Abstract
Background
We updated the 2006 ACCP clinical practice guidelines for management of reflux-cough syndrome.
Methods
Two population, intervention, comparison, outcome (PICO) questions were addressed by systematic review: (1) Can therapy for gastroesophageal reflux improve or eliminate cough in adults with chronic and persistently troublesome cough? and (2) Are there minimal clinical criteria to guide practice in determining that chronic cough is likely to respond to therapy for gastroesophageal reflux?
Results
We found no high-quality studies pertinent to either question. From available randomized controlled trials (RCTs) addressing question #1, we concluded that (1) there was a strong placebo effect for cough improvement; (2) studies including diet modification and weight loss had better cough outcomes; (3) although lifestyle modifications and weight reduction may be beneficial in suspected reflux-cough syndrome, proton pump inhibitors (PPIs) demonstrated no benefit when used in isolation; and (4) because of potential carryover effect, crossover studies using PPIs should be avoided. For question #2, we concluded from the available observational trials that (1) an algorithmic approach to management resolved chronic cough in 82% to 100% of instances; (2) cough variant asthma and upper airway cough syndrome (UACS) (previously referred to as postnasal drip syndrome) from rhinosinus conditions were the most commonly reported causes; and (3) the reported prevalence of reflux-cough syndrome varied widely.
Conclusions
The panelists (1) endorsed the use of a diagnostic/therapeutic algorithm addressing causes of common cough, including symptomatic reflux; (2) advised that although lifestyle modifications and weight reduction may be beneficial in suspected reflux-cough syndrome, PPIs demonstrated no benefit when used in isolation; and (3) suggested that physiological testing be reserved for refractory patients being considered for antireflux surgery or for those in whom there is strong clinical suspicion warranting diagnostic testing.
Key Words: cough, evidence-based medicine, gastroesophageal reflux disease, guidelines
Abbreviations: ACE, angiotensin-converting enzyme; CHEST, American College of Chest Physicians; GER, gastroesophageal reflux; GERD, gastroesophageal reflux disease; H2RA, histamine-2 receptor antagonist; LPR, laryngopharyngeal reflux; PICO, population, intervention, comparison, outcome; PPI, proton pump inhibitor; RCT, randomized controlled trial; UACS, upper airway cough syndrome
Summary of Recommendations and Suggestions
1. In adult patients with chronic cough, we suggest that the cough be managed according to a published management guideline that initially considers the most common potential etiologies as well as symptomatic gastroesophageal reflux (ungraded, consensus based).
Remarks: Common potential etiologies include environmental or occupational irritants, primary or secondary smoking, use of angiotensin-converting-enzyme (ACE) inhibitors, abnormal chest radiographic findings, asthma, upper airway cough syndrome due to a variety of rhinosinus conditions, nonasthmatic eosinophilic bronchitis, and suppurative lung disease. Often, more than one etiology is a contributing factor.
2. In adult patients with chronic cough suspected to be due to reflux-cough syndrome, we recommend that treatment include (1) diet modification to promote weight loss in overweight or obese patients; (2) head of bed elevation and avoiding meals within 3 hours of bedtime; and (3) in patients who report heartburn and regurgitation, proton pump inhibitors (PPIs), H2-receptor antagonists, alginate, or antacid therapy sufficient to control these symptoms (Grade 1C).
Remarks: (1) While it is expected that GI symptoms will respond within 4-8 weeks, the literature suggests that improvement in cough may take up to 3 months.1 b) Head of bed elevation is suggested based on its utility for improving GI GERD symptoms2 while acknowledging that it has not been demonstrated to be beneficial for cough.
3. In adult patients with suspected chronic cough due to reflux-cough syndrome, but without heartburn or regurgitation, we recommend against using PPI therapy alone because it is unlikely to be effective in resolving the cough (Grade 1C).
4. In adult patients with chronic cough potentially due to reflux-cough syndrome who are refractory to a 3-month trial of medical antireflux therapy and are being evaluated for surgical management (antireflux or bariatric), or in whom there is strong clinical suspicion warranting diagnostic testing for gastroesophageal reflux, we suggest that they undergo esophageal manometry and pH-metry with conventional methodology (Grade 2C).
Remarks: Esophageal manometry is done both to evaluate for a major motility disorder and to accurately position the pH electrode for the pH monitoring study. With conventional methodology, the pH electrode is placed 5 cm proximal to the lower esophageal sphincter, and the study is done off antisecretory medications after withholding PPI therapy for 7 days and H2 receptor antagonists for 3 days prior to the study. It was agreed by consensus of the Esophageal Diagnostic Advisory Panel composed of both gastroenterologists and surgeons3 that this is the only methodology with proven validity with respect to surgical outcomes.
5. In adult patients with chronic cough and a major motility disorder (eg, absent peristalsis, achalasia, distal esophageal spasm, hypercontractility) and/or normal acid exposure time in the distal esophagus, we suggest not advising antireflux surgery (Grade 2C).
Remarks: Under the circumstances of a major motility disorder or normal esophageal acid exposure on esophageal pH-metry, there is no supportive controlled data for antireflux surgery and there is quantifiable risk to the procedure making for an unacceptable risk-benefit ratio.3, 4
6. In adult patients with chronic cough, adequate peristalsis, and abnormal esophageal acid exposure determined by pH-metry in whom medical therapy has failed we suggest antireflux (or bariatric when appropriate) surgery for presumed reflux-cough syndrome (Grade 2C).
Remarks: With respect to defining adequate peristalsis, there is no consensus. Some consider any preserved peristalsis to be adequate while others stipulate that it must be at least 30% and others at least 50% of normal.5
The Montreal consensus defined gastroesophageal reflux disease (GERD) as “a condition which develops when the reflux of stomach content causes troublesome symptoms and/or complications.”6 Hence, a GERD diagnosis can be based on either tissue damage (esophagitis, stricture, Barrett’s esophagus, esophageal adenocarcinoma) or “troublesome” symptoms attributable to reflux. Furthermore, the defining symptoms can be esophageal (heartburn, regurgitation, chest pain) or extraesophageal. However, no symptom, not even the most typical one (heartburn) is 100% specific for GERD, and as the specificity of a potential GERD-defining symptom decreases, the challenge of establishing a GERD diagnosis increases. Nowhere is this dilemma more apparent than in the case of reflux-cough syndrome. Compounding the problem, the efficacy of PPI therapy for resolving GERD symptoms is substantially less than it is for healing esophagitis and, within the domain of potential symptoms, much less for atypical symptoms than it is for heartburn.7
Cough is a potential symptom of gastroesophageal reflux (GER), usually listed among the inventory of extraesophageal symptoms in GERD questionnaires. For example, in a population-based study, Locke et al8 used a validated GER questionnaire to survey 2,200 residents of Olmsted County, Minnesota and found a prevalence rate of 18% for frequent heartburn, defined as occurring at least weekly. Among these individuals, 14% also endorsed “bronchitis” defined by cough as often as four to six times a day on 4 or more days of the week. Similarly, from the perspective of a primary care population, El-Serag et al9 accessed data from the UK general practice research database and found that patients with a new diagnosis of GERD had an increased likelihood (OR, 1.7; CI, 1.4-2.1) of subsequently being diagnosed with cough in the next 12 months. Examined from the opposite perspective, a cross-sectional survey of 4,003 residents of West Yorkshire, England aged 40 to 49 years found that 12% reported chronic cough and that regurgitation was a strong predictor of cough (OR, 1.71; 99% CI, 1.20-2.45).10 Other studies of patients with chronic cough conclude that reflux is a contributing factor in 0% to 41% of cases referred to specialty cough clinics, depending on the definitional criteria applied and populations studied.11 Notably, from this perspective, GERD is thought to be “silent” from a GI perspective in up to 75% of cases,12 raising the possibility that nonacidic reflux is a potential cause of cough.
The relationship between reflux and cough is supported by several physiological observations. In patients with chronic cough, acid infusion into the distal esophagus increases the frequency of coughing13 and cough reflex sensitivity.14 During carefully conducted reflux monitoring studies, approximately half of unselected patients with chronic cough exhibit a positive symptom association between cough and acid or weakly acidic reflux events.15 However, the relationship between reflux and cough is particularly complex because other disease processes, issues of cause and effect, and hypersensitivity of both the esophagus and the cough reflex itself all come into play.11 Given the implicit variation in approaches used to identify patients with putative reflux-related cough, it is understandable that a recent Cochrane review found insufficient evidence to conclude that PPI treatment is beneficial in treating nonspecific chronic cough.16, 17
Adding to the complexities of establishing the relationship between GERD and chronic cough is that a major defining criterion has been resolution or substantial improvement in cough with therapy for GER, and the spectrum of that therapy includes antireflux surgery, a fundamentally irreversible and potentially morbid intervention. Hence, it is desirable to have prospective criteria for reflux-cough patient identification to apply in a clinical scenario. This led to the approach advocated in the 2006 ACCP cough guideline of predicting reflux-cough syndrome by a clinical profile aimed at excluding other potential chronic cough causes (Table 1).18 Based on post hoc analyses of four before-and-after intervention studies along with two small prospective before-and-after intervention studies, the clinical profile in Table 1 was estimated to be 91% predictive that a patient’s cough would respond to antireflux treatment, even when there were no GI symptoms.18
Table 1.
