Abstract
Background
Electromagnetic navigational bronchoscopy (ENB) is guided bronchoscopy to pulmonary nodules (PN) that relies on a preprocedural chest CT to create a three-dimensional (3D) virtual airway map. The CT is traditionally done at a full inspiratory breath hold (INSP), but the procedure is performed while the patient tidal breaths, when lung volumes are closer to functional residual capacity. Movement of a PN from INSP to expiration (EXP) has been shown to average 17.6 mm. Therefore, the hypothesis of this study is that preprocedural virtual maps built off a CT closer to physiological lung volumes during bronchoscopy may better represent the actual 3D location of a PN.
Methods
Consecutive patients with a PN needing a histological diagnosis were enrolled. A preprocedure INSP and EXP CT scan were obtained to create two virtual maps. During the airway inspection, the system tracked the sensor probe to collect 3D points that were reconstructed into the lumen registration map. This map is thought to best represent the patient’s airways during bronchoscopy. Predicted PN location on an EXP and INSP map was compared with lumen registration.
Results
Twenty consecutive PN underwent ENB. The predicted PN location, compared with lumen registration, was significantly closer on EXP vs INSP (4.5 mm ± 3.3 mm vs 14.8 mm ± 9.7 mm; p < 0.0001).
Conclusions
Predicted 3D nodule location using an EXP scan for ENB is significantly closer to actual nodule location when compared with an INSP scan, but whether this leads to increased yields needs to be determined.
Key Words: electromagnetic navigational bronchoscopy, lung biopsy, peripheral pulmonary nodule
Abbreviations: 3D, three-dimensional; ENB, electromagnetic navigational bronchoscopy; EXP, end-expiration breath hold; INSP, full-inspiration breath hold; PN, pulmonary nodule; R-EBUS, radial endobronchial ultrasound
Pulmonary nodules (PN) are common and diagnostic algorithms have become especially relevant in “high-risk” population since the United States Preventive Services Task Force gave annual lung cancer screening with a low-dose chest CT a Grade B recommendation for eligible patients based on the National Lung Screening Trial.1, 2 The American College of Chest Physicians’ lung cancer guidelines recommend biopsy in certain populations, such as those with moderate risk of malignancy, or high-risk patients who desire confirmation before surgery.3 There are two main nonsurgical options for tissue acquisition: CT-guided transthoracic needle aspiration and bronchoscopy. Bronchoscopy has the advantage of a substantially lower complication profile but at the expense of a lower yield.4, 5, 6, 7, 8
Electromagnetic navigational bronchoscopy (ENB) is an image-guided technique to aid bronchoscopists along the correct pathway to the PN by aligning a virtual three-dimensional (3D) image, created from a preprocedural chest CT, to the patient’s airways. A sensor probe, inserted through the bronchoscope, is continuously tracked by an electromagnetic field created around the patient, allowing analogous virtual and live endobronchial images to be displayed simultaneously. The bronchoscope is then navigated down a predetermined path to the PN, where biopsies are performed.9 Single-center trials have demonstrated yields of 50% to 85%, with a pooled diagnostic yield of 65%, which has led to guidelines giving this technology a Grade 1C recommendation as an initial diagnostic test.4, 10, 11, 12 More recently, data on ENB have been conflicting: a multicenter registry study suggests that the yield for ENB in routine clinical practice may be significantly lower than previously reported,5 whereas preliminary data from an ongoing multicenter trial are more promising.13
Three main factors contribute to inferior yields with ENB: user characteristics, nodule characteristics (size, relationship to airway),6, 14, 15 and system characteristics. The aim of this study is to investigate possible system factors. The CT is performed at a maximal inspiratory breath hold (INSP) so small airways leading to the PN are maximally visible, but the bronchoscopy is performed during tidal breathing, which may introduce conformational change of the airways, altering 3D nodule location. Presumptively, for electromagnetic navigation to be accurate, the physical shape of the virtual map must closely match the patient’s airways during bronchoscopy. Chen et al16 demonstrated that actual nodule location may differ from the anticipated location on an INSP map. They aligned paired virtual airway maps obtained at INSP and expiration (EXP) at functional residual capacity for patients undergoing ENB and found that the location of a PN differed by almost 2 cm, with lower lobe nodules moving twice as much as upper lobe nodules. Movement of upper lobe nodules was not inconsequential, however, averaging just over a centimeter. In this study, an EXP map was chosen as the comparator because it was believed to best represent the patient’s lung volumes during bronchoscopy, but further evidence is needed to validate this assumption.16
We hypothesize that a virtual airway map based upon a chest CT done at EXP is more representative of the patient’s actual airways and PN location than at INSP. We therefore undertook this study to compare PN location on two virtual airway maps (INSP and EXP) with a lumen registration generated airway map of patients undergoing ENB for PN.
