Abstract
Anaphylaxis is a systemic, life-threatening disorder triggered by mediators released by mast cells and basophils activated via allergic (IgE-mediated) or nonallergic (non-IgE-mediated) mechanisms. It is a rapidly evolving, multisystem process involving the integumentary, pulmonary, gastrointestinal, and cardiovascular systems. Anaphylaxis and angioedema are serious disorders that can lead to fatal airway obstruction and culminate in cardiorespiratory arrest, resulting in hypoxemia and/or shock. Often, these disorders can be appropriately managed in an outpatient setting; however, these conditions can be severe enough to warrant evaluation of the patient in the ED and in some cases, hospitalization, and management in an ICU. Reports suggest that underdiagnosis and undertreatment of anaphylaxis are common. Several new syndromes have been described recently including bird-egg, pork-cat, delayed allergy to mammalian meat and a diverse group of mast cell activation disorders. Conditions such as postural orthostatic tachycardia syndrome, carcinoid syndrome, Munchausen stridor, and factitious anaphylaxis can present similarly and need to be included in the differential diagnosis. Anaphylaxis is a clinical diagnosis, but plasma tryptase and urinary histamine levels are often elevated, allowing diagnostic confirmation; however, diagnostic testing should not delay treatment as results may not be immediately available. The sine qua non of treatment is avoidance of any known triggers and epinephrine, which should never be delayed if this disorder is suspected. Secondary treatments include fluids, bronchodilators, antihistamines, and glucocorticoids. Patients with cardiopulmonary arrest or airway or vascular compromise require mechanical ventilation, vasopressors, and other advanced life support in the ICU.
Key Words: allergy, anaphylaxis, angioedema, shock, urticaria
Abbreviations: alpha-gal, alpha-galactose; ECLS, extracorporeal life support; IA, idiopathic anaphylaxis; IO, intraosseous; NSAID, nonsteroidal antiinflammatory drug; PAF, platelet-activating factor; VLM, vastus lateralis muscle
Anaphylaxis and angioedema are serious disorders that can lead to fatal airway obstruction and culminate in cardiorespiratory arrest, resulting in hypoxemia and/or shock, requiring management in an ICU setting.1, 2, 3 Reports suggest that underdiagnosis and undertreatment of anaphylaxis are common.4 Anaphylaxis is presumably an ancient disease, although several developments in the past century have led to enormous insights and treatment advances.5 In the early 20th century, the French physiologist, Charles Richet, along with Paul Portier, undertook a study of hypnotoxin, an urticaria-inducing toxin and other toxins derived from Physalia (Portuguese man of war or “floating terror” found in the Atlantic, Indian, and Pacific oceans) extracts.6 An important series of experiments were conducted on the dog, Neptune, wherein an initial injection of toxin was followed by a second injection 22 days later. Within minutes of the second injection, Neptune began to gasp, wheeze, and collapsed with bloody emesis, only to die within 25 min. Richet termed the condition “anaphylaxis” as opposed to prophylaxis and was awarded the Nobel Prize in Medicine for this work in 1913.5 e-Table 1 provides a historical perspective of events leading to our current understanding of anaphylaxis.
Anaphylaxis has been defined as a systemic, immediate hypersensitivity reaction mediated by IgE and resulting in mast cell and basophil mediator release. This results in multiple clinical effects leading to the diagnosis (Table 1). Anaphylactic reactions result from release of mediators by mast cells and basophils activated either by IgE, termed “immunologic,” or by direct activation of these cells by certain agents, termed “nonimmunologic anaphylaxis.”1, 7, 8, 9, 10 Although the term “anaphylactoid” was previously used to describe non-IgE-mediated anaphylaxis, this terminology is no longer recommended.11
Table 1.
Criteria for Diagnosis of Anaphylaxis
| Anaphylaxis is highly likely if any one of the following three conditions is satisfied. | |
| 1. | Acute onset of illness with: |
| Mucocutaneous involvement (pruritus, flushing, urticaria, angioedema) and one of the following: | |
| A. Respiratory complications (wheezing, stridor, hypoxemia/cyanosis) | |
| B. Hypotensiona or end-organ damage (encephalopathy, kidney injury, etc.) | |
| 2. | Two or more of the following occurring rapidly after exposure to known or likely allergen: |
| • Mucocutaneous involvement (pruritus, flushing, urticaria, angioedema) | |
| • Respiratory complications (wheezing, stridor, hypoxemia/cyanosis) | |
| • Hypotensiona or evidence of end organ hypoperfusion (encephalopathy, kidney injury, etc.) | |
| • Persistent gastrointestinal symptoms (pain, nausea, vomiting) | |
| 3. | Reduced BP soon after exposure to a known allergen. |
Hypotension in adults is regarded as systolic BP of <90 mm Hg or greater than a 30% decrease in systolic BP from the patient’s baseline. Hypotension in infants and children: systolic BP <70 mm Hg (1-12 months); <(70 mm Hg + [2x age ]) (1-10 years); <90 mm Hg (11-17 years); or >30% decrease in systolic BP.
Anaphylaxis Patterns: Uniphasic, Biphasic, and Protracted
Three patterns of anaphylactic syndromes have been described based on disease expression: uniphasic, biphasic and protracted. Uniphasic type accounts for 70% to 90% of anaphylaxis cases, peaks at 30 to 60 min, and resolves over the next hour with no recurrence of symptoms. Biphasic anaphylaxis is defined by recurrence of symptoms hours after resolution of the initial event in the absence of re-exposure to the trigger.12 Biphasic type has been variably reported to occur in <113 to up to 23% of reactions, with a recent report suggesting that 3% of adults and up to 15% of children experience biphasic anaphylaxis.12 Early administration of epinephrine may be beneficial in preventing biphasic reactions; the role of glucocorticoids in preventing this type is unclear but physiologically reasonable. Protracted or persistent anaphylaxis refers to the rare reaction lasting for days or even weeks.14
Fatal Anaphylaxis and Time to Death
Jerschow et al15 examined rates of fatal anaphylaxis in the United States between 1999 and 2010. Using International Classification of Diseases, version 10, diagnostic codes on death certificates, they identified 2,458 anaphylaxis-related deaths over an 11-year period with a prevalence of 0.69 people per million. In this study population (>96% adult), medication-induced anaphylaxis fatalities were the most frequent (58.8%), followed by “unspecified” (19.3%), venom (15.2%), and food (6.2%). Fatal anaphylaxis in the outpatient setting was most commonly food-induced anaphylaxis, whereas drug-induced anaphylaxis was most frequent in the inpatient setting. Two case series reported median time from clinical manifestation to death as 30 to 35 min for food, 10 to 15 min for insect venom, and 5 min for IV medications.16, 17
Epidemiology: Prevalence, Hospitalization, and Anaphylactic Shock
Determining the prevalence of anaphylaxis is challenging because it is underdiagnosed,18 and estimates are based on studies with variable study designs that are not always comparable.4, 19 In 2006, the American College of Allergy Asthma and Immunology Epidemiology of Anaphylaxis Working Group estimated a 0.05% to 2% lifetime prevalence of anaphylaxis.20 Children and adolescents made up the largest number of cases based on prescription information of epinephrine autoinjectors.20 Multiple studies support the notion that the prevalence of anaphylaxis is increasing, especially in industrialized nations.21 Wood and colleagues21 conducted two nationwide, cross-sectional random-digit-dial surveys to estimate lifetime prevalence of anaphylaxis in the United States: 7.7% of the 1,000 adults included in the public survey reported prior anaphylactic reactions and were classified as “possible” anaphylaxis. The study defined “probable” anaphylaxis as reports of allergic symptoms involving two or more organ systems with respiratory and/or cardiovascular involvement and “very likely anaphylaxis” as “probable anaphylaxis” coupled with hospital presentation and a feeling that one’s life was in danger. Using these more stringent criteria for anaphylaxis, this study estimates between 1.6% and 5.1% of adults surveyed have a history of “probable” or “very likely” anaphylaxis.21 Increasing rates of anaphylaxis have been reported in the United States as well as in Asia.22, 23 From 186 to 225 deaths/year were reported in the United States by Ma and coworkers24 using multiple cause of death databases. Rohacek et al25 reported that of 532 anaphylactic episodes in EDs, 507 (45%) had uniphasic and 25 (<5%) had biphasic disease, with 12 of the latter being clinically significant and one patient being transferred to the hospital for refractory anaphylaxis. Jeppesen et al23 reviewed anaphylactic shock in a cohort study using a national database from Denmark between 1995 and 2012. A total of 6,707 patients had anaphylactic shock, with a 65% hospitalization rate that increased twofold during the observation period in adults and 10 fold in children. Fifteen percent were admitted to the ICU and 0.7% (50 patients) died of the disease. Between 2005 and 2009, there were 81 pediatric and 1,269 adult admissions with anaphylaxis admitted to UK critical care units, with a 90% survival rate.26 The authors noted increased absolute numbers of patients admitted for anaphylaxis each year of the study period, with female predominance in adult patient admissions.
