Abstract
Neuromuscular disorders are increasingly recognized as a cause of both short- and long-term physical morbidity in survivors of critical illness. This recognition has given rise to research aimed at better understanding the risk factors and mechanisms associated with neuromuscular dysfunction and physical impairment associated with critical illness, as well as possible interventions to prevent or treat these issues. Among potential risk factors, bed rest is an important modifiable risk factor. Early mobilization and rehabilitation of patients who are critically ill may help prevent or mitigate the sequelae of bed rest and improve patient outcomes. Research studies and quality improvement projects have demonstrated that early mobilization and rehabilitation are safe and feasible in patients who are critically ill, with potential benefits including improved physical functioning and decreased duration of mechanical ventilation, intensive care, and hospital stay. Despite these findings, early mobilization and rehabilitation are still uncommon in routine clinical practice, with many perceived barriers. This review summarizes potential risk factors for neuromuscular dysfunction and physical impairment associated with critical illness, highlights the potential role of early mobilization and rehabilitation in improving patient outcomes, and discusses some of the commonly perceived barriers to early mobilization and strategies for overcoming them.
Key Words: early mobilization, ICU, physical therapy, rehabilitation
Abbreviations: CIM, critical illness myopathy; CIP, critical illness polyneuropathy; CRRT, continuous renal replacement therapy; ETT, endotracheal tube; ICUAW, ICU-acquired weakness; IQR, interquartile range; LOS, length of stay; MICU, medical ICU; MMT, manual muscle testing; NMB, neuromuscular blocker; NMES, neuromuscular electrical stimulation; OT, occupational therapy; PT, physical therapy; QI, quality improvement; RCT, randomized controlled trial
Early mobilization and rehabilitation of patients who are critically ill have received growing attention because of increasingly recognized sequelae of neuromuscular dysfunction and physical impairment associated with critical illness. This topic will be discussed in the following sections: (1) acquired neuromuscular disorders in the ICU, (2) risk factors for neuromuscular disorders and functional impairment, (3) sequelae of neuromuscular disorders, (4) safety of early mobilization and rehabilitation, (5) benefits of early mobilization and rehabilitation, (6) commonly perceived barriers to early mobilization and rehabilitation, and (7) future directions.
Acquired Neuromuscular Disorders in the ICU
One of the earliest published reports on neuromuscular dysfunction in the ICU was a case series of five patients with flaccid limbs, loss of reflexes, and delayed weaning from mechanical ventilation.1 Electrophysiological testing revealed a primary axonal polyneuropathy, with sparing of the central nervous system. These findings led to the term critical illness polyneuropathy (CIP).2 CIP is characterized by reduced sensory nerve action potentials and compound muscle action potentials, with normal or mildly reduced nerve conduction velocity.3, 4 The complex underlying pathophysiology may include dysfunction of microvascular circulation of the endoneurium with increased permeability and extravasation of activated leukocytes.5
Critical illness myopathy (CIM) is another manifestation of neuromuscular dysfunction that includes thick filament (myosin) loss,6 as well as myofiber necrosis and atrophy.4, 7, 8 This loss of muscle mass may result from increased muscle proteolysis mediated by enhanced ubiquitin-proteasome system activation, combined with decreased muscle protein synthesis.4 Importantly, despite their distinct definitions, CIP and CIM often coexist.9
ICU-acquired weakness (ICUAW) refers to clinically detectable muscle weakness in patients who are critically ill, in the absence of weakness caused by factors other than critical illness.8 Approximately one-third of patients who are critically ill have ICUAW.10 Possible etiologies may include muscle atrophy or wasting, CIP, CIM, or a combination of these factors.11 ICUAW is diagnosed by means of manual muscle testing (MMT), whereby six different muscle groups are assessed bilaterally by using the six-point ordinal Medical Research Council scale,12 with a sum score ranging from 0 (no visible or palpable contraction in all 12 muscle groups) to 60 (normal strength), with ICUAW typically defined as a sum score < 48.3, 8 Although MMT is feasible and easily administered, it requires the patient to be awake, cooperative, and able to follow commands.13 In addition, being an ordinal scale, this measure is limited in its ability to detect clinically meaningful differences in strength.11
Other tests that might play a role in diagnosing muscle weakness include handgrip dynamometry and handheld dynamometry. In a prospective cohort study of 136 patients receiving mechanical ventilation, handgrip strength showed good correlation with MMT and an independent association with in-hospital mortality.14 Handheld dynamometry is a small force measurement device that fits into the palm of the hand of the examiner and is used as part of a standardized physical examination to measure volitional strength on a continuous scale.15, 16 However, it generally requires the tested muscle to move against gravity (minimum score of 3 on MMT) and can be fatiguing when performed in patients who are weak, which can potentially limit feasibility in patients who are critically ill.15
Risk Factors for Neuromuscular Disorders and Functional Impairment
We will briefly summarize available evidence on the following commonly cited risk factors for neuromuscular disorders and functional impairment: bed rest, corticosteroids, and neuromuscular blockers (NMBs).
