Abstract
This study focuses on the behavior of chitosan (CHI) and its polyelectrolyte complexes with carboxymethyl starch (CMS) used as monolithic matrices with acetaminophen as drug tracer. Two different chitosan grades were tested alone or associated in various ratios with CMS as excipients for tablets obtained by direct compression. The degree of deacetylation (DDA) of CHI, estimated from 1H NMR and FTIR data, was correlated with X-ray diffraction and scanning electron microscopy (SEM) to evaluate structural organization of the monolithic matrices. In vitro drug dissolution assays showed major differences in CHI kinetic profiles between tablets exposed to acidic medium for 2h (to mimick gastric passage) prior to dissolution in simulated intestinal fluid (SIF), and those administered directly to SIF. Prior exposure to acidic SGF conducted to longer dissolution profiles (release completed after 16 h) and preservation of tablet shape, whereas tablets directly incubated in SIF were rapidly disintegrated. The improved properties of chitosan matrices exposed to SGF may be related to an outer compact coating layer (visible in SEM). The effect of self-stabilization of chitosan in acidic medium was compared to that due to formation of polyelectrolyte complexes (PEC) in co-processed polymeric systems (CHI:CMS). The self-formed membrane following exposure to gastric acidity appears to help maintaining tablet integrity and allows higher drug loading, recommending CHI and its complexes with CMS as excipients for drug delivery.
Keywords: Chitosan, Self-stabilization, Biomimicking gastro-intestinal transit, Carboxymethylstarch, Self-assembling, Polyelectrolyte complexes, Drug delivery
Abbreviations: CHI, Chitosan; CMS, Carboxymethylstarch; DDA, degree of deacetylation; FTIR, Fourier transformed Infra-Red; GIT, Gastrointestinal tract; 1H NMR, proton nuclear magnetic resonance; HAS, Gelatinized High Amylose Starch; PEC, polyelectrolyte complex; SIF, simulated intestinal fluid; SGF, simulated gastric fluid
Graphical abstract
Highlights
-
•
Chitosan tablets incubated in conditions mimicking gastric residence undergo favorable structural rearrangement.
-
•
The effect is stronger in chitosan with higher degree of deacetylation, with better tablet shape and dissolution profiles.
-
•
Tablets made with chitosan:carboxymethyl starch are stabilized due to polyelectrolyte complex formation.
-
•
Polyelectrolyte complexation is higher at low deacetylation while acidic self-conditioning is stronger at high deacetylation.
-
•
Tablets made of chitosan or chitosan:carboxymethyl starch show promise for colonic drug delivery.
1. Introduction
The number of biocompatible polymers (natural or synthetic) proposed as excipients for the drug formulations by the pharmaceutical industry recorded a huge increase during the last decades.
Chitosan [poly β-(1,4)-2-amino-2-deoxy-d-glucopyranose, CHI] can be obtained by the deacetylation and partial depolymerization of chitin, the most abundant polysaccharide in nature [1]. Until recently, only marine sources (shrimp, prawn, crab) have been used to provide the starting chitin. Chitosans obtained following this difficult to control process [2] contain chains with 2-amino-glucose and with N-acetyl-2-amino-glucose units, in variable ratios. They are obtained in different molecular weights. Recently, new commercial chitosans, better characterized by manufacturers and with enhanced safety characteristics for certain pharmaceutical, cosmetic, and biomedical applications have been produced at lower costs [[3], [4], [5]]. Not affected by seasonal variations, different fungi have also been studied in recent years as a competitive source of chitin/chitosan [6,7].
The interest for chitosan-based materials for pharmaceutics and biomedical applications continues to increase [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. Chitin, the natural source of chitosan, not being of plant origin, generated a certain delay in acceptance by the pharmaceutical industry in many countries. However, the fact that chitosan is nontoxic, biocompatible, biodegradable, antimicrobial, and easy to obtain makes it a good candidate for many applications. There are reports of chitosan used in dietary foods [1,9,10], as films for food packaging and for biomedical applications [11,12], as matrix for immobilization of bacterial cells [13], as implantable delivery systems [14], in bandages [15] and as excipient for oral medication [2,16,17]. Chitosan is considered Generally Recognized As Safe (GRAS) and approved for specific applications [[18], [19], [20]]. Another attractive feature of chitosan is its capability to produce nanostructure using simple procedures conducted under mild conditions [[21], [22], [23], [24], [25]]. Chitosan based nanomaterials mimicking natural structures (crustaceous shells or extracellular matrices) have also been reported [[23], [24], [25], [26]]. When used in tablets, chitosan role can change depending on its proportion. Evaluation of chitosan-microcrystalline cellulose blends as direct compression excipients has shown that chitosan promotes flowability of powder mix and rapid disintegration of the tablet. However, incorporation of equal proportion of microcrystalline cellulose and chitosan affords an extended-release tablet. It was concluded that in combination with microcrystalline cellulose chitosan promotes tablet disintegration at low concentration and enables extended-release at higher concentration [27]. Cross-linked chitosan was investigated as sustained release agent in tablets. In granular form, particles of CHI crosslinked with tripolyphosphate exbibited some limitations like inefficient drug loading and incomplete release [28]. Associated with anionic polymers (i.e. xanthan gum), CHI was used for wet granulation of a model drug with high solubility in gastric fluid (paracetamol) [29]. From the data generated it was concluded that this approach has sufficient potential to sustain the release of paracetamol. Another type of CHI application consists of its incorporation in vaginal tablets. Two-layer vaginal tablets, containing different polymeric ratios (i.e. 20 mg Na-CMC; 50 mg Carbopol® 934; 20 mg chitosan) were selected as optimal due to swelling index and dissolution/erosion capability [30].
