Highlights
-
•
Seroconverters to YF vaccine in reduced doses remained seropositive 8 years later.
-
•
This applies equally well to doses from 27,476 IU down to 31 IU.
-
•
This study supports the minimum dose required by WHO, 1000 IU.
-
•
This information is crucial when use of fractional YF vaccine is considered.
Keywords: Yellow fever vaccine, Immunogenicity, Dose-response study, Duration of immunity, Fractionated vaccine
Abstract
In 2009, Bio-Manguinhos conducted a dose-response study with the yellow fever vaccine, administering the vaccine in the usual mean dose of 27,476 IU (full dose, reference) and in tapered doses (10,447 IU, 3013 IU, 587 IU, 158 IU, and 31 IU) by the usual subcutaneous route and usual volume (0.5 mL). Tapered doses were obtained by dilution in the manufacturer’s laboratory, and the test batches presented industrial quality. Doses down to 587 IU showed similar immunogenicity to the full dose (27,476, reference), while the 158 IU and 31 IU doses displayed lower immunogenicity. Seropositivity was maintained at 10 months, except in the group that received the 31 IU dose. The current study aims to determine whether yellow fever seropositivity was maintained eight years after YF vaccination in non-revaccinated individuals. According to the current study’s results, seropositivity was maintained in 85% of 318 participants and was similar across groups. The findings support the use of the yellow fever vaccine in fractional doses during outbreaks, but each fractional dose should have at least 587 IU. This study also supports the minimum dose required by WHO, 1000 IU.
Clinical trials registration: Clinicaltrials.gov NCT 03338231.
1. Background
There is a global shortage of yellow fever (YF) vaccine, and the problem of vaccine stockpile depletion is recurrent due to a combination of limited production capacity and expanding circulation of the YF virus, with increasing risk of YF urbanization or reurbanization in several countries. An estimated 450 million doses are needed to achieve >80% coverage in YF-affected areas, while current annual YF vaccine production is only 80 million doses [1], [2]. The situation will become even more dramatic if yellow fever spreads to Asia [3].
In 2016, 7509 suspected and 1080 laboratory-confirmed cases of yellow fever, with 171 deaths, were reported to WHO during outbreaks in six countries, including two urban outbreaks, in Angola and Democratic Republic of Congo (DRC). Yellow fever vaccine stockpiles were depleted. To deal with this challenge, WHO proposed to fractionate the YF vaccine doses to address the emergency in Angola and DRC in 2016 [4]. In August 2016, over 7 million people received 1/5 of the 17DD YF vaccine in Kinshasa, and the epidemic was rapidly controlled [5], [6]. Previous dose-response studies were the basis for the WHO recommendation to administer 0.1 mL rather than 0.5 mL, by the usual route (SC or IM). However, due to lack of information on duration of immunity following reduced doses and lack of studies on reduced doses in children and pregnant women, this strategy was only recommended in emergency situations [7].
In Brazil, following YF outbreaks in Greater Metropolitan São Paulo and Rio de Janeiro, a mass vaccination campaign with 1/5 (0.1 mL) of the dose was launched in February 2018. Information on duration of immunity from reduced (diluted or fractional) doses is crucial, considering the possible need to revaccinate these individuals.
Unpredictable YF outbreaks suddenly increase the demand for the vaccine, and production targeted to routine vaccination may not meet the needs for mass vaccination. There are currently only four WHO-prequalified YF vaccine manufacturers, of which only two are large-scale producers. In addition, YF vaccine is produced with traditional labor-intensive methods and is rather inexpensive, so it tends not to attract new producers. Alternative vaccines employing more modern technologies have not been developed to date, although they are the object of active research efforts.
In response to the need to increase production by diluting the vaccine, in 1988 Bio-Manguinhos conducted a 17DD YF vaccine dose-response study in adults, with very high seroconversion rates using doses from 2000 PFU (plaque-forming units) to 200 PFU and lower seroconversion rates below this dose. However, the small number of participants in the study arms precluded the adoption of reduced doses of YF vaccine based on these findings [8].
