Abstract
Hydrothermal synthesis is challenging in metal oxide systems with diverse polymorphism, as reaction products are often sensitive to subtle variations in synthesis parameters. This sensitivity is rooted in the non-equilibrium nature of low-temperature crystallization, where competition between different metastable phases can lead to complex multistage crystallization pathways. Here, we propose an ab initio framework to predict how particle size and solution composition influence polymorph stability during nucleation and growth. We validate this framework using in situ X-ray scattering, by monitoring how the hydrothermal synthesis of MnO2 proceeds through different crystallization pathways under varying solution potassium ion concentrations ([K+] = 0, 0.2, and 0.33 M). We find that our computed size-dependent phase diagrams qualitatively capture which metastable polymorphs appear, the order of their appearance, and their relative lifetimes. Our combined computational and experimental approach offers a rational and systematic paradigm for the aqueous synthesis of target metal oxides.
Minor variations in synthesis conditions can redirect crystallization pathways through different nonequilibrium intermediates. Here, the authors present a theoretical framework to predict which polymorphs appear during MnO2 precipitation, which is validated by in situ X-ray scattering of reaction progression.
Introduction
Rapid improvements in the theory1,2 and characterization3,4 of inorganic compounds have led to tremendous advances in our understanding of structure-property relationships. However, similar quantitative and predictive theories for understanding synthesis–structure relationships remain largely undeveloped. In particular, finding the conditions required to synthesize a particular stable or metastable phase can be a time consuming trial-and-error process. While phase diagrams offer guiding insights on the relative stability of reaction end-products, little is known about the energy landscape that a material traverses as it nucleates and grows. There is growing evidence of the complexity of this energy landscape, as well-crystallized transient metastable phases are often observed to form before the equilibrium phase, during both solid-state5–8 and aqueous synthesis9,10. Furthermore, minor variations in synthesis conditions can affect the lifetimes of these metastable phases11, or even redirect a crystallization pathway through very different metastable phases12,13. Understanding how synthesis parameters influence the formation and persistence of these metastable intermediates offers a roadmap towards rational materials synthesis, as metastable phases can often exhibit superior properties to their stable counterparts14–17.
Recent experimental and theoretical studies indicate that metal oxides can undergo dramatic thermodynamic crossovers in polymorph stability, as a function of both particle size and composition. Small particles exhibit large surface-area-to-volume-ratios, meaning a bulk metastable phase with low surface energy can become thermodynamically-stabilized at the nanoscale18–20. Alkali ions from solution can preferentially intercalate into specific metal-oxygen frameworks, stabilizing a polymorphic structure at off-stoichiometric compositions21. These two properties of size and composition are especially relevant during hydrothermal synthesis; as all materials nucleate and grow through a nanoscale size regime, and the intercalation of impurity ions from solution can stabilize off-stoichiometric polymorphs during materials growth. Here, we construct aqueous phase stability diagrams that explicitly incorporate the thermodynamic effects of particle size and composition22. These size- and composition-dependent Pourbaix diagrams offer a general theoretical framework to rationalize which non-equilibrium phases appear during the aqueous precipitation of transition metal oxides, and the order that they appear; laying an important foundation for a predictive theory of materials synthesis.
To validate our theoretical framework, we experimentally conduct in situ hydrothermal synthesis of manganese oxide (MnO2) at varying conditions, and compare experimentally observed crystallization pathways to the ab initio computed phase diagrams. We choose MnO2 because it exhibits diverse polymorphism23,24 and many of the structural polymorphs are of interest for specific applications, such as Li-ion battery cathodes, catalysts, capacitors, and molecular sieves, etc24–27. Empirical recipes for the synthesis of specific manganese oxide polymorphs have been cataloged27–29, and generally exhibit two common themes; they involve quenching a multistage crystallization at intermediate times to retrieve a metastable phase30, and often require a structure-directing impurity ion to drive the formation of a specific phase21. However, it is difficult to extend these recipes to other metal oxide systems, as these empirical recipes have not been rationalized from general thermochemical principles. Here, we characterize in situ the hydrothermal synthesis of manganese oxides under varying [K+] in solution, to direct the hydrothermal reduction of a MnO4− based precursor down different crystallization pathways. In situ X-ray wide-angle scattering (WAXS) allows us to monitor the evolution of crystalline intermediates at different [K+], and compare the observed crystallization pathways against ab initio computed phase diagrams with axes of particle size and solution [K+].
Under all conditions, crystallization pathways proceed through intermediate metastable phases. We find that the order of observed metastable MnO2 phases corresponds qualitatively with a growth progression through the computed size-dependent phase diagrams, at the respective [K+]. This suggests that the observed metastable phases nucleate under instantaneous local thermodynamic conditions where they were once stable, and then grow into conditions where they become metastable, consistent with a concept of remnant metastability31. Our combined experimental and theoretical study emphasizes the importance of particle size and solution chemistry in influencing the free-energy landscape of non-equilibrium crystallization, which directly governs polymorph-selection during hydrothermal synthesis. In addition, it offers evidence that a concept of remnant metastability can serve as a guiding principle to design rational synthesis pathways; prompting a more deliberate search for relevant thermodynamic parameters that govern structure-selection during materials synthesis.
Results
Theory of crystallization pathways
Crystallization initiates from a high-energy precursor, and traverses through a metastable free-energy landscape in the multistage nucleation and growth of a bulk macroscopic crystal32. Mapping this free-energy landscape lays a conceptual foundation for understanding structure-selection during multistage crystallization. Here, we discuss how surface energies and solution chemistry influence this free-energy landscape, by stabilizing specific MnO2 phases at various sizes and off-stoichiometric compositions.
Before reaching the bulk equilibrium state, all materials first nucleate and grow through the nanoscale regime, where surface energy contributions are significant. Small particle sizes, or large 1/R (R is the radius), can stabilize a bulk-metastable polymorph with low surface energy, leading to preferential nucleation of this metastable phase over the equilibrium phase. Figure 1 shows the size-dependent free-energy (Φ) versus the particle size (1/R), illustrating how the interplay between bulk and surface energies can lead to multistage crystallization. Starting from a supersaturated solution (gray line), a metastable phase with low surface energy, M, is the lowest free-energy phase at small sizes, meaning that M requires the smallest size-fluctuation to nucleate. After its nucleation, M reduces its free energy by crystal growth, consuming metal solute ions in the process. Because the barrier to crystal growth is smaller than the barrier to nucleation of a new phase, M may grow to a size region where the M is no longer size-stabilized. At this point, there is a thermodynamic driving force for a phase transformation from M onto S, the lower free-energy phase. The induction time to nucleate S depends on the surface energy of S and the thermodynamic driving force for transformation, ΔΦM->S11. A smaller ΔΦ leads to longer induction times, which would prolong the persistence of the metastable phase M. Once S nucleates, the entire system can reduce its free energy by the dissolution of M, and subsequent reprecipitation onto S.
