Abstract
Objectives
Melanin-concentrating hormone (MCH) neurons in the lateral hypothalamus (LH) regulate food intake and body weight, glucose metabolism and convey the reward value of sucrose. In this report, we set out to establish the respective roles of MCH and conventional neurotransmitters in these neurons.
Methods
MCH neurons were profiled using Cre-dependent molecular profiling technologies (vTRAP). MCHCre mice crossed to Vglut2fl/flmice or to DTRfl/flwere used to identify the role of glutamate in MCH neurons. We assessed metabolic parameters such as body composition, glucose tolerance, or sucrose preference.
Results
We found that nearly all MCH neurons in the LH are glutamatergic and that a loss of glutamatergic signaling from MCH neurons from a glutamate transporter (VGlut2) knockout leads to a reduced weight, hypophagia and hyperkinetic behavior with improved glucose tolerance and a loss of sucrose preference. These effects are indistinguishable from those seen after ablation of MCH neurons. These findings are in contrast to those seen in mice with a knockout of the MCH neuropeptide, which show normal glucose preference and do not have improved glucose tolerance.
Conclusions
Overall, these data show that the vast majority of MCH neurons are glutamatergic, and that glutamate and MCH signaling mediate partially overlapping functions by these neurons, presumably by activating partially overlapping postsynaptic populations. The diverse functional effects of MCH neurons are thus mediated by a composite of glutamate and MCH signaling.
Keywords: MCH, Sucrose preference, Body weight, Glucose metabolism, Neuropeptide, Glutamate
Highlights
-
•
MCH neurons molecular profile shows coexpression of glutamatergic but not GABAergic markers.
-
•
Both MCH and glutamate in MCH neurons are responsible for the regulation of whole body energy balance.
-
•
Glutamate in MCH neurons is responsible for the control of sucrose preference and glucose metabolism and not the MCH neuropeptide.
1. Introduction
Melanin concentrating-hormone expressing (MCH) neurons located in the lateral hypothalamus (LH) play an important role in the regulation of energy balance metabolism, and other functions [1], [2], [3]. Both an MCH knockout (KO) and ablation of MCH neurons result in reduced food intake and leanness [2], [4]. MCH ablation also enhances glucose disposition in a glucose tolerance test. Recently, MCH neurons have been also implicated in sucrose preference, a reward related process, via projections to dopaminergic neurons in the striatum and midbrain [5]. In previous studies we have noted that there are differences between the effects of MCH neural ablation compared to an MCH knockout, raising the possibility that additional transmitters besides MCH mediate the effects of these neurons.
In this report, we set out to establish the molecular phenotypes of MCH neurons by profiling these neurons. A previous study suggested that a subset of MCH neurons are GABAergic while a different study suggested they are nearly exclusively glutamatergic [6], [7]. Our data indicated that the great majority of MCH neurons are glutamatergic. We then compared the physiologic effects of ablating glutamate signaling in these neurons to either an MCH knockout or MCH neural ablation. We found that the effects of knocking out the glutamate transporter and MCH were different, suggesting that the function of MCH neurons is a composite of the function of a classic neurotransmitter and a neuropeptide.
2. Methods
2.1. Mice and diets
C57BL/6 mice were purchased from Jackson (Jaxmice). The generation of MCH-Cre mice has been previously reported [5]. VGlut2loxp/loxp mice were purchased from Jackson (Jaxmice). To generate MCH-specific VGlut2 knock-out mice (MCHCre;VGlut2fl/fl and MCHCre;VGATfl/fl, respectively), MCH-Cre mice were crossed with VGlut2loxp/loxp. Colonies were maintained by breeding MCH-Cre; loxp/loxp mice with loxp/loxp mice. MCHKO mice were purchased from Jackson (Jaxmice), and MCH neuron-ablated mice were generated as described previously [5]. Mice were maintained on a 12:12 h light–dark cycle with free access to water and standard chow or high-fat diet (60% Kcal fat; Research Diets) for 12 weeks (starting at 6 weeks of age). In vivo studies were performed following the National Institutes of Health Guidelines on the Care and Use of Animals and approved by the Rockefeller University Institutional Animal Care and Use Committee (Protocols 15797-H and 15818-H).