Clinical Profile Championed in the 2006 ACCP Cough Guideline to Predict That Chronic Cough Was Likely Due to GER Even Without Concomitant GI Symptoms
| Chronic cough greater than 8 wk duration |
| Not exposed to environmental irritants nor a present smoker |
| Not taking an ACE inhibitor |
| Chest radiograph is normal or shows nothing more than stable inconsequential scarring |
| Symptomatic asthma has been ruled out: cough has not improved with asthma therapy or methacholine inhalation challenge is negative |
| UACS due to rhinosinus diseases has been ruled out: first-generation H1-antagonist has been used and cough failed to improve, and “silent” sinusitis has been ruled out |
| Nonasthmatic eosinophilic bronchitis has been ruled out: properly performed induced sputum analysis studies are negative, or cough has not improved with inhaled/systemic corticosteroids |
ACE = angiotensin-converting enzyme; GER = gastroesophageal reflux; UACS = upper airway cough syndrome.
As is evident from the preceding discussion, the literature on reflux-cough syndrome can be characterized as having a major internal contradiction. On the one hand, there is strong evidence of biological plausibility that a reflux-cough syndrome exists and that there is therapeutic efficacy for reflux disease interventions when judged retrospectively. On the other hand, there is a general paucity of controlled data in this domain such that attempts by Chang et al16, 17 to establish the efficacy of medical antireflux therapy for suspected reflux-induced cough concluded that there was no efficacy. Kahrilas et al11 looked at the therapeutic trial data a little differently, attempting to identify subgroups of patients with objective evidence of reflux (endoscopy or reflux monitoring) and concluded that there was some indication (albeit not robust) of therapeutic efficacy. It is from this background that the Expert Cough Panel endeavored to update the CHEST evidence-based clinical practice guidelines for chronic cough due to reflux in adults.
Methods
We used the published methodology of the American College of Chest Physicians (CHEST) Guideline Oversight Committee to select the Expert Cough Panel Chair and the International Panel of Experts to perform a systematic review and synthesis of evidence and to develop recommendations and practice management suggestions.19 After generating the key clinical questions for this systematic review, population, intervention, comparison, outcome (PICO) elements were derived to inform the literature review. The questions were formulated after polling the existing writing group for key clinical questions related to chronic cough due to GERD. This returned a list of 20 questions that were then synthesized into two PICO questions that were sufficiently broad to capture most of the detail from those 20 questions. The resultant PICO questions that formed the basis of the subsequent systematic review are stated in Table 2.
Table 2.
PICO Questions Addressed by Systematic Review
| Question #1 |
| Can therapy intended to treat GER improve or eliminate cough in adults with chronic and persistently troublesome cough? |
|
| Question #2 |
| Are there minimal clinical criteria to guide clinical practice in determining that a patient’s chronic cough is likely to respond to therapy for GER? |
|
See Table 1 legend for expansion of abbreviations.
Literature Search
The methods used for this systematic review conformed to those outlined in the article “Methodologies for the Development of CHEST Guidelines and Expert Panel Reports.”19 Systematic reviews and clinical trials were identified from searches of electronic databases (Ovid Medline, and EMBASE) commencing from the earliest available date until May 2015. A search of the Cochrane database returned 82 articles, eight of which were sufficiently relevant for full text review; however, none met criteria for inclusion in this review. The reference lists of retrieved articles were examined for additional citations. The search terms used were (“Gastroesophageal Reflux” [MeSH] OR GERD OR GORD OR reflux OR gastroesophageal reflux disease OR reflux esophagitis OR nonerosive reflux disease OR NERD) AND (“Proton Pump Inhibitors” [MeSH] OR PPI OR omeprazole OR lansoprazole OR pantoprazole OR rabeprazole OR esomeprazole OR tenatoprazole OR “Histamine H2 Antagonists” [MeSH] OR prokinetic OR surgery) AND cough. The titles and abstracts of the search results were independently evaluated by two reviewers (P. J. K. and R. S. I.) to identify potentially relevant articles. The full texts of all potentially relevant articles were retrieved, and two reviewers (P. J. K. and R. S. I.) independently reviewed all retrieved studies. A third reviewer was available to adjudicate any disagreements.
Quality Assessment
Included articles underwent methodological assessment. For the randomized controlled trials (RCTs), quality assessment was carried out with the Cochrane Risk of Bias Tool20 if the following criteria were met: (1) study excluded other common causes of chronic cough (asthma, upper airway cough syndrome [UACS]) by adequate workup and (2) included patients both with and without additional symptoms of GERD or laryngopharyngeal reflux (LPR), or both, or included patients with and without additional test results suggestive of GERD or LPR, or both. For observational studies, quality assessment was done with the Cochrane Risk of Bias Tool for cohort studies.21
Practice Recommendations/Suggestions
The findings of this systematic review were used to support the evidence-graded recommendations or suggestions. A structured consensus-based Delphi approach was used to provide expert advice on guidance statements. In this regard, for a recommendation or suggestion to be approved by the Expert Cough Panel, 75% of the eligible panel members had to vote, and 80% of those voting had to strongly agree or agree with the statement.19 Quality assessment also included grading the strength of recommendations based on consideration of the balance of benefits to harms, patient values and preferences, and the quality of the evidence supporting the recommendation.19 Harms incorporated risks and burdens to the patients that can include convenience or lack of convenience, difficulty of administration, and invasiveness.
Results
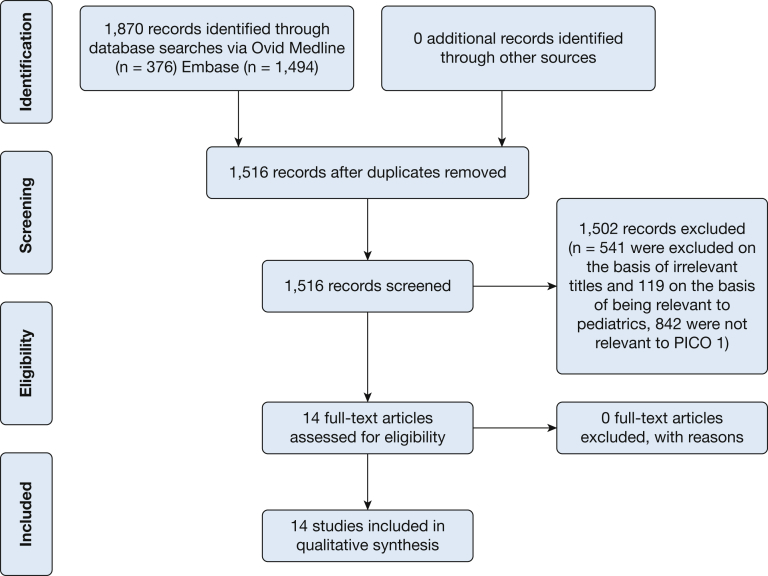
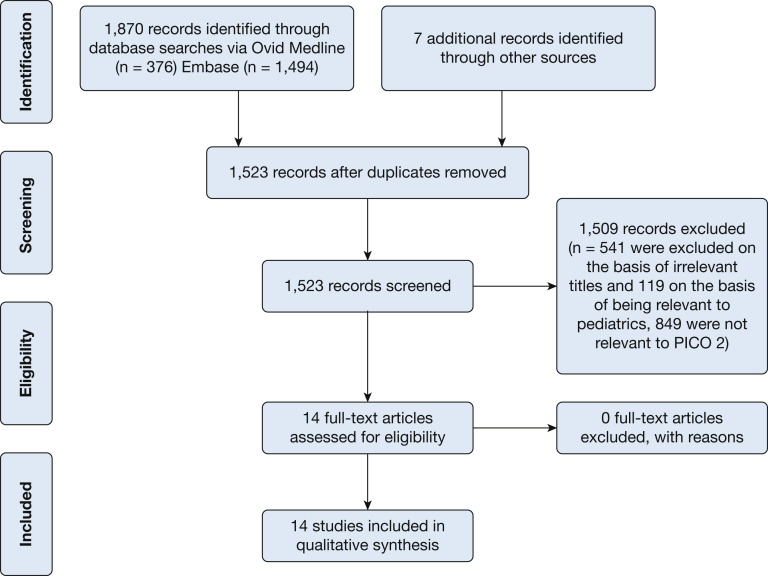
This systematic review addressed two PICO questions. The results of the literature search for the first question appear in Figure 1. The search initially identified 1,870 citations. After the removal of 354 duplicates, 1,516 records were screened, with 541 being excluded on the basis of irrelevant titles and 119 on the basis of being relevant to pediatrics. Hence, 842 abstracts were reviewed. Among these, 14 were controlled medical trials potentially pertinent to PICO question #1. The results of the search for the second question appear in Figure 2. Initial searching identified 1,877 records. After the removal of 354 duplicates, 1,523 records were screened, with 541 being excluded on the basis of irrelevant titles and 119 on the basis of being relevant to pediatrics. Hence, 849 abstracts were reviewed. Among these, 14 full-text articles were assessed for eligibility and deemed pertinent for qualitative synthesis for inclusion. The additional seven studies for PICO question 2 were identified in the recent publication on intervention fidelity.22
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for key question 1: Can therapy intended to treat gastroesophageal reflux improve or eliminate cough in adults with refractory chronic cough? PICO = population, intervention, comparison, outcome.
