Abstract
Background
Sepsis most often presents to the ED, and delayed detection is harmful. WBC count is often used to detect sepsis, but changes in WBC count size also correspond to sepsis. We sought to determine if volume increases of circulating immune cells add value to the WBC count for early sepsis detection in the ED.
Methods
A blinded, prospective cohort study was conducted in two different ED populations within a large academic hospital.
Results
Neutrophil and monocyte volume parameters were measured in conjunction with routine CBC testing on a UniCel DxH 800 analyzer at the time of ED admission and were evaluated for the detection of sepsis. There were 1,320 subjects in the ED consecutively enrolled and categorized as control subjects (n = 879) and those with systemic inflammatory response syndrome (SIRS) (n = 203), infection (n = 140), or sepsis (n = 98). Compared with other parameters, monocyte distribution width (MDW) best discriminated sepsis from all other conditions (area under the curve [AUC], 0.79; 95% CI, 0.73-0.84; sensitivity, 0.77; specificity, 0.73; MDW threshold, 20.50), sepsis from SIRS (AUC, 0.74; 95% CI, 0.67-0.84), and severe sepsis from noninfected patients in the ED (AUC, 0.88; 95% CI, 0.75-0.99; negative predictive value, 99%). The added value of MDW to WBC count was statistically significant (AUC, 0.89 for MDW + WBC vs 0.81 for WBC alone; P < .01); a decision curve analysis also showed improved performance compared with WBC count alone.
Conclusions
The incorporation of MDW with WBC count is shown in this prospective cohort study to improve detection of sepsis compared with WBC count alone at the time of admission in the ED.
Trial Registry
ClinicalTrials.gov; No.: NCT02232750; URL: www.clinicaltrials.gov.
Key Words: biomarker, blood, cell volume, ED, monocyte, sepsis
Abbreviations: AUC, area under the curve; CMH, Cochran-Mantel-Haenszel; CRP, C-reactive protein; MDW, monocyte distribution width; MMV, mean monocyte volume; MNV, mean neutrophil volume; NDW, neutrophil distribution width; NPV, negative predictive value; PCT, procalcitonin; PPV, positive predictive value; SIRS, systemic inflammatory response syndrome
Sepsis contributes significantly to hospital resource utilization and patient mortality.1, 2, 3 Approximately 70% of sepsis cases are admitted through the ED,4 and delays in the identification and treatment of these patients contribute significantly to adverse outcomes.5, 6, 7
Whereas the definition of sepsis was recently revised (Sepsis-3) based on systemic manifestations that portend adverse outcomes,8 most hospital deaths because of sepsis occur in those presenting with milder sepsis, as reflected by Sepsis-2 criteria.3, 5 A standard approach to identifying sepsis includes a clinical suspicion of infection coupled with systemic inflammatory response syndrome (SIRS) criteria, and this approach is shown to perform well relative to Sepsis-3 criteria for predicting hospital mortality or ICU transfer.9 Therefore, the criteria used for the older Sepsis-2 definition performs well in the ED population for predicting adverse sepsis outcomes, and presumably identifies those who would most benefit from early treatment.
In response to the new Sepsis-3 definition, the Surviving Sepsis Campaign10 recommends that the identification and treatment of sepsis should be unchanged, with the first essential step being to identify suspected infection. The early detection and treatment of sepsis remains a major clinical challenge. The detection of infection is typically delayed by > 4 h after admission to the hospital11 and by > 8 h in approximately 30% of cases presenting to the ED.12 Unlike other sepsis biomarkers, such as procalcitonin (PCT) or C-reactive protein (CRP), the WBC count is among the first laboratory tests available to clinicians in the ED. As such, PCT and CRP are not typically used for early detection. Unfortunately, WBC count elevation is nonspecific for sepsis in the ED patient population.13 Therefore, there is a pressing need for a biomarker that is routinely available during the initial ED clinical evaluation to enhance the detection of sepsis.
Circulating neutrophils and monocytes are among the first to respond to pathogenic organisms.14 Recent studies suggest that immune cell volume increases may be useful for the detection of sepsis.14, 15, 16, 17, 18, 19, 20, 21 These recent studies, although encouraging, are limited by their small sample sizes and did not include patients in the ED. Therefore, we sought to determine the utility of measuring volumetric changes of circulating immune cells as predictors of sepsis in a large prospective study of adult patients in the ED. We hypothesized that volume increases in circulating neutrophils and/or monocytes, alone or in combination with other established CBC parameters, such as WBC count, would improve sepsis detection in the ED.
