Abstract
In this study, we precisely showed how the Rg3-enriched red ginseng extract (Rg3-RGE) lowers glucose, triglyceride, and low-density lipoprotein (LDL) levels in LDL–/– mice. Aspartate aminotransferase/serum glutamic-oxaloacetic transaminase), alanine aminotransferase /serum glutamate-pyruvate transaminase, and steatohepatitis were found to be reduced, and atheroma formation was inhibited by Rg3-enriched red ginseng extract.
Keywords: antiatheromatic, antidiabetic, antihypercholesterolemic, Rg3-enriched Korean Red Ginseng extract
Epidemiologically, cardiovascular diseases (CVDs) are the major causes of morbidity and mortality worldwide. The high prevalence of CVDs is primarily attributed to the increased use of nonorganic health products and sedentary lifestyle [1]. A large percentage of the population now suffers from diabetes, which is a risk factor for the occurrence of CVDs [2]. Patients who suffer from diabetes are more prone to myocardial infarction, congestive heart failure, and reinfarction [3]. Diabetes and CVDs show common manifestations, such as arterial dysfunction, dyslipidemia, hyperglycemia, and alteration of cell types in the endothelial layer of vessels, which lead to chronic heart disease [4], [5], [6]. Many allopathic therapies are being used successfully for the treatment of CVDs and diabetes; however, if prolonged, their side effects can become serious. Therefore, managing diabetes by inhibiting atherosclerosis or CVDs using natural herbal medications can drastically improve lifestyle without causing any complications.
Panax ginseng can be considered the “king of herbs” and is a multifunctional supplement that has been reported to possess anti-inflammatory, anticancer, antistress, and antiplatelet activities, as well as to increase longevity and enhance libido [7]. The major active constituents in this herb are divided into three groups: protopanaxadiols, protopanaxatriols, and oleic acids. These components are called ginsenosides. Korean Red Ginseng extract (RGE), which contains these three subcomponents, has been studied extensively for its effects on cardiovascular diseases and diabetes. Rg3-enriched RGE (Rg3-RGE) is the same extract that has been enriched with the ginsenoside Rg3. Briefly, Red ginseng (stem/root = 75:25) was extracted with distilled water proceeding with 55% ethanol extraction. With multiple extractions, a concentrated extract was prepared. Subsequently the extract was then subjected to high performance liquid chromatography (HPLC) and resultant profile of constituents is given in Table 1 with Rg3 in most major quantities. Many of the properties of ginsenoside Rg3 have been elucidated in the past, including its most potent effects on vascular diseases [8], [9], [10], [11], [12].
Table 1.
Profile of Rg3-enriched ginseng extract constituents
| Ginsenosides | Contents (mg/g) |
|---|---|
| Rb1 | 3.86 |
| 20(S)-Rg3 | 44.91 |
| Rc | 1.20 |
| Rb2 | 1.53 |
| Rd | 1.60 |
| Rf | 1.28 |
| Rh1 | 3.71 |
| 20(S)-Rg2 | 3.55 |
| 20(R)-Rg3 | 6.78 |
| Total | 67.41 |
In this study, we report for the first time that Rg3-RGE reduces the levels of low-density lipoprotein (LDL), serum glutamic-oxaloacetic transaminase, and serum glutamate-pyruvate transaminase, as well as steatohepatitis and atheroma formation in LDL–/– mice in a dose-dependent manner. The aim of this study was to characterize the complications associated with diabetes, e.g., atherosclerosis, and to minimize and mitigate the occurrence of diabetes and thereby CVDs.
Male LDL–/– and C57BL/6 mice aged 6 wk were purchased from the Jackson Laboratory (Sacramento, CA, USA). Mice were provided a normal chow diet, while LDL–/– mice were provided a western diet (WD) for 12 wk. Animals were maintained in a pathogen-free facility according to international guidelines for animal care. All in vivo experiments were carried out with the permission and according to the protocols provided by the Institutional Animal Care and Use Committee of Daejeon University. Mice were divided into five groups (n = 6). The first group was fed only the normal chow diet. The second group received a WD, and the third group was treated with 5 mg/kg atorvastatin in addition to the WD. The fourth and fifth groups were administered Rg3-RGE at a dose of 2.5 mg/kg and 5 mg/kg, respectively, over the last 4 wk of WD feeding. At the end of 12 wk, blood and tissue samples were collected and either stored at –70°C or processed to investigate various parameters.
