Abstract
Background
An analysis of cardiac injury markers in patients with OSA who sustain an episode of acute coronary syndrome (ACS) may contribute to a better understanding of the interactions and impact of OSA in subjects with ACS. We compared peak cardiac troponin I (cTnI) levels in patients with OSA and patients without OSA who were admitted for ACS.
Methods
Blood samples were collected every 6 hours from the time of admission until two consecutive assays showed a downward trend in the cTnI assay. The highest value obtained defined the peak cTnI value, which provides an estimate of infarct size.
Results
We included 89 patients with OSA and 38 patients without OSA with an apnea-hypopnea index of a median of 32 (interquartile range [IQR], 20.8-46.6/h and 4.8 [IQR, 1.6-9.6]/h, respectively. The peak cTnI value was significantly higher in patients without OSA than in patients with OSA (median, 10.7 ng/mL [IQR, 1.78-40.1 ng/mL] vs 3.79 ng/mL [IQR, 0.37-24.3 ng/mL]; P = .04). The multivariable linear regression analysis of the relationship between peak cTnI value and patient group, age, sex, and type of ACS showed that the presence or absence of OSA significantly contributed to the peak cTnI level, which was 54% lower in patients with OSA than in those without OSA.
Conclusions
The results of this study suggest that OSA has a protective effect in the context of myocardial infarction and that patients with OSA may experience less severe myocardial injury. The possible role of OSA in cardioprotection should be explored in future studies.
Key Words: ACS, cardiac biomarkers, cardiovascular disease, management, OSA, troponin
Abbreviations: ACS, acute coronary syndrome; AHI, apnea-hypopnea index; cTn, cardiac troponin; cTnI, cardiac troponin I; CVD, cardiovascular disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; SDB, sleep-disordered breathing; STEMI, ST-elevation myocardial infarction
FOR EDITORIAL COMMENT, SEE PAGE 295
Acute coronary syndrome (ACS) affects 1% of the adult global population and is a leading cause of death worldwide, in which one-third of all deaths are due to cardiovascular disease (CVD).1 OSA is a highly prevalent breathing disorder that affects at least 10% of middle-aged men and 3% of middle-aged women.1, 2 The risk of OSA developing increases with age3; moreover, OSA has been associated with major cardiovascular morbidity and mortality and is likely to be an independent risk factor for CVD.3, 4
Patients with ACS are at an increased risk for fatal and nonfatal cardiac events, and the prevalence of OSA has been reported to be as high as 65.7% in patients admitted for ACS.5 However, the impact of OSA on ACS severity and prognosis is mostly unknown. Moreover, despite the existence of closely interrelated and detrimental mechanisms that link OSA and CVD, epidemiologic studies suggest that a protective mechanism may exist in patients with OSA.6 Although such a mechanism may have a pertinent clinical impact, its presence remains under debate. Although several studies described superior postoperative survival in patients with OSA compared with that in patients without OSA7 along with studies that showed increased survival of elderly patients with mild OSA,8 other authors described worse postoperative outcomes after an episode of ACS, such as myocardial infarction (MI) in patients with sleep-disordered breathing (SDB), compared with patients without SDB.9, 10, 11
Cardiac troponin (cTn) is a sensitive marker of cardiac injury and is the preferred clinical biomarker for the diagnosis or exclusion of acute MI in the acute care setting. Thus, cTn has become the biomarker of choice in the assessment and evaluation of myocardial injury.12 The magnitude of cTn elevation correlates with the extent of myocardial necrosis and is related to the subsequent risk of adverse outcomes, thereby predicting poor prognosis.13 Recently, more sensitive cTn assays (high-sensitivity cTn) have been developed. Interestingly, studies suggest that high-sensitivity cardiac troponin I (cTnT) is elevated in patients with OSA.14 A recent study found that based on cTnT levels, patients with OSA had less severe cardiac injury during an acute nonfatal MI than did patients without OSA.15 The presence of OSA might activate mechanisms with cardioprotective effects, which might be reflected by a change in the expression of cardiac damage markers. An analysis of cardiac injury markers in patients with OSA who sustain an episode of ACS may contribute to a better understanding of the interactions and impact of OSA in subjects with ACS. We sought to investigate the impact of OSA on the extent of myocardial damage according to peak cTnI values, the biomarker of choice in the assessment and evaluation of myocardial injury.12 We hypothesized that the presence of chronic intermittent hypoxic episodes during sleep in patients with OSA affects cTn expression in patients who sustain an episode of ACS.
Methods
Study Design and Subjects
This observational prospective study of 127 patients admitted to the University Hospital Arnau de Vilanova (Lleida, Spain) (Fig 1) is an ancillary study of the Continuous Positive Airway Pressure (CPAP) in Patients with Acute Coronary Syndrome and Obstructive Sleep Apnea (ISAACC) cohort (NCT01335087). The aim of that multicenter open-label parallel prospective randomized controlled trial16 was to evaluate the effect of CPAP treatment on the incidence of new cardiovascular events in patients with an episode of ACS and OSA. In this study, we evaluated patients consecutively admitted to the coronary care unit or hospital cardiology room with a diagnosis of ACS. The criteria for inclusion were the detection of a rise or fall, or both, of cardiac biomarker values [preferably cTn] with at least one value greater than the 99th percentile upper reference limit. Additionally, patients were required to have at least one of the following: symptoms of ischemia, new or presumed new significant ST-segment/T wave changes or new left bundle branch block, development of pathologic Q waves on ECG, and imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.17
Figure 1.