Methods
Patient Enrollment
The study, registered with ClinicalTrials.gov (NCT02232750) and approved by the Western Institutional Review Board, Inc (Protocol No. 20141542), was a blinded, prospective cohort study enrolling patients presenting to two different EDs at the Ohio State University Wexner Medical Center. The study enrolled adults (≥ 18-90 years of age) whose initial evaluation included a CBC within 24 h of admission to the ED, and no subject was enrolled more than once. Based on available literature,4, 22 we estimated that approximately 1,350 study subjects in the ED with concurrent CBC testing would be required to achieve the lower limits of 65% for the target sensitivity estimate of 75% using a two-sided 95% CI. These calculations were based on the approach recommended by Burderer.23 Given the large volume of admissions to the two EDs, we enrolled patients using a weekday cap of 25 consecutive patients per day.
WBC Volume Determination
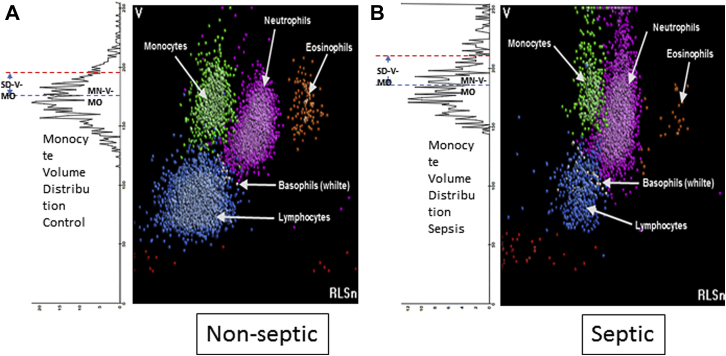
All blood samples were analyzed on a UniCel DxH 800 analyzer (Beckman Coulter, Inc) with version 2.0 software within 8 h of collection. This instrument measures specific cell volume parameters and the distribution of cell volumes within a group of cells (Fig 1) (Cell volume parameters [mean neutrophil volume (MNV), neutrophil distribution width (NDW), mean monocyte volume (MMV), and monocyte distribution width (MDW)] are research use parameters on the DxH 800; their clinical utility has not been established. These parameters are not yet approved for use as sepsis biomarkers.) The clinical research team was blinded to the results at the time of clinical data entry and during assignment of the patients to a clinical category.
Figure 1.
A, B, Cell population distribution analysis. Representative histograms of WBC populations derived from the Beckman Coulter DxH 800 analyzer. (A) Example of a nonseptic donor. (B) Two-dimensional histogram corresponds to an example of a patient with septic shock. (A) The rotated one-dimensional histogram represents the distribution of the monocyte population volumes. The dotted blue line on top of the distribution represents the mean monocyte volume. The dotted red line represents 1 SD from the mean of the distribution (ie, monocyte distribution width), which is shown to be increased in the patient with sepsis.
Clinical Classification of Patients in the ED
Study subjects were categorized as follows based on the Sepsis-2 consensus criteria:24, 25 non-SIRS (ie, zero or one SIRS criterion) and no infection; SIRS (two or more SIRS criteria); sepsis (infection plus SIRS); severe sepsis (sepsis with one or more organ failure); septic shock (sepsis with refractory hypotension); and infection, but no sepsis (ie, zero or one SIRS criterion). SIRS criteria are as follows: WBC > 12,000 or < 4,000 or > 10% bands, pulse > 90 beats per minute, respiratory rate > 20 breaths per minute, and temperature < 96.8°F or > 100.4°F. The presence of infection was determined using all available clinical data available 7 days after ED admission, including results from cultures, molecular tests (eg, polymerase chain reaction, antigens), relevant imaging, and tissue pathology.
Sepsis criteria had to be fulfilled within 6 h of the CBC count, and categorization (as previously discussed) was verified by expert review of the electronic medical record > 7 days after ED admission by two investigators (E. D. C. and D. R. C.). Discordances were adjudicated, and a final determination was made by one investigator (E. D. C.) prior to data analysis.
Preexisting Conditions
We anticipated that certain clinical conditions could confound the results of the study. For example, vitamin deficiencies (eg, B12, folate), primary bone marrow disorders, and certain drugs are common causes of generalized macrocytosis.26 Furthermore, immune suppression could suppress immune cell activation and associated changes in morphology. As such, we performed independent analyses of the data wherein we considered preexisting conditions known to cause macrocytosis or to be associated with impaired immune cell activation (e-Table 1) to determine if these preexisting conditions independently influenced the study results.
