Abstract
Ginsenosides, dammarane-type triterpene saponins obtained from ginseng, have been used as a natural medicine for many years in the Orient due to their various pharmacological activities. However, the therapeutic potential of ginsenosides has been largely limited by the low bioavailability of the natural products caused mainly by low aqueous solubility, poor biomembrane permeability, instability in the gastrointestinal tract, and extensive metabolism in the body. To enhance the bioavailability of ginsenosides, diverse micro-/nano-sized delivery systems such as emulsions, polymeric particles, and vesicular systems have been investigated. The delivery systems improved the bioavailability of ginsenosides by enhancing solubility, permeability, and stability of the natural products. This mini-review aims to provide comprehensive information on the micro-/nano-sized delivery systems for increasing the bioavailability of ginsenosides, which may be helpful for designing better delivery systems to maximize the versatile therapeutic potential of ginsenosides.
Keywords: bioavailability, delivery system, ginsenosides, permeability, solubility
1. Introduction
Ginsenosides are dammarane-type triterpene saponins obtained from ginseng, and they exhibit various pharmacological activities, such as antiinflammatory, antistress, anticancer, antioxidative, and antiaging effects [1], [2], [3], [4], [5], [6], [7]. The mechanisms of the pharmacological activities of ginsenosides are based on diverse bioactivities of the natural products including the induction of apoptosis, stimulation of central nervous system, suppression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, protective effect on DNA, chemoprophylaxis, and antiangiogenesis (Table 1) [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23].
Table 1.
Some pharmacological activities of ginsenosides
| Ginsenosides | Pharmacological effects |
|---|---|
| Rg3 | Induction of apoptosis [9], [10] Anticancer activity [11] Antifatigue effect [12] |
| Rg5 | Anticancer activity [13] Lung inflammation relief [13] |
| Compound K | Anticancer activity [14], [15] Stimulation of chemoprophylaxis [15] Suppression of NF-κB pathway activation [16] Inhibition of mutagenicity [17] |
| Rh2 | Induction of apoptosis [11] Anticancer activity [18] |
| Rg1 | Stimulation of central nervous system [19] Antifatigue effect [20] Antiinflammatory effect [20] Vasodilation effect [20] Sedative effect on central nervous system [20] |
| Rb1 | Sedative effect on central nervous system [19] Antiinflammatory effect [19] Vasodilation effect [19] Protecting and repairing effect on DNA [21], [22] |
| R1 | Neuroprotective effects [23] |
Despite the promising pharmacological efficacies of ginsenosides, the therapeutic potential of ginsenosides has been largely limited by the low oral bioavailability. Many studies investigated pharmacokinetic evaluation of ginsenosides orally administered to rats, and the oral bioavailability of ginsenosides was generally measured to be below 5% as presented in Table 2 [6], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]. The low bioavailability of ginsenosides could be attributed to undesirable physicochemical properties of the natural products such as low aqueous solubility, poor biomembrane permeability, instability in the gastrointestinal (GI) fluid, and extensive metabolism in the body [6], [24], [34], [35], [36], [37].
Table 2.
Some of oral bioavailabilities of ginsenosides evaluated in rats
| Ginsenosides | Oral bioavailability (%) | Dose (mg/kg) | References |
|---|---|---|---|
| Rb1 | 4.35 | 10 | [28] |
| Rd | 2.36 | 10 | [29] |
| Re | 0.19–0.28 | 1 | [30] |
| Rh2 | 4.7, 4.0, 6.4 | 1, 3, 9 | [6] |
| Rg3 | N/A (<LOQ) | 100 | [32] |
| 2.63 | 10 | [33] | |
| 20(S)-Protopanaxadiol | 24.4–49.2 | 2 | [26] |
| 22.37 | 25 | [27] | |
| Compound K | 35.0 | 20 | [25] |
LOQ, limit of quantitation.
Therefore, to enhance the bioavailability of ginsenosides, ginsenosides should be solubilized, efficiently delivered to target tissues, and properly protected from the physiological factors such as low pH in the stomach and enzymes existing in the GI tract. To achieve the goals, various micro-/nano-sized delivery systems have been explored, such as microemulsions/nanoemulsions [38], polymeric microparticles/nanoparticles [39], and vesicular systems including liposomes [40], ethosomes [41], and transfersomes [42]. They have considerably improved the problematic properties of ginsenosides, thereby successfully enhancing their bioavailabilities. The diverse delivery systems have also been administered to the body via different routes to effectively increase the bioavailability of ginsenosides as shown in Table 3 [13], [35], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55].
Table 3.
Various delivery systems for enhancing bioavailability of ginsenosides
| Delivery systems | Administration route | References | |
|---|---|---|---|
| Emulsions | Microemulsion | Intraduodenal | [32], [44] |
| Intranasal | [46] | ||
| Nanoemulsion | Oral | [52] | |
| Polymeric microparticles/nanoparticles | Microparticle | Intramuscular | [47] |
| Topical | [52] | ||
| Oral/Intranasal | [13] | ||
| Nanoparticle | Oral | [50] | |
| Inner ear | [35], [53] | ||
| Nanoparticle | Intravenous | [43] | |
| Vesicular delivery systems | Liposome | Intraperitoneal | [49] |
| Proliposome | Oral | [45] | |
| Modified liposome | Oral/Subcutaneous | [48], [54] | |
| Ethosome and transfersome | Topical | [55] | |
| Niosome | Oral | [51] | |
The works for improving the bioavailability of ginsenosides using various delivery systems have been continuously conducted. However, to the best of our knowledge, there is a paucity of reviews that comprehensively cover the previous studies on delivery systems of ginsenosides. To further advance the delivery systems for enhancing the bioavailability of ginsenosides and maximize the therapeutic potential of ginsenosides, deep understanding and systematic analysis of the previously explored delivery systems of ginsenosides is indispensable. Therefore, this mini-review aims to collectively highlight representative micro-/nano-sized delivery systems for improving the bioavailability of ginsenosides to provide pharmaceutical insight to the readers, which might be useful for designing better delivery systems of ginsenosides.
