Abstract
Background
Bioactive compounds in plant extracts are able to reduce metal ions to nanoparticles through the process of green synthesis. Panax ginseng is an oriental medicinal herb and an adaptogen which has been historically used to cure various diseases. In addition, the P. ginseng leaves-mediated gold nanoparticles are the value-added novel materials. Its potential as a cosmetic ingredient is still unexplored. The aim of this study was to evaluate the antioxidant, moisture retention and whitening properties of gold nanoparticles (PgAuNPs) in cosmetic applications.
Methods
Cell-free experiments were performed to evaluate PgAuNP's antioxidant and moisture retention properties and inhibition activity on mushroom tyrosinase. Furthermore, in vitro cell cytotoxicity was evaluated using normal human dermal fibroblast and murine B16BL6 melanoma cells (B16) after treatment with increasing concentrations of PgAuNPs for 24 h, 48 h, and 72 h. Finally, in vitro cell assays on B16 cells were performed to evaluate the whitening effect of PgAuNPs through reduction of cellular melanin content and tyrosinase activity.
Results
In vitro DPPH radical scavenging assay results revealed that PgAuNPs exhibited antioxidant activity in a dose-dependent manner. PgAuNPs exhibited moisture retention capacity and effectively inhibited mushroom tyrosinase. In addition, 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide results revealed that PgAuNPs were not toxic to human dermal fibroblast and B16 cells; in addition, they significantly reduced melanin content, tyrosinase activity, and mRNA expression of melanogenesis-associated transcription factor and tyrosinase in B16 cells.
Conclusion
Our study is the first report to provide evidence supporting that P. ginseng leaves-capped gold nanoparticles could be used as multifunctional ingredients in cosmetics.
Keywords: antioxidant, antityrosinase activity, gold nanoparticles, moisture retention, Panax ginseng leaves
1. Introduction
Skin researchers are focusing on the development of skin anti-aging and whitening products. Aging is the cumulative result of oxidative damage to the cells and tissues [1]. Free radicals are known to be implicated in skin aging as well as in other diseases affecting the human body [2]. Furthermore, oxidative stress also alters the redox state of cell membrane proteins in skin cells resulting in melanogenesis [3]. Tyrosinase is a crucial enzyme that primarily catalyzes and rate-limits the stages of melanogenesis [4]. Thus, inhibition of tyrosinase activity is the most prominent method for the downregulation of melanogenesis. Antityrosinase agents inhibit melanin biosynthesis, which is related with hyperpigmentation disorders such as freckles and age spots [5].
Human skin is the principal body protection against environmental factors. High importance lies in the lipid matrix in the outer layer of the skin, the stratum corneum. One of its main functions is to prevent disproportionate water loss through the epidermis [6]. Therefore, appropriate hydration of the skin is a key factor for the normal skin function [7]; skin moisturizers hydrate and prevent water loss in the stratum corneum.
To battle skin aging, aging spots, and drying stresses, the antioxidant and antityrosinase activities, and moisture retention capacity of cosmetic ingredients are of paramount importance in the field of cosmetics.
Panax ginseng Meyer is a long-term growing perennial plant of the Araliaceae family [8]. The importance of ginseng lies in its multiple pharmacological functions such as anticancer activity, as well as antistress, antifatigue, antioxidant, and aging inhibitory effects [9]. Most of the research on P. ginseng has focused on the study of the active ingredients in the root. However, a recent report indicates that ginseng leaves contains similar biological activities, and pharmacological and chemical profiles to the root [10]. As a starting point for our study and based on ginseng leaves, particularly the phytochemical profile, we follow our previous work “A strategic approach for rapid synthesis of gold and silver nanoparticles by P. ginseng leaves” [11], in which we described the synthesis and characterization of gold and silver nanoparticles (NPs) from extract of P. ginseng leaves as well as their remarkable biological applications as antimicrobial and potent anticoagulant agents. In this study, regarding gold nanoparticle (AuNP) synthesis from P. ginseng leaves in particular, we concluded that (1) their synthesis is fast, as it takes only 3 min; (2) they are spherical in shape; and (3) their size is between 10 nm and 20 nm. The aim of our new study is to explore AuNPs from P. ginseng leaves as a novel cosmetic material. Recent reports highlight the positive benefit of engineered NPs for use in cosmetics [12], [13]. The cosmetics industry is among the first to benefit from the advantages of nanotechnology through the adoption of tailor-made NPs to enhance the performance and bioavailability of active ingredients in cosmetics products such as perfumes, sunscreens, antiaging creams, and moisturizers [14]. Although AuNPs have shown high antibacterial and antifungal properties and have been used in cosmetic products (e.g., face packs, antiaging creams, anti-inflammation products, and skin wound disinfection products) [12], there are no reports on the application of AuNPs from P. ginseng leaves as antioxidant, antityrosinase, and moisture retention agents for cosmetics. Tests for antioxidant activity by means of DPPH, moisture retention, and antityrosinase effect against mushroom tyrosinase have been performed. These were followed by in vitro safety assessment of P. ginseng Au nanoparticles (PgAuNPs) in a comparative cytotoxicity study between human dermal fibroblast (HDF) and B16 cells. In addition, PgAuNP's whitening effect was confirmed with B16 cells through cellular melanin content, tyrosinase activity, and mRNA tyrosinase expression inhibition assays with the aim of developing a possible new cosmetic material.