2.2. Physiological measurements
Body weights were determined weekly. Blood samples were collected via tail vein or trunk bleeds using a capillary collection system (Sarstedt). Blood glucose was measured using an Ascensia Elite XL glucometer (Bayer Health-Care, Tarrytown, NY). Plasma leptin levels were analyzed by ELISA techniques (Alpco immunoassays). Glucose tolerance tests were performed on overnight fasted mice. d-glucose (2 g/kg) was injected intraperitoneally (i.p.) and blood glucose determined at the indicated time points. Locomotion activity was measured using beam breaks in metabolic cages from TSE systems.
2.3. Construction of AAV-IV-GFPL10
For construction of AAV-IV-GFPL10, see [8]. Briefly, we generated the plasmid for AAV-IV-GFPL10 by synthesizing an inverted EGFP sequence flanked by synthetic introns, lox sites, and forward Rpl10a. This fragment was then subcloned into pAAV-DIO-ChR2-mCherry to establish Cre-dependent expression, and it was then packaged into AAV pseudotype 5 (UNC Vector Core).
2.4. Immunoprecipitation and RNA-seq analyses
Details on GFP immunoprecipitations can be found in [9].
2.5. Stereotaxic surgeries
MCH Cre transgenic mice of 12–16 weeks of age were induced and maintained on isofluorane before being bilaterally injected with 0.5uL of AAV IV-GFPL10 in the LHA (LHA coordinates: −1.4 AP, −/+ 0.7 L and −5.2 DV). After viral injection, the needle was left in place for 10 min before slowly retracting. The skin was closed with a surgical clip.
2.6. Sucrose preference
Animals were acclimated to the chambers until side preference for either bottle was even. When preference was even, mice were studied as follows: first, sucrose in one bottle and water on the other, then, sucralose in one bottle and water in the other, and, lastly, sucrose in one bottle and sucralose in the other. During the acclimation and exposure periods, mice were water-deprived for 16–23 h and then given water through the bottles inside the chamber for half an hour. Two-bottle preference was calculated as the ratio: preference for 1 = number of licks on bottle 1/(number of licks on bottle 1 + number of licks on bottle 2) and expressed as percentage values, with 50% representing the indifference ratio. Behavioral data was analyzed with Excel and Prism and is expressed, as mean ± SEM. Significance tests comparing groups were t tests. The size of each animal group is represented by ‘n’, and each animal was tested three times. The investigator was blind to the genotype. In all cases, concentration of sucrose was 0.4 M, and concentration of sucralose was 1.5 mM. These concentrations were based on previous literature [10]. Briefly, the differences in molarity of sucrose and sucralose reflect differences in ligand-binding affinity of either sweetener to taste receptors and were chosen among the plateau values of behavioral dose–response curves (preference for either sweetener versus water in [10]). Volume dispensed by the lickometers averages 2 μl/lick [10].
2.7. Immunohistochemistry
Mice were first anesthetized with isofluorane followed by transcardial perfusion with PBS and then 10% formalin. Brains were dissected, incubated in 10% formalin overnight at 4 °C, and 40 μm sections were prepared using a vibratome. Free floating sections were blocked for 1 h at room temperature in blocking buffer (PBS, 0.1% Triton-X, 2% goat serum, 3% BSA) and then incubated overnight at 4 °C with primary antibody for rabbit anti-MCH (1:5000; Phoenix Pharmaceuticals). The next day, sections were washed in washing buffer (PBS, 0.1% Triton-X) 3 times for 20 min, incubated with dye-conjugated secondary antibody (1:1000; Alexa Fluor 488 or 594 goat anti-rabbit) for 1 h at room temperature, washed in washing buffer, and then mounted.
2.8. Microscopy and quantification
Stained brain sections were imaged using Zeiss LSM 510 laser scanning confocal microscope. All parameters were kept constant for quantification purposes and were conducted with ImageJ.
2.9. Statistics
Results are expressed as mean ± SEM. Student t test, one-way ANOVA, or two-way ANOVA was used to assess the significance of difference of the mean or the comparative analyses. Sidak post-hoc tests were used for multiple comparisons. Differences were considered significant at p < 0.05.