Figure 2.
PRISMA flow diagram for key question 2: Are there minimal clinical criteria to guide clinical practice in determining that a patient’s chronic cough is likely to respond to therapy for gastroesophageal reflux? See Figure 1 legend for expansion of abbreviations.
PICO Question 1: Can therapy intended to treat gastroesophageal reflux improve or eliminate cough in adults with chronic and persistently troublesome cough?
As is evident from Figure 1, we identified relatively few RCTs addressing the treatment of chronic cough with antireflux therapy. Most of the treatment literature was in the form of uncontrolled trials, retrospective studies, or nonrandomized controlled trials that did not use validated assessment tools of cough as outcome measures. Table 3 summarizes the inclusion criteria, treatment arms, and methods of cough assessment of the identified RCTs.23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 None of these were surgical trials. Particularly problematic when comparing these trials was the variability in how typical features of reflux disease (heartburn, regurgitation) or objective measures of reflux disease (endoscopy, reflux testing) were used as inclusion or exclusion criteria. In some studies (#7, #11, #12, #13, and #14), this was not specified as either a study inclusion or exclusion criterion; in some studies (#1, #3, #4, #5, #8, and #10), typical reflux symptoms were a required inclusion criterion, and in other studies (#2, #6, and #9), typical reflux symptoms were an exclusion criterion. With respect to the treatment arms, they were all placebo controlled with respect to the primary intervention (PPIs in 11 of 14 cases), but most of the trials made no mention of concomitant dietary modifications (#1, #2, #3, #5, #6, #8, #9, #10, #11, and #13), lifestyle modifications (#1, #2, #3, #5, #6, #8, #9, #10, #11, and #13), or use of prokinetic drugs (#1, #2, #3, #8, #9, #10, #11, and #13). At the other extreme, these reflux-directed therapies were either among the interventions under study (#7, #12, and #14) or prohibited (#4).
Table 3.
Description of Randomized Controlled Trials Testing Antireflux Treatments on Chronic Cough or Ear, Nose, and Throat Syndromes Potentially Including Cough
| Study/Year | Study Inclusion | Study Exclusion | Treatment Arms | Method of Cough Assessment |
|---|---|---|---|---|
| 1. Ing et al23/1992 | Chronic cough unexplained after standard diagnostic evaluation Abnormal pH-metry results No mention of workup for other causes of cough |
Smokers | Ranitidine 150 mg bid (n = 11) Placebo (n = 13) for 2 wk then crossed over after -wk washout for additional 2 wk of therapy No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents |
Diaries: linear scale 1-4, mean change in cough score |
| 2. Havas et al24/1999 |
|
Chronic airflow limitation, severe reflux esophagitis, professional voice users (eg, singers) |
|
Mean change in cough score (frequency × severity) scale of 0-12 |
| 3. Kiljander et al25/2000 | ≥ 2 mo of chronic cough, abnormal pH-metry results | Postnasal drip, asthma, abnormal chest radiograph, smokers | Omeprazole 40 mg (n = 9) Placebo (n = 12) × 8 wk then crossover No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents |
Mean change in cough score over final 3 wk |
| 4. Noordzij et al26/2001 | LPR symptoms × 3 mo with abnormal (pharyngeal) pH-metry results Patients included with or without cough (not necessarily chronic) |
Infectious laryngitis, laryngeal cancer, allergies | Omeprazole 40 mg bid (n = 15) Placebo (n = 15) × 2 mo Specified that patients were not allowed to make (unspecified) behavioral changes that could affect results; suggests they could continue to smoke |
Questionnaire: mean change in cough score (frequency × severity) |
| 5. Ours et al27/1999 | Chronic cough > 6 wk, abnormal pH-metry results | Asthma, abnormal chest radiograph, PND, smokers, ACE inhibitors | Omeprazole 40 mg bid (n = 8) Placebo (n = 15) × 12 wk No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents |
Diaries: cough score (frequency × severity, day/night) prespecified criteria |
| 6. Shaheen et al28/2011 | > 8 wk chronic cough of ≥ 2 on Fisman severity score and ≥ 3 on frequency and abnormal pH-metry results Normal pH-metry results All had minimal or no heartburn symptoms |
Postnasal drip, heartburn, abnormal chest radiograph, smokers, patients with asthma, heartburn > 2× per mo, failed PPI trial, ACE inhibitors | Esomeprazole 40 mg bid (n = 10); placebo (n = 7) Esomeprazole 40 mg bid (n = 12); placebo (n = 11) No mention of diet or lifestyle modifications |
CQLQ and Fisman cough severity and frequency score; mean change |
| 7. Steward et al29/2004 | Hoarseness, throat clearing, nonproductive cough, globus sensation, or sore throat > 4 wk and physical examination consistent with LPR (ie, edema, erythema, or pachydermia, or a combination) | Recent PPI or H2RA use, steroid treatment, other laryngoscopic diagnoses | Rabeprazole 20 mg bid and lifestyle modifications (avoid fatty meals, caffeine, alcohol, smoking, eating within 2 h of bedtime, HOB elevation (n = 21 ITT, 16 PP) Placebo and lifestyle modifications (n = 21 ITT, 14 PP) |
Reflux Symptom Questionnaire component: Dry cough defined as more than 4×/d scored 0-4 for frequency and severity |
| 8. Wo et al30/2006 | LPR symptoms with positive LPR findings and abnormal pH-metry results | Prior LPR, GERD, or gastric surgery | Pantoprazole 40 mg (n = 20) Placebo (n = 19) No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents |
Weekly diaries: VAS 0-20, mean change in cough score |
| 9. Vaezi et al31/2006 | > 3 mo of LPR symptoms (throat clearing, cough, globus, sore throat, or hoarseness | Infectious laryngitis, malignancy, sinusitis, frequent heartburn (moderate to severe, ≥ 3× per wk) | Esomeprazole 40 mg bid (n = 11) Placebo (n = 8) No mention of diet, lifestyle modifications |
Diaries: cough severity, prespecified criteria |
| 10. Eherer et al32/2003 | Hoarseness > 2 mo, other laryngeal symptoms, laryngitis, and abnormal pH-metry results (distal or proximal) | Smokers, other obvious cause of laryngitis | Pantoprazole 40 mg bid (n = 10) Placebo bid (n = 10) 3 mo, 2-wk washout, 3-mo crossover No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents |
Mean change in global laryngeal symptom score (frequency × severity) |
| 11. Faruqi et al33/2011 | New primary care referrals with chronic cough > 8 wk, clinical features consistent with reflux-related cough (cough on phonation or bending in association with food or eating) | Smokers, abnormal chest radiograph, obvious lung disease, ACE inhibitors, recent respiratory tract infection or anti-acid medication use | Esomeprazole 20 mg bid (n = 25); placebo (n = 25) × 8 wk No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents |
Numeric 0-9 scale Leicester Cough Questionnaire Hull Airway Reflux Questionnaire |
| 12. Kopec et al34/2001 | Chronic cough due to GERD (by exclusion using standard algorithm) | Patients with other causes of cough were excluded by standard algorithm | Usual care diet + cisapride 10 mg qid Usual care diet + placebo Antireflux diet + counseling + cisapride 10 mg qid Antireflux diet + counseling + placebo 10 mg qid (Total n for 4 groups = 19) |
VAS Flow-volume loops to assess upper airway trauma from coughing |
| 13. Pawar et al35/2007 | Postnasal drip, throat clearing, excessive throat mucus | Smokers, sinusitis, rhinosinusitis, nasal polyposis, GER treatment in past 2 mo | Rabeprazole 20 mg bid (n = 21) Placebo (n = 26) No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents |
RSI component: chronic cough |
| 14. Smith et al36/2013 | Cough secondary to HARQ score > 13 (airway reflux), BMI > 25 | None stated | Reflux diet (n = 15) Energy prescription diet (n = 18) No mention if antireflux (antisecretory or prokinetic) drugs should be avoided. |
Leicester Cough Questionnaire at onset and at 6 mo |
CQLQ = Cough-Specific Quality of Life Questionnaire; GERD = gastroesophageal reflux disease; H2RA = histamine-2 receptor antagonist; HARQ = Hull Airway Reflux Questionnaire; HOB = head of bed; ITT = intent to treat; LPR = laryngopharyngeal reflux; PND = paroxysmal nocturnal dyspnea; PP = per protocol; PPI = proton pump inhibitor; RSI = reflux symptom index; VAS = visual analogue scale. See Table 1 legend for expansion of other abbreviations.