Fig. 1.
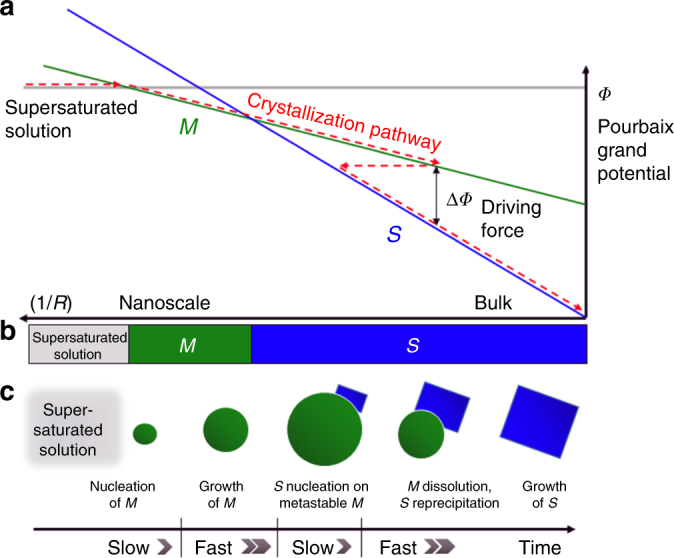
Schematic representation of remnant metastability in a crystallization pathway. a Free-energy of three phases (supersaturated solution (gray), M (green), S (blue)) as a function of the surface-area-to-volume ratio, 1/R (R is a particle radius). The gray line corresponds to the free-energy of a supersaturated solution, green is a metastable phase M that is size-stabilized by a low surface energy (given by the slope), and blue is the bulk equilibrium phase S, with high surface energy. b Phase diagram in the 1/R axis created from the projection of lowest free-energy phases. c A multistage crystallization pathway (red arrow in a) proceeds downhill in energy, but phase transformations are limited by nucleation. Crystal growth of M prior to the induction of S means M can grow into a size-regime where phase M is metastable. S will then nucleate, and quickly grow by consuming M via dissolution-reprecipitation. The characteristic length scale of size-driven phase transitions lies in the 2 nm–50 nm range
In the manganese oxides, we previously showed that the bulk free-energy of each polymorph, Φ, can also be influenced by alkali ion intercalation, which stabilizes different polymorphic frameworks at off-stoichiometric compositions21,23. For example, solution K+ ions can intercalate into the α- (tunnel) and δ- (layered) MnO2 polymorphs, stabilizing them as a function of aqueous K+ activity; and other MnO2 polymorphs can be stabilized by Li+, Na+, Ca2+, etc21. Intercalation of ions into polymorphic frameworks occurs ubiquitously in transition metal oxide systems, meaning solution chemistry can be considered a general handle for tuning bulk polymorph stability. To account for both the size and composition of manganese oxide particles, we can define the free-energy of a manganese oxide particle in water by a full size-dependent Pourbaix grand potential22, Φ, which is a function of the pH; the redox potential, E; the potassium ion activity which can be approximated by the potassium ion concentration, [K+]; and the particle size, signified by the surface-area-to-volume-ratio, or 1/R, where R is the particle radius. The complete equation for Φ is provided in the Methods. The thermochemical data for the K-Mn-O-H system were obtained from a comprehensive set of density functional theory calculations of pristine and off-stoichiometric bulk and surface energies. Our DFT calculations use the recently-developed SCAN metaGGA functional33, which is necessary to accurately reproduce energy differences between MnO2 polymorphs.
Role of [K+] in the crystallization pathways
We perform a joint computational and experimental study to quantify the thermodynamic role of [K+] as a structure-directing agent in the multistage crystallization of manganese oxide polymorphs to examine the robustness of the theoretical framework. Experimentally, we perform in situ WAXS to probe the hydrothermal reduction of a MnO4− precursor at three potassium concentrations: [K+] = 0, 0.2, and 0.33 M, denoted as PK = 0, PK = 0.2, and PK = 0.33, respectively. Crystallization reactions were performed between pH 1–2. We measured the redox potential of these aqueous solutions to be +1.2 V vs the standard hydrogen electrode (SHE), which decreased to +0.5 V after the reaction. The pH values and the change in redox potential are discussed in Supplementary Table 1.
We compute that there are four relevant MnO2 phases at these solution conditions; β-MnO2 (pyrolusite), α-MnO2 (hollandite), R-MnO2 (ramsdellite), and the layered δ-MnO2 (birnessite/vernadite). We note that the layered δ-phase encompasses a large family of structures with varying hydration, ion intercalation, and layer spacings34,35. Throughout this work, we distinguish two of the experimentally-resolved layered structures in this family as δ′ and δ′′. Another family of phases is the so-called intergrowth phases, which is a disordered intergrowth between R-MnO2 and β-MnO2 on a shared oxygen sub-lattice. In this paper, we name the observed intergrowth phase γ′ to distinguish this γ′ phase from the idealized γ-MnO2 structure, which is an ordered 50/50 intergrowth between R-MnO2 and β-MnO221. Detailed information of all the MnO2 phases relevant in this study is provided in Supplementary Table 2, and the structure files can be found in Supplementary Data 1–5.
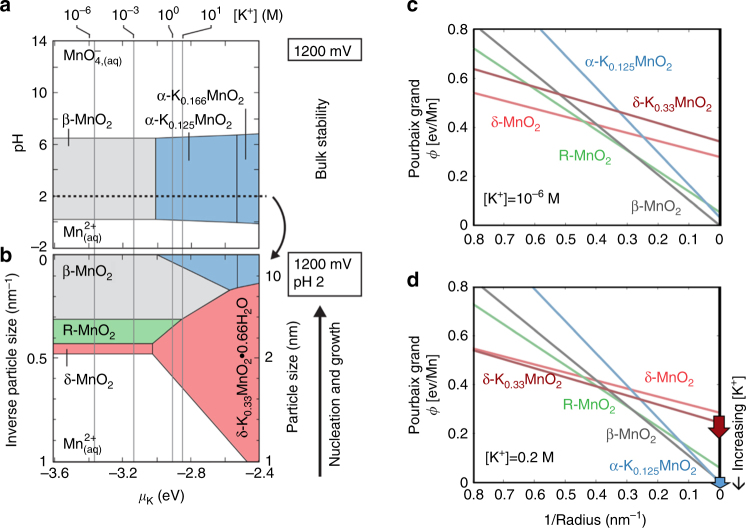
To rationalize the influence of [K+] on MnO2 crystallization, we calculate bulk and size-dependent phase diagrams at varying K+ chemical potential. The bulk phase diagram at E = 1.2 V (Fig. 2(a)) indicates that β-MnO2 is the equilibrium phase at low [K+], and that α-KxMnO2 can be stabilized against β-MnO2 under high [K+]. The size-dependent phase diagram (Fig. 2(b)), taken at E = 1.2 V and fixed pH = 2, reveals a more complicated phase stability landscape, showing that the low surface energies of δ-MnO2 and R-MnO2 can stabilize these bulk-metastable phases at small sizes and low [K+], and that a δ-K0.33MnO2∙0.66H2O phase can be size-stabilized at higher [K+]. Cutaways of Φ as a function of particle size, analogous to Fig. 1, are shown in Fig. 2(c) (low [K+]), and 2(d) (high [K+]). While bulk energetics would only show the stability of β-MnO2 or α-KxMnO2 at these conditions, the size-dependent Pourbaix potential reveals the metastable energy landscape the K-Mn-H2O system traverses as the particles nucleate and grow. Taken together, these diagrams map the free-energy landscape of MnO2 crystallization under the influence of varying [K+] and particle size, and provide a foundation towards rationalizing the order and lifetime of polymorphs in the phase evolution of the reaction pathways.
