Abstract
Manchurian walnut and larch are key timber species of northeast China but information on (fine) root traits of both species is scarce. Plasticity of root traits in mixed plantations has been studied rarely although this could give important insights into mechanisms of root competition. This study examined root traits by branching order in 30-yr-old monocultures and their plasticity in mixed plantations. In monocultures, Manchurian walnut and larch differed in key fine root traits. Larch roots hold more absorptive root orders, larger diameter and lower specific root length/area. Walnut root orders featured greater cortex:stele ratios, N-concentrations and respiration rates. Under interspecific competition, the proportion of walnut root tips increased, the biomass/length of larch root orders 1–3 decreased. Larch possessed a greater morphological and anatomical plasticity of terminal root orders than walnut. Mycorrhizal colonization rates of walnut were reduced. Both species differed fundamentally in their fine root properties. Absorptive fine root orders reacted plastic under interspecific competition while traits of higher root orders remained unchanged. In mixture, larch roots possessed a greater plasticity in traits related to resource uptake (efficiency) than walnut roots whose reaction norm is suggested to be predominantly based on interference competition via juglone exudation.
Introduction
Tree monocultures can provide large quantities of wood per unit area, however, this has often come at an expense, e.g. in terms of biodiversity, ecosystem system services and resilience in regard to climate change and biotic disturbances1,2. In contrast, mixed-species plantations may be less prone to such tradeoffs3, and may even provide increased production relative to monocultures4–6. The higher productivity of mixed-species plantations is the positive net result of negative (i.e. competition) and positive interactions (i.e. facilitation) among combined species including both above- and belowground interactions7–9.
Both Manchurian walnut (Juglans mandshurica) and larch (Larix gmelinii) are key timber species of northeast China and large plantations have been established in the last decades. While the productivity decline of long-term sites planted with Manchurian walnut monocultures remains a significant problem10,11, mixture with larch has been shown to stabilize or even increase the production6,12,13. Due to the economic importance, several previous studies have addressed the influence of larch on different aspects of Manchurian walnut seedlings to shed light on potential mechanisms involved in the inter-specific interactions. For example, extracts of larch root and bark, and root exudates were found to stimulate chlorophyll contents, soluble sugars and overall biomass of Manchurian walnut14. Larch root exudates increased the soil microbial biomass and enzymatic activities in mixed plantations with Manchurian walnuts6,11. In contrast, Manchurian walnut roots produce a highly phytotoxic exudate, juglone (5-hydroxy-1,4-naphthoquinone)15,16; juglone exerts an auto-inhibitory effect on seedlings6 and is likely to be more persistent in the soil of Manchurian walnut monocultures (due to the higher microbial biomass facilitated by larch)17.
Fine roots play a key role for carbon and nutrient cycles in terrestrial ecosystems18,19 and ensure water and nutrient uptake for plant survival, growth, and seed production20. Fine roots of neighboring plants in a mutual soil volume compete for resources, either by exploitative competition (i.e. reduction of water and nutrients resources)21 or through interference competition (i.e. the release of allelopathic chemicals which directly inhibit growth and thus the access to resources)9. Competition below ground is believed to be at least equally intense as shoot competition22,23. Resource competition and allelopathic chemicals, such as juglone, have been shown to reduce the growth of neighboring roots and plants24,25, and to influence traits of competing root systems to different extends26,27. Plants may acclimate to competition below ground by alteration in root biomass, root architecture (e.g. branching frequency), morphology (e.g. specific root length), root anatomy (e.g. cortex:stele ratio), physiology (e.g. maintenance respiration) and potentially intensity of mycorrhizal symbioses to increase the cost/benefit ratio and extend of resource capture28–30.
In the last decade, it has become increasingly clear that root systems of perennial plants consist of individual units (“root orders”) with distinct traits31,32. Especially changes in traits of the 2–3 terminal root orders (i.e. “absorptive fine roots”)31 can be expected to be most sensitive indicators for changes in the nutrient and water uptake capacities of branched root systems29. However, while structural and functional differences between root orders have been studied in some species19,32,33, studies addressing the effects of competition on order-based root traits remain scarce in general and limited to morphological and chemical traits in specific26,34. Similar, previous studies addressing belowground competition focused on biomass, morphology and/or tissue chemistry of whole root branches27,35 but largely neglected anatomical and physiological traits and changes among individual root orders.
Because previous studies on root traits of Manchurian walnut and larch were largely carried out under controlled conditions and focused on seedlings, the first objective of this study was to determine fine root system properties of mature (30 years-old) Manchurian walnut and larch trees in situ. We hypothesize that (i) the different ecology of Manchurian walnut and larch trees is reflected by highly divergent root traits in monocultures. Beyond the scope of previous studies, a wide range of root traits (architectural, morphological, anatomical, root respiration and mycorrhizal colonization rates) was studied across five terminal root orders. The second objective was to determine the plastic acclimation of both species’ fine roots systems to long-term interspecific competition. We hypothesize that (ii) the first 2–3 terminal root orders (i.e. “absorptive fine roots”) will react most plastic to interspecific competition while traits of higher root orders (i.e. “transport fine roots”) possess less adaptive plasticity. We hypothesize further, that (iii) larch roots will possess a greater plasticity in the studied traits (related to C use and resource uptake (efficiency)), under interspecific competition than Manchurian walnut roots whose reaction norm is suggested to be predominantly based on interference competition via juglone exudation.
Results
Root architecture, morphology and anatomy
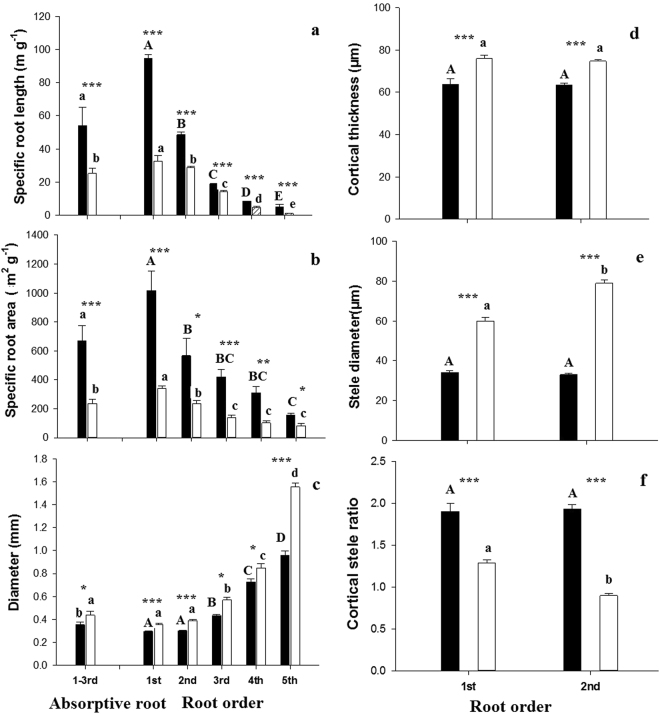
Significant root architectural, morphological, and anatomical differences were found between species and within the branching hierarchies and when comparing the root orders of larch and Manchurian walnut (Table 1, Table S1, Figs 1–2). Length, biomass, diameter, cortical thickness, and stele diameter were significantly greater in larch than in Manchurian walnut root orders. In both species the length per root order decreased with increasing root order (Fig. 1a). While the biomass per root order decreased stepwise towards higher orders of larch, biomass was more equally distributed among root orders of walnut (Fig. 1b). In both species (monocultures), specific root length (SRL) and specific root area (SRA) decreased (Fig. 2a,b), although differences in SRA between orders were less pronounced in larch, while the diameter (Fig. 2c) increased with increasing root order. The stele diameter increased significantly from 1st to 2nd root order in larch only (Fig. 2e). The morphology of root orders 1–5 differed significantly across species: diameters of all root order (and thus cortex and stele diameters) were significantly greater in larch, while SRL and SRA were significantly greater in Manchurian walnut (Fig. 2a–c). The cortex:stele ratio was significant greater in 1st and 2nd order roots of Manchurian walnut than in larch grown in monoculture (Fig. 2f). Calculating the average morphology of root orders 1–3 (“absorptive roots”, i.e. taking the respective biomass frequencies into account) revealed that SRL and SRA were significantly greater in Manchurian walnut, although to a lesser extent than when comparing 1st root orders directly, while root diameter were significantly greater in larch (Fig. 2a–c).
Table 1.