The reported results of the RCTs enumerated in Table 3 are summarized in Table 4. Notably, it is difficult to make comparisons among these trials because no two are the same, or even very similar. However, some observations can be made regarding the study outcomes: (1) there was a strong placebo effect for cough improvement; (2) studies including lifestyle modification and weight loss (albeit mainly uncontrolled) had better cough outcomes (#7, #12, and #14); (3) studies that used a carryover design consistently demonstrated a carryover effect when PPIs were the initial treatment; and (4) only one study (#13) showed a significant benefit in cough outcome when comparing PPI to placebo and that was an LPR study using nonstandard definitions of chronic cough and cough severity. Hence, we conclude that although lifestyle modifications and diet may be beneficial in controlling suspected reflux-cough syndrome, PPIs did not augment this, and PPIs demonstrated no benefit when used in isolation.
Table 4.
Summary of the Outcomes of Randomized Controlled Trials Described in Table 3
| Study/Year | Treatment Arm | Study Outcome |
|---|---|---|
| 1. Ing et al23/1992 | Ranitidine 150 mg bid (n = 11) Placebo (n = 13) |
Significant fall in cough score compared with baseline but not compared with placebo period, which also had an insignificant treatment effect |
| 2. Havas et al24/1999 |
|
Lansoprazole cough improvement +0.90 ± 0.87 Placebo cough improvement +0.86 ± 0.88 |
| 3. Kiljander et al25/2000 | Omeprazole 40 mg (n = 9) Placebo (n = 12) |
No benefit with 8 wk omeprazole |
| 4. Noordzij et al26/2001 | Omeprazole 40 mg bid (n = 15) Placebo (n = 15) |
Coughing showed trend for improvement in omeprazole group only; omeprazole not significantly better than placebo in reducing coughing |
| 5. Ours et al27/1999 | Omeprazole 40 mg bid (n = 8) Placebo (n = 15) |
1 of 8 patients receiving omeprazole and 0 of 9 patients receiving placebo responded to treatment |
| 6. Shaheen et al28/2011 |
|
Both PPI and placebo arms experienced significant improvement in CQLQ, but PPI arm was not greater When only subjects with positive pH-metry results were considered, scores on the CQLQ showed trend for improvement with PPI therapy (mean change of 11.1 for PPI vs 4.7 for placebo; P = .2) |
| 7. Steward et al29/2004 | Rabeprazole 20 mg bid and lifestyle modifications (avoid fatty meals, caffeine, alcohol, smoking, eating within 2 h of bedtime, HOB elevation (n = 21 ITT, 16 PP) Placebo and lifestyle modifications (n = 21 ITT, 14 PP) |
No significant differences between PPI and placebo for change in individual or global reflux symptoms All subjects (100%) with abnormal pH-metry results noted global improvement in symptoms (much better or gone) compared with 50% of subjects with normal pH-metry results, but because of the small number of subjects completing pH-metry, this was not significant |
| 8. Wo et al30/2006 | Pantoprazole 40 mg (n = 20) Placebo (n = 19) |
40% of subjects receiving pantoprazole and 42% receiving placebo reported adequate relief of laryngeal symptoms Heartburn was not predictive of response Subjects randomized to pantoprazole had a significant rebound of laryngeal symptoms and heartburn when pantoprazole was discontinued |
| 9. Vaezi et al31/2006 | Esomeprazole 40 mg bid (n = 11) Placebo (n = 8) |
Primary outcome was resolution of the primary symptom at the final visit; for cough, this was achieved in 1 of 11 subjects receiving esomeprazole and 2 of 8 receiving placebo |
| 10. Eherer et al32/2003 | Pantoprazole 40 mg bid (n = 10) Placebo bid (n = 10) 3 mo, 2-wk washout, 3-mo crossover |
No significant difference between pantoprazole and placebo on esophageal or laryngeal symptom outcome |
| 11. Faruqi et al33/2011 | Esomeprazole 20 mg bid (n = 25); placebo (n = 25) × 8 wk | No significant differences in change in cough scores between PPI and placebo groups When groups were subdivided into patients exhibiting or those not exhibiting “dyspeptic symptoms,” the mean reduction in cough scores tended to be greater in dyspeptic patients |
| 12. Kopec et al34/2001 | Usual care diet + cisapride Usual care diet + placebo Reflux diet + counseling + cisapride Reflux diet + counseling + placebo |
No significant difference in VAS or FIF 50% between any of the 4 groups Improvement in VAS and FIF 50% correlated with > 5 lb weight loss independent of treatment group (P = .037; P = .013, respectively) Improvement in VAS correlated with > 10 lb weight loss independent of treatment group (P = .004) |
| 13. Pawar et al35/2007 | Rabeprazole 20 mg bid (n = 21) Placebo (n = 26) |
Both the placebo and PPI groups had significant improvement in the total RSI score Both groups also had significant improvement in throat clearing and PND symptoms Only the PPI groups showed improvement in chronic cough (P = .045) and heartburn (P = .013) Although no significant difference was noted in the change in total RSI between the placebo and PPI groups, the PPI group had a significantly greater improvement in hoarseness (P = .016) and cough (P = .02) Cough responders had PND (inclusion criterion) and heartburn |
| 14. Smith JE et al36/2013 | Reflux diet (n = 15) Energy prescription diet (n = 18) |
Reflux diet: mean weight reduction = 2.63 kg LCQ improved 2.5 units Energy prescription diet: mean weight reduction 3.2 kg, LCQ improved 3.6 units |
As is evident from the inclusion criteria detailed in Table 3, most of these RCTs focused on patient populations other than “adult patients with more than an 8-week history of refractory chronic cough” intended in PICO question #1; most commonly, these were LPR studies. This attribute severely limited the external validity of the findings when applied to the adult patient population with chronic cough. Table 5 summarizes our analysis of external validity considerations, internal validity considerations, and risk of bias for the studies described in Table 3. Note that the limitations of external and internal validity are such that we could assess the risk of bias in only three of the studies (#6, #11, and #12), and one of these (#12) was an abstract reporting on only 19 patients allocated to four treatment groups, making it severely underpowered. Study #6 reported a negative result for PPI treatment rendered without associated dietary and lifestyle modifications in a “silent reflux population” (the study excluded patients reporting heartburn > 2 times per month). Hence, we concluded that the study had a high risk of bias in patients with frequent heartburn but a low risk of bias in those with infrequent heartburn, regardless of their pH-metry findings. Study #11 reported a strong placebo effect but no treatment benefit in a PPI trial selecting patients with a “reflux-related cough” defined by symptoms including cough on phonation or on bending in association with food and eating with or without heartburn. Those criteria have not been validated, giving this an uncertain risk of bias. Although heavy voice use has been implicated as a cough-provoking irritant, phonation stimulates upper esophageal sphincter contraction, arguing against reflux being the operant mechanism for that.37 Hence, overall we found no studies (positive or negative) pertinent to PICO question #1 with a low risk of bias.
Table 5.
