Abstract
Ginseng is a natural product best known for its curative properties in diverse physiological processes such as cancer, neurodegenerative disorders, hypertension, and maintenance of hemostasis in the immune system. In previous decades, there have been some promising studies into the pharmacology and chemistry of ginseng components and the relationship between their structure and function. The emerging use of modified ginseng and development of new compounds from ginseng for clinical studies have been topics of study for many researchers. The present review deals with the anticancer, anti-inflammatory, antioxidant, and chemopreventive effects, and recent advances in microRNA technology related to red ginseng. The review also summarizes the current knowledge on the effect of ginsenosides in the treatment of cancer.
Keywords: anticancer activity, ginseng-derived compounds, ginsenosides, Panax ginseng
1. Introduction
Cancer is a global health problem that has shown an increase in incidence and mortality rates in recent years. Worldwide, the occurrence of cancer is on the rise because of the increase in population as well as risk factors like smoking, obesity, physical inactivity, and urbanization; the data published by GLOBOCON conclude that ∼14.1 million new cancer cases and ∼8.2 million deaths occurred worldwide in 2012 [1]. Tumor promotion is repeatedly accompanied by cellular, biochemical, and molecular events such as the generation of reactive oxygen species, depletion/suppression of antioxidant defense, acute inflammation followed by skin edema and hyperplasia, induction of cyclooxygenase (COX) activity, and increase in catalytic activity of ornithine decarboxylase and its messenger RNA (mRNA) expression [2], [3]. Tumor promotion is closely related to inflammatory mechanisms that stimulate the proliferation of initiated cells; however, natural compounds isolated form medicinal plants possess extraordinary anti-inflammatory and anticarcinogenic activities. Currently, chemotherapy is the only standby for cancer therapy, which has several disadvantages like the development of chemoresistance. Moreover, cytotoxic agents are effective against cancer but they cause serious damage to normal cells causing severe adverse effects and complications, such as fatigue, pain, diarrhea, nausea, vomiting, and hair loss. These agents cannot be used for cancer treatment; therefore, there is an urgent need to develop a potent medication that is effective for cancer treatment without any adverse effects [4].
Natural compounds derived from plants have recently been gaining popularity because of their pharmacological and clinical effects in various diseases like cancer and neurodegenerative disorders. Therefore, there is a need to develop effective and reliable medication that can be pharmacologically as well as naturally accepted as a source of treating major disorders. Ginseng is the most reliable medicinal herb used in Asia and North America. Ginsenosides isolated form ginseng belongs to the family of steroids with a four trans-ring steroid skeleton divided into several groups, but there are two major functional groups based on their C6 position, namely: (1) protopanaxadoil (PPD) belongs to dammarane-type ginsenosides such as ginsenosides Ra1, Ra2, Ra3, Rb1, Rb2, and Rb3; (2) notoginsenoside R1, R2, Rs1, and Rs2; (3) quinquenoside R1; (4) malonylginsenoside Rb1, Rb2, Rc, and Rd (Fig. 1); and (5) protopanaxatrion (PPT) also belongs to dammarane-type ginsenosides which includes Re, Rf, Rg1, and notoginsenoside Rh1 (Fig. 1), while ocotillol and oleanane groups are the minor groups [5], [6], [7]. The new class of heat-processed Korean ginseng (steamed at 98–100°C for 2–3 h), sun ginseng (steamed at 120°C for 2–3 h), and black ginseng (repeatedly steamed and dried 9 times) appears to have decreased content of common ginsenosides (Rb1, Rc, Rd, Re, and Rg1) but comprises an array of ginsenosides that includes Rg5, Rk1, Rk2, Rk3, Rs4, Rs5, Rs6, and Rs7 [4], [8], [9]. Such changes in composition of ginsenosides give heat-processed ginseng its unique anticancer properties. Recently, a novel glycolipoprotein from Panax ginseng, known as gintonin (Fig. 2), was identified, which consists of a complex of lysophosphatidic acid (LPA) and ginseng proteins [10]. Gintonin consists of approximately 9.5% of LPA which majorly comprising of C18:2 [11], which plays an important role in proliferation and migration of cells in vascular development and neurite reaction. LPA receptor-mediated cellular effects are coupled with brain development, angiogenesis, embryo implantation, spermatogenesis, and wound healing [12]. Gintonin is a potential ginseng compound with diverse molecular mechanisms and could be a breakthrough in pharmacological studies and development of novel drugs.
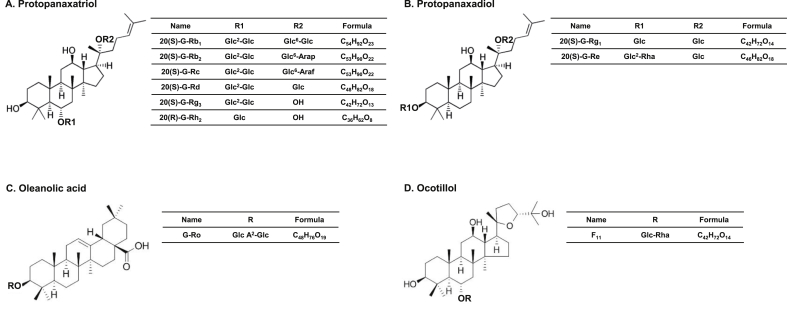
Fig. 1.
Classification and chemical structure of ginsenosides based on sugar attachments on the skeleton and R groups of ginsenosides. (A) Protopanaxatriol, (B) protopanaxadiol, (C) oleanolic acid, and (D) ocotillol.
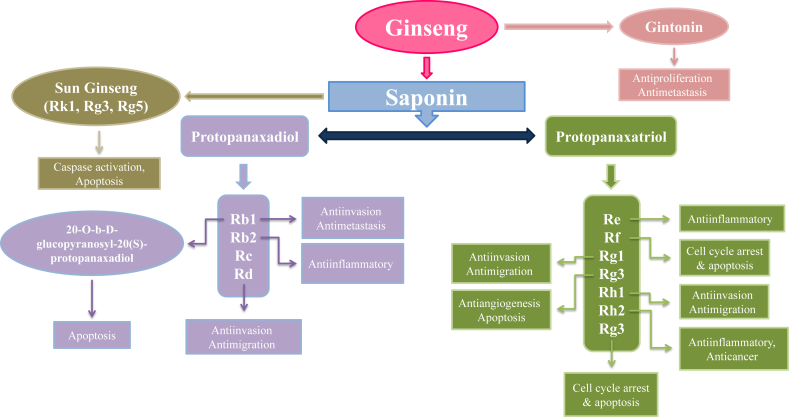
Fig. 2.