Despite largely investigated at laboratory scale, the inexpensive and easily accessible chitosan is still not used as excipient in oral formulations on an industrial scale partially due to concerns related to its solubility: these materials are soluble in acidic media and insoluble in neutral and alkaline fluids. Many formulations found in the literature required addition of different ingredients to enhance compressibility and improve the drug dissolution profiles [31,32]. Chemical procedures targeting advanced deacetylation and reacylation with more hydrophobic groups have been studied together with the interaction of covalently-modified chitosan matrices with both charged and uncharged chemical species. These approaches were recommended for drugs targeting the small intestine or the colon [17].
The novelty of this work is that, to the best of our knowledge, it is the first report showing a rearrangement at the surface of chitosan tablets following exposure to acidic medium, mimicking the physiological gastric residence. This effect of self-stabilization of chitosan was also investigated in monolithic tablets based on CHI: carboxymethylstarch (CMS) mixtures. Used as excipient in tablets, CMS can protect bioactive agents during gastric residence. At pH 1.2, being protonated, it affords a more compact matrix and better control of drug release in the intestinal medium where the carboxyl groups are deprotonated fostering hydration and swelling of the tablet [13]. In the present study the contribution of electrostatic interactions was evaluated in two series of tablets containing: i) a variable ratio of the two polymers CHI:CMS and b) a constant amount of cationic polymer (CHI) combined with various amounts of anionic (CMS) and - neutral high amylose starch (HAS) as filler in order to keep the mass of tablets constant. The structural changes were monitored by spectroscopic analysis. The effects of exposure of tablets based on various polymers to simulated gastric and intestinal fluids were investigated by in vitro dissolution assays and compared.
2. Materials and methods
2.1. Materials
Two different chitosans were used in this study: one (CHIa) obtained in-house by an advanced deacetylation procedure [33] of chitosan from Protan Co. (Drammen, Norway), the other one (CHIb), degree of deacetylation (DDA) 85%, was purchased from Marinard Biotech Inc. (Canada). CMS was prepared starting with high amylose starch from National Starch, USA, and using a procedure previously published [34]. Gelatinized high amylose starch (HAS) used as filler, was prepared following a procedure found in the literature [35]. The other chemicals were from Sigma (USA) and were reagent grades and used without further purification.
2.2. Evaluation of the degree of deacetylation
The estimation of DDA of the two grades of CHI was done using 1H NMR and FT-IR analysis.
2.3. Proton nuclear magnetic resonance (1H NMR)
High resolution 1H NMR spectra were recorded on a 500 MHz Varian Innova NMR spectrometer. CHI samples were dissolved in 1% trifluoroacetic acid (TFA) in D2O.
2.4. Fourier-transform infrared spectroscopy (FTIR)
CHI tablets (6% in KBr pellets of 100 mg) were made in a Carver hydraulic press Model C-3912 (Wabash, IN, USA), at 8 T. For each CHI grade three tablets were made and evaluated by FTIR. Absorbance spectra were recorded in a Bomem MB-100 FT-IR spectrophotometer (Hartmann &Braun) with a deuterated triglycine sulfate (DTGS) detector. The spectra were obtained at a 2 cm−1 resolution as an average of 64 scans, with air as background.
2.5. Tablet preparation, their stability in SGF and dissolution tests in vitro
Monolithic tablets (200 mg, 12 mm diameter) were made by direct compression (in a Carver hydraulic press, at 2.5 T) of CHIa or CHIb with 20% acetaminophen (as model drug) mixed powders.
Two series of tablets (200 mg each), all containing 20% acetaminophen, were made in the same conditions, and contained as follows: Series I) CHI and CMS in different ratios (w/w, 0:3, 1:2, 1:1, 2:1, 3:0), Series II) a constant load of CHI, variable CMS and HAS as filler (as shown in Table 1). To investigate the behavior of preparations in gastric acidity, tablets based on CHI:CMS (1:1) and loaded with 5% bromocresol green (10 mg pH indicator per tablet), were also prepared by direct compression of mixed powders, as described above. Each tablet was incubated for 120 min in 50 mL of SGF at 37 °C and 50 rpm (incubator shaker, series 25D, New Brunswick Scientific Co., NJ, USA). The tablet integrity and color modifications were observed on the whole and on cross-sectioned tablets.
Table 1.
