Abstract
Orally administered ginsengs come in contact with the gut microbiota, and their hydrophilic constituents, such as ginsenosides, are metabolized to hydrophobic compounds by gastric juice and gut microbiota: protopanxadiol-type ginsenosides are mainly transformed into compound K and ginsenoside Rh2; protopanaxatriol-type ginsenosides to ginsenoside Rh1 and protopanaxatriol, and ocotillol-type ginsenosides to ocotillol. Although this metabolizing activity varies between individuals, the metabolism of ginsenosides to compound K by gut microbiota in individuals treated with ginseng is proportional to the area under the blood concentration curve for compound K in their blood samples. These metabolites such as compound K exhibit potent pharmacological effects, such as antitumor, anti-inflammatory, antidiabetic, antiallergic, and neuroprotective effects compared with the parent ginsenosides, such as Rb1, Rb2, and Re. Therefore, to monitor the potent pharmacological effects of ginseng, a novel probiotic fermentation technology has been developed to produce absorbable and bioactive metabolites. Based on these findings, it is concluded that gut microbiota play an important role in the pharmacological action of orally administered ginseng, and probiotics that can replace gut microbiota can be used in the development of beneficial and bioactive ginsengs.
Keywords: fermentation, ginseng, ginsenoside, gut microbiota, metabolism
1. Introduction
Most herbal medicines are orally administered to humans, and the components inevitably come into contact with the gut microbiota in the gastrointestinal tract where trillions of microbes reside. The gut microbiota exhibits diverse physiological activities including the ability to metabolize orally administered and bile-secreted xenobiotics (e.g., drugs, phytochemicals) [1], [2], [3]. Gut microbiota transforms the constituents of orally administered hydrophilic drugs and phytochemicals before absorption by gastrointestinal tract into the blood. Studies on the metabolism of phytochemicals found in natural products, such as ginseng, by the gut microbiota are important in understanding their biological effects [4], [5].
This review describes gut microbiota-mediated metabolism of ginsenosides such as protopanaxadiol- type, protopanaxatriol-type, oleanane-type, and ocotillol-type ginsenosides and their bioactive metabolites.
2. Gut microbiota
The neonate is born in a germ-free state. Immediately after birth, they are exposed to microbes present within the parturient canal, on the skin of mothers and nurses, and in ambient air. These microbes colonize on body surfaces and the gastrointestinal and vaginal tracts [6], [7]. Newer molecular methods have revealed that the gastrointestinal tract hosts over 2,000 species of microbiota in humans. Most species belong to eight dominant phyla: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, Cyanobacteria, and Verrucomicrobia [8], [9]. More than 80% of these microbes belong to the phyla Firmicutes and Bacteroides; Firmicutes includes Clostridia and Bacilli; and Bacteroidetes includes Bacteroides spp. The highly complex gut ecosystem varies between individuals due to factors, such as diet, genetics, hormones, and drugs [10]. It exhibits various physiological actions: fermentation of carbohydrates and proteins that are not digested in the upper gut, production of vitamins B and K, protection against pathogens, stimulation of innate and adaptive immune responses, and metabolism of orally administered hydrophilic phytochemicals and drugs.
3. Ginseng constituents
Ginseng refers to the dried roots of the species Panax sp. (Family Araliaceae), including Panax ginseng Meyer (Korean ginseng or Asian Ginseng), which has been used as a herbal medicine for more than 2000 years [11], Panax quinquefolius L. (American Ginseng), Panax notoginseng (Burk.) FH Chen (Notoginseng), and Panax vietnamensis Ha et Grushv. (Vietnamese Ginseng) [12], [13], [14]. P. ginseng is the most commonly used. Garriques prepared the saponin fraction of P. quinquefolius [1]. Its constituents were not identified until 1963 [15]. Shibata et al. [16], [17], [18] isolated saponins from the root of P. ginseng in 1963 and identified their structures. Since then, many researchers have isolated the constituents including ginsenosides. Approximately 200 substances, such as ginsenosides, polysaccharides, and polyacetylenes have been isolated from Korean ginseng [19], [20] and more than 100 from American ginseng, notoginseng, and Vietnamese ginseng [12], [13], [14].
Shibata et al. [16], [17], [18], [21] established the chemical structures of main prosapogenins 20S-protopanaxadiol, 20S-protopanaxatriol, prosapogenin, and ginsenoside Rg1 found in the dried root of P. ginseng. Kitagawa et al. [22], [23] isolated malonyl ginsenoside Rb1, Rb2, Rc, and Rd; Ruan et al. [24] isolated malonyl ginsenoside Ra3; Zhu et al. [25] isolated six protopanaxatriol-type ginsenosides Re1, Re2, Re3, Re4, Re5, and Re6 and 10 known protopanaxatriol ginsenosides including ginsenoside Rg1.
From red ginseng (steamed P. ginseng), Matsumura et al. [26] isolated ginsenosides Ra1, Ra2, and Ra3 and notoginsenoside R4. Kasai et al. [27] isolated ginsenosides Ra1, Ra2, Ra3, Rs1, and Rs2, notoginsenoside R1, and quinquenoside R1. Thereafter, ginsenosides Ro, Rb1, Rb2, Tc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, Rh2, 20R-ginsenoside Rh1, 20S-ginsenoside Rg3, and 20R-ginsenoside Rg2 were also isolated by [27]. Ryu et al. [28] isolated ginsenoside Rg6 and 20(E)-ginsenoside F4. Baek et al. [29], [30], [31] isolated ginsenoside Rh4 [29] as well as ginsenoside Rs1, Rs2, Rs3, and Rs4, quinoginsenoside R4; ginsenoside Rg3, Rg5, Rg6, F4, and Rf2 [30], [31]. Ginsenosides Rh1, Rg3, and Rg2 were found in large quantities. Under intense steaming or heating, ginsenoside Rg3 can transform into 20S-Rh2 and 20R-Rh2 and subsequently form the aglycone 20S-protopanaxadiol and 20R-protopanaxadiol or even 20-dehydroprotopanaxadiol through chemical degradation [32], [33], [34]. Ginsenoside Rk1 and Rg5 can transform into their degradation products, such as Rk2 and Rh3, and Rh1 into aglycone 20S-protopanaxatriol and 20R-protopanaxatriol or even 20-dehydropropanaxatriol.
In American ginseng (P. quinquefolius), > 60 ginsenosides, including dammarane, ocotillol, and oleanane types, have been isolated: ginsenoside Rb1, Rd, and Re as main constituents, including ocotillol-type ginsenosides (24R-pseudoginsenoside F11, pseudoginsenoside RT5, F-11, 24R-vina-ginsenoside R1) and oleanane-type ginsenosides (ginsenoside Ro, chikusetsusaponin Iva) [4].