Mechanisms
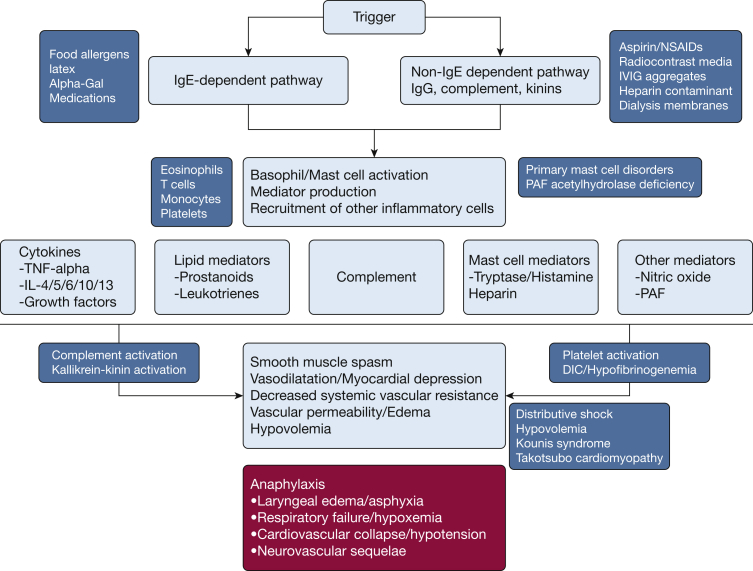
Anaphylaxis results from activation of inflammatory pathways (Fig 1), which are the result of mast cell and/or basophil degranulation.27 The traditional pathway is mediated via T cells, T helper cell 2 cytokines (such as IL-4 and 5), B-cell production of IgE, and subsequent crosslinking of the high-affinity IgE receptor on mast cells and basophils by IgE-antigen complexes. Other triggers could also lead to mast cell and basophil degranulation in a non-IgE-dependent fashion, including IgG immune complexes, complement products, neuropeptides, opiates, and radiocontrast media. Direct activation is secondary to drugs such as icatibant, fluoroquinolones, and several neuromuscular blocking drugs.
Figure 1.
Mechanisms underlying anaphylaxis-IgE and non-IgE-dependent pathway. DIC = disseminated intravascular coagulation; IVIG = intravenous immunoglobulin; NSAIDs = nonsteroidal antiinflammatory drugs; PAF = platelet activating factor; TNF = tumor necrosis factor.
These triggers results in activation of a signaling cascade, culminating in mast cell and basophil degranulation.27, 28 The mediators expressed result in capillary leak, inflammatory cell recruitment, and in the plethora of cardiopulmonary sequelae of anaphylaxis.27 In select situations, activation of the kinin pathway can lead to a mast cell-independent activation of vascular leakage, as described in reactions to contaminated Chinese heparin. Anaphylaxis with resultant inflammation and endothelial activation can result in recruitment of other inflammatory pathways that can amplify the pathophysiological processes. These include complement, kallikrein-kinin, and coagulation pathways.27, 28, 29 The role of platelets and platelet-activating factor (PAF) is receiving renewed interest since publications have suggested that deficiency of PAF acetyl-hydrolase could lead to more severe anaphylaxis in a PAF-dependent manner.30, 31
Anaphylactic Shock and Myocardial Depression
The onset of shock in anaphylaxis is often rapid and multifaceted, comprising features from cardiogenic, hypovolemic, and distributive shock states. Hypovolemia and distributive shock result in the historically termed “empty heart syndrome.” Massive cytokine release results in extensive capillary leak and third space fluid with intravascular volume depletion. Histamine, tumor necrosis factor alpha, prostanoids, and interleukins can also produce vasoplegia with venous fluid sequestration. Taken together, these processes decrease venous return and produce low cardiac filling pressures. Normally in these types of shock, tachycardia and increased stroke volume drives an increased cardiac output to preserve systemic perfusion, but this offers only a partial compensation, and only if sufficient cardiac preload and function exists. Anaphylactic shock is particularly problematic in this setting because myocardial suppression and relative bradycardia are common features.32
Cardiogenic components of anaphylaxis are complex and not completely elucidated in the literature. They include myocardial depression, bradycardia, ischemia, and diastolic and systolic impairment.33 Myocardial depression occurs secondary to the release of vasodepressor substances in anaphylaxis, including prostanoids and leukotrienes or some inflammatory cytokines (proposed mechanism). The role of cardiac mast cells in anaphylaxis has not been rigorously studied, but some data suggest coronary vasospasm with microischemia produce reduced systolic and diastolic function from histamine and PAF release.34 The Kounis syndrome refers to acute coronary syndrome occurring as a complication of anaphylactic reactions and may occur as vasospastic disease or atherothrombotic coronary disease.33 Finally, classical (transient hypokinesis or akinesis of the apical left ventricle) or reverse takotsubo syndromes (transient hypokinesis or akinesis of basal and mid-ventricular segments) have been implicated in case reports and series.33 Combined distributive, hypovolemic, and cardiogenic features can produce a profound state of mixed shock.
Anaphylactic shock refractory to vasopressors such as epinephrine is a rare but ominous occurrence. It is presumably mediated by histamine activation of signaling pathways resulting in the production of endothelium-derived nitric oxide. Nitric oxide increases synthesis of the endogenous vasodilator cyclic guanylate monophosphate by activating guanylate cyclase. Animal studies have shown that this process can be blocked by administering IV methylene blue, which competitively inhibits guanylate cyclase and reduces cyclic guanylate monophosphate production.35 Myocardial depression and hypotension can lead to cerebral and renal hypoperfusion, with the former leading to syncope and seizures and the latter to renal insufficiency.
Classification and Etiology of Anaphylaxis
Table 2 shows the classification and categories of anaphylaxis.36
Table 2.
Classification of Anaphylaxis According to Causative Mechanism
| Mechanism | Examples | Comments |
|---|---|---|
| Immunologic-IgE | Food allergensa | 8 common food allergens listed below |
| (Secondary MCD) | Airborne allergens | Animal dander, aerosolized foods, pollen |
| Latex | Gloves, catheters, masks, medication vials | |
| Hymenoptera venom | Honey bee, wasp, yellow jacket, hornet, IFA | |
| Medication allergy | Antibiotics, biologicals,b vaccines, NSAIDsb | |
| Alpha-gal | Mammalian meat (beef, pork, venison, lamb) | |
| FDEIA | Exercise + food (wheat, nuts, legumes, etc) | |
| Hormones | Progesterone or estrogens (catamenial) | |
| Seminal fluid | Postcoital anaphylaxis | |
| Radiocontrast mediab | IgE-mediated reactions have been reported | |
| Immunologic-non-IgE | Immune aggregates | Includes immune complexes/complement |
| IVIG | From IgG or IgE anti-IgA antibodies | |
| Aspirin and NSAIDsb | Leukotriene-driven and other mechanisms | |
| Dialysis membranes | ||
| Radiocontrast mediab | Complement activation, kinin generation | |
| Dextrans/HMW iron | ||
| Biologicsb | Cytokine inhibitors, omalizumab, etc | |
| Heparin | Generation of kinins by contaminated | |
| Chinese heparin | ||
| Nonimmunologic | ||
| Direct effects | Opiates, physical | Cold, heat, exercise, sunlight |
| Primary MCDc | MMAS and systemic | Genetic defects affect proliferation or activation of mast cells |
| MCASc | Can be associated with germline replications of TPSAB1 gene encoding α-tryptase36 | |
| Idiopathic | Increased mast cell sensitivity/degranulation | |
| T helper cell 2-cytokine polarization | ||
| Unrecognized allergens | ||
| Masqueraders | Munchausen stridor | |
| Undifferentiated somatoform anaphylaxis | ||
| Vocal cord dysfunction |
FDEIA = food-dependent exercise-induced anaphylaxis; HMW = high molecular weight; IFA = imported fire ant; IVIG = immunoglobulin, intravenous; MCD = mast cell activating disorder; MMAS = monoclonal mast cell activation syndrome; MCAS = mast cell activation syndrome; NSAID = nonsteroidal antiinflammatory drugs; TPSAB1 = tryptase alpha/beta 1 (human).