Following the YF vaccine shortage in the 2008 epidemic in Brazil, in 2009 Bio-Manguinhos conducted a randomized dose-response study with the 17DD YF vaccine administered in the usual mean dose of 27,476 IU (full dose, reference) and in tapered doses of 10,447 IU, 3013 IU, 587 IU, 158 IU, and 31 IU, by the usual subcutaneous route and with the usual volume (0.5 mL). Tapered doses were obtained by dilution in the manufacturer’s laboratory, and the test batches presented industrial quality. Doses down to 587 IU showed similar immunogenicity to the full dose, whereas the lowest doses, 158 IU and 31 IU, were less immunogenic. Moreover, seropositivity of volunteers who had seroconverted and had not been revaccinated was maintained for at least 10 months, except in the 31 IU dose group [9]. Thirty days after vaccination, in the groups that received doses of 587 IU or greater, only 2.2% of 509 participants failed to seroconvert, and after 10 months, 2.0% more reverted from seropositive to seronegative. Therefore, 4.2% of the initial cohort that received doses of 587 UI or greater were revaccinated because they were seronegative at 30 days or 10 months. In the 158 IU dose and 31 IU dose groups, 13.5% and 43.9%, respectively, were revaccinated for this same reason.
A complementary study in a subset of volunteers from the previous study evaluated the cellular immune response to the YF vaccine and concluded that doses ≥3013 IU showed immune responses equivalent to that of the standard mean dose of 27,476 IU [10].
The objective of this study was to evaluate the duration of immunity eight years after administration of reduced doses of the 17DD YF vaccine in the dose-response study in 2009, by measuring neutralizing antibody levels, with a view towards supporting the use of fractionated doses.
2. Methods
2.1. Study design
This was a cohort study in healthy young adult males (military recruits) who had received the 17DD YF vaccine during the dose-response study in 2009 [5]. The target group consisted of participants who were seronegative before vaccination in the dose-response study in 2009 and had not been revaccinated. Participants that were YF-seronegative at 30 days and 10 months after vaccination were revaccinated with the standard dose and were not included in the current study. Participants that had gone on military missions or travelled or lived in YF endemic areas since 2009 were analyzed separately.
Participants were contacted by telephone or home visit, and blood samples were collected at Fiocruz, or if necessary at home or in a safe place, following informed consent.
Participants were asked at least twice if they had been revaccinated, during the initial phone call and in the face-to-face interview when the blood sample was taken. They were also asked to confirm that they had participated in the dose-response study in 2009 and about travels on military missions or to YF-endemic areas.
The study was conducted from March 2017 to September 2017, approximately eight years after the dose-response study.
2.2. Laboratory methods
Serology for YF neutralization was performed in all participants, according to the methods described in the dose-response study of 2009 [9], using the same cut-off for seropositivity: >2.7 log10 mIU/mL (501.2 mUI/mL), or about 1/20 in dilution.
2.3. Statistical analysis
Statistical analysis of neutralizing antibodies was performed, first blindly, by comparison of groups by chi-square or Fisher’s exact test, as indicated, or by analysis of variance of log10 titers. After unblinding the codes, each vaccine-dose group’s seroprotection rate and neutralizing antibody level were compared to those of the reference group. Volunteers who had travelled or lived in YF-endemic areas or had participated in military missions to endemic areas were analyzed separately. The statistical analyses used SPSS v.20 and WinPepi v. 11. Neutralizing antibody levels are presented in log10 mIU/mL, and geometric mean titers are presented with 95% CI.
2.4. Ethical approval and good clinical practices
The study protocol and final report were approved by the Institutional Review Board of the Evandro Chagas National Institute of Infectious Diseases and by an independent data safety monitoring committee. All the procedures complied with the Declaration of Helsinki, the Brazilian Code of Research Ethics, the Good Clinical Practices: Document of the Americas and the International Conference on Harmonization. The identification and revaccination of individuals that were YF-seronegative eight years after initial vaccination were clear benefits for participants.