Materials and Methods
Study Design
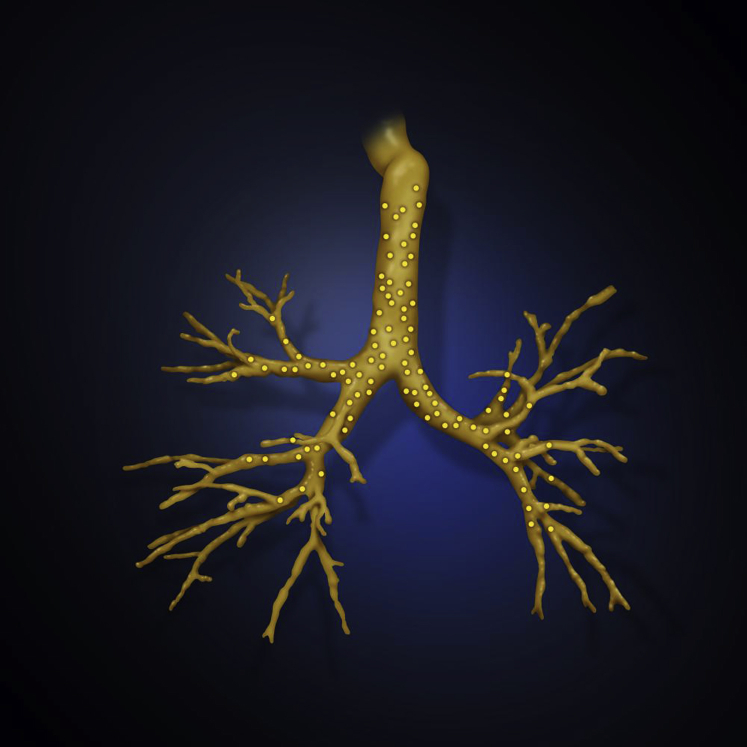
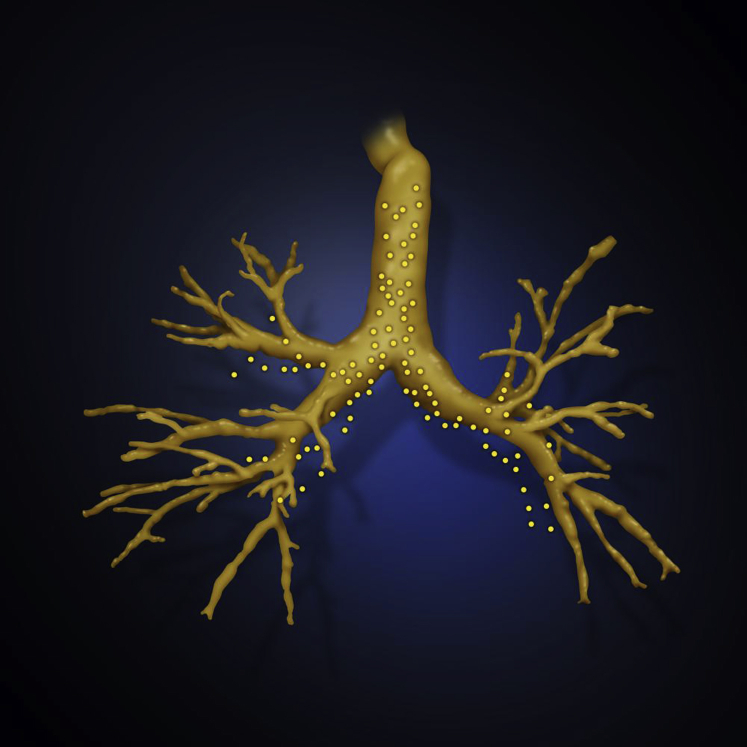
Approval for this study was granted by the Medical University of South Carolina institutional review board (Pro00035915). This was a prospective, single-center, nonrandomized, observational cohort study to compare the difference between predicted PN location on three airway maps at different phases of respiration: tidal breathing, maximal inspiratory breath hold (INSP) and expiratory breath hold (EXP) at functional residual capacity. A lumen registration airway map was generated via a virtual point collection, PointCloud, by tracking a sensor probe during the initial airway inspection on bronchoscopy. The other two maps were reconstructed from chest CT images at two different phases of static respiration, INSP and EXP. The lumen registration map is meant to closely represent the patient’s actual tracheobronchial tree as it is collected during tidal respiration, so it was used as the “gold standard” (Figs 1 and 2). See e-Appendix 1 for full details.
