Abstract
Background
Ginsenosides are the main ingredients of Korean Red Ginseng. They have extensively been studied for their beneficial value in neurodegenerative diseases such as Parkinson's disease (PD). However, the multitarget effects of Korean Red Ginseng extract (KRGE) with various components are unclear.
Methods
We investigated the multitarget activities of KRGE on neurological dysfunction and neurotoxicity in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–induced mouse model of PD. KRGE (37.5 mg/kg/day, 75 mg/kg/day, or 150 mg/kg/day, per os (p.o.)) was given daily before or after MPTP intoxication.
Results
Pretreatment with 150 mg/kg/day KRGE produced the greatest positive effect on motor dysfunction as assessed using rotarod, pole, and nesting tests, and on the survival rate. KRGE displayed a wide therapeutic time window. These effects were related to reductions in the loss of tyrosine hydroxylase–immunoreactive dopaminergic neurons, apoptosis, microglial activation, and activation of inflammatory factors in the substantia nigra pars compacta and/or striatum after MPTP intoxication. In addition, pretreatment with KRGE activated the nuclear factor erythroid 2–related factor 2 pathways and inhibited phosphorylation of the mitogen-activated protein kinases and nuclear factor-kappa B signaling pathways, as well as blocked the alteration of blood–brain barrier integrity.
Conclusion
These results suggest that KRGE may effectively reduce MPTP-induced neurotoxicity with a wide therapeutic time window through multitarget effects including antiapoptosis, antiinflammation, antioxidant, and maintenance of blood–brain barrier integrity. KRGE has potential as a multitarget drug or functional food for safe preventive and therapeutic strategies for PD.
Keywords: Korean Red Ginseng extract; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Multitarget effect
1. Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder. The main symptoms of PD are motor dysfunctions including bradykinesia, tremor, and muscular rigidity and nonmotor-related disorders including genitourinary problems, emotional changes, and cognitive problems [1], [2], [3]. Neuropathological hallmarks of PD are serious loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the midbrain and accumulation of α-synuclein aggregates into Lewy bodies and Lewy neuritis [4], [5]. The loss of dopaminergic neurons is implicated in various other pathogenic mechanisms, including neuroinflammation, glutamate excitotoxicity, oxidative stress, mitochondrial dysfunction, and protein aggregation. The mechanisms share overlapping and complicated features [1], [2], [3], [4], [5]. Although most PD therapies, including levodopa, provide only symptomatic relief in reducing the motor symptoms, patients receiving long-term levodopa therapy must contend with side effects including levodopa-induced dyskinesias and response oscillations [6]. Therefore, development of efficient and safe therapeutic approaches that delay the onset or forestall the progression of PD is critical.
So far, most neuroprotection trials have produced unmet (not satisfied) results in PD when they have targeted only one of the multifactorial pathways that engaged in regulating synaptic function and neuronal survival [7]. On the other hand, the recent advances in medical biology including neuropharmacology have been responsible for new insights into the multifactorial and highly complex pathological hallmarks of various neurodegenerative disorders [7], [8], [9], [10]. The development of multitargeted drugs to prevent and cure PD might be a new paradigm in the discovery and design for safe, effective, and innovative drugs. Although accumulating reports has been emphasized on characterizing and identifying potentially active natural material–derived pharmaceutical components to congruent this unmet demand [11], no approved PD-protective medicines are currently available [12]. Natural products remain an enormous untapped resource for the incessant development and discovery of therapeutic approaches for a wide range of disease conditions [13].
Panax (P.) ginseng Meyer, a perennial herb of the family Araliaceae, has been widely used as an adaptogen, particularly in the countries of East Asia for millennia. The major active constituents of P. ginseng are ginsenosides, which are derivatives of triterpenoid dammarane [14], [15]. More than 100 ginsenosides have been isolated. The most frequently studied ones in PD are ginsenoside Rb1, Rd, and Rg1 [14], [15]. In vitro, ginsenosides Rb1 and Rg1 have protective effects on mesencephalic dopaminergic cells stressed with glutamate [16]. Ginsenoside Rd mitigates neuroinflammation of dopaminergic cells that was evoked by lipopolysaccharide exposure by inhibiting inducible nitric oxide (iNOS) and cyclooxygenase (COX)-2 expressions [17]. Ginsenoside Rg1 mitigates dopamine-induced apoptosis in PC12 cells by reducing oxidative stress [18]. In vivo, Rg1 has positive effects on dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)– and lipopolysaccharide-induced rodent models of PD through antiapoptosis [19] and antiinflammation [20]. Each ginsenoside has different pharmacological effects in vivo and in vitro models of PD, which depend on the diversity of the sugar components and the number and position of the sugar moieties [14], [21], [22]. Korean Red Ginseng extract (KRGE) protected dopaminergic neurons by suppressing the cleavage of p35 to p25 [23] or by alleviating protein expression profiles related to enhancing energy metabolism in the brain of MPTP-treated mice [24]. However, the neuroprotective activities of ginseng extract in PD remain unclear. The purpose of the present study is to evaluate the multitarget effects of KRGE in a MPTP-induced mouse model of PD.
2. Materials and methods
2.1. Animals and ethical statement
Adult male C57BL/6J mice (Narabiotec Co., Ltd., Seoul, Korea, 7–9 weeks old, 22–24 g) were housed at a constant temperature of 23 ± 2°C with a 12-h light–dark cycle (lights on from 07:00 to 19:00) and were provided with food and water ad libitum. All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Kyung Hee University. In this process, proper randomization of laboratory animals and data handling were performed in a blinded manner in accordance with recent recommendations from an National Institutes of Health (NIH) workshop on preclinical models of neurological diseases [25].
2.2. Preparation of KRGE
KRGE (Korea Ginseng Corporation, Daejeon, Korea) was prepared, as previously described [26], [27], from the roots of 6-year-old fresh P. ginseng Meyer. KRGE contained major ginsenosides Rb1 (7.44 mg/g), Rb2 (2.59 mg/g), Rc (3.04 mg/g), Rd (0.91 mg/g), Re (1.86 mg/g), Rf (1.24 mg/g), Rg1 (1.79 mg/g), Rg2s (1.24 mg/g), Rg3s (1.39 mg/g), and Rh1 (1.01 mg/g) and other minor ginsenosides, as determined by high-performance liquid chromatography.
2.3. Experimental groups
First, to confirm the most effective dose and mechanism of administration of KRGE for pretreatment, mice were randomly divided into sham, MPTP, MPTP + KRGE pretreatment (35.7 mg/kg, 75 mg/kg, and 150 mg/kg), and KRGE groups (n = 5–15 per group). Second, to investigate the therapeutic time window of KRGE use, mice were randomly divided into sham, MPTP, MPTP + KRGE treatment pre-, onset-, progression-, and peak-treatment, and KRGE groups (n = 5 per group).
2.4. Treatment with MPTP and KRGE
For MPTP intoxication, mice received four intraperitoneal injection of MPTP-HCl (20 mg/kg body weight; Sigma-Aldrich, St. Louis, MO, USA) dissolved in phosphate-buffered saline at 2 h intervals in accordance with published guidelines [28]. KRGE was dissolved in physiological saline and treated orally at doses of 37.5 mg/kg, 75 mg/kg, or 150 mg/kg once daily from 1 h before the first MPTP intoxication. In addition, mice were treated with 150 mg/kg KRGE (the most effective dose) once daily from 1 h before the first MPTP intoxication and at 24, 72, or 120 h after the last MPTP intoxication. Each experiment was repeated >three times using the same protocol. The total daily dose of KRGE given to mice was determined after considering body weight, metabolic rate, and traditional dose given to humans [26], [27].
