Abstract
Background
Neutrophil to lymphocyte ratio (NLR) in peripheral blood is established to correlate with the morbidity and mortality of heart disease patients. We aimed to define the severity of inflammation (NLR) by observing the association of NLR with cardiac functions or myocardial damage parameters in patients with acute myocardial infarction.
Methods
Data from 715 patients who underwent percutaneous coronary intervention (PCI) within 72 hours of incidence in 2016 were analysed retrospectively.
Results
The NLR ranges from 0.50 to 46 (medium ± SD, 2.76 ± 2.96) in 715 patients. NLR positively correlated with myocardial damage (NLR vs. CK-mB: p < 0.0001) but negatively correlated with myocardial function (NLR vs. EF: p < 0.0001; NLR vs. FS: p < 0.0001). Myocardial damage markers (CK, CK-mB, ASL, LDH) were significantly increased, and cardiac contractile parameters (EF and FS) were reduced at NLR > 2.76 compared to those of NLR < 2.76. ELISA analysis has shown that IL-10 was significantly increased when NLR ≥ 4.6 and TGF-β was increased at NLR > 4. The correlation was diminished between NLR and CK-mB at NLR > 2.76 or at NLR > 4, but that of NLR and EF or FS was maintained in NLR > 2.76 and at NLR > 4. EF and FS were comparable between NLR > 2.76 and NLR > 4. But myocardial damage parameters increased significantly at NLR > 4 compared to those of NLR > 2.76.
Conclusion
NLR is a strong predictor of myocardial damage in acute myocardial patients. High NLR are associated with myocardial dysfunction in all the patients. Severe inflammation (NLR) can predict the consequence of the heart in patients with coronary syndrome.
Keywords: Neutrophil, Lymphocyte, Acute myocardial infarction, Myocardial damage, Dysfunction
1. Introduction
Cardiovascular diseases are the number one causes of mortality in humans worldwide, and coronary syndrome (myocardial infarction, MI) is one of the prevalent conditions those are responsible for fatal heart attack and heart failure.1 Impaired vascular perfusion in MI and reperfusion cause the damage of the myocardium, depending on the duration of ischaemia and metabolic demand of the tissue. As a consequence, systematic and local inflammation can be triggered, which is important in the remodelling and the scar formation of the myocardium.2, 3, 4
There are two main phases of inflammation during MI: the inflammatory phase and the proliferative phase. Neutrophils are the first leukocytes to be found in damaged area. Their activation produces large amounts of inflammatory mediators those regulate the response to tissue injury, demonstrating hypoxic damage, proteolytic enzymes and other mediators.4, 5, 6 At the infarct site, neutrophils release free radicals which act as an injury pathway for cardiomyocytes. The release of proteo-enzymes helps the clearance of the infarct and also amplifies immune cell recruitment (in particular M1 macrophage). As such, neutrophils are involved in not only inducing macrophage to the infarct site, but also allowing the clearance of debris.7, 8, 9, 10
In contrast, lymphocytes play vital roles in the remodelling of the myocardium following inflammation. For example, CD4+ T regulatory cells constitute a particular anti-inflammatory immune regulatory lymphocyte subset which is generated in the thymus and highly enriched for T cells with autoantigen specificity.11 T cells are essential for the recruitment of proangiogenic macrophages and collateral artery formation.11, 12, 13 B cells are involved in monocyte recruitment through the CCL7 pathway.2, 11, 12, 13, 14 The clearance of debris, activation of fibroblasts and collagen deposition for scar formation and neovascularisation (the proliferative phase) occur 3–4 days after MI.8, 14, 15 The release of inflammatory and anti-inflammatory mediators (IL-10, TGF-β and pro-resolving mediators, 8, 14, 16) from neutrophil or lymphocyte cells promotes neutrophil apoptosis and phagocytic uptake by macrophages.9, 10, 15 IL-10 secreted by T lymphocytes inhibits the production of inflammatory cytokines, stabilises the matrix and regulates ECM metabolism. Macrophages engulfing apoptotic neutrophils are a key activator of the anti-inflammatory response and potent inhibitor of pro-inflammatory cytokines.
Neutrophils are seen as a marker of ongoing inflammation and lymphocytes as a marker of regulatory pathways. Neutrophil-to-lymphocyte ratio (NLR) (calculated via dividing neutrophil count by lymphocyte count) as an indication of systemic inflammation has been demonstrated to be associated with poor clinical outcomes in various cardiovascular diseases, including acute coronary syndrome. Recent accumulating evidence points that high NLR to be independently and strongly associated with increased risk of complications and mortality post-acute MI.5, 17, 18, 19, 20, 21, 22, 23, 24 Here, our aim is to evaluate the level of NLR that is associated with myocardial dysfunction (EF and FS from echocardiography) or damage (CK-mB) in 715 myocardial infarction patients. NLR is readily available, so it may be used as a cost-effective adverse predictor.5, 21
2. Methods
2.1. Patients
Data from 1111 patients who underwent PCI 72 hours after the onset at Yanbian University Affiliated Hospital, Jilin province in China (from January 2015 to December 2016) were analysed retrospectively. We excluded 396 patients who had inflammatory diseases such as gastritis, chronic cholecystitis, nephritis, rhinitis, pharyngitis, bronchitis, myocarditis, rheumatoid arthritis, gout, immune system disorders and cancer in analysis group, and the count of cohort in the study was 715. Basic demographics, history, diagnosis at presentation, blood pressure, weight, complete blood count and echocardiogram results were obtained. The study was approved by the Ethical Committee of Yanbian University Hospital with the informed consent to the patients.