Figure 1.
Overlay of an expiratory three-dimensional airway map with the PointCloud (yellow dots) collected during bronchoscopy.
Figure 2.
Overlay of an inspiratory three-dimensional airway map with the PointCloud (yellow dots) collected during bronchoscopy.
The target enrollment was 20 PN. All participants provided written informed consent. Inclusion criteria consisted of a pulmonary nodule ≤3 cm in diameter, intermediate risk for malignancy, or high risk for malignancy but nonoperable candidates who needed a diagnosis for further treatment or refused surgery before biopsy confirmation of malignancy diagnosis. Patients were excluded if they were younger than 18 years of age, had a visible endobronchial nodule, uncorrectable bleeding disorder, unfit to undergo bronchoscopy, or unable to provide informed consent.
On the day of the bronchoscopy, patients underwent a chest CT while wearing registration pads placed across the anterior thorax (vPAD2, Veran Medical Technologies Inc.). Images were obtained by a multidetector CT scanner applying a standard spiral scanning protocol at 0.5-mm intervals with a 0.75-mm thickness. The chest CT images were obtained at INSP, and then EXP, from which virtual airway maps were constructed. The vPADs automatically registered the patient to the virtual airway map and tracked the patient’s respiratory cycle during bronchoscopy. They remained in the same position for the CT scan and the bronchoscopy with six consistent reference points on the body enabling automatic registration, while dynamic referencing maintained registration regardless of patient movement. Respiratory gating also occurs as the inspiration and expiration scan allow the patient's full respiratory cycle to be monitored during the procedure.
Each ENB was performed by one of two interventional pulmonologists, when the patient was under moderate sedation, with standard bronchoscopy monitoring protocols using the SPiN Thoracic Navigation system (Veran Medical Technologies Inc.). Once the initial airway inspection was satisfactorily completed, electromagnetic navigation to the PN was undertaken using EXP map. When the system indicated the sensor probe was at the PN, a virtual 3D point called “target-lock” was acquired, representing PN location on EXP.
The details of calculating respiratory motion of PN between INSP and EXP have previously been described.16 Briefly, the CT scans were overlaid on each other using the main carina as a common point of translation. The physical 3D motion (m) between INSP and EXP for the PN in the two respiratory sates was calculated as: (m) = , where x represents medial/lateral movement, y represents anterior/posterior movement, and z represents cranial/caudal movement. The same method was used to determine the difference between PN location in the lumen registered – EXP scan (using “target-lock”) and lumen registered – INSP scan. In addition, the difference between predicted ENB location and visualized PN location using a 20-MHz radial endobronchial ultrasound probe (R-EBUS; UM-S20-17S, Olympus) was determined by calculating the difference between catheter lengths of the sensor probe – R-EBUS to the nodule (e-Appendix 1).
Data Analysis and Statistical Methods
The main outcome, difference in predicted nodule location between a lumen registration airway map – INSP vs lumen registration – EXP was compared using a nonparametric Wilcoxon signed rank test. Statistical analysis was done with SAS v9.4 (SAS Institute). All reported confidence intervals are two-sided and a P value <.05 was chosen as significant. Continuous variables are expressed as mean ± SD. Secondary outcomes were the difference between ENB predicted nodule location on EXP scan and actual location (visualized by R-EBUS), difference in predicted nodule location between vPAD and lumen registration, procedure duration, and duration of PointCloud collection.