Summary of the influence of monoculture species (Species), competition treatment: (mono or mix) and root order (1–5 Order) and their interactions on the root respiration, N, C/N ratio, root length, specific root area (SRA), specific root length (SRL), root diameter, root order biomass per branch, Cortical thickness, stele diameter and cortical stele ratio.
| Source of variation | df | F | P | F | P | F | P | F | P | F | P | F | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiration | N | C/N | Length | SRL | SRA | ||||||||
| Treatment | 1 | 8.119 | <0.047 | 2.629 | 0.119 | 1.656 | <0.098 | 94.523 | <0.001 | 47.192 | <0.001 | 2.362 | 0.132 |
| Species | 1 | 478.743 | <0.001 | 52.244 | <0.001 | 82.058 | <0.001 | 289.599 | <0.001 | 41.798 | <0.001 | 41.793 | <0.001 |
| Order | 4 | 393.81 | <0.000 | 248.977 | <0.001 | 180.628 | <0.001 | 681.321 | <0.001 | 299.442 | <0.001 | 44.458 | <0.001 |
| Treatment* species | 1 | 3.826 | <0.148 | 0.003 | 0.96 | 0.077 | <0.62 | 6.417 | <0.015 | 2.659 | <0.111 | 3.183 | 0.082 |
| Treatment* order | 4 | 1.053 | 0.392 | 0.278 | 0.891 | 1.113 | 0.097 | 27.717 | <0.001 | 5.859 | <0.001 | 0.757 | 0.559 |
| Species* order | 4 | 18.568 | <0.021 | 1.945 | 0.122 | 13.349 | <0.001 | 76.939 | <0.001 | 48.973 | <0.001 | 7.315 | <0.001 |
| Treatment* species* order | 4 | 2.829 | <0.037 | 3.284 | <0.020 | 5.239 | <0.002 | 7.109 | <0.001 | 2.479 | 0.059 | 0.363 | 0.834 |
| Diameter | Biomass/branch | Cortical thickness | Stele | Cortical stele ratio | |||||||||
| Treatment | 1 | 1.445 | <0.931 | 72.149 | <0.001 | 0.062 | 0.803 | 0.138 | 0.71 | 0.243 | 0.623 | ||
| Species | 1 | 36.595 | <0.001 | 730.251 | <0.001 | 55.555 | <0.001 | 753.007 | <0.001 | 131.951 | <0.001 | ||
| Order | 4 | 215.773 | <0.001 | 81.998 | <0.001 | 0.013 | 0.908 | 76.604 | <0.001 | 6.115 | <0.015 | ||
| Treatment* species | 1 | 1.563 | 0.218 | 78.176 | <0.001 | 0.909 | 0.342 | 1.861 | 0.174 | 0.598 | 0.441 | ||
| Treatment* order | 4 | 2.383 | <0.326 | 17.861 | <0.001 | 0.087 | 0.769 | 2.236 | 0.137 | 0.037 | 0.848 | ||
| Species* order | 4 | 4.674 | <0.003 | 64.162 | <0.001 | 2.528 | 0.114 | 60.046 | <0.001 | 14.504 | <0.001 | ||
| Treatment* species* order | 4 | 1.871 | <0.312 | 15.06 | <0.001 | 1.924 | 0.167 | 0.103 | 0.748 | 1.048 | 0.308 | ||
Shown are the degrees of freedom (df), F and P values (P < 0.0001, P < 0.001, P < 0.05,) of the respective variables and variables with significant influence are printed in bold (P < 0.05).
Figure 1.
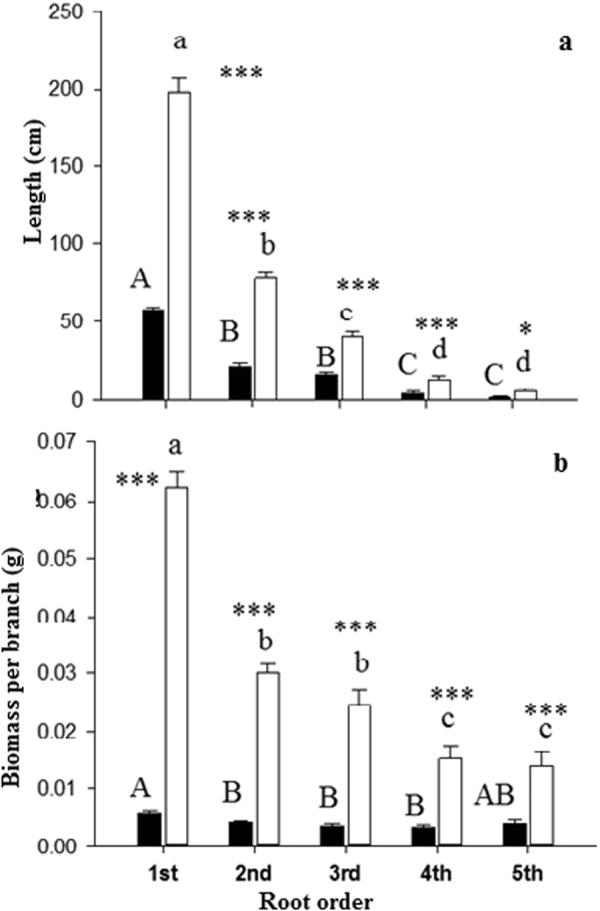
Root architecture (a,b) of root orders 1–5 of Manchurian walnut (black) and larch (white). Different upper case letters indicate significant differences between root orders of Manchurian walnut, lower case letters for larch (P < 0.05, Tukey HSD). Significant differences in a given root order class between species are marked with an asterisks (*P < 0.05; ***P < 0.001, Tukey HSD; n = 10–16; mean ± SE).
Figure 2.
Root morphology (a–c) of individual root orders 1–5 and average of orders 1–3 (absorptive roots) and root anatomy (d–f) of order 1–2 of Manchurian walnut (black) and larch (white). Different upper case letters indicate significant differences between root orders of Manchurian walnut, lower case letters for larch (P < 0.05, Tukey HSD). Significant differences in a given root order class between species are marked with an asterisks (*P < 0.05; **P < 0.01; ***P < 0.001, Tukey HSD; n = 10–16; mean ± SE).
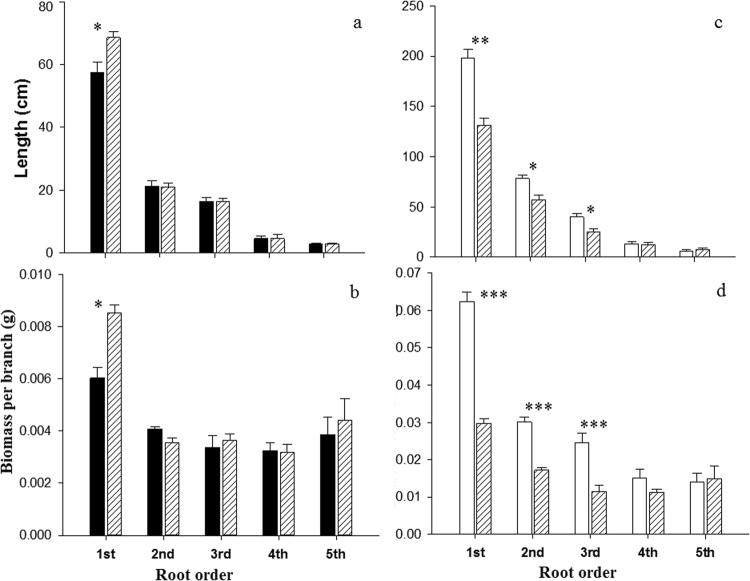
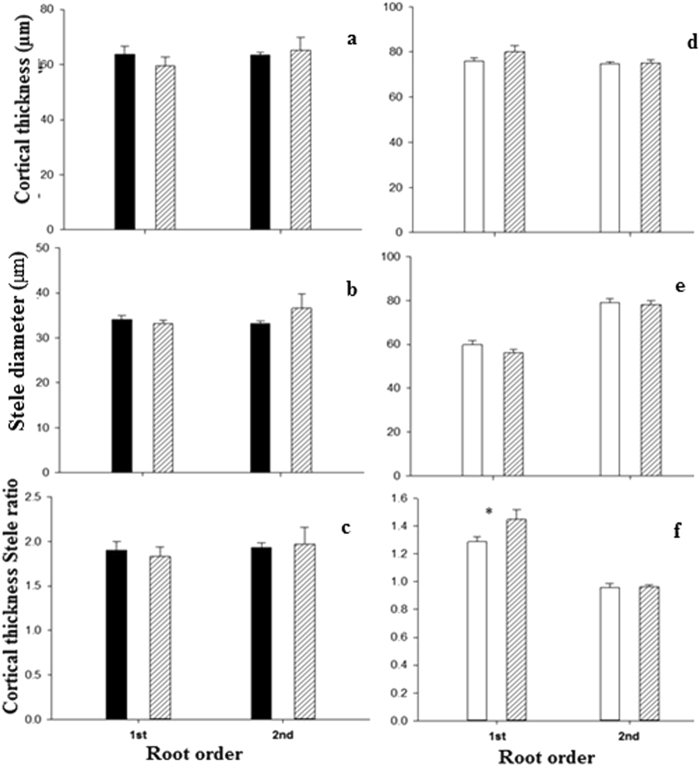
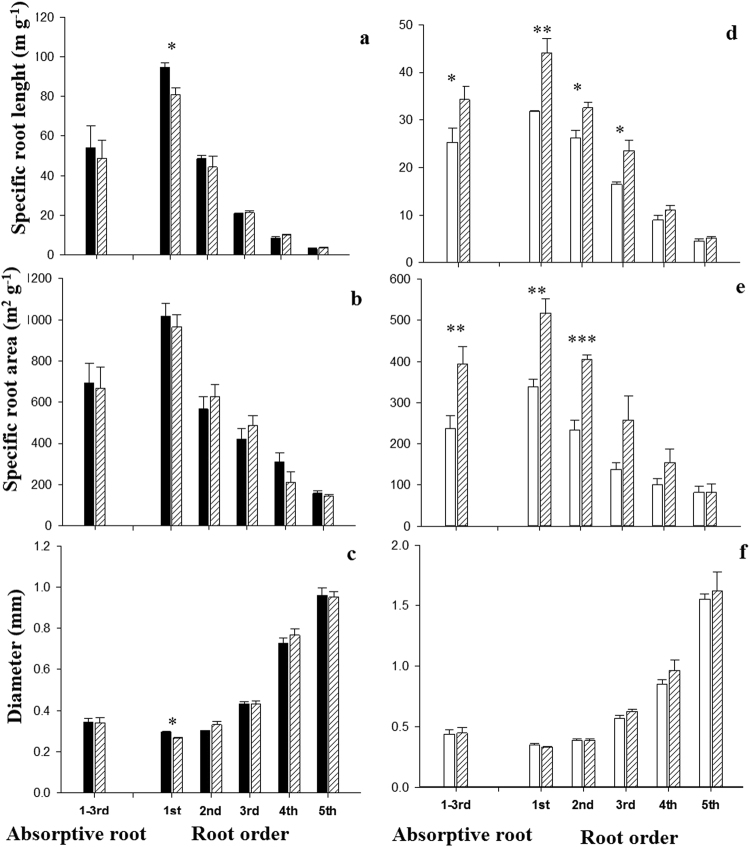
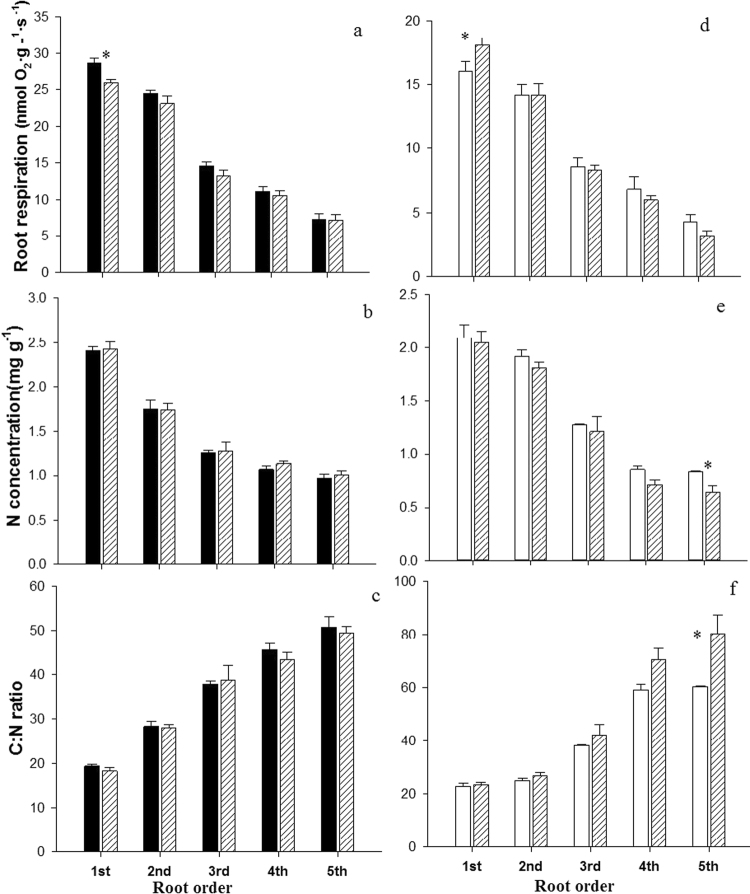