Flowchart of study recruitment. ESS = Epworth Sleepiness Scale; RP = respiratory polygraphy.
After patients agreed to participate and the consent form was signed, all patients underwent respiratory polygraphy in the first 24 to 72 hours after admission to assess the presence of OSA. Those patients with an apnea-hypopnea index (AHI) of ≥ 15 events/h were considered to have OSA and were randomized to conservative treatment or CPAP. Those with an AHI of < 15 events/h were included in the non-OSA group. In the current study, we compared the peak cTnI levels in patients with ACS in the OSA group vs the non-OSA group. The ethics committee of Lleida approved the study (2010-852), and informed consent was obtained from all subjects.
Procedures
Clinical examinations and questionnaires
Demographic and anthropometric characteristics, a medical history, and a detailed medication history were obtained, and questionnaires were administered the day before the sleep study.
Sleep study
The diagnosis of OSA was made according to the guidelines of the national consensus on the apnea-hypopnea syndrome.18 All participants underwent overnight cardiorespiratory polygraphy with the same model of device (Embletta; ResMed, Bella Vista, Australia). The variables measured included oronasal flow and thoracoabdominal movements, and ECG and pulse oximetry were used for analysis. An obstructive apnea episode was scored when a complete cessation of airflow lasted for ≥ 10 s. An episode of hypopnea was defined as a reduction in airflow for ≥ 10 s associated with a > 4% decrease in arterial oxygen saturation. Cardiorespiratory polygraphy studies were performed without supplemental oxygen.
Cardiac biomarkers
We assessed cTnI expression as a marker of myocardial injury. Blood samples were collected at 6-hour intervals from the time of admission until two consecutive cTnI measurements showed a decrease. The highest value obtained defined the peak cTnI value, which provides a relative estimate of infarct size.19 The quantitative determination of cTnI levels was performed by chemiluminescence immunoassay (AccuTnI+3 Beckman-Coulter, Unicel Dxl 600 Beckman-Coulter autoanalyzer). Routine biochemical analyses in patients admitted for ACS were performed during the hospital stay.
Coronary artery disease
The severity of coronary artery disease was based on coronary angiography and the number of stents implanted. The number of affected vessels was calculated as the number of vessels with any stenosis > 50% of the lumen.
Statistical Analysis
Continuous variables are presented as the mean (standard deviation) or median (interquartile range [IQR]) for data with a skewed distribution. Participant characteristics were compared using the Student t test, analysis of variance, or the nonparametric Mann-Whitney and Kruskal-Wallis tests for skewed data.
A multivariable linear regression model was used to evaluate the independent contribution of the presence or absence of OSA to peak troponin levels after adjusting for age, sex, and type of event (non-ST-elevation MI [non-STEMI] and ST-elevation MI [STEMI]). The peak cTnI values and the area under the cTnI curve were log-transformed to obtain a data distribution closer to normal. Another multivariable linear regression model was built to assess the contribution of the non-OSA, OSA (AHI, 15-32 events/h), and OSA (AHI > 32 events/h) groups to the peak cTnI value and the area under the cTnI curve.
All tests were two-sided, and P values < .05 were considered statistically significant. R statistical software, version 3.3.1 (R Project for Statistical Computing) was used for all analyses.
Results
Among the 127 patients enrolled, 89 were found to have OSA (AHI ≥ 15 events/h). The clinical characteristics and demographic variables of the patients are shown in Table 1. No significant differences were observed between patients with OSA and patients without OSA regarding sex, age, or prevalence of cardiovascular risk factors (hypertension, diabetes mellitus, BMI, dyslipidemia, and smoking). Compared with the patients without OSA, the patients with OSA had a higher number of stents placed during percutaneous coronary intervention (PCI) (P = .007). The peak cTn level was not associated with the number of stents (Spearman’s rho correlation coefficient, 0.13; P = .16). We evaluated the distribution of patients with OSA and patients without OSA by the type of ACS (patients with unstable angina, non-STEMI, or STEMI). The results showed a similar distribution of the type of ACS between the non-OSA and OSA groups (P = .36). Moreover, our data showed that the peak cTnI levels in patients with STEMI were significantly higher than those in patients with non-STEMI (P < .001). We evaluated the locations of the lesions undergoing intervention. The results showed that the locations of the lesions undergoing intervention did not differ significantly between groups (P = .29).
Table 1.