In P. notoginseng, a total of 56 dammarane-type saponins have been isolated: protopanaxadiol-type and protopanaxatriol ginsenosides, such as ginsenosides Ra3,RK3, Rh4, Rg3, Rk1, Rg5, F2, Rh1, Rg1, Re, Rd, Rb1, and Rb2, 6′-O-acetylginsenoside Rh1, and another group of saponins, notoginsenosides A – N, R1 – R4, R6 – R9, Fa, Fc, and Fe and gypenoside X VII [14], [35], [36], [37], [38], [39].
From P. vietnamensis, Nguyen et al. [14], [40], [41] isolated ginsenoside Rh1, Rg1, Re, Rd, Rb3, Rb2, and Rb1; pseudoginsenoside RS1; notoginsenosidesR1 and Fa; oleanolic acid; and ocotillol-type saponins (pseudoginsenoside-RT4, 24(S)-pseudoginsenoside F11, majonoside F1, R1, and R2); vinaginsenoside R3, R4, R5, R6, R7, R8, and R9; 20-glucoginsenoside Rf; ginsenoside Rc; notoginsenoside R6; quinquenoside R1; and gypenoside XVII. In addition, Duc et al. [42] isolated 6-O-β-D-glucopyranosyl 20(S),25-epoxydammarane-3β,6α,12β,24α-tetrol, 6-O-β-D-xylopyranosyl-(1-->2)-β-D-glucopyranosyl 20(S),25-epoxydammarane-3β,6α,12β,24α-tetrol; 6-O-β-D-glucopyranosyl dammarane-3β,6α,12β,20(S),24 xi,25-hexol; 3-O-[β-D-glucopyranosyl-(1-->2)-β-D-glucopyranosyl]-20-O-β-D- glucopyranosyl dammarane-3β,12 β,20(S),24 xi,25-pentol; and 6-O-β-D-xylopyranosyl-(1-->2)-β-D-glucopyranosyl 20(S),24(S)-epoxydammarane-3β,6α,12β,25 xi,26-pentol [42].
4. Absorption, distribution, metabolism, and excretion of ginseng phytochemicals
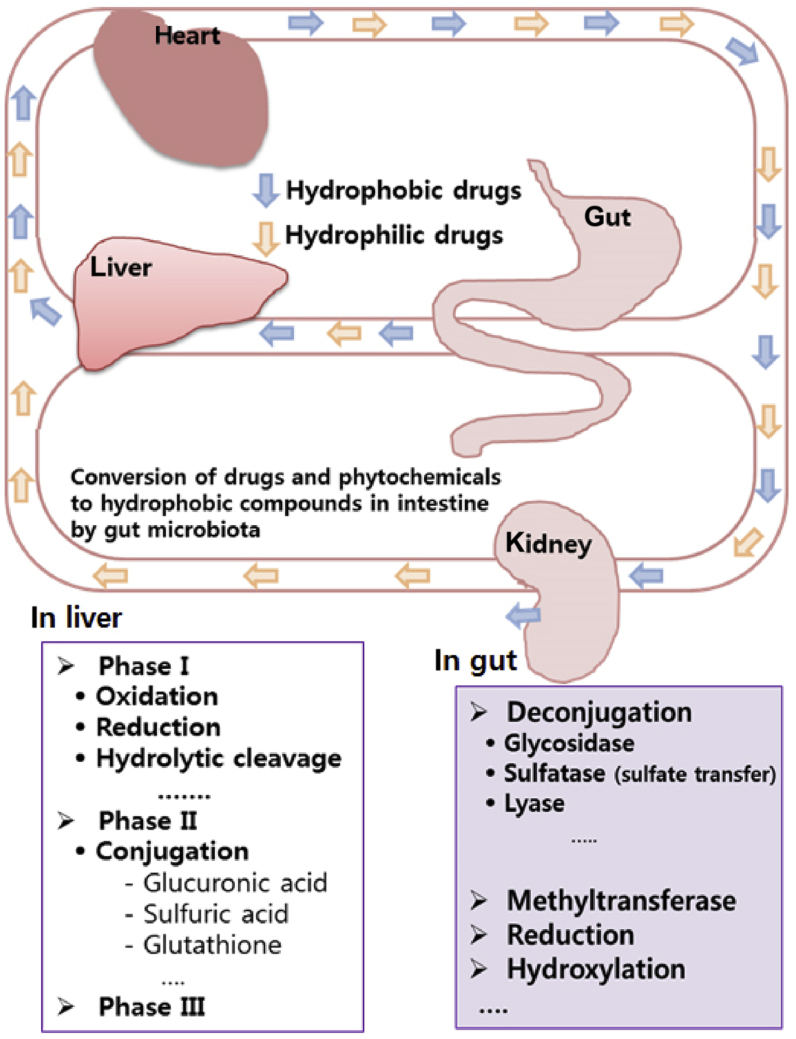
The pharmacological effects of ginsengs, particularly their saponins including ginsenosides, may be dependent on their absorption, distribution, metabolism, and excretion (ADME), similar to drugs (Fig. 1). In a pharmacokinetic study, Tawab et al. [43] investigated the parent ginsenosides and their metabolites in the plasma and urine samples of two individuals orally treated with Ginsana extract (ginseng saponin fraction, Pharmaton S.A., Lugano, Switzerland) by liquid chromatography–mass spectrometry/mass spectrometry. The metabolites ginsenosides Rh1, F1, and compound K were detected in the plasma and urine. However, the metabolites were not detected in Ginsana extract. Therefore, these metabolites (hydrolysates) may be produced for parental ginsenosides by gut microbiota or by the liver. Although ginsenoside Rb1 was detected in the plasma and urine of one individual, it was detected at the lower limit of detection. Akao et al. [44], [45] conducted a pharmacokinetic study of compound K in germ-free and gnotobiotic rats. They could not detect ginsenoside Rb1 in both rats, but they detected compound K in gnotobiotic rats, not in the germ-free rats. In individuals orally administered ginseng extract, Shibata et al. [46] did not detect ginsenoside Rb1, but detected compound K. In our previous studies, we detected compound K in rats orally treated with 0.2 g/kg ginseng extract. Maximum concentration, time to maximum concentration, and area under the curve (AUC) were 24.1 ± 5.5 ng/mL, 15.2 ± 1.8 h, and 153.1 ± 30.6 ng·h/mL, respectively [47], [48], [49]. We found that the absorption of compound K was affected by diets including prebiotic fiber (nutriose). We also performed a pharmacokinetic study of compound K in individuals (n = 34) orally treated with ginseng powder [1], [49]. Maximum concentration, time to maximum concentration, and AUC were found to be 27.89 ± 24.46 ng/mL, 10.76 ± 2.07 h, and 221.98 ± 221.42 ng h/mL, respectively. These findings suggest that compound K may be the main metabolite produced by intestinal bacteria in humans orally administered ginseng.
Fig. 1.
Fates of orally administered drugs and phytochemicals in humans and animals. Orally administered drugs and phytochemicals in humans and animals are converted to hydrophobic compounds in the intestine by enzymes of gut microbiota such as β-D-glucosidase, and delivered into the liver, which metabolizes these hydrophobic metabolites to hydrophilic compounds by enzymes of phase I–III, such as uridine 5′-diphospho-glucuronosyltransferase.
