletal muscle cell cultures
Satellite cells were isolated as previously described73 from the musculus obliquus internus abdominis of lean, healthy volunteers. Donors were 40.3 (±3.7) years old, body mass index 23.6 (±0.8) kg/m2, fasting glucose 5.1 (±0.2) mM, insulin, plasma lipids and blood pressure within normal range and no family history of diabetes. The cells were cultured in DMEM-Glutamax (5.5 mM glucose) with 2% FCS, 2% Ultroser G, HEPES, penicillin/streptomycin, gentamicin, and amphotericin B until 70–80% confluence. Myoblast differentiation to myotubes was then induced by changing medium to DMEM-Glutamax (5.5 mM glucose) with 2% FCS, 25 pM insulin, penicillin/streptomycin, gentamicin, and amphotericin B. Experiments were performed after 7-8 days of differentiation. During the culturing process the muscle cells were incubated in a humidified 5% CO2 atmosphere at 37 °C, and medium was changed every 2-3 days. Growth and differentiation of cells in media containing lactic acid was done by adding DMEM-Glutamax with no glucose, 5 mM L-glutamine, 5 mM lactic acid, 2% FCS, 2% Ultroser G, penicillin/streptomycin, gentamycin, and amphotericin B the day after seeding. At about 70–80% confluence, medium was changed to DMEM with no glucose, 5 mM glutamine, 5 mM lactic acid, 2% FCS, 25 pM insulin, penicillin/streptomycin, gentamycin, and amphotericin B. For comparison, cells were also grown in regular culturing and differentiation media (DMEM-Glutamax, 5.5 mM glucose) without lactic acid.
Lactate treatments
Different concentrations of lactate were used for the purpose of specific experiments in the present study. Initially, the ability of the myotubes to take up and oxidize lactate was assessed by using L-[14C(U)]lactic acid (1 µCi/ml, 100 μM). To verify protein and mRNA expression of the two lactate transporters, MCT1 and MCT4 (representative genes SLC16A1 and SLC16A4, respectively), myotubes were treated with 4 mM or 10 mM lactate for 24 h. Ability to store lactate as glycogen and intracellular lipids, was determined using L-[14C(U)]lactic acid (1 µCi/ml, 20 mM) for 24 h. Concentration of 20 mM was decided based on previous studies investigating incorporation of lactate into triglycerides and glycogen8,51.
To investigate impacts of lactate exposure on metabolism of glucose and oleic acid, we differed between acute, chronic and prolonged lactate treatment. In the acute treatment (4 h), myotubes were exposed to increasing concentrations of lactate (2 mM, 4 mM, 6 mM and 20 mM), covering the range of concentrations from physiological levels to those seen after exercise in vivo74,75. In chronic lactate treatment, glucose in the culturing medium was replaced by the same amount of lactate (5 mM) for 24 h. Finally, prolonged exposure was determined as a condition where glucose in the culturing media was completely replaced by the same concentration of lactate (5 mM) for the entire proliferation and differentiation period.
Substrate oxidation assay
Skeletal muscle cells (7000 cells/well) were cultured on 96-well CellBIND® microplates. Substrate, D-[14C(U)]glucose (1 µCi/ml), [1-14C]oleic acid (1 µCi/ml) or L-[14C(U)]lactic acid (1 µCi/ml), was given during 4 h CO2 trapping as described previously76. Substrates were added in DPBS with 10 mM HEPES, 10 µM BSA and 1 mM L-carnitine (L-carnitine was only added with oleic acid). Oleic acid was bound to BSA at a ratio of 2.5:1 (this was adjusted for in the substrate medium). A 96-well UniFilter® microplate, soaked with NaOH (1 M), was mounted on top of the CellBIND® plate and produced CO2 was trapped during 4 h incubation at 37 °C. CO2 production was measured in the presence or absence of various compounds: insulin (100 nM), glucose (5 mM), oleic acid (100 µM), palmitic acid (100 µM), pyruvate (5 mM), cytochalasin B (10 µM), or CHC (1 µg/ml). CO2 production and cell-associated (CA) radioactivity were assessed using a PerkinElmer 2450 MicroBeta2 scintillation counter. Protein content in each well was determined with the Bio-Rad protein assay using a VICTOR™ X4 Multilabel Plate Reader as described previously77. The sum of 14CO2 and CA was considered as total substrate uptake. Fractional complete oxidation was calculated as CO2/(CO2 + CA).