2. Materials and methods
2.1. Materials
P. ginseng leaves AuNPs were synthetized and characterized as in our previous report [11]. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and penicillin–streptomycin solution were purchased from GenDEPOT (Texas, USA), soluble 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Life Technologies (Texas, USA), arbutin and l-3,4-dihydroxyphenylalanin (l-DOPA) were obtained from Abcam (Cambridge, UK), and á-melanocyte stimulating hormone (á-MSH) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma (St. Louis, MO, USA).
2.2. Free radical scavenging activity of PgAuNPs
The potential antioxidant activity of PgAuNPs was determined on the basis of the free radical scavenging activity of DPPH, and the protocol described by Brand-Williams et al. [15] was used with slight modifications. Aliquots of 180 μL of 0.1mM methanolic solution of DPPH were added to 20 μL of nanoparticle solution containing different concentrations (20–150 μg/mL) of PgAuNPs. Ascorbic acid was used as a positive control. Absorbance values at 517 nm were determined after 30 min using a UV–Vis spectrophotometer. The percent inhibition activity was calculated as follows: [(Absorbance of DPPH − (Absorbance of sample – Absorbance of Control))/Absorbance of DPPH] × 100, where absorbance of sample is the absorbance of the DPPH solution with NPs and absorbance of control is the absorbance of the PgAuNP solution.
2.3. Evaluation of moisture retention
The in vitro moisture retention activity of PgAuNPs was assayed gravimetrically as described in a previous report [16] with slight modifications. One milliliter of PgAuNP solution was incubated for 72 h at 37°C and 55% relative humidity. The weights prior to (Wi) and after (Wf) the incubation were measured by an electronic balance. Glycerin was used as moisture retention control, as it is frequently used in cosmetic preparations. The moisture retention rate (Rr) was evaluated by the weight loss of the sample: Rr (%) = [1 − (Wi, sample − Wf, sample)/(Wi, control − Wf, control)] × 100.
2.4. Effect of PgAuNPs on mushroom tyrosinase activity
The activity of mushroom tyrosinase was spectrophotometrically determined as previously described with minor modifications [17]. l-DOPA (2mM, 0.05 mL) in phosphate-buffered saline (PBS; 50mM, pH 6.8) and 0.05 mL of the same buffer with or without test sample were added to a 96-well microplate; then, 0.05 mL of mushroom tyrosinase (200 U/mL) was mixed in. After incubating the mixture at 37°C for 30 min, the optical density was measured at 492 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Bio-Tek Instrument, Winooski, VT, USA). The inhibition rate of tyrosinase was calculated as follows: % = [(A − B)/A] × 100, where A indicates difference of absorbance without the sample and B indicates absorbance with enzyme or sample.
2.5. Cell culture
HDF and murine melanoma B16BL6 cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). Cell lines were cultured in DMEM and supplemented with 10% of FBS and 1% penicillin–streptomycin at 37°C in a humidified 95% air and 5% CO2 atmosphere as described previously [18], [19].
2.6. Cell viability assay
The effect of PgAuNPs on cell viability was measured using the MTT assay method [20]. Retinoic acid (RA) was used as assay control. Cells were seeded at a density of 1 × 105 in 96-well plates and cultured for 24 h. At 90% confluency, cells were treated with various concentration of PgAuNPs or RA (0.1–100 μg/mL) for 24 h, 48 h, and 72 h. After the incubation period, 10 μL of MTT assay solutions (5 mg/mL in PBS) was added to each well and further incubated at 37°C for 3 h; then finally, 100 μL dimethyl sulfoxide was added to dissolve the formazan crystals. The absorbance was measured at 570 nm with an ELISA reader.