Results
Eighteen consecutive patients with 20 nodules meeting inclusion criteria were enrolled. The mean age was 70.8 ± 11.3 years; 55.6% were women. The mean nodule diameter on an axial view was 19.9 ± 5.8 mm (range, 7-29 mm), with a mean distance from the pleura (measured as the shortest distance either in the anterior, lateral, or posterior direction) of 25.5 ± 18.9 mm, and the majority of the nodules were located in the upper lobes (65%). The mean respiratory motion was 11.2 ± 7.1 mm (range, 0.3-24.5 mm) (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | No. |
|---|---|
| Patient demographics | 18 |
| Man | 8 |
| Woman | 10 |
| Age (y) | 70.8 (54-100) |
| Nodule characteristics | |
| Size (SD) | 19.9 mm (5.8 mm) |
| Distance from pleura (SD) | 25.5 mm (18.9 mm) |
| Nodule location | |
| Right upper lobe | 12 |
| Left upper lobe | 1 |
| Right lower/middle lobe | 4 |
| Left lower lobe | 3 |
| Nodule relation to airway | |
| Concentric | 3 |
| Eccentric | 17 |
| CT bronchus sign + | 7 |
| Respiratory motion (SD) | 11.2 mm (7.1 mm) |
The mean distance between predicted PN location on a lumen registration-generated airway map vs EXP airway map was 4.5 ± 3.3 mm (range, 0-12.7 mm). The mean distance between predicted PN locations on lumen registration vs INSP was 14.8 ± 9.7 mm (range, 4.8-44.3 mm). The predicted PN location, compared with lumen registration location, was significantly closer on an EXP vs INSP airway map (P < .0001). The mean distance between predicted nodule location based on an EXP scan and actual location visualized with R-EBUS was 5 ± 5.8 mm (range, 0-20 mm) (Table 2).
Table 2.
Outcomes
| Comparison | Distance |
|---|---|
| Lumen registration – EXP scan | |
| Mean (SD) | 4.5 mm (3.3 mm) |
| Upper lobe | |
| Mean (SD) | 3.6 mm (2.6 mm) |
| Lower/middle lobe | |
| Mean (SD) | 6.1 mm (4.0 mm) |
| Lumen registration – INSP scan | |
| Mean (SD) | 14.8 mm (9.7 mm) |
| Upper lobe | |
| Mean (SD) | 14.1 mm (8.2 mm) |
| Lower/middle lobe | |
| Mean (SD) | 16.0 mm (12.6 mm) |
| Lumen registration – vPAD | |
| Mean (SD) | 3.0 mm (2.4 mm) |
| ΔR-EBUS – sensor probe | |
| Mean (SD) | 5.0 mm (5.8 mm) |
| SPiN navigation plan time | |
| Mean (SD) | 6.2 min (4.9 min) |
| PointCloud collection time | |
| Mean (SD) | 3.8 min (2.1 min) |
| Total procedure time | |
| Mean (SD) | 49.7 min (13.7 min) |
EXP = end-expiration breath hold; INSP = full-inspiration breath hold; R-EBUS = radial endobronchial ultrasound.
During the bronchoscopy, the vPAD registration of the patient to the EXP virtual airway map as determined by “target-lock” had a mean difference of only 3 ± 2.4 mm, which shows strong correlation for the initial registration method. The average time spent during the planning phase was 6.2 ± 4.9 minutes and on three occasions an additional airway was selected by the bronchoscopist other than what was initially planned by the software platform. The average time to perform a PointCloud collection was 3.8 ± 2.1 minutes and the average duration of the procedure was 49.7 ± 13.7 minutes (Table 2).
Discussion
Previously, the difference in the average motion of pulmonary lesions between INSP and EXP CT scans was shown to be significant (17.6 mm)16; however, until now, the effect of respiratory variation on the creation of virtual airway maps for ENB has not been reported. This study’s finding that predicted pulmonary nodule location during bronchoscopy is better estimated by reformatted images from an EXP scan performed at end-expiration rather than an INSP scan performed during a full inspiratory breath hold is not surprising. The difference between visual confirmation by R-EBUS and the predicted location on EXP scan was similar to EXP – lumen registration (5 mm vs 4.5 mm), which lends credence that the lumen registration map was in fact the most accurate representation. In addition, automatic vPAD registration with the EXP scan may slightly improve predicted nodule location.
Radiation oncologists have been cognizant of the movement of PN during tidal respiration for many years.17, 18, 19 Similarly, work has shown that nodules move on average 1 cm during tidal breathing, with larger variation in the lower lobes. Radiation oncologists have also shown that respiratory motion of a nodule is not necessarily linear but may be hysterical, may change its pattern from day to day, and cannot be assumed based on the respiratory pattern of another part of the lung. This reinforces why a preplanning CT, upon which a virtual 3D airway map is built, should closely approximate the patient’s tracheobronchial tree during bronchoscopy.