Both IgE-mediated and non-IgE-mediated reactions have been reported.
Milk, egg, wheat, soy, tree nuts, peanut, shellfish, and fin fish are commonly incriminated.
Mast cell disorders are associated with both IgE-mediated allergic reactions as well as spontaneous mast cell degranulation.36
Immunologic IgE-Mediated Reactions
IgE-mediated reactions include allergic reactions to food; airborne allergens such as pollen, animal dander, and aerosolized foods; latex; medications (oral or parenteral), food-dependent exercise-induced anaphylaxis; allergy to mammalian sugars such as galactose-1,3-alpha-galactose (alpha-gal); anaphylaxis to seminal fluid and hormones (ie, catamenial or progesterone-related anaphylaxis); and, rarely, radiocontrast media reactions (Table 2). Common foods that trigger anaphylaxis include milk, egg, wheat, soy, fin fish, shellfish, and nuts.37, 38 Venoms (such as hymenoptera or imported fire ant) are common causes of anaphylaxis. Medications associated with IgE-mediated anaphylaxis include beta-lactams, cephalosporins, vancomycin, quinolones, and sulfonamides. Anesthetic drugs such as suxamethonium, pancuronium, and atracurium have also been implicated in perioperative anaphylaxis.39 Rarely, nonsteroidal anti-inflammatory drugs (NSAIDs) can cause anaphylaxis by an IgE-mediated mechanism.40 Fatal and near-fatal allergic reactions occur with 0.1% to 0.4% of all administered injections of subcutaneous immunotherapy with higher rates witnessed in accelerated or rush protocols. Reactions to newer biological agents and monoclonal antibodies have also been described.41
Immunologic Non-IgE-Mediated Reactions
Anaphylaxis has been reported to IV immunoglobulins,42 NSAIDs,40 dialysis membranes,43 dextrans, iron,44 biological agents, and heparin. In the case of dialysis-associated anaphylaxis, most hypersensitivity reactions to components of the dialysis circuit are due to ethylene oxide or complement activating bio-incompatible membranes, whereas anaphylaxis to erythropoietin, latex, heparin, and medications have also been recorded.43
Nonimmunologic Anaphylactic Reactions
Nonimmunologic triggers for anaphylaxis including physical factors (eg, exercise, cold, heat) and iatrogenic agents (including radiocontrast media and opiates) that can stimulate direct mast cell degranulation.45, 46 In primary mast cell disorders, mast cells can degranulate both independently and in response to allergens such as foods and medications.47
Specific Syndromes and Disorders Associated With Anaphylaxis
Varying degrees of anaphylaxis can accompany exercise in some individuals.46 The early prodrome during exercises include a sense of fatigue and warmth, flushing or generalized pruritus followed in sequence by urticaria, angioedema, bronchospasm, airway obstruction, and vascular collapse.48 Some patients experience food-dependent exercise-induced anaphylaxis, in which anaphylaxis occurs only when preceded by food ingestion, especially foods to which the individual is allergic.
Individuals with severe adverse reactions to the oligosaccharide, alpha-gal, present in mammalian meats and in the chemotherapeutic medication cetuximab were recently described. These patients present with urticaria or delayed anaphylactic reactions to red meat and often have a preceding history of tick bites.37, 49, 50 In the United States, these patients often volunteer a history of pruritic tick bites by the Lone Star tick (Amblyomma americanum). The development of urticaria and anaphylaxis 3 to 5 h after mammalian meat ingestion is consistent with the disorder.51, 52 The pork-cat syndrome refers to sensitization to cat albumin that leads to cross-reactivity with pork albumin and resultant allergic reactions and anaphylaxis.53 The bird-egg syndrome refers to cross-reactivity between proteins present in egg yolk and tissue albumin present in muscle tissue of birds.54 The protein alpha-livetin (also referred to as chicken serum albumin [Gal d 5]), is the allergen component in egg yolk that is involved in anaphylactic manifestations of the bird-egg syndrome.
In 2008, a world-wide recall of Chinese heparin was initiated based on severe anaphylactic reactions triggered by a contaminant, oversulfated chondroitin sulfate, in several countries.29, 55 Activation of the contact system culminating in kallikrein pathway activation and resultant bradykinin generation was deemed a likely mechanism.56
It is estimated that 30% to 60% of patients presenting with anaphylaxis may have no obvious etiological trigger to explain the disease and hence are described as having idiopathic anaphylaxis (IA), a diagnosis of exclusion.57, 58, 59 Recent advances in pathophysiology have delineated novel etiologies, including mast cell activation disorders, hormone sensitivity syndromes (including catamenial anaphylaxis), and allergy to alpha-gal.
Mast cell activation disorders may be primary (including clonal disorders such as systemic mastocytosis and monoclonal mast cell activation syndrome), secondary (activation of mast cells by IgE-mediated allergic reactions), or idiopathic (such as the idiopathic mast cell activation syndrome, idiopathic urticaria and idiopathic anaphylaxis).47, 60 Patients with mastocytosis are more likely to develop anaphylactic reactions to hymenoptera stings.61 Confirmation of the diagnosis relies on evaluation of bone marrow and/or extracutaneous tissue biopsies. Indications for bone marrow evaluation include adult patients with urticaria pigmentosa, a baseline tryptase >20 ng/mL, recurrent hypotensive or syncopal episodes regardless of tryptase levels, hymenoptera anaphylaxis with tryptase >11.4 ng/mL, and a Spanish Network on Mastocytosis score ≥2, which is based on sex, clinical symptoms, and tryptase levels. The management of mast cell activation disorders is similar to the management of anaphylaxis in general. e-Table 2 discusses the evaluation of mast cell activation disorders.
Progesterone hypersensitivity can result in symptoms ranging from dermatitis to cyclical anaphylaxis during the luteal phase of the menstrual cycle.62 The patients are usually young women with recurrent perimenstrual anaphylactic events.63 Allergic reactions to human seminal fluid have been described in women manifesting as local urticaria and pruritus to florid anaphylaxis and death.64, 65
Clinical Presentation
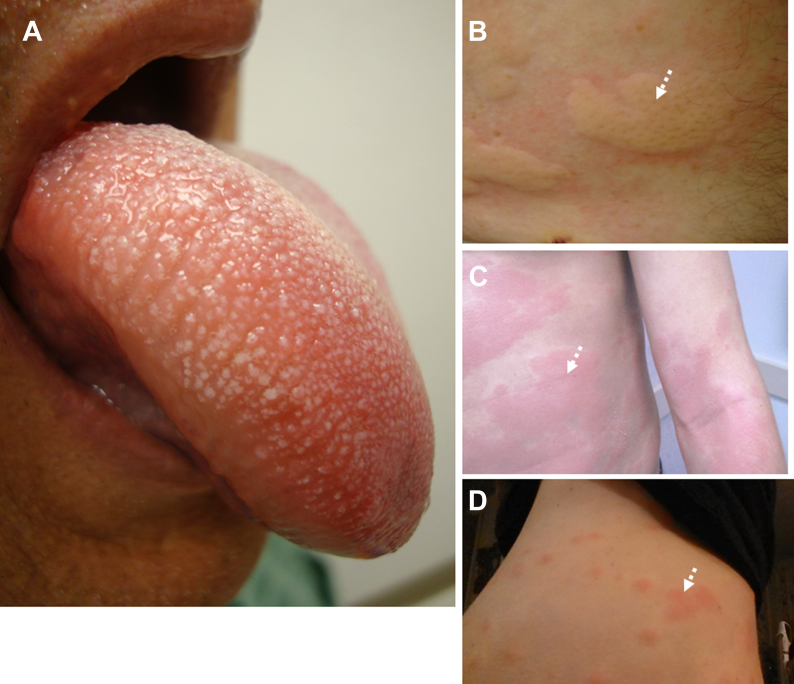
Clinical manifestations are listed in Table 3. Almost every system can be involved. Severe presentations of anaphylaxis characterized by hypotension and/or hypoxemia and indicating cardiovascular and respiratory compromise have been associated with older age, pre-existing lung disease, and antihypertensive medications in one prospective study of anaphylaxis.30 Although mucocutaneous manifestations (Fig 2) occur in the majority of patients, they are absent in 10% to 20% of patients, including those who present with fatal or near-fatal anaphylaxis.66 Risk factors for anaphylaxis are listed in Table 4.67
Table 3.