Major groups of Panax Ginseng along with new compounds isolated from Ginseng, their biological and anti-cancerous activity against different cell lines.
Ginsenosides have numerous pharmaceutical activities, such as enhancing cardiovascular health [13], stimulation of immune function [14], increasing resistance to stress [15], [16], enhancing learning and memory [17], developing mental health, and social functioning in normal adults [18], [19]. Ginsenosides also have shown strong chemoprotective and chemotherapeutic properties in a wide range of experimental studies both in vitro and in vivo [20], [21]. Moreover, researchers have been interested in studying the role of microRNA (miRNA), which act as a group of small noncoding RNAs that regulate gene expression post-transcriptionally, and play an important role in regulating cellular mechanisms such as proliferation, differentiation, cell cycle, and apoptosis [22], [23]. miRNAs appear to be important gene regulators and key players in carcinogenesis by acting as oncogenes or tumor suppressors [24]. Nonetheless, the focus has been directed towards novel antiangiogenic agents, and data obtained from microarray-based miRNA experiments show that ginsenoside Rg1 induces angiogenesis, which increases production of angiogenic factors, endothelial nitric oxide (NO) synthase [25], angiogenic receptors, and vascular endothelial growth factor receptor 2 in human umbilical vein endothelial cells [26]. The data suggest that miRNA-related gene expression plays an important role in ginsenoside-mediated angiomodulation.
There have been efforts to identify compounds in ginseng that possess anticancer activity. The use of some of these compounds is widely accepted and they are available worldwide. Four countries, Korea, Canada, China, and the USA, are the biggest producers and the total production is ∼79,769 tons, and the global ginseng market including roots and other processed parts is estimated to be $2,084 [27]. Our goal is to develop a suitable alternative medicine that has antioxidant as well as anticancer properties; therefore, this review specifically emphasizes the ginseng-derived components, ginsenosides, with anticancer activity, and recent advances in the use of ginsenosides in cancer treatment.
2. Anticancer activities of ginsenosides
Inflammation can derive from both virulent and nonvirulent processes of chronic injury or irritation. The response leads to recruitment of mast cells and leukocytes, with consequent release of free radicals including reactive oxygen species (ROS) that damage macromolecules including DNA and lipids [28]. Cellular and genomic damage occurs as a part of free radical activity and other byproducts. The release of signaling molecules like eicosanoids triggers cell proliferation, which expedites carcinogenesis [28], [29]. Inflammation is considered a well-established cancer risk factor that leads to genetic and epigenetic damage, as well as unnatural activation of oncogenes, which causes cancer progression and malignant phenotypes of remodeling, angiogenesis, metastasis, and suppression of innate immune responses [21], [30], [31]. Therefore, extensive efforts have been made to develop anticancer drugs that show potential effects in vitro as well as in vivo. With regard to this effort, a number of natural compounds extracted from plants, such as ginsenosides, which are excellent candidates because of their low toxicity and antiangiogenic nature. Ginseng extracts have been reported to possess gastroprotective activity [32], suppress allergic inflammation factors such as interleukin (IL)-4 and IL-5 [33], and suppresses expression of tumor necrosis factor-α and IL-8 in HaCaT cells under LPS treatment [34]. Saponins isolated from Korean Red Ginseng have been reported to suppress NO production, mRNA levels of inducible NO synthase, interferon-β, and COX-2, and block the transcriptional activation of cAMP response element binding protein, activating transcription factor 2, and interferon regulatory factor 3 in LPS-treated macrophages [35]. Research on in vivo anticancer studies subjected to Korean wild ginseng (joboksansam), which represses the viability of RMA cells in a dose-dependent manner, implying direct cytotoxicity, suggests that joboksansam acts as one of the major components in anticancer activity [36]. Individual ginseng and extracts like compound K, Rh1, G-F2, G-Rg3, and G-Rp1 possess antiproliferative and anticancer properties [37], [38], [39], [40], [41]. Furthermore, ginsenoside G-Rg3 saponin extracted from ginseng has effective antitumorigenic, antiangiogenic and antifatigue activities [42], [43], and induces apoptosis of human scar fibroblasts [44], [45]. Lee et al. [46] have concluded that G-Rg3 in combination with cisplatin significantly inhibits the growth of cisplatin-resistant bladder cancer cells. Stereoisomers, 20(R)-Rg3 and 20(S)-Rg3, differing in orientation of the OH group on C-20, inhibit tumor metastasis [47]. Studies on cancer invasion and metastasis are of great therapeutic value. Ginsenoside 20(R)-Rg3 potentially inhibits cancer progression in vitro and in vivo by blocking hypoxia-induced epithelial–mesenchymal transition in ovarian cancer cells by reducing expression of hypoxia-inducible factor-1α and activating the proteasome pathway [48]. 20(R)-Rg3 also acts as a dominant inhibitor of gallbladder cancer growth via activation of the p53 pathway and subsequent cellular senescence in vitro and in vivo in a dose-dependent manner [49]. The associated ginseng-derived 20(S)-ginsenoside Rh2 induces apoptosis in Reh leukemia cells through the mitochondrial signaling pathway [50]. Ginsenoside Rh2-B2 exerts an anti-inflammatory response by regulating tumor necrosis factor-α, IL-1β, IL-6, and IL-10, inhibiting phosphorylation of mitogen-activated protein kinase, and activation of nuclear factor (NF)-κB in LPS-treated RAW264.7 cells [51]. Ginsenoside Rk1 suppresses tumor activity in HepG2 cells by arresting the cell cycle at G1 phase (an early stage which proceeds apoptosis) in a dose-dependent manner [52]. Ginsenoside Rd downregulates expression of iNOS, COX-2, and NF-κB, and suppresses phosphorylation of extracellular signal-regulated kinase and C-Jun N-terminal kinase in carrageenan-induced rat paw edema [53]. In