The root architecture, morphology and anatomy of Manchurian walnut and larch changed between monocultures and mixed plantations (Table 1, Figs 3–5). The length and biomass of 1st order roots (i.e. root tips) of Manchurian walnut were significantly greater in the mixed plantation than in the monoculture by +16% and +29%, respectively (Fig. 3a,b). In larch, length and biomass of root orders 1–3 were significantly greater in monoculture, for example by +30% and +49% for 1st order roots, respectively (Fig. 3c,d). Specific root length (SRL, +14%) and root diameter (+8%) were significantly greater in the 1st root order of Manchurian walnut in monoculture compared to the mixture (Fig. 4a,c). An increase in SRL/SRA accompanied by minor changes of root diameter indicates a decrease in tissue density. In larch, SRL and SRA were significantly greater (26% and 38% respectively) in the first three (SRL) or two (SRA) root orders under interspecific competition (Fig. 4d,e). While the anatomy of Manchurian walnut root orders 1 and 2 was unaffected by the competition type (Fig. 5a–c), the cortex:stele ratio of 1st root order of larch significantly increased by 11% in the mixed plantation compared to the monoculture (Fig. 5f). In larch, the average SRL and SRA of root branches, including order 1–3, were significantly higher in mixed plantation as compared to the monoculture (Fig. 4d,e).
Figure 3.
Root architecture of Manchurian walnut (a,b) and larch (c,d) root orders 1–5 in monocultures (walnut: black; larch: white) and mixed plantations (hatched). Please note differences in Y-axis scaling between species. Significant differences in a given root order classes per species between monoculture and mixed plantation are marked with an asterisks (*P < 0.05; **P < 0.01; ***P < 0.001; Tukey HSD; n = 10–16; mean ± SE).
Figure 5.
Root anatomy of Manchurian walnut (a–c) and larch (d–f) root orders 1–5 in monocultures (walnut: black; larch: white) and mixed plantations (hatched). Please note differences in Y-axis scaling between species. Significant differences in a given root order classes per species between monoculture and mixed plantation are marked with an asterisks (*P < 0.05; Tukey HSD; n = 30; mean ± SE).
Figure 4.
Morphology of Manchurian walnut (a–c) and larch (d–f) individual root orders 1–5 and average of orders 1–3 (absorptive roots) in monocultures (walnut: black; larch: white) and the mixed plantation (hatched). Please note differences in Y-axis scaling between species. Significant differences in a given root order classes per species between monoculture and mixed plantation are marked with an asterisks (*P < 0.05, Tukey HSD; n = 10–16; mean ± SE).
Root respiration, C/N stoichiometry and mycorrhizal colonization
Significant root respiration and C/N stiochiometry differences were found between species (Table 1). The biomass-specific root respiration was significantly greater in root orders 1–4 of Manchurian walnut compared to larch and decreased with increasing order (Fig. 6a). Nitrogen concentrations across all five root orders were greater in Manchurian walnut than in larch (Fig. 6b); carbon:nitrogen ratios (C:N) significantly increased from root order 1 to 4 in both species (Fig. 6c).
Figure 6.
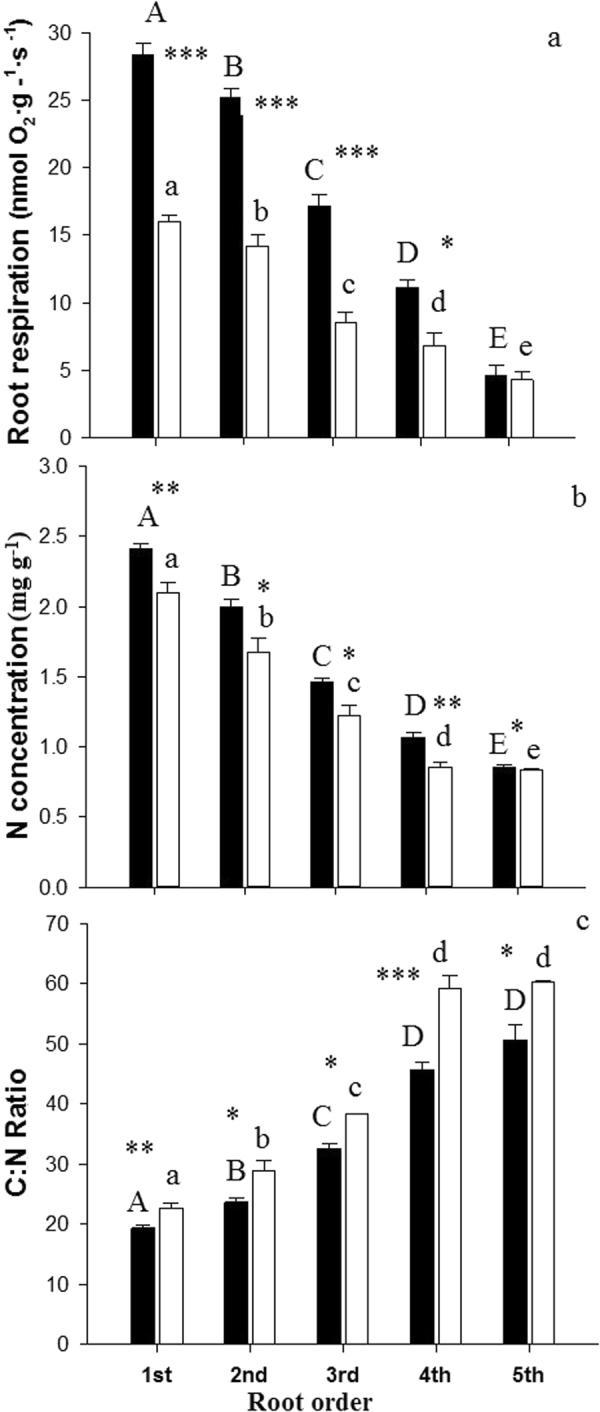
Root respiration (a) and C:N stoichiometry (b,c) of root orders 1–5 of Manchurian walnut (black) and larch (white). Different upper case letters indicate significant differences between root orders of Manchurian walnut, lower case letters for larch (P < 0.05, Tukey HSD). Significant differences in a given root order class between species are marked with an asterisks (*P < 0.05; **P < 0.01; ***P < 0.001, Tukey HSD; n = 27; mean ± SE).