2. Factors to consider for improving bioavailability of ginsenosides
2.1. Physicochemical properties of ginsenosides
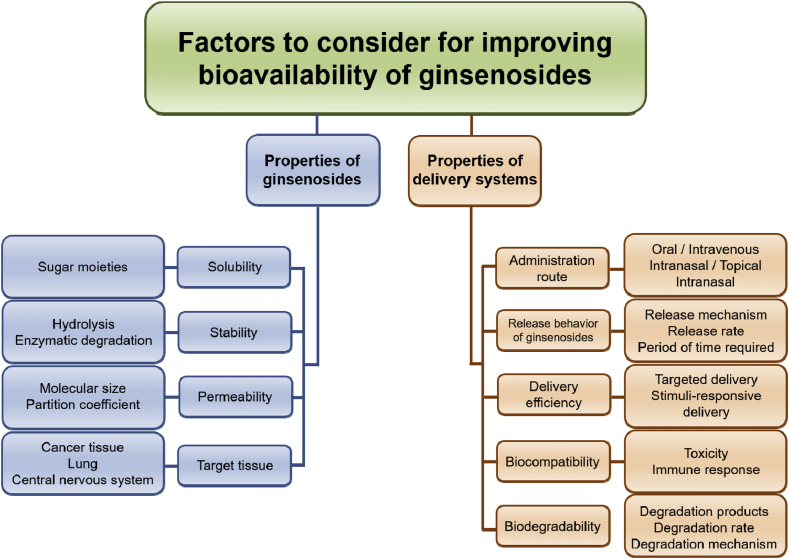
Physicochemical properties of ginsenosides should be carefully considered as shown in Fig. 1 to successfully increase the bioavailability of the natural products. The physicochemical characteristics can be varied depending on the chemical structure of ginsenosides, which typically consists of a dammarane skeleton containing 17 carbons in its four-ring structure and different sugar moieties, such as glucose, xylose, and arabinose, which are bound to the C-3 and C-20 positions [56], [57].
Fig. 1.
The bioavailability of ginsenosides is governed by numerous factors related to delivery carriers and systems as well as physicochemical properties of ginsenosides. The physicochemical properties of ginsenosides such as aqueous solubility, stability, and biomembrane permeability need to be evaluated before design of efficient delivery systems. The delivery systems should be carefully designed to enhance the bioavailability of ginsenosides, considering administration route, release behavior, biocompatibility, and biodegradability. The delivery systems also need to release ginsenosides at the desired sites of the body.
Owing to the large molecular size of the dammarane skeleton, ginsenosides generally exhibit low membrane permeability [36]. For this reason, the absorption of ginsenosides in the GI tract depends largely on energy-dependent transporters such as the sodium-dependent glucose cotransporter 1 [25]. Despite the presence of the transporters, however, the oral bioavailability of ginsenosides and their metabolites has been known to be extremely low [26], [27], [58]. One of the main reasons for this is because p-glycoprotein efflux system causes the efflux of ginsenosides, thereby lowering the bioavailability [23].
In terms of solubility of ginsenosides, it can vary depending on the number of sugar units in the chemical structure of ginsenosides [43]. The sugar moieties exhibiting hydrophilic property facilitate the dissolution of ginsenosides. However, many ginsenosides having anticancer activity have been reported to be aglycones, which means the natural products lack the sugar moieties, thereby showing low aqueous solubility [59].
The stability of ginsenosides is also problematic because ginsenosides can be degraded by acid hydrolysis in the stomach along with the glycosyl elimination and epimerization of C-20 sugar moiety [48], [60]. The intestinal microflora also have been reported to cleave the C-3 or C-20 oligosaccharides of ginsenosides [48]. The degradation of ginsenosides by hydrolysis and enzymes may lead to the loss of therapeutic activities of the natural products. Therefore, suitable delivery systems that can improve the undesirable properties of ginsenosides are indispensable for increasing the oral bioavailability of ginsenosides.
2.2. Pharmaceutical properties of delivery systems and dosage forms
Delivery systems of ginsenosides must be systemically designed with considering various properties that affect resulting bioavailability of ginsenosides as illustrated in Fig. 1.
First of all, fundamental materials constituting the delivery systems should be safe and biocompatible. The materials should not exhibit any toxicity to the human body and affect the physiological functions. In addition, the materials must not undesirably interact with ginsenosides, which can result in the reduction of therapeutic efficacies of the natural products. They also need to be biodegradable and eliminated from the body at a proper rate, and the degradation products also should be safe and properly excreted.
For enhancing the bioavailability of poorly soluble ginsenosides, the delivery systems need to solubilize the ginsenosides because the solubility significantly affect the dissolution rate of the natural products and resulting absorption to the systemic circulation [61].