3. Results
3.1. MCH neuron profiling confirms glutamatergic identity of these neurons
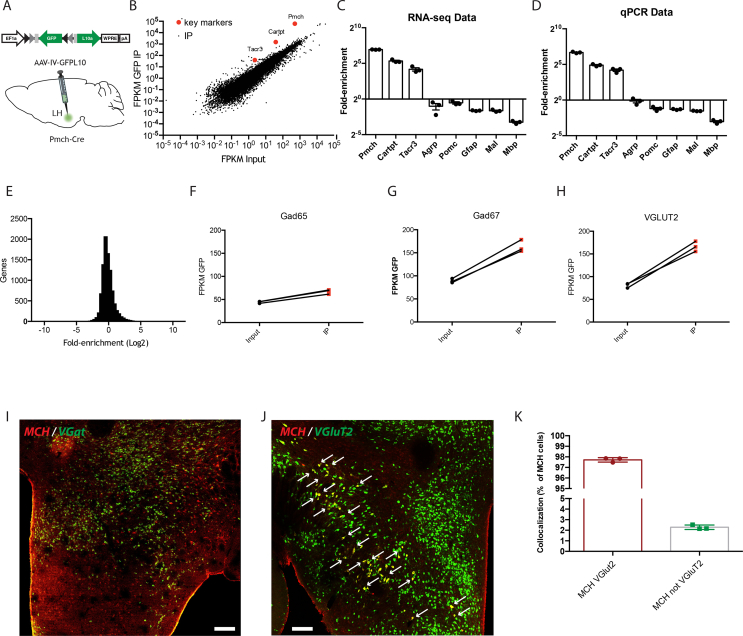
We first set out to establish the phenotypes of MCH neurons in an unbiased manner by molecular profiling using viral TRAP (vTRAP) (short for viral Translating Ribosome Affinity Purification). We injected an adeno-associated virus (AAV) expressing the ribosomal fusion protein L10 flanked by loxp sites (AAV-GFPL10) into the LH of MCH Cre mice. After three weeks, polysomes from hypothalamic blocks were precipitated using an anti-GFP antibody followed by high-throughput RNA sequencing (RNA-seq) of both the precipitated polysomal RNA (IP RNA) and total Input RNA (Figure 1A). We plotted FPKM (fragment per kilobase of transcript per million mapped reads) values for the IP RNA vs Input RNA on a log–log scale (Figure 1B) and found that Pmch itself was 121 Fold differentially enriched (DE is IP/Input) and that Tacr3 (19 Fold DE) and Cartp (40 Fold DE), two RNAs known to be co-expressed in MCH cells, were also highly enriched (Figure 1B–D) [9], [11], [12]. As expected, we also found enrichment of GFP (14 Fold DE). Finally, we plotted the number of differentially enriched genes in a histogram as a function of the fold-enrichment in the IP samples (Figure 1E) and found that in addition to Pmch, which was the most enriched gene, an additional ∼2000 genes were also highly enriched including Mup6 (78.3 fold), Klrg1 (53.91 fold), and Iapp (30.8 fold). The fold enrichment for all of the enriched genes is listed in Supplemental Table 1. We next replicated the RNA-seq data for a subset of genes by performing qPCR on the IP and total RNA and reconfirmed highly significant enrichment of Pmch (102.5 Fold), Tacr3 (18.4 Fold), and Cartp (29.9 Fold) (Figure 1D). As expected, we did not see enrichment of the arcuate markers Agrp and Pomc or glial markers (Gfap, Mal and Mbp) (Figure 1B–D).
Figure 1.
MCH neurons express are uniquely glutamatergic. (A) Mixed illustration. Schema of the adeno-associated virus (AAV) design for capturing transcribing RNA by immunoprecipitation and representation of stereotactic injection of AAVIVGFPL10 into the lateral hypothalamus of the brain. (B) FPKM GFP IP plotted against FPKM input on a Log–Log scale. Red dots highlight differentially enriched markers of MCH cells (Pmch, Tacr3 and Cartp), (C-D) RNAseq data and Taqman analysis of MCH neuron markers (Pmch, Tacr3 and Cartp), glial markers, Agrp and Pomc (Non MCH-expressed genes), (E) Histogram display of number of differentially enriched genes (IP/Input) (F–H) RNAseq enrichment of glutamatergic VGlut2 gene and GABAergic Gad1 and Gad2 genes. (I) RNAscope for VGAT paired to an IHC for MCH showing no colocalization between MCH and VGAT (J–K) IHC of MCH (red) in a VGlut2GFP mouse. Colocalization is shown in yellow and with white arrows pointing to the colocalizing cells. n = 3 in each group. Data are expressed as mean ± SEM. **P < 0.01. Scale bar = 100 nm. See also Supplemental Table 1.
