Abstract
Background
World Trade Center (WTC)-exposed rescue/recovery workers endured massive respiratory insult from inhalation of particulate matter and gases, resulting in respiratory symptoms, loss of lung function, and, for many, bronchial hyperreactivity (BHR). The persistence of respiratory symptoms and lung function abnormalities has been well-documented, whereas persistence of BHR has not been investigated.
Methods
A total of 173 WTC-exposed firefighters with bronchial reactivity measured within 2 years after September 11, 2001 (9/11) (baseline methacholine challenge test), were reevaluated in 2013 and 2014 (follow-up methacholine challenge test). FEV1 measurements were obtained from the late pre-9/11, early post-9/11, and late post-9/11 periods. Respiratory symptoms and corticosteroid treatment were recorded.
Results
Bronchial reactivity remained stable (within 1 doubling dilution) for most (n = 101, 58%). Sixteen of 28 (57%) with BHR (provocative concentration of methacholine producing a 20% decline in FEV1 <8 mg/mL) at baseline had BHR at follow-up, and an additional 27 of the 145 (19%) without BHR at baseline had BHR at follow-up. In multivariable models, we found that BHR baseline was strongly associated with BHR follow-up (OR, 6.46) and that BHR at follow-up was associated with an estimated 15.4 mL/y greater FEV1 decline than experienced by those without BHR at follow-up. Annual FEV1 decline was moderated by corticosteroid use.
Conclusions
Persistent BHR and its deleterious influence on lung function suggest a role for airway inflammation in perpetuation of WTC-associated airway disease. In future massive occupational exposure to inorganic dust/gases, we recommend early and serial pulmonary function testing, including measurements of bronchial reactivity, when possible, and inhaled corticosteroid therapy for those with symptoms or pulmonary function tests consistent with airway disease.
Key Words: airway disease, asthma, epidemiology, firefighting, occupational diseases
Abbreviations: BHR, bronchial hyperreactivity; FDNY, Fire Department of the City of New York; LLN, lower limit of normal; MCT, methacholine challenge test; PC20, provocative concentration of methacholine producing a 20% decline in FEV1; RADS, reactive airway dysfunction syndrome; WTC, World Trade Center
The September 11, 2001 (9/11), terrorist attack on the World Trade Center (WTC) and its consequent collapse exposed thousands of Fire Department of the City of New York (FDNY) firefighters to unprecedented amounts of aerosolized dust and smoke.1 One year later, nearly 20% had abnormal lung function, most with obstructive physiology,2, 3 and without meaningful recovery over the next 13 years.4 Symptoms of airway disease were even more prevalent and also persisted.5 New diagnoses of asthma increased over time, outpacing spirometric abnormalities.6, 7
Bronchial hyperreactivity (BHR) might explain airway disease and the high prevalence of airway symptoms in some subjects.8 Within 2 years of 9/11, we completed more than 400 studies of bronchial reactivity.3, 9, 10 Our current aim is to estimate the persistence of BHR and to explore relationships among BHR, respiratory symptoms, and lung function more than a decade after initial WTC exposure.
Methods
We evaluated bronchial reactivity in 173 WTC-exposed FDNY firefighters between January 10, 2013, and December 9, 2014 (follow-up methacholine challenge test [MCT]). Eligibility was based on: negative pre-9/11 asthma history, normal pre-9/11 spirometry, and availability of baseline MCT (within 2 years post-9/11). The Albert Einstein College of Medicine’s institutional review board (Federal Wide Assurance# 00023382) approved this study (institutional review board #12-08-268) and participant payment of $150. Written informed consent was obtained.
Methacholine Challenge Testing
Baseline MCTs were performed either at FDNY (n = 66, 38%) or at New York University (n = 107, 62%). The technique was similar at the two sites. Preparation included asking participants to discontinue: corticosteroid medications for 4 weeks; antihistamines, long-acting bronchodilators, and leukotriene modifiers for 2 days; and short-acting bronchodilators, coffee, tea, cola, and chocolate for 24 h.11 FEV1 was measured in duplicate at baseline and at 30 and 90 s after each dose of methacholine, using a Koko (FDNY) or Jaeger (New York University) spirometer. At 5-min intervals, increasing doses of methacholine (saline, 0.025, 0.25, 2.5, 10, and 25 mg/mL), nebulized with a DeVilbiss 646 nebulizer, at 10 to 12 per square inch applied pressure, yielding flow rates approximately 7 L/min, were delivered during the first second of each of five inspiratory vital capacities. PC20, the provocative concentration of methacholine producing a 20% decline in FEV1, was estimated by interpolation from the log concentration-response curve. BHR was defined as PC20 <8 mg/mL. Follow-up MCTs were all performed at Montefiore Medical Center, adhering to the dosing regimen described here, using a Koko Spirometer, but using a DeVilbiss 646 nebulizer and Rosenthal dosimeter calibrated to deliver 0.9 μL over 0.6 s.