Comparing monocultures and mixed plantation, the biomass-specific root respiration was significantly greater (9%) in the 1st root order of Manchurian walnut in monoculture compared to mixture (Fig. 7a). In larch, the root respiration was significantly greater (12%) in the 1st root order in mixture compared to the monoculture (Fig. 7d). Nitrogen concentrations and C:N ratios of Manchurian walnut root orders did not differ significantly between monoculture and mixed plantation (Fig. 7c). In larch, nitrogen concentration (−23%) and C:N ratio of the 5th root order were significantly smaller/greater in the mixture, respectively (Fig. 7e,f).
Figure 7.
Root respiration (a,d) and C/N stoichiometry of Manchurian walnut (a–c) and larch (d–f) root orders 1–5 in monocultures (walnut: black; larch: white) and mixed plantation (hatched). Please note differences in Y-axis scaling between species. Significant differences in a given root order classes per species between monoculture and mixed plantation are marked with an asterisks (*P < 0.05, Tukey HSD; n = 10–15; mean ± SE).
Arbuscular mycorrhizal colonization rates of terminal root orders of Manchurian walnut were significantly greater (+28%) in the monoculture compared to the mixed plantation; ecto-mycorrhizal colonization rates of larch root tips did not differ between monoculture and mixture (Fig. 8).
Figure 8.
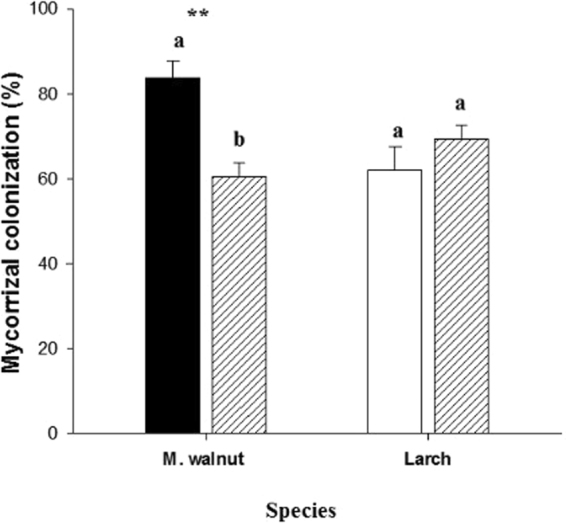
Mycorrhizal colonization rates of Manchurian walnut (AM) and larch (ECM) terminal root orders (first order) in monocultures (walnut: black; larch: white) and the mixed plantation (hatched). Significant differences per species between monoculture and mixed plantation are marked with an asterisks (**P < 0.01, Tukey HSD; n = 3–5; mean ± SE).
Discussion
Inter species differences in fine roots traits of Manchurian walnut and larch
Interspecific differences between tree root system largely affect tree growth by effects on the nutrient and water uptake capacity36 and the C balance37. For example, soil exploitation capacity and exploitation efficiency of the fine-root systems are largely determined by the root surface area/length per soil volume, root distribution within the soil horizons and specific root length (SRL)38,39. The uptake efficiency is determined by C costs related to the construction and maintenance of absorptive fine root surface40 and mycorrhizal symbionts41. In this study, the fine root systems of Manchurian walnut and larch grown in monocultures differed significantly in branching patterns (architecture) and root order-specific morphological, anatomical, chemical and physiological traits. The data on fine root length and biomass of root orders indicates that larch trees, growing under the given edaphoclimatic conditions, have generally more extensive (in terms of length and biomass) fine root branches (order 1–5) than Manchurian walnut trees. In addition, larch root branches feature a much larger proportion of lower, absorptive root orders (1–3) than Manchurian walnut where length and biomass distribution evidence a more homogeneous contribution of individual orders to the architecture of root branches. Both the higher biomass and length of lower (i.e. first two or three orders), absorptive root orders should thus benefit larch’s absolute capacity to forage resources31,42, although the uptake capacity per root surface (of different orders) remains an unknown factor for nutrients with active, transporter-based uptake mechanisms and potentially also for water/nutrients taken up by mass flow43. Variations in root diameter, but also cortex thickness and stele diameter were previously reported to be strongly phylogenetically determined44. Individual larch root orders hold slightly larger diameter but distinctly reduced SRL and SRA values compared to Manchurian walnut root orders, indicating (together with the larger stele) also a higher tissue density of individual larch root orders. Larger diameter and lesser SRL/SRL are often related to a lower efficiency of a species in building root length and surface area for exploration and uptake of resources45.While uncertainties remain in term of root longevity46, these finding indicate that Manchurian walnut trees possesses a more efficient root system than larch in terms of structural C utilized for building root surface/length. Previous studies showed similarly that conifers typically have thicker diameter roots and lower SRL than coexisting deciduous angiosperms38,47. The large root order specific morphological differences in the studied species are partially “balanced” by the divergent frequency of root orders in both species (as evidenced by biomass and length, Fig. 1). However, while differences are reduced when averaging the traits of absorptive root orders (e.g. 2-fold differences for SRL of root branches 1–3 compared to a 3-fold difference in SRL of 1st order roots), SRA and SRL of Manchurian walnut root branches (i.e. as average of orders 1–3) are still significantly greater than of larch absorptive root branches. In contrast to the morphological traits, the lower maintenance respiration of larch (in root orders 1 to 3), potentially related to larger diameter and lower N contents32,48, might serve as an indication of a greater C use efficiency of individual larch roots compared to Manchurian walnut. Root respiration was previously shown to constitute a major component of plant C budgets49,50, especially under suboptimal growing conditions51,52.
Acquisitive (fast growing) species often possess relatively small-diameter, high specific root length (SRL), fast-growing fine roots with short lifespan, high N content, and high rates of respiration and nutrient acquisition in comparison to conservative (slow growing) conifer species19,53. While we found the hypothesized, intrinsic differences of root traits between the two species54, we could not link the root traits in monocultures to the (aboveground) growth performance of both species. Under the given environmental conditions and in monocultures, larch trees possessed a higher aboveground growth than Manchurian walnut during the last three decades. According to previous findings, it is reasonable to speculate that auto-inhibitory effects by juglone in Manchurian walnut monocultures55,56 might have dominantly contributed to the growth depression. We suggest also that the rather a typical root branching pattern of Manchurian walnut, compared to other trees species featuring less even proportions of root orders32,57 might be a direct consequence of the juglone concentration in the soil. Further studies are thus urgently needed to relate root traits of Manchurian walnut to different levels of Juglone present in the soil; these studies should also include information on root system sizes and architecture to calculate realistic, species-specific traits of root branches58 and to identify important species-specific traits of the whole (fine) root systems.
Influence of interspecific competition on fine root traits
Interspecific competition in the mixed plantation resulted in reduced growth of larch trees while Manchurian walnut trees tended to show greater DBH and increased height growth in mixtures compared to monocultures. While this is likely an effect of both above- and belowground interactions between species8,59,60, it is well documented that walnut species release juglone into the soil which inhibit the growth of neighboring plants including seedlings of the own species after a certain accumulation61,62. Thus, in the following we will focus on root traits affected by interspecific competition. Root systems are a quickly responding plant organ that is influenced by a variety of dynamic factor including internal species heredity character54 and external conditions e.g., nutrient availability, soil properties and non-toxic signals/toxic allochemicals released by neighboring plants63. Numerous previous studies have reported on the influence of competing roots on the (root) biomass and biomass distribution of neighboring species64. While root competition intensity is easiest determined by plant responses (e.g. effects on yield, relative growth rate, survival etc.) and potentially best measured by comparing soil resource availability in the presence and absence of competing roots65, few previous studies have reported detailed effects of different competitive environments on root (order) traits of woody species54,66.
Especially root architectural and morphological traits are believed to be important indicators of resources acquisition capability29,67 and construction cost68,69. In this study, the length and biomass and of larch root orders 1–3 were significantly greater, while the SRL and SRA of the first three/two root orders were significantly lesser in monoculture as compared to larch in the mixed stand. In contrast, plasticity of these traits under interspecific competition was limited in Manchurian walnut to the first root order (i.e. root tips). It is noteworthy that especially the terminal, absorptive root orders of the fine root system possessed a different morphology under intra- vs. interspecific competitive environments, while the higher order (“transport” fine roots) did not vary in most assessed traits. However, given that root competition affects the availability of soil resources, this is coherent with earlier finding reporting a greater morphological plasticity of absorptive root orders to changes in soil moisture70,71, nutrient availability72,73, or heavy metal/salt stress43. The reduced frequency of terminal root orders in larch may be at least partially related to a direct growth inhibition by juglone, as shown previously for corn root systems74. In both tree species, modified tissue densities likely caused the changes in SRL/SRL because root diameters were less plastic. In larch, the change in tissue density of 1st root orders (root tips) seem to be largely based on the greater cortex:stele ratio—the cortex holding a lower tissue density than the stele. Kong et al.75 proposed earlier that the allometric relationship of cortex and stele sizes reflects the relative importance of resources absorption and transportation in woody species; as such, the increased cortex of larch may be an indication of increased nutrient uptake capacity (e.g. by increasing exudation capacity76. However, we also deem it possible that an increased cortex holds advantages when facing high juglone concentrations, similar to previous findings of increased cortical tissue thickness under excess NaCl salinity77; however, this and the growth/branching inhibiting effect of juglone on larch root systems need further examination.