Formulations of 200 mg monolithic tablets.
| Sample label | Acetaminophen (mg) | CHIa or CHIb (mg) | CMS (mg) | HAS (mg) | |
|---|---|---|---|---|---|
| Series I |
CHI:CMS 3:0 | 40 | 160 | – | – |
| CHI:CMS 2:1 | 40 | 106.7 | 53.3 | – | |
| CHI:CMS 1:1 | 40 | 80 | 80 | – | |
| CHI:CMS 1:2 | 40 | 53.3 | 106.7 | – | |
| CHI:CMS 0:3 |
40 |
– |
160 |
– |
|
| Series II | CHI:CMS 1:1.00 | 40 | 80 | 80 | 0 |
| CHI:CMS 1:0.75 | 40 | 80 | 60 | 20 | |
| CHI:CMS 1:0.50 | 40 | 80 | 40 | 40 | |
| CHI:CMS 1:0.25 | 40 | 80 | 20 | 60 | |
| CHI:CMS 1:0.00 | 40 | 80 | 0 | 80 |
Tablet shapes and swelling were monitored in a rotary shaker (50 rpm), first for 2 h in 50 mL SGF, and then, after being transferred to 50 mL SIF, they were monitored for up to 20 h (50 rpm) as per recommendations of US Pharmacopeia for dissolution tests. Tablets containing bromocresol green as pH indicator were first exposed to SGF in order to visualize the water front penetration into the tablets. Kinetic studies of the drug release were conducted in a Distek™ dissolution system (2100A paddle system, at 50 rpm), coupled with an UV Hewlett Packard spectrophotometer. Acetaminophen was detected at 280 nm. The dissolution media (SGF, pH 1.2 and SIF, pH 7.2) were prepared in USP (United States Pharmacopeia) conditions [32]. Practically, the SGF was prepared by dissolving 7.0 mL of HCl to 900 mL of deionized water, add 2.0 g of NaCl and complete the solution with water to 1000 mL. SIF was prepared by dissolving 6.8 g of monobasic potassium phosphate in 250 mL of water and then adding 77 mL of 0.2 M sodium hydroxide and 500 mL of water. The resulting solution was adjusted with 0.2 M sodium hydroxide or 0.2 M hydrochloric acid to a pH of 7.2 ± 0.1 and finally diluted to 1000 mL.
The release profiles were recorded at 37 °C in SGF for 2h and then in SIF, with samples taken at 30 min intervals over 24 h. The amount of released drug was expressed as Mt/M∞ (equation (1)) (Korsmeyer – Peppas model [36]):
| (1) |
where Mt/M∞ represents the fraction of drug released at time t from the total amount released M∞k is the release rate constant, n is the release exponent (used to characterize different release mechanism of drug). All dissolution tests were performed in triplicate.
2.6. X-ray diffraction
X-ray diffraction patterns of powders (obtained from tablets dried and ground after exposure to SGF and/or SIF) were recorded using a Siemens D-5000 diffractometer [with silicon detector] in reflectance mode at a Co Kα wavelength of 1.79018 Å over an angular range 5–50°. The spectra obtained were analyzed using Diffract-AT software.
2.7. Scanning electron microscopy (SEM)
Samples were analyzed using a Hitachi S-2300 (VP-SEM) coupled to a Kevex image analysis system and to an EDX x-rays analyzer (by energy dispersion) for dry and conductive samples (a layer of gold was deposited on surface of dry samples by chemical vapor deposition). The vacuum was10−5 torr and the resolution of 4.5 nm at 25 V.
3. Results and discussion
Spectroscopic analysis (1H NMR and FTIR) has shown a higher DDA for the CHIa than for the CHIb. In the 1H NMR spectra (APPENDIX 1_ Fig. 1), a stronger peak at 2.0 ppm was observed for CHIb, ascribed to the three N-acetyl protons of N-acetylglucosamine still present and in a higher percentage due to a lower DDA. The difference in 1H NMR signals at 3.1 ppm (H-2 proton of glucosamine and N-acetylated glucosamine) and at 3.6–4.0 ppm (the cycle protons, H-3,4,5,6, and 6′) as well as the higher integral values for CHIa, support the assignment of a higher DDA of CHIa compared with CHIb. The finding was supported by FT-IR analysis that have shown that the ratio between the FTIR absorption peaks (APPENDIX 1_Fig.2) at 1655 cm−1, assigned to the carbonyl stretching of secondary amides (amide I band), and those at 1570 cm−1 (assigned to the N-H bending vibrations of the non-acylated primary amine) is higher in CHIb, with more N-acetyl groups.
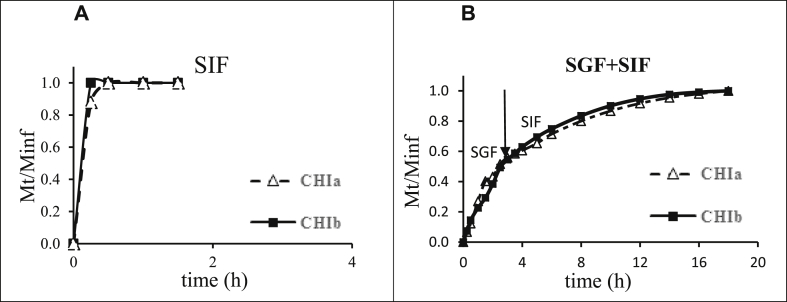
Dissolution kinetics in neutral SIF of the monolithic tablets made with each chitosan as excipient, showed for both of them, a fast release of the entire amount of acetaminophen tracer in less than 1 h (Fig. 1A). The release profiles were markedly different when the tablets were first incubated for 2 h in SGF mimicking the in vivo gastric passage and then transferred in SIF mimicking the behavior of tablets over the whole gastrointestinal tract (GIT). It appeared that the residence in acidic SGF improved the control of the acetaminophen release in SIF, which was completed in about 16 h (Fig. 1B) for tablets based on both CHIa or CHIb excipients. A limited drug dissolution (burst effects) occurred from chitosan tablets in acidic medium (pH 1.2) due to the dissolution of the acetaminophen located in the external layers of the tablet. The dissolution patterns have been followed with both CHIa and CHIb which were close each other (Fig. 1).