Extrapolation Limitations and Risk of Bias Assessment of Randomized Controlled Trials Described in Table 3
| Study/Year | Quality Assessment |
|---|---|
| 1. Ing et al23/1992 |
Study validity considerations: Although it states that it was done, no details provided of workup for other potential causes of cough. Results are not generalizable to patients without abnormal esophageal pH-metry results. Carryover effect of active drug secondary to crossover design. Other extrapolation limitations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 2. Havas et al24/1999 |
Study validity considerations: Not generalizable to a chronic cough population. Results potentially applicable to a laryngoscopically defined LPR population. No power calculation. Other extrapolation limitations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 3. Kiljander et al25/2000 |
Study validity considerations: Carryover effect of active drug secondary to crossover design. No intent-to-treat analysis. Cough workup was adequate to exclude nonreflux conditions but was strongly biased to select reflux patients. Other extrapolation limitations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Results are not generalizable to patients without abnormal pH-metry results. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 4. Noordzij et al26/2001 |
Study validity considerations: Not generalizable to a chronic cough population. Results potentially applicable to a symptomatically defined LPR population. Other extrapolation limitations: Patients were not allowed to make (unspecified) behavioral changes that could affect results; this suggests that they could continue to smoke. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 5. Ours et al27/1999 |
Study validity considerations: Cough workup was not adequate to exclude nonreflux conditions but was strongly biased to select patients with reflux. Results are not generalizable to patients without abnormal pH-metry results. Other extrapolation considerations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 6. Shaheen et al28/2011 |
Study validity considerations: Cough variant asthma ruled out only by history. Suboptimal drug (intranasal steroids) used to exclude postnasal drip syndrome. Strong bias against reflux etiology by excluding patients with heartburn > 2× per month. Other extrapolation considerations: No mention of diet or lifestyle modifications. Results are applicable to a “silent reflux population” (no heartburn, but pH-metry-defined reflux) with chronic cough. Risk of bias assessment: High risk of bias for patients with frequent heartburn; low risk of bias for those with infrequent heartburn. Overall risk of bias is uncertain. |
| 7. Steward et al29/2004 |
Study validity considerations: Patients specifically not instructed on when to take the study medications. Other extrapolation considerations: Not generalizable to a chronic cough population. Results potentially applicable to a laryngoscopically and symptomatically defined LPR population. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 8. Wo et al30/2006 |
Study validity considerations: Not generalizable to a chronic cough population. Results potentially applicable to a laryngoscopically, symptomatically, and pH-metry-defined LPR population. Other extrapolation considerations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 9. Vaezi et al31/2006 |
Study validity considerations: Not generalizable to a chronic cough population. Results potentially applicable to a symptomatically defined LPR population. Other extrapolation considerations: No mention of diet, lifestyle modifications. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 10. Eherer et al32/2003 |
Study validity considerations: Carryover effect of active drug secondary to crossover design. Very underpowered. Not generalizable to a chronic cough population. Results potentially applicable to a laryngoscopically, symptomatically, and pH-metry-defined LPR population. Other extrapolation considerations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 11. Faruqi et al33/2011 |
Study validity considerations: Inadequate workup to exclude nonreflux causes of chronic cough, especially asthma. Other extrapolation considerations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Risk of bias assessment: Uncertain risk of bias because unvalidated criteria for reflux-related cough used. |
| 12. Kopec et al34/2001 |
Study validity considerations: Data available only in an abstract; details of protocol for exclusion of other cough causes not specified but referenced, and R. S. I. provided further data. Underpowered study. Weight loss was not an a priori specified outcome. Risk of bias assessment: Insufficient data available from abstract to assess risk of bias. |
| 13. Pawar et al35/2007 |
Study validity considerations: No intention-to-treat analysis. Not generalizable to a chronic cough population. Results potentially applicable to a UACS population with cough. Other extrapolation considerations: No mention of diet, lifestyle modifications, or concomitant use of prokinetic agents. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
| 14. Smith JE et al36/2013 |
Study validity considerations: Not generalizable to a chronic cough population. Inadequate workup to exclude other nonreflux causes of chronic cough. Results potentially applicable to an overweight or obese reflux population with chronic cough. Other extrapolation considerations: No mention if antireflux (antisecretory or prokinetic) drugs should be avoided. Reflux-cough diagnosis based on a suboptimal HARQ questionnaire. Risk of bias assessment: Not undertaken as did not fulfill criteria.a |
We undertook a Cochrane-based risk of bias table only if the studies fulfilled the following criteria: (1) excluded other common causes of chronic cough (asthma, UACS) by adequate workup and (2) included patients with and those without additional symptoms of GERD or LPR (or both) or included patients with and those without additional test results suggestive of GERD or LPR (or both).
PICO Question 2: Are there minimal clinical criteria to guide clinical practice in determining that a patient’s chronic cough is likely to respond to therapy for gastroesophageal reflux?
Among the 14 studies identified that potentially addressed the question of clinically profiling patients with likely reflux-cough syndrome,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 none fulfilled all PICO criteria in that none of the trials were controlled. Rather, they all reported on the success of an uncontrolled diagnostic/therapeutic algorithm for patients with chronic cough. Consequently, all of them had a high risk of bias and could not be used to make practice recommendations. Additionally, it should be noted that only low-quality evidence supports that effective intervention fidelity strategies were used in conducting these trials (ie, very few studies documented that the intended guidelines or protocols were actually carried out) offering yet another potential explanation for the observed variability in outcomes.22 Nonetheless, Table 6 summarizes the findings and key messages put forth in these studies.38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 As is evident in Table 1, the diagnostic approach varied widely from no evaluation beyond a chest radiograph and pulmonary function testing to a comprehensive evaluation involving barium swallow, manometry, endoscopy and pH-metry. Despite these limitations, some observations can be made regarding the study outcomes: (1) an algorithmic approach to patient management resolved the cause of chronic cough in 82% to 100% of instances among studies; (2) regardless of approach or extent of evaluation, cough variant asthma and UACS due to a variety of rhinosinus conditions were consistently among the most common causes of chronic cough; and (3) the reported prevalence of GERD as a cause or contributing factor to chronic cough varied widely from 2% to 86%. Hence, although the message is clear with respect to UACS and cough variant asthma, it is rather murky with respect to GERD.
Table 6.
Reports Using Diagnostic/Therapeutic Algorithms to Manage Patients With Chronic Cough
| Study/Year | Patients/Algorithm/Intervention | Key Messages | Risk of Bias Assessment |
|---|---|---|---|
| 1. Smyrnios et al38/1998 |
|
|
High risk of bias
|
| 2. McGarvey et al39/1998 |
|
|
High risk of bias
|
| 3. Brightling et al40/1999 |
|
|
High risk of bias
|
| 4. Palombini et al41/1999 |
|
|
High risk of bias
|
| 5. Irwin et al42/2002 |
|
|
High risk of bias
|
| 6. Novitsky et al43/2002 |
|
|
High risk of bias
|
| 7. Kastelik et al44/2005 |
|
|
High risk of bias
|
| 9. Plaza et al46/2006 |
|
|
High risk of bias
|
| 10. Ribeiro et al47/2006 |
|
|
High risk of bias
|
| 11. Yu et al48/2008 |
|
|
High risk of bias
|
| 12. Klochan et al49/2009 |
|
|
High risk of bias
|
| 13. Wei et al50/2009 |
|
|
High risk of bias
|
| 14. Ojoo et al51/2013 |
|
|
High risk of bias
|
CC = chronic cough; EGD = esophagogastroduodenoscopy; nl = normal; PFTs = pulmonary function tests; PNDS = postnasal drip syndrome; RAST = radioallergosorbent test; URI = upper respiratory tract infection. See Table 1 and Table 3 legends for expansion of other abbreviations.
The 2006 ACCP diagnostic algorithm is detailed in Table 1.
No matter how slightly, all the studies in Table 6 found some instances in which GERD therapy was beneficial in resolving adult chronic cough cases. The problem is that there was no diagnostic approach better than the systematic elimination of alternative diagnostic possibilities that consistently isolated this population; neither typical reflux symptoms nor reflux testing had good positive predictive value. There were no controlled data relevant to the use of impedance testing to detect weakly acidic reflux, leaving this as a fundamental knowledge gap. Hence, the series advocating no diagnostic testing for GERD48 reported cough resolution in 88% of the 102 patients managed, a value comparable to that in studies using extensive testing. More impressive was the negative predictive value of pH-metry; both studies that analyzed this39, 41 concluded that normal pH-metry had 100% negative predictive value for a therapeutic response of cough to antireflux therapy. This message was particularly relevant when contemplating antireflux surgery as an intervention, as is evident from the report of Klochan et al.49 Selecting 18 patients for antireflux surgery on the basis of their being medically refractory but with positive pH-metry results irrespective of typical reflux symptoms, they reported resolution in 33%, improvement in 39%, and no change in 28%. Having said that, it should also be pointed out that there is little consistency among studies in either the methodology or the interpretation of pH-metry studies.
Practice Recommendations/Suggestions
As delineated earlier, the findings of this systematic review were most informative with respect to the limitations of the available data pertinent to addressing PICO questions 1 and 2 on the identification and management of patients with suspected reflux-cough syndrome. The evidence described was of low quality and in most instances only tangentially related to the PICO questions. However, simply pointing out the limitations of the evidence base is not much use to the clinician in guiding patient management. Hence, we used the available evidence and a structured consensus-based Delphi approach to formulate the following evidence-graded practice recommendations and suggestions:
1. In adult patients with chronic cough, we suggest that the cough be managed according to a published management guideline that initially considers the most common potential etiologies as well as symptomatic gastroesophageal reflux (ungraded, consensus based).
Remarks: Common potential etiologies include environmental or occupational irritants, primary or secondary smoking, use of ACE inhibitors, abnormal chest radiographic findings, asthma, upper airway cough syndrome due to a variety of rhinosinus conditions, nonasthmatic eosinophilic bronchitis, and suppurative lung disease. Often, more than one etiology is a contributing factor.
2. In adult patients with chronic cough suspected to be due to reflux-cough syndrome, we recommend that treatment include (1) diet modification to promote weight loss in overweight or obese patients; (2) head of bed elevation and avoiding meals within 3 hours of bedtime; and (3) in patients who report heartburn and regurgitation, PPIs, H2- receptor antagonists, alginate, or antacid therapy sufficient to control these symptoms (Grade 1C).