Lung Function Trajectory
We accessed the last pre-9/11 spirometric result from the FDNY database. The first post-9/11 result was obtained from whichever was performed closest to, but after 9/11: either the spirometry performed at the baseline-MCT (n = 65) or the spirometry performed at the routine FDNY-WTC Health Program monitoring examinations (n = 108). The last post-9/11 FEV1 value was obtained on the date of the follow-up MCT. Trans-9/11 FEV1 change was calculated as first post-9/11 FEV1 minus the last pre-9/11 FEV1. The long-term post-9/11 change in FEV1 was calculated as the difference between the last post-9/11 FEV1 and the first post-9/11 FEV1, divided by the time between these two FEV1s, which were expressed as actual values, as percentages of the values predicted from National Health and Examination Survey (NHANES), and dichotomized as at or above vs below the lower limit of normal (LLN) predicted by NHANES.12
Questionnaire Data
We used information from self-administered questionnaires conducted during routine FDNY-WTC Health Program visits to assess WTC exposure based on arrival time (arriving on the morning of 9/11 [high]; arriving in the afternoon of 9/11 or anytime on September 12, 2001 [moderate]; and, arriving on any day between September 13, 2001 and September 24, 2001 [low])2; smoking history; and, atopy. Cough, dyspnea, and wheeze symptoms at baseline were assessed from questionnaires completed closest to the baseline MCT. At follow-up, symptoms of cough, dyspnea, and wheeze were assessed from questionnaires administered at the time of the follow-up MCT. Symptom provocability was assessed only at follow-up as an index from zero (no respiratory symptoms when exposed to strong odors, dust, allergens, temperature, or exercise) to five (respiratory symptoms present at all five exposures). We categorized inhaled or oral corticosteroid use since the first post-9/11 spirometry based on concurrent interviews, prior FDNY-WTC Health Program monitoring questionnaires, and prescription medication records.
Statistical Analyses
Continuous variables are expressed as mean ± SD. Paired categorical data were compared using McNemar test, while we used the Pearson χ2 test for nonpaired categorical data. We used logistic regression models to estimate the effect of predictors such as baseline BHR and symptom provocability (0 to 5) on BHR at follow-up, with trans-9/11 change in FEV1, age on 9/11, baseline abnormal lung function, baseline symptoms, and smoking status considered for inclusion in models as possible confounders. Multiple linear regression analyses were performed to assess associations of BHR and decline in lung function, with trans-9/11 change in FEV1, age on 9/11, height, race, corticosteroid use, weight change, smoking status, and atopy variables considered as potential confounders. Analyses were performed with SAS, version 9.4 (SAS Institute, Inc).
Results
Table 1 shows selected characteristics of the 173 study participants, the 207 eligible nonparticipants, and the source population of 12,948 WTC-exposed firefighters. Characteristics (age, race, smoking status) were generally similar, although eligible participants were about twice as likely to have arrived at the WTC-site during the morning of 9/11 (high exposure). Among eligibles, participants were less likely to be retired than nonparticipants; 48% of retired participants and 69% of retired nonparticipants retired because of a respiratory disability. Among eligibles, participants had, on average, lesser trans-9/11 decline in FEV1 than nonparticipants, but were similar to the source population in decline. Further, at MCT baseline, fewer participants had bronchial hyperreactivity compared with nonparticipants (P = .03). The median dates of the first post-9/11 FEV1 measurement and the baseline MCT were November 15, 2001 (range, September 24, 2001-July 17, 2003) and February 13, 2002 (range, September 24, 2001-September 6, 2003), respectively. Overall, post-9/11 corticosteroid use was reported by two-thirds of participants (117/173); 110 reported inhaled steroid use, 49 oral, and 42 both.
Table 1.
