Abstract
Background
Acute cough associated with the common cold (CACC) causes significant impairment in quality of life. Effective treatment approaches are needed for CACC. We conducted a systematic review on the management of CACC to update the recommendations and suggestions of the CHEST 2006 guideline on this topic.
Methods
This systematic review of randomized controlled trials (RCTs) asked the question: Is there evidence of clinically relevant treatment effects for pharmacologic or nonpharmacologic therapies in reducing the duration/severity of acute CACC? Studies of adults and pediatric patients with CACC were included and assessed for relevance and quality. Based on the systematic review, guideline suggestions were developed and voted on using the American College of Chest Physicians organization methodology.
Results
Six systematic reviews and four primary studies identified from updated literature searches for each of the reviews or from hand searching were included and reported data on 6,496 participants with CACC who received one or more of a variety of interventions. The studies used an assortment of descriptors and assessments to identify CACC.
Conclusions
The evidence supporting the management of CACC is overall of low quality. This document provides treatment suggestions based on the best currently available evidence and identifies gaps in our knowledge and areas for future research.
Key Words: acute cough, common cold, pharmacologic and nonpharmacologic treatment
Abbreviations: CACC, cough associated with the common cold; CHEST, American College of Chest Physicians; FDA, Food and Drug Administration; NSAID, nonsteroidal anti-inflammatory drug; OTC, over the counter; RCT, randomized controlled trial; VAS, visual analogue scale
Summary of Recommendations and Suggestions
1. For adult and pediatric patients with cough due to the common cold, we suggest against the use of over the counter cough and cold medicines until they have been shown to make cough less severe or resolve sooner (Ungraded Consensus-Based Statement).
2. In adult patients with cough due to the common cold, we suggest against the use of nonsteroidal anti-inflammatory agents until they have been shown to make cough less severe or resolve sooner (Ungraded Consensus-Based Statement).
3. In pediatric patients (aged 1-18 years) with cough due to the common cold, we suggest honey may offer more relief for cough symptoms than no treatment, diphenhydramine, or placebo, but it is not better than dextromethorphan (Ungraded Consensus-Based Statement).
Remarks: Infants < 1 year of age should not be administered honey, and children < 2 years of age should not be administered dextromethorphan for cough symptoms.
4. In pediatric patients (aged < 18 years) with cough due to the common cold, we suggest avoiding use of codeine-containing medications because of the potential for serious side effects including respiratory distress (Ungraded Consensus-Based Statement).
The common cold is an acute upper respiratory syndrome, usually due to a viral infection, with symptoms including rhinorrhea and nasal obstruction. Common cold is frequently accompanied by sore throat, sneezing, body aches, low-grade fever, and cough. Cough associated with the common cold is a common and particularly bothersome symptom for patients in the ambulatory setting. A recent Internet survey found that cough outlasted other cold symptoms in 69% of the survey respondents.1 Adults in the United States average two to three colds on an annual basis, and this number is even higher for children.2
Fifty-two percent of adult participants from a recent survey indicated that cough/cold impacted their daily lives a “fair amount to a lot.”3 A total of 74% of survey respondents attempted to treat the cough associated with the common cold (CACC), most commonly with over the counter (OTC) syrup (58%) or a throat lozenge (53%).1 In 2015, the average American household made 26 trips to retail outlets and spent approximately $338 annually on OTC products. OTC medications are sold in 540,000 pharmacies and in > 750,000 retail locations in the United States.4 The Consumer Healthcare Products Association indicates an increasing trend in OTC sales of cough and cold products.5 Total sales of OTC cough, cold, and allergy products was $9.56 billion in the United States in 2015 according to Drug Store News.6
The current Cough Expert Panel believed it would be beneficial to perform a systematic review to update the recommendations of the 2006 guideline.7 The specific aim was to evaluate the evidence for clinically relevant pharmacologic and nonpharmacologic therapies in reducing the duration/severity of acute CACC.
Methods
The methodologies used by the CHEST Guideline Oversight Committee to select the Expert Cough Panel Chair and the international panel of experts perform synthesis of the evidence and develop the recommendations and suggestions that have been published.8, 9 Key questions and parameters of eligibility were developed for this topic. Existing guidelines, systematic reviews, and primary studies were assessed for relevance and quality and were used to support the evidence-based graded recommendations or suggestions. A highly structured consensus-based Delphi approach was used to provide expert advice on all guidance statements. The total number of eligible voters for each guidance statement varied based on the number of managed individuals recused from voting on any particular statements because of their potential conflicts of interest. Transparency of process was documented. Further details of the methods have been published elsewhere.8, 9
Systematic Review Question
The clinical question for this systematic review was generated using the Population, Intervention, Comparison, Outcome format.10 The review question was: Is there evidence of clinically relevant treatment effects for pharmacologic or nonpharmacologic therapies in reducing the duration/severity of acute CACC?
Systematic Literature Search
The methods used for this systematic review conformed to those outlined in the article “Methodologies for the Development of CHEST Guidelines and Expert Panel Reports.”8 The National Guideline Clearinghouse (http://www.guideline.gov) and the Guidelines International Network (http://www.g-i-n.net) were searched for existing guidelines on pharmacologic and nonpharmacologic treatment for CACC. Systematic reviews and clinical trials were identified from searches of electronic databases (PubMed, EMBASE, the Cochrane Central Register of Controlled Trials [Cochrane Library], Google Scholar, and CINAHL, commencing from the earliest available date until April 2014. The reference lists of retrieved articles were examined for additional citations. The search terms used were common cold, cough, antihistamine, decongestant, cough suppressant, antitussive, antibiotic, zinc, expectorant, acute cough, anticholinergic, topical steroids, upper respiratory tract infection. An additional search for cough and cold remedies for the cold, nonpharmacologic therapies, and complementary and alternative therapies was conducted. Since the publication of this review, the databases have been searched periodically to look for additional substantive articles.
The titles and abstracts of the search results were independently evaluated by two reviewers (M. A. M. and P. C. L.) to identify potentially relevant articles based on the eligibility criteria of the study design (randomized controlled trial [RCT], controlled clinical trial, or systematic review) and acute CACC in populations of adults or adolescents (12 years or older) and children (< 12 years) (Table 1). The full text of all potentially relevant articles was retrieved, and two reviewers (M. A. M. and P. C. L.) independently evaluated all the studies retrieved against the criteria.
Table 1.