Bed Rest
Young healthy adults subjected to bed rest can have substantial loss of muscle mass and strength within a short time.17, 18 In patients who are critically ill, bed rest also has a strong association with muscle weakness. A prospective study longitudinally followed 222 survivors of ARDS for 2 years.19 After adjusting for 18 variables, including baseline characteristics and potential risk factors for muscle weakness, the duration of bed rest in the ICU was the only risk factor with a strong, independent, and consistent association with muscle weakness across the entire follow-up. Over 3-, 6-, 12- and 24-month follow-up assessments, there was a 3% to 11% relative decrease in muscle strength for each additional day of bed rest in the ICU.19 Another prospective study longitudinally followed 203 survivors of ARDS from 12 hospitals in the ARDS Network for 12 months after discharge.20 After adjusting for age, sex, comorbidities, and baseline functional status, ICU length of stay (LOS) had a significant and independent association with decreased muscle strength. Importantly, ICU LOS approximated the duration of bed rest because 75% of patients did not routinely receive early mobilization in their ICUs.20
Bed rest is very common in routine clinical practice in the ICU setting. Across point prevalence studies from the United States, Germany, Australia, and New Zealand, the vast majority of patients receiving mechanical ventilation did not receive any form of out-of-bed mobilization.21, 22, 23
Corticosteroids
There are mixed results in studies evaluating systemic corticosteroids and physical sequelae in patients who are critically ill. For instance, in a study of 95 patients receiving mechanical ventilation, administration of corticosteroids before awakening was a strong independent predictor of ICUAW at awakening (OR, 14.9; 95% CI, 3.2-69.8; P < .001); however, there was no association with dose or duration of corticosteroids.3 A prospective study following 203 survivors of ARDS for 1 year reported a significant association between higher mean corticosteroid dosage (up to 40 mg/d of prednisone equivalent) in the ICU and decreased percentage in predicted 6-minute walk distance (P = .032) and ShortForm-36 Health Survey24 physical function domain (P = .005) scores, but not muscle strength (measured via MMT), in a multivariable regression model that adjusted for baseline characteristics, comorbidities, and premorbid functional status.20 In contrast, the previously mentioned 2-year follow-up study of 222 survivors of ARDS found no association between cumulative dose of corticosteroids in the ICU and weakness or any other physical outcome measure.19
Neuromuscular Blockers
Mixed results also have been reported in studies evaluating NMBs. For instance, in a subanalysis of 1,200 patients who were critically ill who were enrolled in a randomized controlled trial (RCT) evaluating intensive insulin therapy, infusion of NMBs was an independent risk factor for the presence of abundant spontaneous activity at electromyography (OR, 2.01; 95% CI, 1.1-3.99; P = .02).25 However, a multicenter RCT randomly assigned 340 patients with severe ARDS to receive either cisatracurium infusion for 48 hours or placebo, starting within 48 hours of ARDS onset. No significant difference was found between the two groups in the median Medical Research Council score or the prevalence of ICUAW at ICU discharge or day 28.26 Moreover, there was no association of NMB use (ever vs never) with muscle weakness in two observational longitudinal follow-up studies of survivors of ARDS described earlier.19, 20 However, only a minority of patients in these observational studies received NMBs.
Sequelae of Neuromuscular Disorders
ICU-associated neuromuscular disorders have short- and long-term sequelae. Several studies reported an association between ICUAW and delayed weaning from mechanical ventilation, increased in-hospital costs, and increased mortality.14, 27, 28, 29 In addition, patients with ICUAW may have a significantly increased risk of death after discharge29, 30, 31 and significant impairments in respiratory muscle strength, physical function, and health-related quality of life that persist for at least 2 years.19 Persistent physical impairment was also reported in other longitudinal studies that followed survivors of ARDS up to 5 years after discharge.32, 33 As discussed in the subsequent sections, increasing recognition of these sequelae and their long-lasting effects has intensified interest in early mobilization and rehabilitation as a potential intervention to improve patient outcomes.
Safety of Early Mobilization and Rehabilitation
Patients receiving mechanical ventilation are often considered to have a vulnerable hemodynamic and respiratory status, among other perceived barriers, that may interfere with mobilization. However, the reported rate of adverse events is very low, mostly in the form of transient physiological derangements that resolve without the need for any additional therapy.34, 35, 36 There is expert consensus that early mobilization and rehabilitation are safe and do not expose patients receiving mechanical ventilation to any significant additional risk, as long as they are closely monitored during and after mobilization.37, 38, 39
Benefits of Early Mobilization and Rehabilitation
There is variability in the results of clinical trials evaluating early mobilization and rehabilitation, with some studies demonstrating substantial benefits and others showing no significant benefit (Table 1).40, 41, 42, 43, 44, 45 For instance, a quasi-RCT showed that early mobilization, initiated within 48 hours of intubation, resulted in a significantly reduced number of days to first mobilization out of bed and reduced ICU and hospital LOS.40 Similarly, in a two-center RCT, starting physical therapy (PT) and occupational therapy (OT) early (at a median of 1.5 days after intubation) resulted in a shorter duration of delirium and mechanical ventilation, as well as a significant increase in functional independence at hospital discharge.41 In addition, as discussed later, trials evaluating the use of rehabilitation devices, such as cycle ergometry and neuromuscular electrical stimulation (NMES), in the ICU setting have demonstrated some evidence of benefit (Table 1).40, 41, 42, 43, 44, 45
Table 1.
