Abstract
Background
Notoginsenoside Ft1 is a promising potential candidate for cardiovascular and cancer disease therapy owing to its positive pharmacological activities. However, the yield of Ft1 is ultralow utilizing reported methods. Herein, an acid hydrolyzing strategy was implemented in the acquirement of rare notoginsenoside Ft1.
Methods
Chemical profiles were identified by ultraperformance liquid chromatography coupled with quadruple-time-of-flight and electrospray ionization mass spectrometry (UPLC-Q/TOF-ESI-MS). The acid hydrolyzing dynamic changes of chemical compositions and the possible transformation pathways of saponins were monitored by ultrahigh-performance LC coupled with tandem MS (UHPLC-MS/MS).
Results and conclusion
Notoginsenoside Ft1 was epimerized from notoginsenoside ST4, which was generated through cleaving the carbohydrate side chains at C-20 of notoginsenosides Fa and Fc, and vina-ginsenoside R7, and further converted to other compounds via hydroxylation at C-25 or hydrolysis of the carbohydrate side chains at C-3 under the acid conditions. High temperature contributed to the hydroxylation reaction at C-25 and 25% acetic acid concentration was conducive to the preparation of notoginsenoside Ft1. C-20 epimers of notoginsenoside Ft1 and ST4 were successfully separated utilizing solvent method of acetic acid solution. The theoretical preparation yield rate of notoginsenoside Ft1 was about 1.8%, which would be beneficial to further study on its bioactivities and clinical application.
Keywords: chemical transformation, notoginsenoside Ft1, Panax notoginseng, saponins
1. Introduction
Panax notoginseng (Burk.) F.H. Chen (Araliaceae), well-known as a highly valuable Chinese medicine herb, has a long history of use in consequence of its paramount pharmacological activities on the treatment of trauma, and cardiovascular and cerebrovascular diseases [1], [2]. Dammarane triterpene saponins are considered to be the major bioactive constituents of P. notoginseng have been mainly classified into three types—protopanaxadiol (PPD), protopanaxatriol (PPT), and octillol—according to their genuine aglycone moieties [3], [4]. The compositions and contents of saponins isolated from the leaves and stems of P. notoginseng (LSPN) are significantly different from underground parts in that LSPN mainly contains plentiful PPD-type saponins, such as ginsenosides Rb3, Rb1, Rc, and notoginsenoside Fc [5], [6], [7], [8].
In recent decades, many researchers have concentrated on the saponin conversion of Panax herbs to reveal effective pharmacological gradients, increase molecular diversity, and obtain rare ginsenosides through processing by various methods, including heating, acid hydrolysis, and microbial and enzymatic transformation [9], [10]. Heating is applied to convert thermally unstable malonyl-ginsenosides into neutral ginsenosides and generate less polar ginsenosides possessing better or other biological activities [11], [12], [13]. A multitude of new dammarane glycosides have been isolated from the acidic deglycosylation of saponins from the roots of P. notoginseng, such as notoginsenosides T1, T3, and T5 [14], [15]. It is reported that treating red ginseng with citric acid significantly increased (> 2-fold) on the yield of ginsenoside Rg3 [16]. In addition, chemical conversion mechanisms of 20(S)-protopanaxatriol type ginsenosides in formic acid solution have been identified, which involve hydrolysis of sugar moieties, dehydration, and hydration addition reactions at corresponding carbon positions [17].
In previous work, our team revealed that notoginsenoside Ft1 had obvious pharmacological activities on angiogenesis promotion, hemostatic effect, vasodilation, and proapoptotic effect of on human neuroblastoma SH-SY5Y cells [18], [19], [20], [21]. Notoginsenoside Ft1 has been firstly found and separated from the crude notoginseng-leaf saponins and further handled with EtOH/AcOH solution to enhance the molecular diversity of ginsenosides [22]. The yield of notoginsenoside Ft1 is very low when using the reported isolation method, seriously hindering further studies on its relevant bioactivities and clinical applications. It is therefore of great value to develop an alternative and sustainable method for scaled preparation of notoginsenoside Ft1. Herein, an acid hydrolyzing strategy was implemented in the acquirement of rare notoginsenoside Ft1. Considering the shortage of time and resource consuming in acidic hydrolysis by employing purified precursor individuals of notoginsenoside Ft1, such as notoginsenoside Fa and Fc, isolated from P. notoginseng, saponins of LSPN were set as the hydrolyzing object to obtain notoginsenoside Ft1 directly. In view of the fact that total saponin of LSPN includes a variety of ginsenosides, it is necessary to clarify the composition of the ginsenosides before and after the acid hydrolysis reaction. To achieve this purpose, we first, we identified the chemical profiles of transformation products and demonstrated possible transformation pathways of saponins by ultraperformance liquid chromatography coupled with quadruple-time-of-flight and electrospray ionization mass spectrometry (UPLC-Q/TOF-ESI-MS). Meanwhile, this study elucidated the dynamic changes of compositions and investigated the crucial influence factors to improve the productivity of notoginsenoside Ft1 in the acidic transformation processing of LSPN saponins. Furthermore, the targeted transformation product notoginsenoside Ft1 and other five compounds were successfully separated and a solvent method of acetic acid solution was developed to achieve the C-20 epimers resolution of notoginsenoside Ft1 and ST4 for the first time.
2. Materials and methods
2.1. Chemicals and reagents
Leaf and stem saponins of P. notoginseng (PNLSS) were supplied by Wenshan Qi Dan Pharmaceutical Co., Ltd. (Wenshan, Yunnan, China). Ginsenoside standards were obtained from the Shanghai R&D Centre for Standardization of Traditional Chinese Medicine (Shanghai, China). Methanol and acetonitrile of highperformance liquid chromatography (HPLC) grade were purchased from Fisher Scientific Co. (Santa Clara, CA, USA). Leucine–enkephalin, formic acid and sodium acetate were purchased from Sigma–Aldrich (St Louis, MO, USA). Glacial acetic acid was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ultrapure water was prepared with the Milli-Q system (Millipore, Bedford, MA, USA). All other chemicals and regents were of analytical grade or better.