2.5. Behavioral assessments
Forelimb and hindlimb motor performance and balance were measured by the pole and rotarod tests. Briefly, mice (n = 5–10 per group) were placed head down near the top of a rough-surfaced wooden pole (40 mm in diameter and 50 cm in height). The time taken to reach the bottom of the pole was measured. One hour after the pole test, the mice were placed on a rotating cylinder (4 cm in diameter) with a coarse surface for firm grip and tested for three trials with an accelerating speed of 16 rpm/s. A cut-off time of 5 min and an intertrial interval of 15 min were used. Latency on the rod before falling was measured. The utility of nest-building behavior was measured as an indicator of health and welfare in mice. Briefly, to test the individual nest-building behavior, mice were individually housed in cages containing wood chip bedding and one square of pressed cotton (Nestlets; Ancare Corp., Bellmore, NY, USA). The following morning, the manipulation of the Nestlet and the constitution of the built nest were assessed manually according to a five-point scale: 0, no nest (>90% intact); 1, flat nest (50–90% remaining intact); 2, nest covering the mouse (<50% remains intact); 4, an identifiable but flat nest (using >90% of Nestlet); 5, perfect nest [29]. The behavioral tests were accomplished by an experimenter who was unaware of the experimental conditions and were done under constant temperature (23 ± 3°C) and humidity (55 ± 4%) in a quiet room, 1, 3, and 7 days after the final MPTP intoxication.
2.6. Histological evaluation
Seven days after the last MPTP intoxication, the brains (n = 5 per group) for histological evaluation were prepared as described previously [26], [27]. Sequential coronal sections (30 μm in thickness) were acquired on a model CM3050S freezing microtome (Leica Biosystems, Wetzlar, Germany) from the level of the SNpc (bregma −2.54 to −3.40 mm) and mid-striatum (bregma +0.26 to +1.10 mm) according to mouse brain atlas [30]. These SNpc and striatal sections were stained with 0.1% cresyl violet dye.
2.7. Immunohistochemistry
Immunohistochemical analysis of SNpc and striatal sections was accomplished as previously described [26], [27]. In brief, the sections (n = 3 per brain) from each group (n = 5-7 per group) were incubated with rabbit anti-tyrosine hydroxylase (TH) (1:1,000; Millipore, Bedford, MA, USA), rabbit anti-cleaved caspase-3 (1:500; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-ionized calcium-binding adapter molecule (Iba)-1 (1:2,000; WAKO, Osaka, Japan), or rabbit anti-glial fibrillary acidic protein (GFAP) (1:5,000; Dako, Carpinteria, CA, USA). The sections were incubated with biotinylated rabbit IgG antibody (1:200; Vector Laboratories Inc, Burlingame, CA, USA), and then avidin-biotinylated horseradish peroxidase complex (1:200; Vector Laboratories), visualized with 3,3′-diamino-benzidine, and coverslipped with Permount.
2.8. Quantification for histological and immunohistochemical staining
The stained sections were captured using a DP70 image analysis system (Olympus, Tokyo, Japan). The density of cresyl violet–stained cells in the SNpc sections (n = 3 per brain) and TH immunoreactivity in the striatum sections (n = 3 per brain) were automatically measured using the NIH Image J program (http://rsb.info.nih.gov/ij/). The number of TH-immunoreactive cells in representative SNpc sections (n = 3 per brain) was semi-manually measured using the NIH Image J program.
2.9. Western blot analysis
Seven days after the last injection of MPTP, the SNpc and striatum of each mouse (n = 5 per group) were immediately removed with lysis buffer (50 mM Tris–Cl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 10% glycerol, and protease inhibitor mixture) under anesthesia. Western blot analysis was accomplished as previously described [26], [27]. Briefly, the polyvinylidene fluoride membranes with protein were probed overnight with rabbit anti-TH (1:1,000; Millipore), rabbit anti-phospho (p)-extracellular signal–regulated kinase, rabbit anti-p-c-Jun N-terminal kinase, p-p38 (1:1,000; Cell Signaling Technology), rabbit anti-p-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) p65 (1:1,000; Cell Signaling Technology), rabbit anti-p-IκBα (1:1,000; Cell Signaling Technology), rabbit anti-p-inhibitor of κBα (IκBα) (1:1,000; Cell Signaling Technology), rabbit anti-nuclear factor erythroid 2–related factor 2 (Nrf2) (1:1,000, Santa Cruz, CA, USA), rat anti-cluster of differentiation molecule 11b (CD11b) (1:500; AbD Serotec, Kidlington, Oxford, UK), mouse anti-GFAP (1:1,000; Millipore), rabbit anti-cleaved caspase-3 (1:500; Cell Signaling Technology), or rabbit anti-p-protein kinase B (AKT) antibody (1:1,000; Cell Signaling Technology) at 4°C, followed by incubation with horseradish peroxidase–conjugated secondary antibody at room temperature for 1 h before enhanced chemiluminescence analysis (Amersham Pharmacia Biotech Inc, Piscataway, New Jersey, USA) and visualized using a super cooled-CCD camera system with a Davinch-K Gel Imaging System (Dvinch-K, Seoul, Korea). Normalization and quantitation of antibody signals were accomplished as previously described [26], [27]. Data are expressed as the ratio of each expression against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for each sample.
2.10. Reverse transcription polymerase chain reaction analysis
Seven days after the MPTP intoxication, the brain of each mouse (n = 3–6 per group) was rapidly removed under anesthesia, 3-mL coronal brain slices were prepared on ice-cold subbed slide glass using a brain matrix device (Roboz Surgical Instrument Co., Gaithersburg, MD, USA), and the SNpc and striatum regions were sampled using microscissors and blade under a dissection microscope. Reverse transcription polymerase chain reaction (RT-PCR) and real-time PCR analyses were accomplished as previously described [26], [27], [31], [32]. Expression levels of each gene were normalized to that of GAPDH. All PCR experiments were performed at least three times, and the mean ± S.E.M. values are presented unless otherwise noted. The primer sequences were as follows: interleukin (IL)-1β-5′-TTG TGG CTG TGG AGA AGC TGT-3′ and 5′-AAC GTC ACA CAC CAG CAG GTT-3′, IL-6-5′-TCC ATC CAG TTG CCT TCT TGG-3′ and 5′-CCA CGA TTT CCC AGA GAA CAT G-3′, tumor necrosis factor (TNF)-α-5′-AGC AAA CCA CCA AGT GGA GGA-3′ and 5′-GCT GGC ACC ACT AGT TGG TTG T-3′, inducible nitric oxide synthase (iNOS)-5′-GGC AAA CCC AAG GTC TAG GTT-3′ and 5′-TCG CTC AAG TTC AGC TTG GT-3′, and GAPDH-5′-AGG TCA TCC CAG AGC TGA ACG-3′ and 5′-CAC CCT GTT GCT GTA GCC GTA T-3′. Heme oxygenase-1 (HO-1)-5′-CCT GGA GCA GGA CAT GGC-3′ and 5′-AAT CAT CCC TTG CAC GCC-3′, nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase 1 (NQO1)-5′-CAT TCT GAA AGG CTG GTT TGA-3′ and 5′-CTA GCT TTG ATC TGG TTG TCA G-3′, γ-glutamate cysteine ligase regulatory subunit (GCLs)-5′-ACA AGC ACC CCC GCT TCG GT-3′ and 5′-CTC CAG GCC TCT CTC CTC CC-3′, endothelial intercellular adhesion molecule-1 (ICAM-1)-5′-TGC GTT TTG GAG CTA GCG GAC CA-3′ and 5′-CGA GGA CCA TAC AGC ACG TGC AG-3′, vascular cell adhesion molecule-1 (VCAM-1)-5′-CCT CAC TTG CAG CAC TAC GGG CT-3′ and 5′-TTT TCC AAT ATC CTC AAT GAC GGG-3′, zona occludens (ZO)-1-5′--3′ and 5′--3′, and claudin-5-5′-AAG GCA ATT CCG TAT CGT TG-3′ and 5′-CCA CAG CTG AAG GAC TCA CA-3′.