Summary of Selected Controlled Clinical Trials of Rehabilitation Interventions in the ICU
| Trials With Positive Outcomes | ||||||
|---|---|---|---|---|---|---|
| Study/Year | Type | No. of patients | Intervention | Control | Key Outcomes | Results (Intervention vs Control) |
| Morris et al40/2008 | Quasi-randomized controlled trial single-center MICU | 330 | A mobility protocol carried out by a dedicated mobility team (ICU nurse, nursing assistant, physical therapist) | PT directed by the primary team |
|
|
| Schweickert et al41/2009 | RCT, MICUs in 2 hospitals | 104 | Early PT and OT intervention starting within 72 h of MV | PT and OT directed by the primary team |
|
|
| Burtin et al42/2009 | RCT, medical and surgical ICUs at 1 hospital | 90 | In-bed cycle ergometry starting at average 14 d from admission | Standard mobilization 5 d/wk |
|
|
| Routsi et al43/2010 | RCT, single-center mixed ICU | 140 | Daily NMES | No NMES |
|
|
| Trials With Negative Outcomes | ||||||
|---|---|---|---|---|---|---|
| Denehy et al44/2013 | RCT, single-center mixed ICU | 150 | Physiology-based mobilization 15 min/d while receiving MV, then 2 × 15 min/d after weaning | PT available 12 h/d, 7 d/wk |
|
|
| Moss et al45/2015 | RCT, MICUs and SICUs in 5 hospitals | 120 | Intensive PT intervention 7 d/wk in ICU and ward, with mean duration of 31 min | ROM, positioning, mobility 3 d/wk in ICU and ward, with mean duration of 21 min |
|
|
ICUAW = ICU-acquired weakness; LOS = length of stay; MICU = medical ICU; MV = mechanical ventilation; NMES = neuromuscular electrical stimulation; NS = not significant; OT = occupational therapy; PFP-10 = Physical Functional Performance 10-item test; PT = physical therapy; RCT = randomized controlled trial; ROM = range of motion; SF-36 = ShortForm-36 Health Survey; SICU = surgical ICU; 6MWD = 6-minute walk distance.
Difference in rate of change in 6MWD between intervention and control groups over 12 months of follow-up.
However, in a single-center RCT in patients in the ICU for ≥ 5 days, intensive exercise in the ICU (started at a median [interquartile range (IQR)] of 7 [5-11] days from admission),46 along with more intensive exercise on the hospital ward and in the outpatient setting, did not improve patient outcomes compared with usual care over a 1-year follow-up.44 Moreover, a five-center RCT, starting an intensive PT program at a median (IQR) of 8 (6-11) days after intubation, also did not improve physical functioning during 1-, 3-, and 6-month follow-up.45 In contrast to the trials in the prior paragraph showing positive outcomes, in both of these trials initiation of the rehabilitation intervention was relatively delayed, with both starting the intervention at the same time as the control group in a prior positive trial.41 In addition, the intensity of PT intervention in the control group in both trials was greater than in the control groups of the positive controlled trials and the usual care practices in many ICUs on the basis of point prevalence studies (Table 2).21, 22, 23, 30, 40, 41, 44, 45 Standardized reporting of usual care in future clinical trials will play an important role in improving comparisons between studies.47
Table 2.
Mobilization of Patients Receiving Mechanical Ventilation Among Selected Observational Studies and Control Groups of Clinical Trials
| Observational Studies of Usual Practice | |||
|---|---|---|---|
| Study/Country/Year | Study Type and No. of Sites | Patients | Mobility Status Achieved and Rehabilitation Delivered |
| Berney et al21/Australia/New Zealand/2013 | 1-d point prevalence study in 38 ICUs in 38 hospitals | N = 200 in ICU for > 2 d |
|
| Nydahl et al,22/Germany/2014 | 1-d point prevalence study in 116 ICUs in 115 hospitals | N = 401 with ETT |
|
| Jolley et al23/United States/2015 | 2-d point prevalence study in 33 ICUs in 17 hospitals | N = 566 (77% with ETT) |
|
| Hodgson et al30/Australia/New Zealand/2015 | Prospective cohort (with up to 14 d of evaluation) in 12 ICUs in 12 hospitals | N = 192 with expected MV > 2 d |
|
| Control Groups From Clinical Trials With Positive Results | |||
|---|---|---|---|
| Morris et al40/United States/2008 | Quasi-RCT: usual care in 7 ICUs in 1 hospital | N = 135 in MICU, enrolled within 2 d of MV |
|
| Schweickert et al41/United States/2009 | RCT: usual care in 2 MICUs in 2 hospitals | N = 55 with ETT, enrolled within 3 d of MV |
|
| Control Groups From Clinical Trials With Negative Results | |||
|---|---|---|---|
| Denehy et al44/Australia/2013 | RCT: usual care in single ICU | N = 76 in ICU for > 5 d (∼92% MV) |
|
| Moss et al45/United States/2015 | RCT: protocolized PT in 5 hospitals | N = 61 enrolled after MV for ≥ 4-5 d |
|
ETT = endotracheal tube; IQR = interquartile range. See Table 1 legend for expansion of other abbreviations.
As reported in the trial, these data are based on a point prevalence evaluation that included 68 patients receiving MV at the study site who were not enrolled in the trial and received usual care.
Multiple quality improvement (QI) projects worldwide have successfully implemented early mobilization and rehabilitation in ICU settings (Table 3).48, 49, 50, 51, 52, 53 Overall, these projects have demonstrated the safety and feasibility of early mobilization and rehabilitation, as well as multiple potential benefits, such as decreasing ICU and hospital LOS.54
Table 3.