Metabolic flexibility parameters
Suppressibility is the ability of the cells to decrease oleic acid oxidation by acutely added glucose, and is calculated as [1 − (oxidation of 100 µM OA at 5 mM glucose/oxidation of 100 µM OA at no glucose added) × 100%]33. Adaptability is the ability to increase oleic acid oxidation with increasing oleic acid concentration, and is calculated as [oxidation of 100 µM OA/oxidation of 5 µM OA]33.
Lipid distribution
Myotubes were incubated with L-[14C(U)]lactic acid (1 µCi/ml, 20 mM) for 24 h. Myotubes were then washed twice with PBS and harvested with 200 μl of 0.1% SDS. Cellular lipids were extracted, as previously described78, from homogenized cell fractions, separated by thin layer chromatography (TLC) and quantified by liquid scintillation (Tri-Carb 1900, PerkinElmer). A non-polar solvent mixture of hexane:diethyl ether:acetic acid (65:35:1) was used to separate the lipids. The amount of lipids was related to total cell protein concentrations. Saponification of the total lipids was performed as described in79 with minor modifications. The cellular total lipid extract from DAG and TAG, respectively, were dried and dissolved in 0.75 ml of 30% KOH and heated at 70 °C for 10 min. An equal volume of 95% ethanol was then added and the mixture was heated at 70 °C for 2 h. After cooling, the aqueous fraction was acidified with 3 M HCl drop-wise and extracted three times with 2 ml light petroleum. The organic fraction was evaporated, redissolved in 1 ml of light petroleum, transferred to scintillation vials and quantified as the acyl fraction using the Tri-Carb scintillation counter. The remaining aqueous fraction contained the glyceryl fraction; 0.5 ml aliquot of this sample was taken and measured for radioactivity using the TriCarb scintillation counter.
Glycogen synthesis
Myotubes were exposed to differentiation media supplemented with L-[14C(U)]lactic acid (1 µCi/ml, 20 mM) for 24 h prior to assay start. At assay start, myotubes were exposed to serum-free DMEM supplemented with L-[14C(U)]lactic acid (1 µCi/ml, 20 mM), in presence or absence of 100 nM insulin (Actrapid® Penfill 100 IE/ml), for 3 h to measure glycogen synthesis. The cells were washed twice with PBS and harvested in 1 M KOH. Protein content was determined by use of the Pierce BCA Protein Assay Kit, before 20 mg/ml glycogen and more KOH (final concentration 4 M) were added to the samples. Then L-[14C(U)]lactic acid incorporated into glycogen was measured as previously described33.
Immunoblotting
Myotubes were cultured in 6-well plates, and two parallell wells of each donor were treated with either 4 mM or 10 mM lactic acid. Samples for immunoblotting were harvested in Laemmli buffer, and aliquots of 15 μg cell protein were electrophoretically separated by SDS-PAGE (Bio-Rad 4–20% Mini Protean® TGX™ precast gels with Tris/glycine buffer) and transferred to nitrocellulose membranes. The membranes were incubated with antibodies against GLUT4 (1:200), MCT1 (1:200), MCT4 (1:200), MHCI (1:10000), MHCIIa (1:1000), and OXPHOS complexes (1:500) overnight. Immunoreactive bands were visualized with Bio-Rad ImmunStar™ WesternC™-kit, detected with Bio-Rad Chemidoc™ XRS + system, and quantified with Image Lab (version 4.0) software. All samples were derived at the same time and processed in parallel. Expression levels were normalized to one sample used as loading control and further normalized to the endogen control α-tubulin (1:1000).