Fig. 2.
Phase diagrams of K-Mn oxide systems at E = 1.2 V. a pH-μK slice at 1/R = 0 indicating bulk stabilized phases. b 1/R-μK slice at pH = 2 illustrating the size-stabilized phases. c, d Pourbaix grand free-energies versus 1/R, showing the metastable energy landscape of crystal growth where [K+] = 10−6 M (c) and [K+] = 0.2 M (d). d is representative of pathways at both [K+] = 0.2 M and [K+] = 0.33 M due to their similar [K+]. The three reaction conditions are referred to as PK = 0, PK = 0.2, and PK = 0.33, respectively, based on their [K+]. , which means that the driving force for the transformation from the δ to the α phase decreases at a larger [K+]. The red and blue arrows indicate the trend that the slope of and changes with increasing [K+]
Crystallization pathway in low [K+] region
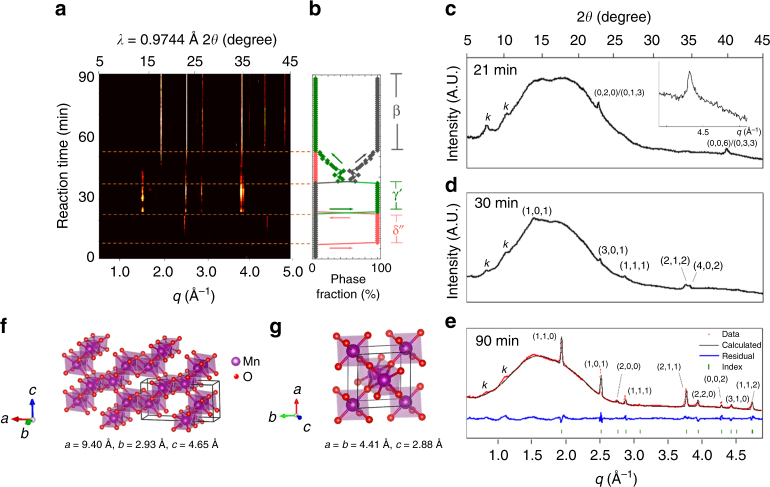
The in situ WAXS results for PK = 0 are summarized in Fig. 3. The evolution of intermediates along the reaction pathways are illustrated by the partially background-subtracted X-ray scattering profiles shown in Fig. 3(a), and the evolution of the phase fractions shown in Fig. 3(b); details of background subtraction and phase fraction calculations can be found in Supplementary Note 3 and 4. We observe a three-stage crystallization along PK = 0, in which δ′′ (belonging to the δ-family), γ′ (belonging to the intergrowth family), and β-MnO2 appear in a successive order that qualitatively corresponds to growth through the size-dependent phase diagram in Fig. 2(b).
Fig. 3.
In situ X-ray scattering analysis of PK = 0. a The evolution of X-ray scattering profiles with time along PK = 0. q is related to the diffraction angle (θ) and incident wavelength (λ) by and λ = 0.9744 Å. b Phase fraction evolution calculated from the intensities of diffraction peaks in a. c–e X-ray scattering profiles of the δ′′ (c), γ′ (d), and β (e) phases, respectively. The inset figure in c shows the asymmetrical tail of the peak at q = 4.45 Å−1. The weak peaks around q = 0.9 and 1.2 are caused by a kapton film covering the capillary reactor (marked by k in c–e). The broad peak around q = 1.5–2.5 Å−1 is caused by imperfect background subtraction (see Supplementary Notes 3 for details). f Structure of a pristine R phase. The γ′ phase discussed in this paper is an R phase with a random amount of β (1 × 1 tunnel) intergrowth. g Refined structure of the β phase (see Supplementary Notes 5 for refinement details)
The first phase we observe is characterized by two diffraction peaks at q = 2.58 and 4.45 Å–1. The position and asymmetrical shape of the peaks are consistent with the intra-layer diffraction peaks of the layered δ′-MnO2 phase observed in PK = 0.2 and PK = 0.33 (Fig. 4(g)) and discussed in detail in the following section. However, the δ′′-phase is missing the two interlayer diffraction peaks present in the δ′ phase. This diffraction pattern suggests that, while the δ′′-phase is structurally related to the δ′-phase, the former may lack the long-ranged interlayer order as in the latter. Most likely, the δ′′-phase consists of small platelet structures, due to the absence of a highly asymmetric peak36 at high q that results from isolated MnO2 layers. This disorder may be caused by the lack of K+ in the system, which promotes strong stacking ordering due to the strong ionic K-O bonds35,37.
Fig. 4.