2.2. Statistical analysis
All analyses were performed using SPSS 23. The main parameters tested were: “NLR ratio” as the independent variables. The dependent variables were “EF”, “FS” and “CK-mB”. Pearson's correlation was performed, and correlation and p values were obtained to assess the strength of any association between variables. The descriptive statistics (on SPSS 23) was used for details on the overall data and the parameters were expressed as means ± SD. Firstly we analysed all the patients together. Next, we divided each of the groups into high NLR and low NLR. CK-mB in different NLR groups was expressed as means ± SE. The cut-off NLR value was 2.76, which was the median value of 715 patients. Student unpaired t-test (followed by Bonferroni correction) was used for testing the significance of the parameters between groups. Scatter plot graphs were used to demonstrate visually whether there are any relationships. p < 0.05 was considered significant.
2.3. Detection of inflammatory and anti-inflammatory cytokines from arterial blood samples
Blood samples were drawn from the coronary artery before PCI procedure. The IL-10 and TGF-β concentration in the plasma was measured by using an enzyme-linked immunosorbent assay (ELISA).
3. Results
A total of 715 patients with myocardial infarction who underwent PCI with stent within 72 hours of symptom onset were enrolled in our study. The mean age was 61.38 (±9.84) years, and 417 (58.32%) of the patients were men. The baseline clinical characteristics of myocardial function (e.g., EF, FS and SV) and myocardial damage (e.g., LDH, CK, CK-mB, AST) were shown in Table 1. The mean NLR of cohort was 2.76 ± 2.96.
Table 1.
Descriptive Statistical Analysis of Patients with Acute Myocardial Infarction
| Variable | Total |
|
|---|---|---|
| Mean (n = 715) | SD | |
| NLR | 2.7607 | 2.95855 |
| Age | 61.3776 | 9.83534 |
| BMI | 25.0484 | 4.10604 |
| WBC (4–10) × 109/L | 7.6625 | 2.64445 |
| HGB (110–160) g/L | 136.7636 | 16.75549 |
| PLT (100–300) × 109/L | 214.4517 | 55.79184 |
| NEU# (2–7.7) × 109/L | 4.7862 | 2.44535 |
| LYM# (0.8–4) × 109/L | 2.1317 | 0.81169 |
| MON# (0.12–0.8) × 109/L | 0.5759 | 0.25681 |
| EOS# (0–0.5) × 109/L | 0.1467 | 0.16398 |
| BAS# (0–0.1) × 109/L | 0.0235 | 0.03995 |
| LDH (115–220) IU/L | 248.9188 | 207.99504 |
| CK (25–196) IU/L | 283.3113 | 649.86892 |
| CK-MB (0–25) IU/L | 31.4671 | 57.60045 |
| AST (0–40) U/L | 43.0198 | 69.86126 |
| ALT (0–40) U/L | 40.5114 | 366.73486 |
| CREA (75–115) μmol/L | 74.1738 | 23.83924 |
| EF (57–75) % | 59.5273 | 6.34161 |
| FS (20–40) % | 29.5972 | 5.08086 |
| SV (60–120) mL | 71.0386 | 8.86597 |
| CO (3.5–8.0) L/min | 4.9013 | 0.70446 |
| E/A | 1.0119 | 0.41200 |
| DT ms | 210.1832 | 46.03374 |
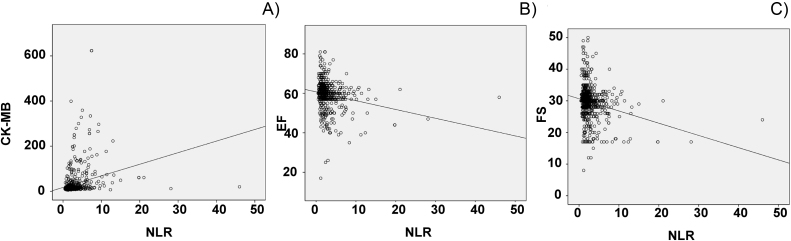
In all the patient groups, there was a positive linear regression of NLR versus CK-mB (r = 0.264, p < 0.0001), negative linear regression of NLR versus EF (r = −0.208, p < 0.0001) or FS (r = −0.225, p < 0.0001) (Table 2). The results suggest that the associations of NLR with these parameters were statistically significant (Fig. 1A–C).
Table 2.
