Abstract
Background
The anti - nociceptive effect of 7, 2′, 3′ – trimethoxy flavone, 7, 2′, 4′ – trimethoxy flavone, 7, 3′, 4′ – trimethoxy flavone and 7, 5, 4′ – trimethoxy flavone against inflammatory, neurogenic and thermal pain in mice was reported earlier. The present study was designed to investigate the effect of the above trimethoxy flavones in amelioration of peripheral neuropathy induced by paclitaxel.
Methods
Peripheral neuropathy was induced in mice by administration of a single i.p. dose (10 mg/kg) of paclitaxel. The manifestations of peripheral neuropathy such as tactile allodynia, cold allodynia and thermal hyperalgesia were assessed 24 h later by employing hair aesthesiometer test, acetone bubble test and hot water tail immersion test respectively. Further, the role of inflammatory cytokines like TNF – α, IL - 1β and free radicals in the action of trimethoxy flavones was investigated using in vitro assays.
Results
The test compounds dose dependently attenuated paclitaxel - induced tactile allodynia, cold allodynia and thermal hyperalgesia in mice. The test compounds inhibited TNF – α, IL - 1β and free radicals in a concentration dependent manner.
Conclusion
The investigated trimethoxy flavones attenuated paclitaxel – induced peripheral neuropathy in mice. The inhibition of cytokines and free radicals in addition to many neuronal mechanisms reported earlier may contribute to this beneficial effect.
Keywords: Peripheral neuropathy, Trimethoxy flavones, Cytokines, Free radicals
1. Introduction
Peripheral neuropathy is a dose - limiting side effect of several classes of anticancer drugs including paclitaxel, oxaliplatin, cisplatin, vincristine and bortezomib1. These drugs produce typical manifestations of neuropathy within a week, which includes spontaneous pain, allodynia and hyperalgesia, that results in discontinuation of chemotherapy schedule by cancer patients. Attenuation of peripheral neuropathy shall ensure greater patient compliance to chemotherapy. Currently available drugs such as anticonvulsants (e.g. gabapentin, carbamazepine), local anesthetics (lidocaine), opioids (e.g. tramadol, morphine), tricyclic antidepressants (amitryptyline) and selective serotonin reuptake inhibitors have limited efficacy in amelioration of peripheral neuropathy because of the diverse aetiology and complex pathophysiology2. In addition, these drugs per se produce numerous side effects3. Thus, there is an unmet clinical need and a challenge to develop more effective therapies for the management of peripheral neuropathy.
A few reports indicate that flavonoids such as myricitrin4, quercetin and rutin5, 6 were inhibit various types of neuropathy in animal models. In a recent study, four trimethoxy flavone derivatives (7, 2′, 3′ – trimethoxy flavone, 7, 2′, 4′ – trimethoxy flavone, 7, 3′, 4′ – trimethoxy flavone and 7, 5, 4′ – trimethoxy flavone) have been shown to possess antinociceptive property in mice7. Based on the aforementioned information, it was considered interesting to explore the potential effect of these trimethoxy flavones in peripheral neuropathy induced by a chemotherapeutic drug, paclitaxel in mice. Proinflammatory cytokines (TNF – α, IL - 1β) and free radicals are implicated in the pathogenesis of peripheral neuropathy.8 Hence, the effect of trimethoxy flavones on the above provocative factors was also considered for investigation
2. Methods
2.1. Animals
Adult Swiss albino mice of either sex weighing 25 – 30 g were used in the present study. These were obtained from the animal house of the Meenakshi Medical College and Research Institute. The animals had free access to food and water and maintained at 24 ± 1° C temperature in a 12 h day/ 12 h night cycle. All the experiments were carried out between 09.00 and 13.00 hours to avoid circadian variation. The experiments were performed after approval of the protocol by the institutional animal ethics committee.
2.2. Drugs and Chemicals
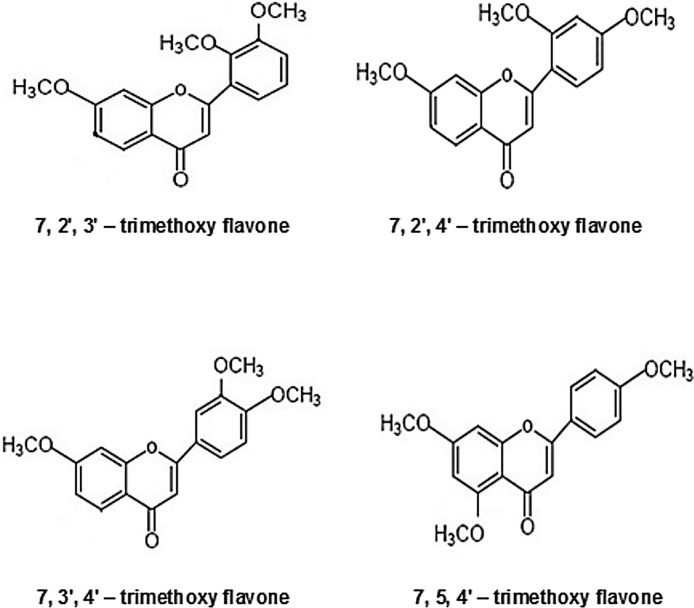
The trimethoxy flavones (Research Organics, Chennai, India) used in the study; 7, 2′, 3′ - trimethoxy flavone, 7, 2′, 4′ - trimethoxy flavone, 7, 3′, 4′ - trimethoxy flavone and 7, 5, 4′ - trimethoxy flavone (Fig. 1) were prepared as a fine suspension in 0.5% carboxy methyl cellulose (CMC) and injected s.c to mice. Morphine sulphate (Pharma Chemico Laboratories, Solan, Himachal Pradesh, India), Paclitaxel (Adley Formulations, Haryana, India) and Acetone (Merck Specialities Private Limited, Mumbai, India) were used in the study. Diagnostic kits (Cayman, USA) were used for in vitro assay of IL - 1β and TNF – α.