2.7. Effect of PgAuNPs on cellular melanin content
B16 melanoma cells were seeded at a density of 2 × 105 cell/well in six-well culture plates and incubated overnight in a humidified atmosphere containing 5% CO2 in air at 37°C. The cells were then treated with medium containing α-MSH and samples for 72 h. Next, 200 μL of the medium was transferred to a 96-well plate, and the amount of melanin in the cell-free culture medium was spectrophotometrically measured at 405 nm and was calculated as follows: % = [(Absorbance of sample/Absorbance of Control)] × 100 [21]. The cellular melanin content of the cultured B16 cells was tested as described previously with slight modifications [22], [23]. In brief, the medium was removed and cells were washed with PBS. Then cells were harvested with trypsin and the cell pellet was solubilized in 500 μL of 1N NaOH containing 10% dimethyl sulfoxide at 80°C for 1 h. The relative melanin content was determined by measuring the absorbance at 475 nm in an ELISA reader. A standard curve for synthetic melanin (0–500 μg/mL) was prepared for each experiment. Melanin production was expressed as percentage of untreated controls.
2.8. Tyrosinase activity assay
Tyrosinase activity was assayed as DOPA oxidase activity [24], and B16 cells were cultured in six-well plates at a density of 4 × 105 cell/well with 100nM α-MSH. After 72 h, the cells were treated with various concentrations of PgAuNPs (1–100 μg/mL); after 72 h, the medium was removed and cells were washed with iced-cold PBS, and lysed with phosphate buffer (pH 6.9) containing 1% Triton X-100. The mixture was freeze-thawed by incubating at −80°C for 15 min and then kept at room temperature for 10 min. The samples were clarified by centrifugation at 12,000g for 15 min. After centrifugation, 10 μL of freshly prepared substrate solution (15mM l-DOPA in 50mM pH 7.1 sodium phosphate buffer) was added to 90 μL of lysate supernatant and incubated at 37°C for 1 h. The absorbance was then measured at 475 nm using an ELISA reader.
2.9. Reverse transcription-polymerase chain reaction
Total RNA was extracted using Trizol reagents solution (Sigma). Reverse transcription (RT) and cDNA amplification were carried with 2 μg of total RNA using a Thermo-Scientific cDNA synthesis kit (Thermo-Scientific, Waltham, MA, USA,) according to the manufacturer's instructions. The cDNA obtained was amplified with the following primer: tyrosinase, forward 5′-ATGGGTCAACACCCATGTTT-3′ and reverse 5′-GGCAAATCCTTC CAGTGTGT-3″; melanogenesis-associated transcription factor (MITF), forward 5′-CGGGATGCCTTGTTTATGGT-3′ and reverse 5′-TGCCTCTGAGCTTGCTGTAT-3′. The reaction was cycled 25 times, for 30 s at 95°C, 30 s at 58°C, and 60 s at 72°C. The amplified RT-polymerase chain reaction (PCR) products were analyzed on 0.8% agarose gels, visualized by ethidium bromide staining, and photographed under ultraviolet light. The quantitative real-time PCR was performed using an R-Corbett Rotor-Gene Model 6000 (Sydney, Australia), with SYBR Green qPCR Super Mix UDG kit (Invitrogen, Carlsbad, CA, USA). The relative expression levels of the target genes against (GAPDH) as reference gene were calculated using the delta cycle threshold (Ct) method [25].
3. Results and discussion
3.1. Scavenging effect of PgAuNPs
The results of our study revealed that PgAuNPs exhibited the activity of DPPH free radical scavenging activity in a dose-dependent manner (Fig. 1). However, PgAuNPs showed moderate free radical scavenging activity (79.2%) compared to the assay control. In vitro DPPH radical scavenging assay showed that PgAuNPs (IC50 = 141.3 ìg/mL) had better antioxidant activity than P. ginseng leaves extract reported previously by Jung et al. [26]. A possible reason for the higher antioxidant activity of PgAuNPs may be correlated with the presence of copious bioactive compounds in P. ginseng leaves extract that enhance PgAuNP's phytochemical properties; the most remarkable compounds are ginsenosides, polysaccharides, triterpenoids, and flavonoids [10]. Moreover, the increased antioxidant activity of PgAuNPs can be credited to the adsorption of existing bioactive compounds of the P. ginseng leaves extract. The interaction of plant metabolites with metal ions during NP formation may result in improved free radical scavenging compounds. In addition, the electrostatic attractions between negatively charged phytochemicals and positively or neutrally charged PgAuNPs act synergistically to improve the bioactivity of plants [27], [28].