2.5. Results and comments
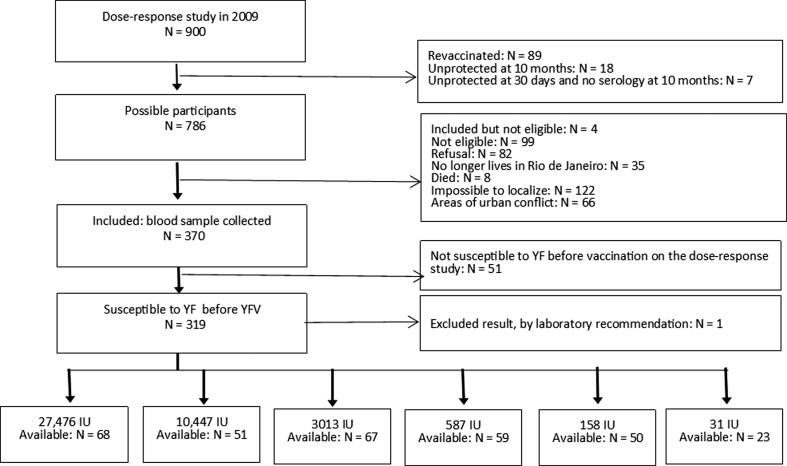
Fig. 1 summarizes the study inclusion steps. 318 participants were eligible according to the study protocol. The lower number of participants in the lowest dose group reflects the high number of primary vaccine seroconversion failures in that group in the dose-response study in 2009 (i.e., with fewer eligible participants for follow-up).
Fig. 1.
Study inclusion steps.
From a total of 370 participants that were included in the 2017 follow-up study, 51 were later found to be ineligible. One other participant was excluded because of a marked rise in antibody levels in 2017, suggesting that he had received a booster dose. Therefore, data from 318 individuals were available for analysis.
The geometric mean titers were similar between the dose groups before vaccination, at one month, and at 10 months after vaccination, but differed substantially at eight years after vaccination (Table 1). From here on, we present only the results at eight years after vaccination.
Table 1.
Geometric mean titers (GMT, International Units) and 95% CI at sequential times on the dose-response study; N (total) = 318.
| Group | N | Before vaccination |
At 30 days |
At 10 months |
At 8 years |
||||
|---|---|---|---|---|---|---|---|---|---|
| GMT | 95% CI | GMT | 95% CI | GMT | 95% CI | GMT | 95% CI | ||
| 27,476 IU | 68 | 154 | 130; 183 | 16,472 | 13,574; 19,990 | 4628 | 3797; 5,640 | 1682 | 1236; 2290 |
| 10,447 IU | 51 | 150 | 122; 184 | 13,346 | 10,716; 16,622 | 4389 | 3573; 5392 | 2167 | 1457; 3221 |
| 3013 IU | 67 | 132 | 111; 158 | 13,609 | 10,936; 16,937 | 4671 | 3840; 5682 | 1603 | 1173; 2189 |
| 587 IU | 59 | 147 | 122; 176 | 13,209 | 10,415; 16,752 | 5053 | 4273; 5975 | 2394 | 1758; 3261 |
| 158 IU | 50 | 140 | 114; 171 | 13,171 | 10,356; 16,751 | 4956 | 3973; 6181 | 1651 | 1145; 2382 |
| 31 IU | 23 | 119 | 87; 164 | 15,898 | 12,251; 20,630 | 4873 | 3571; 6648 | 4087 | 2605; 6410 |
| p (for differences among groups) | 0.639 | 0.570 | 0.940 | 0.025 | |||||
The difference in seropositivity rates between groups are large but statistically non-significant compared to the reference dose (27,476 IU). A high proportion of participants were seropositive, with no consistent pattern according to vaccine dose (Table 2).
Table 2.
Proportion of seropositivity of participants on the dose-response study 8 years after vaccination, by vaccine group.
| Group | Seropositive participants (neutralizing antibodies >2.7 log10 mUI/mL) |
Total tested | p-value (pair-wise comparisons to the reference vaccine) | ||
|---|---|---|---|---|---|
| N | % | 95% CI | N | ||
| 27,476 IU | 56 | 82.4 | 71.2; 90.5 | 68 | Ref |
| 10,447 IU | 44 | 86.3 | 73.7; 94.3 | 51 | 0.563 |
| 3013 IU | 54 | 80.6 | 69.1; 89.2 | 67 | 0.793 |
| 587 IU | 55 | 93.2 | 83.5; 98.1 | 59 | 0.106 |
| 158 IU | 40 | 80.0 | 66.3; 90.0 | 50 | 0.746 |
| 31 IU | 22 | 95.7 | 78.1; 99.9 | 23 | 0.172 |
| Total | 271 | 85.2 | 80.8; 88.9 | 318 | |
P = 0.159 (All groups).