MCF-7 cells, ginsenoside Rg1 inhibits phorbol-myristate-acetate-induced matrix metalloproteinase-9 activity through suppressing NF-κB-mediated migration and invasion of breast cells [54]. A recent study using sun ginseng with a specific formulation of quality-controlled red ginseng containing equal amounts of major ginsenosides (RK1, Rg3, and Rg5) showed marked enhancement of cancer cell death induced by epirubicin or paclitaxel in human cervical adenocarcinoma HeLa cells, human colon cancer SW111C cells, and SW480 cells [55]. Panax ginseng is well known for its control of gene expression. The antiapoptotic and anticancer activity of Korean White Ginseng and Korean Red Ginseng has been a topic of study for many years. Recently, it was found that PPD-derived ginsenosides exhibit more cytotoxic properties, which was observed with Korean Red Ginseng, and inhibit human breast cancer cells (MCF-7), in comparison to PPT-derived ginsenosides [56]. Similarly several ginseng components, such as PPD, PPT, G-Rg3, and G-Rh2 suppress survival and invasiveness, and promote apoptosis in A549 (adenocarcinoma cell line) and SK-MES-1 (a lung squamous cell line) cells. Among these, PPT is a potential extract for addressing lung cancer [57]. Growth inhibition is the key feature in cancer therapy. It has been observed that AMP-activated protein kinase (AMPK) can act as an antiapoptotic molecule. Ginsenoside Rh2, a ginseng saponin, is suggested to have anticancer properties, based on a study performed in HepG2 cells that showed that AMPK activity is caused by ginsenosides-Rh2-mediated ROS generation, which contributes to cancer cell growth and survival [58]. Ginsenoside Rh2 hinders migration of HepG2 carcinoma cells by recruiting histone deacetylase, thus inhibiting activator protein-1 transcription factor [59]. Korean Red Ginseng extract acts as a cytoprotective agent by preventing the induction of mitochondrial dysfunction, which might be associated with AMPK activation in hepatocytes [60]. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginseng saponin, causes apoptosis in colon cancer cells through induction of cytoplasmic Ca2+, decreases cell viability, increases annexin-V-positive early apoptosis, and induces sub-G1 phase accumulation and condensation of CT-26 murine colon cancer cells in vitro, and suppresses tumor growth in vivo [61]. It has been postulated that ginsenosides CK and Rg3 induce apoptosis via the calcium/calmodulin-dependent protein kinase kinase–AMPK pathway in HT-29 colon cancer cells, and these activities have been confirmed through the use of compound C (chemical inhibitor of AMPK) or small interfering RNA for AMPK or STO-609 9 (a chemical inhibitor for calcium/calmodulin-dependent protein kinase kinase) [62], [63]. Thus, further studies are needed to define the stage of cancer progression in which to use AMPK activation for efficient cancer treatment (Table 1).
Table 1.
Anticancer activities of ginsenosides
| Ginsenosides | Functions | Cancer types | Target molecules | Refs |
|---|---|---|---|---|
| Rb1 | Inhibition of invasion and migration | Liver cancer | Nrf2 | [25], [46] |
| Rb2 | Inhibition of metastasis and proliferation | Ovarian cancer | ROS | [26] |
| Rg3 | Induction of apoptosis of cancer cells Inhibition of viability and invasiveness Inhibition of angiogenesis |
Gastric cancer Lung cancer |
COX-2, NF-κB, BCL-2, BAX, c-Fos, c-Jun, VEGF, Caspases | [30], [34], [78] |
| Rh2 | Inhibition of tumor migration, ROS generation, and cancer cell growth Changes in miRNA expression level Inhibition of chemoresistance |
Pancreatic cancer Liver cancer Lung cancer Brest cancer |
MAPK, NF-κB, AP-1, CDKs | [33], [57], [76] |
| Compound K | Induction of apoptosis of cancer cells | Lung cancer Colon cancer |
ROS, c-Jun, JNK, CaMKK-AMPK | [45], [47], [53] |
| Rd | Inhibition of metastasis Decrease in the expression of miRNA-18a |
Liver cancer Mammary cancer |
Smad2, iNOS, COX-2, NF-κB | [75] |
| Rg1 | Inhibition of invasion and migration | Breast cancer | MMP-9, NF-κB | [32] |
| Rk1 | Inhibition of telomerase activity and cell proliferation Induction of apoptosis of cancer cells Induction of G1/S phase arrest and autophagy |
Liver cancer | Caspases, Cyclin | [27] |
| Rg5 | Inhibition of cell cycle | Cervical cancer Gastric cancer Colon cancer Lung cancer Liver cancer |
Cyclin, CDKs | [27] |
| Sun ginseng (Rk1, Rg3, Rg5) | Induction of apoptosis of cancer cells | Cervical cancer Colon cancer |
Caspases, BAX, BAK | [11], [12] |
| Gintonin | Inhibition of metastasis and proliferation | Melanoma | [81] |
AMPK, AMP-activated protein kinase; AP-1, activator protein-1; BCL-2, B-cell lymphoma 2; CaMKK, calcium/calmodulin-dependent protein kinase kinase; Calcium/calmodulin-dependent protein kinase kinase 2 CDK, cyclin-dependent kinase; COX-2, cyclooxygenase-2; iNOS, inducible NO synthase; JNK, C-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP-9, matrix metalloproteinase-9; NF-κB, nuclear factor-κB; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor
3. Chemoprevention and ginsenosides
The incidence of skin carcinogenesis has been rapidly increasing due to depletion of the ozone layer and resulting changes in environmental conditions [64]. It was reported that Korean Red Ginseng had a potentially therapeutic effect on skin cancer [65]. It has also been reported that ginseng root extract causes a significant reduction in oxidative stress and inhibits skin tumors, which proves that it has the potential to act as a chemopreventive agent [66]. Oral ginsenoside Rp1 before and after initiation significantly reduces the incidence and multiplicity of chemically induced mouse skin papilloma [67]. Several ginsenoside, including Rg3, Rh2, Compound K, PPD, and PPT have been observed to inhibit P-gp multidrug resistance, which leads to enhanced activity of chemotherapeutic drugs within cancer cells and increases lifespan in mice [68], [69], [70]. Combination of western medicines and ginseng has been a topic of interest. Consumption of American ginseng with warfarin reduced the effect of the anticoagulant in a double-blind placebo-controlled study [71]. However, to date, there is no convincing study that has proved that ginsenosides diminishes the effect of chemotherapy. Thus, further studies are needed.