Mice were fasted overnight and blood was harvested the following day. Serum samples were obtained in order to investigate glucose, LDL, total cholesterol, triglyceride, glutamic-oxaloacetic transaminase, and glutamate-pyruvate transaminase levels using enzymatic methods (FUJI DRI-CHEM 4000i; FUJI, Shizuoka, Japan). For hematoxylin and eosin staining, liver and aortic tissues were fixed in 10% neutral buffered formalin solution, dehydrated, embedded in paraffin wax, and cut into 4 μm-thick sections. These were later analyzed using a light microscope.
All results are presented as means ± standard deviation. One-way analysis of variance followed by Dunnet's t test was used for significance testing. A p value of <0.05 was considered statistically significant using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA) compared with the atorvastatin-treated group.
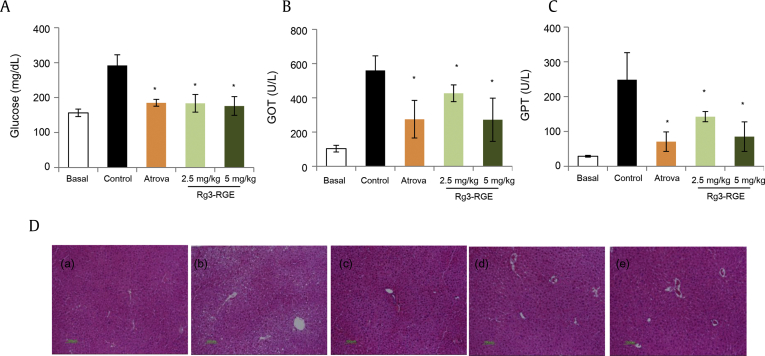
Rg3-RGE treatment was associated with a potent decrease in serum glucose levels in LDL–/– mice, in a dose-dependent manner. As patients suffering from diabetes are at a risk of cardiovascular diseases [13], [14], [15], glucose content was also measured in LDL receptor-knockdown mice. Without atorvastatin or Rg3-RGE treatment, mice showed elevated levels of glucose, as shown in Fig. 1A.
Fig. 1.
Rg3-RGE attenuated serum glucose and liver biomarker levels in LDL–/– mice. (A–C) Serum glucose, GOT, and GPT levels were determined using an ELISA kit after oral treatment of the mice with Rg3-RGE over 4 wk. Bar graphs represent means ± SEM (n = 6). A p value of <0.05 was considered significant compared with the atorvastatin group (positive control). (D) This figure part represents the following: (a) normal liver section of mice treated with regular chow diet; (b) LDL–/– mice fed with a western diet for 12 wk; (c) LDL–/– mice treated with atorvastatin at 5 mg/kg as positive control; and LDL–/– mice given Rg3-RGE at (d) 2.5 mg/kg and (e) 5 mg/kg. GOT, glutamic-oxaloacetic transaminase; GPT, glutamate-pyruvate transaminase; LDL, low-density lipoprotein; Rg3-RGE, Rg3-enriched red ginseng extract; SEM, standard error of the mean.
Alanine aminotransferase/serum, glutamate-pyruvate transaminase and aspartate aminotransferase/serum glutamic-oxaloacetic transaminase are biomarkers for liver function. However, elevated levels of these enzymes indicate hepatic anomaly or related organ diseases [16]. While alanine aminotransferase is a more specific indicator of hepatic inflammation, aspartate aminotransferase is more closely linked with myocardial infarction, renal diseases, acute pancreatitis, and some forms of anemia. However, both these enzymes are equally important parameters for the evaluation of liver function [17]. As shown in Figs. 1B and 1C, serum glutamate-pyruvate transaminase and glutamic-oxaloacetic transaminase were both inhibited by Rg3-RGE in a dose-dependent manner when compared with the control group. Atherosclerosis successfully developed in LDL–/– mice whose liver functions diminished after the consumption of a high-fat diet, which can lead to hepatic pathologies such as steatohepatitis and later to liver cirrhosis [18]. Histological examination of the liver tissue from these mice clearly showed inflammation and fat accumulation leading to steatohepatitis. This eventually decreased by Rg3-RGE treatment, as shown in Fig. 1D (a–e).