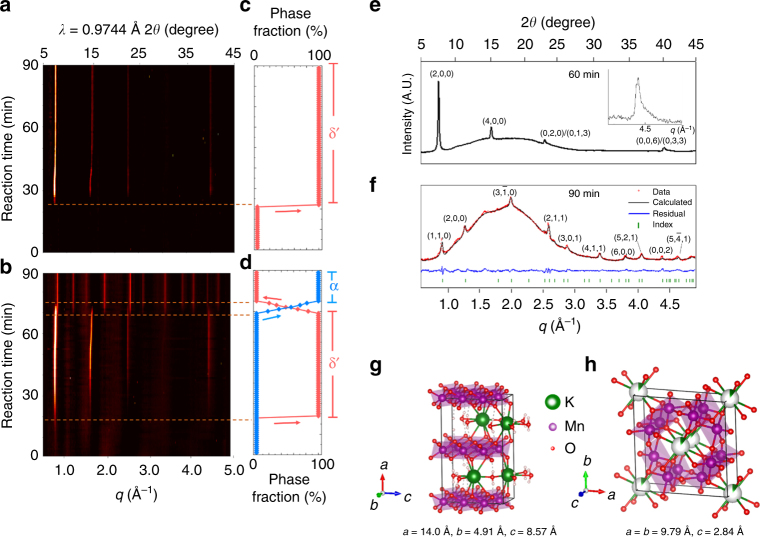
In situ X-ray scattering analysis of PK = 0.33 and PK = 0.2. a, b The evolution of X-ray scattering profile with time during hydrothermal synthesis along PK = 0.33 (a) and PK = 0.2 (b). c, d Phase fraction evolution calculated from the intensities of diffraction peaks in a, b, respectively. e, f X-ray scattering profiles of the δ′ phase (e) and α phase (f). The inset figure in d shows the asymmetrical tail of the peak at q = 4.45 Å−1. The broad peak around q = 1.5–2.5 Å−1 is caused by imperfect background removal, and is discussed in Supplementary Note 3. g, h Refined structures of the δ′ phase (g) and α phase (h). Manganese (purple), potassium (green), and oxygen (red) are represented by spheres, and the unit cell is identified by gray lines (see Supplementary Notes 5 for refinement details). The partially filled spheres represent the site fraction of the atoms
The second intermediate phase, with the diffraction profile shown in Fig. 3(d), is a ramsdellite (R)-like framework (1 × 2) tunnel (Fig. 3(f)) with some amount of random pyrolusite (1 × 1) tunnel intergrowths. We identify this phase as a ramsdellite structure containing de Wolff defects and microtwinning38,39, and refer to it as γ′-MnO2. The fraction of (1 × 1) intergrowth in the γ′-MnO2 that we observed was approximately 30%, and the detailed calculation can be found in Supplementary Note 6.
The final phase to appear has the scattering profile shown in Fig. 3(e) and is refined to be the β-MnO2 phase in Fig. 3(g), consistent with previous reports in low [K+] synthesis conditions40,41. This trend of transformation from δ′′ to γ′ and then to β-MnO2 also agrees with previous reports of Na-incorporated birnessite gradually transforming to (1 × 2) tunnel, and finally a (1 × 1) tunnel or β-MnO2 structure40.
The observed order of MnO2 phases from δ′′ → γ′ → β can be qualitatively mapped onto a growth progression through the size-dependent phase diagram of Fig. 2(b) at [K+] < 10−6 M. δ-MnO2 forms first, because δ-MnO2 is stabilized at small sizes by its low surface energy. The R-, γ′- and β- phases of MnO2 all share the same oxygen sub-lattice, and differ from one another by the manganese occupation of the octahedral lattice sites. The γ′-family represents a disordered structural continuum between the (1 × 2) tunnel R-MnO2 and the (1 × 1) tunnel β-MnO2, and in our experiment the observed γ′-MnO2 has 70% R-MnO2 character. Because the observed γ′-MnO2 is disordered, its thermodynamic properties cannot be directly computed in DFT, but the γ′-MnO2 bulk and surface energies are likely reasonably approximated by the structurally-similar, ordered R-MnO2 phase. In the size-dependent phase diagram, using the ordered R-MnO2 as a proxy for the experimentally observed disordered γ′ phase qualitatively captures the thermodynamic driving force in a size-driven phase progression from δ′′ → γ′. Finally, because β-MnO2 is the lowest bulk energy phase, the disordered γ′ intermediate eventually transforms into the topotactically-related β-phase. This is a transformation pathway similar to previous experimental results42. Therefore, our experimentally observed phase progression qualitatively reflects a remnant metastability interpretation of the size-dependent phase diagram, where the major structural motifs of the experimentally observed δ′′, γ′, and β phases are captured by ordered δ-, R-, and β- phases computed by DFT. The consistency between experiment and the size-dependent phase diagram supports the utility of remnant metastability as a guiding principle for the synthesis planning of a multistage crystallization.
Crystallization pathways in the higher [K+] region
Aqueous K+ ions can be important structure-directing agents in the precipitation of MnO2 phases, as intercalation of potassium into tunnel frameworks changes the equilibrium phase from a (1 × 1) β-MnO2 phase to (2 × 2) α-MnO2 phase21,41,43, and also increases the stability of δ′-MnO2 by K+-intercalation between the layers. We will demonstrate that an intermediate [K+] indeed promotes the crystallization of the α- phase, but if the [K+] is too high, it can increase the persistence of the metastable δ- phase, which illustrates the competition between thermodynamic stability and the kinetics of phase transformations.
To validate the influence of K+ on redirecting the crystallization pathway, we perform in situ hydrothermal synthesis with [K+] = 0.2 M and [K+] = 0.33 M. Figure 4(a–d) show the in situ WAXS data and phase evolution versus time for the first 90 min of the reactions. PK = 0.2 proceeds via two crystallization stages over the course of 90 min; while for PK = 0.33, we observe only the first stage, which does not transform to the second stage even after a 70 h reaction (see Supplementary Figure 2).
We resolve the initial phase to be in the same layered δ-MnO2 phase family as found in PK = 0. This family of compounds is represented by the general chemical formula KxMnO2 ∙ yH2O (x = 0.25–0.75), where the amount of K+ intercalation and hydration changes the interlayer spacing of the MnO2 sheets44. To refine the structure and composition of the observed δ-phase, we first determine the d-spacing and relative intensities of the diffraction peaks from the WAXS data. As extracted from the diffraction profile in Fig. 4(e), the strongest diffraction peak, at q = 0.86 Å−1, corresponds to an interlayer d-spacing of 7.3 Å. We next use DFT to resolve which KxMnO2∙yH2O stoichiometry gives the best agreement with this diffraction profile, predicting a final δ′ stoichiometry of K0.33MnO2-0.66H2O, which is a P2-type layered structure, as shown in Fig. 4(g). Inductively coupled plasma (ICP) measurements on products synthesized ex situ were performed and confirmed the refined stoichiometry (see Supplementary Note 7 and 8 for details).
To confirm the structure of the δ′ phase, we match the plane spacings with the positions of the experimental diffraction profile in Fig. 4(e). The scattering profile of δ′ consists of four major peaks. The two peaks at q = 0.86 Å−1 (2,0,0), and 1.72 Å−1 (4,0,0) correspond to the interlayer spacing, and the two peaks at 2.58 Å−1 ((0,2,0)/(0,1,3)), and 4.45 Å−1 ((0,0,6)/(0,3,3)) correspond to intra-layer spacing of hexagonally close-packed Mn atoms in individual MnO2 layers in the theoretically-predicted K0.33MnO2-0.66H2O structure. For detailed hkl indexing of the δ′ phase, see Supplementary Figure 5. The two peaks at q = 2.58 and 4.45 Å−1 show asymmetric tails (see the insert Fig. 4(e)), suggesting the presence of stacking faults between the MnO2 layers45–47. However, the stacking faults and the limited number of diffraction features in the δ′ phase preclude reliable structure determination with Rietveld refinement.