4. Scavenging activity of ginsenosides
There are different types of antioxidants produced in plants, which makes them interesting to study and use in pharmacological research, and to develop drugs with few or no adverse effects. These plant-based antioxidants play an important role in maintaining human health. It has been suggested that water, methanol, and ethanol extracts of wild ginseng leaves can scavenge free radicals. Ethanol extract showed the highest scavenging of 2, 2-diphenyl-1-picrylhydrazyl and hydroxyl radicals, and ferrous ion chelating activities, whereas water extract showed the highest superoxide radical scavenging activity [72]. Ginseng cultivated and grown naturally under mountainous forests, also known as Lin-Xia-Shan-Shen, showed higher antioxidant activity in comparison with garden ginseng, by inhibiting formation of thiobarbituric acid-reactive substance [73]. Studies in mice have shown that ginsenoside Rg3 has an inhibitory effect on oxidative stress by upregulating the activity of catalase, superoxide dismutase, and lysozyme, and by reducing xanthine oxidase activity and the levels of malondialdehyde and NO in several organs [74]. Ginsenoside Rb1 can directly react with hydroxyl radicals and hypochlorous acid, two of the strongest ROS to protect DNA [75]. It is also reported that ginsenosides have a positive effect by inhibiting hydrogen radicals but the activity has not been determined [76], [77], [78]. Oral ginsenosides have antioxidative activity in humans [79]. One recent study on ginsenosides Rb1, Rg1, and 20S showed activation of the Nrf2-mediated oxidative pathway, which is the key regulatory pathway against oxidative stress [80]. Antioxidative and hepatoprotective effects of red ginseng oil confirm its ability to protect cells/tissue against oxidative damage by activation and induction of antioxidant enzymes through inhibiting the MAPK pathway [81]. Compound K from ginseng enhances gamma-ray-induced apoptosis via generation of ROS and disruption of the mitochondrial membrane in human lung cancer cells [82]. Compound K induces apoptosis and autophagy in HCT-116 colon cells, increases ROS levels, and activates JNK signaling [83].
5. Expression of miRNAs in cancer and in response to ginsenosides
miRNAs are endogenous, single-stranded, noncoding RNAs with 22 nucleotides, which play an important role in gene expression by targeting 3′-untranslated regions, resulting in mRNA degradation or translational repression [22].
Panax notoginseng saponin inhibits breast cancer metastasis in mice [84]. It also suppresses tumor growth and decreases miRNA-18a expression in tumors derived from Lewis lung carcinoma cells [85]. Ginsenoside Rd also has neuroprotective and cardioprotective actions, as well as inhibitory activity against metastasis of hepatocellular carcinoma HepG2 cells [86], [87], [88]. Rd also decreases expression of miRNA-18a and increases expression of Smad2 mRNA and protein expression in 4T1 cells and 4T1-cell-derived tumors [89]. Several studies have shown that ginsenoside Rh2 mediates changes in miRNA expression profiles in human lung cancer A549 cells, and inhibits glioma cell proliferation by targeting miRNA-128 [90], [91]. It is proposed that Rh2 mediates drug-resistance-specific miRNA, which can regulate target genes through gene regulatory networks, and inhibit development of drug-resistant tumor cells, thus improving treatment effect [92]. miRNAs have the potential to regulate multiple functionally related genes, making them new candidates for cancer intervention. Various ginsenosides have recently been studied and postulated to modulate miRNA, which has attracted much attention. For example, ginsenoside Rg3 induces angiosuppression and mediates miR-520h by suppressing EphB2 and EphB4 expression [93]. Rg3 also inhibits leukemia effect because of its antiangiogenic activity, by inhibiting the phosphoinositide 3-kinase/Akt and extracellular signal-regulated kinase 1/2 pathways [94]. Ginsenoside Rg1 downregulates miR15b expression in human umbilical vein endothelial cells, which leads to cell migration, tube formation, and angiogenesis [26]. The focus has been on discovering antiangiogenic agents such as TNP-470 and thalidomide, although the pharmacological mechanisms are still unclear.
6. Pharmacology of gintonin, a novel ginseng-derived compound
Studies have demonstrated that ginseng contains various types of LPA receptors, which are endogenous phospholipid-derived growth factors with diverse biological effects. Gintonin comprises carbohydrates, lipids, and proteins such as ginseng latex-like protein and ginseng ribonuclease-like storage protein, also known as G-protein coupled receptor, which is a novel class of glycolipoprotein in ginseng (ginseng contains 0.2% gintonin) that induces intracellular Ca2+ increase in mammalian cells [11]. Gintonin also provides the diverse effects of ginseng that ginsenosides cannot [95]. Gintonin controls the release of hormones and neurotransmitters, resulting in long-term potentiation and enhancement of synaptic transmission, therefore, gintonin modulates cell–cell communications via neurotransmitters, which leads to cognitive behavior [96], [97]. Gintonin exerts antimetastatic and antitumor activity, blocks cell migration at low concentration, and oral gintonin and LPA decrease metastatic lung nodule formation [98]. Gintonin inhibits the amyloidogenic pathway that produces soluble amyloid precursor protein-α in neurons by activating LPA receptors [99]. Long-term oral administration of gintonin significantly inhibits amyloid plaque deposition and short- and long-term memory impairment in a transgenic mouse model of Alzheimer's disease [99]. Therefore, gintonin may act as a critical molecule for memory improving effects that have been proven in human trials. Gintonin exerts significant neuroprotective, antimetastatic, and antitumor activities, which could make it a major candidate for developing ginseng-derived medicine and alternative products. Gintonin is now regarded as a part of ginseng, in contrast to LPA, which is responsible for different biological events through ginseng-mediated G-protein coupled receptor signaling. Although development of a new drug targeting LPA receptor is in progress, gintonin can provide an alternative for dealing with medical conditions related to LPA.
7. Conclusions and perspectives
Our understanding of ginseng has dramatically advanced in the last few years; a plethora of research related to pharmacology of ginseng has been helpful in identifying the functions and benefits of ginsenosides in the nervous, cardiovascular, and immune systems. Ginseng has been used as a substitute for drugs in the treatment of cancer patients in Asian countries. Based on our review, ginseng has potential as an anticancer agent because of its antiproliferative, anti-inflammatory, and antioxidant effects. Ginseng has great potential for providing diverse mechanisms for cancer treatment. Moreover, the ability to kill cancer cells while causing relatively little toxicity to normal cells makes ginsenosides attractive candidates for drug development. High-throughput expression analysis will help to identify molecular mechanisms and effects of different ginsenosides. We should explore how Chinese medicine can reverse and inhibit chemotherapy resistance from a clinical point of view. Few studies have looked at ginseng-induced molecular alterations, including changes in cytokine secretion, antibody production, marker expression, and cellular functions such as phagocytosis and cytotoxicity. In order to study the molecular mechanisms underlying the effects of ginseng, a more detailed and in-depth analysis is needed. Hundreds of studies have been published to prove the anticancer and anti-inflammatory effects of ginseng and ginsenosides, which are mainly through immunity consisting of cytotoxic cells, while other approaches such as apoptosis, angiogenesis, miRNA activity, and chemopreservation are also involved. Further studies are needed to determine why ginsenosides have toxicity towards tumor cells. Finally, a comprehensive review of anticancer effects from the clinical and therapeutic pharmacological point of view would be useful.