Fig. 1.
Structure of trimethoxy flavones (TMF).
2.3. Induction of peripheral neuropathy by paclitaxel
Peripheral neuropathy was induced9 in mice according to the method described by Hidaka et al., (2009). Mice were injected i.p. with a single dose of 10 mg/kg paclitaxel diluted in normal saline (0.9% NaCl) just before use. The manifestations of peripheral neuropathy (tactile allodynia, cold allodynia and thermal hyperalgesia) were assessed 24 h after paclitaxel administration.
2.4. Tactile allodynia (Hair aesthesiometer test)
The hair aesthesiometer test has been used to explore the dynamic responses to a tactile stimulus. The response to hair aesthesiometer has been described as allodynia because normal mice never withdraw from this stimulus. The mice were housed and habituated for 10 min in a transparent plastic box (7 X 7 X 13 cm) secured on a raised steel frame with the floor made of wire mesh. After the adaptation period, a 15 mm length of hair aesthesiometer was applied five times perpendicularly against the plantar skin of the each hind paw at an interval of 30 seconds. The paw withdrawal response was ranked as follows: 0 - no response, 1 - move away from the stimulus, 2 - immediate flinching of the hind paw 10. The sum of the ten values noted from both hind paws served as the paw withdrawal response score. The paw withdrawal response score was noted prior to the drug treatment and 30 min after administration of various trimethoxy flavones in doses of 25, 50, 100 or 200 mg/kg, s.c. or morphine (10 mg/kg, s.c.).
2.5. Cold allodynia (acetone bubble test)
Cold allodynia test11 was performed according to the method described by Flatters and Bennett, (2004). The mice were housed and habituated for 10 min in a transparent plastic box (7 X 7 X 13 cm) secured on a raised steel frame with the floor made of wire mesh. After the adaptation period, acetone bubble formed at the tip of a one ml syringe was applied to the plantar skin of hind paw and the paw withdrawal response was observed for a period of 20 sec. The withdrawal responses were ranked as follows: 0 - no response, 1 - immediate withdrawal, 2 - prolonged withdrawal, 3 – licking/biting of the hind paw. The response was measured three times in each paw alternatively at an interval of 1 min. The sum of six values served as the paw withdrawal response score. The paw withdrawal response score was noted before drug treatment and 30 min after administration of various trimethoxy flavones in doses of 25, 50, 100 or 200 mg/kg, s.c. or morphine (10 mg/kg, s.c.).
2.6. Thermal hyperalgesia (hot water tail immersion test)
Thermal hyperalgesia12 was assessed using hot water tail immersion method as previously described by Lauren et al., (2009). The mouse was restrained in a mouse holder and the tail (2 - 3 cm) of the mouse was immersed in hot water maintained at 48 ± 0.5 °C temperature. The time taken to flick the tail from the hot water was taken as the reaction time. A cut off time of 20 seconds was maintained. The reaction time was noted prior to the drug treatment and 30 min after drug treatment. Any increase in reaction time between these two readings is considered as anti - nociceptive response. Different trimethoxy flavones in doses of 25, 50, 100 or 200 mg/kg or morphine 10 mg/kg were administered s.c. to various groups of animals. The analgesic response was expressed as % maximum protective effect (MPE), which was calculated using the formula:
2.7. Effect of trimethoxy flavones on tumor necrosis factor alpha (TNF- α) and interleukin-1β (IL-1β)
Freshly heparinised human whole blood was used for this immunometric assay. This assay is based on a double antibody “sandwich” technique. Microwell plate supplied with the commercial kit (Cayman USA) has been coated with monoclonal antibody specific for TNF-α or IL-1β. They will capture any TNF-α or IL-1β introduced in the well. Various concentrations of trimethoxy flavones (dissolved and diluted in DMSO to 20-240 μM) were added to the well. 50 μl of acetylcholinesterase (AChE), which binds selectively to a different epitope on the TNF-α or IL-1β molecule, was also added to the well. When TNF-α or IL-1β was added to the well, the two antibodies form a sandwich by binding on opposite sides of the TNF-α or IL-1β molecule. The concentration of the analyte was then determined by measuring the enzymatic activity of the acetylcholinesterase by adding Ellman's reagent (which contains the substrate for AchE) to each well. The product of the AchE- catalysed reaction has a distinct yellow colour which shows strong absorbance at 412 nm. The intensity of this colour, determined spectrophotometrically is directly proportional to the amount of bound conjugate which in turn is proportional to the concentration of the TNF-α or IL-1β. Dexamethasone was used as a standard for TNF-α and IL-1β assay. The percentage cytokine inhibition was calculated from the following formula
2.8. DPPH radical scavenging activity of trimethoxy flavones 12