Clinical Features of Anaphylaxis
| Organ system | Presentation | Sequelae |
|---|---|---|
| Skin/mucosa | Urticarial eruption | Hypovolemia |
| (80%-90%) | Angioedema | |
| Oropharyngeal | Airway obstruction | |
| Laryngeal | Stridor | |
| Airway obstruction | ||
| Intestinal | Abdominal pain | |
| Flushing | Hypotension | |
| Pruritus (palms/soles/oral/genitalia) | ||
| Bronchopulmonary | Laryngeal edema | Hoarseness/stridor |
| (60%-70%) | Dysphonia | |
| Wheeze/cough | Respiratory failure | |
| Hypoxemia/cyanosis | ||
| Rhinitis | Nasal obstruction | |
| Cardiac | Vasodilation/reduced SVR | Hypotension/shock |
| (40%-50%) | Myocardial vasoconstriction | Reduced CO |
| Myocardial depression | Myocardial ischemia | |
| Arrhythmia | ||
| Cardiac arrest | ||
| Gastrointestinal | Nausea, vomiting | Dehydration |
| (40%-50%) | Diarrhea | Hypovolemia |
| Intestinal edema | Abdominal pain | |
| Neurological | Dizziness | Syncope |
| (<15%) | Confusion | Seizures |
| Headache | ||
| Feeling of impending doom | ||
| Tunnel vision | ||
| Genitourinary | Uterine cramps (♀) | Pain |
| Uterine bleeding (♀) | ||
| Scrotal edema (♂) | Pain | |
| Miscellaneous | Urinary/fecal incontinence |
Prevalence of symptom cluster is showed as percentages (%). CO = cardiac output; SVR = systemic vascular resistance.
Figure 2.
Clinical manifestations of anaphylaxis. A, Angioedema of the tongue and oropharynx. B, Discrete urticarial lesions in a patient with acute allergic reaction. C, Coalescent urticaria and diffuse erythema in a patient with a severe systemic allergic reaction. D, Delayed urticarial reactions in response to beef ingestion in an entomologist who had sustained multiple occupation-related tick bites months earlier. White dotted arrows indicate clinical findings.
Table 4.
Risk Factors for Anaphylaxis and Disease Severity
| Risk factors for anaphylaxis | |
| 1. Age | Boys <15 y and women >15 y of age |
| 2. Route of allergen introduction | Parenteral > ingested |
| 3. Interruption of medication | Example: insulin interruption after desensitization |
| 4. Atopic history | Example: latex anaphylaxis, RCM, EIA, and IA |
| 5. Prior exposure | Example: protamine/zinc insulin (NPH) use and reaction to protamine used for heparin reversal |
| 6. Asthma | More severe asthma increases risk for anaphylaxis |
| 7. Geography | Higher incidence in Northern latitudes |
| 8. Sex | Latex, aspirin, and certain medication reactions more common in women. |
| Venom reactions more common in men. | |
| Risk factors for severe anaphylaxis | |
| 1. Infants and elderly | |
| 2. Comorbidity | Asthma, ischemic dilated cardiomyopathy, CAD |
| 3. Medication use | Antihypertensive medicationsa |
| Monoamine oxidase inhibitors and tricyclic antidepressants | |
| 4. Impaired cognition | Alcohol, sedative medications, recreational drugs |
CAD = coronary artery disease; EIA = exercise-induced anaphylaxis; IA = idiopathic anaphylaxis; NPH = neutral protamine hagedorn; RCM = radiocontrast media.
Beta-adrenergic blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, direct renin inhibitors.
Differential Diagnosis
Many conditions may mimic anaphylaxis or be accompanied by systemic manifestations that resemble allergy and need to be considered in the differential diagnosis. Important disorders that require consideration includes capillary leak, postural orthostatic tachycardia syndrome, carcinoid syndrome, neuroendocrine tumors, factitious anaphylaxis, and undifferentiated somatoform anaphylaxis.68, 69, 70, 71 These disorders and their evaluation are summarized in Table 5.
Table 5.
Differential Diagnosis of Anaphylaxis
| Neurologic/autonomic dysregulation | Suggested tests |
|---|---|
| 1. Vasovagal and vasodepressor reactions | Clinical/ECG/BP |
| 2. Postural orthostatic tachycardia syndrome | Tilt table testing |
| 3. Seizure | EEG |
| 4. CVA | CT or MRI of the brain |
| Cardiovascular | |
| 1. Cardiogenic shock | TTE or RHC |
| 2. Hemorrhagic shock | Clinical bleeding/CBCa |
| 3. Vasodilatory/distributive/endotoxic shock | Bacterial cultures |
| 4. Capillary leak syndrome (hypovolemic shock) | Paraproteinemia |
| Endocrine/flushing | |
| 1. Carcinoid | Urine 5-HIAA |
| 2. Pheochromocytoma | Urine/serum catecholamines |
| 3. VIP-secreting tumors | VIP level |
| 4. Thyroid medullary carcinoma | Serum calcitonin |
| 5. Menopause (flushing, hot flashes) | LH, FSH, estrogen level |
| 6. Hypoglycemia | Blood sugar |
| Iatrogenic/drugs | |
| 1. Vancomycin (Red Man syndrome) | History/clinical |
| 2. Niacin (flushing) | History/clinical |
| 3. General anesthetics (hypotension) | History/clinical |
| Toxic | |
| 1. “Restaurant syndromes” | |
| a. Scombroidosis | History/tryptase/histamine |
| b. MSG | Toxicology |
| 2. Alcohol | EtOH level/osmolar gapb |
| 3. Sulfites | Clinical/toxicology |
| Hematologic/malignantc | |
| 1. Systemic mastocytosis | Tryptase, bone marrow |
| 2. Urticaria pigmentosa | Skin biopsy, tryptase |
| 3. Basophil leukemia | Bone marrow |
| 4. Acute promyelocytic leukemia with tretinoin treatment | Bone marrow |
| Immunologic | |
| 1. Bradykinin-mediated angioedema | C4, C1 inhibitor levels |
| Infection | |
| 1. Hydatid cyst (Echinococcus granulosus) | Clinical, serology, radiology |
| 2. Sepsis/septic shock | Blood cultures |
| Psychosomatic/functional disorders | |
| 1. Panic attack | Psychiatric consultation |
| 2. Factitious anaphylaxis | |
| a. Munchausen stridor | Psychiatric consultation |
| 3. Undifferentiated somatoform anaphylaxis | Psychiatric consultation |
| 4. Vocal cord dysfunction | Spirometry/flow volume loop |
C1 = complement 1; C4 = complement 4; CBC = complete blood count; CVA = cerebrovascular accident; EtOH = ethanol; FSH = follicle-stimulating hormone; 5-HIAA=urine 5-hydroxy indole acetic acid; LH=luteinizing hormone; MSG = monosodium glutamate; RHC = right heart catheterization; TTE = transthoracic echocardiogram; VIP = vasoactive intestinal peptide.
May be normal in acute loss of whole blood.
Osmolar gap allows for the assessment of other toxic alcohols.
Clonal and malignant mast cell disorders often demonstrate mutations of stem cell factor receptor, c-kit.
Diagnostic Testing: Tryptase and Histamine
Total serum tryptase is the biomarker most widely used to confirm a diagnosis of anaphylaxis retrospectively.72 Small amounts of the immature form of tryptase (beta-protryptase) are constitutively secreted into the systemic circulation. Following mast cell and basophil degranulation, total serum tryptase levels increase significantly because of release of mature beta-tryptase. Ideally, serum tryptase should be measured within 1 to 2 h after symptom onset because tryptase levels typically peak within 60 to 90 min after symptom onset but can persist for 6 h.73
Plasma histamine levels rise 5 to 10 min after the onset of anaphylaxis and can also be assayed. However, plasma histamine levels are only transiently elevated, returning to normal within 60 min, making them of little utility if the patient is evaluated greater than 1 h after symptom onset. Twenty-four-hour urinary histamine metabolites may be elevated for up to 24 h after the index event.
Vadas and colleagues31 have shown that serum histamine and tryptase levels are not always elevated, even in patients with severe manifestations of anaphylaxis including cutaneous, gastrointestinal and respiratory, or cardiovascular compromise. In addition, serum tryptase levels are not always elevated during food-induced anaphylaxis.66 As a result, there is growing interest in identifying alternative serum biomarkers such as platelet-activating factor or carboxypeptidase A3 that more accurately confirm the diagnosis of anaphylaxis and correlate with severity, but these have not yet been developed for clinical use.31
Other diagnostic tests to consider are those that help evaluate for mimics of anaphylaxis are listed in Table 5.8 If the clinical history is suspicious for IgE-mediated anaphylaxis, allergy testing (serum or epicutaneous) is indicated to identify the trigger and often requires referral to a board-certified allergist-immunologist.38 This could be accomplished emergently in the ICU or as an outpatient after discharge.