Summary of Selected QI Projects of Early Mobilization and Rehabilitation Interventions in the ICU
| QI Project Location/Year | ICU | Patient Population and Time Frame | Selected Aspects of the QI Intervention | Key Outcomes (Before vs After QI) |
|---|---|---|---|---|
| Johns Hopkins Hospital48/2010 | 16-bed MICU | N = 576, QI executed over 4 mo |
|
|
| University of California San Francisco51/2013 | 16-bed mixed ICU | N = 373, QI executed over 9 mo |
|
|
| University of Alabama at Birmingham52/2013 | 28-bed trauma and burns ICU | N = 2,176, QI executed over 12 mo |
|
|
| University Hospitals Birmingham, England53/2015 | 75-bed mixed ICU | N = 582, MV ≥ 5 d, QI executed over 12 months |
|
PROM = passive range of motion; QI = quality improvement; RN = registered nurse; See Table 1 legend for expansion of other abbreviations.
The 4Es model is part of a structured quality improvement model that consists of engaging and educating stakeholders followed by executing the intervention and then evaluating results in an iterative manner
Commonly Perceived Barriers to Early Mobilization and Rehabilitation
We will discuss some commonly perceived barriers to early mobilization and rehabilitation, as well as potential strategies to overcome them.55
Cultural Barriers
In many centers, current ICU culture is an important and potentially modifiable barrier to early mobilization and rehabilitation. Insufficient coordination, timing conflicts with different procedures, and competing patient priorities are common.21, 46, 56, 57 Overcoming these barriers requires a structured multidisciplinary effort, with clear communication and recognition of the importance of early mobilization and rehabilitation.58 Provision of critical care in an ICU with a culture that prioritizes early rehabilitation increases the number of patients receiving mechanical ventilation ambulating by threefold.59, 60
A structured QI model in the Johns Hopkins Hospital medical ICU (MICU) was used to change the culture and implement early rehabilitation for all patients.48 Key components of this project included creating PT and OT consultation guidelines, as well as changing patient activity and sedation practices (Table 3).48, 49, 50, 51, 52, 53 Ongoing implementation and evaluation of the project was performed through weekly meetings with all members of the multidisciplinary team.48, 49 Through this process, there was a large decrease in the proportion of ICU days in which eligible patients did not receive PT and OT interventions (41% vs 7%; P = .004),48 with improvement in the timing of early PT interventions sustained for at least 5 years after completion of the QI project.61 Qualitative research conducted with the staff in the MICU revealed that this QI project led to early mobilization and rehabilitation being perceived as common sense and was associated with increased staff satisfaction.62
Sedation
The widespread use of sedation in the ICU can be a major barrier to mobilizing patients who are critically ill.23, 30, 63, 64 Sedation minimization can be combined with early mobility via implementing the ABCDE bundle,65 in which all patients undergo daily coordinated spontaneous awakening trials, spontaneous breathing trials, sedation and delirium screening, and early mobility and rehabilitation.66, 67, 68 A pre-post prospective study in 296 patients showed that applying this bundle was feasible and improved patient outcomes, such as reducing delirium and increasing mobilization out of bed.66
Endotracheal Tubes
The presence of an endotracheal tube (ETT) is another commonly perceived barrier.30 To assist with overcoming this barrier, one study detailed the steps undertaken to start PT and OT interventions after a median of only 1.5 days of intubation in 49 patients who had undergone endotracheal intubatation.69 Patients underwent daily screening for 10 contraindications to PT and OT interventions, followed by daily interruption of sedation until they achieved wakefulness, with PT and OT interventions initiated thereafter. Initiation of PT and OT interventions was preceded by securing the ETT, removing unnecessary noninvasive devices, and disconnecting enteral feedings tubes. Rehabilitation interventions were completed on 90% of patient days. One-third of sessions involved patients who had undergone intubation moving from bed to chair and standing, and 15% involved patients who had undergone intubation ambulating. Therapy was stopped prematurely in only 4% of all sessions, mostly because of ventilator dyssynchrony.69 Figure 1 illustrates a patient receiving mechanical ventilation via an ETT and ambulating with a physical therapist in the ICU.
Figure 1.
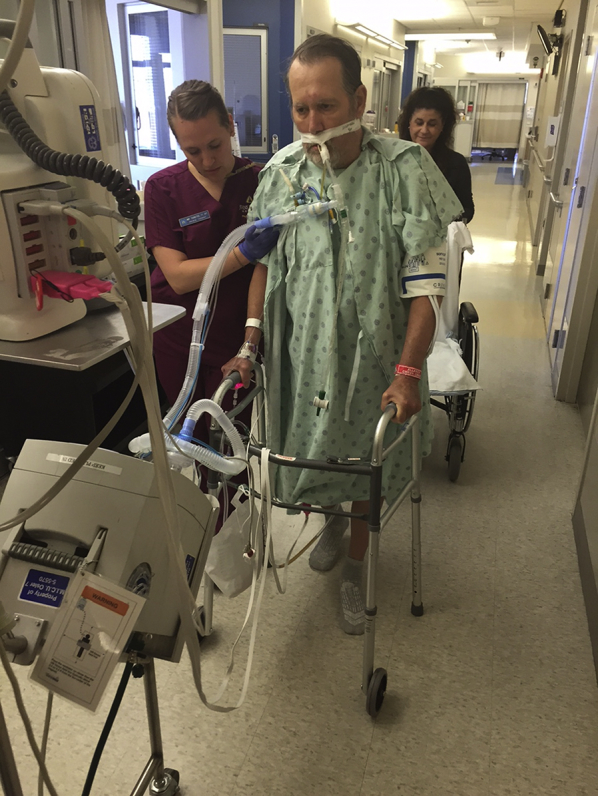
Photo showing a patient receiving mechanical ventilation via an endotracheal tube while ambulating in the ICU with a physical therapist. (The patient provided written consent for the use of this photograph.)