Fig. 1.
Increasing 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical-scavenging activity of different concentrations (1–150 μg/mL) PgAuNPs. Results are expressed as mean ± SD of three separate experiments. For DPPH assay, ascorbic acid (1–150 μg/mL) was used as reference standard. PgAuNPs, Panax ginseng leaves-capped gold nanoparticles; SD, standard deviation.
3.2. Evaluation of moisture retention
Proper hydration of skin is crucial for healthy skin function, and moisturizers are essential components of basic skin care. The in vitro moisture retention property PgAuNPs was examined gravimetrically and compared with that of glycerin. Recent studies report that glycerin exists in the skin's outer layer, the stratum corneum, as a natural endogenous humectant [29]. Glycerin is also a cosmetic ingredient regularly used as a hygroscopic and humectant agent. Fig. 2 shows that PgAuNP solutions (1%, 5%, and 10%) have similar values of moisture retention activities, at 75.6%, 74.3%, and 74.3%, respectively. These values are close to that obtained (79.7%) for glycerin solution at 10%, thereby confirming the moisture retention property of PgAuNPs from P. ginseng leaves.
Fig. 2.
Moisture retentions of PgAuNP solution (1–10%). Results are expressed as mean ± SD of three separate experiments. For moisture retention assay, glycerin solution (10%) was used as reference standard. PgAuNPs, Panax ginseng leaves-capped gold nanoparticles; SD, standard deviation.
3.3. Effect of PgAuNPs on mushroom tyrosinase inhibition
Tyrosinase is a binuclear copper-containing enzyme that catalyzes two different reactions using molecular oxygen. Initial reaction consists in the hydroxylation of tyrosine to DOPA (monophenolase activity) and diphenolase activity catalyzes the oxidation of DOPA to dopaquinone [30]. For this purpose, l-DOPA was used as a substrate to determine the inhibitory effect of PgAuNPs on the diphenolase activity of the mushroom tyrosinase. The inhibitory effect of PgAuNPs on the activity of mushroom tyrosinase for the oxidation of l-DOPA was investigated by UV–Vis spectrophotometer [21]. The inhibitor concentration leading to 50% activity lost (IC50) by PgAuNPs was 16.06 μg/mL, which is similar to that recorded in the control drug, arbutin (14.3 μg/mL). Antityrosinase activity in PgAuNPs synthetized from P. ginseng leaves extract could be from flavonoids absorbed by PgAuNPs, the corresponding flavonoids' ability to chelate two coppers at the active site of tyrosinase enzyme [31]. In their previous work, Seo et al. [32] reported that the crude methanol extract of the fresh leaves of P. ginseng showed the inhibitory activity on mushroom tyrosinase.
3.4. Effect of PgAuNPs on cell viability of B16 and HDF cells
To assess the effects of PgAuNPs on cell viability, mouse B16 and HDF cells were used as in vitro models and RA as assay control. Cells were cultured and treated with different concentrations of PgAuNPs or RA (1–100 μg/mL), and cell viability was measured using the MTT assay after 24 h, 48 h, and 72 h. Control wells were incubated with fresh free serum media, and experiments were repeated in triplicates. As shown in Fig. 3, cells treated with PgAuNPs did not have cytotoxic effects on the viability of HDF and B16 cells at concentrations ≤100 μg/ml. No visible changes in cell morphology were noted between the treated cells and control groups treated at different times and concentrations. By contrast, cell viability of B16 and HDF cells decreased at concentration ≥ 10 μg/mL when treated with RA. This result is in accordance with previous reports [33], [34]. Other researchers have reported that AuNPs were uptaken by cells via the clathrin-mediated endocytosis pathway [25]. This finding suggests that P. ginseng leaves extract capped AuNPs could be taken up by HDF and B16 cells in a similar manner. Based on the results of this preliminary study, it is indicated that inhibition of melanogenesis by PgAuNPs can be attributed to their ability to inhibit tyrosinase activity rather than to their cytotoxic effects.
Fig. 3.