Management
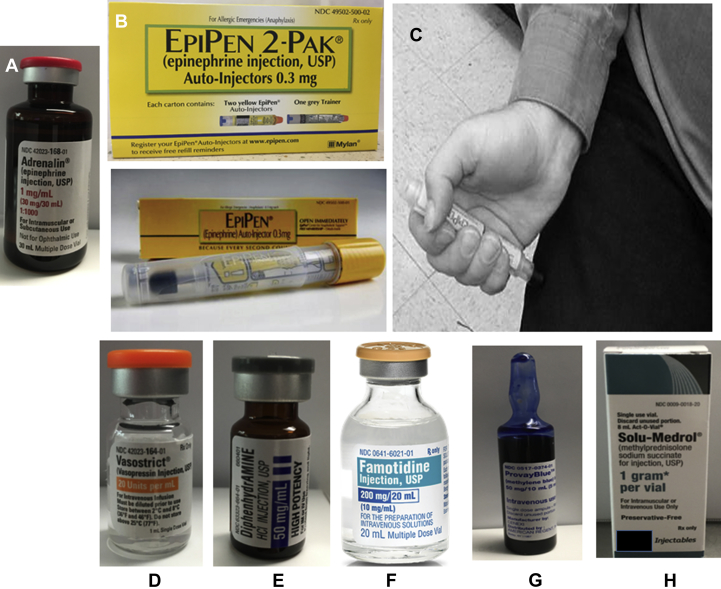
Anaphylaxis is considered a medical emergency with its immediate onset (seconds to minutes) and rapid progression to cardiovascular and/or respiratory collapse resulting in death within minutes of inception. Initial management principles are the same whether the patient is being managed in the outpatient, ED, operating room, or hospital setting because anaphylaxis can occur in any of these locations (Table 6). Patients with more severe cardiorespiratory complications are best managed in an ICU. Tables 6 and 7 provide outlines in management and drug dosages respectively. Figure 3 demonstrates medications commonly used in the management of anaphylaxis that all medical facilities and intensive care unit should have readily available.
Table 6.
Overall Approaches to Managing Systemic Anaphylaxis
| Initial Management (Office/ED/Hospital) | Level of Evidence |
|---|---|
| Base diagnosis on clinical assessment | C |
| Early intervention is recommended | |
| Assess risk for serious/fatal reactions (see Table 7) | C |
| Immediate general measures (first-line treatment) | |
| Place patient in supine position | C |
| Remove inciting trigger/allergen | C |
| Address circulation-airway-breathing | C |
| Epinephrine administration (ALVLM, IM) | B |
| (1 mL/kg of 1:1,000 epinephrine or 0.3-0.5 mg) | |
| 1. Repeat up to 3 injections every 5-15 min | |
| 2. If patient is not responding to IM epinephrine, move to monitored setting and attempt | |
| IV epinephrine | C |
| 3. If IV access is difficult to obtain, then obtain | |
| IO access and administer epinephrine | D |
| Airway management | C |
| Oxygen (up to 100% by face mask) | D |
| Administer nebulized albuterol for bronchospasm | B |
| Prepare for intubation if stridor or airway compromise | |
| Administer IV fluids/crystalloids rapidly | B |
| 30 mL/kg with large-bore catheters in first hour for hypotension; if volume responsive, continue fluids | |
| Adjunctive therapies (after epinephrine is administered) | |
| Administer H1 antihistaminea | B |
| Administer H2 antihistamineb | B |
| Administer corticosteroids | |
| Methylprednisolone (1-2 mg/kg) IV | B |
| Additional measures (hospital/ICU) | |
| Refractory hypotension | |
| After beta-blockade | |
| Glucagon (3-10 mg slow IV in adults) followed by | B |
| IV infusion of 0.05-0.1 mg/kg/h (caution because of emesis) | |
| After several doses of IM epinephrine | |
| Continuous IV infusion of epinephrine | C |
| (Mix 1 mL of 1:1,000 epinephrine in 1,000 mL of 5% dextrose or 0.9 normal saline; infuse at 5-15 mcg/min to maximum of 10 mcg/min) | |
| Bolus epinephrine: for impending cardiovascular collapse (50 mcg/0.5 mL of 1:10,000 slow IV push) | |
| Consider other pressors, including dopamine or vasopressin | |
| Asphyxia/respiratory compromise/respiratory arrest | |
| Oxygenation | |
| Evaluation of airway for extent of edema | |
| Nebulized/racemic epinephrine (0.5 mL of 2.25% every 20 min for stridor) | |
| Intubation/postintubation ventilator management | C |
| Avoid medications that lower BP | |
| Avoid paralytics if possible for intubation | |
| Recommend awake fiberoptic intubation if possible | |
| (if laryngeal involvement is suspected) | |
| Sedate and allow adequate expiration time | |
| Minimize breath stacking and barotrauma | |
| Ketamine use is associated with bronchodilatation | |
| (caution advised with ketamine in cardiac patients) | |
| For difficult airway, consider cricothyroidotomy | |
| Extracorporeal membrane oxygenation | D |
| Consider when response is poor | |
| Consider early and often if patient has limited responsiveness to vasopressors | |
| Postdischarge management | |
| Education | |
| Trigger detection and avoidance | |
| Anaphylaxis action plan | |
| Prescription of an epinephrine autoinjector |
Grades of recommendation are based on levels of evidence: A = randomized studies; B = controlled study without randomization; C = case control, comparative, or correlation studies; D = expert opinion/reports or based on experience of authorities): strong recommendation (grade A), moderate recommendation (grades B and C) and weak recommendation (grade D). Adapted from: Campbell RL et al.3
ALVLM = anterolateral vastus lateralis muscle; IO = intraosseous.
For example, diphenhydramine 1-2 mg/kg.
For example, ranitidine 1-2 mg/kg.
Table 7.
Medications Used in Anaphylaxis Management
| Medication Effects | Concentration | Dose | Route | Frequency | Adverse Effects |
|---|---|---|---|---|---|
| Epinephrine | 1:1,000 (1 mg/mL) | 0.01 mg/kg 0.3 to 0.5 mg |
IM | Every 5-15 min | Tachycardia, palpitations, tachyarrhythmia, anxiety, palpitations, flushing |
| 1:10,000 (0.1 mg/mL) | 0.01 mg/kg 0.5-1.0 mg (5-10 mL) |
IV | Every 5-15 min Push | As above | |
| Vasopressin | NA | 0.04 U | IV | Per minute | Ischemia |
| Dopamine | NA | 1-50 mcg/kg | IV | Per minute | Tachycardia, tachyarrhythmia |
| Norepinephrine | NA | 0.02-1 mcg/kg | IV | Per minute | Tachycardia, tachyarrhythmia |
| Albuterol | |||||
| MDI | 2.5 mg per puff | 1-2 puffs (2.5-5 mg) | INH | Every 2-4 h | Tachycardia, palpitations, anxiety |
| Nebulized | 2.5 mg/3 mL | 3 mL | INH | Every 2-4 h | As above |
| 5 mg/3 mL | 3 mL | INH | Continuous | As above | |
| Glucagon | 3-10 mga 0.05-0.1 mg/kg/h |
IV IV |
Once Continuous | Nausea, vomiting, tachycardia | |
| Diphenhydramine | |||||
| Treatment | NA | 25-50 mg | IV, POb | Once | Drowsiness, sedation |
| Prophylaxis | NA | 25-50 mg | Oncec | ||
| Corticosteroids | |||||
| Hydrocortisone | NA | 100 mg | IV | Every 8 h | Hyperglycemia |
| Prednisoned | |||||
| Treatment | NA | 1-2 mg/kg | POb | Once | Agitation, anxiety, psychosis |
| Prophylaxis | NA | 50 mg | POb | 13 h, then 7 h, then 1 h before | As above |
INH = inhaled; MDI = metered dose inhaler; NA = not available.
Infuse slowly over 2-5 min to minimize nausea and vomiting.
Avoid po medications in patients who are nauseated, vomiting, or unable to protect their airway (unless intubated and gastric tube in place).
1 h before procedure.
IV alternatives should be dosed in prednisone equivalents.
Figure 3.
