Abstract
Background
Pleural effusions are present in 15% to 44% of hospitalized patients with pneumonia. It is unknown whether effusions at first presentation to the ED influence outcomes or should be managed differently.
Methods
We studied patients in seven hospital EDs with International Statistical Classification of Disease and Health Related Problems-Version 9 codes for pneumonia, or empyema, sepsis, or respiratory failure with secondary pneumonia. Patients with no confirmatory findings on chest imaging were excluded. Pleural effusions were identified with the use of radiographic imaging.
Results
Over 24 months, 4,771 of 458,837 adult ED patients fulfilled entry criteria. Among the 690 (14.5%) patients with pleural effusions, their median age was 68 years, and 46% were male. Patients with higher Elixhauser comorbidity scores (OR, 1.13 [95% CI, 1.09-1.18]; P < .001), brain natriuretic peptide levels (OR, 1.20 [95% CI, 1.12-1.28]; P < .001), bilirubin levels (OR, 1.07 [95% CI, 1.00-1.15]; P = .04), and age (OR, 1.15 [95% CI, 1.09-1.21]; P < .001) were more likely to have parapneumonic effusions. In patients without effusion, electronic version of CURB-65 (confusion, uremia, respiratory rate, BP, age ≥ 65 years accurately predicted mortality (4.7% predicted vs 5.0% actual). However, eCURB underestimated mortality in those with effusions (predicted 7.0% vs actual 14.0%; P < .001). Patients with effusions were more likely to be admitted (77% vs 57%; P < .001) and had a longer hospital stay (median, 2.8 vs 1.3 days; P < .001). After severity adjustment, the likelihood of 30-day mortality was greater among patients with effusions (OR, 2.6 [CI, 2.0-3.5]; P < .001), and hospital stay was disproportionately longer (coefficient, 0.22 [CI, 0.14-0.29]; P < .001).
Conclusions
Patients with pneumonia and pleural effusions at ED presentation in this study were more likely to die, be admitted, and had longer hospital stays. Why parapneumonic effusions are associated with adverse outcomes, and whether different management of these patients might improve outcome, needs urgent investigation.
Key Words: brain natriuretic peptide, emergency medicine, pleural effusion, pneumonia
Abbreviations: BNP, brain natriuretic peptide; CURB-65, confusion, uremia, respiratory rate, BP, age ≥ 65 years score; CXR, chest radiograph; eCURB, electronic version of CURB-65 (confusion, uremia, respiratory rate, BP, age ≥ 65 years); HCAP, health-care-associated pneumonia; ICD-9, International Statistical Classification of Disease and Health Related Problems-Version 9
Community-acquired pneumonia is the eighth leading cause of death in the United States.1 It accounts for > 6 million reported cases and up to 1.1 million hospitalizations per year.2, 3 Depending on the modality used, between 15% (with chest radiography) and 44% (with ultrasonography) of patients with pneumonia have a pleural effusion.4 Complicated parapneumonic effusion and empyema occur in approximately 5% of patients with pneumonia, with significant morbidity, prolonged hospital stays, and increased health service consumption, including surgery.5
Accurate severity assessment in patients with pneumonia is crucial to initial management. Numerous studies have found similar predictive utility of the CURB-65 (confusion, uremia, respiratory rate, BP, and age ≥ 65 years) score and the Pneumonia Severity Index.6 eCURB is an electronic version of CURB-65 using the same elements as continuous and weighted data to improve prediction of 30-day mortality and is a valuable real-time, electronic decision support tool.7, 8 Neither CURB-65 nor eCURB considers the impact of pleural effusion on mortality.
Pneumonia treatment guidelines do not address how parapneumonic effusions should influence the decision for hospital admission.6 The ED has become an increasingly common site for the initial diagnosis of pneumonia. All but one study on parapneumonic effusions investigated hospitalized patients, many of whom developed the effusions following hospital admission.4, 9, 10 The Pneumonia Patient Outcomes Research Team (PORT) study cohort enrolled outpatients but did not focus on ED patients.11 The impact of pleural effusion on clinical outcomes among patients with pneumonia at first presentation to the ED is unknown.
The present study, which included a large ED database of patients with pneumonia, investigated pleural effusions at first encounter and subsequent clinical outcomes. We report the severity-adjusted association of both unilateral and bilateral effusions with comorbid illnesses, hospital admission, length of stay, and mortality.