5. Metabolism of protopanaxadiol-type and protopanaxatriol-type ginsenosides in gastrointestinal tract by gastrointestinal juice and gut microbiota
To understand the metabolism of ginsenosides in the gastrointestinal tract, many experiments were conducted in vitro and in vivo [44], [50], [51], [52], [53]. Karikura et al. [33] and Han et al. [51] reported that protopanaxadiol-type ginsenosides Rb1 and Rb2 transformed into ginsenoside Rg3 in diluted hydrochloric acid in vitro. In addition, ginsenoside Rb1 transformed into a 25-hydroperoxy-23-ene derivative. Ginsenoside Rb2 transformed into 25-hydroxyl-23-ene, 24-hydroxy-25-ene, 25-hydroperoxy-23-ene, and 24-hydroperoxy-25-ene derivatives. Thus, protopanaxadiol-type ginsenosides hydrolyzed the C-20 glycosyl moiety and hydrated or oxygenated the side chain. Nevertheless, the amount of their metabolites in rat stomach was negligible.
After our incubation in a diluted condition at 60°C or at boiling temperature, protopanaxadiol-type ginsenosides Rb1, Rb2, and Rc transformed into ginsenoside Rg3, Rg5, and Rk1; however, the transformation was negligible at 37°C [32]. These findings suggest that orally administered protopanaxadiol-type ginsenosides may be resistant to the gastric juice, but can be transformed by heat under acidic condition.
Hydrophilic ginsenosides, when orally ingested by humans and animals, come into contact with gut microbiota in the gastrointestinal tract and can be metabolized to hydrophobic metabolites by the gut microbiota [4], [5]. Hydrophobic metabolites are easily absorbed from the gastrointestinal tract into the blood compared with the parent ginsenosides. When ginseng was orally administered in humans, compound K (a hydrophobic metabolite of ginsenosides) was detected in the blood as the main component [43], [46]; a small quantity of ginsenoside Rb1, but not ginsenoside Rb2, Rc, and Re, was detected in the blood of one of two individuals. Akao et al. [44], [45] and Park et al. [54] also found compound K in the intestinal content, blood, and urine of conventional and gnotobiotic rats orally treated with ginsenoside Rb1. Moreover, Kato et al. [55] detected compound K in the blood of human following the intake of red ginseng powder. Although ginsenosides Rb1, Rb2, Rc, and Re were detected in some studies [43], [44], [45], the levels were insufficient to exhibit show a biological effect.
Protopanaxatriol-type ginsenosides Rg1 and Re are more labile to acidic condition than protopanaxadiol-type ginsenosides. Protopanaxatriol-type ginsenosides are transformed into ginsenosides Rh1 and Rg2 under acidic condition. The metabolite ginsenoside Rh1 was absorbed from the stomach and small intestine. However, ginsenoside Rg2 was not detected in the blood because the rhamnosyl moiety could not be absorbed due to the absence of rhamnose sugar transporter, such as quercetin-4-O-rhamnoglucoside [56]. Ginsenosides Rg1, Rg2, and Re may be metabolized to ginsenoside Rh1 and protopanaxatriol by the gut microbiota. These metabolites are easily absorbed into the blood. These findings suggest that protopanaxatriol-type ginsenosides can be hydrolyzed in the stomach by gastric juice and the metabolites can be absorbed from stomach and small intestine. However, most protopanaxatriol-type ginsenosides are metabolized by the gut microbiota and the metabolites are absorbed from the lower part of the intestine.
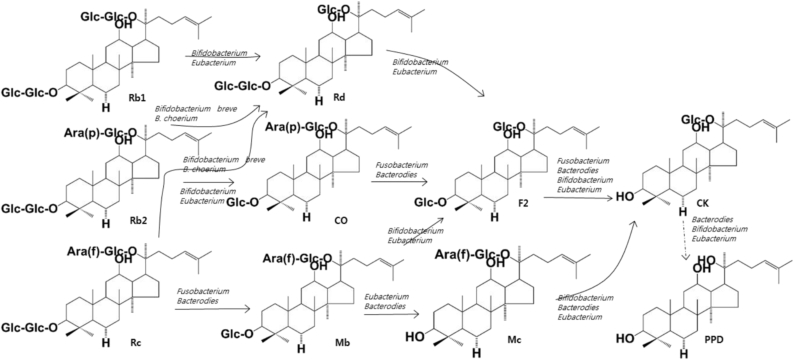
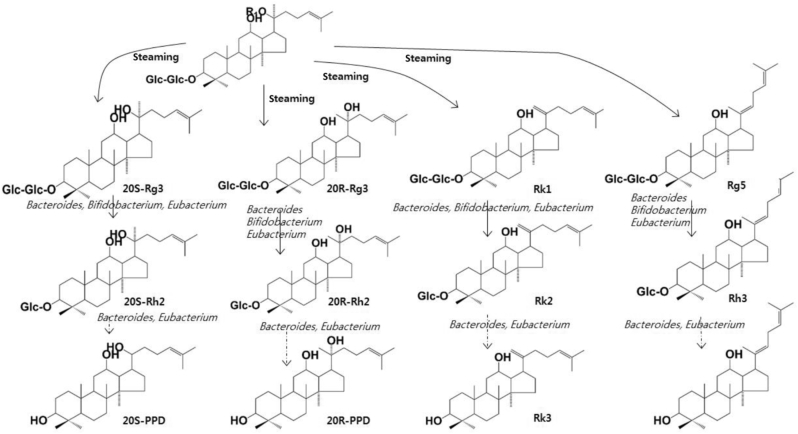
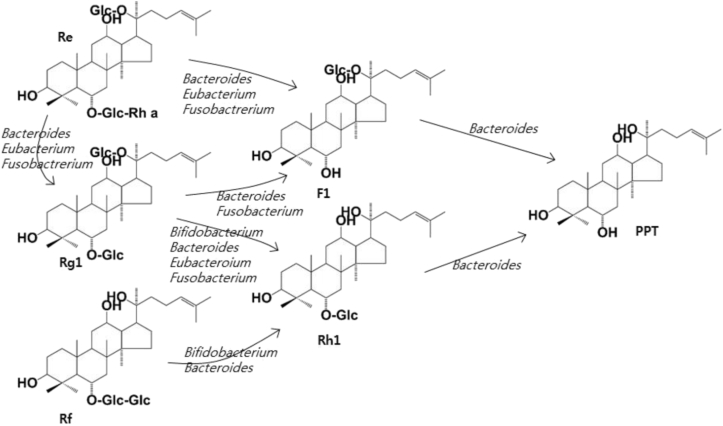
Of the gut microbiota, many bacteria catalyze the metabolic reactions of ginsenosides. Protopanaxadiol-type ginsenoside Rb1, Rb2, and Rc are transformed into the monoglucosylated ginsenoside compound K through the cleavage of the sugar moieties by gut microbiota, which produces β-glucosdiase, β-xylosidase, α-arabinofuranosidase, and/or α-arabinopyranosidase (Fig. 2). These enzymes are produced by Bifidobacterium H-1, Provotella oris, Fusobacterium K-60, Bacteroides JY-6, Eubacterium A-44, Bifidobacterium K-506, and Bifidobacterium K-110, and Fusobacterium K-60 [31], [50], [51], [57], [58], [59], [60], [61], [62]. These bacteria also transform protopanaxadiol-type ginsenoside Rg3 and Rg5 into ginsenosides Rh2 and Rh3 (Fig. 3) [58]. Protopanaxatriol ginsenosides Re and Rg1 are transformed to ginsenoside Rh1 or protopanaxatriol by the gut microbiota producing α-rhamnosidase and β-glucosidase. Fusobacterium K-60, Bacteroides JY-6, Eubacterium A-44, and Bacterodies HJ-15 produce these enzymes (Fig. 4) [57], [63].