Fig. 1.
Drug release profiles from CHIa and CHIb tablets exposed (A) directly to SIF or (B) for 2 h in SGF and then in SIF (mimicking the gastrointestinal tract). Tests were performed in triplicate in USP apparatus II, at 50 rpm and 37 °C using 200 mg tablets and 1000 mL dissolution medium.
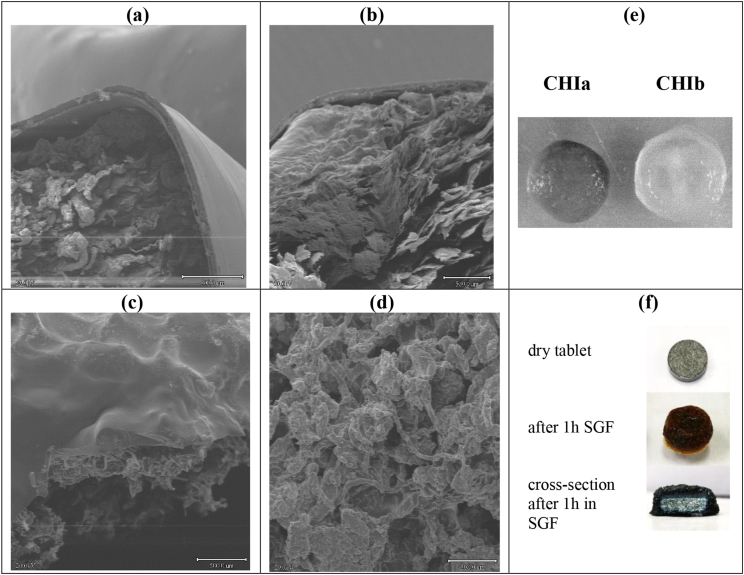
Chitosan tablets incubated for 2h in 50 mL SGF in the rotator shaker swelled visibly and developed an outer gel-like structure. Further evaluation by SEM of the dry tablets after their exposure to SGF and SIF showed that, for both chitosan grades, this gel-like structure was limited to the tablet surface whose consistency clearly differed from that of the tablet core (Fig. 2a and b). This outer coating is probably related to chitosan hydrogen bonding, higher in case of more deacetylated CHIa. When complexed as CHIa:CMS 1:1 the tablets still present a self-induced coating after 2h in SGF (Fig. 2c) whereas no coating at all was found when they were exposed directly to SIF (Fig. 2d). The CHIa maintained better the tablet shape and showed moderate swelling after developing the hydrogel coating, whereas CHIb (more N-acetylated) presented a higher swelling and an almost spherical shape (Fig. 2e) even after the passage from SGF to SIF. These tablet shapes were maintained for both CHI grades even after 22 h spent in SIF, during dissolution tests performed in the Distek™ system, at 37 °C and 50 rpm.
Fig. 2.
SEM micrographs (40 x magnification) of: self-generated coating developed after exposure to SGF of CHIa tablet (a) and CHIb tablet (b); CHIa:CMS 1:1 tablet after 2 h in SGF and then 2 h in SIF (c) and 2 h in SIF only (d) pictures of CHI tablets swollen after 2 h in SGF and then 2 h in SIF (e) and of CHI:CMS (1:1) dry tablet containing bromocresol green and cross-section after its exposure to SGF (f).
To evaluate the pH within the tablets and show to which extent the excipients can protect the drug in SGF, the monolithic devices have been formulated with bromocresol green, a pH indicator changing the color from blue (above pH 5.4) to yellow-orange in acidic media (below pH 3.8). While in the air, before incubation in SGF, the dry tablets based on CHI:CMS (1:1) presented some blue points, the entire surface of the tablets based on CHI:CMS excipients was blue after 1h acidic incubation (Fig. 2f). The gel barrier was better visualized in the tablet cross-section, clearly showing that the core of the tablet was dry. This supports the expectation that the electrostatic association CHI:CMS would have potential as protecting carrier for therapeutic enzymes or of probiotics for colon delivery.
The fast and complete drug release (in less than 1h) from tablets based on both chitosan grades, when incubated directly in SIF (Fig. 1A) fitted well with the SEM observations showing no protective self-coating (Fig. 2d) and, consequently, no barrier to prevent the drug release. In fact, Fig 2c (CHIa:CMS, 1:1) shows part of the gel coating still present while the core is dry.