Next, we analyzed the fold enrichment of markers for glutamate and GABA and found significant enrichment of the vesicular glutamate transporter 2 (vGlut2) RNA (2.1 Fold, p < 0.01) (Figure 1H) and vGlut3 (2,88 Fold, p < 0.01) but not for vGlut1 (Supplemental Table 1). For GABAergic markers, there was a 1.8-fold enrichment for the 67 kD isoform of glutamic acid decarboxylase GAD67 (Gad1, 1.8-fold, p < 0.01) and 1.6-fold enrichment for the 65 KDa isoform, GAD65 (Gad2, 1.6-fold, p < 0.01) (Figure 1F–G) raising the possibility that the MCH neurons could be glutamatergic or GABAergic. However, VGAT, the GABA transporter, was not enriched (VGAT, 1.01-fold, p = 0.95).
We further characterized these neurons by performing dual ISH/IHC for MCH (Red) in a glutamatergic vGlut2 GFP reporter mouse line and found colocalization between vGlut2 and MCH in approximately 97% of the MCH neurons (Figure 1J–K). We also performed in situ hybridization using RNAscope technology for VGAT (green) and MCH (red) and found no colocalization between these two markers (Figure 1I). We next set out to establish the respective functions of glutamate and MCH in these neurons.
3.2. Glutamatergic MCH signaling and energy balance
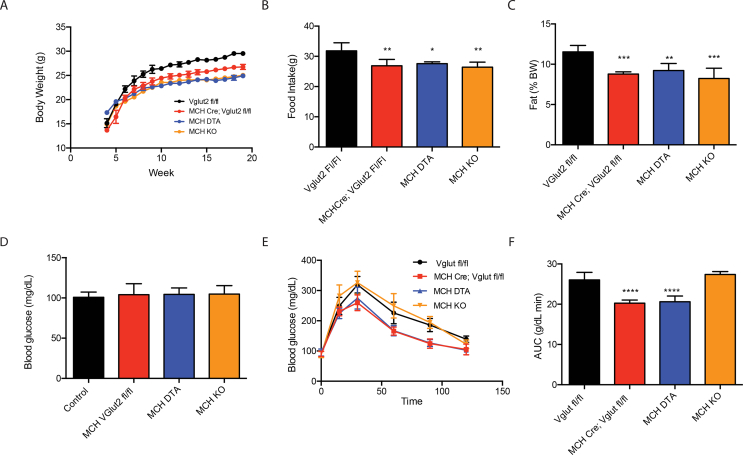
We evaluated the function of glutamate signaling by MCH neurons by generating MCH neuron-specific vGlut2 knockout mice by mating previously validated MCH-Cre mice [2] to animals with a floxed allele of vGlut2 (hereafter referred as MCHCre;vGlut2Fl/Fl) [2]. Immunohistochemical staining for MCH revealed that the vGlut2 knockout in MCH neurons did not alter the MCH population size or somatic area (Figure S1A), indicating that a vGlut2 knockout in these neurons does not alter their differentiation and/or survival. We next compared the metabolic phenotype of these mice with animals to an ablation of MCH neuron using diphtheria toxin (hereafter referred as MCHDTA mice) or a germline knockout of the MCH neuropeptide (MCHKO). VGlut2Fl/Fl mice showed a significant reduction in body weight that was similar in magnitude to those of MCH ablated and MCHKO mice (Figure 2A). Also similar to both, the MCH vGlut2 knockout mice were hyperactive (Figure S1B) and also showed a late onset hypophagia (Figure 2B) resulting in a significant reduction in adiposity (Figure 2C). When fed a HFD, also similar to the MCH KO and MCH ablation, MCHCre; VGlut2Fl/Fl mice gained less weight than controls (Figure S1C) with significantly reduced adiposity (Figure S1D) and food intake (Figure S1E) [13]. Consistent with this, MCH VGLUT2 KO mice had significantly lower leptin levels than control animals (without a knockout) fed either normal chow diet or a high fat diet (HFD) (Figure S1F).
Figure 2.
MCHCre;Vglut2fl/fl mice are lean and show improved glucose tolerance similar to diphtheria toxin ablated MCH mice. (A) Weekly body weight assessment in MCHKO, MCHDTA, and MCHCre;VGlut2 fl/fl mice, (B) Food intake assessment at 12 weeks of age in MCHKO, MCHDTA, and MCHCre;VGlut2 fl/fl mice, (C) Adiposity shown by means of an EchoMRI scan of MCHKO, MCHDTA, and MCHCre;VGlut2 fl/fl, (D) Basal blood glucose levels and (E-F) glucose tolerance test of MCHKO, MCHDTA and MCHCre;VGlut2 fl/fl mice. n = 5–8 in each group. Data are expressed as mean ± SEM. *p < 0,05 **P < 0.01 ***p < 0.001****p < 0,0001. See also Figure S1.