Femoral Catheters
Femoral catheters are a perceived barrier to early rehabilitation because of the risk of accidental removal, bleeding, or infection.30 However, investigators in one study observed 101 patients in the MICU with a femoral catheter (81% venous, 29% arterial, and 6% hemodialysis) who had 253 PT sessions over 210 ICU days. Patients were able to perform in-bed exercises, supine cycle ergometry, and stand or walk on 38%, 12%, and 23% of days, respectively. There were no catheter-related adverse events.70 Similar results were obtained in other studies.71, 72
Continuous Renal Replacement Therapy
The use of continuous renal replacement therapy (CRRT) is another perceived barrier.21 However, a prospective single center study including 57 consecutive patients (79% receiving mechanical ventilation) who received at least one PT session while undergoing CRRT demonstrated no safety events associated with CRRT.73 A total of 268 PT sessions included in-bed exercises (29%), supine cycle ergometry (27%), sitting on the edge of the bed (30%), transferring to a chair (5%), and standing or marching in place (9%). Similar results were obtained in another prospective study that also demonstrated potential improved CRRT filter life with patient mobilization.74
Costs
Increased staffing and costs associated with early rehabilitation are commonly perceived barriers. A controlled trial using a dedicated mobility team did not result in increased overall costs after accounting for extra costs related to the mobility team,40 with evidence of significantly decreased risk of readmission or death in the year after ICU dishcarge.75 Similarly, a QI project demonstrated a significant decrease in average MICU and hospital LOS.48 Using data from the QI project and other publications, a financial model for introducing ICU early mobilization and rehabilitation had 24 possible scenarios (ranging from conservative to best-case scenarios), 83% of which had net savings.76
Future Directions
Novel use of existing equipment and devices may assist with early rehabilitation in the ICU setting. For instance, NMES uses skin electrodes to deliver electrical stimuli to arm and leg muscles,77 with the goal of producing visible contraction.78 Studies using NMES in patients in the ICU have consistently reported that it is safe and feasible and may have potential benefit in improving muscle mass, strength, and function.78, 79, 80, 81 On the basis of evaluation in healthy subjects, the beneficial effects of NMES may be mediated, in part, through changes in cytokines, similar to changes observed after active exercise.82
In-bed cycle ergometry also has been successfully used with patients who are critically ill. An RCT assigned 90 patients who were critically ill to receive either usual care or bedside cycling starting at ≥ 5 days after admission.42 Patients in the intervention group had significantly better outcomes at hospital discharge (Table 1).40, 41, 42, 43, 44, 45 Exercise was terminated early in < 4% of sessions because of transient physiological derangements that resolved within 2 minutes of exercise cessation.42 A prospective cohort study in 181 patients (82% receiving mechanical ventilation) receiving 541 cycle ergometry sessions reported a 0.2% rate of potential safety events or physiological changes.83
Combining NMES with bedside cycling may have the advantage of engaging patients in active cycling at an early stage of critical illness. This technique, called functional electrical stimulation cycling, was evaluated in a small pilot study of eight patients receiving mechanical ventilation,84 with a larger multicenter RCT currently being conducted to assess the efficacy of functional electrical stimulation cycling in improving short- and long-term physical and cognitive outcomes (ClinicalTrials.gov identifier NCT02214823). Single center reports also have highlighted interactive video games and hydrotherapy (ie, rehabilitation in a swimming pool) as innovative ideas for ICU rehabilitation.85, 86
Conclusions
ICU-associated neuromuscular disorders are increasingly being recognized as contributing to short- and long-term physical impairments. Bed rest has a strong association with neuromuscular dysfunction and physical impairments, and early mobilization and rehabilitation may be valuable interventions to mitigate these sequelae. Despite the many perceived barriers, mobilization and rehabilitation, when started shortly after mechanical ventilation, are safe, feasible, and potentially beneficial in improving patients’ functional outcomes and decreasing mechanical ventilation duration and LOS.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
References
- 1.Bolton C.F., Gilbert J.J., Hahn A.F., Sibbald W.J. Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry. 1984;47(11):1223–1231. doi: 10.1136/jnnp.47.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zochodne D.W., Bolton C.F., Wells G.A. Critical illness polyneuropathy: a complication of sepsis and multiple organ failure. Brain J Neurol. 1987;110(pt 4):819–841. doi: 10.1093/brain/110.4.819. [DOI] [PubMed] [Google Scholar]
- 3.De Jonghe B., Sharshar T., Lefaucheur J.P. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 4.Batt J., dos Santos C.C., Cameron J.I., Herridge M.S. Intensive care unit-acquired weakness: clinical phenotypes and molecular mechanisms. Am J Respir Crit Care Med. 2013;187(3):238–246. doi: 10.1164/rccm.201205-0954SO. [DOI] [PubMed] [Google Scholar]
- 5.Fenzi F., Latronico N., Refatti N., Rizzuto N. Enhanced expression of E-selectin on the vascular endothelium of peripheral nerve in critically ill patients with neuromuscular disorders. Acta Neuropathol (Berl) 2003;106(1):75–82. doi: 10.1007/s00401-003-0704-3. [DOI] [PubMed] [Google Scholar]
- 6.Danon M.J., Carpenter S. Myopathy with thick filament (myosin) loss following prolonged paralysis with vecuronium during steroid treatment. Muscle Nerve. 1991;14(11):1131–1139. doi: 10.1002/mus.880141115. [DOI] [PubMed] [Google Scholar]