Anthropometric, Clinical, and Treatment Characteristics of the Non-OSA and OSA Groups
| Variable | Group |
||
|---|---|---|---|
| Non-OSA (AHI < 15 Events/h) (n = 38) |
OSA (AHI ≥ 15 Events/h) (n = 89) |
P Value | |
| Age, mean (SD), y | 64.4 (13.1) | 63.6 (11.5) | .739 |
| Sex, No. (%) | .966 | ||
| Male | 32 (84.2) | 73 (82.0) | |
| Female | 6 (15.8) | 16 (18.0) | |
| Apnea-hypopnea index, median (IQR), events/h | 4.8 (1.6-9.6) | 32.0 (20.8-46.6) | < .001 |
| Oxygen desaturation index > 4%/h, median (IQR) | 4.7 (3.5-10.1) | 20.2 (6.6-38.1) | < .001 |
| Minimum Sao2, %, median (IQR) | 87.0 (84.0-89.0) | 83.0 (78.0-87.0) | .002 |
| Mean Sao2, %, median (IQR) | 93.3 (92.1-94.1) | 93.0 (91.7-94.2) | .614 |
| Time with Sao2 < 90%, %, median (IQR) | 1.6 (0.2-10.0) | 4.20 (0.9-15.6) | .130 |
| Epworth Sleepiness Scale, median (IQR) | 3.0 (3.0-4.3) | 5.0 (3.0-6.0) | .021 |
| Hypertensive patients, No. (%) | 24 (63.2%) | 61 (68.5%) | .701 |
| Systolic blood pressure, median (IQR), mm Hg | 130 (120-145) | 125 (118-137) | .194 |
| Diastolic blood pressure, median (IQR), mm Hg | 76.2 (70-82.6) | 78.0 (65-85) | .66 |
| BMI, median (IQR), kg/m2 | 26.4 (24.6-30.0) | 27.7 (25.0-30.1) | .402 |
| Neck circumference, No. (%), cm | 42.0 (39.5-43.5) | 41.0 (39.5-42.0) | .247 |
| Diabetes mellitus, No. (%) | 10 (26.3%) | 33 (37.5%) | .312 |
| Dyslipidemia, No. (%) | 18 (47.4%) | 53 (59.6%) | .284 |
| Peak cTnI, ng/mL | 10.7 (1.78-40.1) | 3.79 (0.37-24.3) | .04 |
| Area under the peak cTnI | 451 (79.8-1,533) | 143 (14.8-845) | .049 |
| Type of ACS, No. (%) | .36 | ||
| Unstable angina | 4 (10.5) | 9 (10.1%) | |
| Non-STEMI | 15 (39.5) | 47 (52.8) | |
| STEMI | 19 (50.0) | 33 (37.1) | |
| First episode of ACS, No. (%) | 28 (73.7) | 70 (78.7) | .704 |
| Cardiomyopathy, No. (%) | 11 (28.9) | 28 (31.8) | .912 |
| Stroke, No. (%) | 1 (2.7) | 3 (3.5) | 1 |
| Killip class, median (IQR) | 1.0 (1.0-1.0) | 1.0 (1.0-1.0) | .98 |
| No. of affected vessels, median (IQR) | 1.0 (1.0-2.0) | 1.5 (1.0-3.0) | .622 |
| No. of stents, median (IQR) | 1.0 (0.0-1.0) | 1.0 (1.0-2.0) | .007 |
| Location of lesions undergoing intervention | .29 | ||
| No lesion | 3 (9.68) | 5 (13.9) | |
| Proximal | 9 (29.0) | 14 (38.9) | |
| Medial | 16 (51.6) | 11 (30.6) | |
| Distal | 3 (9.68) | 6 (16.7) | |
| Smoker, No. (%) | 0.883 | ||
| Former smoker | 11 (28.9) | 24 (27.0) | |
| No | 13 (34.2) | 28 (31.5) | |
| Yes | 14 (36.8) | 37 (41.6) | |
| Total tobacco exposure, median (IQR), pack-years | 20.0 (15.0-30.0) | 28.2 (17.0-43.8) | 0.354 |
| Alcohol use, No. (%) | .333 | ||
| Former alcohol consumption | 2 (6.5) | 1 (1.56) | |
| No | 24 (77.4) | 54 (84.4) | |
| Yes | 5 (16.1) | 9 (14.1) | |
| Diuretics, No. (%) | 17 (44.7) | 26 (29.9) | .161 |
| Anticoagulants, No. (%) | 6 (15.8) | 16 (18.6) | .902 |
| Antacids, No. (%) | 11 (28.9) | 35 (40.7) | .295 |
| Hypolipidemic agents, No. (%) | 12 (31.6) | 36 (41.4) | .403 |
| β-blockers, No. (%) | 17 (44.7) | 33 (37.9) | .606 |
| Antiplatelet agents, No. (%) | 6 (15.8) | 15 (17.4) | 1 |
| Bronchodilators, No. (%) | 3 (7.9) | 5 (5.9) | .701 |
| Oral antidiabetic drugs, No. (%) | 7 (18.4) | 26 (29.9) | .264 |
| Insulin, No. (%) | 1 (2.6) | 10 (11.8) | .170 |
| Calcium antagonists, No. (%) | 2 (5.3%) | 20 (23.0) | .032 |
ACS = acute coronary syndrome; AHI = apnea-hypopnea index (No. of events/h); cTnI = cardiac troponin I; IQR = interquartile range; Sao2 = arterial oxygen saturation; STEMI = ST-elevation myocardial infarction.
We found that a higher percentage of patients with OSA than patients without OSA were treated with a calcium channel antagonist (P = .032), likely due to the known association between OSA and hypertension.20 Regarding the timing of PCI (before or after the peak cTnI measurement), no difference was observed between the patients without OSA and patients with OSA (P = .85).
No significant differences were observed between patients with OSA and those without OSA regarding the mean values for the time of admission (7:17 am [8 hours 41 min SD] and 8:39 am [8 hours 26 min SD]; P = .58), time of onset of symptoms (10:45 am [6 hours 24 min SD) and 11:35 am [6 hours 13 min SD]; P = .55) or time to peak cTnI levels (after 9 hours 23 min [8 hours 14 min SD] and after 9 hours 19 min [7 h 35 min SD]; P = .97), respectively. The number of cTnI measurements during the hospital stay did not differ significantly between the non-OSA and OSA groups (median, 2.00 [IQR, 2.00-3.00] and median, 2.00 [IQR, 2.00-3.00]; P = .65).