2.2. Acidic hydrolysis of PNLSS
A solution of PNLSS (12 mg) in 6 mL of 25% (w/v) acetic acid was reacted at 55°C. A hydrolytic sample was withdrawn at regular times ranging from 0 h to 24 h, then diluted with a certain amount of methanol and filtered through a syringe filter membrane (0.22 μm) ahead of analysis. To disclose temperature effects on saponin conversion, the reactions were conducted as the conditions before, but at 25°C, 40°C, 55°C, and 70°C for 3 h, respectively. Similarly, the experiments were performed at 55°C for 3 h with different concentrations of acetic acid (2%, 10%, 25%, and 40%).
2.3. Targeted compounds preparation
Column chromatography (CC) was accomplished with silica gel (200−300 mesh; Qingdao Makall Group Co., Ltd., Qingdao, China) and Rp-C8 (40–63 μm; YMC Co., Ltd., Kyoto, Japan). Rp-HPLC was conducted Agilent 1100 systems (Agilent, Santa Clara, CA, USA) equipped with YMC Pack PRO C18 column (250 × 10 mm, inner diameter, 5 μm; YMC Co., Ltd.) and refractive index detector (RID). Nuclear magnetic resonance (NMR) spectra was carried out on a Bruker AV 400 NMR spectrometer in C5D5N.
PNLSS (100 g) was added to 20 L of 25% (w/v) acetic acid and reacted at 55°C for 5 h. The precipitate (10.2 g) was collected by centrifugation at 2500 g for 10 min, and supernatant was evaporated to dryness under vacuum to obtain acid hydrolysates of PNLSS (AHPNLSS, 82.5g). The precipitate (10 g) was chromatographed on a silica gel column by step gradients eluting with CHCl3–MeOH–H2O (9:1:0.1, 8:2:0.2, and 7.5:2.5:0.5; v/v) to obtain compound A (1.5g), compound B (4.4 g), and compound C (580 mg). PNLSS (50 g) was subjected to silica gel CC using the same elute way to obtain mixture 1-1 (10.3 g) and 1-2 (5.2 g). Mixture 1-2 (4.6 g) was chromatographed on Rp-C8 CC eluted with different concentrations of methanol solution (MeOH-H2O, 70% to 100%, v/v) to acquire fractions 1-2-1 (1.5 g) and 1-2-2 (1.4 g). The 1-2-1 fraction (50 mg) was isolated further by Rp-HPLC-RID (CH3CN:H2O, 63:37, v/v) to yield compound E (15 mg) and compound F (22 mg).
The epimer mixture of 1-2-1 was resolved in 0.5 mL of 2%, 5%, 10%, 25%, and 40% acetic acid solutions at the final concentration of 4 mg/mL. Five sample resolutions were stored at 20°C, investigated the process of precipitation and analyzed by UHPLC-ESI-MS at different time points.
2.4. UPLC-Q/TOF-ESI-MS analysis
Chemical profiles of untreated PNLSS and its acetic acid degradation products were assigned by a Waters Acquity UPLC-Q/TOF-ESI-MS (Waters, Milford, MA, USA). The chromatographic separation was conducted on an Acquity UPLC HSS T3 column (100 mm × 2.1 mm inner diameter, 1.8 μm; Waters) with a constant flow rate of 0.4 mL/min at 45°C. The mobile phase of 0.1% formic acid (A) and acetonitrile (B) was programmed as follows: 0–2 min (15–30% B), 2–8 min (30–35% B), 8–10 min (35–42% B), 10–15 min (42–44% B), 15–21 min (44–55% B), 21–22 min (55% B), 22–24 min (55–70% B), 24–25 min (90% B). MS was acquired in negative mode by scanning m/z ranges from 100 Da to 1,500 Da. Acquisition was corrected by an external reference (lock spray) consisting of a solution of (2 μg/mL) leucine enkephalin (m/z 554.2615). The conditions of ESI were set as follows: capillary voltages, 2,600 V; sampling cone, 40 V; source temperature, 120°C; desolvation gas temperature 450°C; desolvation gas, 800 L/h; cone gas, 50 L/h.
2.5. UHPLC-MS/MS analysis
The relatively quantitative analysis procedures were carried out using an Agilent 1290 series UHPLC (Agilent Technologies, Waldbronn, Germany) and an Agilent 6410 Triple Quadrupole mass spectrometer equipped with an electrospray ionization source. Chromatographic separations were conducted on an Acquity UPLC HSS T3 column (100 mm × 2.1 mm inner diameter, 1.8 μm; Waters) with a constant flow rate of 0.4 mL/min at 45°C, using a mobile phase of 0.1% formate acid with 5mM ammonium acetate (A) and acetonitrile (B). Separation was performed by gradient elution: 0–2 min (15–30% B), 2–8 min (30–35% B), 8–10 min (35–42% B), 10–13 min (42–43% B), 14–17 min (43–44% B), 17–23 min (44–55% B), 24–26 min (55–70% B), 26–28 min (70–90% B), 28–30 min (90% B), 30–30.01 min (90–15% B), 30.01–32 min (15% B). MS analysis data were collected in the negative ion multiple reaction monitoring mode with capillary voltage (3,400 V) to determine target compounds. Temperatures of desolvation gas and electrospray source were set at 300°C and 100°C, respectively. The m/z of precursor/product ions, fragment electric voltage and collision energy were summarized in Table 1.
Table 1.
Mass spectrometric parameters for internal standard (digoxin) and analytes
| Analyte | Precursor/product ions | FE (V) | CE (eV) |
|---|---|---|---|
| Digoxin | 779.4/649.4 | 260 | 35 |
| Notoginsenoside Fa | 915.6/621.4 | 255 | 34 |
| Notoginsenoside Fc | 1239.8/1107.6 | 220 | 52 |
| Vina-ginsenoside R7 | 1077.6/945.7 | 205 | 48 |
| Notoginsenoside ST4 | 915.6/621.4 | 255 | 34 |
| Notoginsenoside Ft1 | 915.6/621.4 | 255 | 34 |
| 25-OH (20S/R)-ginsenoside Rg3 | 783.6/621.5 | 210 | 35 |
| 20 (R/S)-Ginsenoside Rg3 | 783.6/621.5 | 210 | 35 |
| 20 (S)-Ginsenoside Rh2 | 783.6/621.5 | 210 | 35 |
CE = collision energy; FE = fragment electric voltage.