In this study, interspecific root competition significantly increased the respiration of root tips in larch and decreased it in Manchurian walnut compared to the respective monocultures. Meier et al.78 have earlier shown a substantial increase in root respiratory O2 uptake when pea plants were exposed to non-self competitors. An increase in respiration may indicate a more active root system40,79 and Volder et al.30 suggested further that high root respiration rates may be an indicator of a higher competitive ability if accompanied by increased nutrient uptake capacities. However, increased root respiration rates can also be a direct driver of competition affecting soil CO2 concentrations and thus inhibiting growth of neighboring roots80. The increased respiration in larch root tips in mixture could also be related to their increased cortical tissue81, or to higher respiratory costs for metabolic defense and repair mechanisms82–84. Concerning the latter, the decrease in root respiration in Manchurian walnut could then be related to the reduced concentration of toxic juglone in mixed plantation soil85–87. However, contrasting results exist on the effect of juglone on O2 consumption rates of other species, reporting to either affect or not affect respiration rates of soybean and corn74,88, thus, further work is necessary to clarify the situation for the given species.
The changes in mycorrhizal infection rates under interspecific competition are likely related to different nutrient availabilities, especially P, in the mixed/monoculture production systems. The microbial biomass (via PLFA) decreases dramatically as the juglone concentration increased in Manchurian walnut stand15. Thus, a potentially higher microbial biomass in monocultures of larch might have facilitated the plant availability of P and other nutrients. Both arbuscular and ectomycorrhizal symbionts have previously been shown to be especially enhance P uptake89,90; however, especially ectomycorrhizal tree species invest more in mycorrhizal maintenance and hyphae proliferations to maximize nutrient foraging91,92. In Manchurian walnut, the higher P availability in mixed plantation compared to monocultures might thus result in a decreased investment into mycorrhizal symbionts, while the ectomycorrhizal colonization rate in larch tended to be increased due to the lower availability of P (but potentially also other nutrients) in mixture compared to larch monocultures.
In general, larch’s absorptive root orders possessed a greater plasticity under interspecific competition than the individual root segments of Manchurian walnut. We speculate, that the greater plasticity of larch, especially in terms of branching patterns, root morphology and anatomy, could have been either caused by the relative conservative morphology of this species in terms of C use efficiency (low SRL, SRA, greater root diameter)40, or is a direct consequence of the accumulated juglone in the shared soil74. Previously, greater SRL and SRA values were reported in mixed species forests relative to monocultures, likely increasing the competitive ability28,93. Conversely, the limited plasticity of Manchurian walnut root orders under interspecific competition, compared to larch, might be due to the increased nutrient availability in mixture with larch, as indirectly evidenced by decreased AMF colonization rates, SRL, and respiration rates of 1st order roots. However, it is also reasonable to speculate that Manchurian walnuts competitive “strategy” may be rather based on interference competition via juglone exudation and not exploitative competition—resulting in a lower plasticity in uptake-related root traits. Further studies are necessary to separate the direct allopatric effects of juglone from resource competition effects on fine root traits, potentially using an inoculation with Pseudomonas sp. strains to degrade the exudated juglone rapidly94 and/or establishing a fertilization gradient.
Material and Methods
Site description
This experiment was conducted at the Maoershan experimental station (45°16′ N, 128°34′ E), located in the temperate forest region of the Heilongjiang Province, NE China. The climate is a continental monsoon climate featuring a strong monsoon in the spring, a warm and humid summer, and a dry and cold winter. The average annual air temperature is 2.8 °C; average temperatures in January and July are -19.6 °C and 20.9 °C, respectively. The frost-free period is 120 to 140 days-long. The annual averages of rel. humidity, precipitation, and potential evapotranspiration are 70%, 724 mm a−1, and 1094 mm a−1, respectively. The parent material is granite bedrock and soils are Boric Luvisols95; the bulk soil density (0–10 cm depth) is 0.83 g cm−3 96; further soil characteristics are listed in Table 2.
Table 2.
Summary of stand and soil characteristics of three plantations used in this study: monocultures (Mono) of Larix gmelinii and Juglan mandshurica and their mixed plantation (Mix) in NE China.
| Plantation type | Species | Stand characteristics | Soil characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Density (n ha−1) |
DBH (cm) | Height (m) | pH | OM (g kg−1) | Total N (g kg−1) |
Hydrolytic N (mg kg−1) | Total P (g kg−1) | Available P (mg kg−1) | ||
| Mono | Larch | 1284 | 80.7 | 13.2 | 4.7 | 6.29 | 5.48 | 409.2 | 0.56 | 11.5 |
| Mono | Walnut | 1500 | 54.9 | 11.8 | 4.9 | 6.63 | 5.72 | 451.3 | 0.59 | 7.5 |
| Mix | Average | 1380 | — | — | 5.1 | 6.41 | 6.47 | 524.1 | 0.61 | 8.5 |
| Mix | Larch | 735 | 41.7 | 13.7 | — | — | — | — | — | — |
| Mix | Walnut | 645 | 65.4 | 13.0 | — | — | — | — | — | — |
Stand characteristics given are tree density, diameter at breast height (DBH) and tree height, soil characteristics (0–10 cm soil depth without litter) given are pH (H2O), organic matter (OM), total and hydrolytic Nitrogen (N), and total and available Phosphorous (P) (mean; nDBH/Height = 13–15, nSoil = 3–5).
Larch (Larix gmelinii Rupr.) and Manchurian walnut (Juglan mandschurica) are dominant species in natural forests, and key species used for plantations in Northeast China. The two species contrast in taxonomy (gymnosperm vs. angiosperm), and type of mycorrhizal symbionts (larch: ectomycorrhizal; Manchurian walnut: arbuscular)97. Due to their economic importance, large monocultures and mixed plantations of the two species were established in the region in 1987; 2-year-old seedlings were planted in a 1.5 m × 1.5 m grid (between rows × within rows) in all three stands. Line mixing (i.e. three rows of Manchurian walnut followed by five rows of larch) was used in the mixed-species plantation. Stand characteristics in the year 2017 are listed in Table 1; differences in tree densities are caused by mortality. Three adjacent stands, two monocultures of larch and Manchurian walnut (intraspecific competition), respectively, and a mixed plantation (interspecific competition) consisting of the two species, were selected for this study. Plots were located on a SW-facing slope (approx. 13°) at 450–500 m a.s.l. In monocultures, three subplots were established at random locations (distance > 10 m) in-between rows; in mixed-plantation, subplots were located between rows holding larch and Manchurian walnut trees respectively. At each subplot, samples were taken at three locations; in monocultures the locations were randomly selected. In mixed stands, the locations were chosen by following roots from opposing larch and Manchurian walnut boles, samples were taken at locations were root systems of both species overlapped in the top soil. In total nine locations were sampled per stand type.
Root architecture and morphology
Nine soil monoliths (15 cm × 15 cm) per stand were carefully sampled at 0–10 cm depth at the end of July after removing the litter from the soil surface, following the method by Guo et al.97. Root samples were stored in plastic bags after coarsely shaking off the soil and transported to the laboratory (8 °C). In the lab, root samples were rinsed in water and adhering soil particles were carefully removed with small brushes; dead roots were discarded. Subsequently, 10–15 intact root branches per soil monolith and species were dissected into five branching orders32,33, first order roots being defined as the root tips. Lateral roots with ≤4 root orders and directly originating from branching orders ≥6 as well as root segments of orders ≥6 were discarded. Root order samples were individually imaged with a flatbed scanner (Expression 10000XL with transparency unit, Epson, Japan; 600 dpi, gray-scale) and analyzed with the software WinRhizo 2004b (Regent Instruments, Québec, Canada) for morphological traits, including total root length (cm), surface area (cm2) and average root diameter (AD; mm). The root segments were dried to constant mass (65 °C) and dry mass was determined to an accuracy of ±0.1 mg (Sartorius BT 125D, Göttingen, Germany). Specific root area (SRA; m2 g−1) and specific root length (SRL; m g−1) were calculated by dividing the surface area/length by the dry mass. Root architecture traits calculated were length (cm) and biomass (g) per root order98,99. Average SRL, SRA and AD for root orders 1–3 were calculated by multiplying the respective values of root orders 1 to 3 (i.e. “absorptive” root segments) with their respective biomass frequency (1–3) in individual root branches.
Root respiration and root chemistry
Small batches of roots were sampled as describes above and immediately transported to the lab where roots were rinsed carefully with de-ionized water to remove adhering soil particles. Three root branches per soil pit were sectioned into root orders as described above; dead roots were discarded. Root respiration measurements were initiated within 40–50 min after root sampling and were completed within 4 hours after sample collection. Individual root order samples (approx. 0.5 g fresh weight) were placed in vented chambers and equilibrated for 20 min to 18 °C; root desiccation was avoided by placing a piece of moist paper towel into each chamber until measurements started. Root respiration was approximated by measuring the O2 consumption for 20 min, using gas-phase O2-electrodes (Hansatech, Norfolk, UK), connected to a circulating water bath (18 °C)100. After measurements, the samples were dried (65 °C, 72 h) and weighed (±0.01 mg). Root respiration was calculated as nmol O2 g−1 s−1 by dividing the respiration rate by the corresponding dry weight. Per soil pit, three respiration measurements per root order and species were conducted, resulting in 27 respiration measurements per root order and species in both mono and mixed plantations (n = 27; 540 measurements in total). The dried samples were individually ground and homogenized; anElemental Analyzer (Vario MACRO; Elementar, Langenselbold, Germany) was utilized to determine N and C concentrations (%). C/N ratios were calculated.