Selected Characteristics of the FDNY Study Participants, Invitees Who Did Not Participate, and the Source Population
| Characteristics | Eligibles (n = 380) |
Source Populationa N = 12,948 |
|
|---|---|---|---|
| Participants n = 173 |
Nonparticipants n = 207 |
||
| WTC Exposure, No. (%) | |||
| High exposure | 51 (29.5) | 61 (29.5) | 1,928 (14.9) |
| Moderate exposure | 105 (60.7) | 136 (65.7) | 9,944 (68.3) |
| Low exposure | 17 (9.8) | 10 (4.8) | 2,176 (16.8) |
| Race, No. (%) | |||
| White | 165 (95.4) | 200 (96.6) | 12,014 (92.8) |
| African American | 8 (4.6) | 7 (3.4) | 366 (2.8) |
| Other | 0 (0.0) | 0 (0.0) | 568 (4.4) |
| Smoking status | |||
| Current | 11 (6.4) | 17 (8.2) | 706 (5.5) |
| Former | 31 (17.9) | 70 (33.8) | 4,384 (33.9) |
| Never | 131 (75.7) | 120 (58.0) | 7,361 (58.6) |
| Missing | 0 (0.0) | 0 (0.0) | 262 (2.0) |
| Duty status by 2013-2014 | |||
| Active | 41 (23.7) | 22 (10.6) | 5,428 (41.9) |
| Retired | 132 (76.3) | 185 (89.4) | 7,347 (56.7) |
| Age at 9/11, mean (SD) | 42.6 (7.0) | 43.3 (6.6) | 41.0 (9.5) |
| Height (cm), mean (SD) | 177.9 (6.5) | 178 (6.8) | |
| Pre-9/11 FEV1 (mL), mean (SD) | 4,280 (670) | 4,200 (600) | 4,390 (810) |
| Pre-9/11 FEV1 (% predicted), mean (SD) | 103.0 (13.2) | 101.8 (12.1) | 102.9 (19.2) |
| Trans-9/11 change FEV1 (mL), mean (SD) | −399.0 (−468.3) | −521.01 (−491) | −377 (−650) |
| BHR in 2001-2003, No. (%) | 28 (16%) | 52 (25%) | N/A |
| Years between early and late post-9/11 PFT, mean (SD) | 11.5 (0.5) | N/A | N/A |
| Past or present atopy | 92 (53.2) | N/A | N/A |
BHR = bronchial hyperreactivity; FDNY = Fire Department of the City of New York; N/A = not applicable; WTC = World Trade Center.
Source population = male firefighters exposed to WTC in the first 2 wk.
Bronchial Reactivity Persistence
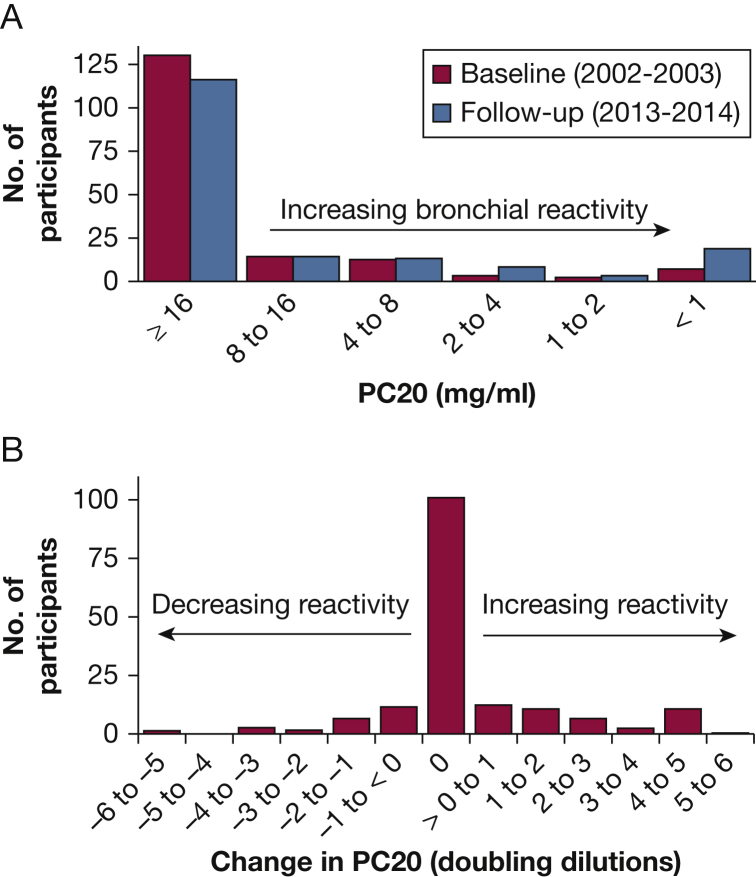
Figure 1A shows the distribution of bronchial reactivity at baseline and at follow-up. At baseline, 16% were classified as BHR (PC20 < 8mg/mL); the proportion increased to 25% at follow-up (P = .016). Figure 1B shows the distribution of change in bronchial reactivity between baseline and follow-up studies. Although the median change in reactivity was zero (58% were within 1 doubling dilution), the mean reactivity increased by 0.38 doubling concentrations. More people (27%) increased reactivity than decreased (14%).
Figure 1.
(A) Histogram showing the number of participants at various levels of PC20, at the 2001-2003 and the 2013-2014 studies. (B) The numbers of participants whose bronchial reactivity decreased, increased, or did not change between the two studies. On average, there was a slight increase in reactivity (decrease in PC20). PC20 = provocative concentration of methacholine producing a 20% decline in FEV1.