Examples of medications commonly used in the management of anaphylaxis (kindly provided by Michael Keens, PharmD, and Johnny Perry, RPh, Wake Baptist Medical Center). A, Epinephrine 1 mg/mL (1:1,000). B, EpiPen Auto-Injector 0.3 mg. C, Proper location for autoinjecting IM administration mid-outer aspect of the thigh (anterolateral vastus lateralis, mid-muscle belly). D, Vasopressin 20 U/mL. E, Diphenhydramine 50 mg/mL. F, Famotidine 20 mg in 50 mL. G, Methylene blue 1 mg/mL concentration. H, Methylprednisolone 1-g vial.
Immediate Measures (First-Line Treatment)
-
1.
The immediate administration of 0.3 to 0.5 mg of epinephrine (1:1,000) in the mid-outer aspect of the thigh (anterolateral vastus lateralis, mid-muscle belly [VLM]) is the most essential intervention (Fig 3). This may need to be repeated every 5 to 15 min.1, 7, 8, 74 Studies show absorption is faster with higher tissue and plasma levels when injected in the VLM compared with other muscles or following subcutaneous administration.1, 7, 8, 74 In emergencies, an epinephrine autoinjector may be used, realizing that the dose is fixed (0.3 mg in adults and 0.15 mg in children weighing <15 kg). In obese individuals, autoinjector needle length may not be sufficient for intramuscular epinephrine delivery.75
-
2.
Removal of the potential triggering antigen, placing the patient in a supine position, and quickly addressing circulation-airway-breathing are critical. The position of the anaphylactic patient can have important implications. Vasodilation and hypovolemia prevail in anaphylaxis. As such, patients are extremely sensitive to fluid shifts, and sudden postural changes can result in fatal cardiac arrest.76 Despite the lack of prospective data, there is uniform agreement that patients should be placed in the supine position unless contraindicated by active vomiting, respiratory distress or pregnancy; in which case, the left lateral decubitus position is more appropriate.3, 8, 76, 77 Elevation of the legs (or the Trendelenburg position using a tilting table) remains controversial. This position may play a role initially while the patient is undergoing fluid resuscitation if no vasopressors are available.78 It is important to note that this position is seldom used in the ICU (other than during procedures) as vasopressors and IV fluids are more effective and readily available.8
-
3.
In the event of respiratory distress, the patient should be placed in a position of comfort and restrictive clothing should be removed or loosened. A short-acting β2 agonist bronchodilator (albuterol) should be administered as 2.5 or 5 mg in 3 mL nebulized or two puffs of a metered dose inhaler every 2 to 4 h until symptomatic relief or patient reaches a higher level of care.10
-
4.
IV access needs to be established using large bore catheters and fluids administered as rapidly as possible. Intraosseous (IO) access is an acceptable alternative.
Adjunctive Therapies
Antihistamines (antagonists H1 and H2) as well as corticosteroids are considered adjunctive treatments; some guideline statements regard them as “optional” therapy. The administration of these treatments should never delay the administration of epinephrine.3, 7, 8, 10 Unfortunately, one of the most common reasons for delay of epinephrine administration is that caretakers “wait to see if the antihistamine will work.” This can be a fatal management error.8 During anaphylaxis, antihistamines are effective only in treating cutaneous symptoms such as pruritus and urticaria.7 A recent retrospective Canadian study demonstrated that the use of H1 antagonists in the ED on patients that had an allergic reaction without evidence of anaphylaxis reduced the likelihood that those patients progressed to anaphylaxis. The limits of this study should be acknowledged, and epinephrine is still the first and only effective pharmacologic treatment for anaphylaxis.10, 79
Additional Measures (In-Hospital/ICU)
Management of Respiratory Complications: Asphyxia/Respiratory Compromise
Any patient with impending respiratory failure, evidence of obstructing oropharyngeal, or laryngeal edema should undergo prompt endotracheal intubation with the most advanced airway tools available by the most experienced provider trained to do so (ideally without paralytic so the patient is still spontaneously breathing).2 No published reports formally support this practice, but we strongly recommend an awake, fiberoptic intubation if laryngeal edema is suspected. Fiberoptic intubation requires special equipment and an operator skilled in this technique. The placement of a surgical airway such as a cricothyrotomy or tracheostomy should be a last resort, but should not be delayed if it is necessary, as in circumstances of significant upper airway obstruction.
We recommend against the use of noninvasive positive pressure ventilation, or a supraglottic airway such as a laryngeal mask airway unless angioedema of the larynx and vocal folds has been ruled out by laryngoscopy (or there are no other airway options). These devices will not bypass the upper airway obstruction and local trauma could exacerbate angioedema of the larynx (if present), worsening the blockage and precipitating respiratory arrest.2
Racemic epinephrine can be administered by nebulizer to decrease laryngeal edema and to facilitate intubation.3 In some reports, epinephrine has been introduced by endotracheal administration or sublingually when there has been a delay in procuring IV access.3 During intubation and mechanical ventilation, care should be taken to avoid use of sedatives or medications that lower BP. Minimizing breath stacking and avoiding barotrauma are also essential to prevent further deterioration. Ketamine use in anaphylaxis has been associated with bronchodilation.
Refractory Hypotension
Volume resuscitation (30 mL/kg) should be initiated immediately with isotonic crystalloid fluid through multiple, large-bore (≥20 gauge) angiocatheters, after which time supplementation with colloid solutions has been suggested.1, 3, 7, 8, 10 During refractory shock in patients using beta blockers, glucagon can be administered. Glucagon bypasses the β2 adrenergic receptor directly activating adenyl cyclase, producing positive inotropy, bronchodilation, and vasoconstriction.8 Glucagon can be administered by slow IV push in doses of 3 to 10 mg in the adult followed by 0.05 to 0.1mg/kg/h by IV infusion.80 Glucagon is associated with nausea, vomiting, and hypoglycemia.
Patients who remain hypotensive or who have a recurrence of symptoms despite more than two doses of bolus epinephrine and adequate fluid resuscitation should be started on an epinephrine infusion, administered cautiously with adequate monitoring. Epinephrine is a α1-, β1-, and β2- adrenergic receptor agonist that increases systemic vascular resistance, enhances cardiac chronotropy as well as inotropy (increasing cardiac output) and produces pulmonary bronchodilation.3, 74 Epinephrine should be infused via a central venous catheter or IO needle if possible.1, 7, 8, 77, 81 A 1:1,000,000 infusion solution needs to be compounded by the pharmacy by diluting 1 mL of a 1:1,000 concentration of epinephrine in 1,000 mL of either 5% dextrose (avoid if using glucagon) or normal saline, resulting in a 1 mcg/mL concentration. This can be infused at 5 to 15 mcg/min, titrating to mean arterial pressure >65 mm Hg.
In rare cases in which rapid deterioration is occurring, epinephrine can be administered by bolus injection (0.5 to 1.0 mg or 5 to 10mL of a 1:10,000 dilution by slow IV/IO push) or 1 mL of 1:1,000 IV/IO bolus in the event of impending or current cardiac arrest. Adverse effects of epinephrine include anxiety, flushing, tachycardia, atrial or ventricular dysrhythmias, cerebrovascular accident, and hypertension. Special preparations may be available in the rare sulfite-allergic individual. Occasionally, patients may benefit from an infusion of adjuvant vasopressor to epinephrine in refractory anaphylactic shock. Vasopressin and/or phenylephrine can be used to increase systemic vascular resistance without contributing to excessive tachycardia. If the patient is relatively bradycardic, then norepinephrine or dopamine can be added. There has been anecdotal success in treating refractory anaphylaxis with methylene blue where synergy with epinephrine has been described, realizing that on rare occasions, methylene blue itself has been incriminated in inducing anaphylaxis.35, 82
Extracorporeal Life Support
Anaphylactic shock refractory to multiple modalities or in situations of catastrophic reductions in cardiac function may be treated effectively with venoarterial extracorporeal life support (ECLS), which produces the best cardiac flow, aortic pressures, and myocardial perfusion.3 There are no prospective or trial data supporting the use of ECLS in patients with anaphylaxis.83 The major limitation of this modality is that it requires advanced equipment and personnel trained in its proper use, which may only be available in tertiary care centers. ECLS also requires use of heparin and possibly protamine, which have both been linked to severe anaphylaxis.7
Conclusion
Anaphylaxis is a rapidly progressive life-threatening disorder. It is often underrecognized and undertreated. Early recognition, high index of suspicion, early removal of potential triggers, and administration of epinephrine can be life-saving. Skilled intervention in ICUs may be required for the patient with complicated, prolonged, or severe anaphylaxis.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: G. K. has received royalties from UpToDate in Medicine, research grants from CSL Behring, and is a grant reviewer for the National Institute of Allergy and Infectious Diseases/National Institutes of Health. None declared (D. L., O. I. I., A. E.).