Statistical Approach
General descriptive statistics and box plots were calculated for cell population distribution parameters. Diagnostic ability was evaluated in terms of the area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) along with their 95% CIs. The score approach was used to calculate CIs for sensitivity, specificity, PPV, and NPV. The initial cutoff values were obtained using the Youden index approach, which were optimized iteratively to maximize the sensitivity and specificity.
Three approaches were used to demonstrate the added value of MDW in comparison with WBC count. The first was the differences in AUCs. The AUC was calculated using a one-predictor variable logistic model with WBC count as the predictor and sepsis status as the response. In addition, the AUC was calculated for the two-predictor variables logistic model with both WBC count and MDW as predictors and sepsis as response. The comparison between the AUC from the two models (WBC count vs WBC count + MDW) along with their CIs were calculated as described by De Long et al.27 The second was the Cochran-Mantel-Haenszel (CMH) approach. Both WBC count and MDW were dichotomized to 0 and 1 based on their values falling into the normal or abnormal category. WBC count was considered to be normal if the recovered value was between 3.6 and 12.0. MDW was considered to be normal when its recovered value was < 20.5. CMH statistics and the common OR for MDW adjusted for WBC count effects were calculated as previously described.28 MDW provided added value if the estimated OR was statistically different from 1 (ie, the 95% CI did not include 1). The third was the decision curve analysis. The number of true positives (TP) and false positives (FP) were calculated from the two logistic models (WBC count alone and WBC count + MDW). The net benefit for a given probability (p, 0 ≤ p ≤ 1) was calculated for each model as follows:
Where N is the total number of subjects in the trial, and p is the threshold probability. Decision curves (net benefit vs threshold probability) were plotted for each model and compared with each other.29, 30 SAS 9.3 (SAS Institute) was used for data analyses.
Results
Patient Demographics
From September 2014 through December 2014, 1,320 patients were enrolled. Demographic features of the patients are shown in e-Table 2. Approximately 30% of the sepsis cases were admitted through a community hospital ED in eastern Columbus, Ohio; approximately 70% were admitted to the ED of a larger tertiary hospital located near the center of the city. The distribution of control subjects and subjects with SIRS, infection, sepsis, severe sepsis, and septic shock is provided in Table 1. The prevalence of sepsis in this ED population was 7.4%, with severe sepsis or septic shock accounting for 18.8% of the patients with sepsis (1.4% of the total ED population). In keeping with other sepsis studies,31 more than one-half of the patients with sepsis (61%) had positive cultures (blood, urine, or other body fluids), with the rest being diagnosed based on clinical criteria (eg, surgical tissue analysis), serological testing, or polymerase chain reaction testing. Among those with positive cultures, diagnostic serologies, or polymerase chain reaction results, the causes of sepsis were categorized as follows: 79% bacterial, 14% viral, and 7% fungal.
Table 1.
ED Demographics Based Upon Presenting Clinical Status
| Patient Categories | No. (%) |
|---|---|
| Total | 1,320 (100) |
| Control | 879 (66.6) |
| SIRS | 203 (15.4) |
| Infection | 140 (10.6) |
| Sepsisa | 98 (7.4) |
| Sepsis | 79 (78.2) |
| Severe Sepsis | 13 (12.9) |
| Septic Shock | 6 (5.9) |
SIRS = systemic inflammatory response syndrome.
Sepsis is further broken down into sepsis, severe sepsis and septic shock categories.