Table 2 shows BHR patterns at baseline and follow-up; 57% of those with BHR at baseline had BHR at follow-up. An additional 19% developed BHR by follow-up. In the logistic models, those with BHR at baseline were more than 6 times more likely to have BHR at follow-up than those without BHR at baseline (OR, 6.46; 95% CI, 2.49-16.77). Corticosteroid therapy was not significantly associated with change in reactivity (OR, 1.79; 95% CI, 0.63-5.12).
Table 2.
Association BHR at Baseline and at Follow-up
| BHR at Follow-up | No BHR at Follow-up | Total | |
|---|---|---|---|
| BHR at baseline | 16 | 12 | 28 |
| No baseline BHR | 27 | 118 | 145 |
| Total | 43 | 130 | 173 |
The proportion of participants with BHR at follow-up is greater than the proportion of participants with BHR at baseline (P = .0163 McNemar test). See Table 1 legend for expansion of abbreviation.
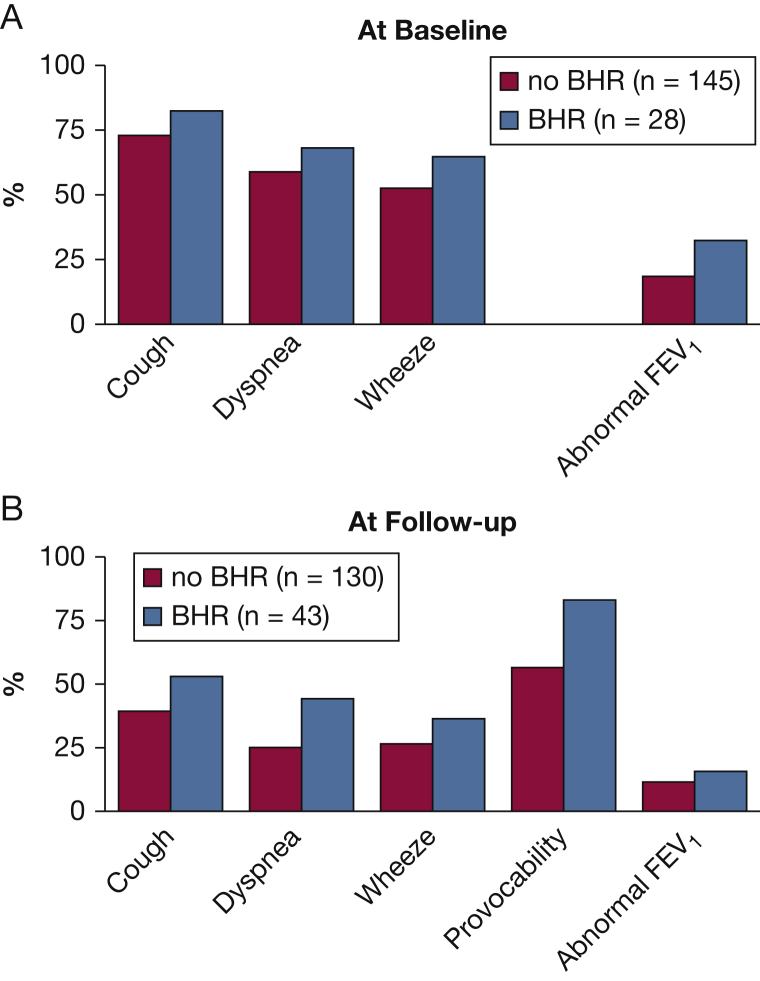
Figure 2A shows the prevalence of symptoms and abnormal lung function at baseline MCT among those with and without BHR. Figure 2B shows the prevalence of symptoms, abnormal lung function, and provocability (≥3 of 5 provocable symptoms) at follow-up among those with and without BHR at follow-up. The prevalence of one or more respiratory symptoms at follow-up-MCT was lower than at baseline, regardless of BHR status (81% vs 52%, P < .001). However, symptom prevalence tended to be higher in those with BHR at each time point, although the difference was statistically significant only for dyspnea at the follow-up time point. At follow-up, provocability of symptoms was higher among those with BHR than among those without BHR (P < .01). In this logistic model, we also found that for every increase in the provocability symptom index (range, 0-5), the odds of BHR at follow-up increased (OR, 1.39; 95% CI, 1.06-1.83), adjusting for BHR at baseline and other covariates.
Figure 2.
(A) Baseline respiratory symptoms and percentage of subjects with abnormal lung function (FEV1 < LLN) among those with and without bronchial hyperreactivity at baseline. Provocability was not available at baseline. (B) Follow-up respiratory symptoms and percentage with FEV1 < LLN among those with and without bronchial hyperreactivity at follow-up. All symptoms were less prevalent at follow-up than at baseline (P < .001). At follow-up, dyspnea and provocability of respiratory symptoms (3 or more vs. less than 3) were significantly more prevalent (OR, 2.28 [1.09-4.73], and OR, 3.89 [1.61-9.39], respectively) among those with BHR than among without BHR. BHR = bronchial hyperreactivity; LLN = lower limit of normal.