The delivery systems should also stabilize ginsenosides from the harsh physiological conditions such as highly acidic environment in the stomach and enzymes existing in the GI tract. Without suitable protection of ginsenosides, the natural products may be degraded before being absorbed, leading to a low bioavailability [6]. In addition, the delivery systems should facilitate the penetration of ginsenosides through the biomembranes at the absorption site because ginsenosides generally exhibit poor permeability as described previously [44], [45], [49], [62].
To maximize the bioavailability of ginsenosides, the release behavior of ginsenosides from the delivery systems also should be considered. Based on physicochemical properties of ginsenosides, the optimization of the release rate of ginsenosides can also be an effective means to increase the bioavailability [20], [44], [46], [47], [63], and it can be achieved by changing various properties of the delivery systems, such as the composition including types and concentration of pharmaceutical materials used and physical features such as the size and shape of the systems.
Depending on the types of diseases and lesions, the delivery systems can be modified to target ginsenosides to the specific sites of the body. The targeted delivery of ginsenosides is able to promote their efficient delivery to the site of interest, thereby maximizing the therapeutic efficacy of ginsenosides and also decreasing their possible systemic toxicity [23], [48], [50], [51]. In addition, the administration route of the delivery systems can be varied to avoid the delivery of ginsenosides to unwanted sites and increase the bioavailability [64], [65]. Preventing possible toxicity of ginsenosides is important because ginsenosides have been known to exhibit some side effects, such as hypertension, insomnia, anxiety, diarrhea, and vomiting, when they are administered to the body at a high dose or for a long period of time [66], [67]. Recently, it has also been newly reported that ginsenosides can even disturb the immune and hematopoietic systems [67]. Therefore, the delivery systems should be carefully designed along with selecting suitable administration routes to maximize the therapeutic efficacies of ginsenosides and minimize the possible toxicities of ginsenosides.
3. Representative micro-/nano-sized delivery systems for improving bioavailability of ginsenosides
3.1. Oil-based delivery systems
3.1.1. Microemulsions
Emulsions are immiscible mixtures of a biphasic oil–water system, in which one liquid phase is dispersed in the other phase. There are two types of emulsions: oil-in-water (o/w) emulsion consisting of oil droplets dispersed in water and water-in-oil (w/o) emulsion where water droplets are dispersed in oil. Emulsions are useful delivery systems for poorly soluble drugs because they can effectively solubilize the drugs and increase the absorption rate and bioavailability of the drugs due to their small droplet size and resulting large surface area of droplets [68], [69].
Among various types of emulsions, microemulsions have been shown to be highly effective for improving the bioavailability of poorly soluble drugs [70]. The main difference of microemulsions compared with conventional emulsions is the thermodynamic stability achieved by optimized composition of the microemulsion including appropriate ratios of oil, water, surfactant, and possibly cosurfactant. Owing to the improved thermodynamic stability, the microemulsions exhibit smaller droplet sizes (typically dozens-hundreds nanometers) than conventional emulsions with droplet sizes of micrometer scale. The microemulsions thus show increased drug dissolution rates than normal emulsions, thereby efficiently facilitating the drug absorption [38]. The microemulsions have also been reported to be less affected by food effects, leading to enhanced reproducibility of drug plasma profiles [71], [72]. In addition, the microemulsions have been known to not depend largely on the lipid digestion process, which is required for the absorption of drugs formulated in other lipid-based drug delivery systems [71]. For the reasons, the microemulsions have been exploited to enhance the bioavailability of ginsenosides.
Han et al [44] have investigated w/o microemulsion of ginsenoside Rg1 and showed that the microemulsion can significantly increase the intestinal absorption of ginsenoside Rg1 after the intraduodenal administration to rats. The increased absorption may be associated with the improved solubility and dissolution rate of ginsenoside Rg1 and enhanced membrane permeability of Rg1 by the microemulsions, which was demonstrated by the parallel artificial membrane permeability assay and in vitro rat skin permeability test. The microemulsion finally increased relative bioavailability of ginsenoside Rg1 ranging from 268% to 1270% in comparison with that of the control ginsenoside solution.
Administration of microemulsions containing ginsenosides through nasal route for brain delivery has also been explored [12]. Nasal delivery of ginsenosides provides the following advantages. First, the first-pass metabolism can be avoided. Second, the enzymatic activity of the nasal route is relatively low compared with that of other organs. Third, the onset of therapeutic effects is comparatively rapid. In addition, ginsenosides administered through the nasal passage can directly enter cerebrospinal fluid, cross the blood–brain barrier, and reach the brain [73]. The nasal mucosa is supplied with sufficient bloodstream, which is advantageous for the absorption of ginsenosides into the systemic circulation [61].
Tao et al [46] investigated the pharmacokinetics and brain uptake of ginsenoside Rg1 and Rb1 contained in o/w microemulsion. The results showed that the relative rates of uptake and the ratio of peak concentration of the ginsenosides in the brain increased by 126.31% and 147.48%, respectively, by the microemulsion compared with those of the ginsenoside solution. These data demonstrated that the intranasal administration of the microemulsion promoted delivery efficiency of the ginsenosides to the brain and enhanced the bioavailability.