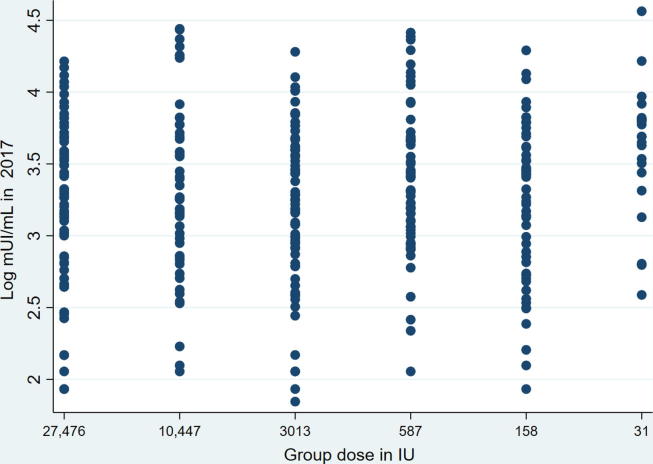
Geometric mean titers were much higher in the 31 IU group, and the discrepancy persisted when the 5% trimmed means were considered in order to reduce the impact of outliers (data not shown). In any case, the lower limits of the 95% CI were above the cut-off of 2.7 log10 mIU/mL (501.2 mIU/mL) in all the groups (Table 3). The scatterplot of log10 neutralizing antibody titers by dose group indeed showed the 31 IU group with a higher concentration of upper-level values when compared to the other groups (Fig. 2).
Table 3.
Geometric mean antibody titers (GMT in mili-International Units per mL), ratios between GMT from lower doses and the full dose, and corresponding 95% C.I., by vaccine group.
| Group | N | GMT | 95% CI | Ratio to reference vaccine | 95% CI of ratio |
|---|---|---|---|---|---|
| 27,476 IU | 68 | 1682.0 | 1235.6; 2289.7 | Ref | Ref |
| 10,447 IU | 51 | 2166.7 | 1457.4; 3221.1 | 1.29 | 0.79; 2.10 |
| 3013 IU | 67 | 1602.7 | 1173.2; 2189.4 | 0.95 | 0.62; 1.47 |
| 587 IU | 59 | 2394.4 | 1758.2; 3260.8 | 1.42 | 0.92; 2.20 |
| 158 IU | 50 | 1651.2 | 1144.6; 2382.1 | 0.98 | 0.61; 1.57 |
| 31 IU | 23 | 4086.6 | 2605.2; 6410.5 | 2.43 | 1.35; 4.36 |
| Total | 318 | 1949.8 | 1698.2; 2290.9 | ||
P = 0.025.
Fig. 2.
Scatterplot of log10 neutralizing antibody titers by dose group. 8 years after vaccination.
2.6. Further analyses
Inadvertent revaccination may have occurred in subjects who participated in military missions or trips to or living in endemic areas, even though they were questioned at least twice on this issue and replied in the negative. Sensitivity analyses are presented below to assess the impact of these and other issues on the results.
Eight years after vaccination, seropositivity rates of subjects that had participated in military missions versus those who had not were similar (p = 0.648), and there was no significant difference in geometric mean neutralizing antibody titers (p = 0.466). The same was true for those who had travelled to or lived in YF-endemic areas, both for seropositivity rates (p = 0.098), and geometric mean titers (p = 0.631).
Seventy volunteers (22%) showed higher neutralizing antibody titers in 2017 than in 2009. The variability in plaque reduction neutralization tests (PRNT) is well-known, and recent validation of the test at our viral technology laboratory demonstrated that variations up to three-fold can be expected in intra- and inter-assays. Therefore, increases above this range may mean exposure to the virus or revaccination. For this reason, the laboratory recommended excluding one PRNT result, reducing the total from 319 to 318.
Assuming that the group with higher antibody titers in 2017 than in 2009 included individuals that were revaccinated or exposed to natural infection, data analysis was conducted disregarding their results. Total seropositivity of the remaining subjects was 81% (200/247), and the size differences among groups were similar to the whole cohort and not statistically significant (p = 0.235). Meanwhile, the analysis of variance of neutralizing antibody titers among groups was not statistically significant (p = 0.171), although the 31 IU group still showed the highest antibody level.