Conflicts of interest
The authors have no conflicts of interest regarding the publication of this paper.
Acknowledgments
This work was supported by Sungkyunkwan University (2016).
Contributor Information
Jong-Hoon Kim, Email: jhkim1@jbnu.ac.kr.
Young-Su Yi, Email: ysyi@cju.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.von Kitzing E., Jonas P., Sakmann B. Europepmc_Citation. Res Vet Sci. 1994;30:364–367. [Google Scholar]
- 3.O'Brien T.G., Simsiman R.C., Boutwell R.K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975;35(7):1662–1670. [PubMed] [Google Scholar]
- 4.Wong A.S.T., Che C.-M., Leung K.-W., Karmazyn M., Moey M., Gan X.T., Liu J., Wang S., Liu H., Yang L. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep [Internet] 2015;32(2):256–272. doi: 10.1039/c4np00080c. http://xlink.rsc.org/?DOI=C4NP00080C Available from: [DOI] [PubMed] [Google Scholar]
- 5.Qu C., Bai Y., Jin X., Wang Y., Zhang K., You J., Zhang H. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem [Internet] 2009;115(1):340–346. Available from: [Google Scholar]
- 6.Wang A., Wang C.-Z., Wu J.-A., Osinski J., Yuan C.-S. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem Anal. 2005;16(4):272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 7.Nag S.A. Ginsenosides as anticancer agents: in vitro and in vivo activities, structure–activity relationships, and molecular mechanisms of action. Front Pharmacol [Internet] 2012 February;3:25. doi: 10.3389/fphar.2012.00025. http://www.ncbi.nlm.nih.gov/pubmed/22403544 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun B.-S., Gu L.-J., Fang Z.-M., Wang C., Wang Z., Lee M.-R., Li Z., Li J.-J., Sung C.-K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009 Aug;50(1):15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y.Y., Luo D., Cheng Y.J., Ma J.F., Wang Y.M., Liang Q.L., Luo G.A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MS n-based multicomponent quantification fingerprint. J Agric Food Chem. 2012;60(33):8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- 10.Pyo M.K., Choi S.-H., Hwang S.H., Shin T.-J., Lee B.-H., Lee S.-M., Lim Y.-H., Kim D.-H., Nah S.-Y. Novel glycolipoproteins from ginseng. J Ginseng Res. 2011;35(1):92–103. [Google Scholar]
- 11.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33(2):151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun J., Hla T., Lynch K.R., Spiegel S., Moolenaar W.H. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid. Pharmacol Rev. 2010;62(4):579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmazyn M., Moey M., Gan X.T. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs. 2011 Oct;71(15):1989–2008. doi: 10.2165/11594300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Liu J., Wang S., Liu H., Yang L., Nan G. Stimulatory effect of saponin from Panax ginseng on immune function of lymphocytes in the elderly. Mech Ageing Dev. 1995;83:43–53. doi: 10.1016/0047-6374(95)01618-a. [DOI] [PubMed] [Google Scholar]
- 15.Rai D., Bhatia G., Sen T., Palit G. Anti-stress effects of Ginkgo biloba and Panax ginseng: a comparative study. J Pharmacol Sci [Internet] 2003;93(4):458–464. doi: 10.1254/jphs.93.458. http://www.ncbi.nlm.nih.gov/pubmed/14737017 Available from: [DOI] [PubMed] [Google Scholar]
- 16.Oliynyk S., Oh S. Actoprotective effect of ginseng: improving mental and physical performance. J Ginseng Res. 2013;37(2):144–166. doi: 10.5142/jgr.2013.37.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao R., McDaniel W.F. Ginseng improves strategic learning by normal and brain-damaged rats. Neuroreport. 1998 May;9(7):1619–1624. doi: 10.1097/00001756-199805110-00066. [DOI] [PubMed] [Google Scholar]
- 18.Coleman C.I., Hebert J.H., Reddy P. Effects of Panax ginseng on stress. J Ginseng Res. 2008;32(1):8–14. [Google Scholar]
- 19.Reay J.L., Scholey A.B., Kennedy D.O. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol. 2010 Aug;25(6):462–471. doi: 10.1002/hup.1138. [DOI] [PubMed] [Google Scholar]
- 20.Shin H.R., Kim J.Y., Yun T.K., Morgan G., Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control [Internet] 2000;11(6):565–576. doi: 10.1023/a:1008980200583. http://www.ncbi.nlm.nih.gov/pubmed/10880039 Available from: [DOI] [PubMed] [Google Scholar]
- 21.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 22.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim V.N., Nam J.W. Genomics of microRNA. Trends Genet. 2006;22:165–173. doi: 10.1016/j.tig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Fiorucci G., Chiantore M.V., Mangino G., Percario Z.A., Affabris E., Romeo G. Cancer regulator MICRORNA: potential relevance in diagnosis, prognosis and treatment of cancer [Internet] Curr Med Chem. 2012;19:461–474. doi: 10.2174/092986712798918798. http://www.eurekaselect.com/node/76151/article Available from: [DOI] [PubMed] [Google Scholar]
- 25.Chan L.S., Yue P.Y.K., Mak N.K., Wong R.N.S. Role of MICRORNA-214 in ginsenoside-Rg1-induced angiogenesis. Eur J Pharm Sci. 2009;38(4):370–377. doi: 10.1016/j.ejps.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Chan L.S., Yue P.Y.K., Wong Y.Y., Wong R.N.S. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2. Biochem Pharmacol. 2013;86(3):392–400. doi: 10.1016/j.bcp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat Rev Cancer. 2003;3(4):276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 29.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria S., Pikarsky E., Karin M., Coussens L.M., Chen Y.-C., El-Omar E.M., Trinchieri G., Dubinett S.M., Mao J.T., Sazbo E. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33(4):335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coussens L.M., Zitvogel L., Palucka A.K. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science [Internet] 2013;339(6117):286–291. doi: 10.1126/science.1232227. http://www.ncbi.nlm.nih.gov/pubmed/23329041%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3591506 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim Y.J., Chung J.W., Lee S.J., Choi K.S., Kim J.H., Hahm K.B. Progression from chronic atrophic gastritis to gastric cancer; tangle, toggle, tackle with Korea red ginseng. J Clin Biochem Nutr. 2010;46(3):195–204. doi: 10.3164/jcbn.10-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung J.H., Kang I.G., Kim D.Y., Hwang Y.J., Kim S.T. The effect of Korean red ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 2013;37(2):167–175. doi: 10.5142/jgr.2013.37.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong C.-E., Lyu S.-Y. Anti-inflammatory and anti-oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154(1):218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Park J.G., Kang W.S., Park K.T., Park D.J., Aravinthan A., Kim J.H., Cho J.Y. Anticancer effect of joboksansam, Korean wild ginseng germinated from bird feces. J Ginseng Res. 2016;40(3):304–308. doi: 10.1016/j.jgr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.S., Lim J.M., Kim J.Y., Kim Y., Park S., Sohn J. Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int J Cancer. 2016;138(6):1432–1441. doi: 10.1002/ijc.29879. [DOI] [PubMed] [Google Scholar]
- 38.Choi K., Choi C. Proapoptotic ginsenosides compound K and Rh2 enhance Fas-induced cell death of human astrocytoma cells through distinct apoptotic signaling pathways. Cancer Res Treat. 2009;41(1):36–44. doi: 10.4143/crt.2009.41.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim M.-Y., Yoo B.C., Cho J.Y. Ginsenoside-Rp1-induced apolipoprotein A-1 expression in the LoVo human colon cancer cell line. J Ginseng Res [Internet] 2014;38(4):251–255. doi: 10.1016/j.jgr.2014.06.003. http://www.sciencedirect.com/science/article/pii/S1226845314000694 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao Q., Zhang P.-H., Wang Q., Li S.-L. Ginsenoside F2 induces apoptosis in humor gastric carcinoma cells through reactive oxygen species-mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine. 2014;21(4):515–522. doi: 10.1016/j.phymed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Wang J.-H., Nao J.-F., Zhang M., He P. 20(s)-Ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumor Biol [Internet] 2014;35(12):11985–11994. doi: 10.1007/s13277-014-2497-5. Available from: [DOI] [PubMed] [Google Scholar]
- 42.Zhao Q., Zheng X., Jiang J., Zhou H., Hu P. Determination of ginsenoside Rg3 in human plasma and urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878(24):2266–2273. doi: 10.1016/j.jchromb.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y.J., Choi W.I., Jeon B.N., Choi K.C., Kim K., Kim T.J., Ham J., Jang H.J., Kang K.S., Ko H. Stereospecific effects of ginsenoside 20-Rg3 inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology. 2014;322:23–33. doi: 10.1016/j.tox.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Yoo H.H., Yokozawa T., Satoh A., Kang K.S., Kim H.Y. Effects of ginseng on the proliferation of human lung fibroblasts. Am J Chin Med. 2006;34(1):137–146. doi: 10.1142/S0192415X06003709. [DOI] [PubMed] [Google Scholar]
- 45.Liu T.-G.G., Huang Y., Cui D.-D.D., Huang X.-B.B., Mao S.-H.H., Ji L.-L.L., Song H.-B., Yi C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009;9(250):250. doi: 10.1186/1471-2407-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y.J., Lee S., Ho J.N., Byun S.S., Hong S.K., Lee S.E., Lee E. Synergistic antitumor effect of ginsenoside Rg3 and cisplatin in cisplatin-resistant bladder tumor cell line. Oncol Rep. 2014;32(5):1803–1808. doi: 10.3892/or.2014.3452. [DOI] [PubMed] [Google Scholar]
- 47.Mochizuki M., Yoo Y.C., Matsuzawa K., Sato K., Saiki I., Tono-oka S., Samukawa K., Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull [Internet] 1995;18(9):1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 48.Liu T., Zhao L., Zhang Y., Chen W., Liu D., Hou H., Ding L., Li X. Ginsenoside 20(S)-Rg3 targets HIF-1α to Block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS One. 2014;9(9):1–12. doi: 10.1371/journal.pone.0103887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F., Li M., Wu X., Hu Y., Cao Y., Wang W., Xiang S., Li H., Jiang L., Tan Z. 20 (S)-ginsenoside Rg3 promotes senescence and apoptosis in gallbladder cancer cells via the p53 pathway. Drug Des Devel Ther. 2015;9:3969–3987. doi: 10.2147/DDDT.S84527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia T., Wang J.-C., Xu W., Xu L.-H., Lao C.-H., Ye Q.-X., Fang J.P. 20(S)-Ginsenoside Rh2 induces apoptosis in human leukaemia Reh cells through mitochondrial signaling pathways. Biol Pharm Bull [Internet] 2014;37(2):248–254. doi: 10.1248/bpb.b13-00667. http://www.ncbi.nlm.nih.gov/pubmed/24492721 Available from: [DOI] [PubMed] [Google Scholar]
- 51.Bi W.Y., Fu B.D., Shen H.Q., Wei Q., Zhang C., Song Z., Qin Q.Q., Li H.P., Lv S., Wu S.C. Sulfated derivative of 20(S)-Ginsenoside Rh2 inhibits inflammatory cytokines through MAPKs and NF-kappa B pathways in LPS-Induced RAW264.7 macrophages. Inflammation. 2012;35(5):1659–1668. doi: 10.1007/s10753-012-9482-1. [DOI] [PubMed] [Google Scholar]