Eligibility Criteria
| Criteria | Study Requirements |
|---|---|
| Inclusion | English-language publication |
| Population | |
| Common cold syndrome: the common cold is an acute upper respiratory syndrome of rhinorrhea and nasal obstruction, frequently accompanied by sore throat, sneezing, and cough | |
| Adult (12 y and older) | |
| Pediatric (< 12 y) | |
| Elderly (65 y and older) | |
| Intervention | Pharmacologic treatment |
| Acetylcysteine | |
| Antihistamine alone | |
| Expectorant alone | |
| Cough suppressant alone | |
| Antihistamine + expectorant | |
| Antihistamine + suppressant | |
| Expectorant + cough suppressant | |
| Antihistamine + suppressant + expectorant | |
| Supplements (zinc, vitamin C) | |
| Antihistamine + decongestants, inhaled nasal steroids, and inhaled nasal antihistamines and inhaled nasal ipratropium bromide | |
| NSAIDs and acetaminophen | |
| OTC cough and cold remedies | |
| Nonpharmacologic therapies | |
| Neti pot | |
| Chest rub (Vicks VapoRub) | |
| Honey | |
| Demulcents | |
| Cough drops | |
| Gelatin | |
| Chicken soup | |
| Complementary/alternative therapies | |
| Comparison/control | Other pharmacologic or nonpharmacologic therapies |
| Placebo | |
| Head to head comparisons | |
| Pharmacologic agents added to antibiotics vs no antibiotics | |
| Outcome | Symptoms |
| Severity of acute cough | |
| Duration of acute cough | |
| Side effects of therapies (benefits vs harms) | |
| Rating scales or VAS or HRQoL | |
| Cough must be mentioned as the primary symptom or can be teased out as part of a symptom complex |
HRQoL = health-related quality of life; NSAID = nonsteroidal anti-inflammatory drug; OTC = over the counter; VAS = visual analogue scale.
Pharmacologic interventions included acetylcysteine, antihistamine monotherapy, expectorant monotherapy, cough suppressant monotherapy, antihistamine + expectorant, antihistamine + suppressant, expectorant + suppressant, antihistamine + suppressant + expectorant, supplements (zinc, vitamin C), antihistamine + decongestants, inhaled nasal steroids, inhaled antihistamines, inhaled ipratropium bromide, nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen, and OTC cough and cold remedies. Nonpharmacologic therapies included a neti pot, mentholated chest rub, honey, demulcents, cough drops, gelatin, chicken soup, and complementary/alternative therapies. For outcome measures, cough must have been mentioned as the primary symptom or was able to be teased out as part of a symptom complex including severity of cough, duration of acute cough, side effects of therapies (benefits vs harms), and rating scales (visual analogue scale [VAS] or health-related quality of life).
Quality Assessment
Included articles underwent quality assessment. For RCTs and controlled clinical trials, quality assessment was conducted using the Cochrane Risk of Bias Tool.11 For systematic reviews, the Documentation and Appraisal Review Tool (Guidelines International Network) was used.12 Studies that were at high risk of bias or of poor quality were excluded.
Grading Recommendations
Recommendations were graded using two dimensions: quality of the body of evidence using three categories—low, moderate, or high—and strength of the supporting evidence, rated as either strong or weak.8 In the context of practice recommendations, a strong recommendation applies to almost all patients, whereas a weak recommendation is conditional and applies only to some patients. In the context of research recommendations (those provided in the present guidelines), we intended for a strong recommendation (grade 1) to imply that we recommended using intervention fidelity strategies in all studies in which patients with CACC are being managed. Intervention fidelity has been identified as an important aspect of acute cough studies and is defined “as the extent to which an intervention was delivered as conceived and planned to arrive at valid conclusions concerning the effectiveness in achieving target outcomes.”13 The strength of recommendations here is based on consideration of three factors: balance of benefits to harms, patient values and preferences, and resource considerations. Harms incorporate risk and burdens to the patients, which can include convenience or lack of convenience, difficulty of administration, and invasiveness. These variables in turn affect patient preferences. The resource considerations extend beyond economics and should also factor in time and other indirect costs. The authors of these recommendations have considered these parameters in determining the strength of the recommendations and associated grades.
The findings of this systematic review were used to support the evidence-graded recommendations or suggestions. A highly structured consensus-based Delphi approach was used to provide expert advice on all guidance statements. The total number of eligible voters for each guidance statement varied based on the number of managed individuals recused from voting on any particular statement because of their potential conflicts of interest. Transparency of process was documented. Further details of the methods related to conflicts of interests have been published elsewhere.8
Results
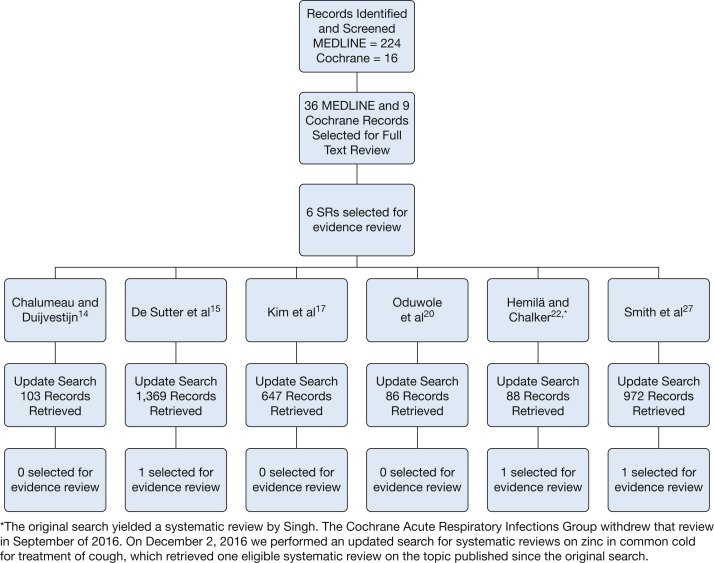
The flowchart in Figure 1 presents the results of the systematic search. Two hundred forty records were identified, and the abstracts were screened (224 RCTs and 16 Cochrane systematic reviews). Thirty-six RCTs and nine potentially relevant systematic reviews were identified for full text review, and six systematic reviews met the inclusion and exclusion criteria. The systematic reviews were mostly of good quality. Generally, however, the relevant study data were quite limited and of low quality. Details of study quality are reflected in Table 2, Table 3, Table 4, Table 5, Table 6, Table 7. An updated search for new studies was conducted in February 2015, and 3,265 records were retrieved. Of those, only three met all inclusion criteria.
Figure 1.
Systematic review flow diagram.14, 15, 17, 20, 21, 22, 27 SRs = systematic reviews.
Table 2.
GRADE Evidence Profile: Common Cold (Acetylcysteine and Carbocysteine for Acute Upper and Lower Respiratory Tract Infections in Children)14
| Quality Assessment |
No. of Patients |
Effect |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | AC or CC | Placebo | Relative (95% CI) | Absolute | ||
| Cough after 6-7 d | ||||||||||||
| 3 studies N = 139 |
RCT | Seriousa | No serious risk | Seriousb | Seriousc | Undetected | 74 | 65 | 0.37 (95% CI, 0.12-1.20) | 10% (–19% to –1%) | Very Low | |
Question: Is there evidence of clinically relevant treatment effects for acetylcysteine and carbocysteine in reducing the duration of cough?
Settings: Many studies included only hospitalized patients.
AC = acetylcysteine; CC = carbocysteine; GRADE = Grading of Recommendations Assessment, Development and Evaluation; RCT = randomized controlled trial.
Studies have an overall high risk of bias.
Many patients were hospitalized, which is not routine in common cold, and included subjects with bronchitis and acute lower respiratory tract infections.
Studies were very small and even when combined contributed only 139 total subjects.