3. Results and discussion
3.1. Chemical profiles of PNLSS and AHPNLSS
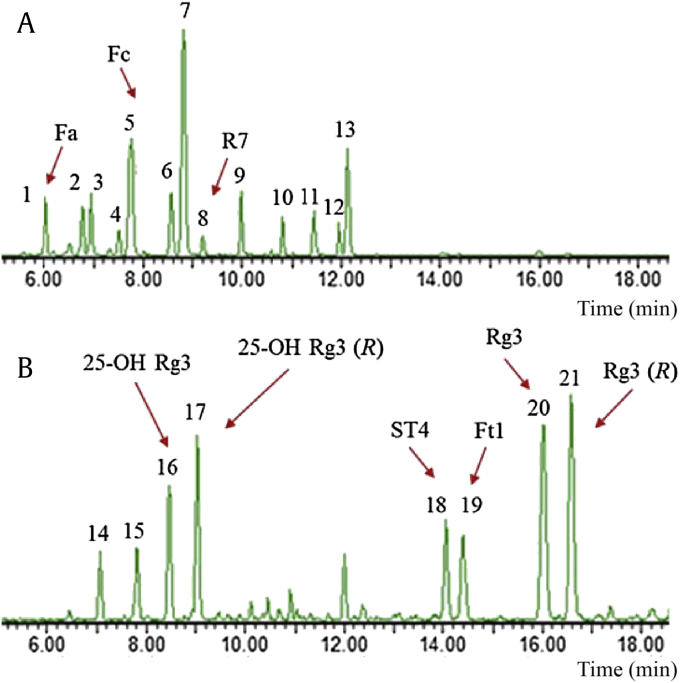
UPLC-Q/TOF-ESI-MS was applied to identify the chemical profiles of PNLSS and AHPNLSS [23], [24], [25]. Fig. 1 shows the typical and well-separated base peak ion chromatogram of the major saponins in PNLSS and AHPNLSS hydrolyzed by acetic acid in the negative ion mode ranging from 6 min to 18 min, which was indicative of an entire difference on saponins between PNLSS and AHPNLSS. The information of original and chemical conversion saponins on the retention time, formula, experimental and calculated mass m/z, ppm error and fragment ions are shown in Table 2. A total of 21 major saponins were detected, 15 of which were undoubtedly assigned by comparison with purchased reference compounds (Table 2, Nos. 1, 3, 5–11, 13, 18–21) and nuclear magnetic resonance (Table 2, Nos. 16–17, isolated by our lab), and others were tentatively identified (Table 2, Nos. 2, 4, 12, 14, 15). The analysis of PNLSS showed that it contained plentiful 20(S)-PPD-type saponins, such as notoginsenoside Fa (Fa), notoginsenoside Fc (Fc) and vina-ginsenoside R7 (R7), which was in accordance with the previous reports. As shown in Fig. 1, comparing the chemical compositions in original saponins and possessed ones, more polar saponins in PNLSS appeared prior to 10 min (Peaks 1–8), while those of less polar ones in AHPNLSS mainly eluted after 13 min (Peaks 18–21). The MS2 spectra (Fig. S1A) demonstrated fragment ion of compound 18 and 19 at [M-H]− m/z 915.5335, 783.4853, 621.4380, 459.3855, 375.2950, 161.0450, and 101.0257. The characteristic fragment ion of PPD at m/z 459.3855 was tentatively generated by consecutive losses of sugar moieties, one Xyl- or Ara- unit (132 Da) and two Glc- units (162 Da). Product ions at m/z 179.0566, 161.0450, 119.0369, 113.0256, and 101.0257 also were suggestive of the presence of glucose residues. Loss of 84 Da from m/z 459.3855 to m/z 375.2950 corresponds to the alkyl side chain moiety of C6H12- at C-20 position. Peak 18 and 19 were assigned to notoginsenoside ST4 and Ft1 basing on 20(R)-PPD type compound eluted later than its 20(S) epimer [24]. The specific product ions at m/z 477 in four compounds (Peaks 14–17) showed the information of aglycone with +18 Da mass different comparing with m/z 459. Meanwhile, no absorptive signals were detected on these four compounds using ultraviolet detector. These results indicated that the hydration reaction occurred on the side chain of PPD aglycone. As shown in Fig. S1B, the observed fragment ions of m/z 801.4989, 639.4484, and 477.3987 (Peaks 16 and 17) were higher by 18 Da than that of corresponding C-20 epimer ginsenoside Rg3 and loss of 102 Da from m/z 477.3987 to m/z 375.2865 refers to the hydration side chain at the position of C-20. Peaks 15 and 16 were confirmed as 20(S) 25-OH ginsenoside Rg3 and 20(R) 25-OH Rg3 [26].
Fig. 1.
UPLC-Q/TOF-MS base peak ion chromatograms of (A) leaf and stem saponins of Panax notoginseng (PNLSS) and (B) acid hydrolysates of PNLSS in the negative ion mode ranging from 6 min to 18 min. Acid hydrolysates of PNLSS were obtained by hydrolyzing in acetic acid for 3 h at 55°C. UPLC-Q/TOF-MS analysis was performed under the conditions described in the Materials and methods section. UPLC-Q/TOF-MS, ultraperformance liquid chromatography coupled with quadruple-time-of-flight mass spectrometry
Table 2.