- 52.Ko H., Kim Y.J., Park J.S., Park J.H., Yang H.O. Autophagy inhibition enhances apoptosis induced by ginsenoside Rk1 in hepatocellular carcinoma cells. Biosci Biotechnol Biochem. 2009;73(10):2183–2189. doi: 10.1271/bbb.90250. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y.X., Wang L., Xiao E.L., Li S.J., Chen J.J., Gao B., Min G.N., Wang Z.P., Wu Y.J. Ginsenoside-Rd exhibits anti-inflammatory activities through elevation of antioxidant enzyme activities and inhibition of JNK and ERK activation in vivo. Int Immunopharmacol. 2013;17(4):1094–1100. doi: 10.1016/j.intimp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Li L., Wang Y., Qi B., Yuan D., Dong S. Suppression of PMA-induced tumor cell invasion and migration by ginsenoside Rg1 via the inhibition of NF- κ B-dependent MMP-9 expression. Ontol Reports. 2014;32:1779–1786. doi: 10.3892/or.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin Y., Jiang D., Li Y., Han X., Yu D., Park J.H., Jin Y.H. Effect of sun ginseng potentiation on epirubicin and paclitaxel-induced apoptosis in human cervical cancer cells. J Ginseng Res. 2015;39(1):22–28. doi: 10.1016/j.jgr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee J.I., Ha Y.W., Choi T.W., Kim H.J., Kim S.M., Jang H.J., Choi J.H., Choi M.H., Chung B.C., Sethi G. Cellular uptake of ginsenosides in Korean white ginseng and red ginseng and their apoptotic activities in human breast cancer cells. Planta Med. 2011;77(2):133–140. doi: 10.1055/s-0030-1250160. [DOI] [PubMed] [Google Scholar]
- 57.Xu F., Shang W., Yu J., Sun Q., Li M., Sun J. The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am J Transl Res. 2016;8(4):1708–1718. [PMC free article] [PubMed] [Google Scholar]
- 58.Kim M.J., Yun H., Kim D.H., Kang I., Choe W., Kim S.S., Ha J. Amp-activated protein kinase deter mines apoptotic sensitivity of cancer cells to ginsenoside-Rh2. J Ginseng Res [Internet] 2014;38(1):16–21. doi: 10.1016/j.jgr.2013.11.010. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Q., Li J., Feng Z., Zhao L., Luo L., You Z., Li D., Xia J., Zuo G., Chen D. Effect of ginsenoside Rh2 on the migratory ability of HepG2 liver carcinoma cells: recruiting histone deacetylase and inhibiting activator protein 1 transcription factors. Mol Med Rep. 2014;10(4):1779–1785. doi: 10.3892/mmr.2014.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong G., Jang E.J., Kang S.H., Cho I.J., Park S., Kim S.C., Kim Y.W. Red ginseng abrogates oxidative stress via mitochondria protection mediated by LKB1-AMPK pathway. BMC Complement Altern Med. 2013;13:64. doi: 10.1186/1472-6882-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang J.A., Hwang M.K., Jang Y., Lee E.J., Kim J.E., Oh M.H., Shin D.J., Lim S., Ji G., Bode A.M. 20-O-b-d-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginseng, inhibits colon cancer growth by targeting TRPC channel-mediated calcium influx. J Nutr Biochem. 2013;24(6):1096–1104. doi: 10.1016/j.jnutbio.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Yuan H.D., Quan H.Y., Zhang Y., Kim S.H., Chung S.H. 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol Med Rep. 2010;3(5):825–831. doi: 10.3892/mmr.2010.328. [DOI] [PubMed] [Google Scholar]
- 63.Kim D.Y., Park M.W., Yuan H.D., Lee H.J., Kim S.H., Chung S.H. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J Agric Food Chem. 2009;57(22):10573–10578. doi: 10.1021/jf902700h. [DOI] [PubMed] [Google Scholar]
- 64.Garbe C., Leiter U. Epidemiology of melanoma and nonmelanoma skin cancer-the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 65.Xiaoguang C., Hongyan L., Xiaohong L., Zhaodi F., Yan L., Lihua T., Rui H. Cancer chemopreventive and therapeutic activities of red ginseng. J Ethnopharmacol. 1998;60(1):71–78. doi: 10.1016/s0378-8741(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 66.Sharma J., Goyal P.K. Chemoprevention of chemical-induced skin cancer by Panax ginseng root extract. J Ginseng Res [Internet] 2015;39(3):265–273. doi: 10.1016/j.jgr.2015.01.005. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar A., Kumar M., Panwar M., Samarth R.M., Park T.Y., Park M.H., Kimura H. Evaluation of chemopreventive action of Ginsenoside Rp1. Biofactors. 2006;26:29–43. doi: 10.1002/biof.5520260104. [DOI] [PubMed] [Google Scholar]
- 68.Choi C.H., Kang G., Min Y.D. Reversal of P-glycoprotein-mediated multidrug resistance by protopanaxatriol ginsenosides from Korean red ginseng. Planta Med. 2003;69(3):235–240. doi: 10.1055/s-2003-38483. [DOI] [PubMed] [Google Scholar]
- 69.Kim S.W., Kwon H.Y., Chi D.W., Shim J.H., Park J.D., Lee Y.H., Pyo S., Rhee D.K. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg3. Biochem Pharmacol. 2003;65(1):75–82. doi: 10.1016/s0006-2952(02)01446-6. [DOI] [PubMed] [Google Scholar]
- 70.Li N., Wang D., Ge G., Wang X., Liu Y., Yang L. Ginsenoside metabolites inhibit P-glycoprotein in vitro and in situ using three absorption models. Planta Med. 2014;80(4):290–296. doi: 10.1055/s-0033-1360334. [DOI] [PubMed] [Google Scholar]
- 71.Yuan C.S., Wei G., Dey L., Karrison T., Nahlik L., Maleckar S., Kasza K., Ang-Lee M., Moss J. Brief communication: American ginseng reduces warfarin's effect in healthy patients. A randomized, controlled trial. Ann Intern Med. 2004;141(1):23–27. doi: 10.7326/0003-4819-141-1-200407060-00011. [DOI] [PubMed] [Google Scholar]
- 72.Jung C.H., Seog H.M., Choi I.W., Park M.W., Cho H.Y. Antioxidant properties of various solvent extracts from wild ginseng leaves. LWT – Food Sci Technol. 2006;39(3):266–274. [Google Scholar]
- 73.Pan H.Y., Qu Y., Zhang J.K., Kang T.G., Dou D.Q. Antioxidant activity of ginseng cultivated under mountainous forest with different growing years. J Ginseng Res. 2013;37(3):355–360. doi: 10.5142/jgr.2013.37.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei X., Su F., Su X., Hu T., Hu S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia. 2012;83(4):636–642. doi: 10.1016/j.fitote.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Lü J.-M., Weakley S.M., Yang Z., Hu M., Yao Q., Chen C. Ginsenoside Rb1 directly scavenges hydroxyl radical and hypochlorous acid. Curr Pharma Des. 2012;18:6339–6347. doi: 10.2174/138161212803832254. [DOI] [PubMed] [Google Scholar]
- 76.Lo Y.-T., Tsai Y.-H., Wu S.-J., Chen J.-R., Chao J.C.-J. Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. J Med Food. 2011;14(10):1135–1143. doi: 10.1089/jmf.2010.1485. [DOI] [PubMed] [Google Scholar]