3.1.2. Nanoemulsions
Nanoemulsions are similar to microemulsions in terms that they are also composed of oil, water, surfactants, and possibly cosurfactants. However, in contrast to microemulsions, the dispersion of the dispersed phase in nanoemulsions is not spontaneously achieved because the dispersion state of nanoemulsions is not thermodynamically stable [74]. The dispersion process of nanoemulsion thus generally requires intensive mechanical forces exerted by some devices, such as high pressure homogenizer and ultrasound generator. Despite the disadvantages, nanoemulsions provide benefits such as less amount of surfactants needed for the preparation and extremely small droplet sizes (generally less than 100 nanometers), which is advantageous for increasing the drug absorption rate [75], [76]. Therefore, the nanoemulsion could be one of the promising delivery systems for improving the bioavailability of ginsenosides.
Nanoemulsion has been studied to improve the oral bioavailability of Panax notoginseng saponins (PNS), which are easily degraded and metabolized by the gastric acid, glycosidase, and intestinal flora existing in the GI tract after oral administration [77]. The nanoemulsions of PNS were successfully prepared by the following procedure. The oil phase, surfactant, and cosurfactant were added to PNS solution and stirred adequately. Then, the mixture was homogenized by a high pressure homogenizer until transparent nanoemulsions of PNS were acquired. The particle characteristics of the nanoemulsion such as particle size, polydispersity index, refractive index, and content of ginsenosides in the emulsion were also optimized. In this study, the nanoemulsion exhibited the improved stability of PNS and released the ginsenosides in a sustained manner. Improved stability of the nanoemulsions of PNS was demonstrated by an accelerated test. Under this test, the nanoemulsions of PNS were still transparent, without any turbidities after 6 months. PNS from the nanoemulsions were released near completely (98.13%) within 48 hours, whereas PNS were released from the solution completely within only 10 hours. In addition, pharmacokinetic study in rats was performed. Compared with PNS solution, the nanoemulsions of PNS showed around fivefold increase in the absorption and 2.58-fold increase in the oral bioavailability [47]. The reason for the increase in oral bioavailability of PNS might be largely attributed to the small droplet size and good permeability of the dispersed phase containing the PNS.
3.2. Polymeric microparticles/nanoparticles
3.2.1. Polymeric microparticles
Polymeric microparticles are small spherical particles, with diameters generally ranging from 1 μm to 1000 μm. The microparticles have large surface area due to their small particle size, thereby increasing the drug absorption rate in the GI tract and also can protect the entrapped drugs from external environments [78]. In addition, the polymeric microparticles can provide controllable drug release behaviors, which is also useful for improving the bioavailability of drugs [47]. Therefore, polymeric microparticles have been used to improve the bioavailability of ginsenosides.
Wei et al [47] investigated the effect of ginsenoside Rg1-loaded gelatin microspheres (GMS) crosslinked with genipin on the angiogenesis in myocardial tissues and the preservation of left ventricular function after injecting the GMS to rats via intramuscular route. They demonstrated the improved stability of Rg1 loaded in GMS by the crosslinking using genipin. In contrast, GMS without the crosslinking became collapsed and agglomerated when exposed to aqueous environment. From the rats administered the Rg1-loaded GMS, the attenuation of the left ventricular hypertrophy and myocardial fibrosis was observed. In addition, the Rg1-loaded GMS were found to significantly promote angiogenesis in myocardial tissue. The results thus imply that Rg1-loaded GMS were stable and successfully improved myocardial perfusion and left ventricular function [47].
Another study investigated GMS-incorporated collagen/chitosan (CC) scaffolds for regeneration of skin tissues [52]. The GMS was used as a controlled release system of ginsenoside Rg1 that can stimulate cell proliferation and angiogenesis, which might be helpful for enhancing the skin regeneration effect of the CC scaffolds. Pharmacological activity of Rg1 could be preserved from external environment due to the protecting function of the GMS. In addition, Rg1 released from the GMS in a controlled manner increased proliferation, migration, and tube formation of human umbilical vein endothelial cell by 267.59%, 186.42%, and 252.73%, respectively, compared with those of the control group devoid of the GMS. Therefore, Rg1-loaded GMS-CC scaffolds were proved to be effective for regeneration of skin tissues.
Zhang et al [12] designed and evaluated Rg3-loaded chitosan microspheres to prolong the residence time of Rg3, which has pharmacological activities such as antifatigue and immune-boosting effects, in the nasal cavity. Despite various advantages aforementioned, the drug administration through nasal route generally does not permit the retention of drugs in the nasal cavity for a long time, leading to rapid clearance of drugs and resulting low bioavailability. The authors assumed that the chitosan microspheres can protect Rg3 from enzymatic degradation and increase the retention time of Rg3 in the nasal cavity and performed pharmacodynamics studies using Rg3-loaded chitosan microspheres. They evaluated the weight-bearing swimming time of mice to assess the antifatigue effect. The result showed that the swimming time was increased by 12.5% after the administration of Rg3-loaded chitosan microspheres compared with that measured with Rg3 aqueous solution. Biochemical parameters associated with antifatigue effect, such as concentration of lactic acid, serum urea nitrogen, hepatic glycogen, and lactate dehydrogenase in the blood, were also examined. Blood lactic acid and serum urea nitrogen were significantly lower in mice administered Rg3-loaded chitosan microspheres than those administered the Rg3 solution. Hepatic glycogen and lactate dehydrogenase were significantly higher in mice administered Rg3-loaded chitosan microspheres than those administered the Rg3 solution. The results might be largely due to the prolonged residence time of Rg3 in the nasal cavity [12].