2.11. Detection of apoptosis with the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The fragmentation of DNA was tested using an ApopTag Peroxidase in situ Apoptosis Detection Kit (S7100) (Millipore) according to the manufacturer's instructions and as previously described [26].
2.12. Statistical analysis
Statistical analyses were performed using the SPSS 21.0 package (SPSS Inc, Chicago, IL, USA) for Windows. Two-sample comparisons were carried out using the Student t test, and multiple comparisons were made using two-way analysis of variance with Tukey's post hoc test. All data are presented as means ± S.E.M., and statistical difference was identified at the 5% level unless otherwise indicated.
3. Results
3.1. Effects of KRGE on neurological behavior and survival rate after MPTP intoxication
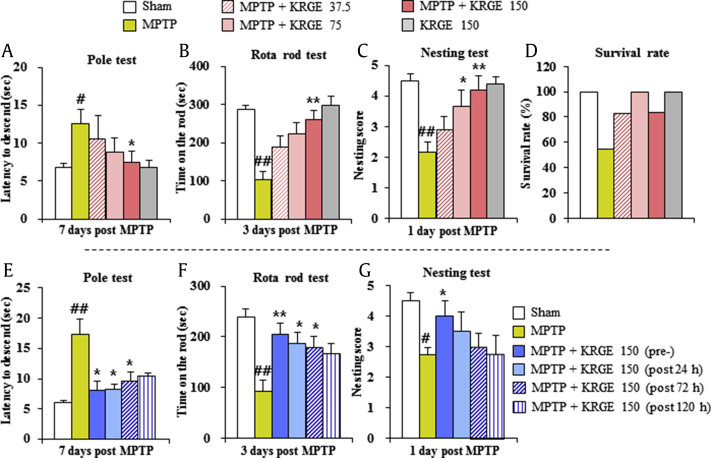
First, we confirmed the effect and the most effective dose of KRGE on MPTP-induced neurological symptoms. The pole test was performed to evaluate mice movement disorder caused by striatal dopamine depletion. Mice from the MPTP group showed enhanced average descent time to the bottom of the pole (12.5 ± 1.9 s) compared with those of the sham group (6.8 ± 0.5 s). Mice in the MPTP + KRGE group displayed significantly decreased average descent time (7.4 ± 1.5 s in the 150 mg/kg/day KRGE groups) (Fig. 1A). One hour after the pole test, rotarod performance was tested. Mice from the MPTP group displayed decreased average latency to fall (101.6 ± 21.7 s) compared with those from the sham group (287.0 ± 11.2 s). Mice in the MPTP + KRGE group displayed increased average latency to fall (260.8 ± 23.9 s in the 150 mg/kg/day KRGE groups, respectively) compared with those in the MPTP group (Fig. 1B). Nest-building behavior was measured as an indicator of health and welfare. Mice from the MPTP group displayed decreased mean score of the quality of the resulting nest (2.2 ± 0.3) compared with those from the sham group (4.5 ± 0.2). Mice in the MPTP + KRGE group displayed significantly improved average score (3.7 ± 0.5 and 4.2 ± 0.3 in the 75 and 150 mg/kg/day KRGE groups, respectively) compared with those in the MPTP group (Fig. 1C). At the end of experiment, the survival rate of MPTP + KRGE group was improved in a dose-dependent manner (82.4%, 100%, and 83.3% in the 37.5, 75, or 150 mg/kg/day KRGE groups, respectively) compared with that of MPTP group (54.5%) (Fig. 1D).
Fig. 1.
KRGE attenuates MPTP-induced locomotor (neurological) impairment. To determine the most effective dose, mice were orally treated with KRGE (37.5 mg/kg, 75 mg/kg, and 150 mg/kg) once daily from 1 h before the first MPTP intoxication (20 mg/kg, every 2 h × 4). (A) Pole test 7 days post MPTP. (B) Performance on the rotarod test 3 days post MPTP. (C) Nest-building behavior 1 day post MPTP scored after 18 h in the cage with a square of cotton Nestlet. (D) Survival rate scored 7 days post MPTP. To investigate its therapeutic time window, mice were orally treated with KRGE (150 mg/kg) once daily from 1 h before the first MPTP intoxication (pretreatment), and 24 h (post 24h), 72 h (post 72h), and 120 h (post 120h) after the last MPTP intoxication. (E) Pole test.(F) Performance on the rotarod test. (G) Nest-building behavior test. ANOVA test; #p < 0.05 and ##p < 0.01 versus Sham group. *p < 0.05 and **p < 0.01 versus MPTP group. ANOVA, analysis of variance; KRGE, Korean Red Ginseng extract; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Consequently, to examine whether KRGE has a wide therapeutic time window against MPTP-induced PD-like symptoms, mice were treated with KRGE (150 mg/kg body weight/day; the most effective dose in Fig. 1A–D) before or at various time points (1, 3, and 5 days) after MPTP intoxication. Groups were designated as pretreated, post 1d-, post 3d-, and post 5d-treated groups. In the pole test, the mean descending time to reach the bottom of the pole was significantly decreased to 8.0 ± 1.6, 8.3 ± 0.9, 9.6 ± 1.6, and 10.5 ± 0.4 s in the KRGE pretreated, post 1d-, post 3d-, and post 5d-treated groups, respectively compared with that in the MPTP group (17.3 ± 2.5 s) (Fig. 1E). In the rotarod test, the mean latency to fall from cylinder was significantly increased in the MPTP + KRGE group, in a dose-dependent manner (204.9 ± 25.5, 186.2 ± 25.7, 178.5 ± 28.7, and 166.8 ± 24.8 s in the KRGE pretreated, post 1d-, post 3d-, and post 5d-treated groups, respectively) compared with that in the MPTP group (92.7 ± 25.6 s) (Fig. 1F). In the nest-building test, the score of the quality of the resulting nest was significantly increased in the KRGE pretreated groups (4.0 ± 0.5) compared with that in the MPTP group (2.7 ± 0.3) (Fig. 1G).