Correlation Between N/L Ratio and Laboratory Characteristics
| Variable | Total |
|
|---|---|---|
| r | p | |
| Age | 0.050 | 0.184 |
| BMI | 0.214 | 0.000 |
| WBC (4–10) × 109/L | 0.479 | 0.000 |
| HGB (110–160) g/L | 0.083 | 0.027 |
| PLT (100–300) × 109/L | −0.031 | 0.410 |
| NEU# (2–7.7) × 109/L | 0.670 | 0.000 |
| LYM# (0.8–4) × 109/L | −0.458 | 0.000 |
| MON# (0.12–0.8) × 109/L | 0.133 | 0.000 |
| EOS# (0–0.5) × 109/L | −0.198 | 0.000 |
| BAS# (0–0.1) × 109/L | −0.008 | 0.836 |
| LDH (115–220) IU/L | 0.312 | 0.000 |
| CK (25–196) IU/L | 0.285 | 0.000 |
| CK-MB (0–25) IU/L | 0.264 | 0.000 |
| AST (0–40) U/L | 0.269 | 0.000 |
| ALT (0–40) U/L | 0.010 | 0.785 |
| CREA (75–115) μmol/L | 0.126 | 0.001 |
| EF (57–75) % | −0.208 | 0.000 |
| FS (20–40) % | −0.225 | 0.000 |
| SV (60–120) mL | −0.086 | 0.021 |
| CO (3.5–8.0) L/min | −0.034 | 0.362 |
| E/A | 0.030 | 0.427 |
| DT ms | −0.015 | 0.679 |
Fig. 1.
Correlation of neutrophil-to-lymphocyte ratio (NLR) with CK-mB (A), EF (B) and FS (C). Pearson rank correlation was used to evaluate the relation between NLR and other parameters.
The patients were divided into low NLR (<2.76, n = 522) and high NLR (>2.76, n = 193) groups. The parameters of cardiac functions and myocardial damage of respective groups were presented in Table 3. EF and FS were not significantly different between NLR low and high groups. LDH, CK, CK-mB and AST, however, were significantly higher in NLR > 2.76 group compared to those of NLR < 2.76 (Table 3). Furthermore, we have noticed that ALT and creatinine were also significantly increased in NLR > 2.76 group (Table 3).
Table 3.
Descriptive Statistical Analysis of Low NLR and High NLR
| Variable | NLR < 2.76, n = 522 |
NLR > 2.76, n = 193 |
p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| NLR | 1.6911 | 0.53428 | 5.6535 | 4.50038 | |
| Age | 61.5000 | 9.46237 | 61.0466 | 10.79896 | 0.607 |
| BMI | 24.9787 | 3.83970 | 25.2368 | 4.75827 | 0.456 |
| WBC (4–10) × 109/L | 6.9209 | 2.02447 | 9.6683 | 3.05693 | 0.000 |
| HGB (110–160) g/L | 136.2395 | 15.97581 | 138.1813 | 18.67327 | 0.169 |
| PLT (100–300) × 109/L | 215.2701 | 54.98789 | 212.2383 | 57.99888 | 0.519 |
| NEU# (2–7.7) × 109/L | 3.8253 | 1.35848 | 7.3851 | 2.81596 | 0.000 |
| LYM# (0.8–4) × 109/L | 2.3639 | 0.78704 | 1.5036 | 0.47554 | 0.000 |
| MON# (0.12–0.8) × 109/L | 0.5388 | 0.20971 | 0.6764 | 0.33470 | 0.000 |
| EOS# (0–0.5) × 109/L | 0.1676 | 0.16565 | 0.0902 | 0.14539 | 0.000 |
| BAS# (0–0.1) × 109/L | 0.0231 | 0.01557 | 0.0245 | 0.07260 | 0.764 |
| LDH (115–220) IU/L | 201.4568 | 110.21854 | 377.0415 | 324.25343 | 0.000 |
| CK (25–196) IU/L | 144.8393 | 309.54999 | 657.1140 | 1056.56799 | 0.000 |
| CK-MB (0–25) IU/L | 19.7414 | 27.09584 | 63.1813 | 94.66274 | 0.000 |
| AST (0–40) U/L | 28.1667 | 33.79825 | 83.3579 | 113.37827 | 0.000 |
| ALT (0–40) U/L | 24.4588 | 24.26591 | 83.6000 | 702.33071 | 0.247 |
| CREA (75–115) μmol/L | 72.3255 | 19.59934 | 79.1905 | 32.22256 | 0.006 |
| EF (57–75) % | 60.3851 | 5.73898 | 57.2073 | 7.26425 | 0.000 |
| FS (20–40) % | 30.1877 | 4.84493 | 28.0000 | 5.36579 | 0.000 |
| SV (60–120) mL | 71.4253 | 8.58150 | 69.9927 | 9.53818 | 0.055 |
| CO (3.5–8.0) L/min | 4.9060 | 0.67550 | 4.8886 | 0.77914 | 0.784 |
| E/A | 0.9816 | 0.35007 | 1.0937 | 0.53802 | 0.008 |
| DT ms | 213.0460 | 44.45652 | 202.4404 | 49.34454 | 0.006 |
In NLR < 2.76 group, NLR positively correlates with CK-mB (r = 0.143, p = 0.001) and negatively associates with EF (r = −0.131, p = 0.003) or with FS (r = −0.102, p = 0.020). Therefore, the associations between NLR and myocardial function were preserved in this patients group (Table 4).
Table 4.