Remarks: (1) While it is expected that GI symptoms will respond within 4-8 weeks, the literature suggests that improvement in cough may take up to 3 months.1 (2) Head of bed elevation is suggested based on its utility for improving GI GERD symptoms2 while acknowledging that it has not been demonstrated to be beneficial for cough.
3. In adult patients with suspected chronic cough due to reflux-cough syndrome, but without heartburn or regurgitation, we recommend against using PPI therapy alone because it is unlikely to be effective in resolving the cough (Grade 1C).
4. In adult patients with chronic cough potentially due to reflux-cough syndrome who are refractory to a 3-month trial of medical antireflux therapy and are being evaluated for surgical management (antireflux or bariatric), or in whom there is strong clinical suspicion warranting diagnostic testing for gastroesophageal reflux, we suggest that they undergo esophageal manometry and pH-metry with conventional methodology (Grade 2C).
Remarks: Esophageal manometry is done both to evaluate for a major motility disorder and to accurately position the pH electrode for the pH monitoring study. With conventional methodology, the pH electrode is placed 5 cm proximal to the lower esophageal sphincter, and the study is done off antisecretory medications after withholding PPI therapy for 7 days and H2-receptor antagonists for 3 days prior to the study. It was agreed by consensus of the Esophageal Diagnostic Advisory Panel composed of both gastroenterologists and surgeons3 that this is the only methodology with proven validity with respect to surgical outcomes.
5. In adult patients with chronic cough and a major motility disorder (eg, absent peristalsis, achalasia, distal esophageal spasm, hypercontractility) and/or normal acid exposure time in the distal esophagus, we suggest not advising antireflux surgery (Grade 2C).
Remarks: Under the circumstances of a major motility disorder or normal esophageal acid exposure on esophageal pH-metry, there is no supportive controlled data for antireflux surgery and there is quantifiable risk to the procedure, making for an unacceptable risk-benefit ratio.3, 4
6. In adult patients with chronic cough, adequate peristalsis, and abnormal esophageal acid exposure determined by pH-metry in whom medical therapy has failed, we suggest antireflux (or bariatric when appropriate) surgery for presumed reflux-cough syndrome (Grade 2C).
Remarks: With respect to defining adequate peristalsis, there is no consensus. Some consider any preserved peristalsis to be adequate while others stipulate that it must be at least 30% and others at least 50% of normal.5
Future Studies to Narrow the Gaps in Knowledge
As is evident from this review, the relationship between reflux and chronic cough is complex with numerous variables at play. Clinical strategies for diagnosis and management remain controversial, and despite the considerable quantity of research reviewed herein, several fundamental questions that are potentially amenable to further studies remain unanswered.
From a diagnostic viewpoint:
-
1.
Are there specific findings related to acid or weakly acidic reflux from pH or pH-impedance studies that either implicate or vindicate reflux as a cause of chronic cough?
-
2.
Is there a symptom profile with respect to typical reflux symptoms or cough characteristics that either implicates or vindicates reflux as a cause of chronic cough?
From a therapeutic viewpoint:
-
1.
Is weight loss the fundamental lifestyle modification of benefit? If so, how is that benefit mediated and when is it operant?
-
2.
Is there a role for prokinetic agents, with or without PPIs, in managing reflux-cough syndrome?
Summary
Our objective was to update the 2006 ACCP clinical practice guidelines for the diagnosis and treatment of chronic cough due to reflux using an evidence-based approach. To do so, we convened an International Panel of Experts to develop practice management suggestions from a systematic review of published evidence. After generating key clinical questions, PICO elements were derived to assist the literature review. The questions that formed the basis of the systematic review were the following: (1) Can therapy intended to treat gastroesophageal reflux improve or eliminate cough in adults with refractory chronic cough? (2) Are there minimal clinical criteria to guide clinical practice in determining that a patient’s chronic cough is likely to respond to therapy for gastroesophageal reflux?
Overall, we found no published studies (positive or negative) pertinent to question #1 with a low risk of bias. From the RCTs available, we concluded that (1) there was a strong placebo effect for cough improvement; (2) studies including diet modification and weight loss had better cough outcomes; (3) because of a potential carryover effect of PPIs, a crossover study design should be avoided when using these medications (unless data become available establishing the duration of a sufficient washout period); and (4) although lifestyle modifications and diet may be beneficial in controlling suspected reflux-cough syndrome, PPIs did not augment this, and PPIs demonstrated no benefit when used in isolation. For question #2, there were no relevant RCTs to review, so our suggestions were based on observational studies only. We again found no high-quality evidence but concluded that (1) an algorithmic approach to patient management was associated with resolution of the cause of chronic cough in 82% to 100% of instances; (2) regardless of approach or extent of evaluation, cough variant asthma and UACS were consistently among the most common causes of chronic cough; and (3) the reported prevalence of GERD as an associated cause or contributing factor to chronic cough varied widely from 2.0% to 86% among studies.
We concluded that the literature addressing these PICO questions was of low quality. However, the panelists agreed on suggestions to (1) endorse a diagnostic/therapeutic algorithm sequentially addressing causes of common cough including symptomatic reflux and (2) advise that patients refractory to the diagnostic therapeutic algorithm may still benefit from therapy for potential reflux-cough syndrome that includes weight reduction when appropriate and lifestyle modifications. Also, for patients with potential reflux-cough syndrome who are refractory to lifestyle modifications ± acid suppressive therapy, a suggestion was made to evaluate them with esophageal manometry and pH-metry and to exclude an esophageal motility disorder and confirm a diagnosis of GERD for patients in whom there is strong clinical suspicion that warrants diagnostic testing or before these patients are considered for antireflux surgery.
Acknowledgements
Author contributions: P. J. K. and R. S. I. reviewed all the articles and independently abstracted data for the systematic review that formed the basis of the guideline portion of this manuscript. P. J. K. wrote the initial draft of this manuscript. All authors participated in the development of the key questions using the PICOTS format, reviewed the systematic review and this manuscript, participated in writing the final manuscript through suggested revisions, and agreed with all recommendations/suggestions.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: P. J. K., K. W. A., S. K. F., S. M. H., A. P. L., K. L., and L. M. report no financial or intellectual conflicts of interest pertinent to either PICO question #1 or #2. A. B. C. reports an intellectual conflict of interest pertinent to PICO question #1 as it it assesses one paper that she authored; she reports no financial conflict of interest pertinent to either PICO question #1 or #2. J. S. is a named inventor on a patent that describes novel techniques for detecting cough from sound recordings pertinent to PICO question #2. This patent is owned by the University Hospital of South Manchester and is licensed to a medical device company. She reports no financial conflict of interest pertinent to either PICO question #1 or #2. R. S. I. discloses that he has no financial or intellectual conflicts of interest pertinent to either PICO question #1 or #2. Moreover, although R. S. I. is the Editor in Chief of CHEST, the review and all editorial decisions regarding this manuscript were made independently by others.