Fig. 2.
Proposed metabolism of protopanaxadiol-type ginsenoside Rb1, Rb2, Rc, and Rd isolated from the root of dried ginseng by the gut microbiota. When fresh or dried ginsengs are orally administered in humans or animals, ginsenosides Rb1, Rb2, Rc, and Rd present in these ginsengs are metabolized to compound K or protopanaxadiol through ginsenoside F2 or Mc by gut microbiota.
Fig. 3.
Proposed metabolism of protopanaxadiol-type ginsenoside Rg3, Rg5, and Rk1 from the root of red ginseng by gut microbiota. Ginsenoside Rb1, Rb2, Rc, and Rd present in fresh and dried ginsengs are transformed into ginsenoside Rg3, Rg5, and Rk1 by heating. When the heat-processed ginsengs, such as red ginseng, are orally treated in humans or animals, the transformed compounds ginsenoside Rg3, Rg5, and Rk1 in these ginsengs are metabolized to ginsenoside Rh2, Rk2, and Rh3 or PPD by gut microbiota.
Fig. 4.
Proposed metabolism of protopanaxatriol ginsenoside Re, Rg1, and Rf from dried ginseng by gut microbiota. When fresh or dried ginsengs are orally administered in humans or animals, ginsenoside Re, Rg1, and Rf in these ginsengs are metabolized to protopanaxatriol, ginsenoside F1 or ginsenoside Rh1 by gut microbiota.
6. Metabolism of ginsenoside Ro, vina-majonoside R2, and majonoside R2 in gastrointestinal tract by gut microbiota
In the fecal suspension of humans and mice, ginsenoside Ro is transformed into oleanolic acid. Furthermore, when it was orally administered to mice and their metabolites were analyzed, oleanolic acid was detected in the small intestine, cecum, and colon.
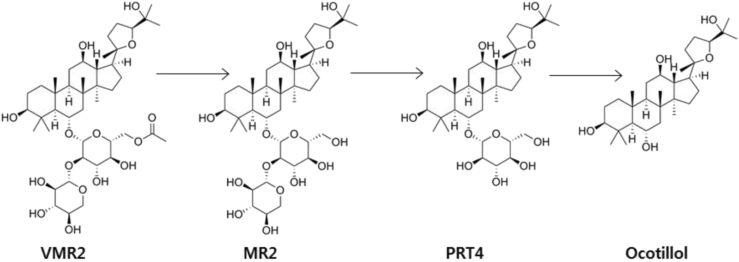
On incubation of VR2 and MR2 with the fecal suspension of humans and mice, they were rapidly transformed into PRT4 and ocotillol [64], [65]. The main metabolite in the fecal suspension was ocotillol, followed by PRT4. Therefore, it was proposed that VR2 and MR2 were metabolized to ocotillol in the intestine by the gut microbiota through PRT4 (Fig. 5). When MR2 was orally administered and its metabolites were analyzed in the intestinal fluid 12 h after the treatment, the dominant metabolite was found to be ocotillol, followed by PRT4.
Fig. 5.
Proposed metabolism of ocotillol-type vinamajonoside R2 and majonoside R2 from Vietnamese ginseng by gut microbiota. When fresh or dried Vietnamese ginseng (VG) is orally administered in humans or animals, vinamajonoside R2 (VMR2) and majonoside R2 (MR2) present in VG are metabolized to ocotillol through pseudoginsenoside RT4 (PRT4) by gut microbiota.
7. Metabolism of ginsenosides in liver
Little is known about the metabolism of ginsenosides in the liver. Wakabayashi et al. [66] reported that orally administered ginsenoside Rb1 was metabolized to compound K in the intestine by the gut microbiota, absorbed into the blood, and metabolized again to stearyl compound K in the liver. Thus, compound K ginsenoside conjugated with fatty acids, such as stearic acid, in the liver, similar to steroids [67]. Moreover, these ginsenosides were metabolized to their hydroxyl derivatives by liver microsomes. The liver microsomal and cytosolic fractions did not hydrolyze β-glucosyl hydrolytic reactions.
8. Pharmacological effects of ginseng constituents and their metabolites
Of Korean, North American, Chinese, and Vietnamese ginsengs, Panax ginseng is the most commonly used and studied; it enhances physical performance, promotes vitality, increases resistance to stress and aging, and possesses immunomodulatory activity [68], [69], [70], [71].
Many researchers have isolated ginsenosides as bioactive compounds from ginsengs. Dammarane oligoglycosides were found to be the major saponins; oleanane- and ocotillol-type were also identified [4], [42], [72]. Malonyl-ginsenoside Rb1, Rb2, Rc, and Rd, and ginsenoside Rb1, Rb2, and Rc belong to protopanaxadiol-type ginsenosides Re, Rf, Rg1, and Rg2 belong to protopanaxatriol-type; ginsenoside Ro belong to oleanane-type; and VR2 and MR2 belong to ocotillol-type.
Ginsenosides exhibit antidiabetic [73], [74], antitumor [66], [75], [76], antiallergic [77], [78], and anti-inflammatory activities [79], endothelium-independent aorta relaxation [80], and neuroprotective [81], [82], adjuvant-like [83], and immunomodulating effects [84]. However, these pharmacological effects of ginsenosides were observed to be different between in vitro and in vivo studies. Ginsenosides exhibited an antitumor effect in in vivo studies, but contradictively a negligible effect in in vitro studies [85], [86]. In a recent study, orally administered ginsenosides were metabolized to compound K, ginsenoside Rh2 and Rh1, and protopanaxatriol by the gut microbiota; compound K, ginsenoside Rh2, and protopanaxatriol exhibited potent cytotoxicity against tumor cells [45]. When antiallergic activity of the parent molecules and transformed metabolites were evaluated in vitro, ginsenoside Rh1 and Rh2, compound K, and protopanaxatriol were found to show potent inhibitory activity [77], [87], [88], [89]. These results suggest that the pharmacological effects of ginsengs may be dependent on the metabolism of ginsenosides to bioactive compounds by the gut microbiota.