RNA isolation and analysis of gene expression by qPCR
Human skeletal muscle cells were washed, trypsinized and pelleted before total RNA was isolated using QIAGEN RNeasy Plus Mini Kit according to the supplier’s protocol. Total RNA was reversely transcribed (25 °C for 10 min, 37 °C for 80 min, 85 °C for 5 min) with a High-Capacity cDNA Reverse Transcription Kit and TaqMan Reverse Transcription Reagents using a 2720 Thermal Cycler. Primers were designed using Primer Express® and real-time qPCR was performed using a StepOnePlus Real-Time PCR system. Each target were quantified in triplicates and carried out in a 25 µl reaction volume in accordance with the supplier’s protocol. All assays were run for 44 cycles (95 °C for 15 s followed by 60 °C for 60 s). The transcription levels were normalized to the housekeeping gene acidic ribosomal phosphoprotein P0 (RPLP0, acc.no. M17885). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH, acc.no. NM_002046) was also analyzed; there were no differences between normalizing for RPLP0 or GAPDH. The following forward and reverse primers were used at concentration of 30 µM: ANGPTL4 (acc.no. NM_139314), CD36 (acc.no. L06850), CPT1A (acc.no. L39211), CPT1B (acc.no. D1852C12) and CYC1 (acc.no. NM_001916), GAPDH, GLUT4 (acc.no. M20747), PDK4 (acc.no. BC040239), RPLP0, SLC16A1 (acc.no. NM_003051, representing MCT1), and SLC16A4 (acc.no. NM_004696, representing MCT4).
Statistics
Data are presented as means ± SEM in nmol/mg protein or as percent of control. The value n represents the number of different donors, each with at least duplicate observations. Statistical comparison between different treatments was performed by Student’s t-test using GraphPad Prism 6 for Windows. The parameter of interest was entered as the dependent variable and pretreatment (lactic acid or no glucose) and acute treatments were entered as fixed variables. Differences were considered statistically significant at p-values < 0.05.
Electronic supplementary material
Acknowledgements
The authors thank Professor Arild C. Rustan for assistance with the thin layer chromatography. This work was supported by grants from the University of Oslo and OsloMet – Oslo Metropolitan University. The sponsors did not have any involvement in study design, collection, analysis or interpretation of data, writing of the manuscript, or decision to submit the manuscript for publication.
Author Contributions
J.L., V.A. and N.N. conception and design of experiments; all authors performed experiments; J.L., V.A. and N.N. analyzed data; J.L., V.A. and N.N. interpreted results of experiments; J.L. and V.A. prepared figures; J.L., V.A. and N.N. prepared manuscript; all authors approved final version of manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28249-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta physiologica. 2010;199:499–508. doi: 10.1111/j.1748-1716.2010.02122.x. [DOI] [PubMed] [Google Scholar]
- 2.Hui S, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BF, et al. Metabolic and cardiorespiratory responses to “the lactate clamp”. Am J Physiol Endocrinol Metab. 2002;283:E889–898. doi: 10.1152/ajpendo.00266.2002. [DOI] [PubMed] [Google Scholar]
- 4.Miller BF, et al. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol. 2002;544:963–975. doi: 10.1113/jphysiol.2002.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller BF, et al. Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J Appl Physiol (1985) 2005;98:856–865. doi: 10.1152/japplphysiol.00753.2004. [DOI] [PubMed] [Google Scholar]
- 6.Fattor JA, Miller BF, Jacobs KA, Brooks GA. Catecholamine response is attenuated during moderate-intensity exercise in response to the “lactate clamp”. Am J Physiol Endocrinol Metab. 2005;288:E143–147. doi: 10.1152/ajpendo.00117.2004. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21:2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Ye X, Xie M, Ye J. Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Sci Rep. 2016;6:33732. doi: 10.1038/srep33732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiological reviews. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 10.van Hall G, et al. Leg and arm lactate and substrate kinetics during exercise. American Journal of Physiology-Endocrinology And Metabolism. 2003;284:E193–E205. doi: 10.1152/ajpendo.00273.2002. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y, et al. Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. The Journal of physiology. 2007;582:1317–1335. doi: 10.1113/jphysiol.2007.135095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahlin K, Fernström M, Svensson M, Tonkonogi M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. The Journal of physiology. 