3.3. Glutamatergic signaling by MCH neurons, but not MCH, regulates glucose metabolism and sucrose preference
Previous studies have shown that MCH neurons are glucose sensitive and that ablation of these neurons alters glucose homeostasis [3], [14], [15]. At baseline, MCHKO, MCHDTA, and MCHCre;VGlut2Fl/Fl mice were normoglycemic (Figure 2D). However, similar to mice with an MCH ablation, MCHCre;VGlut2Fl/Fl mice showed a significant improvement of glucose tolerance with a 21.16% lower area under the curve compared to control littermate mice after a glucose tolerance test with an injection of a 2 g/kg bolus of glucose. In contrast, mice with a knockout of the MCH peptide did not show an altered glucose tolerance test (Figure 2E–F and [4]). These data demonstrate that, similar to MCH ablation but in contrast to an MCHKO, a deletion of vGlut2 in MCH neurons alters glucose metabolism.
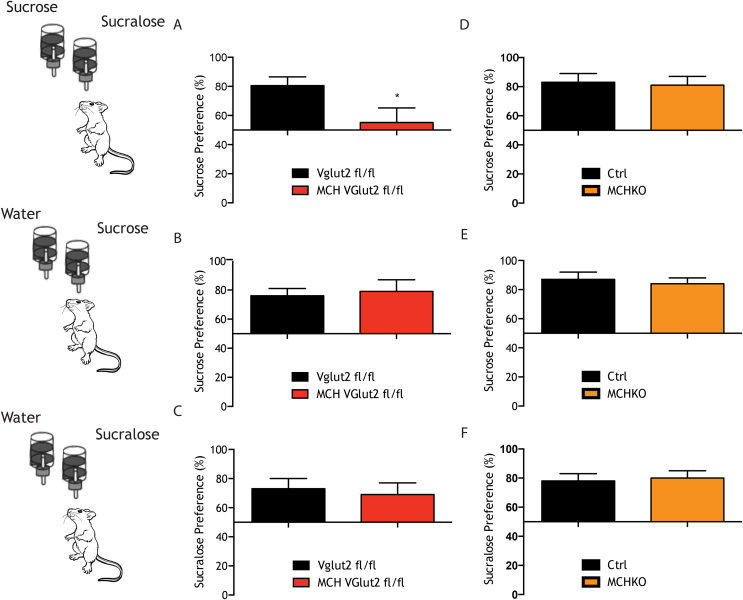
MCH neurons convey the post-ingestive value of sucrose and project to midbrain regions regulating reward [5]. For example, we previously showed that animals with an ablation of MCH neurons no longer prefer sucrose to a sucralose, non-nutritive sweetener. In contrast, MCHKO mice show a normal preference for sucrose. To further assess the roles of glutamate signaling by these neurons, we evaluated the sucrose preference of MCHCre;VGlut2Fl/Fl relative to MCHKO and mice with an MCH neural ablation [10]. The mice were given a sequential set of choices using a two-bottle test as follows: sucrose vs. sucralose, sucrose vs. water and sucralose vs. water, and the preference ratios were computed. Normal mice show a strong preference for sweet taste (either sucralose or sucrose) compared to water and also prefer sucrose to sucralose.
As previously reported, an ablation of MCH neurons reduced an animal's preference for sucrose relative to sucralose while sucrose preference in an MCHKO was unchanged relative to controls (Figure 3A–C). However similar to mice with an MCH ablation, MCHCre;VGlut2Fl/Fl mice did not show a preference for sucrose relative to sucralose (Figure 3A). Thus, the effect of an ablation of MCH neurons on sucrose preference is a result of a loss of glutamate signaling, not MCH itself. A knockout of glutamate signaling in MCH neurons was specific for nutrient preference as the knockout mice still showed a normal preference for either sucrose or sucralose relative to water. (Figure 3B–C).
Figure 3.
Lack of Vglut2 in MCH neurons impairs their sucrose preference but no effect is seen in MCHKO. Mice lacking Vlgut2 (red bars) or global knock out mice (orange bars) in MCH neurons and their respective controls were given the choice of (A,D) Sucrose (0.4 M sucrose) vs Sucralose (1.5 mM sucralose). (B,E) Sucrose vs water. (C,F) Sucralose vs water. All mice preferred either sweetener—sucrose or sucralose—over water. Data are expressed as mean ± SEM. *p < 0,05.