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs LoVerde and Iweala contributed equally to this manuscript.
Supplementary Data
References
- 1.Simons F.E., Ardusso L.R., Bilo M.B. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014;7(1):9. doi: 10.1186/1939-4551-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LoVerde D., Files D.C., Krishnaswamy G. Angioedema. Crit Care Med. 2017;45(4):725–735. doi: 10.1097/CCM.0000000000002281. [DOI] [PubMed] [Google Scholar]
- 3.Campbell R.L., Li J.T., Nicklas R.A. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol. 2014;113(6):599–608. doi: 10.1016/j.anai.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Sclar D.A., Lieberman P.L. Anaphylaxis: underdiagnosed, underreported, and undertreated. Am J Med. 2014;127(1 Suppl):S1–S5. doi: 10.1016/j.amjmed.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Ghably J., Saleh H., Vyas H. Paul Ehrlich's mastzellen: a historical perspective of relevant developments in mast cell biology. Methods Mol Biol. 2015;1220:3–10. doi: 10.1007/978-1-4939-1568-2_1. [DOI] [PubMed] [Google Scholar]
- 6.Ring J., Brockow K., Behrendt H. History and classification of anaphylaxis. Novartis Found Symp. 2004;257:6–16. [PubMed] [Google Scholar]
- 7.Simons F.E., Ebisawa M., Sanchez-Borges M. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8(1):32. doi: 10.1186/s40413-015-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman P., Nicklas R.A., Randolph C. Anaphylaxis–a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015;115(5):341–384. doi: 10.1016/j.anai.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Ventura M.T., Scichilone N., Gelardi M. Management of allergic disease in the elderly: key considerations, recommendations and emerging therapies. Expert Rev Clin Immunol. 2015;11(11):1219–1228. doi: 10.1586/1744666X.2015.1081564. [DOI] [PubMed] [Google Scholar]
- 10.Muraro A., Roberts G., Worm M. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69(8):1026–1045. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]
- 11.Sampson H.A., Munoz-Furlong A., Campbell R.L. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 12.Lee S., Sadosty A.T., Campbell R.L. Update on biphasic anaphylaxis. Curr Opin Allergy Clin Immunol. 2016;16(4):346–351. doi: 10.1097/ACI.0000000000000279. [DOI] [PubMed] [Google Scholar]
- 13.Grunau B.E., Li J., Yi T.W. Incidence of clinically important biphasic reactions in emergency department patients with allergic reactions or anaphylaxis. Ann Emerg Med. 2014;63(6):736–744. doi: 10.1016/j.annemergmed.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Zisa G., Riccobono F., Calamari A.M. A case of protracted hypotension as unique symptom of a biphasic anaphylaxis to amoxicillin. Eur Ann Allergy Clin Immunol. 2009;41(2):60–61. [PubMed] [Google Scholar]
- 15.Jerschow E., Lin R.Y., Scaperotti M.M. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134(6):1318–1328. doi: 10.1016/j.jaci.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pumphrey R.S. Fatal anaphylaxis in the UK, 1992-2001. Novartis Found Symp. 2004;257:116–128. [PubMed] [Google Scholar]
- 17.Pumphrey R.S. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30(8):1144–1150. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 18.Altman A.M., Camargo C.A., Jr., Simons F.E. Anaphylaxis in America: a national physician survey. J Allergy Clin Immunol. 2015;135(3):830–833. doi: 10.1016/j.jaci.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman P. Epidemiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8(4):316–320. doi: 10.1097/ACI.0b013e3283036a69. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman P., Camargo C.A., Jr., Bohlke K. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 21.Wood R.A., Camargo C.A., Jr., Lieberman P. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Yang M.S., Kim J.Y., Kim B.K. True rise in anaphylaxis incidence: epidemiologic study based on a national health insurance database. Medicine (Baltimore) 2017;96(5):e5750. doi: 10.1097/MD.0000000000005750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeppesen A.N., Christiansen C.F., Froslev T. Hospitalization rates and prognosis of patients with anaphylactic shock in Denmark from 1995 through 2012. J Allergy Clin Immunol. 2016;137(4):1143–1147. doi: 10.1016/j.jaci.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Ma L., Danoff T.M., Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(4):1075–1083. doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohacek M., Edenhofer H., Bircher A. Biphasic anaphylactic reactions: occurrence and mortality. Allergy. 2014;69(6):791–797. doi: 10.1111/all.12404. [DOI] [PubMed] [Google Scholar]
- 26.Gibbison B., Sheikh A., McShane P. Anaphylaxis admissions to UK critical care units between 2005 and 2009. Anaesthesia. 2012;67(8):833–839. doi: 10.1111/j.1365-2044.2012.07159.x. [DOI] [PubMed] [Google Scholar]
- 27.Krishnaswamy G., Ajitawi O., Chi D.S. The human mast cell: an overview. Methods Mol Biol. 2006;315:13–34. doi: 10.1385/1-59259-967-2:013. [DOI] [PubMed] [Google Scholar]
- 28.Modena B.D., Dazy K., White A.A. Emerging concepts: mast cell involvement in allergic diseases. Transl Res. 2016;174:98–121. doi: 10.1016/j.trsl.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Warkentin T.E., Greinacher A. Heparin-induced anaphylactic and anaphylactoid reactions: two distinct but overlapping syndromes. Expert Opin Drug Saf. 2009;8(2):129–144. doi: 10.1517/14740330902778180. [DOI] [PubMed] [Google Scholar]
- 30.Brown S.G., Stone S.F., Fatovich D.M. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132(5):1141–1149. doi: 10.1016/j.jaci.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Vadas P., Gold M., Perelman B. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 32.Brown S.G. The pathophysiology of shock in anaphylaxis. Immunol Allergy Clin North Am. 2007;27(2):165–175. doi: 10.1016/j.iac.2007.03.003. v. [DOI] [PubMed] [Google Scholar]
- 33.Gouel-Cheron A., Harpan A., Mertes P.M. Management of anaphylactic shock in the operating room. Presse Med. 2016;45(9):774–783. doi: 10.1016/j.lpm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Kelley J.L., Chi D.S., Abou-Auda W. The molecular role of mast cells in atherosclerotic cardiovascular disease. Mol Med Today. 2000;6(8):304–308. doi: 10.1016/s1357-4310(00)01747-0. [DOI] [PubMed] [Google Scholar]
- 35.Jang D.H., Nelson L.S., Hoffman R.S. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol. 2013;9(3):242–249. doi: 10.1007/s13181-013-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyons J.J., Yu X., Hughes J.D. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48(12):1564–1569. doi: 10.1038/ng.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iweala O.I., Burks A.W. Food allergy: our evolving understanding of its pathogenesis, prevention, and treatment. Curr Allergy Asthma Rep. 2016;16(5):37. doi: 10.1007/s11882-016-0616-7. [DOI] [PubMed] [Google Scholar]
- 38.Jarvinen K.M. Food-induced anaphylaxis. Curr Opin Allergy Clin Immunol. 2011;11(3):255–261. doi: 10.1097/ACI.0b013e32834694d8. [DOI] [PubMed] [Google Scholar]
- 39.Garvey L.H. Old, new and hidden causes of perioperative hypersensitivity. Curr Pharm Des. 2016;22(45):6814–6824. doi: 10.2174/1381612822666161004125143. [DOI] [PubMed] [Google Scholar]
- 40.Dona I., Salas M., Perkins J.R. Hypersensitivity Reactions to non-steroidal anti-inflammatory drugs. Curr Pharm Des. 2016;22(45):6784–6802. doi: 10.2174/1381612822666160928142814. [DOI] [PubMed] [Google Scholar]
- 41.Bonamichi-Santos R, Castells M. Diagnoses and management of drug hypersensitivity and anaphylaxis in cancer and chronic inflammatory diseases: reactions to taxanes and monoclonal antibodies [published online ahead of print June 8, 2016]. Clin Rev Allergy Immunol. https://doi.org/10.1007/s12016-016-8556-5. [DOI] [PubMed]