Patients and Methods
Study Population
ED patients with pneumonia seen in seven Intermountain Healthcare Hospitals in the urban corridor of Utah were studied. Patient enrollment occurred during two 12-month periods: December 2009 through November 2010, and December 2011 through November 2012. Most patients in the present study were originally enrolled in a study of the implementation of a pneumonia electronic clinical decision support tool; the gap year between December 2010 and November 2011 was the period of tool deployment.12
All patients ≥ 18 years old evaluated in the EDs were identified with International Statistical Classification of Disease and Health Related Problems-Version 9 (ICD-9) codes specific for a primary diagnosis of pneumonia (480-487.0) or respiratory failure or sepsis (518.x and 038.x) as the primary diagnosis, with pneumonia secondary. Patients lacking radiographic evidence for pneumonia on ED chest imaging were excluded. Also excluded were patients who received a diagnosis of aspiration (507.0) and immunocompromised conditions, including receipt of antiretroviral therapy (042), solid organ transplants (v420-7), and hematologic malignancies (v106x, 200.x, and 208.x). Patients with health-care-associated pneumonia (HCAP) were identified electronically and were included in the study.11 To exclude patients with recurrent pneumonia often caused by chronic aspiration or structural lung disease, only the first episode in each 12-month period was included. Thirteen ED patients with a primary ICD-9 code for empyema (510.x) and secondary pneumonia codes during the same two periods were added to the original database for this study.
The initial ED chest radiograph (CXR) and CT scans were reviewed for radiographic evidence of pleural effusion by two physician authors (N. D. and P. P. G.), with a weighted κ agreement of 0.91 (P < .001) for a random sample of 50 reports. Patients with effusions were further characterized according to size of effusion (questionable/equivocal/tiny, small, moderate, and large), simple vs loculated effusion, unilateral vs bilateral lung opacities, and unilateral vs bilateral effusion. Effusion size was determined by the radiologist’s report as part of routine practice; physician reviewers translated the report description into small/moderate/large in the few dictations in which these terms were not specifically present. Patients with the presence of pleural effusion for this study were defined as those with small, moderate, or large effusions, excluding patients with tiny or questionable effusions. CT chest imaging was performed within 8 h of ED arrival in 20% of patients. If available, a CT chest scan followed by posterior-anterior upright CXR defined the presence and characteristics of pleural effusion instead of using a portable CXR.
Data Collection
Data elements were extracted from the electronic medical record necessary for measuring severity of illness according to eCURB.7 Demographic characteristics and laboratory test results were obtained from the electronic medical record. Few (< 5%) patients had arterial blood gases measured; Pao2/Fio2 in the remainder was calculated from the percutaneous arterial oxygen saturation and delivered oxygen.13 Six patients missing simultaneous percutaneous arterial oxygen saturation and Fio2 were excluded from analyses that required Pao2/Fio2. We assumed absence of confusion where not documented; manual review of sample patients revealed other missing data to be < 0.1%. Data on brain natriuretic peptide (BNP) levels were more often missing and were recognized to be missing not at random. Expected normal values for bilirubin, creatinine, and BNP levels were imputed according to age and sex where missing, using multiple imputation by chained equations with a pattern mixture model.
Current Procedural Terminology codes were used to identify patients who underwent thoracentesis, chest tube insertion, and thoracotomy. The accuracy of the coded procedure data was confirmed by review of 50 random patient records. We also computed each patient’s Elixhauser comorbidity score, a validated index of 29 comorbidities based on previous ICD-9 coding from inpatient and outpatient encounters.14 The Elixhauser score was used rather than the older Charlson comorbidity index because of better performance in several studies.15, 16, 17
Outcome Measures
Length of stay for hospitalized patients was obtained from the electronic medical record. Thirty-day all-cause mortality was determined by merging electronic medical record data with vital status information from the Utah Population Database.18 We identified secondary admission to any Intermountain Healthcare Hospital within 7 days of the initial ED evaluation through the electronic medical record. This time frame was chosen because most secondary admissions occurred within that period, and later admissions were mostly attributable to diagnoses other than pneumonia. The study was approved by the Intermountain Healthcare Institutional Review Board (1024050) and the Intermountain Privacy Board and was authorized by the Utah Population Database. Individual patient consent was not required.