Collaborators: Todd M. Adams, MD (Webhannet Internal Medicine Associates of York Hospital, Moody, ME), Kenneth W. Altman, MD, PhD (Baylor College of Medicine, Houston, TX), Elie Azoulay, MD, PhD (University of Paris, Paris, France), Alan F. Barker, MD (Oregon Health & Science University, Portland, OR), Fiona Blackhall, MD, PhD (University of Manchester, Department of Medical Oncology, Manchester, England), Donald C. Bolser, PhD (College of Veterinary Medicine, University of Florida, Gainesville, FL), Louis-Philippe Boulet, MD, FCCP (Institut universitaire de cardiologie et de pneumologie de Québec [IUCPQ], Quebec, QC, Canada), Christopher Brightling, MBBS, PhD, FCCP (University of Leicester, Glenfield Hospital, Leicester, United Kingdom), Priscilla Callahan-Lyon, MD (US Food and Drug Administration, Rockville, MD), Brendan J. Canning, PhD (Johns Hopkins Asthma and Allergy Center, Baltimore, MD), Anne B. Chang, MBBS, PhD, MPH (Royal Children’s Hospital, Queensland, Australia), Terrie Cowley (The TMJ Association, Milwaukee, WI), Satoru Ebihara, MD, PhD (Department of Rehabilitation Medicine, Toho University School of Medicine, Tokyo, Japan), Ali A. El Solh, MD, MPH (University at Buffalo, State University of New York, Buffalo, NY), Patricio Escalante, MD, MSc, FCCP (Mayo Clinic, Rochester, MN), Stephen K. Field, MD (University of Calgary, Calgary, AB, Canada), Anthony Feinstein, MPhil, PhD (Sunnybrook Health Sciences Centre, Toronto, ON, Canada), Dina Fisher, MD, MSc (University of Calgary, Respiratory Medicine, Calgary, AB, Canada), Cynthia T. French, PhD, FCCP (UMass Memorial Medical Center, Worcester, MA), Peter Gibson, MBBS (Hunter Medical Research Institute, New South Wales, Australia), Philip Gold, MD, MACP, FCCP (Loma Linda University, Loma Linda, CA), Michael K. Gould, MD, MS, FCCP (Kaiser Permanente, Pasadena, CA), Cameron Grant, MB ChB, PhD (University of Auckland School of Medicine, Auckland, New Zealand), Susan M. Harding, MD, FCCP (Division of Pulmonology, Allergy and Critical Care Medicine, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL), Anthony Harnden, MB ChB, MSc (University of Oxford, Oxford, England), Adam T. Hill, MB ChB, MD (Royal Infirmary and University of Edinburgh, Edinburgh, Scotland), Richard S. Irwin, MD, Master FCCP (UMass Memorial Medical Center, Worcester, MA), Peter J. Kahrilas, MD (Feinberg School of Medicine, Northwestern University, Chicago, IL), Karina A. Keogh, MD (Mayo Clinic, Rochester, MN), Kefang Lai, MD, PhD (First Affiliated Hospital of Guangzhou Medical College, Guangzhou, China), Andrew P. Lane, MD (Johns Hopkins University School of Medicine, Baltimore, MD), Kaiser Lim, MD (Mayo Clinic, Rochester, MN), Mark A. Malesker, PharmD, FCCP (Creighton University School of Pharmacy and Health Professions, Omaha, NE), Stuart Mazzone, PhD, FCCP (University of Queensland, Queensland, Australia), Lorcan McGarvey, MD (The Queen’s University Belfast, Belfast, Northern Ireland), M. Hassan Murad, MD, MPH (Mayo Clinic, Rochester, MN), Huong Q. Nguyen, PhD, RN (Kaiser Permanente, Pasadena, CA), Peter Newcombe, PhD (School of Psychology, University of Queensland, Queensland, Australia), John Oppenheimer, MD (University of Medicine and Dentistry of New Jersey-Rutgers University), Mark Rosen, MD, Master FCCP (American College of Chest Physicians, Glenview, IL), Bruce Rubin, MEngr, MD, MBA (Virginia Commonwealth University, Richmond, VA), Jay H. Ryu, MD, FCCP (Mayo Clinic, Rochester, MN), Jaclyn Smith, MB ChB, PhD (University of Manchester, Manchester, England), Susan M. Tarlo, MBBS, FCCP (Toronto Western Hospital, Toronto, ON, Canada), Anne E. Vertigan, PhD, MBA, BAppSc (SpPath) (John Hunter Hospital, New South Wales, Australia), Gang Wang, MD, PhD (Sichuan University, West China Hospital, Chengdu, China), Miles Weinberger, MD, FCCP (University of Iowa Hospitals and Clinics, Iowa City, IA), Kelly Weir, MsPath (Queensland Children’s Medical Research Institute, Queensland, Australia)
Other contributions: We thank CHEST for supporting this work.
Endorsements: This guideline document has been endorsed by the following organizations: American College of Allergy, Asthma, and Immunology (ACAAI); American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF); American Association for Respiratory Care (AARC); Asian Pacific Society for Respirology (APSR); and the Canadian Thoracic Society.
Role of sponsors: CHEST was the sole supporter of these guidelines, this article, and the innovations addressed within.
Footnotes
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://www.chestnet.org/Guidelines-and-Resources/Guidelines-and-Consensus-Statements/CHEST-Guidelines.
Contributor Information
Peter J. Kahrilas, Email: p-kahrilas@northwestern.edu.
CHEST Expert Cough Panel:
Todd M. Adams, Kenneth W. Altman, Elie Azoulay, Alan F. Barker, Fiona Blackhall, Donald C. Bolser, Louis-Philippe Boulet, Christopher Brightling, Priscilla Callahan-Lyon, Brendan J. Canning, Anne B. Chang, Terrie Cowley, Satoru Ebihara, Ali A. El Solh, Patricio Escalante, Stephen K. Field, Anthony Feinstein, Dina Fisher, Cynthia T. French, Peter Gibson, Philip Gold, Michael K. Gould, Cameron Grant, Susan M. Harding, Anthony Harnden, Adam T. Hill, Richard S. Irwin, Peter J. Kahrilas, Karina A. Keogh, Kefang Lai, Andrew P. Lane, Kaiser Lim, Mark A. Malesker, Stuart Mazzone, Lorcan McGarvey, M. Hassan Murad, Huong Q. Nguyen, Peter Newcombe, John Oppenheimer, Mark Rosen, Bruce Rubin, Jay H. Ryu, Jaclyn Smith, Susan M. Tarlo, Anne E. Vertigan, Gang Wang, Miles Weinberger, and Kelly Weir
References
- 1.Irwin R.S., Madison J.M. Diagnosis and treatment of chronic cough due to gastro-esophageal reflux disease and postnasal drip syndrome. Pulm Pharmacol Ther. 2002;15(3):261–266. doi: 10.1006/pupt.2002.0348. [DOI] [PubMed] [Google Scholar]
- 2.Khan B.A., Sodhi J.S., Zargar S.A. Effect of bed head elevation during sleep in symptomatic patients of nocturnal gastroesophageal reflux. J Gastroenterol Hepatol. 2012;27(6):1078–1082. doi: 10.1111/j.1440-1746.2011.06968.x. [DOI] [PubMed] [Google Scholar]
- 3.Jobe B.A., Richter J.E., Hoppo T. Pre-operative diagnostic work-up prior to antireflux surgery: an “evidence and experience-based” consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg. 2013;217(4):586–597. doi: 10.1016/j.jamcollsurg.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Kahrilas P.J., Shaheen N.J., Vaezi M in collaboration with the AGAI Medical Position Panel on GERD management AGAI medical position statement: management of gastroesophageal reflux disease. Gastroenterology. 2008;135(4):1383–1391. doi: 10.1053/j.gastro.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 5.Pandolfino J.E., Kahrilas P.J. The second American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128(1):209–229. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Vakil N., Van Zanten S.V., Kahrilas P., Dent J., Jones R. Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease (GERD): a global evidence-based consensus. Am J Gastroenterol. 2006;101(8):1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 7.Boeckxstaens G., El-Serag H.B., Smout A.J.P.M., Kahrilas P.J. Symptomatic reflux disease: the present, the past, and the future. Gut. 2014;63(7):1185–1193. doi: 10.1136/gutjnl-2013-306393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locke G.R., III, Talley N.J., Fett S.L., Zinsmeister A.R., Melton L.J., III Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag H., Hill C., Jones R. Systematic review: the epidemiology of gastro-oesophageal reflux disease in primary care, using the UK General Practice Research Database. Aliment Pharmacol Ther. 2009;29(5):470–480. doi: 10.1111/j.1365-2036.2008.03901.x. [DOI] [PubMed] [Google Scholar]
- 10.Ford A.C., Forman D., Moayyedi P., Morice A.H. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax. 2006;61(11):975–979. doi: 10.1136/thx.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahrilas P.J., Howden C.W., Hughes N., Molloy-Bland M. Response of chronic cough to acid-suppressive therapy in patients with gastroesophageal reflux disease. Chest. 2013;143(3):605–612. doi: 10.1378/chest.12-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin R., French C.L., Curley F.J. Chronic cough due to gastroesophageal reflux: clinical, diagnostic, and pathogenetic aspects. Chest. 1993;104(5):1511–1517. doi: 10.1378/chest.104.5.1511. [DOI] [PubMed] [Google Scholar]
- 13.Ing A.J., Ngu M.C., Breslin A.B. Pathogenesis of chronic persistent cough associated with gastroesophageal reflux. Am J Respir Crit Care Med. 1994;149(1):160–167. doi: 10.1164/ajrccm.149.1.8111576. [DOI] [PubMed] [Google Scholar]
- 14.Javorkova N., Varechova S., Pecova R. Acidification of the oesophagus acutely increases the cough sensitivity in patients with gastro-oesophageal reflux and chronic cough. Neurogastroenterol Motil. 2008;20(2):119–124. doi: 10.1111/j.1365-2982.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith J.A., Decalmer S., Kelsall A. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology. 2010;139(3):754–762. doi: 10.1053/j.gastro.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Chang A.B., Lasserson T.J., Kiljander T.O., Connor F.L., Gaffney J.T., Garske L.A. Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ. 2006;332(7532):11–17. doi: 10.1136/bmj.38677.559005.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang A.B., Lasserson T.J., Gaffney J., Connor F.L., Garske L.A. Gastro-oesophageal reflux treatment for prolonged non-specific cough in children and adults. Cochrane Database Syst Rev. 2006;4:CD004823. doi: 10.1002/14651858.CD004823.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Irwin R. chronic cough due to gastroesophageal reflux disease: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):80S–94S. doi: 10.1378/chest.129.1_suppl.80S. [DOI] [PubMed] [Google Scholar]
- 19.Lewis S.Z., Diekemper R., Ornelas J., Casey K.R. Methodologies for the development of CHEST guidelines and expert panel reports. Chest. 2014;146(1):182–192. doi: 10.1378/chest.14-0824. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane-handbook.org. Accessed October 18, 2016.