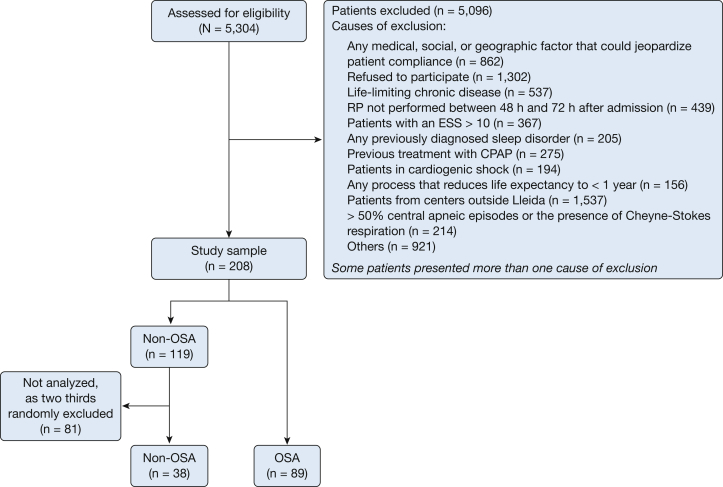
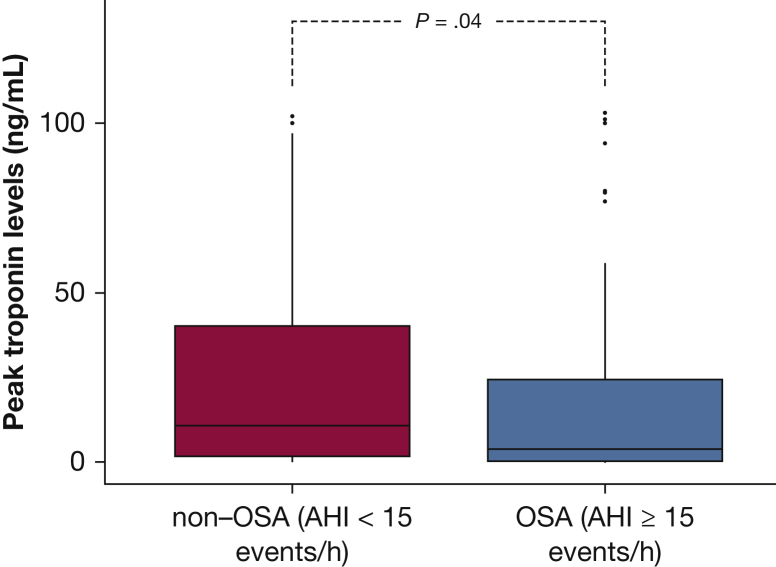
The peak cTnI value was significantly higher in patients without OSA than in patients with OSA (median, 10.7 ng/mL [IQR, 1.78-40.1 ng/mL] vs median, 3.79 ng/mL [IQR, 0.37-24.3 ng/mL]; P = .04) (Fig 2). Moreover, we estimated the infarct size by calculating the area under the cTnI curve, which was significantly different between the patients without OSA and the patients with OSA (median, 451 [IQR, 79.8-1533] vs median, 143 [IQR, 14.8-845]; P = .049) (Fig 3).
Figure 2.
Box plots of peak cardiac troponin I levels in patients with and those without OSA. P values were obtained using the Mann-Whitney test.
Figure 3.
Box plots of the area under the cardiac troponin I curve in patients with and those without OSA. P values were obtained using the Mann-Whitney test.
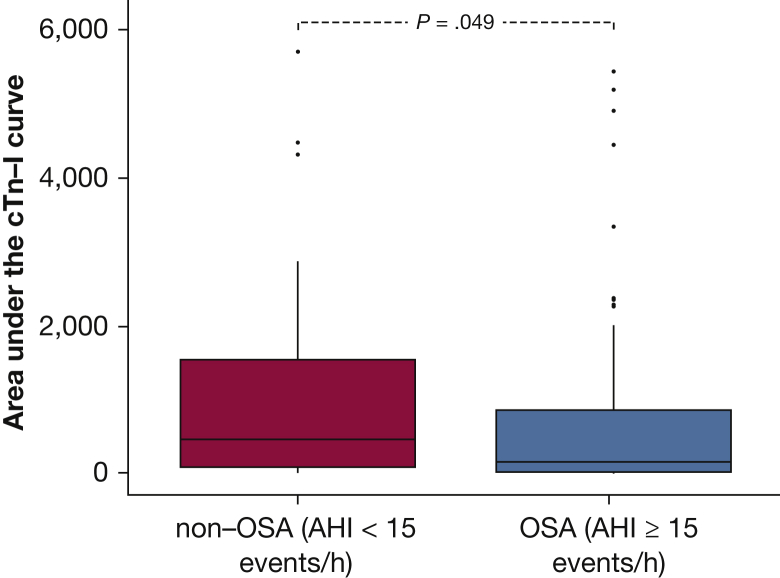
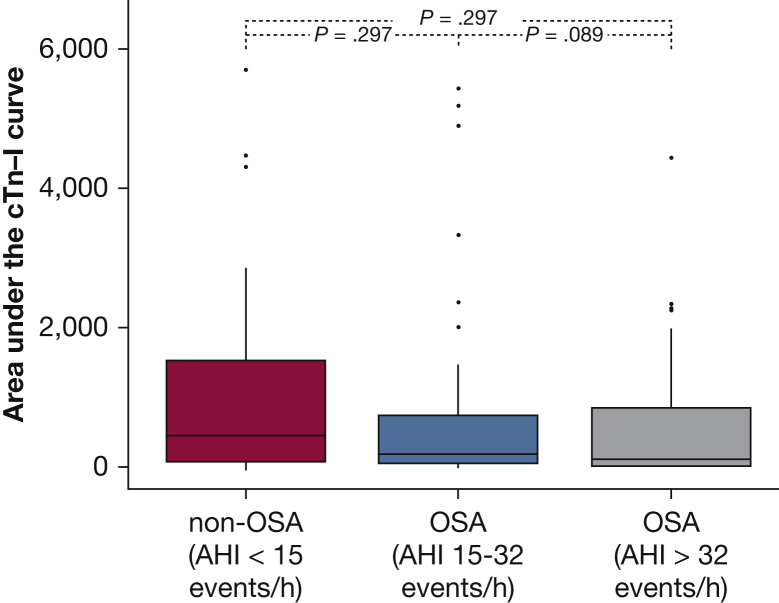
To further investigate the impact of OSA severity on peak cTnI levels, we classified the patients with OSA into two groups according to the observed median AHI value (32 events/h) (e-Appendix 1). The characteristics and demographic variables of the patient groups are shown in e-Table 1. We observed a trend toward a decrease in cTnI levels as the severity of OSA increased (P = .058) (Fig 4). The area under the cTnI curve was not significantly different between the non-OSA (AHI < 15 events/h), mild to moderate OSA (AHI = 15-32 events/h), and severe OSA (AHI > 32 events/h) groups (P = .08) (Fig 5).
Figure 4.
Box plots of peak cardiac troponin I levels in patients without OSA or with mild to moderate or severe OSA. The severity of OSA was based on the apnea-hypopnea index. P values were obtained using the Mann-Whitney and Kruskal-Wallis tests.
Figure 5.
Box plots of the area under the cardiac troponin I curve in patients without OSA or with mild to moderate or severe OSA. The severity of obstructive apnea was based on the apnea-hypopnea index. P values were obtained using the Mann-Whitney and Kruskal-Wallis tests.