- 7.Puthucheary Z.A., Rawal J., McPhail M. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 8.Stevens R.D., Marshall S.A., Cornblath D.R. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37(10 suppl):S299–S308. doi: 10.1097/CCM.0b013e3181b6ef67. [DOI] [PubMed] [Google Scholar]
- 9.Latronico N., Fenzi F., Recupero D. Critical illness myopathy and neuropathy. Lancet. 1996;347(9015):1579–1582. doi: 10.1016/s0140-6736(96)91074-0. [DOI] [PubMed] [Google Scholar]
- 10.Appleton R.T., Kinsella J., Quasim T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care Soc. 2015;16(2):126–136. doi: 10.1177/1751143714563016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kress J.P., Hall J.B. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 12.Kleyweg R.P., van der Meché F.G., Schmitz P.I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 13.Hermans G., De Jonghe B., Bruyninckx F., Van den Berghe G. Clinical review: critical illness polyneuropathy and myopathy. Crit Care. 2008;12(6):238. doi: 10.1186/cc7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali N.A., O’Brien J.M., Hoffmann S.P. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178(3):261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 15.Vanpee G., Hermans G., Segers J., Gosselink R. Assessment of limb muscle strength in critically ill patients: a systematic review. Crit Care Med. 2014;42(3):701–711. doi: 10.1097/CCM.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin C.E., Paratz J.D., Bersten A.D. Muscle strength assessment in critically ill patients with handheld dynamometry: an investigation of reliability, minimal detectable change, and time to peak force generation. J Crit Care. 2013;28(1):77–86. doi: 10.1016/j.jcrc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Jones S.W., Hill R.J., Krasney P.A., O’Conner B., Peirce N., Greenhaff P.L. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18(9):1025–1027. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- 18.Suetta C., Hvid L.G., Justesen L. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 1985. 2009;107(4):1172–1180. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 19.Fan E., Dowdy D.W., Colantuoni E. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Needham D.M., Wozniak A.W., Hough C.L. Risk factors for physical impairment after acute lung injury in a national, multicenter study. Am J Respir Crit Care Med. 2014;189(10):1214–1224. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berney S.C., Harrold M., Webb S.A. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15(4):260–265. [PubMed] [Google Scholar]
- 22.Nydahl P., Ruhl A.P., Bartoszek G. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42(5):1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 23.Jolley SE, Moss M, Needham DM, et al. Point prevalence study of intensive care unit mobility across the acute respiratory distress syndrome network [abstract]. In: Moving the Needle on ICU-associated Neuromuscular Weakness. American Thoracic Society; Am J Respir Crit Care Med. 2015. A6349.
- 24.Ware J.E., Jr., Sherbourne C.D. The M.O.S. 36-item short-form health survey (SF-36). I Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 25.Hermans G., Wilmer A., Meersseman W. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175(5):480–489. doi: 10.1164/rccm.200605-665OC. [DOI] [PubMed] [Google Scholar]
- 26.Papazian L., Forel J.M., Gacouin A. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 27.De Jonghe B., Bastuji-Garin S., Sharshar T., Outin H., Brochard L. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30(6):1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 28.De Jonghe B., Bastuji-Garin S., Durand M.C. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35(9):2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 29.Hermans G., Van Mechelen H., Clerckx B. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 30.TEAM Study Investigators. Hodgson C., Bellomo R. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care. 2015;19:81. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieske L., Dettling-Ihnenfeldt D.S., Verhamme C. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. doi: 10.1186/s13054-015-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herridge M.S., Cheung A.M., Tansey C.M. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 33.Herridge M.S., Tansey C.M., Matté A. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 34.Sricharoenchai T., Parker A.M., Zanni J.M., Nelliot A., Dinglas V.D., Needham D.M. Safety of physical therapy interventions in critically ill patients: a single-center prospective evaluation of 1110 intensive care unit admissions. J Crit Care. 2014;29(3):395–400. doi: 10.1016/j.jcrc.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Zafiropoulos B., Alison J.A., McCarren B. Physiological responses to the early mobilisation of the intubated, ventilated abdominal surgery patient. Aust J Physiother. 2004;50(2):95–100. doi: 10.1016/s0004-9514(14)60101-x. [DOI] [PubMed] [Google Scholar]
- 36.Zanni J.M., Korupolu R., Fan E. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25(2):254–262. doi: 10.1016/j.jcrc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Sommers J., Engelbert R.H., Dettling-Ihnenfeldt D. Physiotherapy in the intensive care unit: an evidence-based, expert driven, practical statement and rehabilitation recommendations. Clin Rehabil. 2015;29(11):1051–1063. doi: 10.1177/0269215514567156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodgson C.L., Stiller K., Needham D.M. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 2014;18(6):658. doi: 10.1186/s13054-014-0658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanekom S., Gosselink R., Dean E. The development of a clinical management algorithm for early physical activity and mobilization of critically ill patients: synthesis of evidence and expert opinion and its translation into practice. Clin Rehabil. 2011;25(9):771–787. doi: 10.1177/0269215510397677. [DOI] [PubMed] [Google Scholar]