Correlation Between N/L Ratio and Laboratory Characteristics
| Variable | NLR < 2.76, n = 522 |
NLR > 2.76, n = 193 |
||
|---|---|---|---|---|
| r | p | r | p | |
| Age | −0.002 | 0.967 | 0.138 | 0.055 |
| BMI | −0.057 | 0.193 | 0.431 | 0.000 |
| WBC (4–10) × 109/L | 0.252 | 0.000 | 0.379 | 0.000 |
| HGB (110–160) g/L | 0.130 | 0.003 | 0.079 | 0.277 |
| PLT (100–300) × 109/L | −0.001 | 0.989 | −0.039 | 0.594 |
| NEU# (2–7.7) × 109/L | 0.586 | 0.000 | 0.515 | 0.000 |
| LYM# (0.8–4) × 109/L | 0.410 | 0.000 | −0.524 | 0.000 |
| MON# (0.12–0.8) × 109/L | 0.170 | 0.000 | −0.051 | 0.428 |
| EOS# (0–0.5) × 109/L | 0.002 | 0.966 | −0.204 | 0.005 |
| BAS# (0–0.1) × 109/L | 0.000 | 0.994 | −0.024 | 0.743 |
| LDH (115–220) IU/L | 0.221 | 0.000 | 0.115 | 0.110 |
| CK (25–196) IU/L | 0.113 | 0.010 | 0.105 | 0.148 |
| CK-MB (0–25) IU/L | 0.143 | 0.001 | 0.083 | 0.253 |
| AST (0–40) U/L | 0.082 | 0.064 | 0.082 | 0.258 |
| ALT (0–40) U/L | 0.003 | 0.938 | −0.041 | 0.572 |
| CREA (75–115) μmol/L | 0.217 | 0.000 | 0.048 | 0.513 |
| EF (57–75) % | −0.131 | 0.003 | −0.128 | 0.076 |
| FS (20–40) % | −0.102 | 0.020 | −0.228 | 0.001 |
| SV (60–120) mL | 0.004 | 0.930 | −0.101 | 0.164 |
| CO (3.5–8.0) L/min | −0.007 | 0.869 | −0.059 | 0.414 |
| E/A | −0.068 | 0.120 | −0.065 | 0.372 |
| DT ms | 0.047 | 0.282 | 0.090 | 0.214 |
In NLR > 2.76 group, the association between NLR and CK-mB was absent (r = 0.083, p = 0.253). The correlation between NLR and EF was reduced (NLR vs. EF: r = −0.128, p = 0.07) but still significant between NLR and FS (NLR vs. FS: r = −0.228, p = 0.001) (Table 4).
It is possible that high NLR (i.e., greater systemic inflammation) and greater damage of myocardium triggered the remodelling and recovering process, which obscures the adverse effects on myocardial function. Anti-inflammatory cytokines (e.g., IL-10 or TGF-β) are known to be induced following inflammation after myocardial infarction, which plays fundamental roles in the proliferative process following MI.
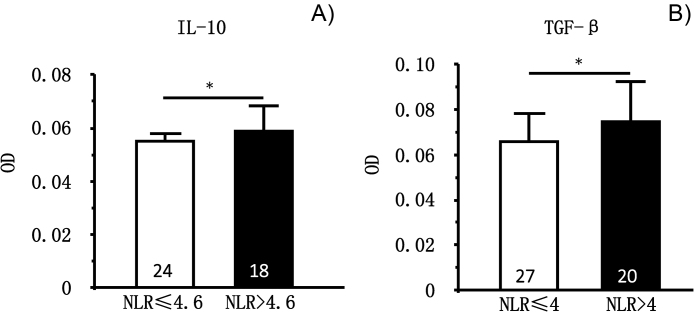
Therefore, we detected IL-10 and TGF-β in the coronary arterial blood samples of the patients and compared between NLR > 2.76 and NLR < 2.76 groups. Results show that IL-10 or TGF-β were not different between NLR > 2.76 and NLR < 2.76 (data not shown). Instead, IL-10 became significantly higher when NLR > 4.6 (p = 0.05, n = 24 vs. n = 18). Similarly, TGF-β became greater when NLR > 4 (p = 0.05, n = 27, n = 20) (Fig. 2).
Fig. 2.
Plasma level of IL-10 and TGF-β in ACS patients at two levels of NLR. (A) IL-10 was significantly increased when NLR > 4.6 (p = 0.05). (B) TGF-b was significantly increased when NLR was >4 (p = 0.05).
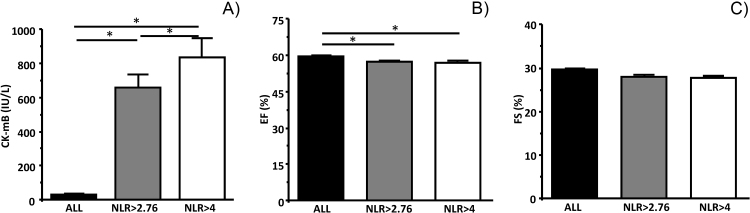
When the patients were divided into NLR > 4 and NLR < 4 groups, the parameters of myocardial damage (LDH, CK, CK-mB, AST) were increased even further, and the concentrations were significantly greater than that of NLR > 2.76 (CK-mB; some examples were shown in Fig. 3 and Table 5). EF and FS were comparable between NLR > 2.76 and NLR > 4 groups (Fig. 3 and Table 5), but were significantly greater than all patients’ data. In NLR > 4 group, the association between NLR and CK-mB was absent (r = 0.024, p = 0.79, Table 6). However, NLR negatively associates with EF (r = −0.19, p = 0.045) or with FS (r = −0.3, p = 0.001) in NLR > 4 group. Therefore, the association between NLR and myocardial function was preserved in all patients’ group (Table 6).