Detection of Sepsis Based on Cell Volume Criteria
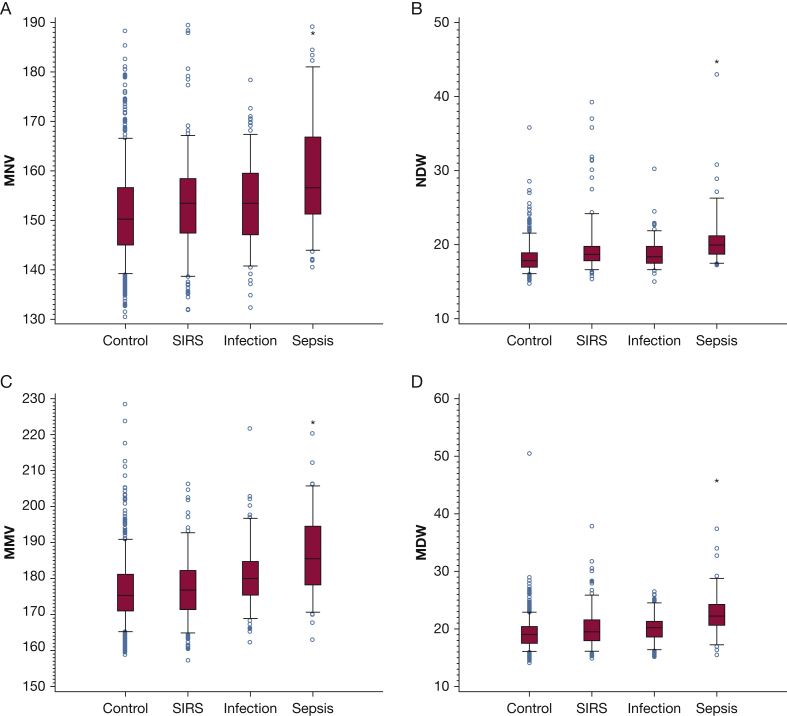
Four independent variables, as well as routine CBC count parameters, were analyzed for their ability to identify patients with sepsis (all sepsis phenotypes were combined for this analysis: sepsis, severe sepsis, and septic shock) in the ED population: MNV, NDW (a measure of size variation within the cell population), MMV, and MDW (e-Table 3, Fig 1). Neutrophil parameters MNV and NDW were significantly higher in the sepsis group compared with all others, as was the case for monocyte parameters MMV and MDW (Fig 2). Although WBC count and the WBC neutrophil percentage were higher in the sepsis group, these parameters were also elevated in the SIRS group (e-Fig 1). After establishing cutoff values best discriminating sepsis from the other conditions for each parameter (e-Table 4), we compared the discrimination of each for sepsis. Overall, MDW best discriminated sepsis from all other conditions based on the AUC (e-Table 5). The NPV of a normal MDW was 97%.
Figure 2.
A-D, Neutrophil and monocyte cell population distribution performance for sepsis in the ED population. (A) MNV is noted to be lowest in the control group, highest in the sepsis group, with intermediate values in the SIRS and infection groups. (B) The NDW shows a similar pattern. *P < .001 for sepsis group vs control group or infection group or SIRS group (individual comparisons) and for sepsis group vs control + infection + SIRS groups combined. (C) MMV is noted to be lowest in the control group, highest in the sepsis group, with intermediate values in the SIRS and infection groups. (D) The MDW shows a similar pattern, and the MDW is statistically higher in the sepsis group compared with each of the other groups. *P < .001 for sepsis group vs control group or infection group or SIRS group (individual comparisons) and for sepsis group vs control + infection + SIRS groups combined. MDW = monocyte distribution width; MMV = mean monocyte volume; MNV = mean neutrophil volume; NDW = neutrophil distribution width; SIRS = systemic inflammatory response syndrome.
The performance of MDW was slightly better when subjects with preexisting conditions associated with macrocytosis or compromised immune cell function, comprising approximately 17% of the total study population, were excluded from the analysis (e-Table 6).
Correlation of MDW With Infection Severity
As shown in e-Figure 2, MDW increases incrementally with progression from milder forms of infection to sepsis, and is highest in those with more organ dysfunction in sepsis.