9. Difference in gut microbiota-mediated ginsenoside-metabolizing enzyme activity between individuals
Gut microbiota transforms ginsenoside Rb1 to compound K in vitro and in vivo and the compound K is absorbed into the blood [48]. The metabolism of ginsenosides to compound K by intestinal bacteria was proportional to the amount of compound K absorbed into in the blood of volunteers who had been administered ginseng [49]. The compound K-forming activity is significantly different between individuals. Their AUCs are also significantly different. Nevertheless, there was a correlation between the compound K-forming activity and AUC for compound K. Cui et al. [90] determined the total amount of protopanaxatriol and protopanaxadiol in the urine of humans orally administered ginseng preparations. They detected approximately 1.2% of the orally treated dose of protopanaxatriol-type ginsenosides and < 0.2% of the orally treated dose of protopanaxadiol-type ginsenosides. However, Hasegawa et al. [67] reported that compound K mono-fatty acid esters such as stearyl compound K, accumulated in mouse liver following intravenous administration. These results suggest that the low absorption of ginsenoside metabolites is dependent on the metabolic activity of gut microbiota and due to the low transforming activity of ginsenosides into hydrophobic metabolites.
10. Fermented/biotransformed ginseng
When medicinal and functional herbs including ginsengs are orally administered to humans, their hydrophilic constituents come in contact with the gut microbiota in the alimentary tract and are transformed into absorbable hydrophobic ginsenosides before absorption from the gastrointestinal tract. All individuals possess characteristic indigenous strains of gut microbiota and the metabolizing activity was found to be significantly different between individuals (Fig. 6) [63], [91], [92], [93], [94], [95], [96], [97], [98], [99]. Thus, the metabolic activity of gut microbiota was affected by environmental factors such diet, drugs, and genetics. When the metabolism of ginsenosides Rb1 and Rb2 to active compound K was measured, a significant variation was observed between individuals. Therefore, bioactive and absorbable ginsenoside metabolites are valuable in the therapy of diverse diseases. Therefore, to develop bioactive and well-absorbable ginsenoside metabolites containing ginsengs, we developed the fermented ginsengs, which contained bioactive ginsenoside metabolites, such as compound K and ginsenoside Rh1. These metabolites were transformed from ginsenoside Rb1, Rb2, Rc, and Rd by probiotics [31], [100]. However, to use these probiotics, their safety and biotransforming activity should be affirmed. Once their safety and efficacy is confirmed, fermentation biotechnology can be valuable in developing novel ginseng preparations. Gut microbiota play an important role in the pharmacological action of ginseng. Therefore, beneficial and bioactive ginsengs can be developed using probiotic fermentation.
Fig. 6.
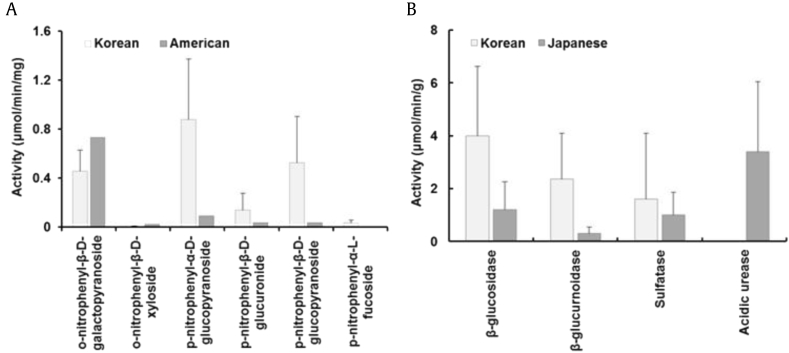
Difference in β-galactosidase, β-xylosidase, β-glucuronidase, β-glucosidase, and α-fucosidase activities in the fecal suspensions of Korean, American, and Japanese. (A) Difference between Korean and American. The metabolic activity of the Korean fecal suspension was measured according to the method described by Tamura et al. [98]. The data of American was obtained from the study conducted by Tamura et al. [98]. (B) Difference between Korean and Japanese. The metabolic activity of the Korean fecal suspension was measured according to the method described by Kobashi et al. [99]. The data of Japanese were obtained from the study conducted by Kobashi et al. [99].
11. Conclusion
Hydrophilic components of orally administered ginsengs, such as ginsenosides Rb1 and Rb2, are metabolized to hydrophobic compounds by gastric juice and gut microbiota: the metabolites of protopanxadiol-type ginsenosides are mainly compound K and ginsenoside Rh2 and protopanaxatriol-type ginsenosides are ginsenoside Rh1 and protopanaxatriol. The absorption of the metabolites, such as compound K, is proportional to fecal gut microbiota-metabolizing activity of ginsenosides to compound K. However, the metabolizing activity varies between individuals. Of these metabolites, absorbable and hydrophobic ones, such as compound K, exhibit potent pharmacological effects, such as antitumor, anti-inflammatory, antidiabetic, antiallergic, and neuroprotective effects, than parent ginsenosides, such as ginsenoside Rb1, Rb2, and Re. These findings suggest that the gut microbiota play an important role in the pharmacological action of orally administered ginseng. Furthermore, some probiotics isolated from human gut microbiota transform hydrophilic ginsenosides to hydrophobic and bioactive ginsenosides, such as compound K, and a novel probiotic fermentation technology has been developed to produce absorbable and bioactive metabolites instead of gut microbiota. These findings suggest that the gut microbiota play an important role in the pharmacological action of orally administered ginseng and probiotics that can replace gut microbiota can be used in the development of beneficial and bioactive ginsengs.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
This research was supported by a grant from Ministry of Food and Drug Safety in 2016 [16182MFD416].