Fig. 3.
Comparison of CK-mB, EF and FS in NLR > 2.76 and NLR > 4 to all patients's data. (A) CK-mB in all patients, NLR > 2.76 and NLR > 4. CK-mB was significantly increased in NLR > 2.76 and in NLR > 4 groups compare to all patients group (p < 0.001, p < 0.001, n = 193, n = 112 and n = 715, respectively). CK-mB was greater in NLR > 4 compare to that in NLR > 2.76 (p < 0.003). (B) EF was significantly lower in NLR > 2.76 and NLR > 4 compare to all patients group (p < 0.001 and p = 0.02). EF was not different between NLR > 2.76 and NLR > 4 (p = 0.6). (C) FS was not different among groups (p = 0.4, p = 0.5 and p = 0.2, respectively).
Table 5.
Descriptive Statistical Analysis of Low NLR and High NLR
| Variable | NLR < = 4, n = 603 |
NLR > 4, n = 112 |
p | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| NLR | 1.9094 | 0.75345 | 7.3629 | 5.28372 | |
| Age | 61.3858 | 9.62852 | 61.3304 | 10.89044 | 0.956 |
| BMI | 25.0056 | 3.86506 | 25.2518 | 5.22747 | 0.568 |
| WBC (4–10) × 109/L | 7.0645 | 2.05207 | 10.8734 | 3.12734 | 0.000 |
| HGB (110–160) g/L | 136.3775 | 15.93488 | 138.6250 | 20.68081 | 0.286 |
| PLT (100–300) × 109/L | 214.3808 | 55.50209 | 214.7946 | 57.34064 | 0.944 |
| NEU# (2–7.7) × 109/L | 4.0560 | 1.48568 | 8.7240 | 2.81531 | 0.000 |
| LYM# (0.8–4) × 109/L | 2.2715 | 0.78454 | 1.3692 | 0.45749 | 0.000 |
| MON# (0.12–0.8) × 109/L | 0.5514 | 0.22043 | 0.7041 | 0.37699 | 0.000 |
| EOS# (0–0.5) × 109/L | 0.1633 | 0.16687 | 0.0738 | 0.12196 | 0.000 |
| BAS# (0–0.1) × 109/L | 0.0231 | 0.01515 | 0.0286 | 0.09627 | 0.556 |
| LDH (115–220) IU/L | 214.2421 | 129.20217 | 434.9732 | 382.02303 | 0.000 |
| CK (25–196) IU/L | 181.5411 | 431.73023 | 830.3839 | 1159.20538 | 0.000 |
| CK-MB (0–25) IU/L | 22.4702 | 34.28610 | 79.9375 | 110.20368 | 0.000 |
| AST (0–40) U/L | 32.4740 | 45.06857 | 100.0818 | 128.72124 | 0.000 |
| ALT (0–40) U/L | 41.5939 | 399.00941 | 34.4091 | 26.19766 | 0.849 |
| CREA (75–115) μmol/L | 72.8111 | 21.74906 | 81.1727 | 32.24387 | 0.011 |
| EF (57–75) % | 59.9719 | 6.17309 | 57.1339 | 6.69475 | 0.000 |
| FS (20–40) % | 29.9487 | 5.00040 | 27.6875 | 5.08846 | 0.000 |
| SV (60–120) mL | 71.1781 | 9.00913 | 70.2589 | 8.00422 | 0.311 |
| CO (3.5–8.0) L/min | 4.8972 | 0.68790 | 4.9205 | 0.78856 | 0.753 |
| E/A | 0.9981 | 0.38590 | 1.0972 | 0.53695 | 0.059 |
| DT ms | 211.4354 | 45.33791 | 203.6964 | 49.20885 | 0.104 |
Table 6.