Table 3.
| Quality Assessment |
No. of Patients |
Effect | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Decongestant or Antihistamine | Comparison | |||
| Antihistamine and decongestant (De Sutter et al)15 | |||||||||||
| 4 studies | RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 672 cold episodes | Pooling not possible Results inconsistent |
Very low | ||
| Antihistamine and analgesic (De Sutter et al15) | |||||||||||
| 2 studies | RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 341 subjects | Pooling not possible No effect observed |
Very low | ||
| Decongestant and analgesic (De Sutter et al15) | |||||||||||
| 2 studies | RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 249 subjects | Pooling not possible No effect observed |
Very low | ||
| Antihistamine decongestant and analgesic (De Sutter et al15) | |||||||||||
| 3 studies | RCT | Serious1 | Serious2 | No serious risk | Seriousc | Undetected | 555 adults, 201 children | Pooling not Possible Results inconsistent |
Very low | ||
| Antihistamine and decongestant with capsaicin challenge (Dicpinigaitis et al16) | |||||||||||
| 1 study | RCT | No serious risk | Seriousc | Seriousd | Seriouse | Undetected | 22 subjects | Mean log C5 (0.4 ± 0.55 SD; P < .01) for diphenhydramine vs placebo | Very low | ||
Question: Is there evidence of clinically relevant treatment effects for decongestants and antihistamines in reducing the duration of CACC?
CACC = cough associated with the common cold. See Table 2 legend for expansion of other abbreviations.
Studies were so poorly reported that risk of bias could not be accurately assessed.
Data were not reported in sufficient detail in the primary studies to evaluate or pool in most cases.
Effect sizes not reported for studies, and data could not be pooled.
Outcome was cough reflex challenge.
Extremely small study.
Table 4.
GRADE Evidence Profile for Common Cold: NSAIDS for Cough17
| Quality Assessment |
No. of Patients |
Effect |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | NSAID | Comparison | Relative (95% CI) | Absolute | ||
| 2 studies | RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 77 | 82 | MD –0.05 (–0.66 to +0.56) |
Very low | ||
Question: Is there evidence of clinically relevant treatment effects for NSAIDS on CACC?
MD = mean duration (of cough). See Table 2 legend for expansion of other abbreviations.
Studies were so poorly reported that most risk of bias categories could not be assessed.
Inconsistency is high as assessed by the I2 statistic.
Studies were very small and the CI was wide and includes both a relative decrease and increase.
Table 5.
GRADE Evidence Profile Common Cold: Honey for Acute Cough in Children20
| Quality Assessment |
No. of Patients |
Effect |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Honey | Comparison | Relative (95% CI) | Absolute | ||
| Honey compared with dextromethorphan for frequency of cough | ||||||||||||
| 2 studies | RCT | Very seriousa | Seriousb | No serious risk | Seriousc | Undetected | 75 | 74 | MD –0.07 (–1.07 to +0.94) |
Very low | ||
| Honey compared with dextromethorphan for severity of cough | ||||||||||||
| 2 studies | RCT | Very seriousa | Seriousb | No serious risk | Seriousc | Undetected | 75 | 74 | MD –0.13 (–1.25 to +0.99) |
Very low | ||
| Honey compared with diphenhydramine for frequency of cough | ||||||||||||
| 1 study | RCT | Very seriousa | Seriousb | No serious risk | Seriousc | Undetected | 40 | 40 | MD –0.57 (–0.90 to –0.24) |
Very low | ||
| Honey compared with diphenhydramine for severity of cough | ||||||||||||
| 1 study | RCT | Very seriousa | Seriousb | No serious risk | Seriousc | Undetected | 40 | 40 | MD –0.60 (–0.94 to –0.26) |
Very low | ||
| Honey compared with no treatment for frequency of cough | ||||||||||||
| 2 studies | RCT | Very seriousa | No serious risk | No serious risk | Seriousc | Undetected | 75 | 79 | MD –1.05 (–1.48 to –0.62) |
Very low | ||
| Honey compared with no treatment for severity of cough | ||||||||||||
| 2 studies | RCT | Very seriousa | No serious risk | No serious risk | Seriousc | Undetected | 75 | 79 | MD –1.03 (–1.59 to –0.47) |
Very low | ||
| Honey compared with placebo for frequency of cough | ||||||||||||
| 1 study | RCT | No serious risk | Seriousb | No serious risk | Seriousc | Undetected | 225 | 75 | MD –1.85 (–3.36 to –0.33) |
Low | ||
| Honey compared with placebo for severity of cough | ||||||||||||
| 1 study | RCT | No serious risk | Seriousb | No serious risk | Seriousc | Undetected | 225 | 75 | MD –1.83 (–3.32 to –0.34) |
Low | ||
Question: Is there evidence of clinically relevant treatment effects for honey in reducing the duration of CACC?
Inconsistency is high as assessed by the I2 statistic or cannot be assessed because it is a single study.
Studies were very small.
Table 6.
GRADE Evidence Profile for Common Cold: Zinc for the Common Cold22
| Quality Assessment |
No. of Patients |
Effect |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Zinc | Placebo | Relative (95% CI) | Absolute | ||
| Cough duration | ||||||||||||
| 3 adult studies | RCT | No serious risk | Seriousa | No serious risk | Seriousb | Undetected | 102 | 97 | Cough duration reduced in subjects receiving zinc Pooled estimate was –46% (95% CI, –28% to –64%) |
Low | ||
Question: Is there evidence of clinically relevant treatment effects for zinc in cough duration?
See Table 2 legend for expansion of abbreviations.
Inconsistency; heterogeneity was 64% as assessed by the I2 statistic.
Individual studies were very small and CIs were wide.
Table 7.
GRADE Evidence Profile for the Common Cold: OTC Medications for Acute Cough in Children and Adults in Community Settings27, 28, 29
| Quality Assessment |
No. of Patients | Effect | Quality | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | |||||
| Antitussive agents (Smith et al27) | |||||||||||
| 6 adult studies 4 child studies |
RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 1,526 adults 327 children |
Pooling not possible Studies had variable results |
Very low | ||
| Expectorants (Smith et al27) | |||||||||||
| 3 studies | RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 604 | Pooling not possible Studies had conflicting results |
Very low | ||
| Mucolytic agents (Smith et al27) | |||||||||||
| 1 adult study 1 child study |
RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 99 adults 40 children |
Pooling not possible | Very low | ||
| Other combinations (Smith et al27) | |||||||||||
| 4 adult studies 2 child studies |
RCT | Seriousa | Seriousb | No serious risk | Seriousc | Undetected | 836 adults 94 children |
Pooling not possible Studies were very small, heterogeneous, and used very different drug preparations and dosing frequency, limiting their comparability |
Very low | ||
| Vicks VapoRub (Paul et al28) | |||||||||||
| 1 child study | RCT | No serious risk | Seriousb | No serious risk | Seriousc | Undetected | 138 children | Change in cough frequency –1.5 placebo vs Vicks VapoRub –2.5; P = .07 | Change in cough severity –1.4 placebo vs Vicks VapoRub –2.1; P = .06 | Low | |
| Agave nectar (Paul et al29) | |||||||||||
| 1 child study | RCT | No serious risk | Seriousb | No serious risk | Seriousc | Undetected | 119 children | Change in cough frequency –2.1 placebo vs agave nectar –2.2; P = .79 | Change in cough severity –2.1 placebo vs agave nectar –2.2; P = .87 | Low | |
Question: Is there evidence of clinically relevant treatment effects for OTC medications in reducing the duration of CACC?