We used a multivariable linear regression model to evaluate relationships between peak cTnI value and patient group, age, sex, and type of ACS. The presence or absence of OSA significantly contributed to the peak cTnI level. Furthermore, the peak cTnI level was 54% lower in patients with OSA than in patients without OSA. Moreover, in the multivariable linear regression model that categorized patients based on OSA severity, patients with severe OSA had 61% lower cTnI levels than did patients without OSA (Tables 2 and 3). In addition, we used a multivariate linear regression model to evaluate the association between area under the troponin curve and patient group, age, sex, and type of ACS. The results were similar to those obtained for peak cTnI levels (Tables 4 and 5).
Table 2.
Multivariable Linear Regression Analysis of Peak cTnI levels in non-OSA vs OSA Groups
| Variable | β (95% CI) | P Value |
|---|---|---|
| Age | –0.02 (–0.05 to 0.01) | .23 |
| Sex | ||
| Female | Referent | |
| Male | 1.05 (0.06-2.04) | .04 |
| Group | ||
| Non-OSA | Referent | |
| OSA | –0.78 (–1.55 to 0.00) | .049 |
| Type of ACS | ||
| Non-STEMI | Referent | |
| STEMI | 2.19 (1.46-2.91) | < .01 |
Coefficients are expressed as the change in log(peak troponin level) per 1 unit change in the independent variable.
See Table 1 legend for expansion of abbreviations.
Table 3.
Multivariable Linear Regression Analysis of Peak cTnI Levels (Non-OSA vs Mild to Moderate OSA vs Severe OSA)
| Variable | β (95% CI) | P Value |
|---|---|---|
| Age | –0.02 (–0.05 to 0.01) | .23 |
| Sex | ||
| Female | Referent | |
| Male | 1.05 (0.06-2.04) | .04 |
| Group | ||
| Non-OSA | Referent | |
| Mild to moderate OSA (AHI 15-32 events/h) | –0.63 (–1.50 to 0.24) | .15 |
| Severe OSA (AHI > 32 events/h) | –0.94 (–1.84 to –0.04) | .04 |
| Type of ACS | ||
| Non-STEMI | Referent | |
| STEMI | 2.17 (1.44-2.90) | < .01 |
Coefficients are expressed as the change in log(peak troponin level) per 1 unit change in the independent variable.
See Table 1 legend for expansion of abbreviations.
Table 4.
Multivariable Linear Regression Analysis of the Area Under the Troponin Curve (Non-OSA vs OSA)
| Variable | β (95% CI) | P Value |
|---|---|---|
| Age | –0.02 (–0.05 to 0.01) | .21 |
| Sex | ||
| Female | Referent | |
| Male | 1.19 (0.20-2.18) | .02 |
| Group | ||
| Non-OSA | Referent | |
| OSA | –0.75 (–1.536 to 0.30) | .05 |
| Type of ACS | ||
| Non-STEMI | Referent | |
| STEMI | 2.07 (1.34-2.80) | < .01 |
Coefficients are expressed as the change in the log(area under the troponin curve) per 1 unit change in the independent variable.