Statistical Analysis
Concordance between authors in assessing radiographic reports for the presence and characteristics of pleural effusions was measured by using κ statistics. The agreement scale developed by Landis and Koch was used to characterize level of agreement.19
Descriptive analyses were conducted by using χ2 and Fisher exact tests for comparison of crude proportions, Mann-Whitney U tests for comparison of continuous distributions, and bootstrapped Kolmogorov-Smirnov tests for discrete, ordinal distributions. Differences were significant based on an α level of 0.05. Inferential analyses for dichotomous outcomes were conducted by using multiple logistic regression analysis and were evaluated by using the Hosmer-Lemeshow goodness-of-fit test and area under the curve of the receiver-operating characteristics. Linear regression was used for continuous outcomes.
To account for interhospital variability between hospital EDs,20 we initially examined the relationship between pleural effusion features and outcomes (30-day mortality, secondary readmissions, and hospital length of stay) with the use of multilevel hierarchical models and Markov chain Monte Carlo techniques, with hospital site treated as the random effect.20, 21 However, after three chains of 150,000 iterative simulations drawing every third observation, each variable’s potential scale reduction factor was > 1.5 (at a convergence of 1.0). Therefore, sensitivity analyses were performed in which the classical models with complete pooling, no pooling, and partial pooling were compared. The estimates and SEs were comparable across pooling strategies; the same conclusions were reached regardless of pooling strategy given a 95% level of confidence computed by classical methods. The simplest model of complete-pooling sufficiently estimated the effects on outcomes of interest and was therefore implemented for all regression analyses in this study.
Results
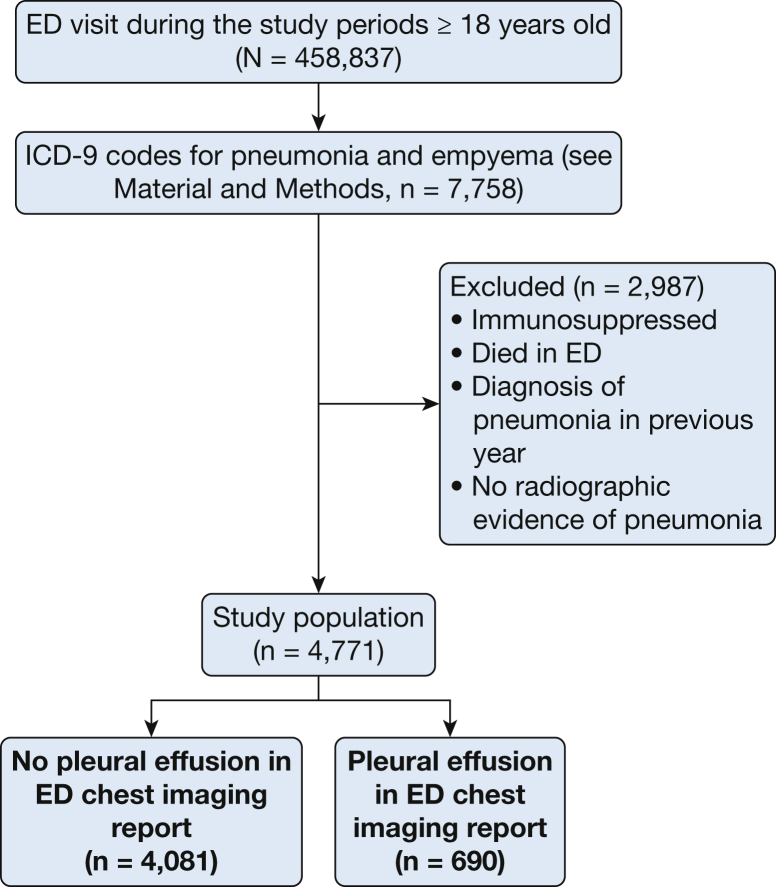
Of the 458,837 adult patients who were admitted to the study EDs, 4,771 had pneumonia and fulfilled the entry criteria. Among these subjects, 690 (14.5%) had a pleural effusion (Fig 1). The effusions were small in 563 (81.6% of all the patients with effusions), moderate in 101 (14.6%), and large in 26 (3.8%) patients. Pleural effusions were defined by using chest CT scans in 263 (38.1%), 280 (40.6%) by upright CXR imaging, and 147 (21.3%) by supine portable CXR imaging in the EDs. Pleural effusions were significantly more common (2.4-fold, 26% vs 11%) in patients with HCAP (n = 673) than in those with community-acquired pneumonia. Thirty-two of the 690 (4.6%) effusions were loculated. Studies other than portable supine imaging had a greater likelihood of diagnosing loculated effusion (two-sided Cochran-Armitage test of trend, P = .001).