Chemical profiles of leaf and stem saponins of Panax notoginseng and acid hydrolysates of leaf and stem saponins of Panax notoginseng
| No. | tR (min) | Identification | Formula | [M–H]− |
Diff. (ppm) | MS/MS fragment ion (m/z) | |
|---|---|---|---|---|---|---|---|
| m/z | Calc. m/z | ||||||
| 1 | 6.03 | Notoginsenoside Fa | C59H100O27 | 1,239.6427 | 1,239.6374 | 4.3 | 1107.5961 [M-H-(Xyl-H2O)]−, 945.5395 [M-H-2(Xyl-H2O)]−, 783.4975 [M-H-2(Glc-H2O)-(Xyl-H2O)]−, 621.4380 [M-H-3(Glc-H2O)-(Xyl-H2O)]−, 459.3855 [M-H-4(Glc-H2O)-(Xyl-H2O)]− |
| 2 | 6.76 | Notoginsenoside Ra1/Ra2/isomer | C58H98O26 | 1,209.6322 | 1,209.6268 | 4.5 | 1077.5870 [M-H-(Xyl-H2O)]−, 945.5466 [M-H-(Xyl-H2O)-(Ara-H2O)]−, 783.4895 [M-H-(Glc-H2O)-(Xyl-H2O)-(Ara-H2O)]−, 621.4404 [M-H-2(Glc-H2O)-2(Xyl-H2O)]−, 459.3842 [M-H-3(Glc-H2O)-2(Xyl-H2O)]− |
| 3 | 6.94 | Ginsenoside Rb1 | C59H92O23 | 1,107.5961 | 1,107.5951 | 0.9 | 945.5395 [M-H-(Glc-H2O)]−, 783.4975 [M-H-2(Glc-H2O)]−, 621.4380 [M-H-3(Glc-H2O)]−, 459.3761 [M-H-4(Glc-H2O)]− |
| 4 | 7.51 | Notoginsenoside Ra1/Ra2/isomer | C58H98O26 | 1,209.6283 | 1,209.6268 | 1.2 | 1077.5830 [M-H-(Xyl-H2O)]−, 945.5395 [M-H-(Xyl-H2O)-(Ara-H2O)]−, 783.4975 [M-H-(Glc-H2O)-(Xyl-H2O)-(Ara-H2O)]−, 621.4380 [M-H-2(Glc-H2O)-(Xyl-H2O)-(Ara-H2O)]−, 459.3855 [M-H-3(Glc-H2O)-(Xyl-H2O)-(Ara-H2O)]− |
| 5 | 7.75 | Notoginsenoside Fc | C58H98O26 | 1,209.6302 | 1,209.6268 | 2.8 | 1077.5864 [M-H-(Xyl-H2O)]−, 945.5421 [M-H-2(Xyl-H2O)]−, 783.4896 [M-H-(Glc-H2O)-2(Xyl-H2O)]−, 621.4360 [M-H-2(Glc-H2O)-2(Xyl-H2O)]−, 459.3808 [M-H-3(Glc-H2O)-2(Xyl-H2O)]−, |
| 6 | 8.56 | Ginsenosdie Rb2 | C53H90O22 | 1,077.5865 | 1,077.5845 | 1.9 | 945.5397 [M-H-(Ara-H2O)]−, 783.4877 [M-H-(Glc-H2O)-(Ara-H2O)]−, 621.4351 [M-H-2(Glc-H2O)-(Ara-H2O)]−, 459.3806 [M-H-3(Glc-H2O)-(Ara-H2O)]− |
| 7 | 8.82 | Ginsenosdie Rb3 | C53H90O22 | 1,077.5850 | 1,077.5845 | 0.5 | 945.5420 [M-H-(Xyl-H2O)]−, 783.4884 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 621.4358 [M-H-2(Glc-H2O)-(Xyl-H2O)]−, 459.3809 [M-H-3(Glc-H2O)-(Xyl-H2O)]− |
| 8 | 9.20 | Vina-ginsenoside R7 | C53H90O22 | 1,077.5830 | 1,077.5845 | −1.4 | 945.5395 [M-H-(Xyl-H2O)]−, 783.4853 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 621.4380 [M-H-2(Glc-H2O)-(Xyl-H2O)]−, 459.3855 [M-H-3(Glc-H2O)-(Xyl-H2O)]− |
| 9 | 9.98 | Ginsenoside Rd | C48H82O18 | 945.5418 | 945.5423 | −0.5 | 783.4891 [M-H-(Glc-H2O)]−, 621.4355 [M-H-2(Glc-H2O)]−, 459.3811 [M-H-3(Glc-H2O)]− |
| 10 | 10.82 | Gypenoside XVII | C48H82O18 | 945.5395 | 945.5423 | −3.0 | 783.4853 [M-H-(Glc-H2O)]−, 621.4380 [M-H-2(Glc-H2O)]−, 459.3855 [M-H-3(Glc-H2O)]− |
| 11 | 11.46 | Notoginsenoside Fe | C47H80O17 | 915.5306 | 915.5317 | −1.2 | 783.4877 [M-H-(Araf-H2O)]−, 621.4353 [M-H-(Glc-H2O)-(Araf-H2O)]−, 459.3826 [M-H-2(Glc-H2O)-(Araf-H2O)]− |
| 12 | 11.96 | Vinaginsenoside R18 | C47H80O17 | 915.5335 | 915.5317 | 2.0 | 783.4853 [M-H-(Xyl-H2O)]−, 621.4380 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 459.3855 [M-H-2(Glc-H2O)-(Xyl-H2O)]− |
| 13 | 12.14 | Notoginsenoside Fd | C47H80O17 | 915.5317 | 915.5317 | 0.0 | 783.4865 [M-H-(Xyl-H2O)]−, 621.4359 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 459.3830 [M-H-2(Glc-H2O)-(Xyl-H2O)]− |
| 14 | 7.06 | Notoginsenoside Ft2 | C47H82O18 | 933.5405 | 933.5423 | 1.8 | 801.4982 [M-H-(Xyl-H2O)]−, 639.4484 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 477.3987 [M-H-2(Glc-H2O)-(Xyl-H2O)]− |
| 15 | 7.80 | 20(R)-Notoginsenoside Ft2 | C47H82O18 | 933.5405 | 933.5423 | 1.8 | 801.4982 [M-H-(Xyl-H2O)]−, 639.4484 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 477.387 [M-H-2(Glc-H2O)-(Xyl-H2O)]− |
| 16 | 8.45 | 25-OH Ginsenoside Rg3 | C42H74O14 | 801.4989 | 801.5000 | 1.1 | 639.4484 [M-H-(Glc-H2O)]−, 477.3987 [M-H-2(Glc-H2O)]− |
| 17 | 9.03 | 20(R)-25-OH Rg3 | C42H74O14 | 801.4984 | 801.5000 | 1.1 | 639.4484 [M-H-(Glc-H2O)]−, 477.3987 [M-H-2(Glc-H2O)]− |
| 18 | 14.05 | Notoginsenoside ST4 | C47H80O17 | 915.5355 | 915.5317 | 3.8 | 783.4853 [M-H-(Xyl-H2O)]−, 621.4380 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 459.3855 [M-H-2(Glc-H2O)-(Xyl-H2O)]− |
| 19 | 14.40 | Notoginsenoside Ft1 | C47H80O17 | 915.5355 | 915.5317 | 3.8 | 783.4853 [M-H-(Xyl-H2O)]−, 621.4380 [M-H-(Glc-H2O)-(Xyl-H2O)]−, 459.3855 [M-H-2(Glc-H2O)-(Xyl-H2O)]− |