- 21.Cochrane Bias Methods Group. The ROBINS-I tool (Risk Of Bias In Non-randomized Studies - of Interventions). https://sites.google.com/site/riskofbiastool/. Accessed October 18, 2016.
- 22.French C.T., Diekemper R.L., Irwin R.S. Assessment of intervention fidelity and recommendations for researchers conducting studies on the diagnosis and treatment of chronic cough in adults. Chest. 2015;148(1):32–54. doi: 10.1378/chest.15-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ing A.J., Ngu M.C., Breslin A.B.X. A randomised double blind placebo controlled cross-over study of ranitidine in patients with chronic persistent cough (CPC) associated with gastro-oesophageal reflux (GOR) Am J Respir Dis. 1992;145:A11. [Google Scholar]
- 24.Havas T., Huang S., Levy M. Posterior pharyngolaryngitis: double-blind randomised placebo-controlled trial of proton pump inhibitor therapy. Aust J Otolaryngol. 1999;3(3):243–246. [Google Scholar]
- 25.Kiljander T.O., Salomaa E.R., Hietanen E.K., Terho E.O. Chronic cough and gastro-oesophageal reflux: a double-blind placebo-controlled study with omeprazole. Eur Respir J. 2000;16(4):633–638. doi: 10.1034/j.1399-3003.2000.16d11.x. [DOI] [PubMed] [Google Scholar]
- 26.Noordzij J.P., Khidr A., Evans B.A. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope. 2001;111(12):2147–2151. doi: 10.1097/00005537-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Ours T.M., Kavuru M.S., Schilz R.J., Richter J.E. A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol. 1999;94(11):3131–3138. doi: 10.1111/j.1572-0241.1999.01504.x. [DOI] [PubMed] [Google Scholar]
- 28.Shaheen N.J., Crockett S.D., Bright S.D. Randomised clinical trial: high-dose acid suppression for chronic cough—a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(2):225–234. doi: 10.1111/j.1365-2036.2010.04511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steward D.L., Wilson K.M., Kelly D.H. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131(4):342–350. doi: 10.1016/j.otohns.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Wo J.M., Koopman J., Harrell S.P., Parker K., Winstead W., Lentsch E. Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol. 2006;101(9):1972–1978. doi: 10.1111/j.1572-0241.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 31.Vaezi M.F., Richter J.E., Stasney C.R. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116(6):254–260. doi: 10.1097/01.mlg.0000192173.00498.ba. [DOI] [PubMed] [Google Scholar]
- 32.Eherer A.J., Haberman W., Hammer H.F., Kiesler K., Friedrich G., Krejs G.J. Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol. 2003;38(5):462–467. doi: 10.1080/00365520310001860. [DOI] [PubMed] [Google Scholar]
- 33.Faruqi S., Molyneux I.D., Fathi H., Wright C., Thompson R., Morice A.X. Chronic cough and esomeprazole: a double-blind placebo-controlled parallel study. Respirology. 2011;16(7):1150–1156. doi: 10.1111/j.1440-1843.2011.02014.x. [DOI] [PubMed] [Google Scholar]
- 34.Kopec S.E., Irwin R.S., French C.L., Wilson M.M., Bol S. A double-blind randomized placebo-controlled trial comparing diet and/or cisapride. Am J Respir Crit Care Med. 2001;163(5 suppl):A64. [Google Scholar]
- 35.Pawar S., Lim H.J., Gill M. Treatment of postnasal drip with proton pump inhibitors: a prospective, randomized, placebo-controlled study. Am J Rhinol. 2007;21(6):695–701. doi: 10.2500/ajr.2007.21.3098. [DOI] [PubMed] [Google Scholar]
- 36.Smith J.E., Morjaria J.B., Morice A.H. Dietary intervention in the treatment of patients with cough and symptoms suggestive of airways reflux as determined by Hull airways Reflux Questionnaire. Cough. 2013;9(1):27. doi: 10.1186/1745-9974-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera L., Kern M., Hofmann C. Manometric evidence for phonation-induced UES contractile reflex. Am J Physiol. 2008;294(4):G885–G891. doi: 10.1152/ajpgi.00470.2007. [DOI] [PubMed] [Google Scholar]
- 38.Smyrnios N.A., Irwin R.S., Curley F.J., French C.L. From a prospective study of chronic cough: diagnostic and therapeutic aspects in older adults. JAMA Intern Med. 1998;158(11):1222–1228. doi: 10.1001/archinte.158.11.1222. [DOI] [PubMed] [Google Scholar]
- 39.McGarvey L.P.A., Heaney L.G., Lawson J.T. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax. 1998;53(9):738–743. doi: 10.1136/thx.53.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brightling C.E., Ward R., Goh K.L., Wardlaw A.J., Pavord I.D. Eosinophilic bronchitis is an important cause of chronic cough. Am J Respir Crit Care Med. 1999;160(2):406–410. doi: 10.1164/ajrccm.160.2.9810100. [DOI] [PubMed] [Google Scholar]
- 41.Palombini B.C., Villanova C.A., Araújo E. A pathogenic triad in chronic cough: asthma, postnasal drip syndrome, and gastroesophageal reflux disease. Chest. 1999;116(2):279–284. doi: 10.1378/chest.116.2.279. [DOI] [PubMed] [Google Scholar]
- 42.Irwin R.S., Zawacki J.K., Wilson M.M., Callery M.P. Chronic cough due to gastroesophageal reflux disease: failure to resolve despite total/near-total elimination of esophageal acid. Chest. 2002;121(4):1132–1140. doi: 10.1378/chest.121.4.1132. [DOI] [PubMed] [Google Scholar]
- 43.Novitsky Y.W., Zawacki J.K., Irwin R.S., French C.T., Hussey V.M., Callery M.P. Chronic cough due to gastroesophageal reflux disease: efficacy of antireflux surgery. Surg Endosc. 2002;16(4):567–571. doi: 10.1007/s00464-001-8328-y. [DOI] [PubMed] [Google Scholar]
- 44.Kastelik J.A., Aziz I., Ojoo J.C., Thompson R.H., Redington A.E., Morice A.H. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J. 2005;25(2):235–243. doi: 10.1183/09031936.05.00140803. [DOI] [PubMed] [Google Scholar]
- 45.Fujimura M., Abo M., Ogawa H. Importance of atopic cough, cough variant asthma, and sinobronchial syndrome as causes of chronic cough in the Hokuriku area of Japan. Respirology. 2005;10(2):201–207. doi: 10.1111/j.1440-1843.2005.00686.x. [DOI] [PubMed] [Google Scholar]
- 46.Plaza V., Miguel E., Bellido-Casado J., Lozano M.P., Rios L., Bolibar I. Usefulness of the Guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) in identifying the causes of chronic cough. Arch Bronconeumol. 2006;42(2):68–73. doi: 10.1016/s1579-2129(06)60120-1. [DOI] [PubMed] [Google Scholar]
- 47.Ribeiro M., De Castro Pereira C.A., Nery L.E., Beppu O.S., Silva O.S. A prospective longitudinal study of clinical characteristics, laboratory findings, diagnostic spectrum and outcomes of specific therapy in adult patients with chronic cough in a general respiratory clinic. Int J Clin Pract. 2006;60(7):799–805. doi: 10.1111/j.1368-5031.2006.00876.x. [DOI] [PubMed] [Google Scholar]
- 48.Yu L., Qiu Z., Lu H., Wei W., Shi C. Clinical benefit of sequential three-step empirical therapy in the management of chronic cough. Respirology. 2008;13(3):353–358. doi: 10.1111/j.1440-1843.2008.01261.x. [DOI] [PubMed] [Google Scholar]
- 49.Klochan C.M., Slaughter J.C., Trawick E.P., Holzman M., Vaezi M.F. Fundoplication in patients with extraesophageal reflux (EER) refractory to PPI therapy: trust clinical judgment over impedance or pH [abstract] Gastroenterology. 2009;136(5 suppl 1):A-107. [Google Scholar]
- 50.Wei W., Yu L., Lu H. Comparison of cause distribution between elderly and non-elderly patients with chronic cough. Respiration. 2009;77(3):259–264. doi: 10.1159/000142942. [DOI] [PubMed] [Google Scholar]
- 51.Ojoo J.C., Everett C., Mulrennan S.A., Faruqi S., Kastelik J.A., Morice A.H. Management of patients with chronic cough using a clinical protocol: a prospective observational study. Cough. 2013;9(1):2–7. doi: 10.1186/1745-9974-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]