Figure 1.
Patient flowchart. ICD-9 = International Statistical Classification of Disease and Health Related Problems-Version 9.
Presence of a pleural effusion was associated with more severe pneumonia as reflected by higher eCURB scores (7% vs 4.7% predicted mortality for patients without effusion) and significantly worse clinical outcomes (Table 1). In patients without effusion, eCURB accurately predicted mortality (4.7% predicted vs 5.0% actual). However, eCURB underestimated mortality in those with effusions (7.0% predicted vs 14.0% actual; P < .001). Patients with pleural effusion at presentation were more likely to be admitted to the hospital (77% vs 57%; P < .001) and stayed longer in the hospital (median, 2.8 vs 1.3 days; P < .001). If initially not admitted to the hospital from the ED, patients were more likely to be secondarily admitted within 7 days (17% vs 5%; P < .001).
Table 1.
Unadjusted Comparisons Between Patients With Any Effusion Vs No Effusion at First Presentation to the ED and Bilateral Effusion Vs Unilateral Effusion
| Variable | Any Effusion | No Effusion | P Value | Bilateral Effusion | Unilateral Effusion | P Value |
|---|---|---|---|---|---|---|
| Elixhauser comorbidity score | 3.49 | 2.40 | < .001 | 3.79 | 3.32 | .020 |
| Multilobar | 57.8% | 42.9% | < .001 | 76.5% | 47.4% | < .001 |
| Bilirubin, mg/dL | 0.8 | 0.7 | .003 | 0.8 | 0.9 | .76 |
| Creatinine, mg/dL | 1.3 | 1.2 | < .001 | 1.4 | 1.3 | .28 |
| BNP, pg/mL | 407 | 214 | < .001 | 511 | 350 | < .001 |
| Median age, y | 68 | 57 | < .001 | 71 | 66 | .035 |
| Median hospital stay, h | 89.5 | 70 | < .001 | 88 | 91 | .86 |
| Male sex | 46.1% | 47.5% | .530 | 46.2% | 46.0% | 1.00 |
| Mean eCURB | 7.0% | 4.7% | < .001 | 8% | 6.4% | .011 |
| Pao2/Fio2 < 300 mm Hg | 58.4% | 44.1% | < .001 | 61.8% | 56.6% | .21 |
| Primary admission | 76.8% | 56.5% | < .001 | 79.8% | 75.2% | .20 |
| Secondary admission | 3.9% | 2.0% | .003 | 5.7% | 2.9% | .12 |
| 30-Day mortality | 14.1% | 5.0% | < .001 | 17% | 12.2% | .12 |
| Thoracentesis | 11.3% | 0.9%a | .012 | 8.9% | 12.6% | .17 |
| Thoracostomy | 8.0% | 0.64%a | < .001 | 5.3% | 9.5% | .07 |
| Thoracotomy | 2.0% | 0.15%a | < .001 | 0% | 3.2% | .003 |
BNP = brain natriuretic peptide; eCURB = electronic version of confusion, uremia, respiratory rate, BP, age ≥ 65 years.
Patients without effusions present on initial ED chest imaging subsequently underwent thoracentesis, tube thoracostomy, and/or thoracotomy for pleural effusions that developed later on.
A bacterial culture of blood, sputum, tracheal aspirate, pleural fluid, and/or urine antigen specimens was performed in 3,172 patients (66.5%), of whom 428 (13.5%) had confirmed or probable bacterial pneumonia. The incidences of unilateral (9.6% vs 11.2%; P = .36) and bilateral (6.8% vs 6.3%; P = .77) effusions were not significantly different between culture-confirmed bacterial pneumonia and culture-negative cases, respectively.