See Table 1 legend for expansion of abbreviations.
Table 5.
Multivariable Linear Regression Analysis of the Area Under the Troponin Curve (Non-OSA vs Mild to Moderate OSA vs Severe OSA)
| Variable | β (95% CI) | P Value |
|---|---|---|
| Age | –0.02 (–0.05 to 0.01) | .21 |
| Sex | ||
| Female | Referent | |
| Male | 1.19 (0.20-2.19) | .02 |
| Group | ||
| Non-OSA | Referent | |
| Mild to moderate OSA (AHI 15-32 events/h) | –0.63 (–1.50 to 0.24) | .16 |
| Severe OSA (AHI > 32 events/h) | –0.88 (–1.79 to –0.01) | .05 |
| Type of ACS | ||
| Non-STEMI | Referent | |
| STEMI | 2.05 (1.32-2.78) | < .01 |
Coefficients are expressed as the change in the log(area under the troponin curve) per 1 unit change in the independent variable.
See Table 1 legend for expansion of abbreviations.
Discussion
The results of this observational study suggest that the presence of OSA has an effect on peak cTnI levels in patients with ACS. We found that patients without OSA exhibited higher peak cTnI levels. These results suggest that patients with a higher AHI are significantly more likely to have low cTnI levels than are patients without evidence of OSA, which could imply that patients with an elevated AHI, particularly those with severe OSA, may experience less severe myocardial injury. Finally, these results suggest that OSA has a protective effect in the context of MI.
The current literature describes controversial benefits of OSA in cardiovascular events. Although some studies have reported that OSA is associated with cardiovascular morbidity and mortality, others have offered the controversial suggestion that the presence of OSA might be protective against myocardial ischemic injury in the setting of acute MI. As described in the literature and in the present study, we observed a significant contribution of the type of ACS to cTnI levels; the patients with STEMI had the highest cTnI levels. Nevertheless, OSA also independently contributed to the peak cTnI levels. In contrast to our results suggesting that OSA is associated with a decrease in the expression of cTnI as a marker of myocardial injury, Belaidi et al21 showed that chronic intermittent hypoxia in patients with OSA resulted in the development of enhanced hypertension and an increase in infarct size. Importantly, the patients studied by Belaidi et al were younger that the patients included in our study. It has been suggested previously that SDB in the elderly and SDB in younger people are two distinct conditions. The current literature suggests that SDB consequences depend on the patient’s age. Thus, cardiovascular risk has been found to be more likely to be elevated in younger (aged < 65 years) than older participants.22 Moreover, moderate and severe levels of sleep apnea are moderately associated with an increased risk of all-cause mortality compared with the general population, particularly in men aged < 50 years.17 Other studies have indicated that in older patients with SDB, OSA was not associated with hypertension,18 and yet others have indicated that the association between OSA and arterial hypertension was stronger in young and middle-aged patients with OSA (compared with elderly subjects).19 Finally, other authors concluded that OSA did not appear to be associated with cardiovascular disease or mortality in older populations .23
Nakashima et al24 reported that after PCI in patients with acute MI, patients with OSA had a left ventricular ejection fraction similar to that of patients without OSA. After 21 days, the left ventricular ejection fraction was significantly different between patients with OSA and control subjects (52% vs 59%; P = .02). These results suggest that OSA may inhibit the recovery of left ventricular function in patients with acute myocardial infarction. Contrary to the findings of Nakashima et al24 and according to the observations in our study, Berger et al6 demonstrated that recurrent episodes of hypoxia/reoxygenation in patients with acute myocardial injury with mild to moderate SDB activated adaptive mechanisms that improved endothelial function, providing cardioprotection in the context of acute myocardial injury. These authors also showed that the proliferative and angiogenic properties of endothelial progenitor cells from healthy individuals were increased after exposure to intermittent hypoxia in vitro, implicating the intermittent hypoxia associated with SDB in the alterations of endothelial progenitor cell numbers and functions. Remarkably, both studies have important differences that deserve comment. Nakashima et al24 studied an Asian population. In addition, Berger et al6 and the present study included patients with overweight or obesity, whereas in the study of Nakashima et al,24 the mean BMI was 23 kg/m2. Therefore, the physiopathologic consequences of the disease could vary between populations with different ethnic origins and different anthropometric characteristics.
In accordance with our results, a previous study15 that included patients with characteristics similar to those of the patients included in our study suggested that patients with OSA had less severe cardiac injury during an acute nonfatal MI than did patients without OSA. The authors postulated a cardioprotective role of sleep apnea during acute MI through ischemic preconditioning in patients with severe OSA.
One of the major studies suggesting a beneficial effect of OSA was reported by Lavie and Lavie.8 These authors showed that elderly people with moderate sleep apnea (20-40 events/h) had significantly lower mortality rates than a matched population cohort. In the present study, we observed decreased myocardial injury, as assessed by cTnI levels, in patients with severe OSA (AHI > 32 events/h). However, it is important to note that Lavie and Lavie8 examined all-cause mortality over a long-term follow-up, an outcome different from the outcome reported in the present study related to myocardial infarct size during ACS. Lavie and Lavie8 hypothesized ischemic preconditioning as a possible explanation for the declining trend in mortality with age in patients with sleep apnea.