MDW Added Value Analysis for Sepsis Detection
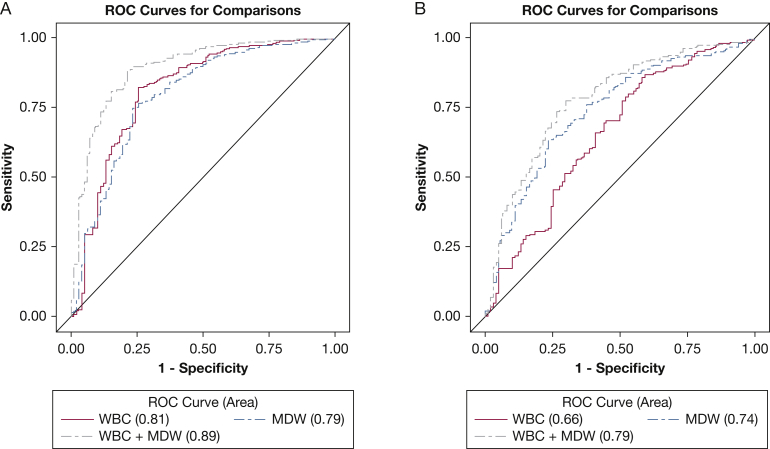
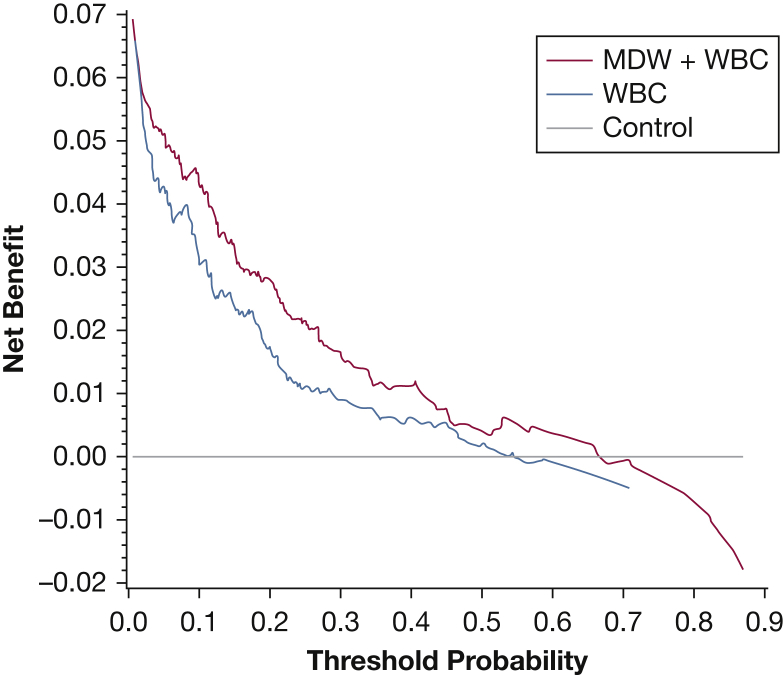
The estimated AUC for WBC count was 0.81 (95% CI, 0.75-0.86) and for MDW + WBC count was 0.89 (95% CI, 0.84-0.92). The difference between the AUCs of the two models was 0.08 (95% CI, 0.02-0.13). The CMH OR was 6.9 (95% CI, 4.2-11.4). The benefit of MDW is reflected when the difference between AUCs is significantly > 0 and the CMH OR is significantly > 1. Inclusion of MDW with WBC count provided an 8% improvement on the AUC (Fig 3A), with P = .0035, and by CMH OR (95% CIs are > 1). Because both criteria were satisfied, the MDW contribution to improved sepsis detection is statistically significant. Although the same assumptions cannot be made for comparisons of the AUC of sepsis and SIRS, MDW is shown to have a higher AUC alone, and in combination with WBC count, compared with WBC count alone (Fig 3B). As shown in Figure 4, the MDW improves sepsis detection relative to WBC count alone across a wide range of decision probability thresholds.
Figure 3.
A, B, ROC for sepsis and SIRS in subjects in the ED using MDW alone and in combination with the WBC count. (A) Comparing sepsis to all other patients in the ED: the area under the curve (AUC) for MDW (cutoff value, 20.5) is comparable with WBC count (cutoff value, 12.0); however, the addition of MDW to WBC count (using 20.5 and 12.2 as the cutoff values for MDW and WBC count, respectively) increases the AUC by 8% relative to WBC count alone, which is statistically significant (see text). (B) Comparing sepsis with SIRS, and using the same cutoff values, the AUC for MDW is higher than for the WBC count and is higher yet when MDW is added to the WBC count. ROC = receiver operating characteristic. See Figure 2 legend for expansion of other abbreviations.
Figure 4.
Decision probability curves for sepsis detection reflecting the added value of MDW compared with WBC count alone. The net benefit of WBC count alone (blue line) and in combination with MDW (red line) for the detection of sepsis was calculated over a range of pretest probabilities for sepsis (see Methods for details). The control (gray line) represents a model that provides no insight into the diagnosis of sepsis. MDW + WBC count show greater benefit than WBC count alone over a wide range of sepsis probabilities (2%-70%). The models converge > 70% sepsis probability, a situation that is not representative of the ED population. See Figure 2 legend for expansion of abbreviation.