3.2. Effects of KRGE on the SNpc and striatal cell death by MPTP intoxication
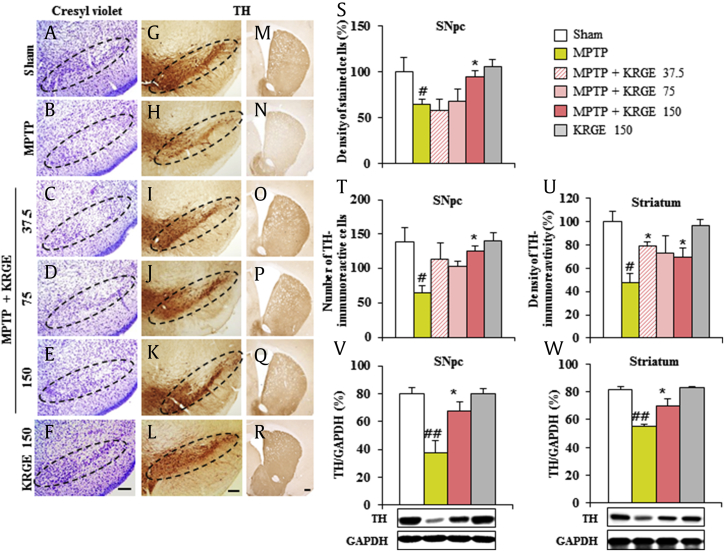
MPTP-induced motor dysfunction results from the loss of dopaminergic neuron in the SNpc and striatum [5]. Therefore, we tested whether the positive effect of KRGE on motor dysfunction is closely related to a reduction of cell loss in the SNpc and striatum. Seven days after the MPTP intoxication, brain sections including the SNpc and striatum were stained with cresyl violet dye (Fig. 2A–F and S). The density of cresyl violet–stained cells in the SNpc was significantly decreased in the MPTP group (64.2 ± 5.5%) compared with that in the sham group (100.0 ± 15.9%). The reduction was significantly inhibited in the MPTP + KRGE group (94.2 ± 6.9 in 150 mg/kg/day KRGE). Subsequently, to investigate whether these cells are dopaminergic neurons, immunohistochemistry and immunoblot analyses were performed using TH antibody (Fig. 2G–R and T–W). The number of TH-immunoreactive cells in the SNpc was significantly reduced in the MPTP group (64.3 ± 10.8) compared with that in the sham group (138.5 ± 21.5). The reduction was significantly inhibited in the MPTP + KRGE group (125.5 ± 7.4 in 150 mg/kg/day KRGE) (Fig. 2G–L and T) and corresponded with the alteration of protein expression for TH by Western blot analysis (Fig. 2V). Because TH-immunoreactive cells in the SNpc project to the striatum [4], [5], we examined the alteration of striatal TH immnunoreactivity (Fig. 2M–R and U). Reduction of striatal TH immnunoreactivity by MPTP intoxication (47.5 ± 7.6%) was also inhibited in the MPTP + KRGE group (79.6 ± 2.7, 72.8 ± 14.7, and 69.4 ± 7.6 in 37.5, 75, and 150 mg/kg/day KRGE, respectively), in agreement with the pattern of protein expression for TH by immunoblot analysis (Fig. 2W). However, KRGE itself did not affect the SNpc and striatal cells. The findings suggest that KRGE can reduce MPTP-mediated dopaminergic degeneration in the SNpc and striatum, related with the improvement of motor impairment.
Fig. 2.
KRGE attenuates MPTP-induced nigrostriatal dopaminergic neuronal damage. Brain sections were prepared 7 days after MPTP intoxication. (A–F) The prepared brain sections were stained with cresyl violet. (G–R) The brain sections were immunostained with TH antibody.(S–U) Quantification. (S) Quantification was carried out by measuring the density of cresyl violet–stained cells. (T) Quantification was carried out by counting the number of TH-immunoreactive cells of the SNpc or striatum.(U) Quantification was carried out by measuring the density of TH immunoreactivity. The SNpc and striatum were sampled 7 days after MPTP intoxication, accomplished by Western blot analysis using TH antibody, and quantified. (V) SNpc. (W) Striatum. The densities were displayed as relative value to that from sham group. Scale bar = 100 μm. ANOVA test; #p < 0.05 and ##p < 0.01 versus Sham group. *p < 0.05 versus MPTP group. ANOVA, analysis of variance; KRGE, Korean Red Ginseng extract; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
3.3. Effect of KRGE on the SNpc and striatal apoptosis by MPTP intoxication
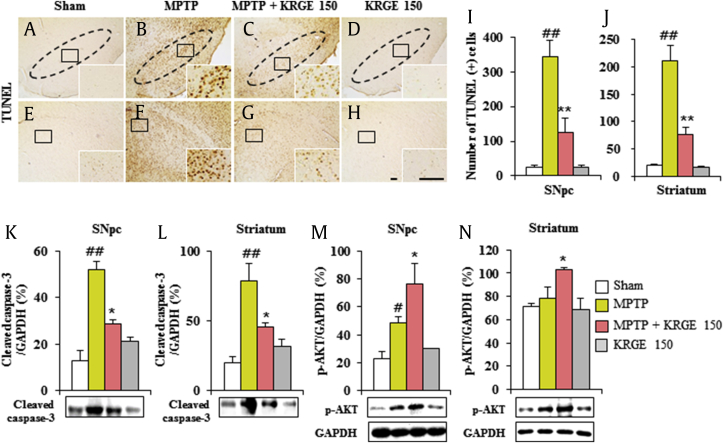
Because MPTP/1-methyl-4-phenylpyridinium (MPP+) leads to apoptosis in the SNpc and striatum [33], we examined whether KRGE can reduce the SNpc and striatal apoptosis 7 days after the MPTP intoxication using TUNEL staining (Fig. 3A–J). The numbers of TUNEL (+) cells were significantly enhanced in the SNpc (343.2 ± 47.9) and striatum (211.6 ± 27.8) in the MPTP group compared with those in the sham group (24.4 ± 5.3 and 19.6 ± 2.6, respectively). The cell numbers were significantly decreased in the MPTP + KRGE group (125.4 ± 41.8 and 75.6 ± 14.4, respectively). In agreement with this result, the protein expression of cleaved caspase-3 was increased in the SNpc (52.2 ± 3.2%) and striatum (78.9 ± 12.2%) after MPTP intoxication, whereas the expressions were inhibited by KRGE pretreatment (28.7 ± 1.9% and 45.6 ± 3.1%, respectively) (Fig. 3K and L). In addition, the expression of p-AKT in the SNpc and striatum after MPTP intoxication was further enhanced by KRGE pretreatment (Fig. 3M and N). Treatment with KRGE alone did not affect the apoptosis in the SNpc and striatum compared with the sham group. Our findings indicate that KRGE may reduce motor deficits and SNpc and striatal toxicity by MPTP intoxication through antiapoptotic activity.
Fig. 3.
KRGE attenuates apoptosis in the SNpc and striatum after MPTP intoxication. (A–H) Brain sections from 7 days after MPTP intoxication were stained using a TUNEL kit. The number of apoptotic cells was manually counted. (I) In SNpc. (J) In striatum. (K–N) The brain was sampled 7 days after MPTP intoxication; immunoblot was performed using cleaved caspase-3 and p-protein kinase B (AKT) antibodies and quantified. The band of GAPDH in graph M and N was shared with graphs K and L. Scale bar = 50 μm. ANOVA test; #p < 0.05 and ##p < 0.01 versus Sham group. *p < 0.05 and **p < 0.01 versus MPTP group. ANOVA, analysis of variance; KRGE, Korean Red Ginseng extract; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNpc, substantia nigra pars compacta.