Root anatomy and Mycorrhizal colonization
Five randomly selected root branches per species and competition situation (sampling scheme see above) were gently washed in de-ionized water and immediately fixed in Formalin-Aceto-Alcohol (FAA) solution (90 ml 50% ethanol, 5 ml 100% glacial acetic acid, 5 ml 37% methanol) and stored in a refrigerator (4 °C). In the lab, thirty segments each of 1st and 2nd order roots were stained with safranin-fast green, dehydrated in 70, 85, 95 and 100% EtOH, and embedded in paraffin. Cross sections (8-μm) were prepared with a microtome and photographed under a compound microscope (BX-51; Olympus Corporation, Tokyo, Japan). Cortex thickness (µm) and stele diameter (µm) were measured97; the cortex:stele ratio was calculated.
The colonization of walnut and larch roots with arbuscular (AM) and ecto (ECM) mycorrhizal fungi, respectively, wasinvestigated on 1st order roots (root tips). For determining the AM colonization rate, walnut root segments were bleached with 10% KOH (90 °C, 50 min), rinsed in distilled water, and acidified (3.7% HCl, 15 min, room temp.). After repeated cleaning with distilled water, the roots were stained for 90–120 s in lactophenol-blue (1 g L−1, pH 2.3; Merck)101. To remove unspecific colorant from the plant tissue, the roots were incubate for 60 min in an acidic glycerin solution (50 ml glycerin, 45 ml H2O, 5 ml 1% HCl) at room temperature; the solution was renewed after 30 min. The roots were then stored in 50% glycerine until mounting on microscope slides as squash preparation and AM colonization rate determination102. The infection rate of ECM on larch roots was determined by counting the root tips with a hyphae mantel under a stereomicroscope (40×); >100 tips were screened per larch root branch.
Statistical analysis
The statistical analyses were carried out with the software IBM SPSS 21.0 and Microsoft Excel 2010. Sigma Plot 12.5 (Systat software Inc.) was used to draw the figures. The effect of species, treatment and root order were analyzed by analysis of variance (ANOVA). Further, subsequent pair-wise comparisons were performed to identify the differences in the root traits using Tukey’s HSD test. Normality of data distribution was checked with a Shapiro-Wilk test. All data is displayed as mean ± standard error (SE). All statistical relationships were considered significant at P < 0.05.
Electronic supplementary material
Acknowledgements
The Fundamental Research Funds for the Central University (2572016EAJ1) and National Natural Science Fundation of China (NSFC31170583) supported this work. The authors thank three anonymous reviewers and the editor for their helpful comments improving the manuscript.
Author Contributions
Y.L. designed and supervised the project and gained financial support, S., M.R. and Z.J. performed the experiment, J.L. and F.K. analyzed the data, S. and B.R. wrote and edited the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27832-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hlásny T, Turcani M. Persisting bark beetle outbreak indicates the unsustainability of secondary Norway spruce forests: case study from Central Europe. Annals of forest science. 2013;70:481–491. doi: 10.1007/s13595-013-0279-7. [DOI] [Google Scholar]
- 2.Lévesque MM, et al. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Global Change Biology. 2013;19:3184–3199. doi: 10.1111/gcb.12268. [DOI] [PubMed] [Google Scholar]
- 3.Felton A, et al. Replacing monocultures with mixed-species stands: Ecosystem service implications of two production forest alternatives in Sweden. Ambio. 2016;45:124–139. doi: 10.1007/s13280-015-0749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen HY, et al. Are mixed-species stands more productive than single-species stands: an empirical test of three forest types in British Columbia and Alberta. Canadian Journal of Forest Research. 2003;33:1227–1237. doi: 10.1139/x03-048. [DOI] [Google Scholar]
- 5.Erickson HE, Harrington CA, Marshall DD. Tree growth at stand and individual scales in two dual-species mixture experiments in southern Washington State, USA. Canadian journal of forest research. 2009;39:1119–1132. doi: 10.1139/X09-040. [DOI] [Google Scholar]
- 6.Yang L, Wang P, Kong C. Effect of larch (Larix gmelini Rupr.) root exudates on Manchurian walnut (Juglans mandshurica Maxim.) growth and soil juglone in a mixed-species plantation. Plant and soil. 2010;329:249–258. doi: 10.1007/s11104-009-0149-0. [DOI] [Google Scholar]
- 7.Falik O, Reides P, Gersani M, Novoplansky A. Self/non‐self discrimination in roots. Journal of Ecology. 2003;91:525–531. doi: 10.1046/j.1365-2745.2003.00795.x. [DOI] [Google Scholar]
- 8.Jose S, Williams R, Zamora D. Belowground ecological interactions in mixed-species forest plantations. Forest ecology and management. 2006;233:231–239. doi: 10.1016/j.foreco.2006.05.014. [DOI] [Google Scholar]
- 9.Schenk HJ, Callaway RM, Mahall B. Spatial root segregation: are plants territorial? Advances in ecological research. 1999;28:145–180. doi: 10.1016/S0065-2504(08)60032-X. [DOI] [Google Scholar]
- 10.Li-xue Y. Effect of water extracts of larch on growth of Manchurian walnut seedlings. Journal of forestry research. 2005;16:285–288. doi: 10.1007/BF02858190. [DOI] [Google Scholar]
- 11.Li J, et al. Larch (larix gmelinii) bulk soil phenolic acids promote manchurian walnut (juglans manshurica) growth and soilmicroorganism manchurian walnut (juglans manshurica) growth and soilmicroorganism biomass. Pakistan Journal of Botany. 2016;48:2549–2556. [Google Scholar]
- 12.Lixue Y. Bioassay of allelopathical activity of larch (Larix gmelini) aqueous extracts against Juglans mandshurica. Journal-Northeast Forestry University-Chinese Edition. 2006;34:15. [Google Scholar]
- 13.Shi F, Chen X, Chen N. Study on the artificial mixed forest of Juglans mandshurica and Larix olgensis. J Northeast Forest Univ. 1991;19:32–43. [Google Scholar]
- 14.Yang L, Yan X, Kong C. Allelopathic potential of root exudates of larch (Larix gmelini) on Manchurian walnut (Juglans mandshurica) Allelopathy Journal. 2007;20:127. [Google Scholar]
- 15.Sun Y, Yang L, Wang Z, Fan J. Temporal variations in soil juglone and soil microbial community structure under Manchurian walnut (Juglans mandshurica Maxim.) plantations. Allelopathy Journal. 2013;31:169–180. [Google Scholar]
- 16.Xu S, Tang W, Han Z. Studies on the toxicity constituents of Juglans mandshurica Maxim. J Shenyang Agric Univ. 1986;17:34–39. [Google Scholar]
- 17.Yang, L., Sun, Y., Wang, D. & Fan, J. Spatial distribution of total phenols and soil microbial populations in Manchurian walnut (Juglans mandshurica Maxim.) and its mixed-species plantations. Allelopathy Journal29 (2012).
- 18.Jackson RB, Mooney H, Schulze E-D. A global budget for fine root biomass, surface area, and nutrient contents. Proceedings of the National Academy of Sciences. 1997;94:7362–7366. doi: 10.1073/pnas.94.14.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luke McCormack M, Adams TS, Smithwick EA, Eissenstat DM. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytologist. 2012;195:823–831. doi: 10.1111/j.1469-8137.2012.04198.x. [DOI] [PubMed] [Google Scholar]
- 20.Paezgarcia A, et al. Root traits and phenotyping strategies for plant improvement. Plants. 2015;4:334–355. doi: 10.3390/plants4020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coomes DA, Grubb PJ. Impacts of root competition in forests and woodlands: a theoretical framework and review of experiments. Ecological monographs. 2000;70:171–207. doi: 10.1890/0012-9615(2000)070[0171:IORCIF]2.0.CO;2. [DOI] [Google Scholar]
- 22.Casper BB, Jackson RB. Plant competition underground. Annual review of ecology and systematics. 1997;28:545–570. doi: 10.1146/annurev.ecolsys.28.1.545. [DOI] [Google Scholar]
- 23.Wilson, J. B. Shoot competition and root competition. Journal of applied ecology, 279–296 (1988).
- 24.Bohanek JR, Groninger JW. Productivity of European black alder (Alnus glutinosa) interplanted with black walnut (Juglans nigra) in Illinois, USA. Agroforestry systems. 2005;64:99–106. doi: 10.1007/s10457-004-0523-0. [DOI] [Google Scholar]
- 25.Schneiderhan, F. The black walnut (Juglans nigra L.) as a cause of the death of apple trees. Phytopathology17 (1927).