Discussion
Volumetric increases are an early manifestation of immune cell responses to severe infections and as such have shown potential as sepsis biomarkers in humans.14, 15, 16, 17, 18, 19, 20, 21 We show that volumetric changes of circulating immune cells, particularly increased MDW, correspond with previously established criteria for sepsis24 in a large patient population in the ED. MDW distinguished sepsis from SIRS, and the magnitude of MDW elevation correlated with infection severity and organ dysfunction, ranging from normal MDW in those with limited infections, and increasing incrementally with sepsis severity. Moreover, the MDW provides added value relative to WBC count alone for early detection of sepsis in the ED. To the extent that earlier detection of sepsis leads to earlier treatment, these findings have implications for reducing sepsis mortality.5, 6, 7
Consistent with the results of recent human studies,14, 15, 16, 17, 18, 19, 20, 21 and the established function of neutrophils as first responders during the innate immune response to invading organisms, both MNV and NDW were reasonably sensitive and specific for the detection of sepsis (e-Table 5). Based on previous studies demonstrating that changes in neutrophil volume and cell activation markers (CD64) can detect sepsis in hospitalized pediatric patients,14, 17, 18, 32 we originally expected that neutrophil volume changes would be superior for the detection of sepsis in the ED population. However, MDW outperformed MNV, NDW, and all other volumetric metrics (MMV, mean corpuscular volume, and mean platelet volume; data not shown) for the detection of sepsis, particularly in the diagnostically challenging subgroup of patients in the ED presenting with SIRS (Fig 3B). We posit that monocyte parameters may perform better because circulating monocytes are first responders to infections,33, 34 and the response is proportional to the intensity of the exposure to either bacterial, fungal, or viral pathogens,35 resulting in an acute increase in monocyte size.36, 37 In contrast, the neutrophil response to certain infections (eg, viral38, 39) is relatively delayed.
The ED population is most appropriate for sepsis screening given that most sepsis cases cared for in the hospital setting meet sepsis criteria at the time of admission,4 and early identification of sepsis is critical in terms of clinical outcomes.5, 6, 7 By recent estimates, > 500,000 cases of severe sepsis, or about two-thirds of all severe sepsis cases, present to the ED each year in the United States.1, 4 This number would be even higher if milder cases of sepsis had been considered, and patients presenting with less severe sepsis make up most of sepsis deaths.1, 3 In keeping with previous studies,4, 22 we found that a minority (7.4%) of ED admissions present with sepsis, and that SIRS of noninfectious etiology was approximately two times more prevalent than sepsis.
Although this study was not designed to compare monocyte and neutrophil volumetric parameters with all other available sepsis biomarkers, MDW is shown to provide added value to WBC count, the biomarker most commonly used to screen for sepsis in the ED. Three different approaches demonstrated added value of MDW in comparison with WBC count alone.30 The area under the receiver operator characteristic curve is often criticized because it provides an overall measure of diagnostic ability and is limited in terms of the detection of positive and negative patients,30 and the test statistics and variances used for comparison of different AUCs may be biased.40 Nonetheless, an increase in the AUC indicates improved diagnostic ability. The CMH OR is also indicative of an overall added value of the diagnostic test and is valid even if data from the other tests used in the model are sparse.28 Decision curves are considered to ultimately quantify the clinical relevance of the new test in comparison with the existing ones over a wide range of clinical situations wherein the risk of sepsis is variable, and all these approaches indicate that MDW adds value to WBC count for the detection of sepsis.
It is important to emphasize that the MDW and WBC count are not comparable with other available sepsis biomarkers (eg, PCT, CRP) because they are available to the health care providers at an earlier stage of the clinical evaluation, at which time the diagnosis of sepsis has not yet been considered. PCT and CRP, which are not incorporated within the routine CBC count, are exclusively used to screen for sepsis in patients who are deemed to have a high clinical likelihood of sepsis by clinicians in the ED based on the available evidence (eg, WBC count, SIRS criteria). Therefore, the MDW and WBC count are more convenient for early sepsis detection and the diagnostic performance is excellent (AUC, 0.89), comparing favorably with that of PCT.41 Furthermore, a normal MDW + WBC count index is associated with a 98% NPV, which may prove clinically useful in excluding a sepsis diagnosis. Although further validation is needed, this relatively large prospective clinical trial that includes two distinct ED patient populations, one a community hospital ED, the other a tertiary hospital ED, provides compelling evidence that MDW in combination with WBC count could improve early sepsis detection in the ED population. A critical distinction of MDW from PCT, CRP, lactate, and other existing sepsis biomarkers is that the integration of MDW with WBC count could occur during the initial phase of ED evaluation, even before the health care provider has considered the diagnosis of sepsis.