Severity-Adjusted Outcomes
Patients with pleural effusion had a greater likelihood of mortality (OR, 2.6 [CI, 2.0-3.5]; P < .001), controlling for eCURB and the Pao2/Fio2 ratio. Additionally controlling for the Elixhauser score lowered the OR to 2.4. Length of stay was longer among those with pleural effusions (coefficient, 0.22 [CI, 0.14-0.29]; P < .001). Presence of an effusion was also associated with increased initial hospital admission (OR, 1.9 [CI, 1.5-2.4]; P < .001). Patients with large effusions (OR, 2.06 [CI, 1.1-3.8]; P = .02) were more likely to be admitted. Those with loculated effusions had ORs for admission similar to those with large effusions (OR, 2.8 [CI, 0.96-8.2]; P = .06), although not statistically significant. Among ED patients managed as outpatients, those with pleural effusions at initial presentation were more likely to be secondarily admitted to the hospital within 7 days (OR, 3.2 [CI, 1.9-5.5]; P < .001).
Unilateral Vs Bilateral Pleural Effusions
Bilateral effusions were identified in 247 (36%) patients with pleural effusions. Compared with patients with unilateral effusions (Table 1), those with bilateral effusions were older and had more bilateral lung opacities (68% vs 3%). They had more severe illness, as well as higher BNP levels and Elixhauser comorbid illness scores. Based on the multiple logistic regression analysis, patients with a higher Elixhauser score (OR, 1.13 [95% CI, 1.09-1.18]; P < .001), higher BNP (OR, 1.20 [95% CI, 1.12-1.28]; P < .001), higher bilirubin level (OR, 1.07 [95% CI, 1.00-1.15]; P = .04), and increased age (OR, 1.15 [95% CI, 1.09-1.21]; P < .001) were associated with an increased likelihood of having a pleural effusion (Table 2).
Table 2.
Logistic Regression Measuring the Probability of Having Effusions Vs No Effusions
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Intercept | 0.05 | 0.04-0.07 | < .001 |
| Elixhauser comorbidity score | 1.13 | 1.09-1.18 | < .001 |
| Bilirubin | 1.07 | 1.00-1.15 | .04 |
| Creatinine | 0.96 | 0.88-1.05 | .39 |
| BNP | 1.21 | 1.12-1.28 | < .001 |
| eCURB (%) | 0.99 | 0.99-1.01 | .87 |
| Male | 0.97 | 0.82-1.15 | .72 |
| Age (decades) | 1.15 | 1.09-1.20 | < .001 |
Values for bilirubin, creatinine, and BNP include imputed values and were centered and scaled in the regression analysis. See Table 1 legend for expansion of abbreviations.
Crude mortality was higher among patients with bilateral vs unilateral effusions. After adjustment of illness severity, mortality in patients with bilateral effusions was not significantly different from those with unilateral effusions (OR, 1.2 [95% CI, 0.74-1.94]; P = .46).
Pleural Fluid Drainage Procedures
Thoracentesis was performed in 78 patients (11.3%) with pleural effusions present at first ED encounter. The proportion of patients undergoing thoracentesis did not significantly change between the two study periods. Thoracentesis was correlated with effusion size (OR, 3.96; P < .001) but not with the presence of loculation. Thoracotomy was performed on 0.4% of all patients, and thoracostomy (insertion of a chest drainage tube) on 1.7%. Thoracostomy was related both to the presence of loculation (OR, 5.8; P < .001) and effusion size (OR, 2.8; P < .001).
Discussion
In this study, patients with pneumonia with radiologically defined pleural effusions at ED presentation were more likely to die by 30 days, more likely to be admitted to the hospital, and had a longer length of stay compared with patients without effusion, even after adjustment for severity of illness. Importantly, eCURB/CURB-65 significantly underestimated the 30-day mortality of patients with pneumonia presenting with a pleural effusion. These data suggest that the presence of a pleural effusion should be incorporated into mortality predictions for ED populations. Why the development of a pleural effusion is associated with adverse prognosis, and whether different management of these patients might improve outcome, needs urgent investigation.
Previous studies on parapneumonic effusions have included inpatients who developed a pleural effusion anytime during their pneumonia course and have focused predominantly on the need of sampling and drainage of the fluid. The present large study revealed for the first time that patients with pneumonia who present with a pleural effusion at the time of ED admission should be recognized for their significantly inferior clinical outcomes and prognosis.