2002;541:569–574. doi: 10.1113/jphysiol.2002.016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks GA. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB. Lactate is always the end product of glycolysis. Front Neurosci. 2015;9:22. doi: 10.3389/fnins.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juel C, Halestrap AP. Lactate transport in skeletal muscle—role and regulation of the monocarboxylate transporter. The Journal of Physiology. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halestrap AP, Wilson MC. The monocarboxylate transporter family–role and regulation. IUBMB Life. 2012;64:109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 17.Thomas C, Bishop DJ, Lambert K, Mercier J, Brooks GA. Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: current status. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2012;302:R1–R14. doi: 10.1152/ajpregu.00250.2011. [DOI] [PubMed] [Google Scholar]
- 18.Ovens MJ, Manoharan C, Wilson MC, Murray CM, Halestrap AP. The inhibition of monocarboxylate transporter 2 (MCT2) by AR-C155858 is modulated by the associated ancillary protein. Biochem J. 2010;431:217–225. doi: 10.1042/BJ20100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. American Journal of Physiology-Endocrinology And Metabolism. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- 20.Pilegaard H, et al. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol. 1999;276:E255–261. doi: 10.1152/ajpendo.1999.276.2.E255. [DOI] [PubMed] [Google Scholar]
- 21.Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- 22.Burgomaster KA, et al. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1970–1976. doi: 10.1152/ajpregu.00503.2006. [DOI] [PubMed] [Google Scholar]
- 23.Perry CG, Heigenhauser GJ, Bonen A, Spriet LL. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metab. 2008;33:1112–1123. doi: 10.1139/H08-097. [DOI] [PubMed] [Google Scholar]
- 24.Benton CR, Campbell SE, Tonouchi M, Hatta H, Bonen A. Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem Biophys Res Commun. 2004;323:249–253. doi: 10.1016/j.bbrc.2004.08.084. [DOI] [PubMed] [Google Scholar]
- 25.Bonen A. Lactate transporters (MCT proteins) in heart and skeletal muscles. Med Sci Sports Exerc. 2000;32:778–789. doi: 10.1097/00005768-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, Reilly JP, Veneman T, Mandarino L. Effects of insulin on skeletal muscle glucose storage, oxidation, and glycolysis in humans. American Journal of Physiology-Endocrinology And Metabolism. 1990;258:E923–E929. doi: 10.1152/ajpendo.1990.258.6.E923. [DOI] [PubMed] [Google Scholar]
- 27.Henriksson J. Muscle fuel selection: effect of exercise and training. Proceedings of the Nutrition Society. 1995;54:125–138. doi: 10.1079/PNS19950042. [DOI] [PubMed] [Google Scholar]
- 28.van Loon LJ, Greenhaff PL, Constantin‐Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. The Journal of physiology. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiological reviews. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. The Journal of clinical investigation. 2005;115:1699–1702. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ukropcova B, et al. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. The Journal of clinical investigation. 2005;115:1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessvik NP, et al. Metabolic switching of human myotubes is improved by n-3 fatty acids. Journal of lipid research. 2010;51:2090–2104. doi: 10.1194/jlr.M003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aas V, et al. Chronic hyperglycemia reduces substrate oxidation and impairs metabolic switching of human myotubes. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2011;1812:94–105. doi: 10.1016/j.bbadis.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Hessvik NP, et al. The liver X receptor modulator 22 (S)-hydroxycholesterol exerts cell-type specific effects on lipid and glucose metabolism. The Journal of steroid biochemistry and molecular biology. 2012;128:154–164. doi: 10.1016/j.jsbmb.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Thoresen GH, Hessvik NP, Bakke SS, Aas V, Rustan A. Metabolic switching of human skeletal muscle cells in vitro. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2011;85:227–234. doi: 10.1016/j.plefa.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Dijk W, Kersten S. Regulation of lipoprotein lipase by Angptl4. Trends Endocrinol Metab. 2014;25:146–155. doi: 10.1016/j.tem.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Sun, J., Ye, X., Xie, M. & Ye, J. Induction of triglyceride accumulation and mitochondrial maintenance in muscle cells by lactate. Scientific Reports6 (2016). [DOI] [PMC free article] [PubMed]