References
- 1.Kim D.H. Chemical diversity of Panax ginseng, Panax quinquefolius, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikov M. The metabolism of drugs by the gut flora. Eur J Drug Metab Pharmacokinet. 1994;19:201–207. doi: 10.1007/BF03188922. [DOI] [PubMed] [Google Scholar]
- 3.Sousa T., Paterson R., Moore V., Carlsson A., Abrahamsson B., Basit A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363:1–25. doi: 10.1016/j.ijpharm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Kim D.H. The possible role of intestinal microflora in pharmacological activities of ginseng. Int Biomed Pharmaceut Sci. 2012;6:90–96. [Google Scholar]
- 5.Kobashi K., Akao T. Relation of intestinal bacteria to pharmacological effects of glycosides. Biosci Microflora. 1987;16:1–7. [Google Scholar]
- 6.Fanaro S., Chierici R., Guerrini P., Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez-Bello M.G., Costello E.K., Contreras M., Magris M., Hidalgo G., Fierer N., Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho I., Blaser M.J. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C.P., Li R.C. An introductory note to ginseng. Am J Chin Med. 1973;1:249–261. doi: 10.1142/s0192415x73000279. [DOI] [PubMed] [Google Scholar]
- 12.Banskota A.H., Tezuka Y., Le Tran Q., Kadota S. Chemical constituents and biological activities of Vietnamese medicinal plants. Curr Top Med Chem. 2003;3:227–248. doi: 10.2174/1568026033392516. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.F., Chiou W.F., Zhang J.T. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 14.Ng T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 15.Garriques S.S. On panaquilon, a new vegetable substance. Ann Chem Pharm. 1954;90:231–234. [Google Scholar]
- 16.Shibata S., Tanaka O., Nagai M., Ishii T. Studies on the constituents of Japanese and Chinese crude drugs. XII. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull. 1963;11:762–765. doi: 10.1248/cpb.11.762. [DOI] [PubMed] [Google Scholar]
- 17.Shibata S., Ando T., Tanaka O., Meguro Y., Sôma K., Iida Y. Saponins and sapogenins of Panax ginseng C.A. Meyer and some other Panax spp. Yakugaku Zasshi. 1965;85:753–755. [In Japanese] [PubMed] [Google Scholar]
- 18.Shibata S., Tanaka O., Soma K., Ando T., Iida Y., Nakamura H. Studies on saponins and sapogenis of ginseng. The structure of panaxatriol. Tetrahedron Lett. 1965;42:207–213. doi: 10.1016/s0040-4039(01)99595-4. [DOI] [PubMed] [Google Scholar]
- 19.Baek S.H., Bae O.N., Park J.H. Recent methodology in ginseng analysis. J Ginseng Res. 2012;36:119–134. doi: 10.5142/jgr.2012.36.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of Panax ginseng (C.A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 21.Shibata S., Fujita M., Itokawa H., Tanaka O. Studies on the constituents of Japanese and Chinese crude drugs. XI. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull (Tokyo) 1963;11:759–761. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa I., Taniyama T., Shibuya H., Noda T., Yoshikawa M. Chemical studies on crude drug processing. V. On the constituents of ginseng radix rubra (2): Comparison of the constituents of white ginseng and red ginseng prepared from the same Panax ginseng root. Yakugaku Zasshi. 1987;107:495–505. doi: 10.1248/yakushi1947.107.7_495. [In Japanese] [DOI] [PubMed] [Google Scholar]
- 23.Kitagawa I., Yoshikawa M., Yoshihara M., Hayashi T., Taniyama T. Chemical studies on crude drug precession. I. On the constituents of ginseng radix rubra (1) Yakugaku Zasshi. 1983;103:612–622. [PubMed] [Google Scholar]
- 24.Ruan C.C., Liu Z., Li X., Liu X., Wang L.J., Pan H.Y., Zheng Y.N., Sun G.Z., Zhang Y.S., Zhang L.X. Isolation and characterization of a new ginsenoside from the fresh root of Panax ginseng. Molecules. 2010;15:2319–2325. doi: 10.3390/molecules15042319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu G.Y., Li Y.W., Hau D.K., Jiang Z.H., Yu Z.L., Fong W.F. Protopanaxatriol-type ginsenosides from the root of Panax ginseng. Agric Food Chem. 2011;59:200–205. doi: 10.1021/jf1037932. [DOI] [PubMed] [Google Scholar]
- 26.Besso H., Kasai R., Saruwatari Y., Fuwa T., Tanaka O. Ginsenoside Ra1 and ginsenoside Ra2, new dammarane-saponins of ginseng roots. Chem Pharm Bull. 1982;30:2380–2385. [Google Scholar]
- 27.Kasai R., Besso H., Tanaka O., Saruwatari Y.I., Fuwa T. Saponins of red ginseng. Chem Pharm Bull. 1983;31:2120–2125. [Google Scholar]
- 28.Ryu J.H., Park T.H., Kim D.H., Sohn J.M., Kim H.M., Park J.H. A genuine dammarane glycoside, (20E)-ginsenoside F4 from Korean red ginseng. Arch Pharm Res. 1996;19:335–336. [Google Scholar]
- 29.Baek N.I., Kim D.S., Lee Y.H., Park J.D., Lee C.B., Kim S.I. Ginsenoside Rh4, a genuine dammarane glycoside from Korean Red Ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- 30.Anufriev V.P., Malinovskaya G.V., Denisenko V.A., Uvaròva N.I., Elyakov G.B., Kim S.I., Baek N.I. Synthesis of ginsenoside Rg3, a minor constituent of ginseng radix. Carbohydr Res. 1997;304:179–182. doi: 10.1016/s0008-6215(97)00217-6. [DOI] [PubMed] [Google Scholar]
- 31.Park J.D., Lee Y.H., Kim S.I. Ginsenoside Rf2, a new dammarane glycoside from Korean red ginseng (Panax ginseng) Arch Pharm Res. 1998;21:615–617. doi: 10.1007/BF02975384. [DOI] [PubMed] [Google Scholar]
- 32.Bae E.A., Han M.J., Kim E.J., Kim D.H. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res. 2004;27:61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- 33.Han B.H., Park M.H., Han Y.N., Woo L.K., Sankawa U., Yahara S., Tanaka O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982;44:146–149. doi: 10.1055/s-2007-971425. [DOI] [PubMed] [Google Scholar]
- 34.Kown S.W., Han S.B., Park I.H., Kim J.M., Park M.K., Park J.H. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J Chromatogr A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- 35.Taniyasu S., Tanaka O., Yang T.R., Zhou J. Dammarane saponins of flower buds of Panax notoginseng (sanchi-ginseng) Planta Med. 1982;44:124–125. doi: 10.1055/s-2007-971420. [DOI] [PubMed] [Google Scholar]
- 36.Yu H.S., Zhang L.J., Song X.B., Liu Y.X., Zhang J., Cao M., Kang L.P., Kang T.G., Ma B.P. Chemical constituents from processed rhizomes of Panax notoginseng. Zhongguo Zhong Yao Za Zhi. 2013;38:3910–3917. [In Chinese] [PubMed] [Google Scholar]
- 37.Zeng J., Cui X.M., Zhou J.M., Jiang Z.Y., Zhang X.M., Chen J.J. Studies on chemical constituents from rhizomes of Panax notoginseng. Zhong Yao Cai. 2007;30:1388–1391. [PubMed] [Google Scholar]