There are > 60 causes of a pleural effusion.22 Most effusions in the present study were not large, and only 11.3% were judged to warrant thoracentesis; hence, the etiology of the majority was not established. Nonetheless, the presence of effusion was a prognostic indicator irrespective of the cause(s). Many pleural effusions in patients with pneumonia develop as a result of pleural extension of lung parenchymal changes. Inflammation of the visceral followed by the parietal surfaces result in exudative fluid formation from plasma extravasation.23 Conversely, comorbidities are common in patients with pneumonia; the subgroup with bilateral effusions were older and had more comorbid illnesses. Previous research by Hasley et al11 showed that outpatients with pneumonia and radiographically defined heart failure, as well as those with bilateral pleural effusions, had higher mortality rates. We found higher crude mortality rates in patients with bilateral effusions, although bilateral effusions were associated with other measures of illness severity that led to mortality being similar after severity adjustment. Patients with HCAP were more likely to have effusions at ED presentation, perhaps a combination of both aforementioned reasons. It can be argued that some effusions in patients with pneumonia reflect more severe lung inflammation, whereas others may reflect concomitant comorbidity in the host; either factor can account for worse outcome.
A number of prognostic models have been used in pneumonia. The CURB-65 (or eCURB) severity score is among the most commonly used instrument in predicting 30-day mortality in clinical practice and in trials. Our study confirmed that eCURB accurately predicted the mortality in patients without an effusion but significantly underestimated mortality in the group with pleural effusion at presentation. Future studies need to evaluate the value of incorporating effusion into CURB-65/eCURB. The Pneumonia Severity Index includes pleural effusion as a predictor, but it adds only 10 points to the multiple other factors. Future clinical studies in pneumonia must recognize the prognostic implication of an effusion to appropriately stratify randomized patients.
We also reported higher rates of initial and secondary hospital admissions in patients with pneumonia with effusions. Whether higher rates of hospital admission improve outcomes in these patients is unclear. Our data do highlight the fact that clinicians must take into account the worse outcomes of patients with pneumonia who have a pleural effusion at time of diagnosis.
There are limitations to our study. Our data were derived from electronic medical records, and they include older and more ill patients with pneumonia compared with prospective studies that require individual patient consent.24 Detecting pleural effusions involved radiographic studies that vary in sensitivity and specificity, although we prioritized the more accurate study when available. Deaths of non-Utah residents who died outside Utah, and patients secondarily admitted to a hospital not affiliated with Intermountain Healthcare Hospitals, may not have been captured, although this total is likely a very small percentage of our cohort and should not alter the conclusions. The etiology of most effusions could not be established due to the low rate of thoracentesis, although the presence alone of an effusion was a prognosticator. We were not able to determine whether the low rate of thoracentesis affected mortality in this study.
Conclusions
Patients with pneumonia presenting with a pleural effusion had more comorbid illnesses, experienced higher rates of mortality and hospital admissions, and had longer stays in the hospital. Clinicians must recognize the implication on clinical outcome conferred by the presence of an effusion. Targeted therapies or increased attention to fluid drainage might be needed to improve outcome in this patient population.
Acknowledgments
Author contributions: N. C. D. conceived the original idea for this study, performed critical revision of the manuscript for intellectual content, obtained funding, and is overall responsible for the content of the manuscript, including the data and analysis. P. P. G. performed data acquisition, analysis, and interpretation of data, and drafted the initial manuscript. J. S. S. was primarily responsible for statistical analysis and performed critical revision of the manuscript for intellectual content. L. M. and B. E. J. performed data acquisition, analysis, and interpretation of data and critically revised the manuscript for intellectual content. Y. C. G. L. performed analysis and interpretation of data and critically revised the manuscript for intellectual content.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Al Jephson, BA, for his work in extracting and cleaning data from the Intermountain Electronic Data Warehouse to create the ED pneumonia database.
Footnotes
FUNDING/SUPPORT: This work was funded in part by the Intermountain Research and Medical Foundation. Dr Jones was supported by a training grant [5 T32–HL-105321-1] from the National Institutes of Health.
References
- 1.Center for Health Statistics. Health, United States, May 20, 2010. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/data/nvsr58/nvsr58_19.pdf. Accessed January 9, 2015.