Studies overall have a high risk of bias.
There are conflicting results between trials in each medication group, or inconsistency cannot be assessed because it was a single study.
Most studies failed to provide quantitative data on cough, and quantitative data that could be combined showed wide ranges, or study sample size was very small.
The key question was: Is there evidence of clinically relevant treatment effects for pharmacologic or nonpharmacologic therapies in reducing the duration/severity of severe CACC? Because of the large number of potential pharmacologic and nonpharmacologic treatments, we further developed six separate questions for each specific therapeutic category (acetylcysteine/carbocysteine, decongestants, antihistamines, acetaminophen as a single product or in combination products, NSAIDs, honey, zinc, and OTC antitussive agents). With respect to voting, no panelist was recused.
Key Clinical Question 1
Is there evidence of clinically relevant treatment effects for acetylcysteine or carbocysteine in reducing the duration of cough (Table 2)?
The Chalumeau and Duijvestijn14 systematic review identified six RCTs comparing acetylcysteine or carbocysteine vs placebo for upper or lower respiratory tract infections in pediatric patients. Only three RCTs assessed cough as a primary outcome, measuring “reduction in cough” after 6 to 7 days. The methods of measuring cough were not discussed. Overall, these studies were very small (139 total subjects in the three RCTs that assessed cough) and had a high risk of bias. It was difficult to account for other therapies and, in many cases, antibiotics were administered. Additionally, many of the patients were hospitalized, which is not routine for the treatment of the common cold.
The Chalumeau and Duijvestijn14 systematic review identified 34 studies, with a total of 2,064 participants, that evaluated product safety. Although limited data are noted for children < 2 years, there were 59 cases of paradoxically increased bronchorrhea in this age group, and many (86%) required hospitalization or extended hospitalization. For other age groups, the products were generally safe, with minor GI symptoms reported in 2% of all participants.
A pooled analysis of the three RCTs that measured cough showed statistically significant effects after 6 to 7 days of treatment. As CACC is generally a self-limited disease process, with most symptoms resolving in 5 to 7 days, no specific recommendations could be made for acetylcysteine or carbocysteine.
Key Clinical Question 2
Is there evidence of clinically relevant treatment effects for decongestants and antihistamines in reducing the duration of CACC (Table 3)?
The De Sutter et al15 systematic review identified four RCTs that evaluated the effects of antihistamine and decongestant combinations on the severity of cough. The data (three studies in adults and one pediatric study) included 672 cold episodes. Data pooling was not possible due to different treatments and combinations. Overall, the results were inconsistent, making it impossible to form any conclusion regarding the effectiveness of this combination of products.
The De Sutter et al15 systematic review identified two RCTs of antihistamine and analgesic combinations. Three hundred forty-one adult subjects had cough symptoms evaluated daily for 5 days. Both trials failed to show an effect from the active treatment compared with placebo or acetaminophen for cough.
The De Sutter et al15 systematic review identified two RCTs with a total of 249 subjects treated with a decongestant and analgesic combination. No effect on cough was observed.
The De Sutter et al15 systematic review evaluated three RCTs of antihistamine, decongestant, and analgesic combinations. Two studies included 555 adult subjects, and one pediatric study included 201 children. Data pooling was not possible, and study results were inconsistent. The two adult studies did demonstrate some treatment effect on cough with the combination of dextromethorphan, doxylamine, paracetamol, and ephedrine. The author concluded that this combination may be effective for CACC in adults. However, there is no commercially marketed product available in the United States that includes this combination of ingredients. The pediatric study using the combination of acetaminophen, diphenhydramine, and pseudoephedrine showed no effect on cough; however, dextromethorphan was not included in the combination product administered in the pediatric study.
A single potentially eligible trial was identified from an updated literature search of the De Sutter review. This trial by Dicpinigaitis et al16 studied the effect of diphenhydramine on cough reflex sensitivity in 22 healthy subjects with acute viral infection by administering a capsaicin challenge on 3 separate days. Diphenhydramine was successful in inhibiting the cough reflex in subjects with acute cough during viral respiratory tract infection. After review, it was determined that this study was not relevant to the clinical question. The available currently marketed products in the United States do not include the combination of ingredients that may be effective for CACC in adults; hence, no specific recommendations could be made for the use of antihistamine, decongestant, and analgesic combinations to treat CACC in adults. In addition, there were no pediatric studies of antihistamine, decongestant, and analgesic combination products that demonstrated efficacy for CACC.
1. For adult and pediatric patients with cough due to the common cold, we suggest against the use of over the counter cough and cold medicines until they have been shown to make cough less severe or resolve sooner (Ungraded Consensus-Based Statement).
Key Clinical Question 3
Is there evidence of clinically relevant treatment effects for NSAIDs on CACC (Table 4)?
The Kim et al17 systematic review identified two RCTs of 77 patients receiving NSAIDs and 82 placebo comparisons. The 2006 cough guidelines recommended a combination of first-generation antihistamine and nasal decongestant or naproxen for CACC.7 The Kim et al systematic review found no clear evidence that treatment with NSAIDs is effective for treatment of CACC. These studies were very small with wide CIs.
2. In adult patients with cough due to the common cold, we suggest against the use of nonsteroidal anti-inflammatory agents until they have been shown to make cough less severe or resolve sooner (Ungraded Consensus-Based Statement).
Key Clinical Question 4
Is there evidence of clinically relevant treatment effects for honey in reducing the duration of CACC in pediatric patients (Table 5)?18, 19
The Oduwole et al20 systematic review identified a total of three RCTs. Two RCTs included a comparison of honey to dextromethorphan for reducing the frequency of cough. Both trials were judged to be at high risk of bias. There were 149 total participants in these study arms (75 received honey and 74 received dextromethorphan). Overall, there was no difference between treatment groups.
One trial also included a comparison of honey to diphenhydramine (40 subjects in each group). This comparison showed honey may be better than diphenhydramine in reducing the frequency of cough and severity of cough. Two other comparisons in the two RCTs showed that honey was probably better compared with no treatment in 154 participants for frequency of cough and cough severity. In the third RCT of 300 pediatric patients, evidence indicated that honey may be better than placebo in reducing cough frequency and severity.
3. In pediatric patients (aged 1-18 years) with cough due to the common cold, we suggest honey may offer more relief for cough symptoms than no treatment, diphenhydramine, or placebo, but it is not better than dextromethorphan (Ungraded Consensus-Based Statement).
Remarks: Infants < 1 year of age should not be administered honey, and children < 2 years of age should not be administered dextromethorphan for cough symptoms
Key Clinical Question 5
Is there evidence of clinically relevant treatment effects for zinc regarding the time to resolution of cough or change in the cough symptom score (Table 6)?
The Singh and Das21 systematic review published in 2013 identified four RCTs in which zinc was evaluated for time to resolution of cough. One study was eliminated because the zinc was administered as syrup, whereas the other three studies used zinc lozenges. In the three lozenge studies (two adult studies, one pediatric study), 122 patients received zinc lozenges and 137 patients received placebo therapy. Data pooling was not possible given the diverse populations and varying dosage frequency. The time for resolution of cough was shorter with zinc treatment. In September 2016, the Singh and Das Cochrane review was withdrawn, and we subsequently performed an updated search for systematic reviews published on the use of zinc. One review by Hemilä and Chalker22 was identified.