- 77.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-κB activation. Neurochem Int. 2011;58(1):119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Kwok H.H., Ng W.Y., Yang M.S.M., Mak N.K., Wong R.N.S., Yue P.Y.K. The ginsenoside protopanaxatriol protects endothelial cells from hydrogen peroxide-induced cell injury and cell death by modulating intracellular redox status. Free Radic Biol Med. 2010;48(3):437–445. doi: 10.1016/j.freeradbiomed.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Lee L.S., Wise S.D., Chan C., Parsons T.L., Flexner C., Lietman P.S. Possible differential induction of phase 2 enzyme and antioxidant pathways by american ginseng, Panax quinquefolius. J Clin Pharmacol. 2008;48(5):599–609. doi: 10.1177/0091270008314252. [DOI] [PubMed] [Google Scholar]
- 80.Saw C.L.L., Yang A.Y., Cheng D.C., Boyanapalli S.S.S., Su Z.Y., Khor T.O., Gao S., Wang J., Jiang Z.H., Tony Kong A.N. Pharmacodynamics of ginsenosides: antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem Res Toxicol. 2012;25(8):1574–1580. doi: 10.1021/tx2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bak M.-J., Jun M., Jeong W.-S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated hepG2 cells and CCl(4)-treated mice. Int J Mol Sci. 2012;13(2):2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chae S., Kang K.A., Chang W.Y., Kim M.J., Lee S.J., Lee Y.S., Kim H.S., Kim D.H., Hyun J.W. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem. 2009;57(13):5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]
- 83.Kim A.D., Kang K.A., Kim H.S., Kim D.H., Choi Y.H., Lee S.J., Kim H.S., Hyun J.W. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P., Cui J., Du X., Yang Q., Jia C., Xiong M., Yu X., Li L., Wang W., Chen Y. Panax notoginseng saponins (PNS) inhibits breast cancer metastasis. J Ethnopharmacol. 2014;154(3):663–671. doi: 10.1016/j.jep.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 85.Yang Q., Wang X., Cui J., Wang P., Xiong M., Jia C., Lui L., Ning B., Li L., Wang W. Bidirectional regulation of angiogenesis and miR-18a expression by PNS in the mouse model of tumor complicated by myocardial ischemia. BMC Complement Altern Med. 2014;14:183. doi: 10.1186/1472-6882-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X., Shi M., Ye R., Wang W., Liu X., Zhang G., Han J., Zhang Y., Wang B., Zhao J. Ginsenoside Rd attenuates tau protein phosphorylation via the PI3K/AKT/GSK-3b pathway after transient forebrain ischemia. Neurochem Res. 2014;39(7):1363–1373. doi: 10.1007/s11064-014-1321-3. [DOI] [PubMed] [Google Scholar]
- 87.Zeng X., Li J., Li Z. Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int J Clin Exp Med. 2015;8(8):14497–14504. [PMC free article] [PubMed] [Google Scholar]
- 88.Yoon J.H., Choi Y.J., Cha S.W., Lee S.G. Anti-metastatic effects of ginsenoside Rd via inactivation of MAPK signaling and induction of focal adhesion formation. Phytomedicine. 2012;19(3–4):284–292. doi: 10.1016/j.phymed.2011.08.069. [DOI] [PubMed] [Google Scholar]
- 89.Wang P., Du X., Xiong M., Cui J., Yang Q., Wang W., Chen Y., Zhang T. Ginsenoside Rd attenuates breast cancer metastasis implicating derepressing microRNA-18a-regulated Smad2 expression. Sci Rep [Internet] 2016 March;6:33709. doi: 10.1038/srep33709. http://www.nature.com/articles/srep33709 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu N., Wu G., Hu R., Li M., Feng H. Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128. Acta Pharmacol Sin. 2011;32(3):345–353. doi: 10.1038/aps.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiao X., Zhao L., Ma M., Bai X., He M., Yan Y., Wang Y., Chen Q., Zhao X., Zhou M. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2) Breast Cancer Res Treat. 2013;139(3):717–730. doi: 10.1007/s10549-013-2607-x. [DOI] [PubMed] [Google Scholar]
- 92.Wen X., Zhang H.Da, Zhao L., Yao Y.F., Zhao J.H., Tang J.H. Ginsenoside Rh2 differentially mediates microRNA expression to prevent chemoresistance of breast cancer. Asian Pacific J Cancer Prev. 2015;16(3):1105–1109. doi: 10.7314/apjcp.2015.16.3.1105. [DOI] [PubMed] [Google Scholar]
- 93.Keung M.-H., Chan L.-S., Kwok H.-H., Wong R.N.-S., Yue P.Y.-K. Role of microRNA-520h in 20(R)-ginsenoside-Rg3-mediated angiosuppression. J Ginseng Res. 2016;40(2):151–159. doi: 10.1016/j.jgr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeng D., Wang J., Kong P., Chang C., Li J., Li J. Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in patient with acute leukemia via inhibiting the activation of PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol. 2014;7(5):2172–2178. [PMC free article] [PubMed] [Google Scholar]
- 95.Nah S.Y. Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front Physiol. 2014 March;5:1–13. doi: 10.3389/fphys.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim S., Kim M.S., Park K., Kim H.J., Jung S.W., Nah S.-Y., Han J.S., Chung C.H. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40(1):55–61. doi: 10.1016/j.jgr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park H., Kim S., Rhee J., Kim H.-J., Han J.-S., Nah S.-Y., Chung C.H. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113(5):1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 98.Hwang S.H., Lee B.H., Kim H.J., Cho H.J., Shin H.C., Im K.S., Choi S.H., Shin T.J., Lee S.M., Nam S.W. Suppression of metastasis of intravenously-inoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: involvement of autotaxin inhibition. Int J Oncol. 2013;42(1):317–326. doi: 10.3892/ijo.2012.1709. [DOI] [PubMed] [Google Scholar]
- 99.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.-J., Kwon S.-H., Jang C.-H., Lee J.-H. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimer's Dis. 2012;31(1):207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]