Cell viability of HDF and B16BL6 cells after treatment with PgAuNPs at different time points: (A) 24 h. (B) 48 h. (C) 72 h. Cell viability of HDF and B16BL6 cells after treatment with RA for different time intervals: (D) 24 h. (E) 48 h. (F) 72 h. Cells (1 × 105 cells/well) were incubated with various concentrations (0.1–100 μg/mL) of PgAuNPs or RA. Cell viability was determined by 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Note that samples did not show any toxicity effect up to 100 μg/mL for PgAuNPs. Results are expressed as a percentage of sample-treated control and presented as mean ± SD of three separate experiments. ***p < 0.001 versus control by Student t test. HDF, human dermal fibroblast; PgAuNPs, Panax ginseng leaves-capped gold nanoparticles; RA, retinoic acid; SD, standard deviation.
3.5. Effect of PgAuNPs on cellular melanin content
Fig. 4A shows the dose-dependent reduction in melanin content of PgAuNPs in cell-free culture medium in B16 cells stimulated with α-MSH and after 72 h treatment with PgAuNPs. B16 cells treated with 100nM of α-MSH significantly increased melanin production as opposed to B16 control cell.
Fig. 4.
Inhibition effect of PgAuNPs on melanin content in B16 cells. Cells (2 × 105 cells/well) were incubated with different concentrations (1–100 μg/mL) of PgAuNPs or arbutin (100 μg/mL) in the presence of 100nM of α-MSH for 72 h. (A) Extracellular melanin content, the melanin levels contained in 100 μL of the medium containing the cells were determined reading the OD value of each sample. Below is a picture of the color intensity of extracellular melanin production in media. (B) Intracellular melanin content was determined as described in Materials and methods. Below is a picture of B16 cell pellet after centrifugation. Results are expressed as a percentage of α-MSH-treated control and presented as mean ± SD of three separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001 versus α-MSH treated control by Student t test. α-MSH, α-melanocyte stimulating hormone; OD, optical density; PgAuNPs, Panax ginseng leaves-capped gold nanoparticles; SD, standard deviation. C stands as for assay control and ###P 0.001 versus assay control (C).
In addition, PgAuNPs led to a dose-dependent decrease in cellular melanin production in α-MSH stimulated cells. B16 cells treated with 100 μg/mL of PgAuNPs show higher inhibition compared with those treated with same concentration of arbutin (Fig. 4B). The antimelanogenic effect of PgAuNPs is probable attributable to the p-coumaric acid, which was identified as a major tyrosinase inhibitor in P. ginseng leaves extract [35]. p-Coumaric acid was identified as an antimelanogenesis agent in B16F10 melanoma cells stimulated by α-MSH, because of its structural similarity with tyrosinase [36]. In addition, phenolic compounds in P. ginseng leaves-capped AuNPs are capable of inhibiting tyrosinase and reducing melanin synthesis, whereas ginsenosides prevent the intracellular increase of reactive oxygen species. Reactive oxygen species are responsible for altering the redox state of cell membrane proteins, resulting in increasing the rates of melanin production and leading to dark skin [3], [37].
3.6. Effect of PgAuNPs on tyrosinase activity in B16BL6 cells
Cellular tyrosinase activity was significantly increased in B16 cells treated with α-MSH alone compare to control cells (Fig. 5). PgAuNPs suppressed tyrosinase activity in a dose-dependent manner. PgAuNP treatment was more effective than arbutin (10 μg/mL) at concentrations of 10 μg/mL and 100 μg/mL. These results are in accordance with a previous report in which p-coumaric acid (mayor tyrosinase inhibitor in P. ginseng leaves extract) strongly inhibited human and murine tyrosinase in comparison with arbutin [38]. Enzyme kinetics analysis indicates that p-coumaric acid is a competitive inhibitor for DOPA for human tyrosinase [38].
Fig. 5.
Inhibitory effect of melanogenesis of PgAuNPs through inhibition of tyrosinase activity. B16 cells (4 × 105 cells/well) were incubated with various concentrations of (1–100 μg/mL) PgAuNPs or arbutin (100 μg/mL) in the presence of 100nM of α-MSH for 72 h. Tyrosinase activity in cellular lysates was determined as described in Material and methods. Results are expressed as a percentage of α-MSH-treated control and presented as mean ± SD of three separate experiments. **p < 0.01, ***p < 0.001 versus α-MSH-treated control by Student t test. α-MSH, α-melanocyte stimulating hormone; PgAuNPs, Panax ginseng leaves-capped gold nanoparticles; SD, standard deviation. C stands as for assay control and ###P 0.001 versus assay control (C).