The Hemilä and Chalker22 systematic review identified three RCTs in adults that addressed the total duration of colds and duration of the symptoms with zinc compared with placebo. The Petrus et al23 1998 study included 101 adult patients, and there was a significant decrease in the mean cough score in the zinc intervention group. The Prasad et al24 2000 study included 48 adult patients, and zinc was associated with reduction in duration and severity of cold symptoms, including cough. The Prasad et al25 2008 study included 50 adult patients, and there was a significant reduction in the duration of cold symptoms and cough. Zinc lozenges (dosed ≥ 75 mg/d) given within 24 hours of symptom onset may reduce the duration of cold symptoms, such as cough, in healthy individuals.22
No systematic review is available regarding the use of zinc in pediatric patients. No recommendation can be made for the use of zinc supplements to reduce the duration and severity of cough in pediatric patients. A trial by Rerksuppaphol26 was identified that assessed the efficacy of 15 mg of chelated zinc (zinc bisglycinate) given once daily vs placebo for 3 months during the winter season to 100 children aged 8 to 13 years in Thailand. There was no significant difference in the incidence of common cold symptoms; however, the authors report that the duration of cough was reduced significantly in the intervention group.26 Similar studies in Turkey and Iran have also shown possible reduction in cold symptoms in children who received prophylactic zinc therapy. Another trial in the United States failed to demonstrate a positive impact. No recommendation can be made for the use of zinc supplement prophylaxis.21, 26
Zinc deficiency is an important cause of childhood morbidity in developing countries; this may explain the differing study results. Although generally well tolerated, some zinc lozenges have a distinctive and unpleasant taste, making subject blinding for these trials very difficult. The bad taste may also affect compliance with zinc therapy. A suggestion for the use of zinc lozenges in healthy adults with cough due to common cold was considered by the expert panel. However, due to weak evidence, the potential side effects of zinc, and the relatively benign and common nature of the condition being treated, the panel did not approve inclusion of this suggestion.
Key Clinical Question 6
Is there evidence of clinically relevant treatment effects for OTC medications in reducing the duration of CACC (Table 7)?
The Smith et al27 systematic review, assessed to be of good quality, included six trials with a total of 1,526 adult patients that compared antitussive agents with placebo. The antitussive agents studied included codeine, dextromethorphan, and moguisteine. Four trials of antitussive studies were identified in 327 pediatric subjects. Overall, the studies had very poor quality with variable results. Data pooling was not possible.
The Smith et al27 systematic review identified three trials comparing the expectorant guaifenesin with placebo in 304 subjects. Data quality was very low, and studies had conflicting results. No studies were identified that reported outcomes with the use of expectorants in pediatric subjects.
The Smith et al27 systematic review identified one trial of 99 subjects that compared the mucolytic agent bromhexine with placebo. One pediatric trial compared the mucolytic letosteine with placebo in 40 subjects. Overall, the data quality for mucolytic agents was very low, and data pooling was not possible. One trial did find reduced cough frequency with mucolytic therapy.
Four studies evaluated other product combinations against placebo in 836 adults; three of the studies found antihistamines to be no more effective than placebo in relieving cough symptoms. Two studies evaluated other product combinations in 99 pediatric patients. Overall, these studies were very small and heterogeneous, using widely varied drug preparations and dosing frequencies. These limitations do not lead to any possible data pooling. None of the pediatric studies showed a benefit over placebo for antitussive therapy, antihistamines, decongestants, or antitussive/bronchodilator treatment.
A trial by Paul et al28 was identified in the updated literature search of Smith et al.27 Paul et al evaluated a single dose of Vicks VapoRub (camphor, menthol, and eucalyptus oils in a petroleum base) compared with petrolatum and no treatment for nocturnal cough caused by respiratory tract infections in 138 children. The study was blinded, but parents who used VapoRub correctly guessed their treatment group 86% of the time, as did 89% of the petrolatum-treating control group. Parents rated VapoRub most favorably for symptomatic relief of nocturnal cough. This study was assessed as low quality. Although the mechanism is not completely clear, menthol has been shown to improve the nasal sensation of airflow and may lead to improved sleep.
Another trial was identified in the update of Smith et al.27 Paul et al29 compared the effects of agave nectar with placebo and no treatment on acute nocturnal cough in 119 infants and toddlers. This study was assessed as low quality. Although a placebo effect was demonstrated, there was no additional benefit from agave nectar.
In adult and pediatric patients with the common cold, we find no evidence to support or refute the use of OTC antitussive agents, expectorants, mucolytic agents, antihistamines, or combination products for reducing cough.
Characteristics of Included Studies
Evidence profiles were created to grade the overall quality of the body of evidence supporting each question. The six evidence profiles can be found in Table 2, Table 3, Table 4, Table 5, Table 6, Table 7.
State of the Available Evidence
Even though the systematic reviews were mostly of good quality,14, 15, 17, 20, 21, 22, 28 in general the only studies identified in the reviews provided low-quality evidence to support a particular strategy for management of cough with the common cold. Therefore, for recommendations, the panel depended heavily on patient values, preferences, and availability of potential therapies. The panel also made several suggestions for future research.
Discussion
We addressed six key clinical questions regarding the treatment of CACC. We could make no specific recommendation regarding the use of acetylcysteine or carbocysteine for key clinical question 1. Regarding key clinical question 2, no specific recommendation can be made based on the role of decongestants and antihistamines. For key clinical question 3, we found no evidence to support the use of nonsteroidal anti-inflammatory agents. For key clinical question 4, we suggested that honey may offer more relief than diphenhydramine, no treatment, or placebo; however, honey is not more effective than dextromethorphan for adults and children. For key clinical question 5, we reviewed evidence on the use of zinc lozenges in healthy adults and children > 2 years of age; some evidence indicates that when administered within 24 hours of symptoms, they may reduce cough duration. However, the evidence was not convincing, and study results are not consistent. Due to the benign nature of the illness, no specific suggestion was made for this question. Finally for key clinical question 6, we found no evidence to support or refute the use of OTC antitussive agents, expectorants, mucolytic agents, antihistamines, or combination products.
Although no significant advancement in treatment has been identified in clinical trial data since the 2006 update,7 there are dozens of pharmacologic and nonpharmacologic treatment options currently available as monotherapy or combination therapy. There have been new prescription products approved by the US Food and Drug Administration (FDA) for the treatment of cough; however, these are primarily new combinations of previously approved products. In addition, concerns about medication safety have led to recommendations regarding the use of some products in specific populations.
The first hydrocodone and guaifenesin combination product (Vituz; Hawthorn Pharmaceuticals)30 was marketed in 2013. No new clinical studies were required by the FDA for approval of this combination product; efficacy was based on the demonstrated bioequivalence of the active ingredients to their respective reference products. The same pathway of approval has been used for several other cough and cold preparations over the past 3 years; hydrocodone bitartrate and guaifenesin oral solution (Obredon; Sovereign Pharmaceuticals),31 (Flowtuss; Mission Pharmacal)32 extended-release codeine and chlorpheniramine (Tuzistra XR; Vernalis Therapeutics, Inc.),33 and hydrocodone bitartrate, pseudoephedrine hydrochloride, and guaifenesin oral solution (Hycofenix; Mission Pharmacal).34 This guideline will be updated when additional studies are reported regarding the efficacy of these or other therapies for CACC.