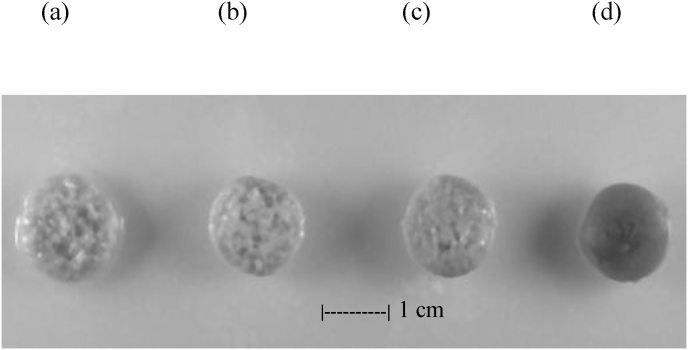
Tablets with different ratios of CHI:CMS were first subjected to a dissolution test in SIF. The presence of CMS was beneficial to the kinetics of the release from tablets based on mixed polymers. This effect varied dependent on the CHI:CMS ratio. Although CHI is largely deprotonated at pH 7.2 (pKa 5.5–6.5), the electrostatic attraction established between anionic (CM) and cationic (amino) groups of CHI helps to maintain the shape of the tablets and controls the drug release. The CMS-only tablets fully disintegrated in less than 2 h in SIF, whereas in the presence of chitosan (CHI:CMS 1:1) they retained their shape for up to 7.5 h. The rapid release of acetaminophen in SIF could be correlated with the porous structure present in the tablets (Fig. 3) as a result of advanced deprotonation at pH 7.2 and subsequent decrease in CHI solubility that leads to formation of large and numerous channels as result of CMS gradual dissolution. A certain porosity was also found for CHI:CMS tablets maintained for 2h in SGF and then in SIF for 16h (Fig. 3a–c). Therefore, the CMS may act as a porogenic agent in CHI:CMS tablets that were not incubated in SGF to get the self-generated coating. The higher the ratio CMS:CHI is, the more porous the structure of the tablet appears. Differently, no porosity at all was found for the CHI tablets (Fig. 3d).
Fig. 3.
Swollen tablets based on physical mixtures CHI:CMS 1:2 (a); CHI:CMS 1:1 (b); CHI:CMS 2:1 (c); CHI:CMS 3:0 (d) after 2 h in SGF followed by 16 h in SIF. The images are obtained with CHIa-based tablets and are representative for CHIb as well.
The dissolution assays done with tablets preexposed for 2 h to SGF, in order to simulate the conditions in the GIT, showed the beneficial effect of chitosan (CHIa) complexation with CMS, as CHI:CMS (Fig. 4 A), providing a better release control when moved to SIF. These observations were also found for CHIb (lower DDA).
Fig. 4.
Dissolution profiles in vitro for acetaminophen released from tablets based on formulations (A) Series I (CHIb:CMS) and (B) Series II - CHIb:CMS:HAS. All tablets were exposed for 2 h to SGF followed by SIF, at 37 °C and 50 rmp.
When chitosan is in an acidic environment, a rate-limiting gel barrier forms, with less defined channels, which results in a slower drug release. At pH 1.2, where ionization of CMS is low due to protonation, the matrix is compacted. Consequently, the formation of PEC can be considered as limited. When CHI:CMS tablets are transferred to SIF, the gel developed in SGF disappears slowly leaving a progressively decreasing barrier for the CMS which is deprotonated, ionized and solubilized tending to leak into the solution. At the same time, upon transfer to SIF, a decrease in the solubility of CHI will occur, possibly with some gain in crystallinity.
While the outer gel is present, the diffusion across it is the rate determining step in drug release. Part of the acetaminophen was released in SGF and the rest is released in SIF with the process being completed after more than 16 h. The presence of CMS in the tablets proportionally increased the release time, presumably due to favorable electrostatic interactions between the two polymers oppositely charged. Except for the CMS-only tablet which disintegrated fast in SIF, the other CHI:CMS tablets preserved their integrity (Fig. 3a–c, Fig. 4A). The swelling was slightly higher in the case of the more N-acetylated CHIb, whereas the impact of the gastric acidity was higher for the CHIa, with a more advanced deacetylation.
Dissolution profiles of CHIa and CHIb were similar. The CHIb was selected for illustration (Fig. 4 A) considering that is a commercial grade and eventual comparison of drug release patterns with other studies could be easier. The hydroxyl groups present in CHI, together with the amino groups, play an important role in the organization of the polymeric matrix as an important parameter in the control of the drug release. The experiments conducted assessed the effect of self-stabilized CHI excipient, on tablet loading and drug delivery profiles and it was compared to the impact on the release of mixed CHI (cationic) with CMS (anionic) polymers, resulting in coulombic interactions.
To better assess the impact of the coulombic attractions on matrix stabilization, parallel dissolution tests were conducted on tablets in which the amount of CHI was kept constant and variable amounts of CMS were added together with uncharged HAS as filler component (Table 1, Series II). The higher the CMS load was, the stronger the control of CHI (more visible for CHIb – Fig 4B) on the release of acetaminophen in SGF appeared to be. Due to the beneficial effect of PEC formation, the tablets with the highest CMS:CHI ratio had the longest release time (∼14h) (Fig 4B). An additional factor in this experiment was the presence of HAS which is not involved in CHI:CMS complexation but it may change the interactions with water leading to higher swelling and less resistant tablets. The dissolution profiles for the CHIa-based formulations are available in APPENDIX 2. The CHI:CMS 1:1 seems to be the optimal ratio, leading to the longest release time (with no HAS in formulation). The same type of dependence was shown by both chitosan grades, both in SGF and in SIF.