3.4. Effect of KRGE on microglial activation in the SNpc and striatum after MPTP intoxication
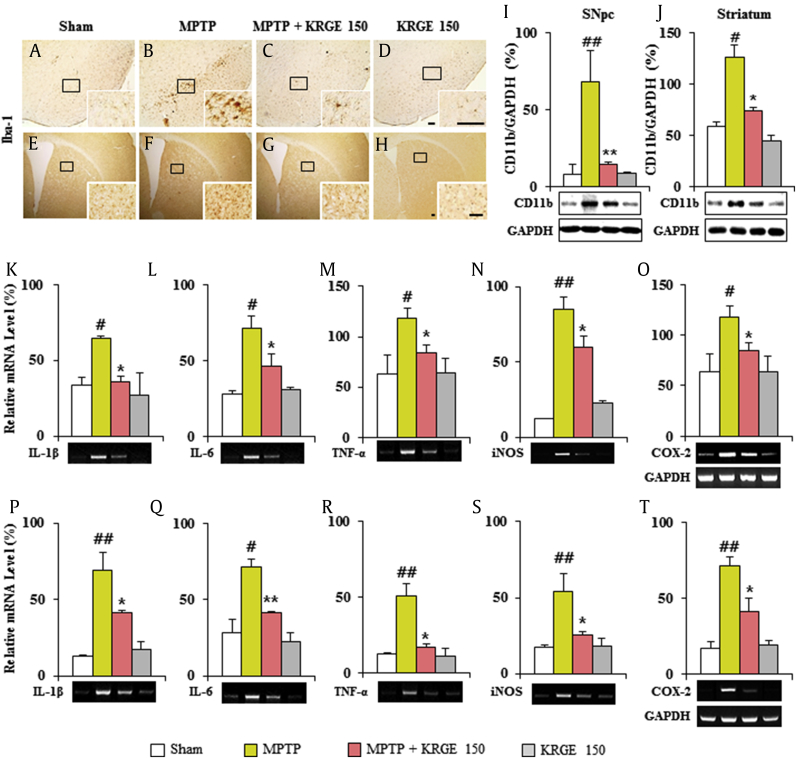
Microglial activation is appeared within or around lesions of neurodegenerative disorders, such as PD, and activated microglia produce proinflammatory and antiinflammatory mediators [34], [35]. Therefore, we tested whether protective effect of KRGE in MPTP-induced SNpc and striatal toxicity (Fig. 2) was closely related with the downregulation of microglial activation (Fig. 4). In the SNpc and striatal tissue from the MPTP group, ionized calcium-binding adapter molecule-1 (Iba-1), a marker for microglia, (+) cells showed typically activated form (Fig. 3B and F) with enlarged cell bodies and short and thick processes [26], [27], [36], compared with those in the sham group (Fig. 3A and E), which generally displayed the typical forms of resting cells, including small cell bodies and thin processes [26], [27], [36]. However, the activation level of Iba-1 (+) cells was significantly decreased in the SNpc and striatum in the MPTP + KRGE group (Fig. 3C and G) compared with that in the MPTP group. The expression trend paralleled the alteration of CD11b protein expression (Fig. 4I and J). The results indicate that KRGE could exert neuroprotective effect in MPTP-induced SNpc and striatal toxicity through the inhibition of the activation/infiltration of microglia.
Fig. 4.
KRGE attenuates the activation of microglia and inflammatory mediators in the SNpc and striatum after MPTP intoxication. Brain sections obtained 7 days after MPTP intoxication were immunostained using Iba-1 antibody. (A–D) SNpc. (E–H) Striata. SNpc and striata from 7 days after MPTP intoxication were analyzed. (I and J) By Western blot. (K–T) By RT-PCR. The band of GAPDH in graphs O and T was shared with graphs K–N and P–S, respectively. CD11b (I and J), IL-1β (K and P), IL-6 (L and Q), TNF-α (M and R), iNOS (N and S), and COX-2 (O and T). Scale bar = 50 μm. ANOVA test; #p < 0.05 and ##p < 0.01 versus Sham group. *p < 0.05 and **p < 0.01 versus MPTP group. ANOVA, analysis of variance; COX, cyclooxygenase; IL, interleukin; iNOS, inducible nitric oxide; KRGE, Korean Red Ginseng extract; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; RT-PCR, reverse transcription polymerase chain reaction; SNpc, substantia nigra pars compacta; TNF, tumor necrosis factor.
3.5. Effect of KRGE on inflammatory mediators in the SNpc and striatum after MPTP intoxication
Because inflammatory mediators secreted from activated microglia may be either beneficial or detrimental to neuronal survival [34], [35], we investigated whether the inhibition of microglial activation by KRGE (Fig. 4A–J) is closely related with the downregulation of inflammatory mediators in the SNpc and striatum after MPTP intoxication. RT-PCR analysis revealed that the relative mRNA expressions of IL-1β, IL-6, TNF-α, iNOS, and COX-2 were upregulated by 1.8-, 2.5-, 1.9-, 4.7-, and 1.9-fold, respectively, in the SNpc and by 5.3-, 2.6-, 4.2-, 3.1-, and 4.1-fold, respectively, in the striatum 7 days after the MPTP intoxication compared with those in the sham group, whereas their upregulation was significantly inhibited by 45%, 35%, 29%, 29%, and 28%, respectively, in the SNpc and by 40%, 42%, 51%, 53%, and 43%, respectively, in the striatum from MPTP + KRGE group (150 mg/kg/day) compared with that in the MPTP group (Fig. 4K–T). RT-PCR analysis displayed little or no mRNA expression of all genes in the SNpc and striatal tissue from the sham groups. Our findings suggest that KRGE might mitigate the neurological dysfunction and SNpc and striatal toxicity by MPTP intoxication through the inhibition of the expression of proinflammatory modulators.
3.6. Protective effect of KRGE on the blood–brain barrier integrity after MPTP intoxication
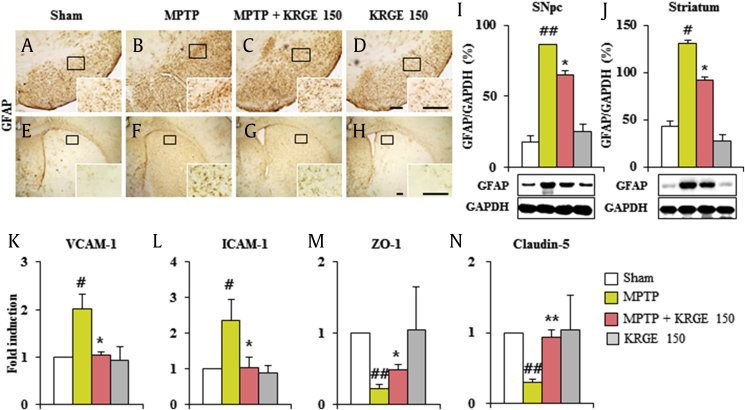
Because astrocytes are implicated in formation of the blood–brain barrier (BBB) [37], we tested the level of astroglial activation in the SNpc and striatum from the MPTP group. Intensity of GFAP-immunoreactive astrocytes (Fig. 5A–H) and the protein expression (Fig. 5I and J) of GFAP were markedly increased in mice of MPTP group. These enhanced expressions were significantly blocked by KRGE pretreatment (Fig. 5A–J). Because the peripheral immune cell for BBB was mediated by increased adhesive molecules and decreased junctional molecules, we investigated the effect of KRGE for their expression. Real-time PCR analysis demonstrated increased mRNA expression of ICAM-1 and VCAM-1 in the SNpc and striatum after MPTP intoxication, whereas these expressions were inhibited by the KRGE pretreatment (Fig. 5K and L). On the other hand, mRNA expressions of ZO-1 and claudin-5 were decreased in the SNpc and striatum after MPTP intoxication, whereas the expressions were increased by the KRGE pretreatment (Fig. 5M and N). Taken together, KRGE could inhibit the disruption of BBB, and its result could contribute to protective effect of KRGE for MPTP intoxication, associated with inhibition of astrocytic activation.
Fig. 5.
KRGE contributes to the maintenance of the BBB after MPTP intoxication. (A–D) SNpc sections from 7 days after MPTP intoxication were immunostained using GFAP antibody. (E–H) Striatal sections from 7 days after MPTP intoxication were immunostained using GFAP antibody. SNpc and striata were analyzed and quantified. (I and J) Western blot. (K–N) Real-time PCR. (K) VCAM-1. (L) ICAM-1. (M) ZO-1. (N) Claudin-5. Scale bar = 100 μm. ANOVA test; #p < 0.05 and ##p < 0.01 versus Sham group. *p < 0.05 and **p < 0.01 versus MPTP group. ANOVA, analysis of variance; BBB, blood–brain barrier; GFAP, glial fibrillary acidic protein; ICAM, intercellular adhesion molecule; KRGE, Korean Red Ginseng extract; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PCR, polymerase chain reaction; SNpc, substantia nigra pars compacta; VCAM, vascular cell adhesion molecule; ZO-1, zona occludens-1.