| 20 | 16.00 | Ginsenoside Rg3 | C42H72O13 | 783.4898 | 783.4895 | −0.8 | 621.4380 [M-H-(Glc-H2O)]−, 459.3855 [M-H-2(Glc-H2O)]− |
| 21 | 16.58 | 20(R)-Ginsenoside Rg3 | C42H72O13 | 783.4898 | 783.4895 | −0.8 | 621.4380 [M-H-(Glc-H2O)]−, 459.3855 [M-H-2(Glc-H2O)]− |
3.2. Studies on the chemical transformation of PNLSS
The relatively quantitative analysis procedure was carried out by UHPLC-ESI-MS with multiple reaction monitoring to study the chemical transformation. To acquire good resolution and sensitivity on saponins in PNLSS and AHPNLSS, UHPLC-MS conditions were optimized by applying different elution gradients and mobile phases. Eventually, as stated in the method of UHPLC-ESI-MS analysis, a mobile phase of 0.1% formate acid with 5mM ammonium acetate and acetonitrile were selected to successfully separate and detect targeted compounds by a binary gradient elution system. The ratio of ESI peak areas of determined ginsenosides to internal standard (Digoxin) was assigned as the relative response values.
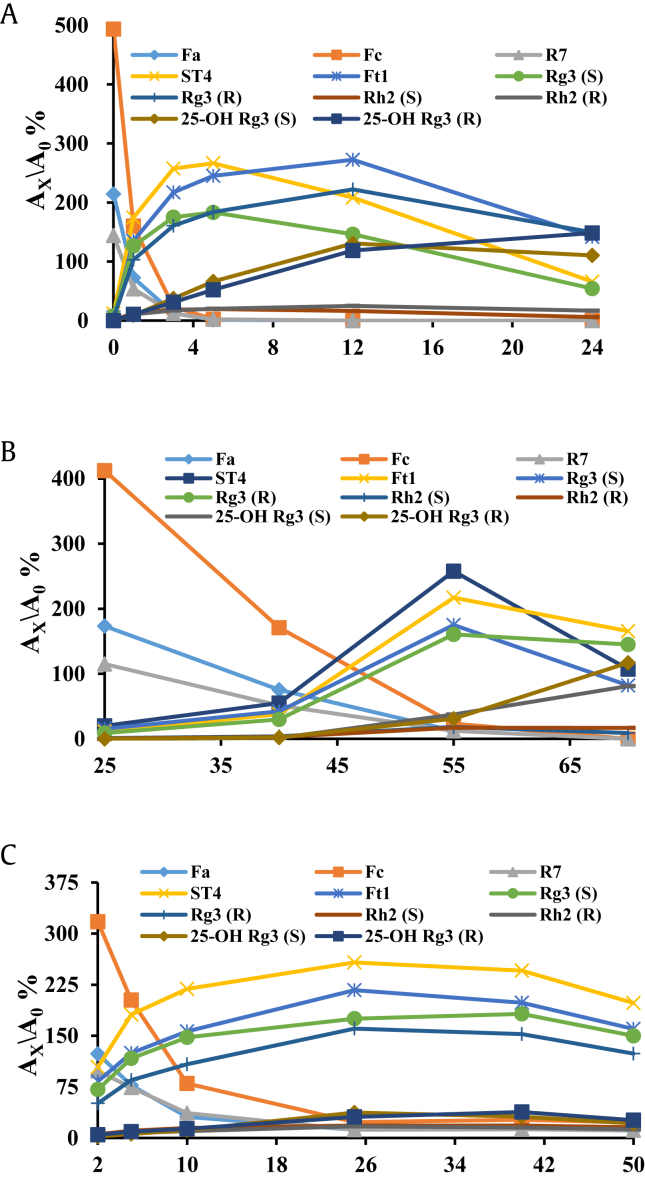
The chemical compositions of AHPNLSS were obviously different from those in PNLSS and saponins conversion started at an early stage during the acid treating process. As shown in Fig. 2A, Fa, Fc, and R7 rapidly decreased and almost disappeared after acid hydrolyzing for 5 h under the given conditions. Meanwhile, four pairs of epimers at C-20 (Ft1, Rg3, 25-OH Rg3 and Rh2), the acid degradation products, steadily accumulated in the first 5 h. Compared to the other three pairs of epimers, 25-OH 20(R/S) Rg3 was significantly increased during the whole acid treating process by the degradation of saponins, and the content of 25-OH 20(R)-Rg3 was a little higher than its epimer at later process of acid treatment. It is worth noting that changeable trends on the contents of Rg3 and Ft1 including their C-20 epimers indicated that the form of 20(S) saponins were epimerized into 20(R) form during the whole process via the configurational conversion. As shown in Fig. 3, 20(S) form saponins in AHPNLSS were firstly dehydrated under the acid conditions, then generated carbocation intermediates at C-20, which next formed an oxonium ion by rehydration reaction, resulting in the inversion of configuration at C-20 and creating the corresponding epimer [27]. In addition, the contents of notoginsenoside ST4 and 20(R)-ginsenoside Rg3 were much higher than their corresponding 20(S) epimer when the reaction lasted for 12 h in acid conditions. Comparing the effects of chemical conversions at different temperatures, it is obvious that temperature directly played an important role in the degradation of PNLSS and the generation of rare valuable secondary glycosides. To speed up the hydrolyzing conversion reaction and demonstrate the temperature effects on targeted chemical compositions, experiments were conducted in various temperatures ranging from 25°C to 70°C in other certain conditions. Fig. 2B revealed that the level of 20(R/S)-notoginsenoside Ft1 and ginsenoside 20(R/S)-Rg3 were increased sharply in AHPNLSS at 55°C as well as 25-OH 20(R/S)-Rg3 at 70°C, from another point of view, which implied that 20(R/S)-Rg3 was beneficially converted to 20(R/S)-Rg3 at higher temperature. Beyond that, the four generated isomer pairs of saponins shown in Fig. 2B were gradually decreased with the temperature increasing from 55°C to 70°C; meanwhile the content of 20(R) former saponins were higher than their C-20 isomers, which might be that the reason for the nucleophile (H2O) attacking the carbocation intermediate more easily, resulting in more inversion of configuration than retention of configuration at relatively high temperature. This phenomenon, to some extent, implies that temperature should be properly controlled to obtain the targeted rare saponins. Given the results presented in Fig. 2C, the contents of all destination products were decreased at higher acetic acid concentrations, which would be transformed other degraded and side chain modified glycosides or aglycones. The acetic acid concentration of 25% (w/v) was sufficient for converting Fa, Fc, and R7 to ST4, Ft1, 20(R/S)-Rg3, and 20-OH (R)-Rg3. These significant chemical constituent changes of AHPNLSS in different acid concentration treating would markedly influence the pharmacological activities.