- 38.Zhou J., Wu M.Z., Taniyasu S., Besso H., Tanaka O., Saruwatari Y., Fuwa T. Dammarane-saponins of sanchi-ginseng, roots of Panax notoginseng (BURK.) F.H. CHEN (Araliaceae): structures of new saponins, notoginsenosides-R1 and -R2, and identification of ginsenosides-Rg2 and -Rh1. Chem Pharm Bull. 1981;29:2844–2850. [Google Scholar]
- 39.Zhao P., Liu Y.Q., Yang C.R. Minor dammarane saponins from Panax notoginseng. Phytochemistry. 1996;41:1419–1422. doi: 10.1016/0031-9422(95)00218-v. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen M.D., Nguyen T.N., Kasai R., Ito A., Yamasaki K., Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. I. Chem Pharm Bull (Tokyo) 1993;41:2010–2014. doi: 10.1248/cpb.41.2010. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen M.D., Kasai R., Ohtani K., Ito A., Nguyen T.N., Yamasaki K., Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis HA et Grushv. Collected in central Vietnam. II. Chem Pharm Bull. 1994;42:634–640. doi: 10.1248/cpb.42.634. [DOI] [PubMed] [Google Scholar]
- 42.Duc N.M., Kasai R., Ohtani K., Ito A., Nham N.T., Yamasaki K., Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. collected in central Vietnam. III. Chem Pharm Bull (Tokyo) 1994;42:115–122. doi: 10.1248/cpb.42.634. [DOI] [PubMed] [Google Scholar]
- 43.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Disp. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 44.Akao T., Kida H., Kanaoka M., Hattori M., Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 45.Akao T., Kanaoka M., Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration—measurement of compound K by enzyme immunoassay. Biol Pharm Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 46.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Kor Med Sci. 2001;16(Suppl.):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim K.A., Yoo H.H., Gu W., Yu D.H., Jin M.J., Choi H.L., Yuan K., Guerin-Deremaux L., Kim D.H. Effect of a soluble prebiotic fiber, NUTRIOSE, on the absorption of ginsenoside Rd in rats orally administered ginseng. J Ginseng Res. 2014;38:203–207. doi: 10.1016/j.jgr.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim K.A., Yoo H.H., Gu W., Yu D.H., Jin M.J., Choi H.L., Yuan K., Guerin-Deremaux L., Kim D.H. A prebiotic fiber increases the formation and subsequent absorption of compound K following oral administration of ginseng in rats. J Ginseng Res. 2015;39:183–187. doi: 10.1016/j.jgr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee J., Lee E., Kim D.H., Lee J., Yoo J., Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Hasegawa H., Sung J.H., Matsumiya S., Uchiyama M. Main ginseng metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–455. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- 51.Karikura M., Miyase T., Tanizawa H., Takino Y., Taniyama T., Hayashi T. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. V. The decomposition products of ginsenoside Rb2 in the large intestine of rats. Chem Pharm Bull. 1990;38:2859–2861. doi: 10.1248/cpb.38.2859. [DOI] [PubMed] [Google Scholar]
- 52.Odani T., Tanizawa H., Takino Y. Studies on the absorption, distribution, excretion and metabolism of ginseng saponins. II. The absorption, distribution and excretion of ginsenoside Rg1 in the rat. Chem Pharm Bull. 1983;31:292–298. doi: 10.1248/cpb.31.292. [DOI] [PubMed] [Google Scholar]
- 53.Strömbom J., Sandberg F., Dencker L. Studies on absorption and distribution of ginsenoside Rg1 by whole-body autoradiobiography and chromatography. Acta Pharmaceut Suecica. 1985;22:113–122. [PubMed] [Google Scholar]
- 54.Park E.K., Shin Y.W., Lee H.U., Kim S.S., Lee Y.C., Lee B.Y., Kim D.H. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 55.Kato H., Shimada F., Yano S., Kanaoka M. Abstract of papers, 11th Symposium of the Medical Society for Red Ginseng Research, Kobe, Japan. 1990. Determination of ginsenoside Rb1 in plasma of human after intake of red ginseng powder; p. 36. [abstract] [Google Scholar]
- 56.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J Nutri. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 57.Bae E.A., Park S.Y., Kim D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 58.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 59.Bae E.A., Choo M.K., Park E.K., Park S.Y., Shin H.Y., Kim D.H. Metabolism of ginsenoside Rc and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 60.Kim D.H. Metabolism of ginsenosides to bioactive compounds by intestinal microflora and its industrial application. J Ginseng Res. 2009;33:165–176. [Google Scholar]
- 61.Park S.Y., Bae E.A., Sung J.H., Lee S.K., Kim D.H. Purification and characterization of ginsenoside Rb1-metabolizing beta-glucosidase from Fusobacterium K-60, a human intestinal anaerobic bacterium. Biosci Biotechnol Biochem. 2001;65:1163–1169. doi: 10.1271/bbb.65.1163. [DOI] [PubMed] [Google Scholar]
- 62.Shin H.Y., Lee J.H., Lee J.Y., Han Y.O., Han M.J., Kim D.H. Purification and characterization of ginsenoside Ra-hydrolyzing beta-D-xylosidase from Bifidobacterium breve K-110, a human intestinal anaerobic bacterium. Biol Pharm Bull. 2003;26:1170–1173. doi: 10.1248/bpb.26.1170. [DOI] [PubMed] [Google Scholar]
- 63.Bae E.A., Shin J.E., Kim D.H. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol Pharm Bull. 2005;28:1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- 64.Jeong J.J., Van Le T.H., Lee S.Y., Eun S.H., Nguyen M.D., Park J.H., Kim D.H. Anti-inflammatory effects of vina-ginsenoside R2 and majonoside R2 isolated from Panax vietnamensis and their metabolites in lipopolysaccharide-stimulated macrophages. Int Immunopharmacol. 2015;28:700–706. doi: 10.1016/j.intimp.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 65.Lee S.Y., Jeong J.J., Le T.H., Eun S.H., Nguyen M.D., Park J.H., Kim D.H. Ocotillol, a majonoside R2 metabolite, ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by restoring the balance of Th17/Treg cells. J Agric Food Chem. 2015;63:7024–7031. doi: 10.1021/acs.jafc.5b02183. [DOI] [PubMed] [Google Scholar]
- 66.Wakabayashi C., Hasegawa H., Murata J., Saiki I. In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol Res. 1998;9:411–417. [PubMed] [Google Scholar]
- 67.Hasegawa H., Lee K.S., Nagaoka T., Tezuka Y., Uchiyama M., Kadota S., Saiki I. Pharmacokinetics of ginsenoside deglycosylated by intestinal bacteria and its transformation to biologically active fatty acid esters. Biol Pharm Bull. 2000;23:298–304. doi: 10.1248/bpb.23.298. [DOI] [PubMed] [Google Scholar]
- 68.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 69.Kennedy D.O., Scholey A.B. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/s0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 70.Scaglione F., Ferrara F., Dugnani S., Falchi M., Santoro G., Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drug Exp Clin Res. 1990;16:537–542. [PubMed] [Google Scholar]