4. Discussion
Numerous studies have shown that MCH neurons in the LH regulate feeding, metabolism, reward, and other biologic processes [1], [2], [3], [5], [16], and many of these effects have been ascribed to MCH itself [1]. However, MCH neurons also express classical neurotransmitter(s) and their functional role compared to the neuropeptide has not been evaluated.
The neurotransmitters co-expressed in MCH neurons have been controversial. While some studies of MCH neurons have only found co-expression of markers for glutamatergic neurotransmission, others have suggested that there is a separate, possibly overlapping, GABAergic subpopulation. For example, a recent report demonstrated expression of glutamate transporter vGlut2 in MCH neurons in 68/69 cells with no co-expression of VGAT [17]. In contrast, another study has shown that MCH neurons co-express the GABA marker GAD67, suggesting that these same neurons may co-release GABA [6]. In yet another publication, the GABAergic LH neurons were reported to project to different VTA regions than the glutamatergic subpopulation and mediate positive reinforcement while LH glutamatergic inputs to the VTA were reported to cause avoidance and suppress DA release in the NAc [18].
To resolve these discrepancies, we used molecular profiling to characterize MCH neurons and found that vGlut2 is expressed in 97% of MCH neurons. In contrast we failed to find expression of the GABA transporter VGAT in these neurons. This suggests that the vast majority of MCH neurons are glutamatergic. However, consistent with other reports, we found a small but significant level of expression of GAD65 and GAD67, which generates GABA by decarboxylation of glutamic acid, in MCH cells (see Figure 1E–G). However, it is unclear what the expression of GAD in these cells means since, in the absence of a reuptake mechanism, GABA release would likely deplete the intracellular pool. Of note, we did not find enrichment for other monoamine transporters, and moreover, any GABA production by these cells would be co-expressed with vGlut2 in the vast majority of MCH cells. Thus while our anatomic data strongly suggest a glutamatergic identity of most MCH cells, we cannot rule out the existence of other MCH cell subtypes or their co-release or reuptake with other neurotransmitters such as GABA through non-canonical mechanisms [19], [20]. Consistent with a more prominent role for glutamate and not GABA signaling by these neurons, our functional studies show that the aggregate effects of the MCH peptide and glutamate signaling by MCH neurons can fully explain the known functions of these neurons. Indeed, in each case where the phenotype of a mouse with an MCH knockout differs from that of mice with ablation of MCH neurons, the discrepancy can be explained by invoking glutamate signaling. For example, while the effect to reduce the body weight of a MCHvGlut2KO is similar to that of an MCHKO, the function of these neurons to control glucose metabolism requires glutamate but not MCH signaling (Figure 2). Similarly while it was hypothesized that the effect of MCH ablation in adult mice to alter glucose tolerance might be a result of disruption of CART, nesfatin-1 or GABA [4], our results show that glutamate signaling is responsible for this effect. Additional reports have established that MCH neurons communicate the reward value of sucrose [5]. Here again, MCHKO mice do not show alterations of sucrose preference suggesting that other transmitters are responsible for this effect and the data in this report show that glutamate in MCH neurons is responsible for conveying the reward value of sucrose. Overall, these data suggest that the function of these neurons is a composite of a distinct set of effects of the neuropeptide (MCH) and the classical neurotransmitter (glutamate).
However, these results do not exclude the possibility that there still might be further heterogeneity of MCH neurons as was suggested by a recent report dissecting the role of MCH neurons that differ with respect to the expression of the insulin receptor (InsR), and showing that the InsR subset regulates glucose metabolism [21]. In line with their results we also found enrichment of InsR in these neurons (Supplemental Table 1). The finding that MCH neurons are heterogeneous with respect to InsR and possibly GAD gene expression suggests that intersectional studies [22] may be necessary to determine the different functions, projection sites, and neurocircuitry of specific subsets of MCH (and other) neural populations. Studies of this sort will be advanced by employing multiple recombinases, such as Dre and Flp, which will enable analyses of specific populations based on their co-expression of two or more marker genes.