Unlike a global positioning satellite system, to which ENB has incorrectly been compared, the airway map is not continuously updated during bronchoscopy. The precision of any tracking system depends upon accurate information input, and the results of this study suggest that an EXP map may better represent nodule location. A meta-analysis of ENB studies that all used an INSP map found a discrepancy between successful electromagnetic navigation to a PN and diagnostic yield of 97.4% and 64.9%, respectively.10 Eberhardt et al20 found that ENB yields for lower lobe nodules were significantly lower than upper lobe nodules (29% vs 77%), suggesting that greater respiratory movement of nodules closer to the diaphragm may adversely affect EMN.16 The difference in yields disappeared with the addition of R-EBUS. This may explain why EBUS has been shown to be complementary to ENB, inferring that readjustment of the biopsy site is needed when using ENB.5, 20 Our study found a significant discordance in predicted nodule location during bronchoscopy compared with INSP in all locations, not just the lower lobes. Whether this is clinically significant remains to be determined.
An interesting incidental finding in this study was the discrepancy between CT bronchus sign found on CT and the view obtained on R-EBUS. A bronchus sign on CT imaging is the airway leading directly to the nodule, which has been associated with increase in yield in ENB.15 This would suggest a concentric view on R-EBUS; however, that may not be the case if CT slice selection hides that the airway deviates around the nodule rather than travels through it. This may not be appreciated on CT because of small airways being extrinsically compressed by the tumor or from the inability to appreciate small airways beyond a certain point. These potential pitfalls may explain why the ultrasonic view obtained on R-EBUS (eccentric vs concentric) has more consistently been shown to be associated with yield than CT bronchus sign.14, 21
There are several limitations to this study. The sample size was small, with the majority of nodules in the right upper lobe. A more even distribution would have been ideal, with a greater percentage in the lower lobes, where respiratory variation potentially has a larger impact. Second, there are still limitations to ENB for which bronchoscopists may not be able to compensate (ie, the act of “wedging” a bronchoscope can significantly distort the lung, potentially displacing a PN).22 This is another factor that can introduce sampling error that cannot be overcome by a preprocedural CT scan regardless of the phase of respiration. Third, it would be injudicious to generalize the findings of this study to other proprietary ENB systems on the market. Last, the science is still evolving and not perfect. We had two cases with technical errors in registration and tracking in which we could not complete navigation. Although this relative failure rate may seem quite high, a small sample size can artificially magnify the result. Literature shows that higher levels of registration error are associated with lower yields.23 It may be that some bronchoscopists are comfortable with more discordance than others and chose to continue with the procedure when others would not, including taking biopsies on the basis of ENB location even if the PN is unable to be visualized by R-EBUS.20 Further evaluation of ENB using an EXP map is needed, paying particular attention to procedure failure rates.
Our study was not powered sufficiently to accurately report yield, and there were patients with biopsies suggesting benign nodules that declined follow-up chest CT scans to document at least 1-year stability or a decrease in nodule size. Therefore, although ENB using an EXP map directs the bronchoscopist closer to actual nodule location, no conclusions about incremental increase in yields can be drawn currently. Future directions of this technology should include a multicenter randomized controlled trial to properly evaluate yield.
Conclusion
In summary, our study demonstrates that the predicted 3D nodule location using an EXP scan for ENB is significantly closer to actual nodule location when compared with an INSP scan. This may partly account for discrepancies between successful navigation and making a diagnosis in some cases as well as the drop off in yields for lower lobe nodules. R-EBUS should be considered as an adjunct to ENB to enhance the accuracy of nodule localization.
Acknowledgments
Author contributions: B. S. F. takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis. N. J. P., N. T. T., A. C., and G. A. S. contributed to study conception and design, or acquisition of data, or analysis and interpretation of data; drafted the submitted article or revised it critically for important intellectual content; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: N. J. P. has received grant funding from Veran Medical Technologies for this study and honoraria from Olympus for teaching at peripheral EBUS courses. N. T. T. has received grant funding from Cook Medical, Olympus, Integrated Diagnostics, and Exact Sciences Veracyte, along with consulting fees from Cook Medical, OncoCyte, Integrated Diagnostics, and Veran Medical Technologies; she is also on the advisory board for Veracyte. A. C. has received grant funding from Veran Medical Technologies and honoraria from Olympus for teaching at peripheral EBUS courses. G. A. S. has received grant funding from Veran Medical Technologies for this study. None declared (B. S. F.).
Role of sponsors: The sponsor had no role in the design of the study or the preparation of the manuscript. The sponsor did assist with use of the software for the collection of data on nodule position but not in the interpretation of any data.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by Veran Medical Technologies, St. Louis, MO.