Lung Function Trajectory
Over the study period, the unadjusted average decline in FEV1 was 32 mL/y, similar to the rates we previously reported in WTC-exposed firefighters,2 but less than the 45 mL/y average observed in a younger cohort of non-WTC-exposed, newly hired firefighters.13
As shown in Figure 2, the percentage with abnormal lung function (<LLN) decreased between baseline and follow-up (20% to 13%) and was slightly higher among those with BHR, although the difference was nonsignificant at either period (P = .09 and P = .42, respectively). Among the 10 participants who developed abnormal lung function during the study, only one had BHR at baseline. At both periods, symptom prevalence was 2 to 4 times as high as the prevalence of abnormal lung function; at follow-up, provocable symptom prevalence was approximately 5 times as high as the prevalence of abnormal lung function.
Table 3 shows results of the multiple regression models assessing the association between BHR at follow-up and annual change in FEV1, controlling for trans-9/11 change, age, height, race, and steroid use. BHR at follow-up was associated with an estimated 15.39 mL/y more rapid decline rate, compared with those without BHR (P < .01). Controlling for BHR status at follow-up, steroid use reduced the rate of decline by 13.01 mL/y. Weight change (0.70 mL/y/kg; P = .055), smoking status, and atopy variables did not improve the model fit and were excluded.
Table 3.
Outcome Is Post-9/11 Change FEV1 (mL/y)
| Post-9/11 Change in FEV1 (mL/y) | Estimate | 95% CI | P Value |
|---|---|---|---|
| Intercept | −36.78 | (−45.80 to −27.76) | < .0001 |
| BHR status follow-up | |||
| Present | −15.38 | (−27.10 to −3.66) | .0104 |
| Not present | Reference | ||
| Steroid use | |||
| Ever | +13.01 | (2.10-23.93) | .0197 |
| Never | Reference | ||
| Trans 9/11 FEV1 change (post-pre per L, centered at −0.4 L) | −51.96 | (−62.42 to −41.50) | < .0001 |
| Age on 9/11 (centered at 42.6 y) | −0.01 | (−0.73 to 0.71) | .9704 |
| Height (centered at 177.9 cm) | −1.09 | (−1.87 to −0.31) | .0066 |
| Race | |||
| African American | −6.40 | (−30.29 to 17.50) | .5978 |
| White | Reference |
The intercept for this model is the estimated rate of FEV1 change (last post-first post-9/11 difference divided by time difference) for a 177.9-cm tall white participant age 42.6 y on 9/11, without BHR at follow-up, who did not report using steroids, and who had a FEV1 change of −0.4 L first post-9/11 to pre- 9/11 measurement. Negative numbers indicate decline in FEV1, positive numbers indicate increase in FEV1. See Table 1 legend for expansion of abbreviation.
Discussion
Our study uniquely examines bronchial reactivity 10 to 12 years after inhalation of WTC particulates and gases in a sample of highly exposed, highly symptomatic firefighters, who were first evaluated for reactivity within 2 y of 9/11. We found that BHR persisted in the majority of initially BHR-positive participants and was newly recognized in a few previously BHR-negative participants more than a decade after exposure.
At the baseline MCT shortly after 9/11, 16% had PC20 <8 mg/mL (BHR), which increased to 25% by the follow-up study, both substantially higher than would have been predicted in a healthy, young-to-middle aged, largely white male cohort14; in FDNY firefighter control subjects9; or in Swiss firefighters without WTC exposure.15 In our logistic models, we found the single most important predictor of BHR at follow-up was BHR at baseline.
Others have reported on irritant-induced occupational asthma and bronchial reactivity 216 and 13 y17 after inhalation accidents,17 finding little change in bronchial reactivity, although the former study was conducted only 2 y after exposure and the latter, which was of comparable duration to ours, included fewer participants (n = 35). Although BHR persistence is our most notable finding, mean bronchial reactivity and the overall prevalence of BHR increased rather than decreased 10 to 12 y after exposure, contrasting with the decline in BHR prevalence expected after removal from noxious exposures in occupational asthma.18, 19, 20
For the first time, we demonstrate that BHR was associated with a greater decline in FEV1 after controlling for trans-9/11 change, demographics, and steroid use. BHR at follow-up was associated with an estimated 15.4 mL/y more rapid decline compared to the rate in those without BHR.