2, 2 diphenyl-1-picryl hydrazyl (DPPH) is a stable free radical, showing a deep violet colour, characterized by an absorption band in ethanol solution at 517 nm. DPPH is reduced to corresponding hydrazine in presence of a substance that can donate a hydrogen atom. Stock solution of DPPH was prepared by dissolving 25 mg of DPPH in 100 ml of ethanol. Two ml of reaction mixtures containing 1.9 ml of DPPH and 0.1 ml of various trimethoxy flavones (dissolved in ethanol) in concentrations of 20, 30, 60, 120 and 240 μM were prepared. Control reaction mixture without the test compound was prepared in an identical manner. The reaction was allowed to proceed in the dark for about 20 min. Then the absorbance of test mixture was read at 517 nm. The activity was compared with Vitamin C, which was used as a standard antioxidant. The percentage DPPH inhibition was calculated from the following formula
2.9. Nitrogen derived radical scavenging activity of trimethoxy flavones 14
This assay is based on the principle that sodium nitroprusside in aqueous solution at physiological pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions. This can be estimated colorimetrically with Griess reagent. 3 ml of reaction mixture containing 2 ml of sodium nitroprusside in phosphate buffered saline and 1 ml of various concentrations (20, 30, 60, 120 and 240 μM) of the trimethoxy flavones dissolved in ethanol were incubated at 37o C for 4 hours. A control sample without test compound was kept in an identical manner. After incubation, 0.5 ml of Griess reagent (100 ml of 1.0% sulfanilamide prepared in 3 M HCl) was added to the reaction mixture. The absorbance of the chromophore formed was read at 546 nm. The percentage inhibition of nitric oxide generation was measured by comparing the absorbance values of control and test compound. A standard antioxidant Vitamin C was used for comparison. The formula used for calculation was
2.10. Statistical analysis
The results were analysed by one way analysis of variance (ANOVA) followed by Dunnett's ‘t’ test for multiple comparison or paired ‘t’ test using SPSS 16 version software. A p value less than 0.05 was considered statistically significant. For in-vitro assays a mean of three observations has been presented. The IC 50 values of trimethoxy flavones for inhibition of cytokines and free radicals were obtained by linear regression analysis.
3. Results
3.1. Effect of trimethoxy flavones on paclitaxel - induced peripheral neuropathy
3.1.1. Tactile allodynia:
Paclitaxel administration resulted in the development of tactile allodynia as reflected by an increase in the paw withdrawal response score to an innocuous mechanical stimulus. The mean paw withdrawal response score in vehicle treated animals was not significantly different from their pretreatment value. However, a significant reduction in paw withdrawal response score was observed in morphine treated mice when compared to the pretreatment value (Table 1). A dose dependent attenuation of paw withdrawal response score was also recorded after treatment with different trimethoxy flavones when compared to their respective pretreatment values (Table 1). The reduction in score was statistically significant in doses of 100 mg/kg and 200 mg/kg of trimethoxy flavones when compared to their pretreatment values. In a dose of 200 mg/kg, 7, 2’, 3’ – trimethoxy flavone and 7, 3′, 4′ – trimethoxy flavone showed maximum inhibition of paw withdrawal response score when compared to 7, 2′, 4′ – trimethoxy flavone or 7, 5, 4′ – trimethoxy flavone (Table 1).
Table 1.
Effect of trimethoxy flavones (TMF) on paclitaxel – induced tactile allodynia in mice@
| Dose of test compounds mg/kg, s.c. | Paw withdrawal response score |
|||||||
|---|---|---|---|---|---|---|---|---|
| 7, 2′,3′ – TMF |
7, 2′,4′ – TMF |
7, 3′,4′ – TMF |
7, 5, 4′ – TMF |
|||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| 25 | 18.33 ± 0.42 | 18.66 ± 0.61 | 19.00 ± 0.36 | 18.66 ± 0.42 | 19.16 ± 0.44 | 19.00 ± 0.30 | 20.00 ± 0.00 | 19.33 ± 0.42 |
| 50 | 19.33 ± 0.42 | 17.33 ± 0.86 | 19.33 ± 0.42 | 17.33 ± 0.66 | 19.33 ± 0.42 | 17.00 ± 0.68 | 19.16 ± 0.40 | 18.00 ± 0.73 |
| 100 | 19.00 ± 0.68 | 12.66 ± 1.33* | 19.66 ± 0.33 | 12.50 ± 0.71* | 19.00 ± 0.68 | 13.00 ± 0.73* | 19.33 ± 0.42 | 14.66 ± 0.42* |
| 200 | 19.00 ± 0.68 | 4.33 ± 1.28* | 19.33 ± 0.42 | 10.00 ± 0.57* | 18.33 ± 0.61 | 4.00 ± 0.51* | 19.53 ± 0.42 | 12.33 ± 0.95* |
Each value represents the mean ± SEM of six observations.
The paw withdrawal response score was 19.66 ± 0.33 and 19.50 ± 0.34 before and after treatment with vehicle
The paw withdrawal response scores in morphine (10 mg/kg) treated mice were, 20.00 ± 0.00 before treatment and 3.16 ± 1.24* after treatment.
P < 0.05 compared to respective value before treatment
All treatment groups received paclitaxel (10 mg/kg, i.p.) 24 h prior to the test. Tactile allodynia was determined before and 30 min after vehicle/morphine/trimethoxy flavones treatment on the next day.
3.1.2. Cold allodynia:
Paclitaxel administration resulted in the development of cold allodynia as reflected by an increase in the paw withdrawal response score to a non - noxious cold stimulus. While vehicle treatment did not alter the response, morphine (10 mg/kg, s.c) treatment resulted in a significant reduction in the paw withdrawal response score when compared to its pretreatment value (Table 2). A dose dependent reduction of paw withdrawal response score was also observed in animals treated with different doses (25 – 200 mg/kg) of trimethoxy flavones when compared to their pretreatment values (Table 2). Compounds 7, 2′, 3′ – trimethoxy flavone and 7, 3′, 4′ – trimethoxy flavone showed maximum reduction in paw withdrawal response score in a dose of 200 mg/kg when compared to other two investigated compounds (Table 2).