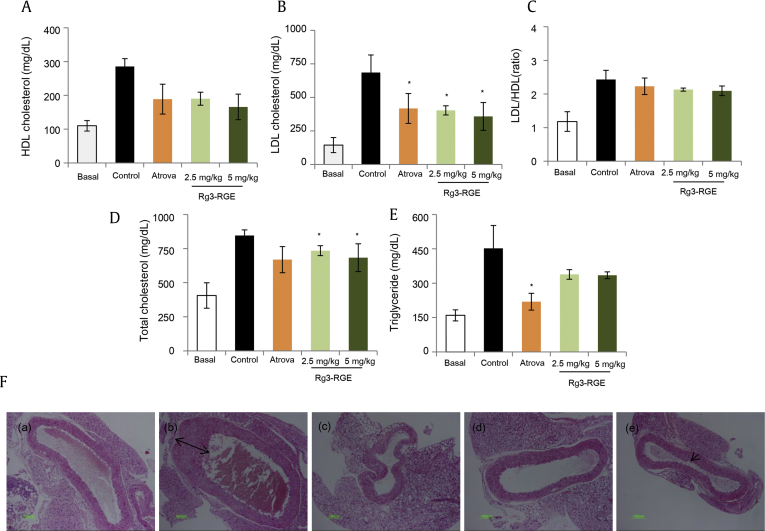
In this study, we used an LDL–/– mouse model to investigate atherosclerosis and relate it to diabetes [19]. We evaluated the levels of high-density lipoproteins, LDL, triglycerides, and total cholesterol in serum samples. As indicated in Figs. 2A–E, Rg3-RGE decreased total cholesterol and LDL levels. Moreover, the histological examination of the aortas also showed that endothelial thickening (as indicated by black arrows), associated with a high-fat diet and leading to atheromatic plague formation [20], potentially decreased by Rg3-RGE treatment [Fig. 2F (a–e)]. In summary, we can conclude that our study has demonstrated a comprehensive link between diabetes and atherosclerosis, and showed their alleviation using a natural herbal product, i.e., Rg3-RGE. With future studies, we will be able to further clarify the link between diabetes and CVDs.
Fig. 2.
Inhibition of cholesterol parameters by Rg3-RGE. (A–E) The levels of LDL, HDL, total cholesterol, and triglycerides were measured in the serum of normal and LDL–/– mice fed a WD, and treated with Rg3-RGE and atorvastatin. Bar graphs represent means ± SEM (n = 6). A p value of <0.05 was considered significant compared with the atorvastatin group (positive control). The H&E staining of aortic sections indicates thickening of the endothelial layer in WD-fed LDL–/– mice, which was attenuated by Rg3-RGE treatment. (F) This figure part represents the following: (a) normal aortic sections of mice fed with a regular chow diet; (b) LDL–/– mice fed a WD for 12 wk; (c) LDL–/– mice treated with atorvastatin 5 mg/kg as positive control; and LDL–/– mice treated with Rg3-RGE at (d) 2.5 mg/kg and (e) 5 mg/kg. HDL, high-density lipoprotein; H&E, hematoxylin and eosin; LDL, low-density lipoprotein; Rg3-RGE, Rg3-enriched red ginseng extract; SEM, standard error of the mean; WD, western diet.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Korean Society of Ginseng (2015).