- 42.Williams S.J., Gupta S. Anaphylaxis to IVIG. Arch Immunol Ther Exp (Warsz) 2017;65(1):11–19. doi: 10.1007/s00005-016-0410-1. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez-de Lara M.A., Martin-Malo A. Hypersensitivity reactions to synthetic haemodialysis membranes an emerging issue? Nefrologia. 2014;34(6):698–702. doi: 10.3265/Nefrologia.pre2014.Jul.12682. [DOI] [PubMed] [Google Scholar]
- 44.Wang C., Wong S., Graham D.J. Risk of anaphylaxis with intravenous iron products. JAMA. 2016;315(20):2232–2233. doi: 10.1001/jama.2016.0965. [DOI] [PubMed] [Google Scholar]
- 45.Lee S.Y., Ahn K., Kim J. A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy Asthma Immunol Res. 2016;8(6):535–540. doi: 10.4168/aair.2016.8.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller C.W., Guha B., Krishnaswamy G. Exercise-induced anaphylaxis: a serious but preventable disorder. Phys Sportsmed. 2008;36(1):87–94. doi: 10.3810/psm.2008.12.16. [DOI] [PubMed] [Google Scholar]
- 47.Schuch A., Brockow K. Mastocytosis and Anaphylaxis. Immunol Allergy Clin North Am. 2017;37(1):153–164. doi: 10.1016/j.iac.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Pravettoni V., Incorvaia C. Diagnosis of exercise-induced anaphylaxis: current insights. J Asthma Allergy. 2016;9:191–198. doi: 10.2147/JAA.S109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Commins S.P., Satinover S.M., Hosen J. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleh H., Embry S., Nauli A. Anaphylactic reactions to oligosaccharides in red meat: a syndrome in evolution. Clin Mol Allergy. 2012;10(1):5. doi: 10.1186/1476-7961-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Commins S.P., Jerath M.R., Cox K. Delayed anaphylaxis to alpha-gal, an oligosaccharide in mammalian meat. Allergol Int. 2016;65(1):16–20. doi: 10.1016/j.alit.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platts-Mills T.A., Schuyler A.J., Hoyt A.E. Delayed anaphylaxis involving IgE to galactose-alpha-1,3-galactose. Curr Allergy Asthma Rep. 2015;15(4):12. doi: 10.1007/s11882-015-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabbah A., Lauret M.G., Chene J. [The pork-cat syndrome or crossed allergy between pork meat and cat epithelia (2)] Allerg Immunol (Paris) 1994;26(5):173–180. [PubMed] [Google Scholar]
- 54.Hemmer W., Klug C., Swoboda I. Update on the bird-egg syndrome and genuine poultry meat allergy. Allergo J Int. 2016;25:68–75. doi: 10.1007/s40629-016-0108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hedlund K.D., Coyne D.P., Sanford D.M. The heparin recall of 2008. Perfusion. 2013;28(1):61–65. doi: 10.1177/0267659112462274. [DOI] [PubMed] [Google Scholar]
- 56.Kishimoto T.K., Viswanathan K., Ganguly T. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358(23):2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenny N., Grammer L.C. Idiopathic anaphylaxis. Immunol Allergy Clin North Am. 2015;35(2):349–362. doi: 10.1016/j.iac.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Greenberger P.A., Lieberman P. Idiopathic anaphylaxis. J Allergy Clin Immunol. Pract. 2014;2(3):243–250. doi: 10.1016/j.jaip.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Kuhlen J.L., Virkud Y.V. Pathogenesis, newly recognized etiologies, and management of idiopathic anaphylaxis. Discov Med. 2015;19(103):137–144. [PMC free article] [PubMed] [Google Scholar]
- 60.Akin C. Mast cell activation syndromes presenting as anaphylaxis. Immunol Allergy Clin North Am. 2015;35(2):277–285. doi: 10.1016/j.iac.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Bonadonna P., Bonifacio M., Lombardo C. Hymenoptera allergy and mast cell activation syndromes. Curr Allergy Asthma Rep. 2016;16(1):5. doi: 10.1007/s11882-015-0582-5. [DOI] [PubMed] [Google Scholar]
- 62.Snyder J.L., Krishnaswamy G. Autoimmune progesterone dermatitis and its manifestation as anaphylaxis: a case report and literature review. Ann Allergy Asthma Immunol. 2003;90(5):469–477. doi: 10.1016/S1081-1206(10)61838-8. [DOI] [PubMed] [Google Scholar]
- 63.Bauer C.S., Kampitak T., Messieh M.L. Heterogeneity in presentation and treatment of catamenial anaphylaxis. Ann Allergy Asthma Immunol. 2013;111(2):107–111. doi: 10.1016/j.anai.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Lee J., Kim S., Kim M. Anaphylaxis to husband's seminal plasma and treatment by local desensitization. Clin Mol Allergy. 2008;6:13. doi: 10.1186/1476-7961-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman S.A., Bernstein I.L., Enrione M. Successful long-term immunotherapy for human seminal plasma anaphylaxis. JAMA. 1984;251(20):2684–2687. [PubMed] [Google Scholar]
- 66.Burks A.W., Jones S.M., Boyce J.A. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128(5):955–965. doi: 10.1542/peds.2011-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zogaj D., Ibranji A., Hoxha M. Exercise-induced anaphylaxis: the role of cofactors. Mater Sociomed. 2014;26(6):401–404. doi: 10.5455/msm.2014.26.401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Druey K.M., Parikh S.M. Idiopathic systemic capillary leak syndrome (Clarkson disease) J Allergy Clin Immunol. 2016;140(3):663–670. doi: 10.1016/j.jaci.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng C., Teuber S., Gershwin M.E. Histamine (scombroid) fish poisoning: a comprehensive review. Clin Rev Allergy Immunol. 2016;50(1):64–69. doi: 10.1007/s12016-015-8467-x. [DOI] [PubMed] [Google Scholar]
- 70.Choy A.C., Patterson R., Patterson D.R. Undifferentiated somatoform idiopathic anaphylaxis: nonorganic symptoms mimicking idiopathic anaphylaxis. J Allergy Clin Immunol. 1995;96(6 Pt 1):893–900. doi: 10.1016/s0091-6749(95)70225-3. [DOI] [PubMed] [Google Scholar]
- 71.Bahna S.L., Oldham J.L. Munchausen stridor-a strong false alarm of anaphylaxis. Allergy Asthma Immunol Res. 2014;6(6):577–579. doi: 10.4168/aair.2014.6.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Waterfield T., Dyer E., Wilson K. How to interpret mast cell tests. Arch Dis Child Educ Pract Ed. 2016;101(5):246–251. doi: 10.1136/archdischild-2015-309887. [DOI] [PubMed] [Google Scholar]
- 73.Vitte J. Human mast cell tryptase in biology and medicine. Mol Immunol. 2015;63(1):18–24. doi: 10.1016/j.molimm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Simons F.E., Ardusso L.R., Bilo M.B. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4(2):13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsai G., Kim L., Nevis I.F. Auto-injector needle length may be inadequate to deliver epinephrine intramuscularly in women with confirmed food allergy. Allergy Asthma Clin Immunol. 2014;10(1):39. doi: 10.1186/1710-1492-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pumphrey R.S. Fatal posture in anaphylactic shock. J Allergy Clin Immunol. 2003;112(2):451–452. doi: 10.1067/mai.2003.1614. [DOI] [PubMed] [Google Scholar]
- 77.Commins S.P. Outpatient emergencies: anaphylaxis. Med Clin North Am. 2017;101(3):521–536. doi: 10.1016/j.mcna.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown S.G. Cardiovascular aspects of anaphylaxis: implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol. 2005;5(4):359–364. doi: 10.1097/01.all.0000174158.78626.35. [DOI] [PubMed] [Google Scholar]
- 79.Zilberstein J., McCurdy M.T., Winters M.E. Anaphylaxis. J Emerg Med. 2014;47(2):182–187. doi: 10.1016/j.jemermed.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 80.Thomas M., Crawford I. Best evidence topic report. Glucagon infusion in refractory anaphylactic shock in patients on beta-blockers. Emerg Med J. 2005;22(4):272–273. doi: 10.1136/emj.2005.023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sicherer S.H., Simons F.E.R., Section on Allergy and Immunology Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017;139(3):e1–e9. doi: 10.1542/peds.2016-4006. [DOI] [PubMed] [Google Scholar]
- 82.Zheng F., Barthel G., Collange O. Methylene blue and epinephrine: a synergetic association for anaphylactic shock treatment. Crit Care Med. 2013;41(1):195–204. doi: 10.1097/CCM.0b013e318267667b. [DOI] [PubMed] [Google Scholar]
- 83.Chan-Dominy A.C., Anders M., Millar J. Extracorporeal membrane modality conversions. Perfusion. 2015;0(4):291–294. doi: 10.1177/0267659114544486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.