Correlation Between N/L Ratio and Laboratory Characteristics
| Variable | NLR < 4, n = 602 |
NLR > 4, n = 113 |
||
|---|---|---|---|---|
| r | p | r | p | |
| Age | −0.015 | 0.712 | 0.177 | 0.063 |
| BMI | −0.011 | 0.789 | 0.569 | 0.000 |
| WBC (4–10) × 109/L | 0.287 | 0.000 | 0.242 | 0.010 |
| HGB (110–160) g/L | 0.087 | 0.032 | 0.091 | 0.338 |
| PLT (100–300) × 109/L | −0.026 | 0.542 | −0.095 | 0.319 |
| NEU# (2–7.7) × 109/L | 0.629 | 0.000 | 0.384 | 0.000 |
| LYM# (0.8–4) × 109/L | −0.476 | 0.000 | −0.567 | 0.000 |
| MON# (0.12–0.8) × 109/L | 0.201 | 0.000 | −0.124 | 0.194 |
| EOS# (0–0.5) × 109/L | −0.059 | 0.149 | −0.122 | 0.256 |
| BAS# (0–0.1) × 109/L | −0.060 | 0.144 | −0.055 | 0.570 |
| LDH (115–220) IU/L | 0.297 | 0.000 | 0.026 | 0.783 |
| CK (25–196) IU/L | 0.205 | 0.000 | 0.024 | 0.799 |
| CK-MB (0–25) IU/L | 0.200 | 0.000 | −0.010 | 0.916 |
| AST (0–40) U/L | 0.215 | 0.000 | 0.005 | 0.957 |
| ALT (0–40) U/L | 0.065 | 0.116 | 0.000 | 0.996 |
| CREA (75–115) μmol/L | 0.166 | 0.000 | 0.019 | 0.843 |
| EF (57–75) % | −0.207 | 0.000 | −0.190 | 0.045 |
| FS (20–40) % | −0.158 | 0.000 | −0.300 | 0.001 |
| SV (60–120) mL | −0.054 | 0.188 | −0.198 | 0.036 |
| CO (3.5–8.0) L/min | −0.033 | 0.412 | −0.114 | 0.231 |
| E/A | 0.041 | 0.315 | −0.098 | 0.305 |
| DT ms | −0.047 | 0.253 | 0.121 | 0.206 |
These results confirm that high NLR is strongly associated with the extent of myocardial damage. Inflammatory and anti-inflammatory cytokines are significantly increased when NLR is larger than 4. The correlation between myocardial function and NLR was preserved in all patient groups.
4. Discussion
NLR is an established biomarker for systemic inflammation. Accumulating evidence has demonstrated the strong link between high NLR and increased morbidity and mortality in a wide range of cardiovascular diseases, including acute coronary syndrome.25, 26, 27, 28, 29, 30 In this study, we have analysed the data retrospectively from 715 patients who underwent PCI 72 hours from onset. Our results showed that NLR was strongly associated with myocardial damage (CK-mB) and negatively associated with cardiac contractility (EF and FS). High NLR (either >2.76 or >4) strongly predicts the severity of myocardial damage. The correlations between NLR and myocardial contraction (EF or FS), however, were maintained, suggesting that the extent of inflammation is associated with contractile dysfunction of the heart. We also found that anti-inflammatory cytokines (IL-10 and TGF-β) were significantly increased in high NLR group >4.6 or >4, respectively. It is possible that the anti-inflammatory mechanisms may have prevented the functional deterioration of the myocardium.
Our results are in agreement with previous studies suggesting that elevated leucocyte counts do associate with worse clinical indexes in patients with acute coronary syndromes (ACS).16 Dogan et al showed that increased neutrophil count positively correlates with increased myocardial damage, evidenced by CK-mB, cardiac troponin and scintigraphic infarct size.31 Furthermore, previous studies have shown both neutrophilia and lymphocytopenia to be independently and strongly associated with an increased risk of complications and mortality post-AMI.5, 6, 17, 18, 19, 20, 21, 22, 23, 24 Similar to our study, Horne et al investigated patients with coronary artery disease (CAD) (without MI) to check whether inflammatory markers can help to identify the risk of death/MI. The study followed up over 3000 patients on a long-term basis. Cox regression was used to assess the predictive ability of the WBC, neutrophil, lymphocyte and monocyte counts and NLR. Their study showed that patients in the top quartile had a 2.2-fold increased risk compared to that of the lower quartile.24
There have been many studies on the predictive value of NLR for adverse outcome and mortality post-MI. Higher NLR values seem to be more predictive.18, 29, 32, 33, 21, 23, 34 Shen et al investigated the association of NLR with long-term mortality after STEMI treated with primary PCI. The study tested the hypothesis that in the acute phase of STEMI, whether NLR could be used to predict long-term prognosis. Their results indicate that patients in the highest NLR quartile were 4 times more likely to die during hospitalisation and during long-term follow-up.35 Arbel et al explored whether NLR provides additive prognostic value in STEMI.29 Patients undergoing STEMI were put into two groups (high/low NLR), and logistic regression was used to assess in-hospital complications and LVEF based on NLR. The results showed that higher NLR values (>6.5) were associated with lower EF and increased 30 days and 5-year mortality.29 Oncel et al studied the relationship of NLR with GRACE risk score to in-hospital cardiac events in patients with STEMI.33 The results showed that GRACE risk score was over 100 when NLR > 2.65 and over 140 when NLR > 6.48 at admission. Therefore, NLR was significantly associated with adverse in-hospital effects, indicating that NLR at this level can be used as a predictor of negative cardiac outcomes.33 The mean NLR in 715 patients enrolled in our study was 2.76, and myocardial damage (CK-mB) was greater in NLR high group (p < 0.0001). The strong association between NLR and CK-mB suggests that the level of inflammation is predictable of the extent of myocardial damage, which may account for the adverse outcome of myocardial functions. At NLR > 2.76, inflammation affected not only heart, but kidney or liver functions also, since ALT and creatinine were also increased significantly. These results indicate that patients with NLR > 2.76 should raise alarm for multiple organ damage. Large cohort from wider range of communities is necessary to set the level of NLR for clinical indications.