Table 2.
Effect of trimethoxy flavones (TMF) on paclitaxel – induced cold allodynia in mice@
| Dose of test compounds mg/kg, s.c. | Paw withdrawal response score |
|||||||
|---|---|---|---|---|---|---|---|---|
| 7, 2′,3′ – TMF |
7, 2′,4′ – TMF |
7, 3′,4′ – TMF |
7, 5,4′ – TMF |
|||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| 25 | 17.16 ± 0.30 | 17.00 ± 0.51 | 17.16 ± 0.30 | 16.50 ± 0.42 | 17.66 ± 0.21 | 17.00 ± 0.44 | 16.83 ± 0.49 | 16.66 ± 0.47 |
| 50 | 17.50 ± 0.84 | 16.33 ± 0.33 | 17.50 ± 0.22 | 14.66 ± 0.42* | 17.16 ± 0.16 | 10.00 ± 1.25* | 17.33 ± 0.33 | 15.83 ± 0.65* |
| 100 | 17.16 ± 0.54 | 9.16 ± 0.87* | 16.66 ± 0.61 | 10.50 ± 0.56* | 17.00 ± 0.68 | 6.16 ± 1.07* | 17.83 ± 0.47 | 12.66 ± 0.61* |
| 200 | 17.66 ± 0.98 | 2.50 ± 0.42* | 17.83 ± 0.16 | 7.83 ± 0.98* | 16.83 ± 0.40 | 3.50 ± 1.05* | 16.83 ± 0.47 | 8.50 ± 0.56* |
Each value represents the mean ± SEM of six observations.
The paw withdrawal response scores were 17.66 ± 0.33 and 17.50 ± 0.34 before and after vehicle treatment.
The paw withdrawal response scores in morphine (10 mg/kg) treated mice were 18.00 ± 0.00 before treatment and 1.83 ± 0.87* after treatment.
P < 0.05 compared to respective score before treatment.
All treatment groups received paclitaxel (10 mg/kg, i.p.) 24 h prior to the test. Cold allodynia was determined before and 30 min after vehicle/morphine/trimethoxy flavones treatment on the next day.
3.2. Thermal hyperalgesia
In vehicle treated animals, the mean increase in reaction time was 0.06 ± 0.44 sec. This was significantly increased to 15.09 ± 0.88 sec in morphine (10 mg/kg) treated animals, thus offering 75.37% protection against paclitaxel - induced thermal hyperalgesia (Table 3). A dose dependent prolongation of thermal latency was observed in mice treated with different doses (25 – 200 mg/kg) of trimethoxy flavones (Table 3). A maximum increase in mean reaction time of 10.17 ± 1.09 sec was observed with 200 mg/kg of 7, 2′, 3′ – trimethoxy flavone offering 50% protection from thermal hyperalgesia. The peak effect of 7, 3′, 4′ – trimethoxy flavone and 7, 2′, 4′ – trimethoxy flavone were almost similar with a mean increase of 8.23 ± 0.84 sec and 7.80 ± 0.50 sec respectively offering nearly 40% protection. The mean increase in reaction time after 7, 5, 4′ – trimethoxy flavone (200 mg/kg) was 5.86 ± 0.84 sec offering only 29% protection.
Table 3.
Effect of trimethoxy flavones (TMF) on paclitaxel – induced thermal hyperalgesia in mice@
| Dose of test compounds (mg/kg, s.c) | Mean increase in reaction time (seconds) |
|||
|---|---|---|---|---|
| 7, 2′,3′ – TMF | 7, 2′,4′ – TMF | 7, 3′,4′ – TMF | 7, 5, 4′ – TMF | |
| 25 | 1.83 ± 0.55 (8.87) | 1.12 ± 0.31(5.31) | 2.27 ± 0.41*(11.08) | 1.04 ± 0.23(4.91) |
| 50 | 5.62 ± 0.57* (27.88) | 2.98 ± 0.37* (14.64) | 3.64 ± 0.49* (17.95) | 1.47 ± 0.37(7.07) |
| 100 | 8.52 ± 0.56* (42.42) | 5.69 ± 0.74* (28.23) | 5.42 ± 0.91* (26.88) | 3.97 ± 0.44*(19.60) |
| 200 | 10.17 ± 1.09* (50.70) | 7.80 ± 0.50* (38.81) | 8.23 ± 0.84* (40.97) | 5.86 ± 0.84*(29.08) |
Each value represents the mean ± S.E.M of six observations.
The values in parenthesis indicate the percentage of maximal protective effect.
The mean increase in reaction time in vehicle treated animals was 0.06 ± 0.04 sec.
The mean increase in reaction time after morphine (10 mg/kg) treatment was 15.09 ± 0.88 sec* (75.37%).
P < 0.05 compared with vehicle treatment.
All treatment groups received paclitaxel (10 mg/kg, i.p.) 24 h prior to the test. Thermal hyperalgesia was determined before and 30 min after vehicle/morphine/TMF treatment on the next day. The mean increase in reaction time represents the difference in time latency recorded before and after treatment with the vehicle / test compounds.