We observed that, on average, after the approximately −400 mL trans-9/11 change in FEV1, there was no evident lung function recovery. Table 3 indicates that a firefighter who experienced the average trans-9/11 FEV1 change of −400 mL, had BHR at follow-up, and had not been treated with corticosteroids could expect, over the subsequent decade, an average change of −52 mL/y (−37 mL for the intercept plus −15 mL for BHR), as compared to −37 mL/y in those without BHR at follow-up. However, that estimate is substantially impacted by the trans-9/11 FEV1 change—again, from Table 3, that same firefighter, with BHR at the follow-up study, would have an expected annual change of −73 mL/y had he experienced no trans-9/11 change (−37 mL for the intercept, plus −15 mL for BHR, plus [0.4 L × −52 mL] for 400 mL greater trans-9/11 change than the average of −400 mL). That those who had greater loss of lung function trans-9/11 had (on average) lesser subsequent decline in FEV1 than did those who lost less lung function over 9/11 suggests that partial recovery occurred, for at least some firefighters. We also note that all of these annual decline estimates are within the expected range for nonsmoking, urban-dwelling, middle-aged men.21
Consistent with prior studies,22, 23 we showed that asthma symptoms were far more common than overt lung function abnormalities. Asthma symptoms were more prevalent among those with BHR than without BHR, although, despite persistence and new onset of BHR, symptoms decreased substantially by follow-up.
Measured bronchial reactivity can be influenced by baseline lung function—a given change in airway smooth muscle length will produce a greater fractional change in airway caliber if initial caliber is below normal.8, 24 Thus, one might infer that some of the associations we observed between BHR and FEV1 could reflect an influence of FEV1 on the measurement of bronchial reactivity, rather than the reverse. However, at the range of FEV1s observed in our subjects, changes in airway caliber would not have been enough to explain the increase in bronchial reactivity.24
More than two-thirds of the participants were treated with corticosteroids, most by inhalation. Despite the probability that those with symptoms or lung function impairment were more likely than others to have corticosteroids prescribed, we did not find an association of corticosteroid therapy with improvement in bronchial reactivity. However, controlling for BHR status at follow-up, we did find an association of steroids with an estimated 13 mL/y lower FEV1 decline rate over the subsequent decade. This effect is consistent with prior studies in asthma25 and COPD.26
Occupational airway disease is heterogeneous, including: work-exacerbated asthma (in those with preexisting asthma)27; occupational asthma, which may be immunologic (characterized by a latent period), or irritant-induced27, 28, 29; reactive airway dysfunction syndrome (RADS), an acute form of irritant asthma usually associated with single, high-level exposures28, 30, 31; and occupational COPD.31, 32 Prognosis is variable,28 although a substantial fraction of those with occupational asthma may improve after discontinuation of exposure,17, 18, 19, 20 or with inhaled corticosteroid therapy, even without discontinuation of exposure.29 Persons with RADS may or may not improve,18, 20, 30 whereas those with occupational COPD typically do not improve.32 Occupational asthma and RADS are usually characterized by BHR—required by most definitions of RADS.31
Although our participants’ BHR was persistent, its prevalence was substantially lower than reported in persons with asthma,33 suggesting that WTC-associated airway disease does not follow the classic trajectory of occupational asthma. On the other hand, the higher prevalence of asthma symptoms, especially provocability, and the decreased prevalence of abnormal lung function that we observed between baseline and follow-up post-9/11 periods suggest at least an element of irritant-induced occupational asthma or RADS. The significant association of bronchial reactivity with FEV1 decline rate in the multiple regression model, and the apparent beneficial effect of corticosteroid therapy (despite the likelihood that corticosteroid therapy was given, at least in part, because of symptoms), suggest that WTC-associated airway disease includes an element of asthmatic inflammation.
A major limitation of our study is our use of differing methods to measure bronchial reactivity at baseline and follow-up. We acknowledge that baseline but not follow-up MCTs were obtained without the use of dosimeters to control delivered doses of methacholine, which may have resulted in overestimation of bronchial reactivity at baseline. If that were the case, however, we would have expected to see a decrease rather than persistence or increase in the proportion of participants with BHR at follow-up, when a dosimeter was used. Furthermore, our finding of an association between BHR and FEV1 decline was based on measurement of BHR at follow-up and therefore was not influenced by this difference in methodology between initial and follow-up challenge tests.
Additional limitations include lack of an unexposed control group, possible selection bias (selection for study inclusion, selection by indication for corticosteroid use), possible misclassification of corticosteroid treatment, and inadequate statistical power for some analyses. Regarding selection bias, study participants were not a random sample of WTC-exposed firefighters. Those with baseline MCTs were about twice as likely as the source population to have arrived at the WTC during the morning of 9/11 when exposure was most intense. However, we note that participants were similar to the source population in trans-9/11 decline and pre-9/11 FEV1. We are also aware that corticosteroid use was likely prescribed for the most highly symptomatic persons, and also that our partial reliance on recall for corticosteroid histories may have led to either nondifferential or differential inaccuracies in reporting. Finally, we were unable to examine baseline BHR as a predictor of COPD because only one individual with baseline BHR developed FEV1 < LLN by follow-up.