Our findings that there are distinct effects of MCH ablation, MCH knockout and a knockout of glutamate signaling in MCH Neurons also shows that the function of neuropeptide producing neurons is likely to be a composite of roles of classic neurotransmitters and neuropeptides. In some cases, these processes are redundant, i.e. weight, food intake, etc., and in other cases non-redundant, i.e. in glutamate producing MCH neurons roles in sucrose preference and glucose metabolism control. The most likely possibility to explain these differences is that the peptides and classical neurotransmitter project to and act at partially overlapping sites. Consistent with this, the pattern of expression of MCH receptor (MCHR) is only partially overlapping relative to the sites of projections of MCH neurons. It is thus likely that some of these projections rely on glutamate rather than MCH signaling. For instance, there is dense labeling of MCH projections in the hippocampal formation, subiculum, basolateral amygdala, and the shell of the nucleus accumbens, all of which express the MCHR [23]. Similarly, MCHR mRNA is expressed at hypothalamic and brainstem projection sites of MCH neurons including the VMH, ARC, and zona incerta, locus coeruleus, and in orofacial nuclei, [23]. In contrast, consistent with our finding that glutamate is required for sucrose preference, MCH neurons project to the VTA, where MCHR expression is not seen [23]. The disparate effects of MCH and glutamate may also be a result of the fact that glutamate acts at synapses while MCH can signal in a volumetric manner which also would result in signaling at partially overlapping cell types.
In summary, molecular, anatomic and genetic analyses were performed to establish the expression and function of classical neuro-transmitters by MCH neurons. Molecular profiling showed that nearly MCH neurons co-express the vGlut2 transporter but not VGAT. While MCH neurons also express GAD65 and GAD67, the function of GABA is uncertain in the absence of a reuptake mechanism and, since ∼97% of MCH neurons express vGlut2, GABAergic MCH neurons would co-express glutamate in the vast majority of these neurons. Consistent with a prominent function of glutamate signaling by these neurons, comprehensive genetic analyses indicate that either MCH and/or glutamate are sufficient to mediate all of the known functions of these neurons. These data suggest that the effects of MCH neurons are a composite of the effects of these two neurotransmitters.
Author contributions
MS, KT, LP, and AN designed and performed experiments and analyzed data. MS and JF wrote the manuscript. EA and AD performed sucrose preference experiments. ZL and CC provided experimental support and intellectual input. JF conceived the study, supervised research, and wrote the manuscript with input from all authors.
Acknowledgements
This work has been supported by funding from The JPB foundation (CEN 5402134; J.M.F.). M.S.P. acknowledges support from the KAVLI NSI Neuroscience Institute at Rockefeller University. A.R.N. acknowledges support from the David Rockefeller Fellowship and the Brain and Behavior Research Foundation NARSAD Young Investigator Award. E.A. acknowledges support from the Human Frontier in Science Fellowship. We would like to thank Friedman laboratory members for helpful discussions and G. Luzia for assistance with preparation and submission of the manuscript. We thank the Rockefeller University Genomics Core for help with RNA-seq. Imaging was performed at the Rockefeller University Bio-Imaging Resource Center.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.05.001.
Conflicts of interest
Authors declare no conflicts of interest.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Further characterization of a MCHCre;Vglut2 fl/fl mouse line. (A) MCH neurons are viable and produce MCH neuropeptide after knocking out Vglut2. (B) Locomotion activity of MCHCre;Vglut2 fl/fl mice and littermate controls. (C) MCHCre;Vglut2 fl/fl mice body weight assessment in HFD. (D) Adiposity of MCHCre;VGlut2 fl/fl mice and littermate controls in HFD. (E) Weekly food intake assessment of MCHCre;VGlut2 fl/fl mice and littermate controls in HFD. (F) Plasma leptin levels of MCHCre;VGlut2 fl/fl mice and littermate controls in chow diet and HFD. Data are expressed as mean ± SEM. *p < 0.05 **P < 0.01.
Gene expression and fold change from RNAseq experiment using vTRAP.