3.2.1. Effect of trimethoxy flavones on TNF α
All the four trimethoxy flavones inhibited TNF α in a concentration dependent manner (Table 4). In a concentration of 20 μM the investigated trimethoxy flavones inhibited TNF α to an extent of 18.04% to 29.10%. In the maximum concentration employed (240 μM) all the test compounds produced almost a similar degree of inhibition (80% – 86%) of TNF α. Dexamethasone used as a standard drug exhibited an IC50 value of 31.32 μM to inhibit TNF α. The IC50 values of investigated trimethoxy flavones ranged from 60.13 μM, to 90.82 μM (Table 4).
Table 4.
Effect of various trimethoxy flavones (TMF) on TNFα
| Concentration of trimethoxy flavones (μM) | % inhibition of TNF α activity |
|||
|---|---|---|---|---|
| 7,2′,3′ TMF | 7,2′,4′ TMF | 7,3′,4′TMF | 7,5,4′ TMF | |
| 20 | 24.18 | 28.48 | 29.10 | 18.04 |
| 30 | 33.42 | 35.14 | 36.82 | 26.42 |
| 60 | 45.64 | 49.64 | 49.64 | 42.70 |
| 120 | 60.32 | 64.82 | 64.32 | 56.36 |
| 240 | 80.48 | 82.24 | 86.02 | 80.82 |
| IC 50 μM | 76.23 | 60.13 | 60.14 | 90.82 |
Each value represents the mean of three observations. The IC 50 value was calculated by linear regression analysis The IC 50 value of dexamethasone was 31.32 μM
3.2.2. Effect of trimethoxy flavones on IL - 1β
A concentration dependent inhibition of IL - 1β was exhibited by all the tested trimethoxy flavones (Table 5). In a concentration of 20 μM all the trimethoxy flavones inhibited IL - 1β activity to an extent of 27% – 37%. However, the above compounds when employed in a concentration of 240 μM produced nearly 80% – 90% inhibition of IL - 1β activity. Dexamethasone used as a standard drug exhibited an IC50 value of 35.21 μM, The IC50 value of investigated trimethoxy flavones ranged from 33 μM,- 72 μM, (Table 5).
Table 5.
Effect of various trimethoxy flavones (TMF) on IL - 1β
| Concentration of trimethoxy flavones (μM) | % inhibition of IL - 1β activity |
|||
|---|---|---|---|---|
| 7,2′,3′ TMF | 7,2′,4′ TMF | 7,3′,4′ TMF | 7,5,4′ TMF | |
| 20 | 35.14 | 27.48 | 36.86 | 27.18 |
| 30 | 48.26 | 37.68 | 48.24 | 38.62 |
| 60 | 56.44 | 46.34 | 58.48 | 48.42 |
| 120 | 63.54 | 62.36 | 68.44 | 68.36 |
| 240 | 88.38 | 80.11 | 90.68 | 85.48 |
| IC 50 μM | 33.91 | 72.62 | 33.32 | 62.43 |
Each value represents the mean of three observations. The IC 50 value was calculated by linear regression analysis The IC 50 value of dexamethasone was 35.21 μM
3.2.3. Effect of trimethoxy flavones on DPPH scavenging
A potent DPPH scavenging activity was evident for all the trimethoxy flavones (Table 6). The inhibition of DPPH radical by trimethoxy flavones was concentration dependent and the activity ranged from 28 – 84 percent. The IC50 of 7, 2′, 4′ – trimethoxy flavone was 48.62 μM, which is almost close to the IC50 of ascorbic acid (42.12 μM). The IC50 of other compounds (Table 6) although slightly higher, were in a similar range (58.82 μM - 61. 21 μM,).
Table 6.
Effect of various trimethoxy flavones (TMF) on DPPH
| Concentration of trimethoxy flavones (μM) | % inhibition of DPPH activity |
|||
|---|---|---|---|---|
| 7,2′,3′ TMF | 7,2′,4′ TMF | 7,3′,4′ TMF | 7,5,4′ TMF | |
| 20 | 30.18 | 39.82 | 29.82 | 28.12 |
| 30 | 39.46 | 43.44 | 38.44 | 38.54 |
| 60 | 51.24 | 56.68 | 50.18 | 49.35 |
| 120 | 63.82 | 64.12 | 62.48 | 62.84 |
| 240 | 84.24 | 83.32 | 80.34 | 83.16 |
| IC 50 μM | 58.82 | 48.62 | 59.91 | 61.21 |
Each value represents the mean of three observations. The IC 50 value was calculated by linear regression analysis The IC50 of Ascorbic acid was 42.12 μM
3.2.4. Effect on nitric oxide generation
A concentration dependent inhibition of nitric oxide generation was exhibited by various trimethoxy flavones (Table 7). In the lowest concentration employed (20 μM) all the trimethoxy flavones inhibited nitric oxide generation to an extent of 28 – 30%. In a maximum concentration of 240 μM these compounds inhibited nitric oxide generation by 75 – 90%. Maximum inhibition was observed for 7, 2′, 3′ – trimethoxy flavone. The IC50 values of these compounds were in the range of 32.39 μM, to 74.59 μM, while that of ascorbic acid was 41.22 μM (Table 7).
Table 7.