3.7. Effect of PgAuNPs on gene expression of tyrosinase
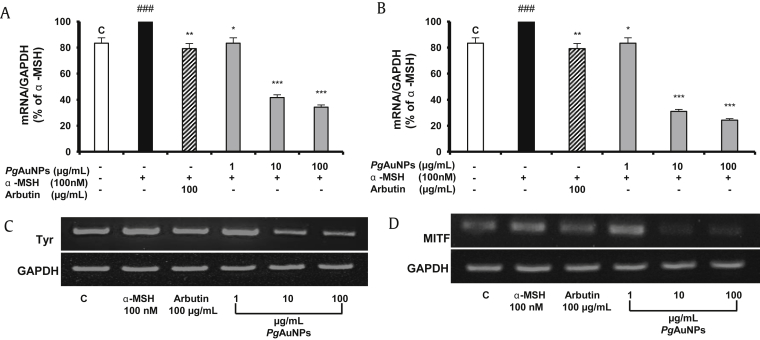
TYR gene encodes an enzyme called tyrosinase. Tyrosinase is a copper-containing enzyme responsible for the pigmentation in mammals [39]. Increase in the production of melanin has been involved in hyperpigmentation or darkening of the skin, and this can be avoid by controlling the activity of tyrosinase and microphthalmia-associated transcription factor, which is crucial in the regulation of melanocyte proliferation, melanogenesis, and a major regulator of tyrosinase [19]. As proof-of-concept, we study the effect of PgAuNPs induced inhibitory effect on melanogenic related genes. We further examined the effect of PgAuNPs on the mRNA expression level of tyrosinase transcription and MITF genes.
The RT-PCR and PCR data for our experiments proved that, in the case of cells treated with PgAuNPs, in comparison with the ones without any therapy (á-MSH stimulated cells), the expression levels of the TYR gene responsible for the synthesis of the tyrosinase protein and MITF gene major regulator of tyrosinase are downregulated, as shown in Fig. 6A–D. The expression level is further decreased when the PgAuNP concentrations are increased and compared to cells treated with arbutin (control drug), and the difference is statistically significant (p < 0.001). These results indicate that PgAuNPs inhibited tyrosinase at the transcriptional level, suggesting its role in skin whitening.
Fig. 6.
Effect of on PgAuNPs mRNA expression of melanogenesis-related genes. Cells (4 × 105 cells/well) were treated with various concentrations of (1–100 μg/mL) PgAuNPs or arbutin (100 μg/mL) in the presence of 100nM of α-MSH for 72 h. Then cells were harvested and total RNA was extracted. mRNA expression was visualized by RT-PCR. (A) Tyrosinase (TYR). (B) Microphthalmia-associated transcription factor (MITF). The size of amplified gene products was 528 bp for GAPDG. (C) Tyrosinase, 563 bp.(D) MITF, 216 bp. Results as a percentage of α-MSH-treated control and presented as mean ± SD of three separate experiments. *p < 0.05, **p < 0.01, ***p < 0.001 versus α-MSH-treated control by Student t test. α-MSH, α-melanocyte stimulating hormone; PgAuNPs, Panax ginseng leaves-capped gold nanoparticles; RT-PCR, reverse transcription-polymerase chain reaction; SD, standard deviation. C stands as for assay control and ###P 0.001 versus assay control (C).
4. Conclusion
The study highlights the evaluation of P. ginseng leaves-capped AuNPs as material for cosmetic applications. P. ginseng leaves extract, a notable bioactive constituent, produces AuNPs with multifunctional properties and effects for cosmetic applications. PgAuNPs can effectively and gradually scavenge the radical cations, have moisture retention properties, and are able to suppress mushroom tyrosinase. In addition, PgAuNPs are nontoxic to HDF and B16 cell and are capable of reducing melanin content in stimulated α-MSH B16 cells as well as cellular tyrosinase activity. The whitening effect of PgAuNPs is mediated trough the suppression of mRNA expression levels of tyrosinase enzyme and MITF. These results suggest that P. ginseng leaves-capped AuNPs could be a promising multifunctional ingredient in cosmetics. However, it should be noted that this study uses PgAuNPs as a proof-of-concept investigation. Further studies should be performed on cosmetic formulations containing PgAuNPs as an ingredient.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ0128132018)” Rural Development Administration, Republic of Korea.