- 39.Kamijo Y, et al. Plasma lactate concentration and muscle blood flow during dynamic exercise with negative-pressure breathing. Journal of applied physiology. 2000;89:2196–2205. doi: 10.1152/jappl.2000.89.6.2196. [DOI] [PubMed] [Google Scholar]
- 40.Hughson RL, Weisiger KH, Swanson GD. Blood lactate concentration increases as a continuous function in progressive exercise. Journal of Applied Physiology. 1987;62:1975–1981. doi: 10.1152/jappl.1987.62.5.1975. [DOI] [PubMed] [Google Scholar]
- 41.Walenta S, Schroeder T, Mueller-Klieser W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Current medicinal chemistry. 2004;11:2195–2204. doi: 10.2174/0929867043364711. [DOI] [PubMed] [Google Scholar]
- 42.McGinley C, Bishop DJ. Distinct protein and mRNA kinetics of skeletal muscle proton transporters following exercise can influence interpretation of adaptations to training. Exp Physiol. 2016;101:1565–1580. doi: 10.1113/EP085921. [DOI] [PubMed] [Google Scholar]
- 43.Perry CG, et al. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588:4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. American Journal of Physiology-Endocrinology And Metabolism. 1999;276:E843–E848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- 45.Aas V, et al. Are cultured human myotubes far from home? Cell and tissue research. 2013;354:671–682. doi: 10.1007/s00441-013-1655-1. [DOI] [PubMed] [Google Scholar]
- 46.Benton CR, et al. PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol Genomics. 2008;35:45–54. doi: 10.1152/physiolgenomics.90217.2008. [DOI] [PubMed] [Google Scholar]
- 47.Nikolic N, et al. Overexpression of PGC-1alpha Increases Fatty Acid Oxidative Capacity of Human Skeletal Muscle Cells. Biochemistry research international. 2012;2012:714074. doi: 10.1155/2012/714074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks GA. Intra-and extra-cellular lactate shuttles. Medicine and science in sports and exercise. 2000;32:790–799. doi: 10.1097/00005768-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Gladden LB. Muscle as a consumer of lactate. Medicine and Science in Sports and Exercise. 2000;32:764–771. doi: 10.1097/00005768-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Baldwin K, Hooker A, Herrick R. Lactate oxidative capacity in different types of muscle. Biochemical and biophysical research communications. 1978;83:151–157. doi: 10.1016/0006-291X(78)90410-2. [DOI] [PubMed] [Google Scholar]
- 51.McLane JA, Holloszy J. Glycogen synthesis from lactate in the three types of skeletal muscle. Journal of Biological Chemistry. 1979;254:6548–6553. [PubMed] [Google Scholar]
- 52.Pagliassotti MJ, Donovan CM. Role of cell type in net lactate removal by skeletal muscle. American Journal of Physiology-Endocrinology And Metabolism. 1990;258:E635–E642. doi: 10.1152/ajpendo.1990.258.4.E635. [DOI] [PubMed] [Google Scholar]
- 53.Pagliassotti MJ, Donovan CM. Influence of cell heterogeneity on skeletal muscle lactate kinetics. American Journal of Physiology-Endocrinology And Metabolism. 1990;258:E625–E634. doi: 10.1152/ajpendo.1990.258.4.E625. [DOI] [PubMed] [Google Scholar]
- 54.Rooney K, Trayhurn P. Lactate and the GPR81 receptor in metabolic regulation: implications for adipose tissue function and fatty acid utilisation by muscle during exercise. British journal of nutrition. 2011;106:1310–1316. doi: 10.1017/S0007114511004673. [DOI] [PubMed] [Google Scholar]
- 55.Fournier P, et al. Glycogen resynthesis in the absence of food ingestion during recovery from moderate or high intensity physical activity: novel insights from rat and human studies. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2002;133:755–763. doi: 10.1016/S1095-6433(02)00254-4. [DOI] [PubMed] [Google Scholar]
- 56.Jin ES, Sherry AD, Malloy CR. Lactate contributes to glyceroneogenesis and glyconeogenesis in skeletal muscle by reversal of pyruvate kinase. Journal of Biological Chemistry. 2015;290:30486–30497. doi: 10.1074/jbc.M115.689174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer C, et al. A potential important role of skeletal muscle in human counterregulation of hypoglycemia. The Journal of Clinical Endocrinology & Metabolism. 2005;90:6244–6250. doi: 10.1210/jc.2005-0225. [DOI] [PubMed] [Google Scholar]
- 58.Chen, Y.-J. et al. Lactate metabolism is associated with mammalian mitochondria. Nature Chemical Biology (2016). [DOI] [PMC free article] [PubMed]