Effect of various trimethoxy flavones (TMF) on Nitric oxide
| Concentration of trimethoxy flavones (μM) | % inhibition of Nitric oxide activity |
|||
|---|---|---|---|---|
| 7,2′,3′ TMF | 7,2′,4′ TMF | 7,3′,4′ TMF | 7,5,4′ TMF | |
| 20 | 39.24 | 38.44 | 39.62 | 28.92 |
| 30 | 48.68 | 44.20 | 45.24 | 40.68 |
| 60 | 58.14 | 57.24 | 53.76 | 48.24 |
| 120 | 72.36 | 64.36 | 59.08 | 53.86 |
| 240 | 90.18 | 85.28 | 75.34 | 76.14 |
| IC 50 μM | 32.39 | 40.29 | 46.91 | 74.59 |
Each value represents the mean of three observations. The IC 50 value was calculated by linear regression analysis The IC50 of Ascorbic acid was 41.22 μM
4. Discussion
Previous studies have indicated a significant effect of flavonoids in various types of neuropathy. Quercetin5, 15, naringin16, rutin17 and nobiletin18 have been reported to offer significant protection against diabetic peripheral neuropathy in experimental animals. Another study by Azevedo et al., (2013) reported significant protective effect of rutin and quercetin against oxaliplatin induced peripheral neuropathy and this effect was attributed to their antioxidant property6.
Based on the above few reports and earlier results on the effectiveness of trimethoxy flavones in attenuating visceral, neurogenic and inflammatory pain7, it was considered interesting to evaluate the possible role of trimethoxy flavones in ameliorating paclitaxel – induced peripheral neuropathy.
In the present study, the investigated trimethoxy flavones dose dependently suppressed neuropathic manifestations induced by paclitaxel such as mechanical allodynia (Table 1), cold allodynia (Table 2) and thermal hyperalgesia (Table 3). However, a statistically significant response was mostly observed only with 100 and 200 mg/kg doses of these compounds. Among the tested trimethoxy flavones, 7, 2′, 3′ – trimethoxy flavone and 7, 3′, 4′ – trimethoxy flavone produced maximum inhibition of mechanical allodynia, cold allodynia and thermal hyperalgesia. A maximum protection against thermal hyperalgesia ranging between 29 and 50 percent was observed with 200 mg/kg dose of different trimethoxy flavones. Thus, the present study further more establishes the efficacy of trimethoxy flavones in amelioration of paclitaxel - induced peripheral neuropathy in addition to their antinociceptive effect in other types of pain reported earlier7.Neuropathic pain resulting from the use of cancer chemotherapeutic drugs is highly refractory to currently employed analgesic drugs. Newer, safe analgesic drugs that can provide adequate relief from the debilitating type of pain are urgently needed which can ensure proper completion of chemotherapy. More expanded studies to investigate the effectiveness of these trimethoxy flavones in neuropathy induced by other cancer chemotherapeutic drugs shall further strengthen their usefulness.
Various mechanisms like, enhanced cytokine release, changes in voltage gated ion channels, altered neurotransmission and cellular signaling pathways have been implicated in the development of chemotherapy induced peripheral neuropathy 19 .The effect of trimethoxy flavones on a few cytokines and free radicals was analysed in the present study.
Effect of trimethoxy flavones on TNF- α and IL-1β: Several studies have identified the over expression of inflammatory cytokines such as TNF-α and IL-1β in paclitaxel induced peripheral neuropathy20, 21, 22. Peripheral neuropathy appears quickly after the first dose of paclitaxel due to sensitization of nociceptors by the inflammatory cytokines23, 24. The level of TNF-α is increased in the sciatic nerve and spinal cord of a vincristine animal model25 and the administration of TNF α neutralizing antibody reduced vincristine - induced allodynia and hyperalgesia significantly26. These findings provide evidence that cytokines are involved in the pathogenesis of chemotherapy - induced painful peripheral neuropathy.
Several flavonoids such as luteolin, quercetin and genistein were found to inhibit cytokines such as TNF – α and IL-1β in a concentration-dependent manner27, 28, 29. Recent studies by Vidyalakshmi et al., (2012) and Kamalakannan et al., (2015) reported the inhibition of TNF –α and IL- 1β by certain dihydroxy and dimethoxy flavones respectively30, 31. In the present study, the investigated trimethoxy flavones also inhibited IL-1β and TNF- α in a concentration dependent manner. Compounds 7, 2′, 4′ – trimethoxy flavone and 7, 3′, 4′ – trimethoxy flavone exhibited a maximum inhibition of TNF-α compared to other trimethoxy flavones. The highest inhibition of IL-1 β was evident with 7, 2′, 3′ – trimethoxy flavone and 7, 3′, 4′ – trimethoxy flavone which exhibited 90% inhibition. The significant inhibition of cytokines TNF - α and IL -1β by the investigated trimethoxy flavones may contribute to the amelioration of paclitaxel – induced peripheral neuropathic manifestations.
Free radical scavenging activity of trimethoxy flavones: Increased formation of free radicals has been attributed to the development of hyperalgesia associated with acute and chronic inflammation32, 33. It has been demonstrated that, paclitaxel treatment promotes mitochondrial injury, which leads to an increase in the release of free radicals resulting in peripheral neuropathy8. Several studies have shown that, administration of free radical scavengers such as phenyl N - tert – butyl nitrone34, vitamin C and N - acetyl - L – cysteine35 significantly reduced mechanical allodynia in paclitaxel - induced neuropathy. Overall inhibition of reactive oxygen species has been shown to inhibit neuropathic manifestations due to paclitaxel 36.