Concerning the impact of the polyelectrolyte complexation as CHI:CMS on the behavior of the tablets made with chitosans with different degrees of substitution, the favorable influence of the electrostatic attractions on the kinetics of acetaminophen release is stronger in the case of more N-acetylated chitosan (CHIb) than for CHIa (with more primary amine groups). Pretreatment in SGF has a stronger favorable effect on the latter.
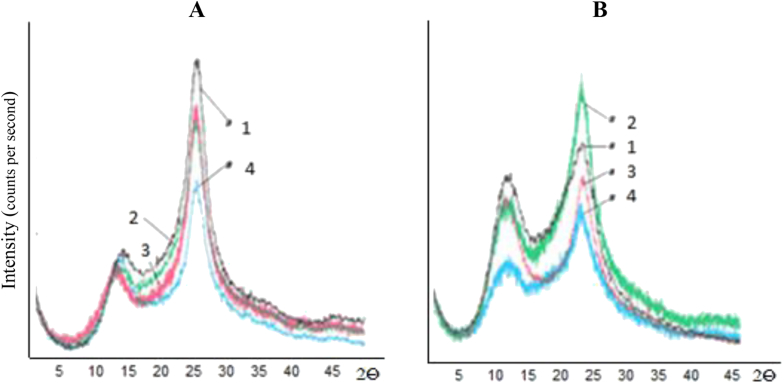
To better understand the role of the matrix characteristics, an X-ray investigation was carried out for both CHIa/CHIb in powder form (Fig. 5). The powders were obtained from tablets, by grinding (Fig. 5 patterns 1 A/B -powders from dry tablets; patterns 2 – after 2 h in SGF; patterns 3 – after 2h in SIF; patterns 4 – after SGF (2h) followed by SIF (2h). Each of them presented major peaks at about 4.3–4.4 Å and around 7.2–7.8 Å. For CHIa, the higher degree order (Fig. 5A) was explained by the enhanced hydrogen bonding afforded by the larger number of primary amine groups.
Fig. 5.
X-rays diffraction patterns of CHIa (A) and CHIb (B) dry (1), after 2 h SGF (2), after SIF only (3), after SGF+SIF (4).
Some differences in the diffraction patterns were found for both CHIa and CHIb before and after acidic pretreatment (Fig. 5). CHIa seems more stabilized by interchain H-bonding, with a higher peak at 4.3 Å. No major differences were found for both chitosans after 2h in SGF but after SGF + SIF treatments a moderate loss of order appeared for both types of chitosan, more pronounced for CHIb.
X-ray diffraction spectra of powders originating from tablets submitted to conditions mimicking the physiological gastric residence and intestinal transit showed peaks at about 4.3 Å and 7.7 Å (Fig. 5). The two CHIa and CHIb chitosans showed in general similar behavior with a more important loss in crystallinity at 4.3 Å in SIF. An overall loss of order was found for both CHI grades after exposure to SGF followed by SIF, more pronounced for CHIb which is less stabilized by hydrogen association (Fig. 5B–4).
The diffraction spectra taken from powders of polyelectrolytic CHI:CMS (PEC) complexes obtained from tablets after 2 h in SGF and then 2h in SIF, where they deprotonate, show a lesser order compared to the tablet based on CHI only (data not shown). This could be due to the disruption of packing and H-bonding upon formation of ionic complexes. However, a better release control was found with the CHI:CMS excipient with lesser order. This observation fits well with the data of Ispas-Szabo et al. [37], showing better release control for moderately ordered cross-linked starch matrices. As a general effect of PEC formation, a decrease in order for both types of chitosans used as mixed powders, with CMS for complexation, is noted in aqueous SGF and SIF media. A feature present in all spectra is the decreased crystallinity around 4.3 Å upon mixing, when compared to the corresponding CHI alone.
It is well known [38] that chitosan as excipient performs better if purified or washed by solubilization in acidic medium and then precipitated with acetone. It is not excluded that the washing and purification procedure based on the solubilization of chitosan in dilute acid solutions (2% acetic acid) can also induce a type of preconditioning like the one shown in the present report for tablet forms. The difference is that in our case, the preconditioning was done in situ, due to the strong acidic medium, mimicking the gastric acidity.
4. Conclusions
The clearly longer and controlled release profiles for CHI tablets exposed to gastric acidity suggest favorable structural rearrangements following self-conditioning of CHI in acidic media. Considering the solubility of chitosan at low pH, the possible result of the incubation in conditions mimicking gastric residence would be an outer gel formation coating the CHI tablets.
The CHIa (higher DDA) showed lower swelling in SGF and in SIF compared to the commercial CHIb, probably due to a better stabilization by H-bonding afforded by the primary amine group and reflected in a good preservation of tablet shape. CHIb, with more N-acetyl groups, was less stabilized, allowing easier water access into the tablets, which resulted in higher swelling and less preservation of the tablet shape.
Adding an anionic starch derivative to the cationic chitosans resulted in a CHI:CMS polyelectrolyte complexation in simulated gastric and intestinal media, with beneficial effects in the dissolution curves. The CHI:CMS matrix forms an outer gel barrier, affording thus a certain protection against gastric acidity, despite the fact that the tablets are not coated with gastro-protective materials.