In determining the end product for PK = 0.2 (Fig. 4(f)), we refine the scattering profile to α- MnO2. The refined chemical formula of the α phase is K0.10 ± 0.02MnO2, which matches the stoichiometry of the computationally predicted α-K0.125 MnO2 phase, shown in Fig. 4(h). ICP confirms that the α phase synthesized ex situ has a K to Mn ratio of 0.10 ± 0.01, consistent with the occupancy obtained from the Rietveld refinement (see Supplementary Note 5 and 8 for the details in refinements and ICP measurements, respectively).
On the computed size-dependent phase diagram, the stable phases as a function of increasing size at [K+] ~10−1 M proceeds as δ′-K0.33MnO2 → R-MnO2 → β-MnO2 → α-K0.125MnO2. The in situ observation of an intermediate δ-phase, followed by an α-phase, is consistent with the order progression as seen on the size-dependent phase diagram. However, why does neither the R-MnO2 nor the β-MnO2 phases form? At [K+] ~10−1 M, both R-MnO2 and β-MnO2 are size-stabilized between 2 and 10 nm, as seen in the size-dependent phase diagram in Fig. 2(b). The crystallites in the observed δ-K0.33MnO2 phase are estimated to be at least 30 nm in diameter by Scherrer analysis; much larger than the R-MnO2 and β-MnO2 stability regimes. Because the energy barrier to nucleation is larger than the barrier to crystal growth, the δ-phase likely nucleated and grew beyond the size-stability regime of R- and β-MnO2, so that these intermediate size-stabilized phases were skipped during multistage crystallization. Similar phenomena occur in other metal oxide systems, for example, in TiO2, the brookite polymorph is size-stabilized between anatase and rutile48, while in Fe2O3, the ϵ-Fe2O3 phase is size stabilized between γ-Fe2O3 and α-Fe2O349. Although experimentally, the formation of brookite TiO2 and ϵ-Fe2O3 during aqueous precipitation is rare, both brookite TiO2 and ϵ-Fe2O3 can be stabilized by annealing at constrained size regimes50,51. Hence, if crystal growth in our MnO2 system can be inhibited, it is plausible that a phase evolution of δ → R → β → α can be observed here as well.
From thermodynamic considerations, the δ′-phase is clearly metastable with respect to the α- phase, and increasing [K+] should only further increase the stability of the α phase. However, our experimental results demonstrate that the δ′ to α transformation occurs in only 20 min in the lower [K+] pathway (PK = 0.2) but that the δ′-phase can persist for more than 70 h in the higher [K+] pathway (PK=0.33). While a higher aqueous [K+] lowers the free-energy of both α-K0.125MnO2 and δ′-K0.33MnO2, the free-energy of the potassium-rich δ′-K0.33MnO2 phase is lowered more than that of the α-K0.125MnO2. This indicates that higher [K+] reduce the driving force (ΔΦ) required for the δ′-K0.33MnO2 to α-K0.125MnO2 transformation (blue/red arrows Fig. 2(d)). The result is that even though a high K+ activity should ultimately make α-K0.125MnO2 more stable, it concurrently increases the stability of the metastable δ′-K0.33MnO2 intermediate, inhibiting the kinetics of the δ′-to-α transformation, and prolonging the persistence of the δ′-K0.33MnO211. Our observations illustrate the competing effects of bulk thermodynamics and transformation kinetics in the progression of a multistage crystallization pathway, and highlights the importance of determining free-energy differences between reactants, metastable intermediates, and equilibrium end-products when understanding multistage crystallization.
Synthesizing desired intermediate phases
We have so far shown that it is possible to predict which metastable phases appear during crystallization. Next, to demonstrate if these metastable phases are obtainable, we isolate desired metastable phases by quenching the solution during a crystallization reaction. We quench PK = 0 and PK = 0.2 by stopping the reaction by breaking the glass capillary in half at appropriate reaction times to stop the reaction. The time points for harvesting the δ′ and δ′′ phases are determined by the progression of intermediate phases observed in the in situ scattering experiments, as shown in Fig. 4(b) and Fig. 3(a).
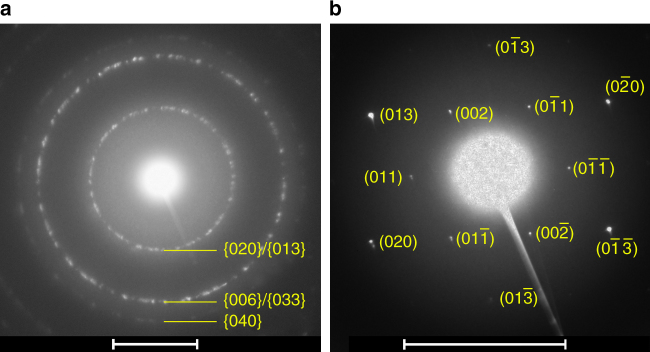
Using transmission electron microscopy (TEM), we confirm that we successfully isolated the transient metastable phases δ′ and δ′′. Figure 5 shows the selected area electron diffraction (SAED) patterns of the quenched δ′ and δ′′ phases. The reflections in Fig. 5(a) located on the diffraction rings labeled {020}/{013} and {006}/{033} were measured at q = 2.60 and 4.49 Å−1, respectively. These reflections are consistent with the d-spacing of the corresponding X-ray diffraction peaks in Fig. 4(e). The diffraction pattern from the δ′′ phase, shown in Fig. 5(b), exhibits similar d-spacings to that of the δ′ phase. However, the diffraction pattern for the δ′′ phase does not exhibit the same extent of hexagonal-like symmetry that is seen in the δ′ phase. This trend is consistent with the hypothesis that the δ′′ phase is lacking the interlayer order of the δ′ phase. Thus, we are able to demonstrate that is possible to not only predict which structures appear during the reaction, but that the metastable phases can in fact be isolated from multistage crystallization and retained.
Fig. 5.
Quenched phases. Selected area electron diffraction (SAED) patterns of the quenched δ′ (a) and δ′′ (b) phases quenched from PK=0.2 and PK=0, respectively. The scale bars are 5 nm−1 in both of the figures
Overall, in this work, we performed in situ WAXS to monitor the multistage crystallization of manganese oxides under the hydrothermal synthesis condition of [K+] = 0, 0.2, and 0.33 M. We demonstrated that particle size and off-stoichiometric composition can affect the free-energy landscape of metal oxide precipitation, influencing which metastable phases appear during multistage crystallization, the order in which they appear, and the persistence of these metastable phases. Furthermore, we showed that while a calculated, size-dependent phase diagram qualitatively maps out crystallization pathways under specific reaction conditions, the driving force (ΔΦ) between two phases can determine the observable progression of crystalline phases by controlling the induction times of various phases. Finally, we successfully isolated the predicted intermediate phases by quenching the reaction at designated time points. Our work provides evidence for the predictive capability of remnant metastability as a general framework not only for predicting synthetically-accessible metastable materials, but also as a practical tool to guide predictive synthesis.