Baek et al [20] developed polymeric microparticles of ginsenosides composed of enteric-coating and mucoadhesive polymer. Eudragit L100-55, an anionic polymer of methacrylic acid that dissolves at pH ranging from 5.5 to 7, was used to prepare the microparticles, and chitosan was used to coat the microparticles to confer them a mucoadhesive property. The study aimed to overcome the low oral bioavailability of ginsenosides caused by degradation in the acidic environment of the stomach. The use of the enteric-coating polymer and mucoadhesive polymer was expected to avoid the hydrolysis of the microparticles in the stomach and to promote the localization of the particles in the intestinal membranes. The results showed that the microparticles slowly released ginsenosides at pH 1.2, followed by rapid release of the ginsenosides at pH 6.8, implying that the microparticles could control the release rate of the ginsenosides depending on the variation of pH. The mucoadhesive property of the microparticles was also demonstrated by the changes in zeta potential values by chitosan. Therefore, the authors showed the possibility of the microparticles to be used as a useful delivery system of ginsenosides.
3.2.2. Polymeric nanoparticles
Polymeric nanoparticles are very small particles of which particle size generally ranges from 10 nm to 1000 nm. Because of their extremely small particle size, the nanoparticles provide improved drug dissolution rates and enhanced drug absorption. The nanoparticles also can release the drugs in a controlled manner, similar to the polymeric microparticles. Furthermore, the polymeric nanoparticles can protect the entrapped drugs from external environments [39]. In some cases, the nanoparticles can easily reach the target sites compared with microparticles due their small particle size. For the advantages, polymeric nanoparticles have been exploited to enhance the bioavailability of ginsenosides.
Voruganti et al [53] identified a novel ginsenoside 25-OCH3-PPD (GS25), which has been known to exhibit high anticancer activity. The authors demonstrated the inhibitory effect of GS25 on mouse double minute 2, an important negative regulator of the p53 tumor suppressor and its anticancer activity in various human cancer models. However, the oral bioavailability of GS25 was highly limited due to its hydrophobic property; therefore, GS25-loaded polyethylene glycol (PEG)-poly(lactic-co-glycolic acid) (PLGA) nanoparticles (GS25NP) were designed to improve the oral bioavailability of GS25. PLGA nanoparticles have been reported to be useful for enhancing solubility and bioavailability of drugs, but they commonly exhibit a short residence time in the systemic circulation due to their rapid clearance. Therefore, the authors coated the nanoparticles with a hydrophilic polymer, PEG, which can stabilize the nanoparticles and increase their residence time. Compared with unencapsulated GS25, GS25NP demonstrated significantly increased oral bioavailability of GS25 and enhanced anticancer activity both in vitro and in vivo models [53].
Zhang et al prepared PLGA nanoparticles loaded with salvianolic acid B, tanshinone IIA, and PNS by a double emulsion/solvent evaporation method and administered the nanoparticles to guinea pigs [23]. Distribution of the ginsenosides within the inner ear, cerebrospinal fluid, and brain of the animals was found to be improved after intratympanic administration compared with that after intravenous injection. In vitro release tests also demonstrated that PLGA nanoparticles showed a good sustained release behavior of ginsenosides. Ginsenoside R1 was completely released from the nanoparticles within 72 hours, whereas it was released completely within only 6 hours without using the nanoparticles. Pharmacodynamic studies also showed that the nanoparticles markedly inhibited oxidizing reactions and protected the brain from cerebral ischemia-reperfusion injury through an upregulation of superoxide dismutase activity in serum and the brain. The levels of malondialdehyde and nitric oxide synthase were also significantly reduced. The preliminary toxicity studies proved that intratympanic delivery of the nanoparticles did not affect cochlear function. Collectively, these findings suggested that ginsenoside-loaded PLGA nanoparticles can be effectively used for the treatment of brain diseases via inner ear administration [23].
Mathiyalagan et al developed hydrophilic polymer-conjugated ginsenosides that can self-assemble to form nanoparticles [50]. The hydrophobic ginsenoside compound K (CK) was covalently conjugated to the backbone of hydrophilic glycol chitosan (GC) through an acid-labile linkage. The conjugates formed self-assembled nanoparticles in a buffer at physiological pH 7.4 and were found to be highly stable in the condition. The nanoparticles could be dissolved when exposed to mildly acidic conditions such as tumor tissues and inflammatory sites, thereby selectively releasing the incorporated ginsenosides to the target lesions. The GC-CK conjugates also exhibited high cytotoxicity in various cell lines. The results imply that the self-assembled GC-CK nanoparticles can be utilized as a pH-responsive delivery system of ginsenosides [50].
Another research group developed nanoparticles composed only of therapeutic agents including ginsenosides [43]. This was achieved by binding ginsenoside Rb1 to natural anticancer drugs such as betulinic acid, dihydroartemisinin, and hydroxycamptothecin. The authors demonstrated that the ginsenoside Rb1 conjugated to the natural anticancer drugs self-assembled to form stable nanoparticles. The nanoparticles exhibited stronger anticancer effects both in vitro and in vivo models than the respective free drugs. Moreover, the nanoparticles, owing to their particle sizes of approximately 100 nm, showed better tumor selectivity and longer half-life in the systemic circulation. Furthermore, the nanoparticles showed decreased side effects in a mouse tumor xenograft model than each free drug. The results therefore strongly support the usefulness of the self-assembled nanoparticles as a novel and efficient nano-sized delivery system of ginsenosides for cancer therapy [43].