Contributor Information
Dong-Hyun Kim, Email: yeonjukim@khu.ac.kr.
Deok Chun Yang, Email: dcyang@khu.ac.kr.
References
- 1.Wickens A.P. Ageing and the free radical theory. Respir Physiol. 2001;128:379–391. doi: 10.1016/s0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 2.Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci. 1994;91:8731–8738. doi: 10.1073/pnas.91.19.8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y.J., Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci. 2005;62:1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solano F., Briganti S., Picardo M., Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Frenk E. Melasma: new approaches to treatment. Martin Dunitz Ltd; London: 1995. Treatment of melasma with depigmenting agents; pp. 9–15. [Google Scholar]
- 6.Van Smeden J., Bouwstra J. Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol. 2016;49:8–26. doi: 10.1159/000441540. [DOI] [PubMed] [Google Scholar]
- 7.Vyumvuhore R., Tfayli A., Biniek K., Duplan H., Delalleau A., Manfait M., Dauskardt R., Baillet-Guffroy A. The relationship between water loss, mechanical stress, and molecular structure of human stratum corneum ex vivo. J Biophotonics. 2015;8:217–218. doi: 10.1002/jbio.201300169. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y.J., Jeon J.N., Jang M.-G., Oh J.Y., Kwon W.-S., Jung S.-K., Yang D.-C. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 2014;38:66–72. doi: 10.1016/j.jgr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang H., Peng D., Xie J. Ginseng leaf–stem: bioactive constituents and pharmacological functions. Chin Med. 2009;4:1. doi: 10.1186/1749-8546-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh P., Kim Y.J., Yang D.C. A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif Cell Nanomed B. 2016;44:1949–1957. doi: 10.3109/21691401.2015.1115410. [DOI] [PubMed] [Google Scholar]
- 12.Lohani A., Verma A., Joshi H., Yadav N., Karki N. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014;2014:843687. doi: 10.1155/2014/843687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiechers J.W., Musee N. Engineered inorganic nanoparticles and cosmetics: facts, issues, knowledge gaps and challenges. J Biomed Nanotechnol. 2010;6:408–431. doi: 10.1166/jbn.2010.1143. [DOI] [PubMed] [Google Scholar]
- 14.Patel A., Prajapati P., Boghra R. Overview on application of nanoparticles in cosmetics. Asian J Pharm Clin Res. 2011;1:40–55. [Google Scholar]
- 15.Brand-Williams W., Cuvelier M.-E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 16.Tsai C.C., Chan C.F., Huang W.Y., Lin J.S., Chan P., Liu H.Y., Lin Y.S. Applications of Lactobacillus rhamnosus spent culture supernatant in cosmetic antioxidation, whitening and moisture retention applications. Mol Cells. 2013;18:14161–14171. doi: 10.3390/molecules181114161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tada T., Ohnishi K., Komiya T., Imai K. Synthetic search for cosmetic ingredients: preparations, tyrosinase inhibitory and antioxidant activities of caffeic amides. J Oleo Sci. 2002;51:19–27. [Google Scholar]
- 18.Lee J., Jung E., Lee J., Huh S., Kim J., Park M., So J., Ham Y., Jung K., Hyun C.-G. Panax ginseng induces human Type I collagen synthesis through activation of Smad signaling. J Ethnopharmacol. 2007;109:29–34. doi: 10.1016/j.jep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Yoo D.S., Rho H.S., Lee Y.G., Yeom M.-H., Kim D.H., Lee S.J., Hong S.Y., Lee J.H., Cho J.Y. Ginsenoside F1 modulates cellular responses of skin melanoma cells. J Ginseng Res. 2011;35:86–91. [Google Scholar]
- 20.Jiménez-Pérez Z.E., Mathiyalagan R., Markus J., Kim Y.J., Kang H.M., Abbai R., Seo K.H., Wang D., Soshnikova V., Yang D.C. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int J Nanomed. 2017;12:709–723. doi: 10.2147/IJN.S118373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang H.M., Chien Y.C., Wu C.-H., Kuo Y.H., Wu W.C., Pan Y.Y., Su Y.H., Wen K.C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem Toxicol. 2014;65:129–139. doi: 10.1016/j.fct.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Hosoi J., Abe E., Suda T., Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1α,25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985;45:1474–1478. [PubMed] [Google Scholar]