References
- 1.Haskell W.L. Cardiovascular disease prevention and lifestyle interventions: effectiveness and efficacy. J Cardiovasc Nurs. 2003;18:245–255. doi: 10.1097/00005082-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Grundy S.M., Benjamin I.J., Burke G.L., Chait A., Eckel R.H., Howard B.V., Mitch W., Smith S.C., Jr., Sowers J.R. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 3.Malmberg K., Yusuf S., Gerstein H.C., Brown J., Zhao F., Hunt D., Piegas L., Calvin J., Keltai M., Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non–Q-wave myocardial infarction. Circulation. 2000;102:1014–1019. doi: 10.1161/01.cir.102.9.1014. [DOI] [PubMed] [Google Scholar]
- 4.Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., Pober J.S., Wick T.M., Konkle B.A., Schwartz B.S. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 5.Verma S., Anderson T.J. The ten most commonly asked questions about endothelial function in cardiology. Cardiol Rev. 2001;9:250–252. doi: 10.1097/00045415-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Wilson P.W. Diabetes mellitus and coronary heart disease. Am J Kidney Dis. 1998;32:S89–S100. doi: 10.1053/ajkd.1998.v32.pm9820468. [DOI] [PubMed] [Google Scholar]
- 7.Coon J.T., Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chan G.H., Law B.Y., Chu J.M., Yue K.K., Jiang Z.H., Lau C.W., Huang Y., Chan S.W., Ying-Kit Yue P., Wong R.N. Ginseng extracts restore high-glucose induced vascular dysfunctions by altering triglyceride metabolism and downregulation of atherosclerosis-related genes. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/797310. 797310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Im E.J., Yayeh T., Park S.J., Kim S.H., Goo Y.K., Hong S.B., Son Y.M., Kim S.D., Rhee M.H. Antiatherosclerotic effect of Korean Red Ginseng extract involves regulator of g-protein signaling 5. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/985174. 985174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J.B., Kwon S.K., Nagar H., Jung S.B., Jeon B.H., Kim C.S., Oh J.H., Song H.J., Kim C.S. Rg3-enriched Korean Red Ginseng improves vascular function in spontaneously hypertensive rats. J Ginseng Res. 2014;38:244–250. doi: 10.1016/j.jgr.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho Y.S., Kim C.H., Kim H.N., Ha T.S., Ahn H.Y. Ginsenoside Rg3 inhibits lipopolysaccharide-induced adhesion molecule expression in human umbilical vein endothelial cell and C57BL/6 mice. Pharmazie. 2014;69:818–822. [PubMed] [Google Scholar]
- 12.Jovanovski E., Bateman E.A., Bhardwaj J., Fairgrieve C., Mucalo I., Jenkins A.L., Vuksan V. Effect of Rg3-enriched Korean Red Ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: a randomized controlled trial. J Am Soc Hypertens. 2014;8:537–541. doi: 10.1016/j.jash.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 14.McGill H.C., Jr., McMahan C.A. Determinants of atherosclerosis in the young. Pathobiological determinants of atherosclerosis in Youth (PDAY) Research Group. Am J Cardiol. 1998;82 doi: 10.1016/s0002-9149(98)00720-6. 30T–6T. [DOI] [PubMed] [Google Scholar]
- 15.Feskens E.J., Kromhout D. Glucose tolerance and the risk of cardiovascular diseases: the Zutphen study. J Clin Epidemiol. 1992;45:1327–1334. doi: 10.1016/0895-4356(92)90173-k. [DOI] [PubMed] [Google Scholar]
- 16.Vozarova B., Stefan N., Lindsay R.S., Saremi A., Pratley R.E., Bogardus C., Tataranni P.A. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51 doi: 10.2337/diabetes.51.6.1889. 1889–95. [DOI] [PubMed] [Google Scholar]
- 17.Ghouri N., Preiss D., Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology. 2010;52:1156–1161. doi: 10.1002/hep.23789. [DOI] [PubMed] [Google Scholar]
- 18.Xu X., Lu L., Dong Q., Li X., Zhang N., Xin Y., Xuan S. Research advances in the relationship between nonalcoholic fatty liver disease and atherosclerosis. Lipids Health Dis. 2015;14:158. doi: 10.1186/s12944-015-0141-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97 doi: 10.1161/01.cir.97.18.1837. 1837–47. [DOI] [PubMed] [Google Scholar]
- 20.De Vriese A.S., Verbeuren T.J., Van de Voorde J., Lameire N.H., Vanhoutte P.M. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]