There was no statistically significant association between subjects that had participated in military missions and those with higher antibody titers in 2017 than in 2009 (p = 0.484). The same was true for those who had travelled to or lived in YF-endemic areas (p = 0.118).
After excluding all these groups from the analysis (i.e., military missions since the 2009 study, travel to or living in a municipality with recommendation for YF vaccination, and higher GMT in 2017 than in 2009), 156 participants remained. The analysis of seropositivity and GMTs comparing dose groups showed similar results, consistent with what has already been presented. Therefore, the inclusion of all these groups according to protocol analysis is justified.
3. Discussion
From the initial cohort of 900 participants, 370 were included, and 52 were excluded for the following reasons: 51 because they were seropositive to YF before the initial vaccination and one because of an incongruent neutralization test. Overall, 85.2% of participants (95% CI 80.8; 88.9) remained seropositive to YF eight years after initial vaccination, with no significant differences between the reduced-dose and full-dose groups. Geometric mean titers were also similar between groups, except for the group that received the lowest dose, which showed higher antibody levels and seropositivity rates than those receiving the reference vaccine. The lower geometric mean titer confidence intervals were above the cut-off level for seropositivity in all the groups.
The group that received the 31 IU dose showed lower immunogenicity in the dose-response study in 2009 and the highest primary seroconversion failure rate. Therefore, this was the group with the least participants in the 2017 study. The finding that the group showed higher antibody levels than the other groups eight years later was unexpected. No participants were ever informed about their vaccine dose. They were only told whether they had seroconverted or not, which rules out the possibility that these participants took the initiative to obtain revaccination because they had received a low dose. Possible causes could be statistical distortion, induced by the small sample size in the low-dose group, or inadvertent selection of a group of individuals that were high responders to YF vaccine and could thus respond adequately to a very low dose of vaccine. Theoretically, these individuals could also respond better to YF immune boosters induced by new infections with other flaviviruses.
Reduced doses appeared to have induced immunity as durable as that of the full dose. Those classified as low-dose groups in the 2009 study (158 IU and 31 IU) remained seropositive eight years later, as long as they had seroconverted. It thus appears that once seroconversion has been achieved one month after vaccination and seropositivity is sustained for 10 months, it is also sustained in the long term.
Based on the available literature, evaluation of the duration of immunity after yellow fever vaccination is a challenging task. Gotuzzo [11] conducted an extensive review identifying eight studies that evaluated duration of immunity ≥10 years after vaccination, with seropositivity rates ranging from 74.5% to 100%. Assessment of the duration of immunity after reduced doses of YF vaccine had never been conducted before. The seropositivity rates obtained in the 2009 dose-response study are similar to those of a recent evaluation of fractional-dose immunogenicity in Africa, including both sexes and all ages above 2 years, showing 98% seroconversion 30 days after vaccination in individuals seronegative to yellow fever virus at baseline [12].
A major difficulty for evaluation of duration of immunity is the diversity of methodologies used for evaluation of seroprotection or seropositivity: intracerebral inoculation in weaned mice, neutralization test in suckling mice, protection test in mice, log neutralization index (LNI), and plaque reduction neutralization test (PRNT). PRNT may have different endpoints (% reduction of plaques): 90, 80, 75, or 50%. The cut-offs for seropositivity in general are 1/10, but 1/20, 1/50, or 2.9 mIU/mL were also used. Only one study investigated the contribution of cellular immune responses to duration of immunity [13].
We thus considered it more adequate to compare the current study with another study by our own group, in which all participants received the full dose of YF vaccine [14]. That study used the same laboratory method, PRNT50, but with a seropositivity cut-off of 2.9 log10 mIU/mL or 1/50 in dilution. Using the database from that study, the results were recalculated using the same cut-off as in the current study, i.e. >2.7 log10 mIU/mL or 1/20 in dilution (Table 4, A and B).
Table 4.
A and B. Seropositivity and mean level of neutralizing antibodies with 95% CI according to time after yellow fever vaccination in 2 studies employing identical laboratorial methods.
 |
The seropositive rates and log means were similar between the two studies, considering the time interval since vaccination that is closest to this study.
Importantly, 2.7 mIU/mL (about 1/20 in dilution in our laboratory) is a conservative cut-off, so seropositivity may have been underestimated.