- 23.Wang D.D., Jin Y., Wang C., Kim Y.J., Perez Z.E.J., Baek N.I., Mathiyalagan R., Markus J., Yang D.C. Rare ginsenoside Ia synthesized from F1 by cloning and overexpression of the UDP-glycosyltransferase gene from Bacillus subtilis: synthesis, characterization, and in vitro melanogenesis inhibition activity in BL6B16 cells. J Ginseng Res. 2018;42:42–49. doi: 10.1016/j.jgr.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong Y.M., Oh W.K., Tran T.L., Kim W.-K., Sung S.H., Bae K., Lee S., Sung J.-H. Aglycone of Rh4 inhibits melanin synthesis in B16 melanoma cells: possible involvement of the protein kinase A pathway. Biosci Biotechnol Biochem. 2013;77:119–125. doi: 10.1271/bbb.120602. [DOI] [PubMed] [Google Scholar]
- 25.Schefe J.H., Lehmann K.E., Buschmann I.R., Unger T., Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s C T difference” formula. J Mol Med. 2006;84:901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 26.Jung C.H., Seog H.M., Choi I.W., Cho H.Y. Antioxidant activities of cultivated and wild Korean ginseng leaves. Food Chem. 2005;92:535–540. [Google Scholar]
- 27.Kumar B., Smita K., Cumbal L., Debut A. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J Biol Sci. 2014;21:605–609. doi: 10.1016/j.sjbs.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swamy M.K., Akhtar M.S., Mohanty S.K., Sinniah U.R. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim Acta A Mol Biomol Spectrosc. 2015;151:939–944. doi: 10.1016/j.saa.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Verdier-Sévrain S., Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007;6:75–82. doi: 10.1111/j.1473-2165.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 30.Xing R., Wang F., Dong L., Zheng A.-P., Wang L., Su W.-J., Lin T. Inhibitory effects of Na 7 PMo 11 CuO 40 on mushroom tyrosinase and melanin formation and its antimicrobial activities. Food Chem. 2016;197:205–211. doi: 10.1016/j.foodchem.2015.10.119. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q.X., Kubo I. Kinetics of mushroom tyrosinase inhibition by quercetin. J Agric Food Chem. 2002;50:4108–4112. doi: 10.1021/jf011378z. [DOI] [PubMed] [Google Scholar]
- 32.Seo S.Y., Sharma V.K., Sharma N. Mushroom tyrosinase: recent prospects. J Agric Food Chem. 2003;51:2837–2853. doi: 10.1021/jf020826f. [DOI] [PubMed] [Google Scholar]
- 33.Lotan R., Giotta G., Nork E., Nicolson G.L. Characterization of the inhibitory effects of retinoids on the in vitro growth of two malignant murine melanomas. J Natl Cancer Inst. 1978;60:1035–1041. doi: 10.1093/jnci/60.5.1035. [DOI] [PubMed] [Google Scholar]
- 34.Nelson D.L., Balian G. The effect of retinoic acid on collagen synthesis by human dermal fibroblasts. Coll Relat Res. 1984;4:119–128. doi: 10.1016/s0174-173x(84)80020-5. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.H., Lee B.S., Yang M.S., Byun B.S., Kim W.G., Kim B.H., Lee S.J. Prevention of photoaging and wrinkle formation in hairless mice dorsal skin by APB-03. Korean J Food Sci Technol. 2005;37:989–996. [Google Scholar]
- 36.An S., Lee S., Choi S., Moon S.W., Boo Y. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by α-melanocyte stimulating hormone. Br J Dermatol. 2008;159:292–299. doi: 10.1111/j.1365-2133.2008.08653.x. [DOI] [PubMed] [Google Scholar]
- 37.Hwang E.Y., Kong Y.H., Lee Y.C., Kim Y.C., Yoo K.M., Jo Y.O., Choi S.Y. Comparison of phenolic compounds contents between white and red ginseng and their inhibitory effect on melanin biosynthesis. J Ginseng Res. 2006;30:82–87. [Google Scholar]
- 38.An S.M., Koh J.S., Boo Y.C. p-Coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother Res. 2010;24:1175–1180. doi: 10.1002/ptr.3095. [DOI] [PubMed] [Google Scholar]
- 39.Balu K., Purohit R. Mutational analysis of TYR gene and its structural consequences in OCA1A. Gene. 2013;513:184–195. doi: 10.1016/j.gene.2012.09.128. [DOI] [PubMed] [Google Scholar]