The tablets made from the more deacetylated CHIa maintained the longest monolithic shape and exhibited good controlled release properties. In this case, the favorable influence of ionic associations is much lower compared to the enhancement of release properties afforded by the transit in the high acidity of the SGF. The influence of the self-conditioning may offset the beneficial effect of the ionic interactions in the case of more deacetylated chitosan (having more primary amine groups available for H-bonding with water and gel formation).
The findings reported herein may be of interest for pharmaceutical formulations since tablets based only on chitosan with relatively high DDA seem to undergo a self-conditioning in situ, during the gastric residence. This results in improved mechanical characteristics and sustained drug release profiles for intestinal and colon delivery and eliminates the need to use additives. Such formulations should be administered before meals (gastric pH ∼ 1.2) when they would perform better than after meals (gastric pH ∼ 3.0–4.0). Moreover, the association of CMS to CHI to form in situ the polyelectrolyte complex CHI:CMS seems of interest for colonic delivery of biopharmaceutics, therapeutic enzymes, probiotics. The biodegradable CMS (due to ionization and to the intestinal alpha-amylase) will favor the colonic delivery of the bioactive agents.
Conflicts of interest
None.
Acknowledgements
Financial support from NSERC (Natural Sciences and Engineering Research Council) of Canada (Discovery Grant 06912) and from Foundation Courtois (Canada), is gratefully acknowledged. Thanks are due to Mr M.A. Labelle for help in manuscript preparation.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bioactmat.2018.04.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Hirano S., Hutadilok K.I., Hayashi K., Hirochi A., Usutni H. ALT Press; Madison: 1993. Carbohydrate and Carbohydrate Polymers: Analysis, Biotechnology, Modification, Antiviral, Biomedical and Other Applications. [Google Scholar]
- 2.Rege P.R., Shukla D.J., Block L.H. Chitinosans as tableting excipients for modified release delivery systems. Int. J. Pharm. 1999;181:49–60. doi: 10.1016/s0378-5173(98)00416-5. [DOI] [PubMed] [Google Scholar]
- 3.Cheung R.C., Ng T.B., Wong J.H., Chan W.Y. Chitosan: an update on potential biomedical and pharmaceutical applications. Mar. Drugs. 2015;13:5156–5186. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirano S., Hirochi K., Hayashi K.-I., Mikami T., Tachibana H. Springer; Boston: 1991. Cosmetic and Pharmaceutical Uses of Chitin and Chitosan. in: Cosmetic and Pharmaceutical Applications of Polymers; pp. 95–104. [Google Scholar]
- 5.Chen P.Y., Lin A.Y., McKittrick J., Meyers M.A. Structure and mechanical properties of crab exoskeletons. Acta Biomater. 2008;4:587–596. doi: 10.1016/j.actbio.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Latge J.P. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 7.Feofilova E.P. The fungal cell wall: modern concepts of its composition and biological function. Microbiology. 2010;79:711–720. [PubMed] [Google Scholar]
- 8.Ruel-Gariépy E., Leroux J.-C. Polysaccharides for Drug Delivery and Pharmaceutical Applications. Oxford University Press; Washington DC: 2006. Chitosan: a natural polycation with multiple applications; pp. 243–259. [Google Scholar]
- 9.Muzzarelli R. Chitosan-based dietary foods. Carbohydr. Polym. 1996;29:309–316. [Google Scholar]
- 10.Vargas M., Gonzalez-Martinez C. Recent patents on food applications of chitosan. Recent patents on food. Nutrition & Agriculture. 2010;2:121–128. doi: 10.2174/2212798411002020121. [DOI] [PubMed] [Google Scholar]
- 11.Kolhe P., Kannan R.M. Improvement in ductility of chitosan through blending and copolymerization with PEG: FTIR investigation of molecular interactions. Biomacromolecules. 2003;4:173–180. doi: 10.1021/bm025689+. [DOI] [PubMed] [Google Scholar]
- 12.Macleod G.S., Collett J.H., Fell J.T. The potential use of mixed films of pectin, chitosan and HPMC for bimodal drug release. J. Contr. Release. 1999;58:303–310. doi: 10.1016/s0168-3659(98)00168-0. [DOI] [PubMed] [Google Scholar]
- 13.Assaad E., Blemur L., Lessard M., Mateescu M.A. Polyelectrolyte complex of carboxymethyl starch and chitosan as protein Carrier: oral administration of ovalbumin. J. Biomater. Sci. Polym. Ed. 2012;23:1713–1728. doi: 10.1163/092050611X597771. [DOI] [PubMed] [Google Scholar]
- 14.Muzzarelli C., Muzzarelli R.A.A. Natural and artificial chitosan–inorganic composites. J. Inorg. Biochem. 2002;92:89–94. doi: 10.1016/s0162-0134(02)00486-5. [DOI] [PubMed] [Google Scholar]
- 15.Waibel K.H., Haney B., Moore M., Whisman B., Gomez R. Safety of chitosan bandages in shellfish allergic patients. Mil. Med. 2011;176:1153–1156. doi: 10.7205/milmed-d-11-00150. [DOI] [PubMed] [Google Scholar]
- 16.Muzzarelli R.A.A. Polymeric Biomaterials. Marcel Dekker, Inc; New York: 1994. In vivo biochemical significance of chitin-based medical items; pp. 181–193. [Google Scholar]
- 17.Le Tien C., Lacroix M., Ispas-Szabo P., Mateescu M.A. N-acylated chitosan: hydrophobic matrices for controlled drug release. J. Contr. Release. 2003;93:1–13. doi: 10.1016/s0168-3659(03)00327-4. [DOI] [PubMed] [Google Scholar]
- 18.FDA, Chitosan Derived from A. Niger,GRAS Notice 000397. Accessed October 17, 2017, https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm277279.