There are several limitations of the study. First, there is no gold standard for the diagnosis of sepsis, and misclassification of sepsis and nonsepsis SIRS inevitably limits the accuracy of the biomarker.42 Furthermore, it is unclear how the MDW will perform for the newly established Sepsis-3 criteria.8 In that regard, it is notable that a subgroup of patients with more severe sepsis presentations (ie, equivalent to Sepsis-3) had the highest MDW values (e-Fig 2), and Sepsis-2 criteria (SIRS) may be superior to Sepsis-3 criteria for identifying high risk patients in non-ICU populations.9 A larger study is needed to confirm that MDW results can be standardized across multiple sites using multiple instruments and to determine if MDW + WBC count perform better when monitored over time. Ultimately, it will be important to determine how the availability of the MDW influences the management of sepsis in the ED, including early and appropriate antibiotic treatments, and sepsis-related clinical outcomes, such as hospital and ICU lengths of stay, morbidity (eg, organ failures), and mortality. The study was conducted over a 4-month period spanning the fall and early winter seasons during which a seasonal effect on sepsis was evident (more cases in late fall/early winter), emphasizing the diversity of sepsis cases and the need to consider how the test performs in each sepsis subset based on the infectious etiology (eg, viral, bacterial, fungal). Finally, host factors, as shown in e-Table 1, could influence the immune cell volumetric analysis. Whereas the inclusion of these preexisting conditions (present in 17% of patients in the ED) in our data analysis had only a small effect on the overall study results (e-Table 6), the effects of these factors may need to be considered on a case-by-case basis in the ED setting.
Conclusions
This study shows that the MDW, a measure of a change in the size distribution of circulating monocytes, provides significant added value to WBC count for the detection of sepsis in the ED population. From a practical perspective, when validated by a larger prospective study, incorporation of MDW and WBC count parameters during initial CBC count analysis could be readily used in the ED to provide a timely and convenient sepsis diagnostic tool and lead to early initiation of antimicrobial therapy.
Acknowledgments
Author contributions: E. D. C. assisted with study design and interpretation of the data, had full access to the study data, assumes responsibility for the integrity of the data and the accuracy of the analysis, and drafted the manuscript. J. E. P., C. S., D. C. A., K. B., L. T., D. C., J. W., M. S., and F. C. assisted with study design and interpretation of the data and edited the initial draft of the manuscript. L. H. and E. R. contributed to data collection and management. R. M. conducted all the statistical analyses for the manuscript. D. R. C. contributed to categorization of patients with sepsis.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor endorsed the study design, contributed to the statistical analysis of the cell volume data, and final editing of the manuscript.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by Beckman Coulter, Inc. grant to E. D. C.
Supplementary Data
References
- 1.Liu V., Escobar G.J., Greene J.D. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 2.Torio CM, Andrews RM. National inpatient hospital costs: the most expensive conditions by payer, 2011. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf. Accessed March 1, 2017. [PubMed]
- 3.Jones S.L., Ashton C.M., Kiehne L.B. Outcomes and resources of sepsis-associated stays by presence on admission, severity, and hospital time. Med Care. 2016;54(3):303–310. doi: 10.1097/MLR.0000000000000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H.E., Shapiro N.I., Angus D.C., Yealy D.M. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer R., Martin-Loeches I., Phillips G. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi: 10.1097/CCM.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Roberts D., Wood K.E. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 7.Gaieski D.F., Mikkelsen M.E., Band R.A. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 8.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(19):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churpek M.M., Snyder A., Han X. Quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores for detecting clinical deterioration in infected patients outside the ICU. Am J Respir Crit Care Med. 2017;195(7):906–911. doi: 10.1164/rccm.201604-0854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surviving Sepsis Campaign. Surviving Sepsis Campaign Responds to Sepsis-3. http://survivingsepsis.org/SiteCollectionDocuments/SSC-Statements-Sepsis-Definitions-3-2016.pdf. Accessed March 1, 2017.