Fig. 2.
The acid hydrolyzing dynamic changes of chemical compositions in leaf and stem saponins of Panax notoginseng (PNLSS). The reaction conditions of acidic hydrolysis of PNLSS were described in the Materials and methods section. Y axis label (Ax/A0 %) represents the ratio of electrospray ionization peak areas of determined ginsenosides to internal standard (digoxin) and assigned as the relative response values. (A) Chemical transformation of PNLSS was monitored at 0 h, 1 h, 3 h, 5 h, 12 h, 24 h, 36 h, and 60 h, respectively. (B) Effect of temperatures on transformation of saponins at 25°C, 40°C, 55°C, and 70°C for 3 h, respectively. (C) Effect of acetic acid concentrations on conversion of saponins at 55°C for 3 h with 2%, 10%, 25% and 40% concentrations of acetic acid
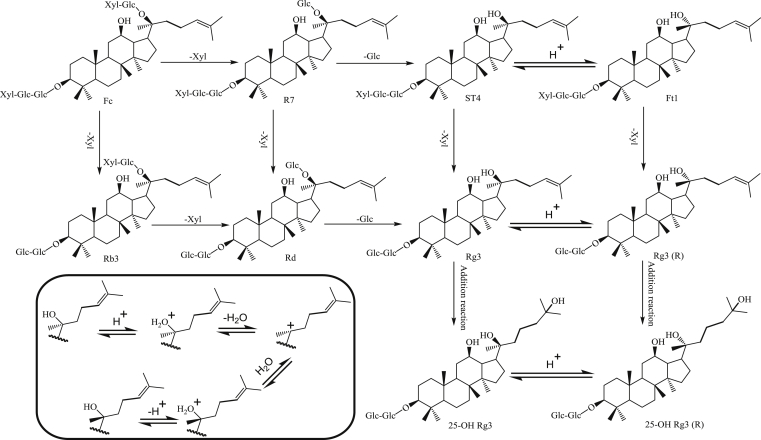
According to degradation products, transformation mechanisms of PNLSS under predefined acidic conditions were deduced mainly involving in the hydrolysis of sugar moieties at C-3/C-20, epimerization of C-20 configuration and hydration addition reactions at C-24/C-25. It is possible that Fa, Fc, and R7 could be transformed into notoginsenoside ST4 in acetic acid resolutions based on the sugar moieties. To illustrate acid degradation mechanism of saponins with detail, the chemical transformation pathways of Fc are shown in Fig. 3. Fc might be converted to ST4 by gradually cleaving the xylose and glucose linked at C-20 or directly hydrolyzing the disaccharide unit but retain sugar moieties at C-3. Ginsenoside Rg3 could be generated through multiple degradation sources, such as Rb3, Rd and the intermediate product of Fc, Fa, and R7, which explains why AHPNLSS was abundant in Rg3 and implied sugars linked at C-20 were more easily hydrolyzed than C-3 in PPD-type saponins [28].
Fig. 3.
Chemical transformation pathways of saponins in leaf and stem saponins of Panax notoginseng under acidic conditions. The reaction conditions of acidic hydrolysis of leaf and stem saponins of Panax notoginseng are described in the Materials and methods section
3.3. Targeted compounds preparation
According to the reported NMR data, compounds A–E were identified to 20(R) ginsenoside Rh2, 20(R) ginsenoside Rg3, notoginsenoside Ft1, 20(S) 25-OH ginsenoside Rg3 and 20(R) 25-OH ginsenoside Rg3, respectively [29], [30], [31]. The epimer mixture of 1-2-1 was composed of notoginsenoside Ft1 and ST4 according to MS/MS.