Despite these limitations, the strengths of this study are notable. We evaluated bronchial reactivity in 173 participants soon after 9/11 and again more than a decade later, and had access to pre-9/11 and post-9/11 spirometry. Our findings underscore the importance of having preexposure lung function data. Being able to take trans-9/11 FEV1 decline into account allowed us to find statistical relationships between bronchial reactivity and post-9/11 lung function decline that were equivocal when only post-9/11 data were analyzed.
In conclusion, we have shown that BHR was present in a significant percentage of WTC-exposed firefighters and that, overall, BHR persisted or increased over the subsequent decade, and was independently associated with worsening lung function. Continued follow-up is required to determine whether persistent symptoms are most consistent with irritant-associated asthma or occupational COPD. In any future massive occupational exposure to inorganic dust, we recommend early and serial pulmonary function testing, including measurements of bronchial reactivity, when possible, and inhaled corticosteroid therapy for those with symptoms or pulmonary function tests consistent with developing airway disease.
Acknowledgments
Author contributions: This study was designed by T. K. A., C. B. H., M. D. W., H. W. C., M. P. W., G. I., and D. J. P. Data were collected by T. K. A., J. W., S. D., T. C., G. I. B., M. D. W., C. K., W. M., A. N., K. J. K., and V. C. All authors participated in analysis of results. The manuscript was prepared by T. K. A., J. W., S. D., A. G., M. D. W., R. Z. O., M. P. W., and D. J. P. T. K. A. and D. J. P. take full responsibility for the entire manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was funded by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health [Grants 1U01OH010411, 200-2011-39383, and 200-2011-39378].
References
- 1.Rom W.N., Reibman J., Rogers L. Emerging exposures and respiratory health: World Trade Center dust. Proc Am Thorac Soc. 2010;7(2):142–145. doi: 10.1513/pats.200908-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrich T.K., Gustave J., Hall C.B. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362(14):1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiden M.D., Ferrier N., Nolan A. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137(3):566–574. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldrich T.K., Vossbrinck M., Zeig-Owens R. Lung function trajectories in WTC-exposed NYC Firefighters over 13 years: the roles of smoking and smoking cessation. Chest. 2016;149(6):1419–1427. doi: 10.1016/j.chest.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weakley J., Webber M.P., Gustave J. Trends in respiratory diagnoses and symptoms of firefighters exposed to the World Trade Center disaster: 2005-2010. Prev Med. 2011;53(6):364–369. doi: 10.1016/j.ypmed.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Niles J.K., Webber M.P., Cohen H.W. The Respiratory Pyramid: from symptoms to disease in World Trade Center exposed firefighters. Am J Ind Med. 2013;56(8):870–880. doi: 10.1002/ajim.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall C.B., Liu X., Zeig-Owens R. The duration of an exposure response gradient between incident obstructive airways disease and work at the World Trade Center Site: 2001-2011. PLoS Curr. 2015;20 doi: 10.1371/currents.dis.8a93e7682624698558a76a1fa8c5893f. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockcroft D.W. Direct challenge tests: airway hyperresponsiveness in asthma: Its measurement and clinical significance. Chest. 2010;138(2 suppl):18S–24S. doi: 10.1378/chest.10-0088. [DOI] [PubMed] [Google Scholar]
- 9.Banauch G.I., Alleyne D., Sanchez R. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am J Respir Crit Care Med. 2003;168(1):54–62. doi: 10.1164/rccm.200211-1329OC. [DOI] [PubMed] [Google Scholar]
- 10.Banauch G.I., Dhala A., Alleyne D. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33(1 Suppl):S102–S106. doi: 10.1097/01.ccm.0000151138.10586.3a. [DOI] [PubMed] [Google Scholar]
- 11.Crapo R.O., Casaburi R., Coates A.L. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161(1):309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 12.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 13.Aldrich T.K., Ye F., Hall C.B. Longitudinal pulmonary function in newly hired, non-World Trade Center-exposed fire department City of New York firefighters: the first 5 years. Chest. 2013;143(3):791–797. doi: 10.1378/chest.12-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britton J., Pavord I., Richards K. Factors influencing the occurrence of airway hyperreactivity in the general population: the importance of atopy and airway caliber. Eur Respir J. 1994;7(5):881–887. [PubMed] [Google Scholar]