- 40.Morris P.E., Goad A., Thompson C. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 41.Schweickert W.D., Pohlman M.C., Pohlman A.S. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burtin C., Clerckx B., Robbeets C. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 43.Routsi C., Gerovasili V., Vasileiadis I. Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care. 2010;14(2):R74. doi: 10.1186/cc8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denehy L., Skinner E.H., Edbrooke L. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17(4):R156. doi: 10.1186/cc12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moss M, Nordon-Craft A, Malone D, et al. A randomized trial of an intensive physical therapy program for acute respiratory failure patients. Am J Respir Crit Care Med. In press. [DOI] [PMC free article] [PubMed]
- 46.Berney S., Haines K., Skinner E.H., Denehy L. Safety and feasibility of an exercise prescription approach to rehabilitation across the continuum of care for survivors of critical illness. Phys Ther. 2012;92(12):1524–1535. doi: 10.2522/ptj.20110406. [DOI] [PubMed] [Google Scholar]
- 47.Parker A., Tehranchi K.M., Needham D.M. Critical care rehabilitation trials: the importance of “usual care.”. Crit Care. 2013;17(5):183. doi: 10.1186/cc12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Needham D.M., Korupolu R., Zanni J.M. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Needham D.M., Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17(4):271–281. doi: 10.1310/tsr1704-271. [DOI] [PubMed] [Google Scholar]
- 50.Pronovost P.J., Berenholtz S.M., Needham D.M. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337:a1714. doi: 10.1136/bmj.a1714. [DOI] [PubMed] [Google Scholar]
- 51.Engel H.J., Tatebe S., Alonzo P.B., Mustille R.L., Rivera M.J. Physical therapist-established intensive care unit early mobilization program: quality improvement project for critical care at the University of California San Francisco Medical Center. Phys Ther. 2013;93(7):975–985. doi: 10.2522/ptj.20110420. [DOI] [PubMed] [Google Scholar]
- 52.Clark D.E., Lowman J.D., Griffin R.L., Matthews H.M., Reiff D.A. Effectiveness of an early mobilization protocol in a trauma and burns intensive care unit: a retrospective cohort study. Phys Ther. 2013;93(2):186–196. doi: 10.2522/ptj.20110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McWilliams D., Weblin J., Atkins G. Enhancing rehabilitation of mechanically ventilated patients in the intensive care unit: a quality improvement project. J Crit Care. 2015;30(1):13–18. doi: 10.1016/j.jcrc.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Engel H.J., Needham D.M., Morris P.E., Gropper M.A. ICU early mobilization: from recommendation to implementation at three medical centers. Crit Care Med. 2013;41(9 suppl 1):S69–S80. doi: 10.1097/CCM.0b013e3182a240d5. [DOI] [PubMed] [Google Scholar]
- 55.Dubb R, Nydahl P, Hermes C, et al. Barriers and strategies for early mobilization in the ICU: a narrative review. Ann Am Thorac Soc. In press. [DOI] [PubMed]
- 56.Bakhru R.N., Wiebe D.J., McWilliams D.J., Spuhler V.J., Schweickert W.D. An environmental scan for early mobilization practices in U.S. ICUs. Crit Care Med. 2015;43(11):2360–2369. doi: 10.1097/CCM.0000000000001262. [DOI] [PubMed] [Google Scholar]
- 57.Leditschke I.A., Green M., Irvine J., Bissett B., Mitchell I.A. What are the barriers to mobilizing intensive care patients? Cardiopulm Phys Ther J. 2012;23(1):26–29. [PMC free article] [PubMed] [Google Scholar]
- 58.Hopkins R.O., Spuhler V.J., Thomsen G.E. Transforming ICU culture to facilitate early mobility. Crit Care Clin. 2007;23(1):81–96. doi: 10.1016/j.ccc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Thomsen G.E., Snow G.L., Rodriguez L., Hopkins R.O. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med. 2008;36(4):1119–1124. doi: 10.1097/CCM.0b013e318168f986. [DOI] [PubMed] [Google Scholar]
- 60.Hopkins R.O., Spuhler V.J. Strategies for promoting early activity in critically ill mechanically ventilated patients. AACN Adv Crit Care. 2009;20(3):277–289. doi: 10.1097/NCI.0b013e3181acaef0. [DOI] [PubMed] [Google Scholar]
- 61.Dinglas V.D., Parker A.M., Reddy D.R.S. A quality improvement project sustainably decreased time to onset of active physical therapy intervention in patients with acute lung injury. Ann Am Thorac Soc. 2014;11(8):1230–1238. doi: 10.1513/AnnalsATS.201406-231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eakin M.N., Ugbah L., Arnautovic T., Parker A.M., Needham D.M. Implementing and sustaining an early rehabilitation program in a medical intensive care unit: a qualitative analysis. J Crit Care. 2015;30(4):698–704. doi: 10.1016/j.jcrc.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Mendez-Tellez P.A., Dinglas V.D., Colantuoni E. Factors associated with timing of initiation of physical therapy in patients with acute lung injury. J Crit Care. 2013;28(6):980–984. doi: 10.1016/j.jcrc.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dinglas V.D., Colantuoni E., Ciesla N., Mendez-Tellez P.A., Shanholtz C., Needham D.M. Occupational therapy for patients with acute lung injury: factors associated with time to first intervention in the intensive care unit. Am J Occup Ther. 2013;67(3):355–362. doi: 10.5014/ajot.2013.007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morandi A., Brummel N.E., Ely E.W. Sedation, delirium and mechanical ventilation: the “ABCDE” approach. Curr Opin Crit Care. 2011;17(1):43–49. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 66.Balas M.C., Vasilevskis E.E., Olsen K.M. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. 2014;42(5):1024–1036. doi: 10.1097/CCM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vasilevskis E.E., Ely E.W., Speroff T., Pun B.T., Boehm L., Dittus R.S. Reducing iatrogenic risks: ICU-acquired delirium and weakness—crossing the quality chasm. Chest. 2010;138(5):1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ICU Liberation website. http://www.iculiberation.org/Pages/default.aspx. Accessed April 1, 2016.