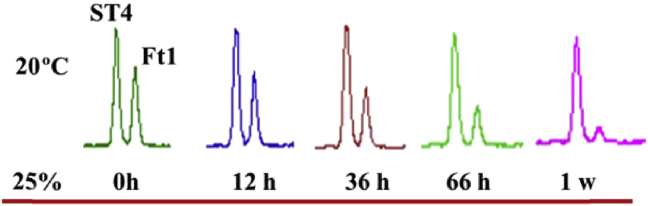
In the process of acid hydrolysis, white precipitate consisting of notoginsenoside Ft1 and other compounds was observed and separated, which prompted us to consider whether notoginsenoside Ft1 would also be precipitated from mixtures at the special condition of acid solution. Excited by this unusual finding, we have investigated on the formation of precipitate in further detail. The precipitation has been found in 25% acetic acid solution at 12 h, while not in other diverse concentration acetic acid solutions. Monitoring by UHPLC-MS/MS analysis, the relative contents of notoginsenoside Ft1 epimer were 58.2%, 63.5%, 68.8%, 75.4%, and 87.1% at 0 h, 12 h, 36 h, 66 h, and 1 wk, respectively, which gradually decreased with the extension of time in 25% acetic acid solution at 20°C (Fig. 4). Ft1 was obtained by centrifugation, washed in distilled water and further died under reduced pressure. As expected, the purity is about 95.2% of Ft1 identified by UHPLC-MS/MS, which testified the effective and simplified epimer separation method of Ft1 and ST4. Fraction 1-2-2 (100 mg) was further subjected to the solvent method of 25% acetic acid to yield notoginsenosides Ft1 (51 mg) and ST4 (compound F, 35 mg) [32]. According to this paper's described preparation method, the theoretical preparation yield rate of notoginsenoside Ft1 is about 1.8% from the leaf and stem extracts of P. notoginseng.
Fig. 4.
Extracted chromatograms of supernatant by UHPLC-MS/MS at different times. UHPLC-MS/MS analysis was performed under the conditions described in the UHPLC-MS/MS analysis section of Materials and methods. UHPLC-MS/MS, ultrahigh-performance liquid chromatography coupled with tandem mass spectrometry
4. Conclusions
Compared to other acid hydrolysis approaches on ginsenosides, this study demonstrated possible transformation pathways of saponins, elucidated the dynamic changes of compositions, and successfully separated C-20 epimers of notoginsenosides Ft1 and ST4 for the first time. Notoginsenoside Ft1 was epimerized from notoginsenoside ST4 generated by cleaving the carbohydrate side chains at C-20 of Fa, Fc, and R7 and further converted to other compounds via hydroxylation at C-25 or hydrolysis of the carbohydrate side chains at C-3 under the acid conditions. High temperature contributed to the hydroxylation reaction at C-25 and 25% acetic acid concentration and is conducive to the preparation of notoginsenoside Ft1. Notoginsenoside Ft1 is gradually precipitated from epimer mixtures in 25% acetic acid solution at 20°C. In conclusion, a strategy was developed on convenient and effective preparation of notoginsenoside Ft1 from PNLSS, which would provide sufficient Ft1 for further study of its pharmacological activities and clinical application.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (U1032604), the Program for Changjiang Scholars and Innovative Research Team in University (IRT1071) and China Postdoctoral Science Foundation (2015M581654).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2016.08.009.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
MS2 spectra of (A) notogisenoside Ft1/ST4 and (B) 20(R/S) 25-OH ginsenoside Rg3 by Q/TOF-MS in negative ion mode. UPLC-Q/TOF-MS analysis was performed under the conditions described in the Materials and methods section. UPLC-Q/TOF-MS, ultraperformance liquid chromatography coupled with quadruple-time-of-flight mass spectrometry.
References
- 1.Cicero A.F.G., Vitale G., Savino G., Arletti R. Panax notoginseng (Burk.) effects on fibrinogen and lipid plasma level in rats fed on a high-fat diet. Phytother Res. 2003;17:174–178. doi: 10.1002/ptr.1262. [DOI] [PubMed] [Google Scholar]
- 2.Ng T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 3.Qi L.W., Wang C.Z., Yuan C.S. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Wan J.B., Yang F.Q., Li S.P., Wang Y.T., Cui X.M. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharmaceut Biomed Anal. 2006;41:1596–1601. doi: 10.1016/j.jpba.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Liu C., Han J.Y., Duan Y.Q., Huang X., Wang H. Purification and quantification of ginsenoside Rb3 and Rc from crude extracts of caudexes and leaves of Panax notoginseng. Sep Purif Technol. 2007;54:198–203. [Google Scholar]
- 7.Xiang H., Liu Y.X., Zhang B.B., Huang J.H., Li Y., Yang B., Huang Z.X., Xiang F.J., Zhang H.L. The antidepressant effects and mechanism of action of total saponins from the caudexes and leaves of Panax notoginseng in animal models of depression. Phytomedicine. 2011;18:731–738. doi: 10.1016/j.phymed.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Mao Q., Yang J., Cui X.M., Li J.J., Qi Y.T., Zhang P.H., Wang Q. Target separation of a new anti-tumor saponin and metabolic profiling of leaves of Panax notoginseng by liquid chromatography with eletrospray ionization quadrupole time-of-flight mass spectrometry. J Pharmaceut Biomed. 2012;59:67–77. doi: 10.1016/j.jpba.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Park C.S., Yoo M.H., Noh K.H., Oh D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biot. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang D., Liao P.Y., Zhu H.T., Chen K.K., Xu M., Zhang Y.J., Yang C.R. The processing of Panax notoginseng and the transformation of its saponin components. Food Chem. 2012;132:1808–1813. [Google Scholar]
- 11.Kwon S.W., Han S.B., Park I.H., Kim J.M., Park M.K., Park J.H. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J Chromatogr A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- 12.Du X.W., Wills R.B.H., Stuart D.L. Changes in neutral and malonyl ginsenosides in American ginseng (Panax quinquefolium) during drying, storage and ethanolic extraction. Food Chem. 2004;86:155–159. [Google Scholar]