3.3. Vesicular delivery systems
3.3.1. Liposomes
Liposomes are one of the most extensively studied nano-sized vesicular delivery systems. They have lipid bilayer vesicles with similarities to the biological membrane. They can trap both hydrophobic and hydrophilic compounds and protect the entrapped compounds from external environment. Liposomes are also biocompatible and biodegradable because they are generally prepared using phospholipids that can be utilized by the human body [40].
Liposomes can incorporate poorly soluble drugs in their lipid bilayer, which can lead to improved dissolution behavior of the drugs [64]. The small size and resulting large surface area of liposomes are also advantageous for increasing the absorption rate of the entrapped drugs. In addition, liposomes can protect the drugs from harsh external environment such as gastric acid and enzymes present in the GI tract, thereby increasing the bioavailability of the drugs. For the advantages, liposomes have been exploited to improve the bioavailability of ginsenosides.
Yu et al evaluated the pharmacokinetic parameters of Rg3 loaded in liposomes and their anticancer activity after administering the liposomal Rg3 to Wistar rats compared with those of Rg3 solutions [49]. The liposomal Rg3 showed considerably improved pharmacokinetic parameters such as Cmax and area under the curve (AUC) compared with those assessed with the Rg3 solution, which might be attributed to the improved dissolution behavior and enhanced permeability of Rg3 by the liposomes. In addition, the liposomal Rg3 significantly reduced the tumor growth rate compared with the Rg3 solution [49].
However, liposomes generally exhibit a poor colloidal stability in the suspension state. They can aggregate each other and also leak drugs incorporated in their internal spaces. In addition, the lipids constituting the liposomes can be oxidized and hydrolyzed, which are not undesirable for maintaining the stability of liposomes [64]. To overcome these limitations, liposomes have been solidified to proliposome by removing water in the liposomal suspension using various methods, such as freeze-drying, vacuum drying, fluidized bed drying, or spray drying [42], [79].
Among the methods, freeze-drying has been shown to be promising for increasing the stability of liposomes, maintaining the biological activity of drugs, and preventing the degradation of drugs by hydrolysis. However, the freeze-drying can cause increase in the particle size of liposomes, which might be caused by the physical stress and formation of ice crystals occurring during the freeze-drying process. Li et al therefore used lyoprotectants such as glucose, trehalose, maltose, lactose, mannitol, inositol, and PEG and compared their protective effects on ginsenoside Rg3-loaded liposomes during the freeze-drying procedure [80]. The results showed that the best protective effect was observed with the combined used of glucose and mannitol, exhibiting the most improved stability of the liposomes in terms of the particle size and drug retention rate. Moreover, the lyophilization of Rg3-loaded liposomes with the lyoprotectants did not significantly affect the anticancer activity of Rg3 [80].
Hao et al [45] also prepared ginseng fruit saponin-loaded proliposomes (GFS-P) incorporating sodium deoxycholate, which is a bile salt that can improve the stability of the liposomes in the GI tract and permeability through the biomembrane. In vitro release profiles of GFS from reconstituted GFS-P and GFS solution were comparatively assessed in artificial gastric fluid at pH 1.2 and simulated intestinal fluid at pH 6.8. The result showed that the reconstituted proliposomes exhibited sustained release of GFS at both pH 1.2 and pH 6.8 compared with the GFS solution. The stability of the proliposomes was evaluated by examining the particle size distribution and drug entrapment efficiency after storage at room temperature (37°C) and refrigerated temperature (4°C) for 90 days. As a result, no significant changes in the particle size and drug entrapment efficiency were observed, implying that the stability of the liposomes was well maintained [45]. The GFS-P could also increase the permeability of ginsenoside Re (GRe) in the GI tract owing to the bile salt contained in the liposomes. Among GFS-P, GFS solution, and Zhenyuan tablets, a commercial product of GFS, the pharmacokinetic study in rats indicated that GFS-P presented the greatest Cmax, longest half-life, and highest AUC. Therefore, the results demonstrated that proliposomes successfully increased the oral availability of GFS.
3.3.2. Modified liposomes
Despite various advantages of liposomes as described previously, conventional liposomes show limitations such as the poor drug delivery efficiency to the lesions and rapid clearance from the systemic circulation caused by the reticuloendothelial system. To address the problems, lipids constituting liposomes have been modified with various polymers [81], [82]. For example, PEGylated liposomes, liposomes consisting of lipids conjugated with PEG, have been extensively investigated for prolonging the residence time of the liposomes in the bloodstream. Some liposomes were modified with cationic materials and were used to increase delivery efficiency of anticancer agents to tumor tissues because the positive charge of the liposomes can strongly interact with the tumor cell membrane [83]. Owing to the advantages, the modified liposomal systems have also been investigated to enhance the bioavailability of ginsenosides.