- 59.Ahmed K, et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell metabolism. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Zuurveld JG, Oosterhof A, Veerkamp JH, van Moerkerk HT. Oxidative metabolism of cultured human skeletal muscle cells in comparison with biopsy material. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1985;844:1–8. doi: 10.1016/0167-4889(85)90226-5. [DOI] [PubMed] [Google Scholar]
- 61.Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Progress in biophysics and molecular biology. 2000;73:195–262. doi: 10.1016/S0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 62.Michael, L. F. et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA98, 3820–3825, 10.1073/pnas.061035098 (2001). [DOI] [PMC free article] [PubMed]
- 63.Al-Khalili, L., Cartee, G. D. & Krook, A. RNA interference-mediated reduction in GLUT1 inhibits serum-induced glucose transport in primary human skeletal muscle cells. Biochem Biophys Res Commun 307, 127–132, doi:S0006291X03011240 [pii] (2003). [DOI] [PubMed]
- 64.Gaster M, et al. GLUT11, but not GLUT8 or GLUT12, is expressed in human skeletal muscle in a fibre type-specific pattern. Pflugers Arch. 2004;448:105–113. doi: 10.1007/s00424-003-1219-4. [DOI] [PubMed] [Google Scholar]
- 65.Kase ET, et al. Remodeling of oxidative energy metabolism by galactose improves glucose handling and metabolic switching in human skeletal muscle cells. PloS one. 2013;8:e59972. doi: 10.1371/journal.pone.0059972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, et al. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 2017;7:5384. doi: 10.1038/s41598-017-05541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tobar N, Porras O, Smith PC, Barros LF, Martinez J. Modulation of Mammary Stromal Cell Lactate Dynamics by Ambient Glucose and Epithelial Factors. J Cell Physiol. 2017;232:136–144. doi: 10.1002/jcp.25398. [DOI] [PubMed] [Google Scholar]
- 68.Ziegler K, Kerimi A, Poquet L, Williamson G. Butyric acid increases transepithelial transport of ferulic acid through upregulation of the monocarboxylate transporters SLC16A1 (MCT1) and SLC16A3 (MCT4) Archives of biochemistry and biophysics. 2016;599:3–12. doi: 10.1016/j.abb.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nikolic N, et al. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One. 2012;7:e33203. doi: 10.1371/journal.pone.0033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eilertsen M, et al. Monocarboxylate transporters 1-4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS One. 2014;9:e105038. doi: 10.1371/journal.pone.0105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laye MJ, Nielsen MB, Hansen LS, Knudsen T, Pedersen BK. Physical activity enhances metabolic fitness independently of cardiorespiratory fitness in marathon runners. Disease markers. 2015;2015:806418. doi: 10.1155/2015/806418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakke SS, et al. Myotubes from severely obese type 2 diabetic subjects accumulate less lipids and show higher lipolytic rate than myotubes from severely obese non-diabetic subjects. PLoS One. 2015;10:e0119556. doi: 10.1371/journal.pone.0119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaster M, Kristensen S, Beck‐Nielsen H, Schrøder H. A cellular model system of differentiated human myotubes. Apmis. 2001;109:735–744. doi: 10.1034/j.1600-0463.2001.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 74.Cheetham ME, Boobis LH, Brooks S, Williams C. Human muscle metabolism during sprint running. J Appl Physiol (1985) 1986;61:54–60. doi: 10.1152/jappl.1986.61.1.54. [DOI] [PubMed] [Google Scholar]
- 75.Ohkuwa T, Kato Y, Katsumata K, Nakao T, Miyamura M. Blood lactate and glycerol after 400-m and 3,000-m runs in sprint and long distance runners. Eur J Appl Physiol Occup Physiol. 1984;53:213–218. doi: 10.1007/BF00776592. [DOI] [PubMed] [Google Scholar]
- 76.Wensaas A, et al. Cell-based multiwell assays for the detection of substrate accumulation and oxidation. Journal of lipid research. 2007;48:961–967. doi: 10.1194/jlr.D600047-JLR200. [DOI] [PubMed] [Google Scholar]
- 77.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 78.Gaster M, Rustan AC, Aas V, Beck-Nielsen H. Reduced lipid oxidation in skeletal muscle from type 2 diabetic subjects may be of genetic origin Evidence from cultured myotubes. Diabetes. 2004;53:542–548. doi: 10.2337/diabetes.53.3.542. [DOI] [PubMed] [Google Scholar]
- 79.Saggerson ED, Greenbaum AL. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970;119:193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.