Supplementary Data
References
- 1.Moyer V.A., Force U.S.P.S.T. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 2.National Lung Screening Trial Research Trial. Aberle D.R., Adams A.M. Reduced lung-cancer mortality with low-dose computed tomographic screening. New Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould M.K., Donington J., Lynch W.R. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera M.P., Mehta A.C., Wahidi M.M. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–e165S. doi: 10.1378/chest.12-2353. [DOI] [PubMed] [Google Scholar]
- 5.Ost D.E., Ernst A., Lei X. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med. 2016;193(1):68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Memoli J.S., Nietert P.J., Silvestri G.A. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142(2):385–393. doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiener R.S., Schwartz L.M., Woloshin S., Welch H.G. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155(3):137–144. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baaklini W.A., Reinoso M.A., Gorin A.B., Sharafkaneh A., Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117(4):1049–1054. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 9.Chenna P., Chen A.C. Radial probe endobronchial ultrasound and novel navigation biopsy techniques. Semin Respir Crit Care Med. 2014;35(6):645–654. doi: 10.1055/s-0034-1395499. [DOI] [PubMed] [Google Scholar]
- 10.Gex G., Pralong J.A., Combescure C., Seijo L., Rochat T., Soccal P.M. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration. 2014;87(2):165–176. doi: 10.1159/000355710. [DOI] [PubMed] [Google Scholar]
- 11.Mahajan A.K., Patel S., Hogarth D.K., Wightman R. Electromagnetic navigational bronchoscopy: an effective and safe approach to diagnose peripheral lung lesions unreachable by conventional bronchoscopy in high-risk patients. J Bronchology Interv Pulmonol. 2011;18(2):133–137. doi: 10.1097/LBR.0b013e318216cee6. [DOI] [PubMed] [Google Scholar]
- 12.Lamprecht B., Porsch P., Wegleitner B., Strasser G., Kaiser B., Studnicka M. Electromagnetic navigation bronchoscopy (ENB): increasing diagnostic yield. Respir Med. 2012;106(5):710–715. doi: 10.1016/j.rmed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Khandhar S, Bowling M, Gildea T, et al. Electromagnetic navigation bronchoscopy for lung lesion evaluation in 500 subjects: first interim analysis of the prospective, multicenter NAVIATE Study [abstract]. Paper presented at: Annual Meeting of the American College of Chest Physicians; October 22–25, 2016; Los Angeles, CA.
- 14.Chen A., Chenna P., Loiselle A., Massoni J., Mayse M., Misselhorn D. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thorac Soc. 2014;11(4):578–582. doi: 10.1513/AnnalsATS.201311-384OC. [DOI] [PubMed] [Google Scholar]
- 15.Seijo L.M., de Torres J.P., Lozano M.D. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest. 2010;138(6):1316–1321. doi: 10.1378/chest.09-2708. [DOI] [PubMed] [Google Scholar]
- 16.Chen A., Pastis N., Furukawa B., Silvestri G.A. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy. Chest. 2015;147(5):1275–1281. doi: 10.1378/chest.14-1425. [DOI] [PubMed] [Google Scholar]
- 17.Shen G., Wang Y.J., Sheng H.G. Double CT imaging can measure the respiratory movement of small pulmonary tumors during stereotactic ablative radiotherapy. J Thorac Disease. 2012;4(2):131–140. doi: 10.3978/j.issn.2072-1439.2012.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seppenwoolde Y., Shirato H., Kitamura K. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53(4):822–834. doi: 10.1016/s0360-3016(02)02803-1. [DOI] [PubMed] [Google Scholar]
- 19.Shirzadi Z., Sadeghi-Naini A., Samani A. Toward in vivo lung's tissue incompressibility characterization for tumor motion modeling in radiation therapy. Med Phys. 2013;40(5):051902. doi: 10.1118/1.4798461. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt R., Anantham D., Ernst A., Feller-Kopman D., Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176(1):36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]
- 21.Yamada N., Yamazaki K., Kurimoto N. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132(2):603–608. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 22.Leira H.O., Lango T., Sorger H., Hofstad E.F., Amundsen T. Bronchoscope-induced displacement of lung targets: first in vivo demonstration of effect from wedging maneuver in navigated bronchoscopy. J Bronchol Intervention Pulmonol. 2013;20(3):206–212. doi: 10.1097/LBR.0b013e31829cb2b5. [DOI] [PubMed] [Google Scholar]
- 23.Makris D., Scherpereel A., Leroy S. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J. 2007;29(6):1187–1192. doi: 10.1183/09031936.00165306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.