- 13.Sun S., Wang C.Z., Tong R., Li X.L., Fishbein A., Wang Q., He T.C., Du W., Yuan C.S. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010;118:307–314. [Google Scholar]
- 14.Teng R.W., Li H.Z., Wang D.Z., Yang C.R. Hydrolytic reaction of plant extracts to generate molecular diversity: new dammarane glycosides from the mild acid hydrolysate of root saponins of Panax notoginseng. Helv Chim Acta. 2004;87:1270–1278. [Google Scholar]
- 15.Cao J.Q., Peng F., Zhao Y.Q. Isolation and identification of a new compound from acid hydrolysate of saponin in stems and leaves of Panax notoginseng. Chin Tradit Herb Drugs. 2013;44:137–140. [Google Scholar]
- 16.Yi J.H., Kim M.Y., Kim Y.C., Jeong W.S., Bae D.W., Hur J.M., Jun M. Change of ginsenoside composition in red ginseng processed with citric acid. Food Sci Biotechnol. 2010;19:647–653. [Google Scholar]
- 17.Wu W., Qin Q.J., Guo Y.Y., Sun J.H., Liu S.Y. Studies on the chemical transformation of 20(S)-protopanaxatriol (PPT)-type ginsenosides Re, Rg2, and Rf using rapid resolution liquid chromatography coupled with quadruple-time-of-flight mass spectrometry (RRLC-Q-TOF-MS) J Agr Food Chem. 2012;60:10007–10014. doi: 10.1021/jf302638f. [DOI] [PubMed] [Google Scholar]
- 18.Shen K.K., Ji L.L., Gong C.Y., Ma Y.B., Yang L., Fan Y., Hou M.Q., Wang Z.T. Notoginsenoside Ft1 promotes angiogenesis via HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways. Biochem Pharmacol. 2012;84:784–792. doi: 10.1016/j.bcp.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Shen K.K., Leung S.W.S., Ji L.L., Huang Y., Hou M.Q., Xu A.M., Wang Z.T., Vanhoutte P.M. Notoginsenoside Ft1 activates both glucocorticoid and estrogen receptors to induce endothelium-dependent, nitric oxide-mediated relaxations in rat mesenteric arteries. Biochem Pharmacol. 2014;88:66–74. doi: 10.1016/j.bcp.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Gao B., Huang L., Liu H., Wu H., Zhang E., Yang L., Wu X., Wang Z. Platelet P2Y12 receptors are involved in the haemostatic effect of notoginsenoside Ft1, a saponin isolated from Panax notoginseng. Br J Pharmacol. 2014;171:214–223. doi: 10.1111/bph.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao B., Shi H.L., Li X., Qiu S.P., Wu H., Zhang B.B., Wu X.J., Wang Z.T. p38 MAPK and ERK1/2 pathways are involved in the proapoptotic effect of notoginsenoside Ft1 on human neuroblastoma SH-SY5Y cells. Life Sci. 2014;108:63–70. doi: 10.1016/j.lfs.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Chen J.T., Li H.Z., Wang D., Zhang Y.J., Yang C.R. New dammarane monodesmosides from the acidic deglycosylation of notoginseng-leaf saponins. Helv Chim Acta. 2006;89:1442–1448. [Google Scholar]
- 23.Dan M., Xie G.X., Gao X.F., Long X.B., Su M.M., Zhao A.H., Zhao T., Zhou M.M., Qiu Y.P., Jia W. A rapid ultra-performance liquid chromatography–electrospray ionisation mass spectrometric method for the analysis of saponins in the adventitious roots of Panax notoginseng. Phytochem Analysis. 2009;20:68–76. doi: 10.1002/pca.1099. [DOI] [PubMed] [Google Scholar]
- 24.Qi L.W., Wang H.Y., Zhang H., Wang C.Z., Li P., Yuan C.S. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J Chromatogr A. 2012;1230:93–99. doi: 10.1016/j.chroma.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 25.Chu C., Xu S.J., Li X.N., Yan J.Z., Liu L. Profiling the ginsenosides of three ginseng products by LC-Q-TOF/MS. J Food Sci. 2013;78:C653–C659. doi: 10.1111/1750-3841.12102. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Song F.R., Cui M., Liu Z.Q., Liu S.Y. Investigation of the hydrolysis of ginsenosides by high performance liquid chromatography-electrospray ionization mass spectrometry. Planta Med. 2007;73:1225–1229. doi: 10.1055/s-2007-981590. [DOI] [PubMed] [Google Scholar]
- 27.Lee S.M., Kim S.C., Oh J., Kim J.H., Na M. 20(R)-Ginsenoside Rf: a new ginsenoside from red ginseng extract. Phytochem Lett. 2013;6:620–624. [Google Scholar]
- 28.Sun C.P., Gao W.P., Zhao B.Z., Cheng L.Q. Optimization of the selective preparation of 20(R)-ginsenoside Rg3 catalyzed by d, l-tartaric acid using response surface methodology. Fitoterapia. 2013;84:213–221. doi: 10.1016/j.fitote.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Chen G.T., Yang M., Song Y., Lu Z.Q., Zhang J.Q., Huang H.L., Wu L.J., Guo D.A. Microbial transformation of ginsenoside Rb1 by Acremonium strictum. Appl Microbiol Biotechnol. 2008;77:1345–1350. doi: 10.1007/s00253-007-1258-4. [DOI] [PubMed] [Google Scholar]
- 30.Dong A., Ye M., Guo H.Z., Zheng J.H., Guo D.A. Microbial transformation of ginsenoside Rb1 by Rhizopus stolonifer and Curvularia lunata. Biotechnol Lett. 2003;25:339–344. doi: 10.1023/a:1022320824000. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y.Q., Yuan C.L., Lu H.R. Isolation and identification of 20(R)-ginsenoside-Rh2: (an Anti-cancer constituent) from the fruits of Panax ginseng C. A. Meyer. Chin J Chin Mat Med. 1991;16:678–679. [PubMed] [Google Scholar]
- 32.Pei Y., Du Q., Liao P.Y., Chen Z.P., Wang D., Yang C.R., Kitazato K., Wang Y.F., Zhang Y.J. Notoginsenoside ST-4 inhibits virus penetration of herpes simplex virus in vitro. J Asian Nat Prod Res. 2011;13:498–504. doi: 10.1080/10286020.2011.571645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MS2 spectra of (A) notogisenoside Ft1/ST4 and (B) 20(R/S) 25-OH ginsenoside Rg3 by Q/TOF-MS in negative ion mode. UPLC-Q/TOF-MS analysis was performed under the conditions described in the Materials and methods section. UPLC-Q/TOF-MS, ultraperformance liquid chromatography coupled with quadruple-time-of-flight mass spectrometry.