In January 2008, the FDA released a consumer update strongly recommending that “over-the-counter (OTC) cough and cold products should not be used for infants and children under 2 years of age because serious and potentially life-threatening side effects could occur.”35
In March 2011, the FDA took action against unapproved prescription oral cough, cold, and allergy products secondary to a concern about potential risks with extended-release formulations and irrational combinations of active ingredients.36
In July 2015, the FDA issued a news release warning that caregivers should not use codeine-containing medications to treat coughs and colds in children < 18 years of age because of the potential for serious side effects, including slowed or difficult breathing.37
In August 2016, the FDA issued a news release warning that the combination of opioids (those prescribed for pain and found in cough medicines) should not be combined with benzodiazepines or other central nervous depressants.38 Prescribers are advised to stay abreast of FDA communications and warning statements.
Both prescription and OTC products contain active ingredients that may be abused. For example, high doses of dextromethorphan can produce euphoria and dissociative effects. The ingestion of large doses of dextromethorphan (Robitussin) cough syrup is referred to as “robo-tripping.”39 “Purple drank” is a cocktail used by teens or young adults in which promethazine with codeine syrup is combined with soda, fruit candy, or even alcohol. This mixture produces a sensation of relaxation, euphoria, and intoxication.40 These medication misadventures can lead to serious complications and even death.
4. In pediatric patients (aged < 18 years) with cough due to the common cold, we suggest avoiding use of codeine-containing medications because of the potential for serious side effects including respiratory distress (Ungraded Consensus-Based Statement).
Areas for Future Research
To improve therapeutic options for treatment of cough due to common cold, potential research endeavors include the following:
-
•
Development of validated models to demonstrate that mucoactive drug efficacy can accelerate the clinical development of mucoactive drugs for symptomatic respiratory tract infections41
-
•
Development of clinical studies to validate the use of multi-ingredient prescription and nonprescription cough therapies42
-
•
Development of effective antitussive medications that are safe for children and adults
-
•
RCTs with appropriate comparators
Conclusions
Unfortunately, there has been little change in the treatment choices for cough due to the common cold since publication of the 2006 CHEST cough guidelines. Many of the published studies are small and have significant limitations and potential biases. Data pooling was generally not possible, making it difficult to provide definitive recommendations. Cold symptoms are one of the most common reasons for seeking medical attention, and cough is one of the most irritating and persistent cold symptoms. We have reviewed the available literature and, when possible, provided treatment recommendations. This article also identified knowledge gaps and suggests areas for future research.
Acknowledgments
Author contributions: M. A. M. and P. C.-L. were the topic editors for this article and developed the key question using the Population, Intervention, Comparator, Outcome (PICO) format in collaboration with R. S. I. B. I. was the appointed methodologist and was among the investigators who conducted the systematic review that formed the basis for the suggestions. M.A.M. wrote the first draft; P. C.-L., B. I., and R. S. I. reviewed and contributed to subsequent versions.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. S. I. reports that he has no financial or intellectual conflicts of interest regarding the content of this manuscript. Although R. S. I. is the Editor in Chief of CHEST, he discloses that he had nothing to do with the review of this manuscript and all decisions regarding the review process and acceptance of this manuscript were independently made by others. None declared (M. A. M., P. C. L., B. I.).
Collaborators: Todd M. Adams, MD (Webhannet Internal Medicine Associates of York Hospital, York, ME), Kenneth W. Altman, MD, PhD (Baylor College of Medicine, Houston, TX), Elie Azoulay, MD, PhD (University of Paris, Paris, France), Alan F. Barker, MD (Oregon Health & Science University, Portland, OR), Surinder S. Birring, MBChB, MD (Division of Asthma, Allergy and Lung Biology, King’s College London, Denmark Hill, London, England), Fiona Blackhall, MD, PhD (Department of Medical Oncology, University of Manchester, Manchester, England), Donald C. Bolser, PhD (College of Veterinary Medicine, University of Florida, Gainesville, FL), Louis-Philippe Boulet, MD, FCCP (Institut universitaire de cardiologie et de pneumonlogie de Québec, Quebec, [IUCPQ], QC, Canada), Sidney S. Braman, MD, FCCP (Mount Sinai Hospital, New York, NY), Christopher Brightling, MBBS, PhD, FCCP (University of Leicester, Glenfield Hospital, Leicester, United Kingdom), Priscilla Callahan-Lyon, MD (Adamstown, MD), Anne B. Chang, MBBS, PhD, MPH (Royal Children’s Hospital, Queensland, Australia), Andréanne Coté, MD (Institut universitaire de cardiologie et de pneumologie de Québec [IUCPQ], Quebec, QC, Canada), Terrie Cowley (The TMJ Association, Milwaukee, WI), Paul Davenport, PhD (Department of Physiological Sciences, University of Florida, Gainesville, FL), Satoru Ebihara, MD, PhD (Department of Rehabilitation Medicine, Toho University School of Medicine, Tokyo, Japan), Ali A. El Solh, MD, MPH (University at Buffalo, State University of New York, Buffalo, NY), Patricio Escalante, MD, MSc, FCCP (Mayo Clinic, Rochester, MN), Stephen K. Field, MD (University of Calgary, Calgary, AB, Canada), Dina Fisher, MD, MSc (University of Calgary, Respiratory Medicine, Calgary, AB, Canada), Cynthia T. French, PhD, FCCP (UMass Memorial Medical Center, Worcester, MA), Cameron Grant, MBChB, PhD (University of Auckland, Auckland, New Zealand), Peter Gibson, MBBS (Hunter Medical Research Institute, New South Wales, Australia), Philip Gold, MD, MACP, FCCP (Loma Linda University, Loma Linda, CA), Susan M. Harding, MD, FCCP (Division of Pulmonary, Allergy and Critical Care Medicine University of Alabama at Birmingham, Birmingham, AL), Anthony Harnden, MBChB, MSc (University of Oxford, Oxford, England), Adam T. Hill, MBChB, MD (Royal Infirmary and University of Edinburgh, Edinburgh, Scotland), Richard S. Irwin, MD, Master FCCP (UMass Memorial Medical Center, Worcester, MA), Peter J. Kahrilas, MD (Feinberg School of Medicine, Northwestern University, Chicago, IL), Joanne Kavanagh, MBChB, (Division of Asthma, Allergy and Lung Biology, King’s College London, Denmark Hill, London, England), Karina A. Keogh, MD (Mayo Clinic, Rochester, MN), Kefang Lai, MD, PhD (First Affiliated Hospital of Guangzhou Medical College, Guangzhou, China), Andrew P. Lane, MD (Johns Hopkins University School of Medicine, Baltimore, MD), Kaiser Lim, MD (Mayo Clinic, Rochester, MN), J. Mark Madison, MD, FCCP, (UMass Memorial Medical Center, Worcester, MA), Mark A. Malesker, PharmD, FCCP (Creighton University School of Pharmacy and Health Professions, Omaha, NE), Stuart Mazzone, PhD, FCCP (University of Queensland, Queensland, Australia), Lorcan McGarvey, MD (The Queens University Belfast, Belfast, Ireland), Alex Molassoitis, PhD, MSc, RN (Hong Kong Polytechnic University, Hong Kong, China), M. Hassan Murad, MD, MPH (Mayo Clinic, Rochester, MN), Mangala Narasimhan, DO, FCCP (Hofstra-Northwell Health, Manhasset, NY), Peter Newcombe, PhD (School of Psychology University of Queensland, Queensland, Australia), Huong Q. Nguyen, PhD, RN (Kaiser Permanente, Pasadena, CA), John Oppenheimer, MD (University of Medicine and Dentistry of New Jersey -Rutgers University), Marcos I. Restrepo, MD, MSc, FCCP (South Texas Veterans Health Care System, San Antonio), Mark Rosen, MD, Master FCCP (Icahn School of Medicine at Mount Sinai, New York, NY), Bruce Rubin, MEngr, MD, MBA (Virginia Commonwealth University, Richmond, VA), Jay H. Ryu, MD, FCCP (Mayo Clinic, Rochester, MN), Jaclyn Smith, MB ChB, PhD (University of Manchester, Manchester, England), Susan M. Tarlo, MBBS, FCCP (Toronto Western Hospital, Toronto, ON, Canada), Julie Turmel, PhD (Quebec Heart and Lung Institute, Laval University, Quebec City, QC, Canada), Anne E. Vertigan, PhD, MBA, BAppSc (SpPath) (John Hunter Hospital, New South Wales, Australia), Gang Wang, MD, PhD (Sichuan University, West China Hospital, Chengdu, China), Miles Weinberger, MD, FCCP (University of Iowa Hospitals and Clinics, Iowa City, IA), Kelly Weir, BSpThy, MspPath, PhD, CPSP (Menzies Health Institute Queensland/Griffith and Gold Coast, Australia).