Given that NLR is a strong and independent predictor of mortality in patients with coronary syndrome, incorporating echocardiographic measures should aid in the management of the patients under risk. The changes in the systolic and diastolic function and their correlation to NLR may improve the prognostic value of clinical assessment of the risk, which will be important in providing a better understanding of the patients. Our results indicate that NLR is associated with adverse LV function in coronary syndrome patients. When the group of patients were divided into high and low NLR (i.e., larger or lower than medium NLR > 2.76 or <2.76), EF and FS were indeed reduced in high NLR group. In addition, the correlation between NLR and myocardial contraction was maintained throughout NLR group. These results demonstrate that inflammation is associated with myocardial dysfunction.
It is possible that pro-inflammatory cytokines exert negative inotropic effects in cardiac tissue.36, 37 High cytokines levels, such as IL-1, are associated with ventricular diastolic diameter increase and collagen deposition in the infarcted area after several weeks of MI, and as such, IL-1 induces abnormalities in cardiac metabolism and promotes myocardial remodelling leading to heart failure.36, 38, 39 Therefore, the pro-inflammatory cytokines (TNF-α levels, IL-6, etc.) at high NLR may play crucial roles in the development of heart failure. In contrast, anti-inflammatory cytokines are involved in the reparatory process following myocardial infarction. Our results showed that IL-10 and TGF-β were increased in NLR high group (>4.6 or >4), indicating that cytokine production could be the consequence of the level of inflammation following infarction. Recent evidence has shown that IL-10 infusion at day-7 post-MI attenuated inflammation and significantly decreased LV dilation and improved ejection fraction (both p < 0.05) following myocardial infarction.40 Another inflammatory cytokine, TGF-β, is markedly induced and rapidly activated in the infarcted myocardium. Experimental studies suggest that TGF-β signalling may be crucial for repression of inflammatory gene synthesis in healing infarcts mediating resolution of the inflammatory infiltrate. In addition, TGF-β may promote extracellular matrix deposition in the infarct by upregulating collagen and fibronectin synthesis.41, 42 The changes of inflammatory cytokines and their impacts on myocardium following MI and the downstream signalling pathways are needed to study in more detail in order to design safe and effective therapeutic strategies.
There are increased numbers of patients admitted to hospital with cardiovascular diseases each year. It is vital for early diagnosis to prevent deterioration of heart function. The study helps to define inflammation as an important risk factor; NLR level > 2.76 can point to myocardial damage and reduced heart function in these patients. A delayed diagnosis can result in worse prognosis, so an early assessment with easily available NLR may be vital. In our study, we confirm that NLR can be used as a predictor for myocardial damage and cardiac dysfunction. High level of NLR (e.g. >4) is linked with elevated anti-inflammatory cytokines. Comprehensive studies need to be undertaken for better understandings of the inflammatory profiles and their influences during the process of pathology following MI.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Yanbian University Hospital, Yanbian University Cardiovascular Translational Innovation Centre for the resources and funding for the research.
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart disease and stroke statistics – 2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epelman S., Liu P.P., Mann D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol. 2015;15:117–129. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruparelia N., Chai J.T., Fisher E.A., Choudhury R.P. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017;14:133–144. doi: 10.1038/nrcardio.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong S.B., Hernández-Reséndiz S., Crespo-Avilan G.E., Mukhametshina R.T., Kwek X.Y., Cabrera-Fuentes H.A. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018 Jan 9 doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karakas M.S., Korucuk N., Tosun V., Altekin R.E., Koc F., Ozbek S.C. Red cell distribution width and neutrophil-to-lymphocyte ratio predict left ventricular dysfunction in acute anterior ST-segment elevation myocardial infarction. J Saudi Heart Assoc. 2016;28:152–158. doi: 10.1016/j.jsha.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams B.A., Merhige M.E. Association between neutrophil–lymphocyte ratio and impaired myocardial perfusion in patients with known or suspected coronary disease. Heart Lung: J Crit Care. 2013;42:436–441. doi: 10.1016/j.hrtlng.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Frodermann V., Nahrendorf M. Neutrophil-macrophage cross-talk in acute myocardial infarction. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangogiannis N.G. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 10.Horckmans M., Ring L., Duchene J., Santovito D., Schloss M.J., Drechsler M. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187–197. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson E., Savvatis K., Mohiddin S.A., Marelli-Berg F.M. T-cell immunity in myocardial inflammation: pathogenic role and therapeutic manipulation. Br J Pharmacol. 2017;174:3914–3925. doi: 10.1111/bph.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan X., Anzai A., Katsumata Y., Matsuhashi T., Ito K., Endo J. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Tang T.T., Yuan J., Zhu Z.F., Zhang W.C., Xiao H., Xia N. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res Cardiol. 