- 15.Miedinger D., Chhajed P.N., Stolz D. Respiratory symptoms, atopy and bronchial hyperreactivity in professional firefighters. Eur Respir J. 2007;30(3):538–544. doi: 10.1183/09031936.00015307. [DOI] [PubMed] [Google Scholar]
- 16.Gautrin D., Leroyer C., Infante-Rivard C. Longitudinal assessment of airway caliber and responsiveness in workers exposed to chlorine. Am J Res Crit Care Med. 1999;160(4):1232–1237. doi: 10.1164/ajrccm.160.4.9811074. [DOI] [PubMed] [Google Scholar]
- 17.Malo J.-L., L’archevêque J., Castellanos L. Long-term outcomes of acute irritant-induced asthma. Am J Respir Crit Care Med. 2009;179(10):923–928. doi: 10.1164/rccm.200810-1550OC. [DOI] [PubMed] [Google Scholar]
- 18.Malo J.-L., Ghezzo H. Recovery of methacholine responsiveness after end of exposure in occupational asthma. Am J Respir Crit Care Med. 2004;169(12):1304–1307. doi: 10.1164/rccm.200312-1749OC. [DOI] [PubMed] [Google Scholar]
- 19.Padoan M., Pozzato V., Simoni M. Long-term follow-up to toluene-diisocyanate-induced asthma. Eur Respir J. 2003;21(4):637–640. doi: 10.1183/09031936.03.00060703. [DOI] [PubMed] [Google Scholar]
- 20.Lemiere C., Cahboillez S., Welman M., Maghni K. Outcome of occupational asthma (OA) after removal from exposure. A follow-up study. Can Respir J. 2010;17(2):61–66. doi: 10.1155/2010/509807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tashkin D.P., Detels R., Simmons M. The UCLA population studies of chronic obstructive respiratory disease: XI. Impact of air pollution and smoking on annual change in forced expiratory volume in one second. Am J Respir Crit Care Med. 1994;149(5):1209–1217. doi: 10.1164/ajrccm.149.5.8173761. [DOI] [PubMed] [Google Scholar]
- 22.Falliers C.J. Ventilatory impairment in asthma: perceptions vs measurements. Chest. 1998;113(2):265–267. doi: 10.1378/chest.113.2.265. [DOI] [PubMed] [Google Scholar]
- 23.Sistek D., Wickens K., Amstrong R. Predictive value of respiratory symptoms and bronchial hyperresponsiveness to diagnose asthma in New Zealand. Respir Med. 2006;100(12):2107–2111. doi: 10.1016/j.rmed.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Dirksen A., Madsen F., Engel T. Airway calibre as a confounder in interpreting bronchial responsiveness in asthma. Thorax. 1992;47(9):702–706. doi: 10.1136/thx.47.9.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Asthma Education and Prevention Program. Expert Panel Report 3 Guidelines for the Diagnosis and Management of Asthma. NIH Publication Number 08-5846, October 2007.
- 26.Calverley P.M.A., Anderson J.A., Celli B. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 27.Tan J., Bernstein J.A. Occupational asthma: an overview. Curr Allerg Asthma Rep. 2014;14(5):431. doi: 10.1007/s11882-014-0431-y. [DOI] [PubMed] [Google Scholar]
- 28.Tarlo S.M., Lemière C. Occupational asthma. New Engl J Med. 2014;370(7):640–649. doi: 10.1056/NEJMra1301758. [DOI] [PubMed] [Google Scholar]
- 29.Marabini A., Siracusa A., Stopponi R. Outcome of occupational asthma in patients with continuous exposure: a 3-year longitudinal study during pharmacologic treatment. Chest. 2003;124(6):2372–2376. doi: 10.1378/chest.124.6.2372. [DOI] [PubMed] [Google Scholar]
- 30.Brooks S.M., Weiss M.A., Bernstein I.L. Reactive airways dysfunction syndrome (RADS). persistent asthma syndrome after high-level irritant exposures. Chest. 1985;88(3):376–384. doi: 10.1378/chest.88.3.376. [DOI] [PubMed] [Google Scholar]
- 31.Alberts W.M., doPico G.A. Reactive airways dysfunction syndrome. Chest. 1996;109(6):1618–1626. doi: 10.1378/chest.109.6.1618. [DOI] [PubMed] [Google Scholar]
- 32.Hodgins P., Henneberger P.K., Wang M.-L., Petsonk E.L. Bronchial responsiveness and five-year FEV1 decline. A study in miners and non-miners. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1390–1396. doi: 10.1164/ajrccm.157.5.9701123. [DOI] [PubMed] [Google Scholar]
- 33.Sumino K., Sugar E.A., Irvin C.G. Variability of methacholine bronchoprovocation and the effect of inhaled corticosteroids in mild asthma. Ann Allerg Immunol. 2014;112:354–360. doi: 10.1016/j.anai.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]