- 71.Singh V.K., Agarwhal S.S., Gupta B.M. Immunomodulatory activity of Panax ginseng extract. Planta Med. 1984;50:462–465. doi: 10.1055/s-2007-969773. [DOI] [PubMed] [Google Scholar]
- 72.Matsuda H., Namba K., Fukuda S., Tani T., Kubo M. Pharmacological study on Panax ginseng C.A. Meyer. IV. Effects of red ginseng on experimental disseminated intravascular coagulation. (3). Effect of ginsenoside-Ro on the blood coagulative and fibrinolytic system. Chem Pharm Bull. 1986;34:2100–2104. doi: 10.1248/cpb.34.2100. [DOI] [PubMed] [Google Scholar]
- 73.Yokozawa T., Kobayashi T., Oura H., Kawashima Y. Studies on the mechanism of the hypoglycemic activity of ginsenoside-Rb2 in streptozotocin-diabetic rats. Chem Pharm Bull. 1985;33:869–872. doi: 10.1248/cpb.33.869. [DOI] [PubMed] [Google Scholar]
- 74.Xie J.T., Mehendale S.R., Li X., Quigg R., Wang X., Wang C.Z., Wu J.A., Aung H.H., Rue P.A., Bell G.I. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim Biophys Acta. 2005;1740:319–325. doi: 10.1016/j.bbadis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Chang Y.S., Seo E.K., Gyllenhaal C., Block K.I. Panax ginseng: a role in cancer therapy? Integr Cancer Therap. 2003;2:13–33. doi: 10.1177/1534735403251167. [DOI] [PubMed] [Google Scholar]
- 76.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 77.Choo M.K., Park E.K., Han M.J., Kim D.H. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522. doi: 10.1055/s-2003-40653. [DOI] [PubMed] [Google Scholar]
- 78.Park E.K., Choo M.K., Kim E.J., Han M.J., Kim D.H. Antiallergic activity of ginsenoside Rh2. Biol Pharm Bull. 2003;26:1581–1584. doi: 10.1248/bpb.26.1581. [DOI] [PubMed] [Google Scholar]
- 79.Park E.K., Choo M.K., Han M.J., Kim D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 80.Kim N.D., Kang S.Y., Kim M.J., Park J.H., Schini-Kerth V.B. The ginsenoside Rg3 evokes endothelium-independent relaxation in rat aortic rings: role of K+ channels. Eur J Pharmacol. 1999;367:51–57. doi: 10.1016/s0014-2999(98)00899-1. [DOI] [PubMed] [Google Scholar]
- 81.Park E.K., Choo M.K., Oh J.K., Ryu J.H., Kim D.H. Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol Pharm Bull. 2004;27:433–436. doi: 10.1248/bpb.27.433. [DOI] [PubMed] [Google Scholar]
- 82.Shieh P.C., Tsao C.W., Li J.S., Wu H.T., Wen Y.J., Kou D.H., Cheng J.T. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes. Neurosci Lett. 2008;434:1–5. doi: 10.1016/j.neulet.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 83.Su X., Pei Z., Hu S. Ginsenoside Re as an adjuvant to enhance the immune response to the inactivated rabies virus vaccine in mice. Int Immunopharmacol. 2014;20:283–289. doi: 10.1016/j.intimp.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 84.Lee E.J., Ko E., Lee J., Rho S., Ko S., Shin M.K., Min B.I., Hong M.C., Kim S.Y., Bae H. Ginsenoside Rg1 enhances CD4(+) T-cell activities and modulates Th1/Th2 differentiation. Int Immunopharmacol. 2004;4:235–244. doi: 10.1016/j.intimp.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Lee S.J., Ko W.G., Kim J.H., Sung J.H., Moon C.K., Lee B.H. Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochem Pharmacol. 2000;60:677–685. doi: 10.1016/s0006-2952(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 86.Tatsuka M., Maeda M., Ota T. Anticarcinogenic effect and enhancement of metastatic potential of BALB/c 3T3 cells by ginsenoside Rh(2) Jpn J Cancer Res. 2001;92:1184–1189. doi: 10.1111/j.1349-7006.2001.tb02138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shin Y.W., Kim D.H. Antipruritic effect of ginsenoside rb1 and compound k in scratching behavior mouse models. J Pharm Sci. 2005;99:83–88. doi: 10.1254/jphs.fp0050260. [DOI] [PubMed] [Google Scholar]
- 88.Shin Y.W., Bae E.A., Kim S.S., Lee Y.C., Kim D.H. Effect of ginsenoside Rb1 and compound K in chronic oxazolone-induced mouse dermatitis. Int Immunopharmacol. 2005;5:1183–1191. doi: 10.1016/j.intimp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 89.Choo M.K., Sakurai H., Kim D.H., Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappa B signaling in murine colon cancer cells. Oncol Rep. 2008;19:595–600. [PubMed] [Google Scholar]
- 90.Cui J.F., Björkhem I., Eneroth P. Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations. J Chromatogr B Biomed Sci Appl. 1997;689:349–355. doi: 10.1016/s0378-4347(96)00304-0. [DOI] [PubMed] [Google Scholar]
- 91.Bae E.A., Hyun Y.J., Choo M.K., Oh J.K., Ryu J.H., Kim D.H. Protective effect of fermented red ginseng on a transient focal ischemic rats. Arch Pharm Res. 2004;27:1136–1140. doi: 10.1007/BF02975119. [DOI] [PubMed] [Google Scholar]
- 92.Kim K.A., Jung I.H., Park S.H., Ahn Y.T., Huh C.S., Kim D.H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS One. 2013;8:e62409. doi: 10.1371/journal.pone.0062409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee D.S., Kim Y.S., Ko C.N., Cho K.H., Bae H.S., Lee K.S., Kim J.J., Park E.K., Kim D.H. Fecal metabolic activities of herbal components to bioactive compounds. Arch Pharm Res. 2002;25:165–169. doi: 10.1007/BF02976558. [DOI] [PubMed] [Google Scholar]
- 94.Yim J.S., Kim Y.S., Moon S.K., Cho K.H., Bae H.S., Kim J.J., Park E.K., Kim D.H. Metabolic activities of ginsenoside Rb1, baicalin, glycyrrhizin and geniposide to their bioactive compounds by human intestinal microflora. Biol Pharm Bull. 2004;27:1580–1583. doi: 10.1248/bpb.27.1580. [DOI] [PubMed] [Google Scholar]
- 95.Choi J.R., Hong S.W., Kim Y., Jang S.E., Kim N.J., Han M.J., Kim D.H. Metabolic activities of ginseng and its constituents, ginsenoside rb1 and rg1, by human intestinal microflora. J Ginseng Res. 2011;35:301–307. doi: 10.5142/jgr.2011.35.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim D.H. Herbal medicines are activated by intestinal microflora. Nat Prod Sci. 2002;8:35–43. [Google Scholar]
- 97.Kim D.H. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab Dispos. 2015;43:1581–1589. doi: 10.1124/dmd.115.063867. [DOI] [PubMed] [Google Scholar]
- 98.Tamura G., Gold C., Ferro-Luzzi A., Ames B.N. Fecalase: a model for activation of dietary glycosides to mutagens by intestinal flora. Proc Natl Acad Sci USA. 1980;77:4961–4965. doi: 10.1073/pnas.77.8.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobashi K., Nakata H., Takebe H., Terasawa K. Relation of intestinal microflora to Syo. Wakan-iyaku-kaishi. 1984;1:166–167. [Google Scholar]
- 100.Trinh H.T., Han S.J., Kim S.W., Lee Y.C., Kim D.H. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbiol Biotechnol. 2007;17:1127–1133. [PubMed] [Google Scholar]