- 19.FDA, Shrimp-derived chitosan,GRAS Notice 000170. Accessed October 17, 2017, https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm268859.
- 20.FDA, Shrimp-derived chitosan,GRAS Notice 000073. Accessed October 17, 2017, https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm266601.
- 21.Leonida M.D., Kumar I. Springer-Nature; Heidelberg/New York/London: 2016. Bionanomaterials for Skin Regeneration. [Google Scholar]
- 22.Leonida M.D., Banjade S., Vo T., Anderle G., Haas G.J. Nanocomposite materials with antimicrobial activity based on chitosan. Int. J. Nano Biomaterials (IJNBM) 2011;3:316. [Google Scholar]
- 23.Ruan Q., Liberman D., Zhang Y., Ren D., Zhang Y. Assembly of layered monetite-chitosan nanocomposite and its transition to organized hydroxyapatite. ACS Biomater. Sci. Eng. 2016;2:1049–1058. doi: 10.1021/acsbiomaterials.6b00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brzozowska A.M., Parra-Velandia F.J., Quintana R., Xiaoying Z., Lee S.S. Biomimicking micropatterned surfaces and their effect on marine biofouling. Langmuir. 2014;30:9165–9175. doi: 10.1021/la502006s. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K., Qian Y., Wang H., Fan L., Huang C. Electrospun silk fibroin-hydroxybutyl chitosan nanofibrous scaffolds to biomimic extracellular matrix. J. Biomater. Sci. Polym. Ed. 2011;22:1069–1082. doi: 10.1163/092050610X498204. [DOI] [PubMed] [Google Scholar]
- 26.Lee M.H., Ahluwalia A., Chen J.Z., Shih N.L., Lin H.Y. Synthesis of magnetic cytosine-imprinted chitosan nanoparticles. Nanotechnology. 2017;28 doi: 10.1088/1361-6528/aa5641. 085705. [DOI] [PubMed] [Google Scholar]
- 27.Olorunsola E.O., Akpan G.A., Adikwu M.U. Evaluation of chitosan-microcrystalline cellulose blends as direct compression excipients. J Drug Deliv. 2017;2017 doi: 10.1155/2017/8563858. 8563858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto C.A., Saripella K.K., Loka N.C., Neau S.H. Development and characterization of chitosan cross-linked with tripolyphosphate as a sustained release agent in tablets, Part I: design of experiments and optimization. J Pharm Sci. 2018;107:1063–1075. doi: 10.1016/j.xphs.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Lal N., Dubey J., Gaur P., Verma N., Verma A. Chitosan based in situ forming polyelectrolyte complexes: a potential sustained drug delivery polymeric Carrier for high dose drugs. Mater Sci Eng C Mater Biol Appl. 2017;79:491–498. doi: 10.1016/j.msec.2017.05.051. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez M.T., Ruiz M.A., Castan H., Morales M.E. A novel double-layer mucoadhesive tablet containing probiotic strain for vaginal administration: design, development and technological evaluation. Eur. J. Pharmaceut. Sci. 2018;112:63–70. doi: 10.1016/j.ejps.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Badwan A.A., Rashid I., Omari M.M., Darras F.H. Chitin and chitosan as direct compression excipients in pharmaceutical applications. Mar. Drugs. 2015;13:1519–1547. doi: 10.3390/md13031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The Unites States Pharmacopoeia 41 and the National Formulary 36, Updated November 13, 2017.
- 33.Neugebauer W.A., Neugebauer E., Brzezinski R. Determination of the degree of N-acetylation of chitin-chitosan with picric acid. Carbohydr. Res. 1989;189:363–367. [Google Scholar]
- 34.Mateescu M.A., Ispas-Szabo P., Assaad E. first ed. Woodhead Publishing, Elsevier; London (UK): 2014. Controlled Drug Delivery: the Role of Self-Assembling Multi-task Excipients; pp. 21–84. Chapter Starch and derivatives as pharmaceutical excipients. [Google Scholar]
- 35.Nagahata Y., Kobayashi I., Goto M., Nakaura Y., Inouchi N. the formation of resistant starch during acid hydrolysis of high-amylose corn starch. J. Appl. Glycosci. 2013;60:123–130. [Google Scholar]
- 36.Korsmeyer R.W., Gurny R., Doelker E., Buri P., Peppas N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- 37.Ispas-Szabo P., Ravenelle F., Hassan I., Preda M., Mateescu M.A. Structure–properties relationship in cross-linked high-amylose starch for use in controlled drug release. Carbohydr. Res. 2000;323:163–175. doi: 10.1016/s0008-6215(99)00250-5. [DOI] [PubMed] [Google Scholar]
- 38.Rajasree R., Rahatev K.P. An overview on various modifications of chitosan and its applications. Int. J. Pharma Sci. Res. 2013;12:4175–4193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.