3.7. Antioxidative and antiinflammatory effects of KRGE after MPTP intoxication
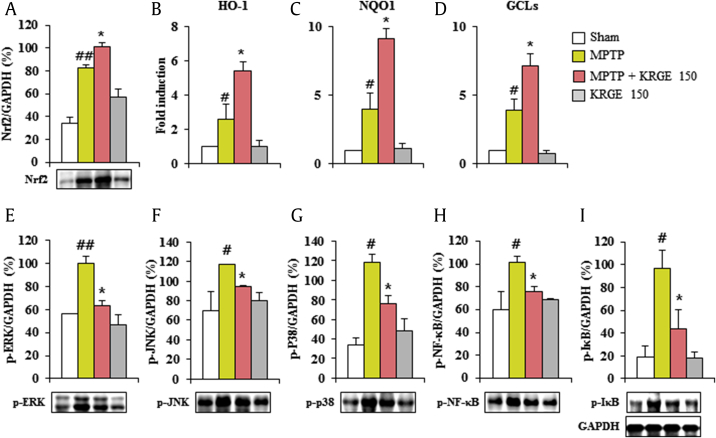
Although ginsenosides have antioxidative effects through Nrf2 transcriptional activation in neurological disorders [15], the antioxidative effects of KRGE are unclear. Therefore, we investigated whether KRGE might regulate Nrf2 pathway in the MPTP-induced PD model. Nrf2 protein expression was significantly upregulated in the striatum by MPTP intoxication compared with that in the sham group, while it was further enhanced by pretreatment with KRGE (150 mg/kg) compared with that in the MPTP group (Fig. 6A). Consequently, the mRNA expression of the phase II enzymes HO-1, NAD(P)H quinone oxidoreductase-1, and GCLs from real-time PCR analysis was significantly increased by 2.6-, 3.9-, and 4.0-fold, respectively, in the striatum of MPTP group, while the increases in their expressions were further increased by 56.0%, 45.1%, and 51.9%, respectively, in the MPTP +KRGE group (Fig. 6B–D). Taken together, our results suggest that KRGE exerts its antioxidant effect through stimulation of the Nrf2 pathway in the MPTP-induced neurotoxicity.
Fig. 6.
KRGE activates Nrf2 pathway and inhibits MAPKs and NFκB signaling pathways after MPTP intoxication. Striata from 7 days after MPTP intoxication were used for Western blot. (A) Nrf2. (E) p-ERK. (F) p-JNK. (G) p-p38. (H) p-NF-κB. (I) p-IκB. The band of GAPDH in graph I was shared with graphs E–H. Striata from 7 days after MPTP intoxication were used for real-time PCR analysis. (B) HO-1. (C) NQO1 (D) GCLs. ANOVA test; #p < 0.05 and ##p < 0.01 versus Sham group. *p < 0.05 versus MPTP group. ANOVA, analysis of variance; ERK, extracellular signal–regulated kinase; GCLs, γ-glutamate cysteine ligase regulatory subunit; JNK, Jun N-terminal kinase; KRGE, Korean Red Ginseng extract; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; Nrf2, nuclear factor erythroid 2–related factor 2; NQO1, NAD(P)H quinone oxidoreductase 1; PCR, polymerase chain reaction.
Ginsenosides (Rb1, Rd, Rg1, etc.) exhibit antiinflammatory activity through inhibition of mitogen-activated protein kinases (MAPKs) and NF-kB pathways, representative inflammation mechanisms in neurodegenerative diseases such as AD [14], [38], [39]. However, the activity of KRGE remains largely unknown. Therefore, we investigated the regulation effect of KRGE on these pathways in the striatum after MPTP intoxication. The phosphorylation of extracellular signal–regulated kinase, Jun N-terminal kinase, and p38 proteins was significantly increased by 1.8-, 1.7-, and 3.5-fold, respectively, in the striatum 7 days after the MPTP intoxication compared with that in the sham group; however, the activation was significantly decreased by 36.3%, 18.8%, and 35.6%, respectively, by pretreatment with KRGE (150 mg/kg/day; Fig. 6E–G). Subsequently, we investigated whether KRGE regulates the activation of NF-κB in the striatum after MPTP intoxication. Phosphorylation of p-NF-κB and p-IκBα was significantly increased by 1.7- and 5.1-fold, respectively, in the striatum 7 days after the MPTP intoxication compared with that in the sham group, whereas the phosphorylation was significantly reduced by 54.6% and 24.9%, respectively, by pretreatment with KRGE (150 mg/kg/day) (Fig. 6H and I). The phosphorylation of MAPKs or the NF-κB pathway was neither increased nor decreased by KRGE treatment alone (Fig. 6E–I). Our findings suggest that KRGE can diminish striatal toxicity and behavioral disorder after MPTP intoxication by inhibiting MAPKs and the NF-κB pathways.
4. Discussion
Cellular and molecular mechanisms underlying the loss of dopamine (DA) neuron remain unclear. They are multifactorial, including apoptosis, oxidative stress, and inflammation [40]. Most PD drugs, such as levodopa, are single-target drugs with limited effects. They have adverse effects when patients receive long-term therapy [41]. Recently, to overcome these limitations, the desire to develop more effective treatment agents for PD seems to be pushing drug design toward the multitarget approach. The shifting from the single to the multitarget paradigm in drug discovery will transform the chimera of neuroprotection into a real benefit for patients with PD [7], [8], [9], [10]. Here, we demonstrated that KRGE has protective effects in the MPTP-induced SNpc and striatal toxicity through multifunctional activities including antiapoptotic, antioxidative, antiinflammatory, and BBB maintenance activities. The findings suggest that KRGE might be a potential candidate for development of new drugs and functional foods from natural products to preventive and treat PD.
The apoptotic death of dopaminergic neurons in the SNpc plays an initial role in the development and progression of PD [33]. Caspases are primary mediators of apoptosis through DNA fragmentation and cleavage of various key cellular proteins. Caspase-3, the major member of this cascade, can be activated by oxidative stress, release of mitochondrial cytochrome C, and caspase-9 activation [42]. Ginsenosides (Rd, Rg1, or 20(R)-Rg3) attenuate mitochondria-mediated apoptosis in a middle cerebral artery occlusion (MCAO)-induced stroke model or glutamate/dopamine-induced primary cortical neurons/PC12 cells through the suppression of caspase-3 activation [38], [43], mitochondrial release of cytochrome C, apoptosis-inducing factor [44], [45], or oxidative stress [46]. Ginseng extracts inhibit apoptosis and cisplatin-induced ototoxicity in MPP (+)-stimulated PC12 cells by inhibiting caspase-3 or poly adenosine diphosphate -ribose polymerase (PARP) [47], [48]. Consistent with these reports, KRGE reduced the number of TUNEL (+) cells associated with the caspase-3 reduction and AKT activation in MPTP-induced PD model. The evidence supports the view that the antiapoptotic activity of KRGE might contribute to the protection of dopaminergic neurons from MPTP-induced SNpc and striatal toxicity.
Oxidative stress is resulted from dopamine metabolism, mitochondrial dysfunction, iron homeostasis, neuroinflammation, and aging [15], [40]. The transcription factor Nrf2 is responsible for strong antioxidant activity which protects neurons from the oxidative stress induced by reactive oxygen species (ROS); therefore, targeting Nrf2 might exert a critical role in protecting against various inflammatory disorders [40]. Given that KRGE activated Nrf2 in the present study (Fig. 6), it is conceivable that triggered Nrf2 pathway by KRGE suppresses MAPKs and NF-κB pathways, contributing to the KRGE's antiinflammatory effect to protect dopaminergic neurons against MPTP-induced neurotoxicity. The results might be supported by antioxidant effect of ginsenoside Rg1 and saponin metabolite Rh3 in cultured cells by Nrf2 activation [49], [50] and antiinflammatory effect (reduced expression of cytokines and chemokines) by suppression of MAPKs and NF-κB pathways through Nrf2 activation [15], [18], [46]. Although our results suggest the possibility that KRGE suppresses the activation of MAPKs and NF-κB pathways via Nrf2 mechanism, the reciprocal interaction between Nrf2 and MAPKs/NF-κB activities remains to be clarified. Nevertheless, our findings indicate that KRGE directly or indirectly exerts a potent neuroprotective effect in MPTP-induced animal model of PD by antiinflammatory and antioxidative activity possibly through both activating Nrf2 pathway and suppressing MAPKs and NF-κB pathways.