Several flavonoids have been shown to inhibit reactive oxygen species and many of their beneficial effects have been attributed to the antioxidant / free radical scavenging properties37. In the present study, a dose dependent inhibition of DPPH free radical generation activity was recorded for all the tested trimethoxy flavone compounds with a maximum effect ranging from 80.34% – 84.24% (Table 6). The free radical scavenging activity was further corroborated by the ability of the tested trimethoxy flavones in scavenging nitric oxide. Almost 90% inhibition was recorded in nitric oxide scavenging activity (Table 7). Among the tested compounds, 7, 2′, 3′ – trimethoxy flavone and 7, 2′, 4′ – trimethoxy flavone exhibited highest inhibition of DPPH and nitric oxide free radical generation. The antinociceptive and antiinflammatory activity of many dihydroxy and dimethoxy flavone compounds has been attributed to their antioxidant / free radical scavenging activity30, 31. The potent antioxidant / free radical scavenging activity of trimethoxy flavones observed in the present study suggests that, this may be one of the important mechanisms contributing to the amelioration of paclitaxel – induced peripheral neuropathy.
Alterations in many neurotransmitters, neuronal pathways and ion channels are implicated in the development of neuropathy. Currently employed drugs like opioids target opioid pathways, anticonvulsants act through GABA and antidepressant drugs act through monoaminergic mechanisms to alleviate the symptoms of neuropathic pain. In an earlier study, these trimethoxy flavones were subjected to detailed investigation to explore their interaction with various neuronal systems in mediating their antinociceptive action7. Involvement of opioid, GABAergic, tryptaminergic, adrenergic, dopaminergic and K+ATP channel pathways in the antinociceptive action of trimethoxy flavones was established in that study7. The above mechanisms are also implicated in the development/manifestations of neuropathy38, 39, 40. Modulation of these pathways may also be suggested as plausible mechanisms leading to protection against paclitaxel – induced peripheral neuropathy.
In conclusion, single administration of paclitaxel caused peripheral neuropathy in mice and the investigated trimethoxy flavones dose dependently attenuated the neuropathic manifestations induced by paclitaxel. The ameliorative effect of trimethoxy flavones on paclitaxel – induced peripheral neuropathy may be through many neuronal mechanisms reported earlier7 and may also involve an interaction with cytokines and reactive oxygen species found in the present study. The efficacy of trimethoxy flavones against neuropathy induced by other chemotherapeutic drugs is also worth investigating.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
The facilities extended by Meenakshi Academy of Higher Education and Research for carrying out the animal experiments are gratefully acknowledged.
References
- 1.Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy induced peripheral neuropathy: prevention and treatment strategies. Eur. J. Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 3.Attal N., Cruccu G., Haanpaa M., Hansson P., Jensen T.S., Nurmikko T., Sampaio C., Sindrup S., Wiffen P. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 4.Meotti F.C., Luiz Ap, Pizzolathi M.G., Kasuya C.A., Calixto J.B., Santos A.R. Analysis of the antinociceptive effects of flavonoid myricitrin; evidence for the role of L-arginine – nitric oxide and protein kinase C pathways. J pharmacol Exp Ther. 2006;316:789–796. doi: 10.1124/jpet.105.092825. [DOI] [PubMed] [Google Scholar]
- 5.Anjaneyulu M., Chopra K. Quercetin, a bioflavonoid attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog Neuro Physcho pharmacol Biol Psychiatry. 2003;27:1001–1005. doi: 10.1016/S0278-5846(03)00160-X. [DOI] [PubMed] [Google Scholar]
- 6.Azevedo M.I., Anamaria F.P., Ricardo B.N., Flavio E.R., Gerly A.B., Deysi V.T.W., Roberto C.P.L., Ronaldo A.R., Mariana L.V. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin - induced chronic painful peripheral neuropathy. Molecular Pain. 2013;9:53. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagan N., Vijaykumar S., Parimala K., Jaikumar S., Binoy Varghese Cheriyan, Viswanathan S. Anti - nociceptive activity of a few structurally related trimethoxy flavones and possible mechanisms involved. J Basic Clin Physiol Pharmacol. 2016;27(2):109–119. doi: 10.1515/jbcpp-2015-0079. [DOI] [PubMed] [Google Scholar]
- 8.Xiao W.H., Zheng H., Bennett G.J. Characterization of oxaliplatin - induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;17:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidaka T., Shima T., Nagira K., Masahiro L., Takafumi N., Yukiko A., Yasushi K., Takashi A., Shigeru S. Herbal medicine Shakuyakukanzo - to reduce paclitaxel - induced painful peripheral neuropathy in mice. Eur J Pain. 2009;13:22–27. doi: 10.1016/j.ejpain.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Takasaki I., Andoh T., Shiraki K., Kuraishi Y. Allodynia and hyperalgesia induced by herpes simplex virus type-1 infection in mice. Pain. 2000;86:95–101. doi: 10.1016/s0304-3959(00)00240-2. [DOI] [PubMed] [Google Scholar]
- 11.Flatters S.J., Bennett G.J. Ethosuximide reverses paclitaxel and vincristine - induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Lauren Ta E., Low Philip A., Windebank Anthony J. Mice with Cisplatin and Oxaliplatin - induced Painful Neuropathy Develop Distinct Early Responses to Thermal Stimuli. Molecular Pain. 2009;5(1):9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sreejayan N., Rao M.N.A. Nitric oxide scavenging by curcuminoids. Journal of Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 15.Jamshid N., Mehrdad R., Hanieh A., Farnoosh S. The effect of the flavonoid quercetin on pain sensation in diabetic rats. Basic and Clinical Neuro Science. 2011;2:51–57. [Google Scholar]