References
- 1.Shimada M., Tritos N.A., Lowell B.B., Flier J.S., Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396(December):670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 2.Alon T., Friedman J.M. Late-onset leanness in mice with targeted ablation of melanin concentrating hormone neurons. Journal of Neuroscience. 2006;26(2):389–397. doi: 10.1523/JNEUROSCI.1203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong D., Vong L., Parton L.E., Ye C., Tong Q., Hu X. Glucose stimulation of hypothalamic MCH neurons involves KATP channels, is modulated by ucp2, and regulates peripheral glucose homeostasis. Cell Metabolism. 2010;12(5):545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiddon B.B., Palmiter R.D. Ablation of neurons expressing melanin-concentrating hormone (MCH) in adult mice improves glucose tolerance independent of MCH signaling. Journal of Neuroscience [Internet] 2013;33:2009–2016. doi: 10.1523/JNEUROSCI.3921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domingos A.I., Sordillo A., Dietrich M.O., Liu Z.W., Tellez L.A., Vaynshteyn J. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife. 2013;2013 doi: 10.7554/eLife.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jego S., Glasgow S.D., Herrera C.G., Ekstrand M., Reed S.J., Boyce R. The role of Hcrt/Orx and MCH neurons in sleep-wake regulation. Nature Neuroscience [Internet] 2013;16(11):1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee M.J.S., Arrigoni E., Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. Journal of Neuroscience [Internet] 2015;35(8):3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nectow A.R., Moya M.V., Ekstrand M.I., Mousa A., McGuire K.L., Sferrazza C.E. Rapid molecular profiling of defined cell types using viral TRAP. Cell Reports. 2017;19(3):655–667. doi: 10.1016/j.celrep.2017.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekstrand M.I., Nectow A.R., Knight Z.A., Latcha K.N., Pomeranz L.E., Friedman J.M. Molecular profiling of neurons based on connectivity. Cell. 2014;157(5):1230–1242. doi: 10.1016/j.cell.2014.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domingos A.I., Vaynshteyn J., Voss H.U., Ren X., Gradinaru V., Zang F. Leptin regulates the reward value of nutrient. Nature Neuroscience [Internet] 2011;14(12):1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight Z.A., Tan K., Birsoy K., Schmidt S., Garrison J.L., Wysocki R.W. Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell. 2012;151(5):1126–1137. doi: 10.1016/j.cell.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croizier S., Franchi-Bernard G., Colard C., Poncet F., la Roche A., Risold P.Y. A comparative analysis shows morphofunctional differences between the rat and mouse melanin-concentrating hormone systems. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0015471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneeberger M., Gomis R., Claret M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. Journal of Endocrinology. 2014 doi: 10.1530/JOE-13-0398. [DOI] [PubMed] [Google Scholar]
- 14.Burdakov D. Unraveling electrical signaling strategies in hypothalamic feeding circuits. Trends in Endocrinology and Metabolism. 2005:202–203. doi: 10.1016/j.tem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Burdakov D., Gerasimenko O., Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. Journal of Neuroscience. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu D., Ludwig D.S., Gammeltoft S., Piper M., Pelleymounter M.A., Cullen M.J. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 17.Mickelsen L.E., Kolling F.W., Chimileski B.R., Fujita A., Norris C., Chen K. Neurochemical heterogeneity among lateral hypothalamic hypocretin/orexin and melanin-concentrating hormone neurons identified through single cell gene expression analysis. Eneuro [Internet] 2017;4(5) doi: 10.1523/ENEURO.0013-17.2017. ENEURO.0013-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieh E.H., Vander Weele C.M., Matthews G.A., Presbrey K.N., Wichmann R., Leppla C.A. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron. 2016;90(6):1286–1298. doi: 10.1016/j.neuron.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tritsch N.X., Ding J.B., Sabatini B.L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490(7419):262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tritsch N.X., Oh W.J., Gu C., Sabatini B.L. Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. Elife. 2014;3:e01936. doi: 10.7554/eLife.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hausen A.C., Ruud J., Jiang H., Hess S., Varbanov H., Kloppenburg P. Insulin-dependent activation of MCH neurons impairs locomotor activity and insulin sensitivity in obesity. Cell Reports. 2016;17(10):2512–2521. doi: 10.1016/j.celrep.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Fenno L.E., Mattis J., Ramakrishnan C., Hyun M., Lee S.Y., He M. Targeting cells with single vectors using multiple-feature Boolean logic. Nature Methods [Internet] 2014;11(7):763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito Y., Cheng M., Leslie F.M., Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. Journal of Comparative Neurology [Internet] 2001;435(1):26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Further characterization of a MCHCre;Vglut2 fl/fl mouse line. (A) MCH neurons are viable and produce MCH neuropeptide after knocking out Vglut2. (B) Locomotion activity of MCHCre;Vglut2 fl/fl mice and littermate controls. (C) MCHCre;Vglut2 fl/fl mice body weight assessment in HFD. (D) Adiposity of MCHCre;VGlut2 fl/fl mice and littermate controls in HFD. (E) Weekly food intake assessment of MCHCre;VGlut2 fl/fl mice and littermate controls in HFD. (F) Plasma leptin levels of MCHCre;VGlut2 fl/fl mice and littermate controls in chow diet and HFD. Data are expressed as mean ± SEM. *p < 0.05 **P < 0.01.
Gene expression and fold change from RNAseq experiment using vTRAP.