Methods
Thermodynamics of the size-dependent Pourbaix grand potential
The size-dependent Pourbaix grand potential is an extension of the Pourbaix potential22, but incorporating surface energy contributions, and transformed into a grand potential µBulk = μf−μKNK with respect to externalK+ activity:
| 1 |
The first term is the bulk free energy, and the second term is the surface energy contribution. μf is the molar Gibbs formation energy, NMn, NK, NO, and NH represent the composition of a K-Mn-O-H phase; and Q is the charge of an aqueous ion. In our phase diagrams, the chemical potential of potassium is μK = μ°K + RTln[K+], where the activity of K+ is approximated by its aqueous concentration ([K+]). The water chemical potential is referenced using the method reported previously52. In the surface energy term, γ is the surface energy, 1/R represents the specific surface area in units of Area/Volume, η is the unitless shape factor of the equilibrium particle morphology, and ρ is the molar volume. Because reaction temperature was not modified between the three pathways, the potential does not include the effect of temperature. We omit pressure, as the pressures in hydrothermal synthesis (P < 30 MPa) are too low to influence the relative enthalpies between metal oxides.
DFT calculation of surface and bulk energies
DFT calculations were performed using the Vienna Ab-Initio Simulation Package (VASP)53, using the projector augmented wave (PAW) method with the Strongly Constrained and Appropriately Normed (SCAN)33 exchange-correlation functional, a reciprocal scape discretization of at least 25 Å−1, and convergence to 2 × 10−7 eV/atom and 0.02 eV/A on energy and interatomic forces, respectively, following earlier benchmarking on the MnO2 system23. Surface energies are computed using surface slabs generated using the efficient creation and convergence scheme developed by Sun and Ceder54. KxMnO2-yH2O phases are prepared using the techniques described in Ref. 21. Vibrational entropy contributions to the free-energy are obtained from experimental data, and the formation free-energy is referenced to hydrothermal synthesis conditions (160 °C, see below). Detailed benchmarking of results can be found in Supplementary Note 10 and 11.
Sample preparation for hydrothermal synthesis
The reaction conditions on PK = 0.2 and PK = 0.33 were adapted from a previously reported procedure40, in which 1 mmol (PK = 0.2) or 3 mmol (PK = 0.33) of KMnO4 (Sigma-Aldrich, 99.0%) was dissolved in 5 mL of 0.2 M HNO3. The purple KMnO4 solution was immediately loaded into a soda lime capillary tube (Kimble 2502, 0.1 mm wall thickness, 75 mm long) which is used as the hydrothermal reactor by syringe. The filling volume of the capillary was approximately 75%. Then, both of the ends of the capillary were flame-sealed. For PK = 0, the preparation procedure was rather different. In order to create a low-K environment, 0.25 M KMnO4 solution was passed through a column filled with Amberlite® 120 ion-exchange resin in H+ form55. As determined by inductively coupled plasma (ICP), [MnO4−] in the ion-exchanged KMnO4 solution were ~0.08 M, and [K+] is below the detection limit (see Supplementary Table 4 and 5).
Before running in situ reactions, we also ran ex situ hydrothermal syntheses in conventional autoclaves parallel to the in situ reaction conditions. We confirmed the consistency in the end product between the in situ and ex situ experiments. For details of the sample preparation method, see Supplementary Note 7. Additionally, the pH and oxidation reduction potentials (ORP) of the solutions are measured before and after the reaction with a pH/ORP meter and the result is shown in Supplementary Table 1. The pH/ORP values are used as the input parameters in constructing the size-dependent phase diagram and induction time theory.
In situ X-ray scattering study of reaction pathways
The in situ wide-angle scattering (WAXS) results were obtained at beamline 11–3 at the Stanford Synchrotron Radiation Lightsource (SSRL), with an incident wavelength of 0.9744 Å. Experimental data were collected by using a Rayonix 225 detector, and calibrated using 325-mesh Si powder. During the hydrothermal reaction, 2D scattering patterns were collected consecutively, with each image exposed for 1 min. X-ray scattering profiles were acquired by integrating individual 2D scattering patterns over polar angles, 30° ≤ χ ≤ 150°. To hold the hydrothermal capillary reactor at the beamline, we have designed a sample holder/heater. Similar designs of the reactor56 has also been applied to other synchrotron facilities for in situ reaction studies57. For detailed measurements set-ups, please see Supplementary Figure 1 in Supplementary Note 3. During the hydrothermal reaction, the temperature was kept at 160 °C for 24 h.
TEM measurements of quenched products
To study the microstructure of the intermediate phases, we forced the hydrothermal reaction to stop at designated time points by stopping the reaction, opening the reactor and extracting the contents inside. The solution and precipitates in the capillary were dispersed in DI water and then sonicated for 2 min. To prepare the samples for TEM, drops of the dilute solution were transferred onto silicon-nitride TEM grids (SimPore Inc.). TEM analysis was conducted using an FEI Co. Talos F200X transmission electron microscope operating at an accelerating voltage of 200 keV. Structural characterization was performed by acquiring selected area electron diffraction patterns on an FEI Co. Ceta 16 M pixel CMOS camera at a camera length of 660 mm. A platinum standard was used to calibrate the camera constant, allowing SAED reflections to be accurately measured and indexed.
Data availability
All data needed to evaluate the conclusions in the paper, including the cifs of the crystalline structures discussed in this article, are present in the paper and/or the Supplementary Information. Additional data related to this paper are available as requested from the authors.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
This work was supported by Center for Next Generation Materials by Design: Incorporating Metastability, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award No. DE-AC36-08GO28308. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was supported by the U.S. Department of Energy, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. D.K. acknowledges computational resources provided by the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231, as well computational resources sponsored by the Department of Energy’s Office of Energy Efficiency and Renewable Energy and located at the National Renewable Energy Laboratory. The authors would like to thank Charles Troxel Jr. and Ross Arthur for helping build the hydrothermal reactor, and Tim Dunn for beamline support.
Author contributions
W.S., L.S., G.C., M.T., K.S., D.K., L.G., and D.G. originated the research. B.-R.C., L.S., and K.S. performed in situ X-ray diffraction and data analysis. K.S. designed the hydrothermal reactor, then K.S., V.T., and L.S. tested it. W.S., D.K., and G.C. developed the theory and computational tools for the size-dependent phase diagram and crystallization pathways. J.M. and B.G. contributed to the TEM characterization. The manuscript was primarily written by B.-R.C., W.S., L.S., and K.S. All authors contributed to discussions of data and manuscript review.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Bor-Rong Chen, Wenhao Sun.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04917-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gerbrand Ceder, Email: gceder@berkeley.edu.