The main difficulty in this study was to avoid the inclusion of individuals that had been revaccinated since the 2009 study. This issue was emphasized, starting with the field research team’s initial training. Participants were asked at least twice about revaccination, once by telephone and again during the face-to-face interview. Several analyses were performed that supported the methods employed.
A group of participants showed higher neutralizing antibody levels in 2017 than in 2009 (following vaccination). The laboratory staff that performed the neutralization tests and conducted further careful analysis of those cases recommended excluding only one result, which was too high and likely indicated immune boosters to yellow fever. Plausible explanations for increased antibody titers eight years after vaccination include inherent variability of the neutralization test, undisclosed revaccination, wild virus infection, and immune boosters via new infections with other flaviviruses such as dengue or Zika. The latter possibility was raised in the background document of the SAGE working group on yellow fever vaccine [15]. Moreover, several analyses were performed that supported the decision to include this group.
In addition to these internal validity issues, the study’s external validity was reduced by the limited representativeness of the participants in the 2009 dose-response study, who constituted a selected sample of young healthy males. Since children under 5 years of age and adults over 50 that received the fractional dose in the YF vaccination campaign in Kinshasa had lower geometric mean titers than young adults [12], it is plausible that duration of immunity will not be as good as in this study. Importantly, although all groups had similar seropositivity eight years after vaccination, the 158 IU/dose and 31 IU/dose groups were still lower, because they showed more primary vaccination failures (especially the lowest dose group) [9]. However, among those that seroconverted and remained seropositive 10 months later, whichever the dose, about 85% remained seropositive, ranging from 80% to 95% across the vaccine dose groups.
For groups with doses ≥587 IU, 4.4% of the participants in the 2009 study were revaccinated due to seronegativity at 30 days or 10 months after vaccination, so the remaining participants included in the current study are not strongly biased towards higher seroconversion. Importantly, sustained seropositivity with reduced doses was similar to that of the reference vaccine.
In conclusion, this study supports the use of doses of ≥587 IU for adequate and sustainable immune response to yellow fever vaccine. As we did not explore subgroups who could respond less well to the vaccine, the recommendation by WHO of a minimum dose of 1000 IU should be followed [16].
4. Conclusions
-
–
At least 80% of the subjects who had seroconverted after yellow fever vaccination with doses from 27,476 IU down to 31 IU showed seropositivity comparable to that of the full dose after eight years. Consistently, antibody titers in the reduced-dose groups were also comparable to those of the full-dose group. However, the lowest doses (158 IU and 31 IU) had more primary seroconversion failures, so they should still be considered inferior to the full dose vaccine.
-
–
Groups of decreasing doses from the dose-response study in 2009 showed YF seropositivity rates ranging from 80.0% to 95.7%, eight years later. All the rates were acceptable and comparable to other studies on duration of immunity in adults receiving the full dose.
-
–
Geometric mean neutralizing antibody titers to yellow fever were similar across groups, except for the 31 IU group, in which the GMT was higher. All groups had the lower limit of the 95% confidence interval above the cut-off for seropositivity.
-
–
To the extent that protection can be inferred from these immunological parameters, the current study supports the use of yellow fever vaccine in fractionated doses and the minimum dose recommended by WHO, 1000 IU.
Funding
The research was funded by a grant from Wellcome Trust and did not receive any specific funding from other sources in the public, commercial, or non-profit sectors.
The collaborative group for studies on duration of immunity from yellow fever vaccine: Suelen Manhães Pessanha, Maria Letícia Borges dos Santos, Robson Leite de Souza Cruz, Dayana Cristina Vieira de Souza, Ricardo Cristiano Brum, Clara Lucy de Vasconcelos Ferroco, Deborah Araújo da Conceição, Leonardo Secundino, Olindo Assis Martins Filho, and Ana Carolina Campi Azevedo.
Conflicts of interest
All the authors work or worked at the time of the study at Bio-Manguinhos/Fiocruz or at the Oswaldo Cruz Foundation (Fiocruz), a non-profit, government-owned institution with which Bio-Manguinhos is affiliated, with the exception of Roberto Henrique Guedes Farias, a member of the Brazilian Army Health Service.