- 26.Hajek P, Hertel D, Leuschner C. Root order-and root age-dependent response of two poplar species to belowground competition. Plant and soil. 2014;377:337–355. doi: 10.1007/s11104-013-2007-3. [DOI] [Google Scholar]
- 27.Rewald B, Leuschner C. Belowground competition in a broad-leaved temperate mixed forest: pattern analysis and experiments in a four-species stand. European Journal of Forest Research. 2009;128:387–398. doi: 10.1007/s10342-009-0276-4. [DOI] [Google Scholar]
- 28.Fujii S, Kasuya N. Fine root biomass and morphology of Pinus densiflora under competitive stress by Chamaecyparis obtusa. Journal of Forest Research. 2008;13:185–189. doi: 10.1007/s10310-008-0063-y. [DOI] [Google Scholar]
- 29.Ostonen I, et al. Specific root length as an indicator of environmental change. Plant Biosystems. 2007;141:426–442. doi: 10.1080/11263500701626069. [DOI] [Google Scholar]
- 30.Volder A, Smart DR, Bloom AJ, Eissenstat DM. Rapid decline in nitrate uptake and respiration with age in fine lateral roots of grape: implications for root efficiency and competitive effectiveness. New Phytologist. 2005;165:493–502. doi: 10.1111/j.1469-8137.2004.01222.x. [DOI] [PubMed] [Google Scholar]
- 31.McCormack ML, et al. Redefining fine roots improves understanding of below‐ground contributions to terrestrial biosphere processes. New Phytologist. 2015;207:505–518. doi: 10.1111/nph.13363. [DOI] [PubMed] [Google Scholar]
- 32.Pregitzer KS, et al. Fine root architecture of nine North American trees. Ecological Monographs. 2002;72:293–309. doi: 10.1890/0012-9615(2002)072[0293:FRAONN]2.0.CO;2. [DOI] [Google Scholar]
- 33.Wang Z, Guo D, Wang X, Gu J, Mei L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant and Soil. 2006;288:155–171. doi: 10.1007/s11104-006-9101-8. [DOI] [Google Scholar]
- 34.Bu W, et al. Interspecific and intraspecific variation in specific root length drives aboveground biodiversity effects in young experimental forest stands. Journal of Plant Ecology. 2017;10:158–169. doi: 10.1093/jpe/rtw096. [DOI] [Google Scholar]
- 35.Lei P, Scherer-Lorenzen M, Bauhus J. The effect of tree species diversity on fine-root production in a young temperate forest. Oecologia. 2012;169:1105–1115. doi: 10.1007/s00442-012-2259-2. [DOI] [PubMed] [Google Scholar]
- 36.Silvertown J, Silvertown J. Plant coexistence and the niche. Trends Ecol Evol 19: 605-611. Trends in Ecology & Evolution. 2004;19:605–611. doi: 10.1016/j.tree.2004.09.003. [DOI] [Google Scholar]
- 37.Strand AE, Pritchard SG, Mccormack ML, Davis MA, Oren R. Irreconcilable Differences: Fine-Root Life Spans and Soil Carbon Persistence. Science. 2008;319:456–458. doi: 10.1126/science.1151382. [DOI] [PubMed] [Google Scholar]
- 38.Bauhus J, Messier C. Soil exploitation strategies of fine roots in different tree species o. Canadian Journal of Forest Research. 1999;29:260–273. [Google Scholar]
- 39.Kenny M. Fine-root biomass and production in Scots pine stands. Tree Physiology. 2001;21:193–198. doi: 10.1093/treephys/21.2-3.193. [DOI] [PubMed] [Google Scholar]
- 40.Pregitzer KS, Laskowski MJ, Burton AJ, Lessard VC, Zak DR. Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiology. 1998;18:665–670. doi: 10.1093/treephys/18.10.665. [DOI] [PubMed] [Google Scholar]
- 41.Guo D, Mitchell RJj. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia. 2004;140:450–457. doi: 10.1007/s00442-004-1596-1. [DOI] [PubMed] [Google Scholar]
- 42.Rewald B, Ephrath JE, Rachmilevitch S. A root is a root is a root? Water uptake rates of Citrus root orders. Plant Cell & Environment. 2015;34:33–42. doi: 10.1111/j.1365-3040.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 43.Rewald B, Leuschner C, Wiesman Z, Ephrath JE. Influence of salinity on root hydraulic properties of three olive varieties. Giornale Botanico Italiano. 2011;145:12–22. [Google Scholar]
- 44.Kong D, et al. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytologist. 2014;203:863–872. doi: 10.1111/nph.12842. [DOI] [PubMed] [Google Scholar]
- 45.Liu B, et al. Complementarity in nutrient foraging strategies of absorptive fine roots and arbuscular mycorrhizal fungi across 14 coexisting subtropical tree species. New Phytologist. 2015;208:125–136. doi: 10.1111/nph.13434. [DOI] [PubMed] [Google Scholar]
- 46.Guo D, et al. Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytologist. 2008;177:443–456. doi: 10.1111/j.1469-8137.2007.02331.x. [DOI] [PubMed] [Google Scholar]
- 47.Reich PB, Walters MB, Tjoelker MG, Vanderklein D, Buschena C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Functional Ecology. 1998;12:395–405. doi: 10.1046/j.1365-2435.1998.00209.x. [DOI] [Google Scholar]
- 48.Jia S, Wang Z, Li X, Zhang X, Mclaughlin NB. Effect of nitrogen fertilizer, root branch order and temperature on respiration and tissue N concentration of fine roots in Larix gmelinii and Fraxinus mandshurica. Tree Physiology. 2011;31:718–726. doi: 10.1093/treephys/tpr057. [DOI] [PubMed] [Google Scholar]
- 49.Hanson PJ, Edwards NT, Garten CT, Andrews JA. Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry. 2000;48:115–146. doi: 10.1023/A:1006244819642. [DOI] [Google Scholar]
- 50.King JS, Pregitzer KS, Zak DR, Holmes WE, Schmidt K. Fine root chemistry and decomposition in model communities of north-temperate tree species show little response to elevated atmospheric CO2 and varying soil resource availability. Oecologia. 2005;146:318–328. doi: 10.1007/s00442-005-0191-4. [DOI] [PubMed] [Google Scholar]
- 51.Buchmann N, Ehleringer JR. CO2 concentration profiles, and carbon and oxygen isotopes in C3 and C4 crop canopies. Agricultural & Forest Meteorology. 1998;89:45–58. doi: 10.1016/S0168-1923(97)00059-2. [DOI] [Google Scholar]
- 52.Prueger JH, Hatfield JL, Parkin TB, Kustas WP, Kaspar TC. Carbon Dioxide Dynamics During a Growing Season in Midwestern Cropping Systems. Environmental Management. 2004;33:330–343. doi: 10.1007/s00267-003-9142-1. [DOI] [Google Scholar]
- 53.Reich PB. The world‐wide ‘fast–slow’plant economics spectrum: a traits manifesto. Journal of Ecology. 2014;102:275–301. doi: 10.1111/1365-2745.12211. [DOI] [Google Scholar]
- 54.Valverde-Barrantes OJ, Smemo KA, Feinstein LM, Kershner MW, Blackwood CB. The distribution of below-ground traits is explained by intrinsic species differences and intraspecific plasticity in response to root neighbours. Journal of Ecology. 2013;101:933–942. doi: 10.1111/1365-2745.12087. [DOI] [Google Scholar]
- 55.Pedlar JH, Mckenney DW, Fraleigh S. Planting black walnut in southern Ontario: midrotation assessment of growth, yield, and silvicultural treatments. Canadian Journal of Forest Research. 2006;36:495–504. doi: 10.1139/x05-256. [DOI] [Google Scholar]
- 56.Xu, S., Tang, W. & Han, Z. Studies on the Toxicity Constituents of Juglans mandshurica Maxim. Journal of Shenyang Agricultural University (1986).
- 57.Rewald B, Rechenmacher A, Godbold DL . Focus Issue on Roots: It’s Complicated: Intraroot System Variability of Respiration and Morphological Traits in Four Deciduous Tree Species. Plant Physiology. 2014;166:736–745. doi: 10.1104/pp.114.240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rewald B, Godbold DL. Focus Issue on Roots: It’s Complicated: Intraroot System Variability of Respiration and Morphological Traits in Four Deciduous Tree Species. Plant Physiology. 2014;166:736–745. doi: 10.1104/pp.114.240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guariguata MR, Rheingans R, Montagnini F. Early Woody Invasion Under Tree Plantations in Costa Rica: Implications for Forest Restoration. Restoration Ecology. 1995;3:252–260. doi: 10.1111/j.1526-100X.1995.tb00092.x. [DOI] [Google Scholar]
- 60.Binkley D, Senock R, Bird S, Cole TG. Twenty years of stand development in pure and mixed stands of Eucalyptus saligna and nitrogen-fixing Facaltaria moluccana. Forest Ecology & Management. 2003;182:93–102. doi: 10.1016/S0378-1127(03)00028-8. [DOI] [Google Scholar]
- 61.Beineke, W. F. Black walnut plantation management. FNT - Purdue University Cooperative Extension Service (USA) (1987).
- 62.Willis RJ. Juglans spp., juglone and allelopathy. Allelopathy Journal. 2000;7:1–55. [Google Scholar]
- 63.Rietveld, W. J. The significance of allelopathy in black walnut cultural systems [Juglans nigra, toxicity, associated forest trees, herbaceous vegetation]. Usda Forest Service General Technical Report Nc (1982).
- 64.Kroon, H. D., Mommer, L. & Nishiwaki, A. In APS Division of Atomic, Molecular & Optical Physics Meeting (2003).