- 69.Pohlman M.C., Schweickert W.D., Pohlman A.S. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38(11):2089–2094. doi: 10.1097/CCM.0b013e3181f270c3. [DOI] [PubMed] [Google Scholar]
- 70.Damluji A., Zanni J.M., Mantheiy E., Colantuoni E., Kho M.E., Needham D.M. Safety and feasibility of femoral catheters during physical rehabilitation in the intensive care unit. J Crit Care. 2013;28(4):535.e9–535.e15. doi: 10.1016/j.jcrc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Perme C., Lettvin C., Throckmorton T.A., Mitchell K., Masud F. Early mobility and walking for patients with femoral arterial catheters in intensive care unit: a case series. J Acute Care Phys Ther. 2011;2(1):32–36. [Google Scholar]
- 72.Perme C., Nalty T., Winkelman C., Kenji Nawa R., Masud F. Safety and efficacy of mobility interventions in patients with femoral catheters in the ICU: a prospective observational study. Cardiopulm Phys Ther J. 2013;24(2):12–17. [PMC free article] [PubMed] [Google Scholar]
- 73.Toonstra AL, Zanni JM, Sperati C, et al. Feasibility and safety of physical therapy during continuous renal replacement therapy in the intensive care unit. Ann Am Thorac Soc. In press. [DOI] [PubMed]
- 74.Wang Y.T., Haines T.P., Ritchie P. Early mobilization on continuous renal replacement therapy is safe and may improve filter life. Crit Care. 2014;18(4):R161. doi: 10.1186/cc14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris P.E., Griffin L., Berry M. Receiving early mobility during an intensive care unit admission is a predictor of improved outcomes in acute respiratory failure. Am J Med Sci. 2011;341(5):373–377. doi: 10.1097/MAJ.0b013e31820ab4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lord R.K., Mayhew C.R., Korupolu R. ICU early physical rehabilitation programs: financial modeling of cost savings. Crit Care Med. 2013;41(3):717–724. doi: 10.1097/CCM.0b013e3182711de2. [DOI] [PubMed] [Google Scholar]
- 77.Needham D.M., Truong A.D., Fan E. Technology to enhance physical rehabilitation of critically ill patients. Crit Care Med. 2009;37(10 suppl):S436–S441. doi: 10.1097/CCM.0b013e3181b6fa29. [DOI] [PubMed] [Google Scholar]
- 78.Kho M.E., Truong A.D., Zanni J.M. Neuromuscular electrical stimulation in mechanically ventilated patients: a randomized, sham-controlled pilot trial with blinded outcome assessment. J Crit Care. 2015;30(1):32–39. doi: 10.1016/j.jcrc.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parry S.M., Berney S., Granger C.L., Koopman R., El-Ansary D., Denehy L. Electrical muscle stimulation in the intensive care setting: a systematic review. Crit Care Med. 2013;41(10):2406–2418. doi: 10.1097/CCM.0b013e3182923642. [DOI] [PubMed] [Google Scholar]
- 80.Wageck B., Nunes G.S., Silva F.L., Damasceno M.C.P., de Noronha M. Application and effects of neuromuscular electrical stimulation in critically ill patients: systematic review. Med Intensiva Soc Esp Med Intensiva Unidades Coronarias. 2014;38(7):444–454. doi: 10.1016/j.medin.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 81.Williams N., Flynn M. A review of the efficacy of neuromuscular electrical stimulation in critically ill patients. Physiother Theory Pract. 2014;30(1):6–11. doi: 10.3109/09593985.2013.811567. [DOI] [PubMed] [Google Scholar]
- 82.Truong AD, Kho ME, Brower RG, Feldman DR, Colantuoni E, Needham DM. Effects of neuromuscular electrical stimulation on cytokines in peripheral blood for healthy participants: a prospective, single-blinded study [published online ahead of print October 16, 2015]. Clin Physiol Funct Imaging.http://dx.doi.org/10.1111/cpf.12290. [DOI] [PubMed]
- 83.Kho M.E., Martin R.A., Toonstra A.L. Feasibility and safety of in-bed cycling for physical rehabilitation in the intensive care unit. J Crit Care. 2015;30(6):1419.e1–1419.e5. doi: 10.1016/j.jcrc.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 84.Parry S.M., Berney S., Warrillow S. Functional electrical stimulation with cycling in the critically ill: a pilot case-matched control study. J Crit Care. 2014;29(4):695.e1–695.e7. doi: 10.1016/j.jcrc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 85.Kho M.E., Damluji A., Zanni J.M., Needham D.M. Feasibility and observed safety of interactive video games for physical rehabilitation in the intensive care unit: a case series. J Crit Care. 2012;27(2):219.e1–219.e6. doi: 10.1016/j.jcrc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 86.Felten-Barentsz K.M., Haans A.J.C., Slutsky A.S., Heunks L.M.A., van der Hoeven J.G. Feasibility and safety of hydrotherapy in critically ill ventilated patients. Am J Respir Crit Care Med. 2015;191(4):476–477. doi: 10.1164/rccm.201408-1559LE. [DOI] [PubMed] [Google Scholar]