In a study conducted by Xu et al, three types of liposomes, namely conventional liposomes, methoxy PEG-polylactic acid–modified liposomes (PLP), and cationic liposomes (CLP) with octadecylamine, were prepared and evaluated [48]. The results showed that CLP and PLP showed similar physicochemical properties such as average particle size, particle size distribution, and zeta potential value to conventional liposomes. The PLP and CLP also released Rh2 in a sustained manner compared with free Rh2, and CLP exhibited more pH-sensitive release of Rh2 than other liposomes. In vivo test, PLP, and CLP significantly increased the accumulation of a fluorescent cyanine dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide (DiR) in tumor tissues xenografted to nude mice more than conventional liposomes. PLP and CLP also prolonged the residence time of DiR in tumors. When comparing the PLP and CLP, PLP exhibited more increased accumulation and longer residence time of DiR in the tumors. The results might be attributed to decreased elimination of ginsenoside Rh2 by the PEGylation and resulting in much more accumulation of the PLP in tumor tissues by the enhanced permeability and retention effect [62]. Furthermore, the PLP also showed the highest suppressive effect on the tumor growth, without causing any systemic toxicity. The results together suggested that PLP and CLP could release ginsenoside Rh2 in a controlled manner, prolong the resident time of the ginsenoside in the bloodstream, and enhance the bioavailability [62], [84].
In another study, poorly soluble drug, ginsenoside compound K (GCK) was loaded in liposomes modified with tocopheryl polyethylene glycol succinate (TPGS) to enhance the solubility of the ginsenoside, improve the drug encapsulation efficiency, and inhibit efflux of GCK from tumor cells caused by P-glycoprotein [49], [85], [86]. The amphiphilic property of TPGS confers the liposomes good surfactivity because of the polar head portion of PEG and the lipophilic alkyl tail of tocopherol succinate. Therefore, it could increase solubility and membrane permeability of GCK. In vitro release studies showed the improvement of dissolution behavior and controlled release of GCK from the liposomes due to increased hydrophobic interaction between the aromatic ring of TPGS and phospholipids constituting the liposomes. In addition, the liposomes exhibited a strong hypersensitization effect on A549 cells, which enhanced their cellular uptake. Furthermore, the IC50 of GCK from the liposomes was significantly lower than that of free GCK. Moreover, the liposomes could be selectively accumulated in tumor tissues by the inhibition of P-glycoprotein pump and the enhanced permeability and retention effect [49].
Owing to comparatively poor drug-loading efficiency and possible drug leakage of liposomes, liposomes are generally considered to be not proper for achieving controlled drug release [64]. To overcome the drawbacks of liposomes, PNS-loaded core-shell hybrid liposomal vesicles (PNS-HLV) were developed [54]. The PNS-HLV consisted of a core nanoparticle composed of methyl ether PEG conjugated to PLGA and lipid bilayers surrounding the core nanoparticle. Therefore, the PNS-HLV were expected to exhibit the combined advantages of polymeric nanoparticles and liposomes, thereby achieving the controlled drug release, high drug loading efficiency, and good biomembrane permeability.
When evaluating the drug-loading efficiency of PNS, the PNS-HLV exhibited the highest value among a PNS solution, conventional PNS-loaded nanoparticles, and liposomes. In addition, the PNS-HLV showed the best controlled drug release profiles at physiological pH condition (pH 7.4) and acidic condition (pH 2). Furthermore, the stability of PNS-loaded nanoparticles, PNS-loaded liposomes, and PNS-HLV in terms of particle size and drug entrapment efficiency was evaluated at 4°C under application of light. No significant change was observed from PNS-HLV, indicating that the stability of PNS-HLV was well preserved. In the case of in vivo test, PNS-HLV significantly decreased the infarction volume and exhibited the highest antioxidant effect [54].
3.3.3. Ethosomes and transfersomes
Topical delivery of ginsenosides such as Rh1 has exhibited antiallergic and antiinflammatory effects on the skin. However, conventional liposomes containing ginsenosides generally do not penetrate deeply into the skin, and therefore, advanced liposomes such as ethosomes and transfersomes have been studied to enhance the topical delivery of ginsenosides. Ethosomes, consisting of phospholipid, ethanol and water, show increased skin permeability because ethanol makes the lipid bilayer flexible and disrupts the skin barrier [41]. Transfersomes are ultraflexible liposomes composed of phospholipids and edge activator (surfactants) present within the lipid matrix, which render the lipid bilayer flexibility. The flexibility of transfersomes is advantageous for penetrating into the skin tissues [87]. Therefore, ethosomes and transfersomes were used to enhance the dermal delivery of ginsenosides.
Choi et al investigated ginsenoside Rh1-loaded vesicular systems such as liposomes, ethosomes, and transfersomes [55]. They demonstrated that the flexibility of the vesicles affected the entrapment of ginsenoside Rh1. The transfersomes exhibited the highest entrapment efficiency of ginsenoside Rh1, followed by ethosomes and liposomes. The authors then evaluated the skin permeability of the Rh1-loaded vesicles using Franz diffusion cells where the human cadaver skin was inserted. The result showed that the fraction of ginsenoside Rh1 that penetrated the skin was the greatest with the transfersomes, followed by the ethosomes and liposomes. Therefore, it has been proven that flexible liposomes could be used to enhance the topical delivery of ginsenosides [55].
3.3.4. Niosomes
Niosomes are nonionic surfactant-based vesicles and can encapsulate drugs with a wide range of solubility due to the presence of amphiphilic moieties in the structure [88], [89]. Therefore, niosomes are promising as a carrier of ginsenosides, which exhibit a broad range of aqueous solubility.