- 2.Marston B.J., Plouffe J.F., File T.M., Jr., the Community-Based Pneumonia Incidence Study Group Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. Arch Intern Med. 1997;157(15):1709–1718. [PubMed] [Google Scholar]
- 3.Niederman M.S., McCombs J.S., Unger A.N., Kumar A., Popovian R. The cost of treating community-acquired pneumonia. Clin Ther. 1998;20(4):820–837. doi: 10.1016/s0149-2918(98)80144-6. [DOI] [PubMed] [Google Scholar]
- 4.Falguera M., Carratala J., Bielsa S. Predictive factors, microbiology, and outcome of patients with parapneumonic effusion. Eur Resp J. 2011;38(5):1173–1179. doi: 10.1183/09031936.00000211. [DOI] [PubMed] [Google Scholar]
- 5.Skouras V., Awdankiewicz A., Light R.W. What size parapneumonic effusions should be sampled? Thorax. 2010;65(1):91. doi: 10.1136/thx.2008.112797. [DOI] [PubMed] [Google Scholar]
- 6.Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Inf Clin Infect Dis. 2007;44(suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones B.E., Jones J., Bewick T. CURB-65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163. doi: 10.1378/chest.10-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones B.E., Jones J.P., Vines C.G., Dean N.C. Validating hospital admission criteria for decision support in pneumonia. BMC Pulm Med. 2014;14:149. doi: 10.1186/1471-2466-14-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed R.A., Marrie T.J., Huang J.Q. Thoracic empyema in patients with community acquired pneumonia. Am J Med. 2006;119(10):877–883. doi: 10.1016/j.amjmed.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers J.D., Singanayagam A., Murray M.P., Scally C., Fawzi A., Hill A.T. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax. 2009;64(7):592–597. doi: 10.1136/thx.2008.105080. [DOI] [PubMed] [Google Scholar]
- 11.Hasley P.B., Albaum M.N., Li Y.H. Do pulmonary radiographic findings at presentation predict mortality in patients with community-acquired pneumonia? Arch Intern Med. 1996;156(19):2206–2212. [PubMed] [Google Scholar]
- 12.Dean N.C., Jones B.E., Jones J.P. Impact of an electronic clinical decision support tool for emergency department patients with pneumonia. Ann Emerg Med. 2015;66(5):511–520. doi: 10.1016/j.annemergmed.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Sanz F., Dean N.C., Dickerson J. Accuracy of PaO2/FiO2 calculated from SpO2 for severity assessment in emergency department patients with pneumonia. Respirology. 2015;20(5):813–818. doi: 10.1111/resp.12560. [DOI] [PubMed] [Google Scholar]
- 14.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Southern D.A., Quan H., Ghali W.A. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Medical Care. 2004;42(4):355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 16.Lieffers J.R., Baracos V.E., Winget M., Fassbender K. A comparison of Charlson and Elixhauser comorbidity measures to predict colorectal cancer survival using administrative health data. Cancer. 2011;117(9):1957–1965. doi: 10.1002/cncr.25653. [DOI] [PubMed] [Google Scholar]
- 17.Menendez M.E., Neuhaus V., van Dijk C.N., Ring D. The Elixhauser comorbidity method outperforms the Charlson index in predicting inpatient death after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(9):2878–2886. doi: 10.1007/s11999-014-3686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skolnick M. The Utah Genealogical Database: a resource for genetic epidemiology. In: Cairns J.L., Skolnick M., editors. Banbury Report No 4: Cancer Incidence in Defined Populations. Cold Spring Harbor Laboratory; New York, NY: 1980. pp. 285–297. [Google Scholar]
- 19.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1997;33(1):159–174. [PubMed] [Google Scholar]
- 20.Fitzmaurice G.M., Laird N.M., Ware J.H. 2nd ed. John Wiley & Sons, Inc; Hoboken, NJ: 2012. Applied Longitudinal Analysis. [Google Scholar]
- 21.Bolker B.M., Brooks M.E., Clark C.J. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24(3):127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Sahn S.A., Heffner J.E. Pleural fluid analysis. In: Light R.W., Lee Y.C.G., editors. Textbook of Pleural Diseases. 2nd ed. Arnold Press; London, England: 2008. pp. 209–226. [Google Scholar]
- 23.McCauley L., Dean N. Pneumonia and empyema: causal, casual or unknown. J Thorac Dis. 2015;7(6):992–998. doi: 10.3978/j.issn.2072-1439.2015.04.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S., Self W.H., Wunderink R.G., the CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]