In conclusion, the current literature suggests that a possible cardioprotective effect of OSA could be dependent on age, anthropometric characteristics, and ethnic origin. Moreover, the current evidence highlights the need to clearly identify the deleterious consequences of OSA in each population of patients and the a priori delineation of the optimal candidates who will benefit from CPAP therapy.
Several limitations of this analysis should be noted. First, we excluded patients with more severe ACS, as these patients represented a small proportion of the patient population (3.6% of patients assessed). Second, this study excluded sleepy subjects (Epworth Sleepiness Scale > 10), which could have included patients who exhibited the most severe OSA. However, the number of patients excluded for these causes was relatively low (6.9% of patients assessed). Third, we assessed the extent of myocardial damage according to peak cTnI values, the biomarker of choice in the assessment and evaluation of myocardial injury.12 No data (eg, from echocardiography) were available to evaluate infarct size.
Conclusions
Patients with ACS and OSA had overall lower peak cTnI levels than did patients without OSA, suggesting that patients with OSA may experience less severe myocardial injury. These findings indicate that OSA has a protective effect in the context of MI. The possible role of OSA in cardioprotection should be explored in future studies.
Acknowledgments
Author contributions: M. S. T. is the guarantor of the study. A. S. T., X. S., F. B., A. M., and M. S. T. contributed to study design. A. S. T., F. B., M. F., A. A., F. W., C. T., E. G., J. B., and M. S. T. contributed to data acquisition. A. S. T., X. S., F. B., A. M., M. R., S. B., A. A., F. W., J. V., C. H. L., J. B., and M. S. T. contributed to data analysis and interpretation. A. S. T., X. S., F. B., A. M., and M. S. T. contributed to drafting of the manuscript. A. S. T., X. S., F. B., M. F., A. M., M. R., S. B., A. A., F. W., J. V., C. H. L., C. T., E. G., J. B. and M. S. T. contributed to revision of the manuscript for intellectual content and approval of the final version.
Financial/nonfinancial disclosures: None declared.
∗Writing Committee Members for Spanish Sleep Network: Gerard Castellà (IRBLleida. Lleida, Catalonia, Spain), Anunciación Cortijo (Hospital Santa María. Lleida, Catalonia, Spain), Jorge Abad (Hospital Germans Trias i Pujol. Barcelona, Catalonia, Spain), Aida Muñoz (Hospital Germans Trias i Pujol. Barcelona, Catalonia, Spain), Laura Abad (Hospital Germans Trias i Pujol. Barcelona, Catalonia, Spain), Miguel Cervantes (Hospital Germans Trias i Pujol. Barcelona, Catalonia, Spain), Joaquín Durán (Hospital Araba. Vitoria-Gasteiz, Álava, Spain), Carlos Egea (Hospital Araba. Vitoria-Gasteiz, Álava, Spain), Sandra Inglés (Hospital Araba. Vitoria-Gasteiz, Álava, Spain), Berenice Muria (Hospital Araba. Vitoria-Gasteiz, Álava, Spain), Olga Mediano (Hospital de Guadalajara. Guadalajara, Spain), José Román-Sánchez (Hospital de Guadalajara. Guadalajara, Spain), Maribel Valiente (Hospital de Guadalajara. Guadalajara, Spain), Valentín Cabriada (Hospital de Cruces. Vizcaya, Spain), Jose Amibilia (Hospital de Cruces. Vizcaya, Spain), Amaia Urrutia (Hospital de Cruces. Vizcaya, Spain), Ruth Arnesto (Hospital de Cruces. Vizcaya, Spain), María José Masdeu (Hospital Parc Taulí. Barcelona, Catalonia, Spain), María Piñar (Hospital Parc Taulí. Barcelona, Catalonia, Spain), Enriqueta Ramínez (Hospital Parc Taulí. Barcelona, Catalonia, Spain), Isabel Rodríguez (Hospital Parc Taulí. Barcelona, Catalonia, Spain), Joaquín Terán (Hospital de Burgos. Burgos, Spain), Mari Luz Alonso (Hospital de Burgos. Burgos, Spain), Juan Fernando Masa (Hospital San Pedro Alcántara. Cáceres, Spain), Jaime Corral (Hospital San Pedro Alcántara. Cáceres, Spain), Mónica de la Peña (Hospital Son Espases. Palma, Islas Baleares, Spain), Andrés Carrillo (Hospital Son Espases. Palma, Islas Baleares, Spain), Ramón Coloma (Hospital General de Albacete. Albacete, Spain), Mercè Mayos (Hospital de la Santa Creu i Sant Pau. Barcelona, Catalonia, Spain), Patricia Peñacoba (Hospital de la Santa Creu i Sant Pau. Barcelona, Catalonia, Spain), Josep María Montserrat (Hospital Clinic. Barcelona, Catalonia, Spain), Ornintza Garmendia (Hospital Clinic. Barcelona, Catalonia, Spain), Gil Bonet (Hospital Universitari de Tarragona Joan XXIII. Tarragona, Catalonia, Spain) and Eusebi Chiner (Hospital General Universitario de Alicante. Alicante, Spain).
Other contributions: The authors thank Maricel Arbonés, MMM, Cristina Girón, MLT, Montserrat Martínez, PhD, Ana Martínez, MLT, Olga Mínguez, MLT, Lydia Pascual, CNS, and Silvia Ortega, MLT, for technical contributions to this study and Unión Europea: Fondo Europeo de Desarrollo Regional (FEDER): “Una manera de hacer Europa.”