All the authors hereby attest that they meet the Vaccine criteria for authorship. RMM, MLSM, SMBL, TGN, JRX, LABC, EMA, RHGF, TMC, and AH participated in the study protocol’s conception and development, field research, data analysis, and writing of the report and article.
Acknowledgments
Acknowledgements
The study received funding from the Wellcome Trust (grant 206523/Z/17/Z).
The authors wish to thank the volunteers for their generous collaboration.
References
- 1.Shearer F.M., Moyes C.L., Pigott D.M., Brady O.J., Marinho F., Deshpande A. Lancet Infect Dis. 2017 Nov;17(11):1209–1217. doi: 10.1016/S1473-3099(17)30419-X. Epub 2017 Aug 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilder-Smith A. Yellow fever vaccination: estimating coverage. Lancet Infect Dis. 2017 Nov;17(11):1109–1111. doi: 10.1016/S1473-3099(17)30494-2. [DOI] [PubMed] [Google Scholar]
- 3.Monath T.P., Woodall J.P., Gubler D.J., Yuill T.M., Mackenzie J.S., Martins R.M. Yellow fever vaccine supply: a possible solution. The Lancet. 2016 Apr;387(10028):1599–1600. doi: 10.1016/S0140-6736(16)30195-7. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Fractional dose yellow fever vaccine as a dose-sparing option for outbreak response. WHO Secretariat information paper. WHO; 20 July 2016.
- 5.http://www.who.int/immunization/sage/meetings/2016/october/Session11-Part2-Feedback-from-the-yellow-fever-mass-vaccination-campaign-using-fractional-dose.pdf.
- 6.http://www.who.int/immunization/sage/meetings/2016/october/Session11-Part2-Fractional-dosing-of-Yellow-fever-vaccines.pdf?ua=1
- 7.Yellow fever vaccine: WHO position on the use of fractional doses – June 2017. Wkly Epidemiol Rec. 2017 23;92(25):345–50. [PubMed]
- 8.de S Lopes O., Guimarães S.S., de Carvalho R. Studies on yellow fever vaccine. III–Dose response in volunteers. J Biol Stand. 1988 Apr;16(2):77–82. doi: 10.1016/0092-1157(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 9.Martins R.M., Maia de M.L.S., Farias R.H.G., Camacho L.A.B., Freire M.S., Galler R. 17DD yellow fever vaccine: a double-blind, randomized clinical trial of immunogenicity and safety in a dose-response study. Human Vaccines Immunother. 2013 Apr;9(4):879–888. doi: 10.4161/hv.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campi-Azevedo AC, de Almeida Estevam P, Coelho-dos-Reis JG, Peruhype-Magalhães V, Villela-Rezende G, Quaresma PF, et al. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Inf Dis [Internet]. 2014 Dec [cited 2018 Jan 30];14(1). Available from: <http://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-14-391>. [DOI] [PMC free article] [PubMed]
- 11.Gotuzzo E., Yactayo S., Cordova E. Review article: efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg. 2013;89:434–444. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahuka-Mundeke S., Casey R.M., Harris J.B., Dixon M.G., Nsele P.M., Kizito G.M. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak – preliminary report. N Engl J Med. 2018 Feb;14 doi: 10.1056/NEJMoa1710430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campi-Azevedo A.C., Costa-Pereira C., Antonelli L.R., Fonseca C.T., Teixeira-Carvalho A., Villela-Rezende G. Booster dose after 10 years is recommended following 17DD-YF primary vaccination. Human Vaccines Immunotherapeutics. 2016 Feb;12(2):491–502. doi: 10.1080/21645515.2015.1082693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collaborative group for studies on yellow fever vaccines Duration of post-vaccination immunity against yellow fever in adults. Vaccine. 2014;32:4977–4984. doi: 10.1016/j.vaccine.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 15.SAGE Working Group. Background paper on yellow fever vaccine. Version: 2013, March, 19th. Obtained at <http://www.who.int/immunization/sage/meetings/2013/april/1_Background_PaperYellow_Fever_Vaccines.pdf> [2018/02/04], at 3:19 PM.
- 16.WHO. Recommendations to assure the quality, safety and efficacy of live attenuated yellow fever vaccines. Replacement of the annex 2 of WHO Technical Report series, N° 872 and of the amendment to the annex in WHO Technical Report series, N° 2012;944.