Other contributions: The authors thank Nancy Harger, MLS, and Judy Nordberg, MLS, Education and Clinical Service Librarians working at in the University of Massachusetts Medical Library who undertook all the searches for this systematic review. The authors also thank Tamara Pringsheim, MD, and Rebecca L. Diekemper, MPH, for methodology support.
Endorsements: This guideline has been endorsed by the American Association for Respiratory Care (AARC), American Bronchoesophological Association (ABEA), American College of Allergy, Asthma, and Immunology (ACAAI), Asian Pacific Society for Respirology (APSR), and Irish Thoracic Society (ITS).
Role of Sponsors: The American College of Chest Physicians was the sole supporter of these guidelines, this article, and the innovations addressed within.
Footnotes
DISCLAIMER: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care or physician advice, which should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://www.chestnet.org/Guidelines-and-Resources/Guidelines-and-Consensus-Statements/CHEST-Guidelines.
FUNDING/SUPPORT: CHEST was the sole supporter of these guidelines, this article, and the innovation addressed within.
Contributor Information
Mark A. Malesker, Email: markmalesker@creighton.edu.
CHEST Expert Cough Panel:
Todd M. Adams, Kenneth W. Altman, Elie Azoulay, Alan F. Barker, Surinder S. Birring, Fiona Blackhall, Donald C. Bolser, Louis-Philippe Boulet, Sidney S. Braman, Christopher Brightling, Priscilla Callahan-Lyon, Anne B. Chang, Andréanne Coté, Terrie Cowley, Paul Davenport, Satoru Ebihara, Ali A. El Solh, Patricio Escalante, Stephen K. Field, Dina Fisher, Cynthia T. French, Cameron Grant, Peter Gibson, Philip Gold, Susan M. Harding, Anthony Harnden, Adam T. Hill, Richard S. Irwin, Peter J. Kahrilas, Joanne Kavanagh, Karina A. Keogh, Kefang Lai, Andrew P. Lane, Kaiser Lim, J. Mark Madison, Mark A. Malesker, Stuart Mazzone, Lorcan McGarvey, Alex Molassoitis, M. Hassan Murad, Mangala Narasimhan, Peter Newcombe, Huong Q. Nguyen, John Oppenheimer, Marcos I. Restrepo, Mark Rosen, Bruce Rubin, Jay H. Ryu, Jaclyn Smith, Susan M. Tarlo, Julie Turmel, Anne E. Vertigan, Gang Wang, Miles Weinberger, and Kelly Weir
References
- 1.Druce H.M., Dicpinigaitis P., Eccles R., Turner R., Adeleke M. Nationwide internet survey on the symptom of cough associated with common cold. J Gen Intern Med. 2016;31(2 suppl):S309–S310. doi: 10.1080/00325481.2016.1185376. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Common colds: protect yourself and others. http://www.cdc.gov/features/rhinoviruses/. Accessed April 30, 2016.
- 3.Dicpinigaitis P.V., Eccles R., Blaiss M.S., Wingertzahn M.A. Impact of cough and common cold on productivity, absenteeism, and daily life in the United States: ACHOO survey. Curr Med Res Opin. 2015;31(8):1519–1525. doi: 10.1185/03007995.2015.1062355. [DOI] [PubMed] [Google Scholar]
- 4.Consumer Healthcare Products Association. Statistics on OTC use. http://www.chpa.org/marketstats.aspx. Accessed May 14, 2016.
- 5.Consumer HealthCare Products Association. Statistics on OTC use. OTC Sales by Category 2013-2016. Available at: http://www.chpa.org/OTCsCategory.aspx. Accessed June 08, 2016.