- 11.Seymour C.W., Liu V.X., Iwashyna T.J. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filbin M.R., Arias S.A., Camargo C.A., Barche A., Pallin D.J. Sepsis visits and antibiotic utilization in U.S. emergency departments. Crit Care Med. 2014;42(3):528–535. doi: 10.1097/CCM.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 13.Horeczko T., Green J.P., Panacek E.A. Epidemiology of the systemic inflammatory response syndrome (SIRS) in the emergency department. West J Emerg Med. 2014;15(3):329–336. doi: 10.5811/westjem.2013.9.18064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raimondi F., Ferrara T., Capasso L. Automated determination of neutrophil volume as screening test for late-onset sepsis in very low birth infants. Pediatr Infect Dis J. 2010;29(3):288. doi: 10.1097/INF.0b013e3181c37fb4. [DOI] [PubMed] [Google Scholar]
- 15.Dilmoula A., Kassengera Z., Turkan H. Volume, conductivity and scatter properties of leukocytes (VCS technology) in detecting sepsis in critically ill adult patients. Blood. 2011;118(21):4729. [abstract] [Google Scholar]
- 16.Celik I.H., Demirel G., Askoy H.T. Automated determination of neutrophil VCS parameters in diagnosis and treatment efficacy of neonatal sepsis. Pediatr Res. 2012;71(1):121–125. doi: 10.1038/pr.2011.16. [DOI] [PubMed] [Google Scholar]
- 17.Bhargava M, Saluja S, Sindhuri U, Saraf A, Sharma P. Elevated mean neutrophil volume + CRP is a highly sensitive and specific predictor of neonatal sepsis. Int J Lab Hematol. In press. [DOI] [PubMed]
- 18.Lee A.J., Kim S.G. Mean cell volumes of neutrophils and monocytes are promising markers of sepsis in elderly patients. Blood Res. 2013;48(3):193–197. doi: 10.5045/br.2013.48.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaves F., Tierno B., Xu D. Quantitative determination of neutrophil VCS parameters by the Coulter automated hematology analyzer: new and reliable indicators for acute bacterial infection. Am J Clin Pathol. 2005;124(3):440–444. doi: 10.1309/LLF7-5W0F-WQQ8-TCC5. [DOI] [PubMed] [Google Scholar]
- 20.Chaves F., Tierno B., Xu D. Neutrophil volume distribution width: a new automated hematologic parameter for acute infection. Arch Pathol Lab Med. 2006;130(3):378–380. doi: 10.5858/2006-130-378-NVDWAN. [DOI] [PubMed] [Google Scholar]
- 21.Mardi D., Fwity B., Lobmann R., Ambrosch A. Mean cell volume of neutrophils and monocytes compared with C-reactive protein, interleukin-6 and white blood cell count for prediction of sepsis and nonsystemic bacterial infections. Int J Lab Hematol. 2010;32(4):410–418. doi: 10.1111/j.1751-553X.2009.01202.x. [DOI] [PubMed] [Google Scholar]
- 22.Rezende E., Silva J.M., Isola A.M., Campos E.V., Amendola C.P., Almeida S.L. Epidemiology of severe sepsis in the emergency department and difficulties with initial assistance. Clinics (Sao Paulo) 2008;63(4):457–464. doi: 10.1590/S1807-59322008000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burderer N.M. Statistical methodology. I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med. 1996;3(9) doi: 10.1111/j.1553-2712.1996.tb03538.x. 895-890. [DOI] [PubMed] [Google Scholar]
- 24.Levy M.M., Fink M.P., Marshall J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 25.Members of the American College of Chest Physicians/Society of Crit Care Med Consensus Conference Committee: American College of Chest Physicians/Society of Crit Care Med Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 26.Aslinia F., Mazza J.J., Yale S.H. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4(3):236–241. doi: 10.3121/cmr.4.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Long ER, DeLong DM, Clarke-Pearson DL Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 28.Agresti A. John Wiley & Sons, Inc; Hoboken, New Jersey: 2002. Categorical Data Analysis. [Google Scholar]
- 29.Vickers A.J., Elkin E.B. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moons K.G., de Groot J.A., Linnet K., Reitsma J.B., Bossuyt P.M. Quantifying the added value of a diagnostic test or marker. Clin Chem. 2012;58(10):1408–1417. doi: 10.1373/clinchem.2012.182550. [DOI] [PubMed] [Google Scholar]
- 31.Martin G.S., Mannino D.M., Easton S., Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 32.Fitrolaki D.M., Dimitriou H., Kalmanti M., Biassoulis G. CD64-neutrophil expression and stress metabolic patterns in early sepsis and severe traumatic brain injury in children. BMC Pediatr. 2013;13:31. doi: 10.1186/1471-2431-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson R.B., Hobbs J.A., Mathies M., Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102(1):328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 34.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages, phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu D. Clinical applications of leukocyte morphological parameters. Int J Clin Res. 2015;1:1. [Google Scholar]
- 36.McCullough K.C., Basta S., Knotig S. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98(2):203–212. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S.Y., Mak K.L., Chen L.Y., Chou M.P., Ho C.K. Heterogeneity of human blood monocyte: two subpopulations with different sizes, phenotypes and functions. Immunology. 1992;77(2):298–303. [PMC free article] [PubMed] [Google Scholar]
- 38.Iwasaki A., Pillai P.S. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou W., Gibbs J.S., Lu X. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119(13):3128–3131. doi: 10.1182/blood-2011-09-379479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seshan V.E., Gonen M., Begg C.B. Comparing ROC curves derived from regression models. Stat Med. 2013;32(9):1483–1493. doi: 10.1002/sim.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kibe S., Adams K., Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66(suppl 2):ii30–ii40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]
- 42.Lee T.S., Hui Q. Testing the assumption of non-differential misclassification in case-control studies. J Mod Appl Stat Methods. 2013;12(2):211–230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.