2012;107:232. doi: 10.1007/s00395-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 14.Prabhu S.D., Frangogiannis N.G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zlatanova I., Pinto C., Silvestre J.S. Immune modulation of cardiac repair and regeneration: the art of mending broken hearts. Front Cardiovasc Med. 2016;3:40. doi: 10.3389/fcvm.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erkol A., Oduncu V., Turan B., Kilicgedik A., Karabay C.Y., Akgun T. Neutrophil to lymphocyte ratio in acute ST-segment elevation myocardial infarction. Am J Med Sci. 2014;348:37–42. doi: 10.1097/MAJ.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 18.Arruda-Olson A.M., Reeder G.S., Bell M.R., Weston S.A., Roger V.L. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2:656–662. doi: 10.1161/CIRCOUTCOMES.108.831024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudiger A., Burckhardt O.A., Harpes P., Muller S.A., Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24:451–454. doi: 10.1016/j.ajem.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Ertem A.G., Ozcelik F., Kasapkara H.A., Koseoglu C., Bastug S., Ayhan H. Neutrophil lymphocyte ratio as a predictor of left ventricular apical thrombus in patients with myocardial infarction. Korean Circ J. 2016;46:768–773. doi: 10.4070/kcj.2016.46.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gul U., Kayani A.M., Munir R., Hussain S. Neutrophil lymphocyte ratio: aprognostic marker in acute st elevation myocardial infarction. J Coll Phys Surg Pakistan. 2017;27:4–7. [PubMed] [Google Scholar]
- 22.Ghaffari S., Nadiri M., Pourafkari L., Sepehrvand N., Movasagpoor A., Rahmatvand N. The predictive value of total neutrophil count and neutrophil/lymphocyte ratio in predicting in-hospital mortality and complications after STEMI. J Cardiovasc Thorac Res. 2014;6:35–41. doi: 10.5681/jcvtr.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazi E., Bayram B., Gazi S., Temiz A., Kirilmaz B., Altun B. Prognostic value of the neutrophil–lymphocyte ratio in patients with ST-elevated acute myocardial infarction. Clin Appl Thromb Hemost. 2015;21:155–159. doi: 10.1177/1076029613492011. [DOI] [PubMed] [Google Scholar]
- 24.Horne B.D., Anderson J.L., John J.M., Weaver A., Bair T.L., Jensen K.R. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 25.Azab B., Zaher M., Weiserbs K.F., Torbey E., Lacossiere K., Gaddam S. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 26.Núñez J., Núñez E., Bodí V., Sanchis J., Miñana G., Mainar L. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Park J.J., Jang H.J., Oh I.Y., Yoon C.H., Suh J.W., Cho Y.S. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Kaya A., Kurt M., Tanboga I.H., Işık T., Günaydın Z.Y., Kaya Y. Relation of neutrophil to lymphocyte ratio with the presence and severity of stable coronary artery disease. Clin Appl Thromb Hemost. 2014;20:473–477. doi: 10.1177/1076029612473517. [DOI] [PubMed] [Google Scholar]
- 29.Arbel Y., Shacham Y., Ziv-Baran T., Laufer Perl M., Finkelstein A., Halkin A. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. Can J Cardiol. 2014;30:1177–1182. doi: 10.1016/j.cjca.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Kurtul A., Murat S.N., Yarlioglues M., Duran M., Celik I.E., Kilic A. Increased neutrophil-to-lymphocyte ratio predicts persistent coronary no-flow after wire insertion in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clinics (Sao Paulo) 2015;70:34–40. doi: 10.6061/clinics/2015(01)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogan I., Karaman K., Sonmez B., Celik S., Turker O. Relationship between serum neutrophil count and infarct size in patients with acute myocardial infarction. Nucl Med Commun. 2009;30:797–801. doi: 10.1097/MNM.0b013e32832e3a16. [DOI] [PubMed] [Google Scholar]
- 32.Jones H.R., Robb C.T., Perretti M., Rossi A.G. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28:137–145. doi: 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Oncel R.C., Ucar M., Karakas M.S., Akdemir B., Yanikoglu A., Gulcan A.R. Relation of neutrophil-to-lymphocyte ratio with GRACE risk score to in-hospital cardiac events in patients with ST-segment elevated myocardial infarction. Clin Appl Thromb Hemost. 2015;21:383–388. doi: 10.1177/1076029613505763. [DOI] [PubMed] [Google Scholar]
- 34.Nunez J., Nunez E., Bodi V., Sanchis J., Minana G., Mainar L. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Shen X.H., Chen Q., Shi Y., Li H.W. Association of neutrophil/lymphocyte ratio with long-term mortality after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Chin Med J. 2010;123:3438–3443. [PubMed] [Google Scholar]
- 36.Bezerra O.C., França C.M., Rocha J.A., Neves G.A., Souza P.R.M., Teixeira Gomes M. Cholinergic stimulation improves oxidative stress and inflammation in experimental myocardial infarction. Sci Rep. 2017;7:13687. doi: 10.1038/s41598-017-14021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kain V., Prabhu S.D., Halade G.V. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res Cardiol. 2014;109:444. doi: 10.1007/s00395-014-0444-7. [DOI] [PubMed] [Google Scholar]
- 38.Nian M., Lee P., Khaper N., Liu P. Infammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 39.Ono K., Matsumori A., Shioi T., Furukawa Y., Sasayama S. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodelling. Circulation. 1998;98:149–156. doi: 10.1161/01.cir.98.2.149. [DOI] [PubMed] [Google Scholar]
- 40.Jung M., Ma Y., Iyer R.P., DeLeon-Pennell K.Y., Yabluchanskiy A., Garrett M.R. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112:33. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bujak M., Frangogiannis N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frangogiannis N.G. The role of transforming growth factor (TGF)-β in the infarcted myocardium. J Thorac Dis. 2017;9(Suppl 1):S52–S63. doi: 10.21037/jtd.2016.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]