Michael F. Toney, Email: mftoney@slac.stanford.edu
Laura T. Schelhas, Email: schelhas@slac.stanford.edu
References
- 1.Curtarolo S, et al. The high-throughput highway to computational materials design. Nat. Mater. 2013;12:191–201. doi: 10.1038/nmat3568. [DOI] [PubMed] [Google Scholar]
- 2.Jain A, Shin Y, Persson KA. Computational predictions of energy materials using density functional theory. Nat. Rev. Mater. 2016;1:15004. doi: 10.1038/natrevmats.2015.4. [DOI] [Google Scholar]
- 3.Baumgartner J, et al. Nucleation and growth of magnetite from solution. Nat. Mater. 2013;12:310–314. doi: 10.1038/nmat3558. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen MH, Aloni S, De Yoreo JJ. In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Sci. (80-.). 2014;345:1158–1162. doi: 10.1126/science.1254051. [DOI] [PubMed] [Google Scholar]
- 5.Stein A, Keller SW, Mallouk TE. Turning down the heat: design and mechanism in solid-state synthesis. Science. 1993;259:1558–1564. doi: 10.1126/science.259.5101.1558. [DOI] [PubMed] [Google Scholar]
- 6.Chung SY, Kim YM, Kim JG, Kim YJ. Multiphase transformation and Ostwald’s rule of stages during crystallization of a metal phosphate. Nat. Phys. 2009;5:68–73. doi: 10.1038/nphys1148. [DOI] [Google Scholar]
- 7.Shoemaker DP, et al. In situ studies of a platform for metastable inorganic crystal growth and materials discovery. Proc. Natl Acad. Sci. USA. 2014;111:10922–10927. doi: 10.1073/pnas.1406211111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birgisson S, Saha D, Iversen BB. Formation mechanisms of nanocrystalline MnO2 polymorphs under hydrothermal conditions. Cryst. Growth Des. 2018;18:827–838. doi: 10.1021/acs.cgd.7b01304. [DOI] [Google Scholar]
- 9.Gopalakrishnan J. Chimie douce approaches to the synthesis of metastable oxide materials. Chem. Mater. 1995;7:1265–1275. doi: 10.1021/cm00055a001. [DOI] [Google Scholar]
- 10.Lee S, et al. Multiple pathways of crystal nucleation in an extremely supersaturated aqueous potassium dihydrogen phosphate (KDP) solution droplet. Proc. Natl Acad. Sci. USA. 2016;113:13618–13623. doi: 10.1073/pnas.1604938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun W, Ceder G. Induction time of a polymorphic transformation. CrystEngComm. 2017;19:4576–4585. doi: 10.1039/C7CE00766C. [DOI] [Google Scholar]
- 12.Navrotsky A. Nanoscale effects on thermodynamics and phase equilibria in oxide systems. Chemphyschem. 2011;12:2207–2215. doi: 10.1002/cphc.201100129. [DOI] [PubMed] [Google Scholar]
- 13.Sun W, et al. Nucleation of metastable aragonite CaCO3 in seawater. Proc. Natl Acad. Sci. USA. 2015;112:E2735–E2735. doi: 10.1073/pnas.1506100112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ANGUS JC, HAYMAN CC. Low-pressure, metastable growth of diamond and ‘diamondlike’ phases. Science. 1988;241:913–921. doi: 10.1126/science.241.4868.913. [DOI] [PubMed] [Google Scholar]
- 15.Sclafani A, Herrmann JM. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996;100:13655–13661. doi: 10.1021/jp9533584. [DOI] [Google Scholar]
- 16.Nagabhushana GP, Shivaramaiah R, Navrotsky A. Direct calorimetric verification of thermodynamic instability of lead halide hybrid perovskites. Proc. Natl Acad. Sci. USA. 2016;113:7717–7721. doi: 10.1073/pnas.1607850113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuech TF, et al. Growth far from equilibrium: examples from III-V semiconductors. Appl. Phys. Rev. 2016;40801:0–12. [Google Scholar]
- 18.Kitchaev DA, Ceder G. Evaluating structure selection in the hydrothermal growth of FeS2 pyrite and marcasite. Nat. Commun. 2016;7:13799. doi: 10.1038/ncomms13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navrotsky A, Ma C, Lilova K, Birkner N. Nanophase transition metal oxides show large thermodynamically driven shifts in oxidation-reduction equilibria. Science. 2010;330:199–201. doi: 10.1126/science.1195875. [DOI] [PubMed] [Google Scholar]
- 20.McHale JM. Surface energies and thermodynamic phase stability in nanocrystalline aluminas. Science. 1997;277:788–791. doi: 10.1126/science.277.5327.788. [DOI] [Google Scholar]
- 21.Kitchaev DA, Dacek ST, Sun W, Ceder G. Thermodynamics of phase selection in MnO2 framework structures through alkali intercalation and hydration. J. Am. Chem. Soc. 2017;139:2672–2681. doi: 10.1021/jacs.6b11301. [DOI] [PubMed] [Google Scholar]
- 22.Sun, W., Kitchaev, D., Kramer, D., Ceder, G. Non-equilibrium crystallization pathways of manganese oxides in aqueous solution. Preprint at 10.26434/chemrxiv.6300959 (2018). [DOI] [PMC free article] [PubMed]
- 23.Kitchaev DA, et al. Energetics of MnO2 polymorphs in density functional theory. Phys. Rev. B - Condens. Matter Mater. Phys. 2016;93:1–5. doi: 10.1103/PhysRevB.93.045132. [DOI] [Google Scholar]
- 24.Post JE. Manganese oxide minerals: crystal structures and economic and environmental significance. Proc. Natl Acad. Sci. USA. 1999;96:3447–3454. doi: 10.1073/pnas.96.7.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suib SL. Porous manganese oxide octahedral molecular sieves and octahedral layered materials. Acc. Chem. Res. 2008;41:479–487. doi: 10.1021/ar7001667. [DOI] [PubMed] [Google Scholar]
- 26.Wei W, Cui X, Chen W, Ivey DG. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011;40:1697–1721. doi: 10.1039/C0CS00127A. [DOI] [PubMed] [Google Scholar]
- 27.Robinson DM, et al. Photochemical water oxidation by crystalline polymorphs of manganese oxides: structural requirements for catalysis. J. Am. Chem. Soc. 2013;135:3494–3501. doi: 10.1021/ja310286h. [DOI] [PubMed] [Google Scholar]
- 28.Portehault D, Cassaignon S, Baudrin E, Jolivet JP. Selective heterogeneous oriented attachment of manganese oxide nanorods in water: Toward 3D nanoarchitectures. J. Mater. Chem. 2009;19:7947–7954. doi: 10.1039/b909761a. [DOI] [Google Scholar]
- 29.McKenzie RM. The synthesis of birnessite, cryptomelane, and some other oxides and hydroxides of manganese. Mineral. Mag. 1971;38:493–502. doi: 10.1180/minmag.1971.038.296.12. [DOI] [Google Scholar]