Niosomes with pH-sensitive core materials could be particularly useful for delivery of ginsenosides with anticancer activity. For example, Chen et al [51] have designed niosomes having double pH-sensitive mixed micelles containing ginsenoside Rh2. The pH-sensitive micelles were prepared with hydrazine-based pH-responsive methoxy PEG2000-hydrazone-cholesteryl hemisuccinate (mPEG2000-Hz-CHEMS) and methoxy PEG2000 (mPEG2000) [90]. The pH-sensitive micelles containing ginsenoside Rh2 were mixed with Pluronic F-68 used as a nonionic surfactant in an aqueous medium to fabricate niosomes to prepare Rh2-loaded multicore niosomes (Rh2-MCN).
In vitro drug release study showed that ginsenoside Rh2 was rapidly released from the MCN at pH 5.0 compared with at pH 7.4. Evaluation of cytotoxicity of Rh2-MCN was carried out using MCF-7 cells and MTT assay. As a result, Rh2-MCN exhibited greater cytotoxicity than free Rh2, possibly due to the better permeability of Rh2-MCN than free Rh2. In vivo study also showed the selective accumulation of Rh2-MCN in tumor tissues and resulting reduced growth of the tumor tissues after 2 weeks [51].
4. Conclusion
The importance of ginsenosides as a natural medicine has been recognized for a long time due to their versatile therapeutic activities that have been continuously proven in vitro and in vivo models. Despite the promising therapeutic potentials of ginsenosides, however, the use of ginsenosides has been largely limited by the low bioavailability, which might be attributed to the undesirable physicochemical properties of ginsenosides. Pharmaceutical scientists have made many efforts to improve the bioavailability of ginsenosides by developing efficient delivery systems and examining various administration routes of ginsenosides, leading to significant improvement of the bioavailability of ginsenosides, showing some degree of success. Despite the presence of the accumulated works on the development of delivery systems of ginsenosides, only a few reviews that summarized the information on specific delivery systems of ginsenosides such as polymeric nanoparticles are available, and such articles generally lacked the comprehensive and systematic analysis of previously studied delivery systems of ginsenosides. Therefore, in this mini-review, representative delivery systems for enhancing the bioavailability of ginsenosides were covered along with providing factors to consider for improving the bioavailability of ginsenosides such as properties of ginsenosides and delivery systems.
However, the delivery systems were designed and evaluated only for a single type of ginsenoside, and therefore, their performance might be limited for other ginsenosides exhibiting different physicochemical properties. In addition, the delivery systems were studied to enhance the bioavailability of only a few representative types of ginsenosides, which is also problematic for expanding the application range of the delivery systems into further diverse types of ginsenosides.
Furthermore, in general, the delivery systems explored for enhancing the bioavailability of ginsenosides were confined to some dosage forms depending on comparatively simple working mechanisms, and thereby efficient delivery of ginsenosides to target tissues or lesions was largely limited although they exhibited enhanced systemic bioavailability of ginsenosides. Recently, some studies have reported further advanced delivery systems of ginsenosides such as targeted and stimuli-responsive systems; the accumulated data are still insufficient for clinical translation.
Therefore, further studies need to be conducted by pharmaceutical scientists to develop better drug delivery systems based on systematic analysis of different physicochemical properties of ginsenosides, along with pursuing enhancement of the delivery efficiency of ginsenosides to target tissues. The future delivery systems will maximize the potential of ginsenosides as a natural medicine, thereby further contributing to promoting global health.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2015R1A5A1008958). This work was also supported by the Industry Technology Development Program (10077593) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Contributor Information
Ji-Yun Lee, Email: jylee98@cau.ac.kr.
Jaehwi Lee, Email: jaehwi@cau.ac.kr.
References
- 1.Wang C.Z., Zhang B., Song W.X., Wang A., Ni M., Luo X. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 2.Park E.K., Shin Y.W., Lee H.U., Kim S.S., Lee Y.C., Lee B.Y. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 3.Rai D., Bhatia G., Sen T., Palit G. Anti-stress effects of Ginkgo biloba and Panax ginseng: a comparative study. J Pharmacol Sci. 2003;93:458–464. doi: 10.1254/jphs.93.458. [DOI] [PubMed] [Google Scholar]
- 4.Keum Y.S., Park K.K., Lee J.M., Chun K.S., Park J.H., Lee S.K. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 6.Gu Y., Wang G.J., Sun J.G., Jia Y.W., Wang W., Xu M.J. Pharmacokinetic characterization of ginsenoside Rh2, an anticancer nutrient from ginseng, in rats and dogs. Food Chem Toxicol. 2009;47:2257–2268. doi: 10.1016/j.fct.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Qi L.W., Wang C.Z., Yuan C.S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms S. Cancer prevention and therapeutics: panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 9.Yuan H.D., Quan H.Y., Zhang Y., Kim S.H., Chung S.H. 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol Med Rep. 2010;3:825–831. doi: 10.3892/mmr.2010.328. [DOI] [PubMed] [Google Scholar]
- 10.Kim B.J., Nah S.Y., Jeon J.H., So I., Kim S.J. Transient receptor potential melastatin 7 channels are involved in ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic Clin Pharmacol Toxicol. 2011;109:233–239. doi: 10.1111/j.1742-7843.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 11.Yun T.K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Wang X., