Abstract
Sickle cell disease (SCD), the most common genetic hemolytic anemia worldwide, affects 250,000 births annually. In the United States, SCD affects approximately 100,000 individuals, most of African descent. Hemoglobin S (HbS) results from a glutamate-to-valine mutation of the sixth codon of the β-hemoglobin allele; the homozygous genotype (HbSS) is associated with the most prevalent and severe form of the disease. Other SCD genotypes include HbSC, composed of one HbS allele and one HbC (glutamate-to-lysine mutation) allele; and HbS-β-thalassemia0 or HbS-β-thalassemia+, composed of one HbS allele and one β-thalassemia allele with absent or reduced β-chain production, respectively. Despite advances in care, median survival remains in the fifth decade, due in large part to chronic complications of the disease. Chronic pulmonary complications in SCD are major contributors to this early mortality. Although our understanding of these conditions has improved much over the past 10 to 15 years, there remains no specific treatment for pulmonary complications of SCD. It is unclear whether conventional treatment regimens directed at non-SCD populations have equivalent efficacy in patients with SCD. This represents a critical research need. In this review, the authors review the state-of-the-art understanding of the following pulmonary complications of SCD: (1) pulmonary hypertension; (2) venous thromboembolic disease; (3) sleep-disordered breathing; (4) asthma and recurrent wheezing; and (5) pulmonary function abnormalities. This review highlights the advances as well as the knowledge gaps in this field to update clinicians and other health care providers and to garner research interest from the medical community.
Key Words: airway hyperresponsiveness, pulmonary embolism, pulmonary hypertension, sickle cell disease, sleep-disordered breathing
Abbreviations: ACS, acute chest syndrome; AHI, apnea-hypopnea index; AHR, airway hyperresponsiveness; Dlco, diffusion capacity of the lung for carbon monoxide; Hb, hemoglobin; HbC, hemoglobin C; HbS, hemoglobin S; NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; PAH, pulmonary arterial hypertension; PE, pulmonary embolism; PFT, pulmonary function testing; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RCT, randomized controlled trial; RV, right ventricular; SCD, sickle cell disease; SCD-PH, sickle cell disease-associated pulmonary hypertension; SDB, sleep-disordered breathing; TRV, tricuspid regurgitant jet velocity; VOE, vaso-occlusive event
Sickle cell disease (SCD) is clinically heterogeneous, characterized pathogenically by recurrent episodes of hemolysis and vaso-occlusion. During hypoxia, the hemoglobin (Hb) tetramer () polymerizes, leading to the classic inflexible crescent shape of the erythrocyte. Hb polymerization and abnormal interactions between erythrocytes and leukocytes, platelets, and the vascular endothelium lead to increased viscosity, impaired microvascular blood flow, tissue ischemia-reperfusion injury, and promotion of inflammation, thrombosis, and oxidant stress.1
Despite advances in care, median survival in SCD remains in the fifth decade. Acute and chronic pulmonary complications are among the most common causes of morbidity and mortality, yet our understanding of each of these conditions remains limited. We are focusing the current review on the chronic pulmonary complications of SCD, which affect practically every cell type and structure of the lungs.2 This is an update for clinicians and researchers to improve the care for these patients and to foster research interests.
Pulmonary Hypertension
Definitions
Pulmonary hypertension (PH), defined hemodynamically as a mean pulmonary arterial pressure ≥ 25 mm Hg at rest, occurs in 6% to 10.4% of adults with SCD.3, 4, 5, 6 Right-sided heart catheterization is the gold standard for the diagnosis of PH3 and assessment of prognosis. The hemodynamics of PH in patients with SCD are heterogeneous (Table 1). PH may be precapillary (pulmonary arterial hypertension [PAH]), postcapillary (pulmonary venous hypertension), or have features of both.7 Clinical guidelines from the American Thoracic Society’s expert panel define elevated pulmonary vascular resistance (PVR) in patients with SCD as different from other types of precapillary PH because of the anemia-induced elevation of cardiac output, and consequent reduction in PVR that occurs at baseline.8 In idiopathic PAH, increased PVR is defined as ≥ 240 dynes·s/cm5 (3 Wood Units), which is 2 standard deviations above the PVR of ≤ 80 to 120 dynes·s/cm5 (1 Wood Unit) that is observed in healthy volunteers. In adults with SCD, the baseline mean cardiac output is 8 to 9 L/min with a PVR of 0.90 to 0.93 (± 0.31-0.48) Wood Units.5, 6, 7 This suggests that in SCD, ≥ 2 Wood Units is consistent with elevated PVR.8
Table 1.
Hemodynamic Profiles and Mortality Risk for Pulmonary Hypertension in Sickle Cell Disease
| Precapillary PH | Postcapillary PH | PH with Features of Both Precapillary and Postcapillary PH | |
|---|---|---|---|
| mPAP | ≥ 25 mm Hg | ≥ 25 mm Hg | ≥ 25 mm Hg |
| PAWP or LVEDP | ≤ 15 mm Hg | > 15 mm Hg | > 15 mm Hg |
| PVR | ≥ 2 Wood Units | < 2 Wood Units | ≥ 2 Wood Units |
| Survival | Reduced | Reduced | Reduced |
LVEDP = left ventricular end-diastolic pressure; mPAP = mean pulmonary arterial pressure; PAWP = pulmonary arterial wedge pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance.
Hemodynamics consistent with precapillary PH occur in approximately 40% to 50% of those with PH and in 2.5% to 5.9% of adults with SCD.5, 7 Although these hemodynamics are typically observed in PAH, in SCD they can also be observed in patients with chronic thromboembolic PH and, at times, in those with an anemia-induced hyperdynamic state. When compared with associated forms of pulmonary arterial hypertension classified as group 1 PAH, this is second only to systemic sclerosis (12%) in terms of frequency.9 Despite the hemodynamic similarities to group 1 PAH, the most recent PH classification guidelines have placed the PH of SCD into group 510 on the basis of (1) features of both precapillary and postcapillary PH7, 11, 12 in these patients, (2) a lower PVR compared with group 1 PAH, and (3) coexistent thromboses in some patients similar to group 4 PH.13 This unfortunately reflects the imprecision of the current PH classification system and stresses the need to better define PH subgroups.8
The complex hemodynamics of PH in SCD emphasize the need for right-sided heart catheterization for diagnostic confirmation, and to accurately stratify patients prior to consideration of treatment options. Although the hemodynamics of PH in SCD can differ across patients, all have reduced exercise capacity and survival.4, 14
Diagnostic Evaluation
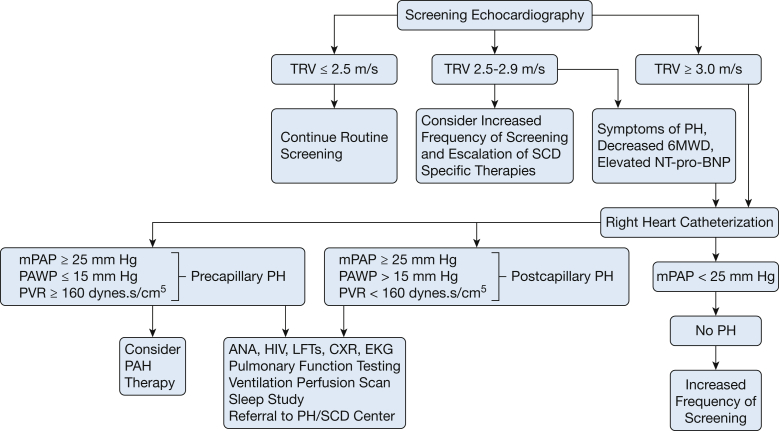
In 2014, the American Thoracic Society published evidence-based consensus guidelines for the diagnosis and treatment of sickle cell disease-associated pulmonary hypertension (SCD-PH).8 The diagnostic evaluation of a patient with suspected PH is similar to that of a patient without SCD (Fig 1).3
Figure 1.
Diagnostic algorithm for the evaluation of patients with sickle cell disease and suspected of having pulmonary hypertension. 6MWD = 6-minute walk distance; ANA = anti-nuclear antibody; CXR = chest X-ray; LFTs = liver function tests; mPAP = mean pulmonary arterial pressure; NT-pro-BNP = N-terminal pro-brain natriuretic peptide; PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; PH = pulmonary hypertension; PVH = pulmonary venous hypertension; PVR = pulmonary vascular resistance; SCD = sickle cell disease; TRV = tricuspid regurgitant jet velocity.
Patients with SCD and symptoms suggestive of PH should be evaluated initially by echocardiography.8 Elevated right ventricular (RV) systolic pressure (reflective of elevated tricuspid regurgitant jet velocity [TRV]), RV dilation or dysfunction, right atrial enlargement, tricuspid insufficiency, and interventricular septum flattening are all suggestive of PH.15 While a TRV ≥ 2.5 m/s is associated with 40% 40-month mortality, it only has a sensitivity of 78%, specificity of 19%, and positive predictive value of 25% for PH.5 Using a TRV of > 2.9 m/s decreased the sensitivity to 67% but increased the specificity to 81%.16
Serum N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels, reflective of right and left ventricular strain, can be used for mortality risk stratification and to improve the positive predictive value of echocardiography in SCD.8 An NT-pro-BNP level of ≥ 160 pg/mL increases the mortality risk in SCD three to six fold.17 In addition, serial levels may be helpful in assessing prognosis and response to therapy.
The 6-minute walk test is an important measure of functional capacity in non-SCD PAH.18 In SCD-PH, although concomitant SCD-related musculoskeletal or neurologic disease may reduce its reliability, 6-minute walk distance (6MWD) correlates with PH severity and improves with therapy.12 Patients with SCD-PH had significant limitations in 6MWD (358 ± 115 vs 437 ± 108 m; P = .004) compared with those without PH, reflective of reduced exercise capacity.7 The combination of a TRV of ≥ 2.5 m/s with either an NT-pro-BNP level > 164 pg/mL or a 6MWD < 333 m improved the positive predictive value of echocardiography to 62%.5
SCD-PH: Survival and Prognosis
Hemodynamically, patients with SCD-PH have relatively modest elevations of mean pulmonary arterial pressure and PVR, and often have preserved cardiac output.12 However, worsening PH severity, as determined by hemodynamics, is a mortality risk factor in this population.7 Although the severity of hemodynamic derangements is important, all patients with SCD-PH have reduced functional capacity, increased mortality, and severe histopathologic changes, suggesting that other factors impact outcomes.7 After a median follow-up of 4.7 years, all-cause mortality was higher in patients with PH compared with those without PH (log-rank test, P < .001). Survival estimates for the patients with SCD with PH compared with those without PH were 89% vs 100% at 1 year, 76% vs 93% at 3 years, and 63% vs 83% at 5 years from diagnosis, respectively. Several hemodynamic variables were associated with increased mortality in the patients with SCD-PH, although a reduced cardiac index, typical of idiopathic PAH, was not (Table 2).7 In PH of SCD, the transpulmonary pressure gradient and PVR were independent mortality risk factors, whereas the pulmonary arterial wedge pressure was not, suggesting that precapillary PH may be a larger determinant of mortality risk.7 Of note, during vaso-occlusive events19 and acute chest syndrome (ACS) an acute rise in pulmonary arterial pressure occurs, which carries an additional mortality risk for these patients.20
Table 2.
Hemodynamic Variables Associated with Increased Mortality Risk in Pulmonary Hypertension of Sickle Cell Diseasea
| Variable | Adjusted HR | 95% CI | P Value |
|---|---|---|---|
| mPAP | 1.61/10 mm Hg | 1.05-2.45 | .027 |
| dPAP | 1.83/10 mm Hg | 1.09-3.08 | .022 |
| TPG | 1.78/10 mm Hg | 1.14-2.79 | .011 |
| PVR | 1.44/Wood Unit | 1.09-1.89 | .009 |
| dPAP – PAWP | 2.19/10 mm Hg | 1.23-3.89 | .008 |
Treatment of PH in SCD
SCD-Specific Therapy
Patients with SCD-PH should undergo intensification of SCD-specific therapy to reduce the hemolytic anemia that may contribute to disease pathogenesis. Erythrocyte damage-associated molecular pattern molecules, including cell-free hemoglobin, arginase, and reactive oxygen species, are released into the bloodstream during hemolysis and contribute to endothelial dysfunction.21 Hydroxyurea is the first-line treatment to reduce hemolytic anemia.22 In patients who cannot tolerate hydroxyurea, the use of chronic red blood cell transfusions can be considered.22 Although neither hydroxyurea nor chronic transfusions have been specifically tested in randomized controlled trials (RCTs) in patients with SCD-PH, hydroxyurea increases fetal Hb concentration, decreases the frequency of vaso-occlusive events and ACS, and improves survival of HbSS patients. Vaso-occlusive events and ACS increase the risk of death in patients with SCD-PH related to acute right-sided heart failure both during and after hospitalization, and this indirectly justifies the use of hydroxyurea. The American Thoracic Society guidelines committee strongly recommended the use of hydroxyurea for patients with SCD-PH or those with increased mortality risk based on an elevated TRV or NT-pro-BNP level.5, 8, 19 Chronic transfusion therapy can improve tissue oxygen delivery by decreasing the synthesis of sickle RBCs and the hemolytic rate.23 The benefits of its use must be balanced against the risks of iron overload and alloimmunization, and this treatment can be considered for HbSS patients with PH who are not candidates for hydroxyurea.8
Treatment of Associated Conditions
Patients with SCD-PH should be screened and treated for VTE and sleep-disordered breathing (SDB) because these may worsen cardiopulmonary function.8 Supplemental oxygen should be used to maintain an arterial oxyhemoglobin saturation of at least 90% at rest, with exertion, and during sleep.24 Diuretics are used to treat volume overload,24 but this must be done carefully to minimize the risk of volume depletion-induced erythrocyte sickling.
Pulmonary Vasodilator Therapy
Pulmonary vasodilators have not been well studied in SCD-related PH. Two RCTs compared bosentan with placebo in patients with SCD with precapillary PH (ASSET-1) or postcapillary PH with a PVR of > 100 dynes·s/cm5 (ASSET-2).25 After randomization of only 14 subjects in ASSET-1 and 12 patients in ASSET-2, the trials were prematurely terminated because of withdrawal of sponsor support. Although few patients were enrolled, there was no apparent bosentan-related toxicity. A third RCT, Walk-PHaSST (Treatment of PH and SCD with Sildenafil Therapy),26 compared sildenafil with placebo in patients with SCD with a TRV of ≥ 2.7 m/s. After 74 (of a targeted 132) subjects were enrolled, the study was prematurely discontinued because of increased serious adverse events in the sildenafil group, primarily hospitalization for vaso-occlusive events.
These RCTs were insufficient to determine whether or not patients with SCD and precapillary PH should receive pulmonary vasodilator therapy because of the small sample sizes, the relatively few events, and the imprecision of the estimated effects.8 Anecdotally, however, some patients with SCD with precapillary PH do respond well to these therapies. In support of this, four case series in which patients with SCD and precapillary PH received bosentan, sildenafil, and/or epoprostenol demonstrated an increase in 6MWD of 41 to 144 m beyond baseline.27, 28, 29 Hemodynamics also improved, but these were ascertained only in a small number of subjects.29 The most common adverse effects were headache (15%) and peripheral edema (21%); transaminase elevation occurred in 14% of patients during bosentan therapy.29 On the basis of these findings, pulmonary vasodilator therapy can be considered on a case-by-case basis for patients with SCD with precapillary PH. We do not recommend phosphodiesterase-5 inhibitor therapy as first-line treatment and typically start with an oral endothelin receptor antagonist because they are generally well tolerated. Patients with SCD with only an elevated TRV or postcapillary PH should not receive these medications.8
Overall, treatment of patients with SCD-PH is complex, and we advocate for referral to a center with expertise in these disciplines. Ideally, these patients need to be enrolled in clinical trials so that we can better understand their response to therapy. What we learned from the Walk-PHaSST trial is that the underlying SCD may have important impacts on medication response and toxicity that may not be detected outside of a clinical trial setting.
Venous Thromboembolism in SCD
VTE (deep vein thrombosis and pulmonary embolism [PE]) affects about 25% of adults with SCD and is a risk factor for death.30, 31 Despite the known hypercoagulability of SCD, VTE is often overlooked as a major complication of SCD,30 in part because in situ microvascular thrombosis occurs in these patients and large-vessel thromboses have been recognized only with diagnostic advances.
The cause of increased VTE risk in SCD is multifactorial. Patients with SCD have traditional VTE risk factors and SCD-specific factors contributing to VTE risk. In fact, every aspect of Virchow’s triad is present in patients with SCD, resulting in a highly thrombogenic environment (Table 3).32
Table 3.
Risk Factors for Venous Thromboembolism in Sickle Cell Disease
| Traditional VTE Risk Factors | SCD-Related VTE Risk Factors |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
SCD = sickle cell disease; VOE = vaso-occlusive event; vWF = von Willebrand factor.
Prevalence of VTE in SCD and Link to ACS
An evaluation of 1,804,000 SCD admissions between 1979 and 2003 revealed that patients with SCD under the age of 40 years had a risk for PE approximately 3.5 times higher than that of African-American control subjects.33 A second inpatient study demonstrated a nearly 50- to 100-fold increased annual incidence of PE in hospitalized 50-year-old patients with SCD compared with patients without SCD.31 More recently, Naik et al reported a history of VTE in 25% of adult patients with SCD; the median age at first VTE was 30 years.30 These data are similar to those observed in family cohorts of patients with high-risk thrombophilias,34 and underscore the potent thrombophilia of SCD.
Although the cause of ACS is often multifactorial, a link between PE and ACS has been reported.35 In a study of 144 ACS events, 17% had pulmonary thrombi without coexistent fat emboli on CT scanning; primarily filling defects in the main pulmonary artery or its segmental branches.36 No proximal deep vein thrombosis was observed, although two patients did have an infrapopliteal thrombosis. This suggests that pulmonary thrombosis during ACS may be an in situ, rather than embolic phenomenon.36 In addition, in the Cooperative Study of SCD, PE was the most common cause of death, although thrombi were not differentiated from fat emboli.35 Given the uncertainty concerning the cause of PEs in ACS, anticoagulation practices vary widely. Pediatricians do not typically prescribe anticoagulation in ACS,37 whereas in adults, treatment dose anticoagulation is frequently used and continued for a standard course of therapy.36
VTE and Pulmonary Hypertension
Thrombosis is likely a disease modulator in PH of SCD. In one study, six of 27 patients with SCD-PH who underwent ventilation-perfusion scans had high-probability results; three scans were suggestive of chronic thromboembolic PH.12 In a small autopsy study, up to 50% of patients with SCD with RV hypertrophy had coexistent proximal-vessel pulmonary thrombi.38 Successful pulmonary endarterectomy has been described in an HbSC patient with chronic thromboembolic PH, suggesting that this is a large-vessel clot potentially amenable to surgical treatment.39
VTE Treatment
Small pilot studies have investigated the use of anticoagulants to prevent vaso-occlusive events (VOEs) in SCD,40 but trials evaluating their use for VTE treatment and prevention are lacking. Anticoagulation treatment of VTE in SCD, therefore, follows established guidelines for VTE treatment in the general population.41 As recurrent VTE is common in SCD,30 care should be taken to elicit a comprehensive history to inform treatment duration decisions, and indefinite anticoagulation for patients with SCD with recurrent VTE should be considered.41 Patients with SCD-PH, VTE suggestive of a possible thromboembolic component to their PH, and no additional risk factors for bleeding should be considered for indefinite anticoagulant therapy.8 The use of D-dimer levels is unreliable in SCD. Patients with SCD have elevated baseline D-dimer levels compared with control subjects because of coagulation cascade activation, and these fluctuate during vaso-occlusive events.42
It is likely that the presence of SCD should impact decisions to prescribe VTE prophylaxis. Patient demographics are often used to determine VTE risk but, in SCD, may not be appropriate. In addition, the degree of anemia and potential bleeding risk needs to be addressed in conjunction with knowledge of the patient’s baseline Hb values. Prophylactic anticoagulation for orthopedic surgery or pregnancy should be prescribed on the basis of general current practices.43, 44
Although transfusion decreases VTE risk in patients with β-thalassemia,45 chronic transfusion therapy is not currently used for VTE treatment in SCD. Hydroxyurea use is associated with decreased coagulation markers46; however, its effect on VTE risk is unknown.
Sleep-Disordered Breathing and Nocturnal Hypoxemia
SDB, particularly OSA and intermittent oxyhemoglobin desaturation, are potential contributors to cardiopulmonary compromise in SCD, possibly via increasing vaso-occlusion.47, 48 Despite this, SDB is underappreciated and, consequently, often undertreated; this is due, in part, to a lack of understanding of its prevalence and natural history in SCD.
Much of what is known about SDB in SCD stems from retrospective cohort studies in which up to 79% of symptomatic children and adolescents were affected.47, 49 In two small cohort studies of asymptomatic children and adolescents, 10% to 40% of patients with SCD had OSA, based on an apnea-hypopnea index (AHI) ≥ 1/h; 10.6% had an AHI ≥ 5/h.50, 51 It is unclear whether an AHI between 1/h and 5/h has clinical relevance, particularly in adults. This is an area in need of greater investigation. In patients without SCD, the prevalence of OSA increases with age, yet, in adults with SCD, SDB is rarely considered by clinicians. However, one study of 32 consecutive HbSS adults reported SDB in 44% with a mean AHI of 17/h (95% CI, 10-24/h).52 These studies, although small in size, suggest that SDB is common in SCD and may occur in the absence of symptoms.
The cause of SDB is not well understood in SCD. Children and adolescents with SCD have reduced upper airway diameters and increased adenoid and tonsillar hypertrophy compared with normal volunteers,48 both of which are known OSA risk factors. Although it has been presumed that OSA is the primary cause of nocturnal hypoxemia in SCD, this is not always the case. In one study of patients with SCD irrespective of SDB symptoms, 20 of 38 (53%) had an oxyhemoglobin saturation of < 92% for > 5% of total sleep time, but 12 of those 20 patients did not have OSA,53 which may explain why adenotonsillectomy is ineffective in some patients.47 Oxyhemoglobin desaturations in patients with SCD are often multifactorial (Table 4).54 In addition, gas exchange abnormalities in SCD go beyond hypoxemia. In one study comparing 12 children with SCD and OSA with 10 age-, sex-, and racially matched children with OSA alone, the children with SCD and OSA not only had longer periods of oxygen desaturation, but also a higher percentage of sleep time with end-tidal carbon dioxide levels > 50 mm Hg.49 Interestingly, oxygen desaturations with sleep are associated with daytime hypoxemia in SCD,55 suggesting a more widespread pathologic impact.
Table 4.
Mechanisms of Nocturnal Oxygen Desaturation in Sickle Cell Disease
|
|
|
|
|
|
The clinical consequences of SDB in SCD may go beyond the cardiopulmonary system. There is some evidence linking nocturnal hypoxemia with an increased rate of both vaso-occlusive and cerebrovascular events,56 but these data are inconsistent. Epidemiologic associations between OSA and nocturnal enuresis57 and between nocturnal hypoxemia and priapism58 in SCD have been reported.
It is uncertain whether the same risk factors and symptoms associated with SDB in patients without SCD are relevant in SCD and, as such, it is difficult to make recommendations in terms of screening.59 Habitual snoring and lower waking oxygen saturation appear to be strong risk factors for OSA in children with SCD, but it is not clear whether this is true in adults.59 We recommend that evaluation for SDB should also be considered for patients with SCD with unexplained hypoxemia, recurrent vaso-occlusive events, or enuresis. The treatment of SDB complicating SCD is essentially the same as that in patients without SCD. In a small case series, treatment of children with SCD and OSA by adenotonsillectomy produced a reduction in TRV,60 suggesting a potential benefit on cardiopulmonary outcomes. All children and adolescents with SCD and OSA should be evaluated for adenotonsillectomy.61 For those in whom this is not an option, the use of oxygen supplementation and/or bilevel or continuous noninvasive positive airway pressure with sleep is recommended.
Asthma and Recurrent Wheezing
In the United States, African Americans and people of mixed race have a higher prevalence of asthma than do white individuals with an increased mortality rate.62 One of the difficulties in diagnosing asthma in SCD stems from the overlap of clinical features that may be due solely to SCD63, 64 and those of asthma in the non-SCD population (Table 5). Koumbourlis et al reported that 35% of 5- to 18-year-old patients with SCD had lower airway obstruction.64 In addition, up to 77% of children and adolescents had airway hyperresponsiveness (AHR),65 and there was a 20% to 48% prevalence of asthma.64, 66 Of patients with SCD, 54% had a positive bronchodilator response64 and AHR by cold air challenge irrespective of findings on spirometry or asthma symptoms. This and the lack of relationship between methacholine-induced AHR and either traditional symptoms or a physician diagnosis of asthma suggest a potential novel mechanism for AHR in SCD.63 Interestingly, AHR is associated with increased serum lactate dehydrogenase levels, suggesting a link to hemolysis in SCD.
Table 5.
Comparison of Clinical Features of Recurrent Wheezing vs Asthma in Sickle Cell Disease
| Patient Characteristics | Recurrent Intermittent Wheezing | Asthma |
|---|---|---|
| Typical asthma symptoms (nocturnal cough, positive family history, atopy, elevated IgE) |
|
|
| Potential risk factors | ||
| Pathophysiologic mechanisms |
|
|
| Risk for VOE and/or acute chest syndrome |
|
|
| Risk of death |
|
|
| Treatment | Optimal treatment is less clear:
|
NIH guidelines:
|
LDH = lactate dehydrogenase; NIH = National Institutes of Health; Th2 = helper T-cell type 2.
Expert opinion.
There has been no standard diagnostic approach to asthma in SCD clinical studies,63 making comparison across studies difficult. In 187 children with SCD, the presence of a parent with asthma (P = .006), and of wheezing associated with dyspnea and with exercise (P = .001), was 100% sensitive for an asthma diagnosis.67 Patients with SCD with asthma had nearly twice as many episodes of ACS (0.39 vs 0.20 events/patient-year) and more frequent vaso-occlusive events (1.39 vs 0.47 events/patient-year). In addition, these patients had a more than two-fold increased risk of mortality (hazard ratio, 2.36; 95% CI, 1.21-4.62; P = .01) when compared with those without asthma.2, 68 Possible explanations for these findings include the following: (1) tissue hypoxia due to bronchoconstriction-induced ventilation-perfusion mismatch,54 and (2) increased inflammation and oxidative stress with increased endothelial adhesion molecule expression and vaso-occlusion.69
However, some patients with SCD have recurrent wheezing without a personal or familial history of asthma.70 Importantly, recurrent, severe wheezing regardless of an asthma history was associated with increased morbidity and mortality.71, 72 In one study, 19% of 262 patients with SCD visiting an ED had at least one episode of physician-documented wheeze, but 53% did not have a history of asthma.72 Wheezing was associated with an 118% increase in vaso-occlusive events and a 158% increase in ACS, which was greater than the risk in patients with asthma. This suggests that recurrent wheezing and asthma are possibly independent risk factors for ACS in SCD.72 It is recommended that all children and adolescents with SCD undergo a complete evaluation for asthma if the diagnosis is suspected, as should all those with multiple episodes of VOE or ACS; the association between asthma and SCD in adults is less clear.
Asthma treatment in SCD should follow the National Institutes of Health guidelines for asthma treatment in the general population.73 Although allergen avoidance is essential, medications remain the cornerstone of treatment. Inhaled corticosteroids should be the first-line treatment for chronic persistent asthma, with the use of inhaled β2-agonists as a rescue medication. Systemic corticosteroids should be used in the treatment of acute asthma exacerbations even though there is some concern that they may increase the frequency of rebound pain in SCD and may place patients with SCD at increased risk for avascular necrosis.74 A pulmonary and hematology specialist should be consulted when systemic steroids are used in these patients. Patients with SCD may have increased urinary leukotrienes, suggesting that leukotriene antagonists may have additional benefit in this population, but this has not been well studied.75
Pulmonary Function in SCD
Adults
Pulmonary function testing (PFT) often produces abnormal results in adults with SCD.76, 77, 78 A cross-sectional analysis of 310 HbSS adults demonstrated abnormal pulmonary function in 90%, predominantly restrictive physiology (mildly decreased total lung capacity and diffusion capacity of the lung for carbon monoxide [Dlco]).77 Longitudinal studies of adults with SCD demonstrate an average decline in forced expiratory volume in 1 second of 49 mL/y (unrelated to smoking status) compared with 20 to 26 mL/y in the general population; this decline is an independent risk factor for mortality.79
The exact mechanism of restrictive physiology in SCD is likely multifactorial (Table 6). Historically, this was thought to be due to acute on chronic inflammation, intensified by ACS episodes, and parenchymal fibrosis; but more recently, no correlation between pulmonary fibrosis on CT scanning and abnormal respiratory physiology was observed.78 However, increased small-vessel pulmonary vascular dimensions on CT scanning were associated with reduced air flow, increased air trapping, hypoxemia, and lung function decline, suggesting a link between lung function and the vasculature.78
Table 6.
Possible Factors Associated with Restrictive Physiology in Sickle Cell Disease
|
|
|
|
Extrapulmonary conditions, such as VOE-related respiratory muscle ischemia and rib infarcts,80 are associated with abnormal lung volumes and Dlco values in SCD, but the causation is unclear. Cardiomegaly is associated with decreased lung volumes in patients without SCD with congestive heart failure81; 16 of 33 patients with SCD had an elevated cardiac-to-thoracic ratio on chest radiography and this was associated with reduced lung volumes.82
Oxygen saturation is reduced in patients with SCD, with baseline values below 96% in many patients and significant desaturations with both exertion and sleep. One mechanism by which this occurs is via the rightward shift of the oxyhemoglobin dissociation curve. A widened alveolar-arterial oxygen gradient occurs both at rest and with exercise and the Dlco is reduced, even when corrected for race and degree of anemia (Table 7).
Table 7.
Potential Mechanisms Leading to Reduced Diffusion Capacity in Sickle Cell Disease
|
|
|
Children and Adolescents
Children and adolescents with SCD also have abnormal PFT results. Although restrictive physiology can occur, multiple studies report a predominance of obstructive disease.83 A retrospective study of 127 children and adolescents with SCD demonstrated obstructive physiology in 35% and restrictive physiology in 26%.83 A subsequent prospective study of 146 HbSS or HbS-β-thalassemia0 patients, 7 to 20 years old, demonstrated abnormal PFT results in 39%, most commonly in an obstructive pattern.84 Increasing age, a family or patient history of asthma or wheezing, and increased hemolysis were all associated with obstructive physiology. In addition, up to 70% of children with SCD have AHR on methacholine challenge testing.64
Recommendations Concerning PFT in SCD
Despite the frequency of abnormal PFT results in SCD, the most recent National Heart, Lung, and Blood Institute-sponsored SCD guidelines do not recommend screening by PFT, because of the lack of impact on outcomes. Dyspnea occurs in up to 50% of HbSS adults and in up to 40% of HbSC adults,85 and we believe that PFT is an essential part of evaluating patients with SCD with dyspnea regardless of genotype. The usefulness of PFT in asymptomatic adults is unclear. In children and adolescents, the observed frequency of obstructive disease and AHR leads us to suggest performing spirometry in children with SCD every 1 to 3 years, using the more frequent intervals for those with dyspnea, a history of asthma or recurrent wheezing, or marked elevations in hemolytic markers.84
Summary and Future Directions
Pulmonary complications of SCD represent a diverse group of diseases affecting the large and small airways, parenchyma, and vasculature. Although pulmonary disease clearly has an adverse impact on outcomes in SCD, much needs to be learned about the natural history of these conditions across the life span of patients with SCD to better understand the potential impacts of screening and prevention. Moreover, clinical trials that target this population specifically are needed, because it is not appropriate to assume that medications that are efficacious in the general population will have similar safety and efficacy profiles in patients with SCD. To advance this field, a collaborative effort between clinicians and researchers from the adult and pediatric pulmonary, cardiology, and hematology fields will be necessary.
Acknowledgment
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: E. S. K. has received grant support from Actelion Pharmaceuticals and Pfizer. None declared (A. M.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: Dr Mehari is supported by National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number P50HL118006 and Department of Medicine Academic Enrichment Fund.
References
- 1.Bunn H.F. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Miller A.C., Gladwin M.T. Pulmonary complications of sickle cell disease. Am J Respir Crit Care Med. 2012;185(11):1154–1165. doi: 10.1164/rccm.201111-2082CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeper M.M., Bogaard H.J., Condliffe R. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Mehari A., Gladwin M.T., Tian X. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307(12):1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parent F., Bachir D., Inamo J. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca G.H.H., Souza R., Salemi V.M.C. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39(1):112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 7.Mehari A., Alam S., Tian X. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187(8):840–847. doi: 10.1164/rccm.201207-1222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klings E.S., Machado R.F., Barst R.J. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014;189(6):727–740. doi: 10.1164/rccm.201401-0065ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukerjee D., St George D., Coleiro B. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Ann Rheum Dis. 2003;62(11):1088–1093. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonneau G., Gatzoulis M.A., Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Klings E.S. Pulmonary hypertension of sickle cell disease: more than just another lung. Am J Hematol. 2008;83(1):4–5. doi: 10.1002/ajh.21083. [DOI] [PubMed] [Google Scholar]
- 12.Anthi A., Machado R.F., Jison M.L. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175(12):1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naeije R. Physiology of the pulmonary circulation and the right heart. Curr Hypertens Rep. 2013;15(6):623–631. doi: 10.1007/s11906-013-0396-6. [DOI] [PubMed] [Google Scholar]
- 14.Castro O., Hoque M., Brown B.D. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101(4):1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 15.Forfia P.R., Vachiery J.L. Echocardiography in pulmonary arterial hypertension. Am J Cardiol. 2012;110(6 suppl):16S–24S. doi: 10.1016/j.amjcard.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald M., Fagan K., Herbert D.E. Misclassification of pulmonary hypertension in adults with sickle hemoglobinopathies using Doppler echocardiography. South Med J. 2012;105(6):300–305. doi: 10.1097/SMJ.0b013e318256b55b. [DOI] [PubMed] [Google Scholar]
- 17.Machado R.F., Hildesheim M., Mendelsohn L. NT-pro brain natriuretic peptide levels and the risk of death in the cooperative study of sickle cell disease. Br J Haematol. 2011;154(4):512–520. doi: 10.1111/j.1365-2141.2011.08777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto S., Nagaya N., Satoh T. Clinical correlates and prognostic significance of Six-Minute Walk Test in patients with primary pulmonary hypertension: comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161(2):487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 19.Machado R.F., Mack A.K., Martyr S. Severity of pulmonary hypertension during vaso-occlusive pain crisis and exercise in patients with sickle cell disease. Br J Haematol. 2007;136(2):319–325. doi: 10.1111/j.1365-2141.2006.06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mekontso Dessap A., Leon R., Habibi A. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177(6):646–653. doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- 21.Potoka K.P., Gladwin M.T. Vasculopathy and pulmonary hypertension in sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):L314–L324. doi: 10.1152/ajplung.00252.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yawn B.P., Buchanan G.R., Afenyi-Annan A.N. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 23.Lezcano N.E., Odo N., Kutlar A. Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 2006;37(6):1424–1426. doi: 10.1161/01.STR.0000221173.97108.01. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin V.V., Archer S.L., Badesch D.B. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Barst R.J., Mubarak K.K., MacHado R.F. Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies. Br J Haematol. 2010;149(3):426–435. doi: 10.1111/j.1365-2141.2010.08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado R.F., Barst R.J., Yovetich N.A. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118(4):855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machado R.F., Martyr S., Kato G.J. Sildenafil therapy in patients with sickle cell disease and pulmonary hypertension. Br J Haematol. 2005;130(3):445–453. doi: 10.1111/j.1365-2141.2005.05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derchi G., Forni G.L., Formisano F. Efficacy and safety of sildenafil in the treatment of severe pulmonary hypertension in patients with hemoglobinopathies. Haematologica. 2005;90(4):452–458. [PubMed] [Google Scholar]
- 29.Minniti C.P., MacHado R.F., Coles W.A. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol. 2009;147(5):737–743. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik R.P., Streiff M.B., Haywood C., Jr. Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. Am J Med. 2013;126(5):443–449. doi: 10.1016/j.amjmed.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novelli E.M., Huynh C., Gladwin M.T. Pulmonary embolism in sickle cell disease: a case-control study. J Thromb Haemost. 2012;10(5):760–766. doi: 10.1111/j.1538-7836.2012.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ataga K.I. Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica. 2009;94(11):1481–1484. doi: 10.3324/haematol.2009.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein P.D., Beemath A., Meyers F.A. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. Am J Med. 2006;119(10):897.e7–897.e11. doi: 10.1016/j.amjmed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Lijfering W.M., Brouwer J.L., Veeger N.J. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood. 2009;113(21):5314–5322. doi: 10.1182/blood-2008-10-184879. [DOI] [PubMed] [Google Scholar]
- 35.Vichinsky E.P., Neumayr L.D., Earles A.N. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342(25):1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 36.Mekontso Dessap A., Deux J.F., Abidi N. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2011;184(9):1022–1029. doi: 10.1164/rccm.201105-0783OC. [DOI] [PubMed] [Google Scholar]
- 37.Miller S.T. How I treat acute chest syndrome in children with sickle cell disease. Blood. 2011;117(20):5297–5305. doi: 10.1182/blood-2010-11-261834. [DOI] [PubMed] [Google Scholar]
- 38.Adedeji M.O., Cespedes J., Allen K. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125(11):1436–1441. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- 39.Jerath A., Murphy P., Madonik M. Pulmonary endarterectomy in sickle cell haemoglobin C disease. Eur Respir J. 2011;38(3):735–737. doi: 10.1183/09031936.00192910. [DOI] [PubMed] [Google Scholar]
- 40.Ataga K.I., Key N.S. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am Soc Hematol Educ Program. 2007;2007(1):91–96. doi: 10.1182/asheducation-2007.1.91. [DOI] [PubMed] [Google Scholar]
- 41.Kearon C., Akl E.A., Comerota A.J. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):e419S–e494S. doi: 10.1378/chest.11-2301. [published correction appears in Chest. 2012;142(6):1698-1704]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kabrhel C., Mark Courtney D., Camargo C.A., Jr. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17(6):589–597. doi: 10.1111/j.1553-2712.2010.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falck-Ytter Y., Francis C.W., Johanson N.A. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naik R.P., Lanzkron S. Baby on board: what you need to know about pregnancy in the hemoglobinopathies. Hematology Am Soc Hematol Educ Program. 2012;2012:208–214. doi: 10.1182/asheducation-2012.1.208. [DOI] [PubMed] [Google Scholar]
- 45.Taher A.T., Musallam K.M., Karimi M. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115(10):1886–1892. doi: 10.1182/blood-2009-09-243154. [DOI] [PubMed] [Google Scholar]
- 46.Colella M.P., De Paula E.V., Conran N. Hydroxyurea is associated with reductions in hypercoagulability markers in sickle cell anemia. J Thromb Haemost. 2012;10(9):1967–1970. doi: 10.1111/j.1538-7836.2012.04861.x. [DOI] [PubMed] [Google Scholar]
- 47.Rogers V.E., Lewin D.S., Winnie G.B. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6(4):374–381. [PMC free article] [PubMed] [Google Scholar]
- 48.Strauss T., Sin S., Marcus C.L. Upper airway lymphoid tissue size in children with sickle cell disease. Chest. 2012;142(1):94–100. doi: 10.1378/chest.11-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaleyias J., Mostofi N., Grant M. Severity of obstructive sleep apnea in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30(9):659–665. doi: 10.1097/MPH.0b013e31817eb7ef. [DOI] [PubMed] [Google Scholar]
- 50.Salles C., Ramos R.T., Daltro C. Prevalence of obstructive sleep apnea in children and adolescents with sickle cell anemia. J Bras Pneumol. 2009;35(11):1075–1083. doi: 10.1590/s1806-37132009001100004. [DOI] [PubMed] [Google Scholar]
- 51.Rosen C.L., Debaun M.R., Strunk R.C. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014;134(2):273–281. doi: 10.1542/peds.2013-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma S., Efird J.T., Knupp C. Sleep disorders in adult sickle cell patients. J Clin Sleep Med. 2015;11(3):219–223. doi: 10.5664/jcsm.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Needleman J.P., Franco M.E., Varlotta L. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28(6):418–422. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 54.Caboot J.B., Allen J.L. Hypoxemia in sickle cell disease: significance and management. Paediatr Respir Rev. 2014;15(1):17–23. doi: 10.1016/j.prrv.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Spivey J.F., Uong E.C., Strunk R. Low daytime pulse oximetry reading is associated with nocturnal desaturation and obstructive sleep apnea in children with sickle cell anemia. Pediatr Blood Cancer. 2008;50(2):359–362. doi: 10.1002/pbc.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirkham F.J., Hewes D.K., Prengler M. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357(9269):1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 57.Lehmann G.C., Bell T.R., Kirkham F.J. Enuresis associated with sleep disordered breathing in children with sickle cell anemia. J Urol. 2012;188(4 suppl):1572–1576. doi: 10.1016/j.juro.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roizenblatt M., Figueiredo M.S., Cancado R.D. Priapism is associated with sleep hypoxemia in sickle cell disease. J Urol. 2012;188(4):1245–1251. doi: 10.1016/j.juro.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 59.Marcus C.L., Brooks L.J., Draper K.A. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 60.Ambrusko S.J., Gunawardena S., Sakara A. Elevation of tricuspid regurgitant jet velocity, a marker for pulmonary hypertension in children with sickle cell disease. Pediatr Blood Cancer. 2006;47(7):907–913. doi: 10.1002/pbc.20791. [DOI] [PubMed] [Google Scholar]
- 61.Roland P.S., Rosenfeld R.M., Brooks L.J. Clinical practice guideline: polysomnography for sleep-disordered breathing prior to tonsillectomy in children. Otolaryngol Head Neck Surg. 2011;145(1 suppl):S1–S15. doi: 10.1177/0194599811409837. [DOI] [PubMed] [Google Scholar]
- 62.Akinbami L.J., Moorman J.E., Bailey C. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 63.Field J.J., Stocks J., Kirkham F.J. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139(3):563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koumbourlis A.C., Zar H.J., Hurlet-Jensen A. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. J Pediatr. 2001;138(2):188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- 65.Ozbek O.Y., Malbora B., Sen N. Airway hyperreactivity detected by methacholine challenge in children with sickle cell disease. Pediatr Pulmonol. 2007;42(12):1187–1192. doi: 10.1002/ppul.20716. [DOI] [PubMed] [Google Scholar]
- 66.Knight-Madden J.M., Barton-Gooden A., Weaver S.R. Mortality, asthma, smoking and acute chest syndrome in young adults with sickle cell disease. Lung. 2013;191(1):95–100. doi: 10.1007/s00408-012-9435-3. [DOI] [PubMed] [Google Scholar]
- 67.Strunk R.C., Cohen R.T., Cooper B.P. Wheezing symptoms and parental asthma are associated with a physician diagnosis of asthma in children with sickle cell anemia. J Pediatr. 2014;164(4):821–826.e1. doi: 10.1016/j.jpeds.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyd J.H., Macklin E.A., Strunk R.C. Asthma is associated with increased mortality in individuals with sickle cell anemia. Haematologica. 2007;92(8):1115–1118. doi: 10.3324/haematol.11213. [DOI] [PubMed] [Google Scholar]
- 69.Gladwin M.T., Machado R.F. Pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(17):1646–1647. doi: 10.1056/NEJMc1109130. author reply 1648-1649. [DOI] [PubMed] [Google Scholar]
- 70.Knight-Madden J.M., Forrester T.S., Lewis N.A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60(3):206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen R.T., Madadi A., Blinder M.A. Recurrent, severe wheezing is associated with morbidity and mortality in adults with sickle cell disease. Am J Hematol. 2011;86(9):756–761. doi: 10.1002/ajh.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glassberg J.A., Chow A., Wisnivesky J. Wheezing and asthma are independent risk factors for increased sickle cell disease morbidity. Br J Haematol. 2012;159(4):472–479. doi: 10.1111/bjh.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 74.Darbari D.S., Castro O., Taylor JGt. Severe vaso-occlusive episodes associated with use of systemic corticosteroids in patients with sickle cell disease. J Natl Med Assoc. 2008;100(8):948–951. doi: 10.1016/S0027-9684(15)31410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joos S., Miksch A., Szecsenyi J. Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: a systematic review. Thorax. 2008;63(5):453–462. doi: 10.1136/thx.2007.081596. [DOI] [PubMed] [Google Scholar]
- 76.Knight-Madden J.M., Forrester T.S., Lewis N.A. The impact of recurrent acute chest syndrome on the lung function of young adults with sickle cell disease. Lung. 2010;188(6):499–504. doi: 10.1007/s00408-010-9255-2. [DOI] [PubMed] [Google Scholar]
- 77.Klings E.S., Wyszynski D.F., Nolan V.G. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173(11):1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lunt A., Desai S.R., Wells A.U. Pulmonary function, CT and echocardiographic abnormalities in sickle cell disease. Thorax. 2014;69(8):746–751. doi: 10.1136/thoraxjnl-2013-204809. [DOI] [PubMed] [Google Scholar]
- 79.Field J.J., Glassberg J., Gilmore A. Longitudinal analysis of pulmonary function in adults with sickle cell disease. Am J Hematol. 2008;83(7):574–576. doi: 10.1002/ajh.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ong B.A., Caboot J., Jawad A. Respiratory muscle force and lung volume changes in a population of children with sickle cell disease. Br J Haematol. 2013;163(1):112–117. doi: 10.1111/bjh.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hosenpud J.D., Stibolt T.A., Atwal K. Abnormal pulmonary function specifically related to congestive heart failure: comparison of patients before and after cardiac transplantation. Am J Med. 1990;88(5):493–496. doi: 10.1016/0002-9343(90)90428-g. [DOI] [PubMed] [Google Scholar]
- 82.van Beers E.J., van der Plas M.N., Nur E. Exercise tolerance, lung function abnormalities, anemia, and cardiothoracic ratio in sickle cell patients. Am J Hematol. 2014;89(8):819–824. doi: 10.1002/ajh.23752. [DOI] [PubMed] [Google Scholar]
- 83.Intzes S., Kalpatthi R.V., Short R. Pulmonary function abnormalities and asthma are prevalent in children with sickle cell disease and are associated with acute chest syndrome. Pediatr Hematol Oncol. 2013;30(8):726–732. doi: 10.3109/08880018.2012.756961. [DOI] [PubMed] [Google Scholar]
- 84.Arteta M., Campbell A., Nouraie M. Abnormal pulmonary function and associated risk factors in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2014;36(3):185–189. doi: 10.1097/MPH.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klings E.S., Anton Bland D., Rosenman D. Pulmonary arterial hypertension and left-sided heart disease in sickle cell disease: clinical characteristics and association with soluble adhesion molecule expression. Am J Hematol. 2008;83(7):547–553. doi: 10.1002/ajh.21187. [DOI] [PubMed] [Google Scholar]
- 86.Brousseau D.C., Owens P.L., Mosso A.L. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 87.Piccin A., Murphy C., Eakins E. Protein C and free protein S in children with sickle cell anemia. Ann Hematol. 2012;91(10):1669–1671. doi: 10.1007/s00277-012-1447-9. [DOI] [PubMed] [Google Scholar]
- 88.Raj A., Bertolone S., Bond S. Cathlink 20: a subcutaneous implanted central venous access device used in children with sickle cell disease on long-term erythrocytapheresis—a report of low complication rates. Pediatr Blood Cancer. 2005;44(7):669–672. doi: 10.1002/pbc.20252. [DOI] [PubMed] [Google Scholar]
- 89.Kucuk O., Gilman-Sachs A., Beaman K. Antiphospholipid antibodies in sickle cell disease. Am J Hematol. 1993;42(4):380–383. doi: 10.1002/ajh.2830420409. [DOI] [PubMed] [Google Scholar]
- 90.Noubouossie D.F., Le P.Q., Corazza F. Thrombin generation reveals high procoagulant potential in the plasma of sickle cell disease children. Am J Hematol. 2012;87(2):145–149. doi: 10.1002/ajh.22206. [DOI] [PubMed] [Google Scholar]
- 91.Caboot J.B., Jawad A.F., McDonough J.M. Non-invasive measurements of carboxyhemoglobin and methemoglobin in children with sickle cell disease. Pediatr Pulmonol. 2012;47(8):808–815. doi: 10.1002/ppul.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gladwin M.T., Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 93.Blake K., Lima J. Asthma in sickle cell disease: implications for treatment. Anemia. 2011;2011:740235. doi: 10.1155/2011/740235. [DOI] [PMC free article] [PubMed] [Google Scholar]