Microglia play crucial roles in the development and maintenance of the central nervous system microenvironment through the regulation of neuroimmune system [34], [35]. Activation of microglial cells are widely considered to participate in the pathology of PD through production of proinflammatory and antiinflammatory mediators [34], [35]. Therefore, it is considered as an attractive trial to prevent and treat protect dopaminergic neurons in the SNpc of the animal model of PD and patients with PD [35]. In the present study, the activated microglial cells were observed in the SNpc and striatum from the MPTP group, whereas the increased level of microglial activation was significantly blocked by KRGE, which is consistent with alteration of mRNA expressions of proinflammatory cytokines (IL-1β, IL-6, and TNF-α), COX-2, and iNOS in the SNpc and striatum from the MPTP model. These results could be indirectly explained by the effect of ginseng's components (gisenosides, polysaccharide, gintonin, etc.) in neurological disorders such as PD [14], [15]. Collectively, our findings suggest that KRGE may attenuate the microglial activation and its inflammatory responses in the MPTP-induced PD model mice and that KRGE can contribute to dopaminergic neurodegeneration from MPTP toxicity via antiinflammatory activity.
Migrated immune cells from periphery by BBB leakage exert the protective or detrimental roles dopaminergic neurons in the SNpc of in vivo models of PD [51]. Therefore, agents protecting BBB disruption may be an attractive therapeutic tool for PD. In the present study, KRGE blocked increase in the mRNA expression of endothelial adhesion molecules (ICAM-1 and VCAM-1) in the SNpc of MPTP model, whereas it inhibited the decrease in the mRNA expression of junctional molecules (ZO-1 and claudin-5) (Fig. 5). The maintenance effect of KRGE on BBB integrity might be supported by the observations that ginsenoside Rb1 reduces the expression of tight junction proteins (ZO-1 and occludin), Evans blue extravasation, and brain edema in the ischemic brain. Collectively, KRGE could attenuate MPTP-induced BBB disruption, and it could prevent the degeneration of dopamine neurons in the SNpc of MPTP-treated mice.
Pharmacokinetics and central nervous system distribution of ingredients and metabolites of herbal medicines are very important for explaining and predicting their efficacy and toxicity. After oral or intranasal administration of ginseng and pure ginsenosides, ginsenoiside Rb1, Rd, Re, and Rg1 were detected in brain tissues in various concentrations [52], [53]. Effects of these ginsenosides have also been well demonstrated both in vivo and in vitro [16], [17], [18], [19], [20]. Although the beneficial effect of KRGE in MPTP-induced PD model might be explained directly and indirectly by previous reports, details need to be further studied.
5. Conclusions
The discovery of a multifunctional therapy targeting both symptomatic treatment and neuroprotection is the attractive challenge of PD therapy. Presently, KRGE protected dopaminergic neurons from MPTP-induced neurotoxicity, possibly through multifunctional mechanisms including antineuronal apoptosis, antioxidation by activation of the Nrf2 pathway, antiinflammation by inhibition of the MAPKs and NF-kB pathways, and maintenance of BBB integrity. Our findings suggest that KRGE may provide symptom-relieving benefits and a disease-modifying potential concurrently for the treatment of multifactorial PD.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Authors' contributions
Jong Hee Choi performed the behavioral experiments, immunohistochemistry, and Western blots analysis. Minhee Jang carried out TUNEL analysis. Seikwan Oh and Seung-Yeol Nah contributed to draft of article and critical revision for important intellectual content. Ik-Hyun Cho conceived all experiments, analyzed the results, and wrote the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (NRF-2017R1A2A2A05069493) and by the grants from the Korean Society of Ginseng and the Korea Ginseng Cooperation (2014–2015).
References
- 1.Kalia L.V., Lang A.E. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 3.Shulman J.M., De Jager P.L., Feany M.B. Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 4.Kalinderi K., Bostantjopoulou S., Fidani L. The genetic background of Parkinson's disease: current progress and future prospects. Acta Neurol Scand. 2016;134:314–326. doi: 10.1111/ane.12563. [DOI] [PubMed] [Google Scholar]
- 5.Gubellini P., Kachidian P. Animal models of Parkinson's disease: an updated overview. Rev Neurol (Paris) 2015;171:750–761. doi: 10.1016/j.neurol.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Marsden C.D. Problems with long-term levodopa therapy for Parkinson's disease. Clin Neuropharmacol. 1994;17(Suppl. 2):S32–S44. [PubMed] [Google Scholar]
- 7.Calabresi P., Di Filippo M. Multitarget disease-modifying therapy in Parkinson's disease? Lancet Neurol. 2015;14:975–976. doi: 10.1016/S1474-4422(15)00227-6. [DOI] [PubMed] [Google Scholar]
- 8.Dias K.S., Viegas C., Jr. Multi-target directed drugs: a modern approach for design of new drugs for the treatment of Alzheimer's disease. Curr Neuropharmacol. 2014;12:239–255. doi: 10.2174/1570159X1203140511153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajda M., Guzior N., Ignasik M., Malawska B. Multi-target-directed ligands in Alzheimer's disease treatment. Curr Med Chem. 2011;18:4949–4975. doi: 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H., Fridkin M., Youdim M. From single target to multitarget/network therapeutics in Alzheimer's therapy. Pharmaceuticals (Basel) 2014;7:113–135. doi: 10.3390/ph7020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X.Z., Zhang S.N., Liu S.M., Lu F. Recent advances in herbal medicines treating Parkinson's disease. Fitoterapia. 2013;84:273–285. doi: 10.1016/j.fitote.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Athauda D., Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2015;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- 13.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho I.H. Effects of Panax ginseng in neurodegenerative diseases. J Ginseng Res. 2012;36:342–353. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y.M., Yoon H., Park H.M., Song B.C., Yeum K.J. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J Ginseng Res. 2017;41:113–119. doi: 10.1016/j.jgr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radad K., Gille G., Moldzio R., Saito H., Rausch W.D. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Lin W.M., Zhang Y.M., Moldzio R., Rausch W.D. Ginsenoside Rd attenuates neuroinflammation of dopaminergic cells in culture. J Neural Transm Suppl. 2007:105–112. doi: 10.1007/978-3-211-73574-9_13. [DOI] [PubMed] [Google Scholar]
- 18.Chen X.C., Zhu Y.G., Zhu L.A., Huang C., Chen Y., Chen L.M., Fang F., Zhou Y.C., Zhao C.H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol. 2003;473:1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- 19.Chen X.C., Chen Y., Zhu Y.G., Fang F., Chen L.M. Protective effect of ginsenoside Rg1 against MPTP-induced apoptosis in mouse substantia nigra neurons. Acta Pharmacol Sin. 2002;23:829–834. [PubMed] [Google Scholar]