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Sánchez-de-la-Torre and Soler are co-first authors.
FUNDING/SUPPORT: This work was supported by ResMed Ltd. (Australia), Fondo de Investigación Sanitaria (Fondo Europeo de Desarrollo Regional (FEDER) [PI10/02763 and PI10/02745]), the Spanish Respiratory Society (SEPAR), the Catalonian Cardiology Society, Esteve-Teijin (Spain), Oxigen Salud (Spain), and ALLER.
Contributor Information
Manuel Sánchez-de-la-Torre, Email: sanchezdelatorre@gmail.com.
Spanish Sleep Network∗:
Gerard Castellà, Anunciación Cortijo, Jorge Abad, Aida Muñoz, Laura Abad, Miguel Cervantes, Joaquín Durán, Carlos Egea, Sandra Inglés, Berenice Muria, Olga Mediano, José Román-Sánchez, Maribel Valiente, Valentín Cabriada, Jose Amibilia, Amaia Urrutia, Ruth Arnesto, María José Masdeu, María Piñar, Enriqueta Ramínez, Isabel Rodríguez, Joaquín Terán, Mari Luz Alonso, Juan Fernando Masa, Jaime Corral, Mónica de la Peña, Andrés Carrillo, Ramón Coloma, Mercè Mayos, Patricia Peñacoba, Josep María Montserrat, Ornintza Garmendia, Gil Bonet, and Eusebi Chiner
Supplementary Data
References
- 1.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duran J., Esnaola S., Rubio R., Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez-de-la-Torre M., Campos-Rodriguez F., Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 4.Kohler M., Stradling J.R. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677–685. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 5.Lee C.-H., Khoo S.-M., Tai B.-C. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135(6):1488–1495. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 6.Berger S., Aronson D., Lavie P., Lavie L. Endothelial progenitor cells in acute myocardial infarction and sleep-disordered breathing. Am J Respir Crit Care Med. 2012;187(1):90–98. doi: 10.1164/rccm.201206-1144OC. [DOI] [PubMed] [Google Scholar]
- 7.Mokhlesi B., Hovda M.D., Vekhter B., Arora V.M., Chung F., Meltzer D.O. Sleep-disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144(3):903–914. doi: 10.1378/chest.12-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavie P., Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18(4):397–403. doi: 10.1111/j.1365-2869.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 9.Buchner S., Satzl A., Debl K. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J. 2014;35(3):192–199. doi: 10.1093/eurheartj/eht450. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima H., Katayama T., Takagi C. Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J. 2006;27(19):2317–2322. doi: 10.1093/eurheartj/ehl219. [DOI] [PubMed] [Google Scholar]
- 11.Buchner S., Eglseer M., Debl K. Sleep disordered breathing and enlargement of the right heart after myocardial infarction. Eur Respir J. 2015;45(3):680–690. doi: 10.1183/09031936.00057014. [DOI] [PubMed] [Google Scholar]
- 12.Antman E.M., Anbe D.T., Armstrong P.W. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110(9):e82–e292. [PubMed] [Google Scholar]
- 13.Antman E.M., Tanasijevic M.J., Thompson B. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335(18):1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 14.Querejeta Roca G., Redline S., Punjabi N. Sleep apnea is associated with subclinical myocardial injury in the community. The ARIC-SHHS study. Am J Respir Crit Care Med. 2013;188(12):1460–1465. doi: 10.1164/rccm.201309-1572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah N., Redline S., Yaggi H.K. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2013;17(2):819–826. doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 16.Esquinas C., Sánchez-de-la-Torre M., Aldomá A. Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC Trial. Clin Cardiol. 2013;36(9):495–501. doi: 10.1002/clc.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavie P., Lavie L., Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25(3):514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]
- 18.Haas D.C., Foster G.L., Nieto F.J. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–621. doi: 10.1161/01.CIR.0000154540.62381.CF. [DOI] [PubMed] [Google Scholar]
- 19.Hua-Huy T., Rouhani S., Nguyen X.-Y., Luchon L., Meurice J.-C., Dinh-Xuan A.T. Cardiovascular comorbidities in obstructive sleep apnoea according to age: a sleep clinic population study. Aging Clin Exp Res. 2015;27(5):611–619. doi: 10.1007/s40520-015-0318-3. [DOI] [PubMed] [Google Scholar]
- 20.Somers V.K., White D.P., Amin R. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 21.Belaidi E., Joyeux-Faure M., Ribuot C., Launois S.H., Lévy P., Godin-Ribuot D. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J Am Coll Cardiol. 2009;53(15):1309–1317. doi: 10.1016/j.jacc.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 22.Newman A.B., Nieto F.J., Guidry U. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154(1):50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Johansson P., Alehagen U., Svanborg E., Dahlström U., Broström A. Clinical characteristics and mortality risk in relation to obstructive and central sleep apnoea in community-dwelling elderly individuals: a 7-year follow-up. Age Ageing. 2012;41(4):468–474. doi: 10.1093/ageing/afs019. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima H., Muto S., Amenomori K., Shiraishi Y., Nunohiro T., Suzuki S. Impact of obstructive sleep apnea on myocardial tissue perfusion in patients with ST-segment elevation myocardial infarction. Circ J. 2011;75(4):890–896. doi: 10.1253/circj.cj-10-0768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.