- 30.Hem JD, Lind CJ. Nonequilibrium models for predicting forms of precipitated manganese oxides. Geochim. Cosmochim. Acta. 1983;47:2037–2046. doi: 10.1016/0016-7037(83)90219-3. [DOI] [Google Scholar]
- 31.Sun W, et al. The thermodynamic scale of inorganic crystalline metastability. Sci. Adv. 2016;2:e1600225–e1600225. doi: 10.1126/sciadv.1600225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen M, Pentin IV, Schön JC. A universal representation of the states of chemical matter including metastable configurations in phase diagrams. Angew. Chem. Int. Ed. Engl. 2012;51:132–135. doi: 10.1002/anie.201106220. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Ruzsinszky A, Perdew JP. Strongly constrained and appropriately normed semilocal density functional. Phys. Rev. Lett. 2015;115:36402. doi: 10.1103/PhysRevLett.115.036402. [DOI] [PubMed] [Google Scholar]
- 34.Chukhrov FV. Manganese minerals in clays: a review. Clays Clay Miner. 1980;28:346–354. doi: 10.1346/CCMN.1980.0280504. [DOI] [Google Scholar]
- 35.Giovanoli R. Vernadite is random-stacked birnessite. Miner. Depos. 1980;15:251–253. doi: 10.1007/BF00206520. [DOI] [Google Scholar]
- 36.Horn PM, Birgeneau RJ, Heiney P, Hammonds EM. Melting of submonolayer krypton films on graphite. Phys. Rev. Lett. 1978;41:961–964. doi: 10.1103/PhysRevLett.41.961. [DOI] [Google Scholar]
- 37.Brock SL, et al. Particle size control and self-assembly processes in novel colloids of nanocrystalline manganese oxide. J. Phys. Chem. B. 1999;103:7416–7428. doi: 10.1021/jp991009u. [DOI] [Google Scholar]
- 38.de Wolff PM. Interpretation of some γ-MnO2 diffraction patterns. Acta Crystallogr. 1959;12:341–345. doi: 10.1107/S0365110X59001001. [DOI] [Google Scholar]
- 39.Chabre Y, Pannetier J. Structural and electrochemical properties of the proton / γ-MnO2 system. Prog. Solid State Chem. 1995;23:1–130. doi: 10.1016/0079-6786(94)00005-2. [DOI] [Google Scholar]
- 40.Yin B, Zhang S, Jiang H, Qu F, Wu X. Phase-controlled synthesis of polymorphic MnO2 structures for electrochemical energy storage. J. Mater. Chem. A. 2015;3:5722–5729. doi: 10.1039/C4TA06943A. [DOI] [Google Scholar]
- 41.Duan X, et al. Controllable hydrothermal synthesis of manganese dioxide nanostructures: shape evolution, growth mechanism and electrochemical properties. CrystEngComm. 2012;14:4196. doi: 10.1039/c2ce06587h. [DOI] [Google Scholar]
- 42.Shen XF, Ding YS, Hanson JC, Aindow M, Suib SL. In situ synthesis of mixed-valent manganese oxide nanocrystals: an in situ synchrotron X-ray diffraction study. J. Am. Chem. Soc. 2006;128:4570–4571. doi: 10.1021/ja058456+. [DOI] [PubMed] [Google Scholar]
- 43.Yuan Y, et al. The influence of large cations on the electrochemical properties of tunnel-structured metal oxides. Nat. Commun. 2016;7:13374. doi: 10.1038/ncomms13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R, Zavalij P, Whittingham MS. Hydrothermal synthesis and characterization of KxMnO2-yH2O. Society. 1996;4756:1275–1280. [Google Scholar]
- 45.Nagler SE, et al. Orientational order in xenon fluid monolayers on single crystals of exfoliated graphite. Phys. Rev. B. 1985;32:7373–7383. doi: 10.1103/PhysRevB.32.7373. [DOI] [PubMed] [Google Scholar]
- 46.Hollandt KL, Walker JR. Crystal structure modeling of a highly disordered potassium birnessite. Clays Clay Miner. 1996;44:744–748. doi: 10.1346/CCMN.1996.0440604. [DOI] [Google Scholar]
- 47.Liu J, et al. Quantification of honeycomb number-type stacking faults: application to Na3Ni2BiO6 cathodes for Na-ion batteries. Inorg. Chem. 2016;55:8478–8492. doi: 10.1021/acs.inorgchem.6b01078. [DOI] [PubMed] [Google Scholar]
- 48.Ranade MR, et al. Energetics of nanocrystalline TiO2. Proc. Natl Acad. Sci. USA. 2002;99:6476–6481. doi: 10.1073/pnas.251534898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machala L, Tuček J, Zbořil R. Polymorphous transformations of nanometric iron(III) oxide: a review. Chem. Mater. 2011;23:3255–3272. doi: 10.1021/cm200397g. [DOI] [Google Scholar]
- 50.Sakurai S, Namai A, Hashimoto K, Ohkoshi S. First observation of phase transformation of all four Fe 2 O 3 phases (γ→ε→β→α-Phase) J. Am. Chem. Soc. 2009;131:18299–18303. doi: 10.1021/ja9046069. [DOI] [PubMed] [Google Scholar]
- 51.Haggerty JES, et al. High-fraction brookite films from amorphous precursors. Sci. Rep. 2017;7:15232. doi: 10.1038/s41598-017-15364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Persson KA, Waldwick B, Lazic P, Ceder G. Prediction of solid-aqueous equilibria: Scheme to combine first-principles calculations of solids with experimental aqueous states. Phys. Rev. B - Condens. Matter Mater. Phys. 2012;85:1–12. doi: 10.1103/PhysRevB.85.235438. [DOI] [Google Scholar]
- 53.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 54.Sun W, Ceder G. Efficient creation and convergence of surface slabs. Surf. Sci. 2013;617:53–59. doi: 10.1016/j.susc.2013.05.016. [DOI] [Google Scholar]
- 55.Kappenstein, C. et al. Permanganic acid: A novel precursor for the preparation of manganese oxide catalysts. Prep. Catal. VI Sci. Bases Prep. Heterog. Catal. 91, 699–706 (1995).
- 56.Becker J, et al. Experimental setup for in situ X-ray SAXS/WAXS/PDF studies of the formation and growth of nanoparticles in near-and supercritical fluids. J. Appl. Crystallogr. 2010;43:729–736. doi: 10.1107/S0021889810014688. [DOI] [Google Scholar]
- 57.Chupas PJ, et al. A versatile sample-environment cell for non-ambient X-ray scattering experiments. J. Appl. Crystallogr. 2008;41:822–824. doi: 10.1107/S0021889808020165. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data needed to evaluate the conclusions in the paper, including the cifs of the crystalline structures discussed in this article, are present in the paper and/or the Supplementary Information. Additional data related to this paper are available as requested from the authors.