- 20.Hu J.F., Song X.Y., Chu S.F., Chen J., Ji H.J., Chen X.Y., Yuan Y.H., Han N., Zhang J.T., Chen N.H. Inhibitory effect of ginsenoside Rg1 on lipopolysaccharide-induced microglial activation in mice. Brain Res. 2011;1374:8–14. doi: 10.1016/j.brainres.2010.11.069. [DOI] [PubMed] [Google Scholar]
- 21.Radad K., Moldzio R., Rausch W.D. Ginsenosides and their CNS targets. CNS Neurosci Ther. 2011;17:761–768. doi: 10.1111/j.1755-5949.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nah S.Y., Kim D.H., Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jun Y.L., Bae C.H., Kim D., Koo S., Kim S. Korean red ginseng protects dopaminergic neurons by suppressing the cleavage of p35 to p25 in a Parkinson's disease mouse model. J Ginseng Res. 2015;39:148–154. doi: 10.1016/j.jgr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D., Jeon H., Ryu S., Koo S., Ha K.T., Kim S. Proteomic analysis of the effect of Korean red ginseng in the striatum of a Parkinson's disease mouse model. PLoS One. 2016;11:e0164906. doi: 10.1371/journal.pone.0164906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landis S.C., Amara S.G., Asadullah K., Austin C.P., Blumenstein R., Bradley E.W., Crystal R.G., Darnell R.B., Ferrante R.J., Fillit H. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang M., Lee M.J., Kim C.S., Cho I.H. Korean red ginseng extract attenuates 3-nitropropionic acid-induced Huntington's-like symptoms. Evid Based Complement Alternat Med. 2013;2013:237207. doi: 10.1155/2013/237207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M.J., Jang M., Choi J., Chang B.S., Kim D.Y., Kim S.H., Kwak Y.S., Oh S., Lee J.H., Chang B.J. Korean red ginseng and ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Mol Neurobiol. 2016;53:1977–2002. doi: 10.1007/s12035-015-9131-4. [DOI] [PubMed] [Google Scholar]
- 28.Jackson-Lewis V., Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 29.Deacon R.M. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 30.Franklin K.B.J., Paxinos G. Elsevier Academic Press; San Iego: 2008. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 31.Choi J.H., Jang M., Kim E.J., Kim H., Ye S.K., Cho I.H. Oriental medicine Woohwangchungsimwon attenuates Kainic acid-induced seizures and neuronal cell death in the Hippocampus. Rejuvenation Res. 2016 doi: 10.1089/rej.2015.1779. [DOI] [PubMed] [Google Scholar]
- 32.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch E.C., Hunot S., Faucheux B., Agid Y., Mizuno Y., Mochizuki H., Tatton W.G., Tatton N., Olanow W.C. Dopaminergic neurons degenerate by apoptosis in Parkinson's disease. Mov Disord. 1999;14:383–385. doi: 10.1002/1531-8257(199903)14:2<383::aid-mds1037>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 34.Lobsiger C.S., Cleveland D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Du L., Zhang Y., Chen Y., Zhu J., Yang Y., Zhang H.L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0245-0. [DOI] [PubMed] [Google Scholar]
- 36.Jang M., Cho I.H. Sulforaphane ameliorates 3-nitropropionic acid-induced striatal toxicity by activating the Keap1-nrf2-are pathway and inhibiting the MAPKs and NF-kappaB pathways. Mol Neurobiol. 2016;53:2619–2635. doi: 10.1007/s12035-015-9230-2. [DOI] [PubMed] [Google Scholar]
- 37.Willis C.L. Glia-induced reversible disruption of blood-brain barrier integrity and neuropathological response of the neurovascular unit. Toxicol Pathol. 2010;39:172–185. doi: 10.1177/0192623310385830. [DOI] [PubMed] [Google Scholar]
- 38.He B., Chen P., Yang J., Yun Y., Zhang X., Yang R., Shen Z. Neuroprotective effect of 20(R)-ginsenoside Rg(3) against transient focal cerebral ischemia in rats. Neurosci Lett. 2012;526(2):106–111. doi: 10.1016/j.neulet.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q., Kou J.P., Yu B.Y. Ginsenoside Rg1 protects against hydrogen peroxide-induced cell death in PC12 cells via inhibiting NF-kappaB activation. Neurochem Int. 2011;58:119–125. doi: 10.1016/j.neuint.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V.R. Oxidative stress and Parkinson's disease. Front Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guneysel O., Onultan O., Onur O. Parkinson's disease and the frequent reasons for emergency admission. Neuropsychiatr Dis Treat. 2008;4:711–714. doi: 10.2147/ndt.s3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen G.M. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X.Y., Liang J., Tang Y.B., Zhou J.G., Guan Y.Y. Ginsenoside Rd prevents glutamate-induced apoptosis in rat cortical neurons. Clin Exp Pharmacol Physiol. 2010;37:199–204. doi: 10.1111/j.1440-1681.2009.05286.x. [DOI] [PubMed] [Google Scholar]
- 44.Hu G., Wu Z., Yang F., Zhao H., Liu X., Deng Y., Shi M., Zhao G. Ginsenoside Rd blocks AIF mitochondrio-nuclear translocation and NF-kappaB nuclear accumulation by inhibiting poly(ADP-ribose) polymerase-1 after focal cerebral ischemia in rats. Neurol Sci. 2013;34:2101–2106. doi: 10.1007/s10072-013-1344-6. [DOI] [PubMed] [Google Scholar]
- 45.Ye R., Zhang X., Kong X., Han J., Yang Q., Zhang Y., Chen Y., Li P., Liu J., Shi M. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Nabavi S.F., Sureda A., Habtemariam S., Nabavi S.M. Ginsenoside Rd and ischemic stroke; a short review of literatures. J Ginseng Res. 2015;39:299–303. doi: 10.1016/j.jgr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Im G.J., Chang J.W., Choi J., Chae S.W., Ko E.J., Jung H.H. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother Res. 2010;24:614–621. doi: 10.1002/ptr.3082. [DOI] [PubMed] [Google Scholar]
- 48.Kim E.H., Jang M.H., Shin M.C., Shin M.S., Kim C.J. Protective effect of aqueous extract of Ginseng radix against 1-methyl-4-phenylpyridinium-induced apoptosis in PC12 cells. Biol Pharm Bull. 2003;26:1668–1673. doi: 10.1248/bpb.26.1668. [DOI] [PubMed] [Google Scholar]
- 49.Du X., Xu H., Jiang H., Xie J. Akt/Nrf2 activated upregulation of heme oxygenase-1 involves in the role of Rg1 against ferrous iron-induced neurotoxicity in SK-N-SH cells. Neurotox Res. 2013;24:71–79. doi: 10.1007/s12640-012-9362-3. [DOI] [PubMed] [Google Scholar]
- 50.Lee Y.Y., Park J.S., Lee E.J., Lee S.Y., Kim D.H., Kang J.L., Kim H.S. Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: critical role of 5′-adenosine monophosphate-activated protein kinase signaling pathway. J Agric Food Chem. 2015;63:3472–3480. doi: 10.1021/jf506110y. [DOI] [PubMed] [Google Scholar]
- 51.Kortekaas R., Leenders K.L., van Oostrom J.C., Vaalburg W., Bart J., Willemsen A.T., Hendrikse N.H. Blood-brain barrier dysfunction in Parkinsonian midbrain in vivo. Ann Neurol. 2005;57(2):176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 52.Guo Q., Li P., Wang Z., Cheng Y., Wu H., Yang B., Du S., Lu Y. Brain distribution pharmacokinetics and integrated pharmacokinetics of Panax notoginsenoside R1, ginsenosides Rg1, Rb1, Re and Rd in rats after intranasal administration of Panax notoginseng saponins assessed by UPLC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:264–271. doi: 10.1016/j.jchromb.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 53.Li M., Guan Y.M., Liu N., Shao C., Liu Z.B., Chen J.B., Wang Q., Pan X., Sun H., Zhang Y. Brain concentration of ginsenosides and pharmacokinetics after oral administrationof mountain-cultivated ginseng. J Chin Chem Soc. 2017;64(4):395–403. [Google Scholar]