- 16.Kandhare A.D., Raygude K.S., Ghosh P., Ghule A.E., Bodhankar S.L. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659. doi: 10.1016/j.fitote.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Maher M.A.E. Ameliorative potential of rutin on streptozotocin - induced neuropathic pain in rat. Afr. J. Pharm. Pharmacol. 2013;7(41):2743–2754. [Google Scholar]
- 18.Parkar N., Addepalli V. Effect of Nobiletin on Diabetic Neuropathy in Experimental Rats. J Pharmacol Ther. 2014;2(5):1028. [Google Scholar]
- 19.Boyette-Davis J.A., Walters E.T., Dougherty P.M. Mechanisms involved in the development of Chemotherapy- induced neuropathy. Pain Manag. 2015;5(4):285–296. doi: 10.2217/pmt.15.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledeboer A., Jekich B.M., Sloane E.M., Mahoney J.H., Langer S.J., Milligan E.D., Martin D., Maier S.F., Johnson K.W., Leinwand L.A., Chavez R., Watkins L.R. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav. Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dina O.A., Chen X., Reichling D., Levine J.D. Role of protein kinase - C epsilon and protein kinase - A in a model of paclitaxel - induced painful peripheral neuropathy in the rat. Neuroscience. 2001;108:507–515. doi: 10.1016/s0306-4522(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 22.Schafers M., Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008;437:188–193. doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Bennett G.J., Liu G.K., Xiao W.H., Jin H.W., Siau C. Terminal arbor degeneration - a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. 2011;33:1667–1676. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loprinzi C.L., Reeves B.N., Dakhil S.R., Sloan S.A., Wolf S.L., Burger K.N., Arif Kamal, Le – Lindqwister N.A., Soori G.S., Jaslowski A.J., Novotny P.J., Lachance D.H. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol. 2011;29:1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthuraman A., Singh N., Jaggi A.S. Protective effect of Acorus calamus L. in rat model of vincristine - induced painful neuropathy: an evidence of anti-inflammatory and anti - oxidative activity. Food Chem Toxicol. 2011;49:2557–2563. doi: 10.1016/j.fct.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 26.Kiguchi N., Maeda T., Kobayashi Y., Kishioka S. Up - regulation of tumor necrosis factor - alpha in spinal cord contribute to vincristine - induced mechanical allodynia in mice. Neurosci Lett. 2008;445:140–143. doi: 10.1016/j.neulet.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Comalada M., Ballester I., Bailon E., Sierra S., Xaus J., Galvez J., de Medina F.S., Zarzuelo A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure–activity relationship. Biochem. Pharmacol. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Sharma V., Mamata M., Sowmya G., Richa T., Anirban B., Pankaj S., Ellora S. Modulation of interleukin-1β mediated inflammatory responses in human astrocytes by flavonoids: Implications in neuroprotection. Brain Research Bulletin. 2007;73:55–63. doi: 10.1016/j.brainresbull.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Nair M.P., Mahajan S., Reynolds J.L., Ravikumar A., Nair H., Schwartz S.A., Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (Tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF- kβ system. Clin Vacc Immunology. 2006;13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidyalakshmi K., Kamalakannan P., Viswanathan S., Ramaswamy S. Anti-inflammatory effect of certain dihydroxy flavones and the mechanisms involved. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry. 2012;11:253–261. doi: 10.2174/1871523011202030253. [DOI] [PubMed] [Google Scholar]
- 31.Kamalakannan P., Vidyalakshmi K., Viswanathan S., Ramaswamy S. Anti-inflammatory effect of certain dimethoxy flavones. Inflammopharmacol. 2015;23(6):307–317. [Google Scholar]
- 32.Salvemini D., Neumann W.L. Targeting peroxynitrite driven nitroxidative stress with enzymes: a novel therapeutic approach in chronic pain management. Lif sci. 2010;86:604–614. doi: 10.1016/j.lfs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Bezerra M.M., Brain S.D., Girao V.C., Greenacre S., Keeble J., Rocha F.A. Neutrophils – derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn – Schmiedbergs Arch. Pharmacol. 2007;374:265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim H.K., Zhang Y.P., Gwak Y.S., Abdi S. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology. 2010;112:432–439. doi: 10.1097/ALN.0b013e3181ca31bd. [DOI] [PubMed] [Google Scholar]
- 35.Nakano A., Abe M., Oda A., Amou H., Hiasa M., Nakamura S., Miki H., Harada T., Fujii S., Kagawa K., Takeuchi K., Watanabe T., Ozaki S., Matsumoto T. Delayed treatment with vitamin - C and N – acetyl – l – cysteine protects schwann cells without compromising the anti - myeloma activity of bortezomib. Int. J. Hematol. 2011;93:727–735. doi: 10.1007/s12185-011-0850-7. [DOI] [PubMed] [Google Scholar]
- 36.Fidanboylu M., Griffiths L.A., Flatters S.J. Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel- induced painful peripheral neuropathy. PLoS ONE. 2011;6(9):e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burda S., Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 38.Wolfgang S., Jean D.V., Thomas M.T., Ulrich J., Thomas C. Differential contribution of opioid and noradrenergic mechanisms of tapentadol in rat model of nociceptive and neuropathic pain. Eur J Pain. 2010;14:814–821. doi: 10.1016/j.ejpain.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Kagiava A., Tsingotjidou A., Emmanouilides C., Theophilidis G. The effects of oxaliplatin, an anticancer drug, on potassium channels of the peripheral myelinated nerve fibres of the adult rat. Neurotoxicology. 2008;29:1100–1106. doi: 10.1016/j.neuro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Alessandra D.L., Dietmar B., Marie B., Jules D., Youssef D., Zhi-jian W., Rahul E., James M.C., Hanns U.Z. HZ166, a novel GABAA receptor subtype - selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharm. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]