- 65.Schenk HJ. Root competition: beyond resource depletion. Journal of Ecology. 2006;94:725–739. doi: 10.1111/j.1365-2745.2006.01124.x. [DOI] [Google Scholar]
- 66.Hajek P, Hertel D, Leuschner C. Root order- and root age-dependent response of two poplar species to belowground competition. Plant & Soil. 2014;377:337–355. doi: 10.1007/s11104-013-2007-3. [DOI] [Google Scholar]
- 67.Leuschner C, et al. Stand fine root biomass and fine root morphology in old-growth beech forests as a function of precipitation and soil fertility. Plant and Soil. 2004;258:43–56. doi: 10.1023/B:PLSO.0000016508.20173.80. [DOI] [Google Scholar]
- 68.Eissenstat D, Yanai R. The ecology of root lifespan. Advances in ecological research. 1997;27:1–60. doi: 10.1016/S0065-2504(08)60005-7. [DOI] [Google Scholar]
- 69.Ostonen I, et al. Morphological adaptations of fine roots in Scots pine (Pinus sylvestris L.), silver birch (Betula pendula Roth.) and black alder (Alnus glutinosa (L.) Gaertn.) stands in recultivated areas of oil shale mining and semicoke hills. Oil Shale. 2006;23:187–202. [Google Scholar]
- 70.Gambetta GA, et al. Water uptake along the length of grapevine fine roots: developmental anatomy, tissue-specific aquaporin expression, and pathways of water transport. Plant Physiology. 2013;163:1254–1265. doi: 10.1104/pp.113.221283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Dong X, Wang H, Wang Z, Gu J. Root tip morphology, anatomy, chemistry and potential hydraulic conductivity vary with soil depth in three temperate hardwood species. Tree Physiology. 2016;36:99–108. doi: 10.1093/treephys/tpv094. [DOI] [PubMed] [Google Scholar]
- 72.Chen HB, Wei X, Wang J, Wang ZQ. Morphological and anatomical responses of Fraxinus mandshurica seedling roots to different nitrogen concentrations. Scientia Silvae Sinicae. 2010;46:61–66. [Google Scholar]
- 73.Wahl S, Ryser P, Edwards PJ. Phenotypic Plasticity of Grass Root Anatomy in Response to Light Intensity and Nutrient Supply. Annals of Botany. 2001;88:1071–1078. doi: 10.1006/anbo.2001.1551. [DOI] [Google Scholar]
- 74.Hejl AM, Koster KL. Juglone Disrupts Root Plasma Membrane H+-ATPase Activity and Impairs Water Uptake, Root Respiration, and Growth in Soybean (Glycine max) and Corn (Zea mays) Journal of Chemical Ecology. 2004;30:453–471. doi: 10.1023/B:JOEC.0000017988.20530.d5. [DOI] [PubMed] [Google Scholar]
- 75.Kong, D. L. et al. Economic strategies of plant absorptive roots vary with root diameter. Biogeosciences13 (2016).
- 76.Zhang J, et al. Effects of larch (Larix gmelinii) phenolic acids on manchurian ash (Fraxinus mandshurica) soil microbial community structure. Allelopathy Journal. 2016;37:123–135. [Google Scholar]
- 77.Rewald, B., Shelef, O., Ephrath, J. E. & Rachmilevitch, S. Adaptive Plasticity of Salt-Stressed Root Systems (2013).
- 78.Meier IC, Leuschner C. Genotypic variation and phenotypic plasticity in the drought response of fine roots of European beech. Tree Physiology. 2008;28:297–309. doi: 10.1093/treephys/28.2.297. [DOI] [PubMed] [Google Scholar]
- 79.Day DA, Wilson D, Lambers H. Regulation of Respiration in the Leaves and Roots of Two Lolium perenne Populations with Contrasting Mature Leaf Respiration Rates and Crop Yields. Plant Physiology. 1985;78:678–683. doi: 10.1104/pp.78.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qi JG, Marshall JD, Mattson KG. High soil carbon dioxide concentrations inhibit root respiration of Douglas fir. New Phytologist. 1994;128:435–442. doi: 10.1111/j.1469-8137.1994.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 81.Jia, S., Mclaughlin, N. B., Gu, J., Li, X. & Wang, Z. Relationships between root respiration rate and root morphology, chemistry and anatomy in and Tree Physiology, 33(6), 579–589 (2013). [DOI] [PubMed]
- 82.Laureano RG, et al. The cost of stress resistance: construction and maintenance costs of leaves and roots in two populations of Quercus ilex. Tree Physiology. 2008;28:1721–1728. doi: 10.1093/treephys/28.11.1721. [DOI] [PubMed] [Google Scholar]
- 83.Simons, B. H. & Lambers, J. T. The alternative oxidase: is it a respiratory pathway allowing a plant to cope with stress? (1999).
- 84.Atkin OK. Irradiance, Temperature and Rainfall Influence Leaf Dark Respiration in Woody Plants: Evidence from Comparisons across 20 Sites. New Phytologist. 2006;169:309–319. doi: 10.1111/j.1469-8137.2005.01590.x. [DOI] [PubMed] [Google Scholar]
- 85.Alías JC, Sosa T, Escudero JC, Chaves N. Autotoxicity Against Germination and Seedling Emergence in Cistus ladanifer L. Plant & Soil. 2006;282:327–332. doi: 10.1007/s11104-005-6066-y. [DOI] [Google Scholar]
- 86.von Kiparski GR, Lee LS, Gillespie AR. Occurrence and fate of the phytotoxin juglone in alley soils under black walnut trees. Journal of Environmental Quality. 2007;36:709. doi: 10.2134/jeq2006.0231. [DOI] [PubMed] [Google Scholar]
- 87.Yang LX, Sun YZ, Wang DL, Fan J. Spatial distribution of total phenols and soil microbial populations in Manchurian walnut (Juglans mandshurica Maxim.) and its mixed-species plantations. Allelopathy Journal. 2012;29:263–269. [Google Scholar]
- 88.Peñuelas J, Ribascarbo M, Giles L. Effects of allelochemicals on plant respiration and oxygen isotope fractionation by the alternative oxidase. Journal of Chemical Ecology. 1996;22:801–805. doi: 10.1007/BF02033587. [DOI] [PubMed] [Google Scholar]
- 89.Lambers, H., Shane, M. W., Spiertz, J. H. J., Struik, P. C. & Laar, H. H. V. Role of root clusters in phosphorus acquisition and increasing biological diversity in agriculture. 237–250 (2007).
- 90.Smith, S. E. & David Read, F. Mycorrhizal Symbiosis (Third Edition) (2008).
- 91.Marschner H, Häussling M, George E. Ammonium and nitrate uptake rates and rhizosphere pH in non-mycorrhizal roots of Norway spruce [Picea abies (L.) Karst.] Trees. 1991;5:14–21. doi: 10.1007/BF00225330. [DOI] [Google Scholar]
- 92.Orwin KH, et al. Linkages of plant traits to soil properties and the functioning of temperate grassland. Journal of Ecology. 2010;98:1074–1083. doi: 10.1111/j.1365-2745.2010.01679.x. [DOI] [Google Scholar]
- 93.Fitter, A. H. Characteristics and functions of root systems. Plant Roots the Hidden Half (1991).
- 94.Schmidt SK. Degradation of juglone by soil bacteria. Journal of Chemical Ecology. 1988;14:1561–1571. doi: 10.1007/BF01012522. [DOI] [PubMed] [Google Scholar]
- 95.Gong Z, Zhang X, Chen J, Zhang G. Origin and development of soil science in ancient China. Geoderma. 2003;115:3–13. doi: 10.1016/S0016-7061(03)00071-5. [DOI] [Google Scholar]
- 96.Razaq M, Shen H-l, Sher H, Zhang P. Influence of biochar and nitrogen on fine root morphology, physiology, and chemistry of Acer mono. Scientific reports. 2017;7:5367. doi: 10.1038/s41598-017-05721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo D, et al. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty‐three Chinese temperate tree species. New Phytologist. 2008;180:673–683. doi: 10.1111/j.1469-8137.2008.02573.x. [DOI] [PubMed] [Google Scholar]
- 98.Rewald, B., Rechenmacher, A. & Godbold, D. L. It’s Complicated: Intraroot System Variability of Respiration and Morphological Traits in Four Deciduous (2014). [DOI] [PMC free article] [PubMed]
- 99.Comas L, Eissenstat D. Patterns in root trait variation among 25 co‐existing North American forest species. New Phytologist. 2009;182:919–928. doi: 10.1111/j.1469-8137.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 100.Burton A, Pregitzer K, Ruess R, Hendrick R, Allen M. Root respiration in North American forests: effects of nitrogen concentration and temperature across biomes. Oecologia. 2002;131:559–568. doi: 10.1007/s00442-002-0931-7. [DOI] [PubMed] [Google Scholar]
- 101.Schmitz O, Danneberg G, Hundeshagen B, Klingner A, Bothe H. Quantification of vesicular-arbuscular mycorrhiza by biochemical parameters. Journal of Plant Physiology. 1991;139:106–114. doi: 10.1016/S0176-1617(11)80174-4. [DOI] [Google Scholar]
- 102.McGonigle T, Miller M, Evans D, Fairchild G, Swan J. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New phytologist. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.