- 6.Johnsen M. Cough-Cold Report 2016. Drug Store News. February 2016 http://www.drugstorenews.com/sites/drugstorenews.com/files/Cough-Cold_020816.pdf Accessed June 8, 2016. [Google Scholar]
- 7.Pratter M.R. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 suppl):72S–74S. doi: 10.1378/chest.129.1_suppl.72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis S.Z., Diekemper R., Ornelas J., Casey K.R. Methodologies for the development of CHEST guidelines and expert panel reports. Chest. 2014;146(1):182–192. doi: 10.1378/chest.14-0824. [DOI] [PubMed] [Google Scholar]
- 9.Lewis S.Z., Diekemper R.L., French C.T., Gold P.M., Irwin R.S., Chest Expert Cough Panel Methodologies for the development of the management of cough: CHEST guideline and expert panel report. Chest. 2014;146(5):1395–1402. doi: 10.1378/chest.14-1484. [DOI] [PubMed] [Google Scholar]
- 10.Huang X., Lin J., Demner-Fushman D. Evaluations of PICO as a knowledge representation for clinical questions. AMIA Annu Symp Proc. 2006:359–363. [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diekemper R., Ireland B., Merz L. Development of the documentation and appraisal review tool (DART) for systematic reviews. BMJ Qual Saf. 2013;22:61–62. [Google Scholar]
- 13.Song M., Happ M., Sandelowski M. Development of a tool to assess fidelity to a psycho-educational intervention. J Adv Nurs. 2010;66(3):673–682. doi: 10.1111/j.1365-2648.2009.05216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalumeau M., Duijvestijn Y.C.M. Acetylcysteine and carbocysteine for acute upper and lower respiratory tract infections in paediatric patients without chronic broncho-pulmonary disease. Cochrane Database Syst Rev. 2013;(5):CD003124. doi: 10.1002/14651858.CD003124.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Sutter A.I.M., Saraswat A., van Driel M.L. Antihistamines for the common cold. Cochrane Database Syst Rev. 2015;(11):CD009345. doi: 10.1002/14651858.CD009345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dicpinigaitis P.V., Dhar S., Johnson A., Gayle Y., Brew J., Caparros-Wanderley W. Inhibition of cough reflex sensitivity by diphenhydramine during acute viral respiratory tract infection. Int J Clin Pharm. 2015;37(3):471–474. doi: 10.1007/s11096-015-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S.Y., Chang Y.J., Cho H.M., Hwang Y.W., Moon Y.S. Non-steroidal anti-inflammatory drugs for the common cold. Cochrane Database Syst Rev. 2015;9:CD006362. doi: 10.1002/14651858.CD006362.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul I.M., Beiler J., McMonagle A., Shaffer M.L., Duda L., Berlin C.M. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161(12):1140–1146. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 19.Shadkam M.N., Mozaffari-Khosravi H., Mozayan M.R. A comparison of the effect of honey, dextromethorphan, and diphenhydramine on nightly cough and sleep quality in children and their parents. J Altern Complement Med. 2010;16(7):787–793. doi: 10.1089/acm.2009.0311. [DOI] [PubMed] [Google Scholar]
- 20.Oduwole O., Meremikwu M.M., Oyo-Ita A., Udoh E.E. Honey for acute cough in children. Cochrane Database Syst Rev. 2014;12 doi: 10.1002/14651858.CD007094.pub4. CD007094.0.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 21.Singh M., Das R.R. Zinc for the common cold. Cochrane Database Syst Rev. 2013;6:CD001364. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 22.Hemilä H., Chalker E. The effectiveness of high dose zinc acetate lozenges on various common cold symptoms: a meta-analysis. BMC Family Pract. 2015;16:24. doi: 10.1186/s12875-015-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrus E.J., Lawson K.A., Bucci L.R., Blum K. Randomized, double-masked, placebo-controlled clinical study of the effectiveness of zinc acetate lozenges on common cold symptoms in allergy tested subjects. Curr Ther Res. 1998;59:595–607. doi: 10.1016/S0011-393X(98)85058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad A.S., Fitzgerald J.T., Bao B., Beck FWJ Chandrasekar P.H. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate: a randomized double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:245–252. doi: 10.7326/0003-4819-133-4-200008150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Prasad A.S., Beck F.W.J., Bao B., Smel D., Fitzgerald J.T. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J Infect Dis. 2008;197:795–802. doi: 10.1086/528803. [DOI] [PubMed] [Google Scholar]
- 26.Rerksuppaphol S., Rerksuppaphol L. A randomized controlled trial of chelated zinc for prevention of the common cold in Thai school children. Paediatr Int Child Health. 2013;33(3):145–150. doi: 10.1179/2046905513Y.0000000064. [DOI] [PubMed] [Google Scholar]
- 27.Smith S.M., Schroeder K., Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database Syst Rev. 2014;11:CD001831. doi: 10.1002/14651858.CD001831.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul I.M., Beiler J.S., King T.S., Clapp E.R., Vallati J., Berlin C.M. Vapor Rub, petrolatum, and no treatment for children with nocturnal cough and cold symptoms. Pediatrics. 2010;126(6):1092–1099. doi: 10.1542/peds.2010-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul I.M., Beiler J.S., Vallati J.R., Duda L.M., King T.S. Placebo effect in the treatment of acute cough in infants and toddlers: a randomized clinical trial. JAMA Pediatr. 2014;168(12):1107–1113. doi: 10.1001/jamapediatrics.2014.1609. [DOI] [PubMed] [Google Scholar]
- 30.US National Library of Medicine. Daily Med. Vituz (hydrocodone bitartrate and chlorpheniramine) package insert. Morristown, NJ: Hawthorn Pharmaceuticals, Inc. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35ecdec3-4a59-479f-a3cb-4ffe0d4ab85e. Accessed May 12, 2016.
- 31.US National Library of Medicine. Daily Med. Obredon (hydrocodone bitartrate and chlorpheniramine) package insert. East Windsor, NJ: Accelis Pharma, Inc. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=31c14c1c-a250-4891-b6ab-9c5961fc8a39. Accessed May 12, 2016.
- 32.US National Library of Medicine. Daily Med. Flowtuss (hydrocodone bitartrate and guaifenesin) package insert. San Antonio, TX: Mission Pharmacal. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1d0c2c03-ca11-30b1-e054-00144ff8d46c. Accessed May 12, 2016.
- 33.US National Library of Medicine. Daily Med. Tuzistra XR (codeine polistirex and chlorpheniramine polistirex) package insert. Berwyn, PA; Vernalis Therapeutics, Inc. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aebefc5e-5487-42ef-98f7-01d26eb38362. Accessed May 12, 2016.
- 34.US National Library of Medicine. Daily Med. Hycofenix (hydrocodone bitartrate, pseudoephedrine, and guaifenesin) package insert. San Antonio, TX: Mission Pharmacal. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1cfa7c8f-0309-3591-e054-00144ff8d46c. Accessed May 12, 2016.
- 35.US Food and Drug Administration. Most young children with a cough or cold don't need medicines. FDA consumer update. January 17, 2008. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm048682.htm. Accessed May 12, 2016.
- 36.Ostroff C., Lee C.E., McMeekin J. Unapproved prescription cough, cold, and allergy products. Recent US Food and Drug Administration regulatory action on unapproved cough, cold, and allergy medications. Chest. 2011;140:295–300. doi: 10.1378/chest.11-0981. [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration. Codeine cough-and-cold medicines in children: drug safety communication - FDA evaluating potential risk of serious side effects, July 7, 2015. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm453379.htm. Accessed May 12, 2016.
- 38.Opioid pain or cough medicines combined with benzodiazepine: drug safety communication—FDA requiring boxed warning about serious risks and death, August 31, 2016. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm518710.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery. Accessed August 31, 2016.
- 39.Linn K.A., Long M.T., Pagel P.S. “Robo-tripping”: dextromethorphan abuse and its anesthetic implications. Anesth Pain Med. 2014;4(5):e20990. doi: 10.5812/aapm.20990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burns J.M., Boyer E.W. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75–82. doi: 10.2147/SAR.S36761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albrecht H.H. Can big data analysis help speed up the clinical development of mucoactive drugs for symptomatic RTIs? Lung. 2016;194:31–34. doi: 10.1007/s00408-016-9846-7. [DOI] [PubMed] [Google Scholar]
- 42.Eccles R., Turner R.B., Dicpinigaitis P.V. Treatment of acute cough due to the common cold: multi-component, multi-symptom therapy is preferable to single-component, single-symptom therapy—a pro/con debate. Lung. 2016;194:15–20. doi: 10.1007/s00408-015-9808-5. [DOI] [PubMed] [Google Scholar]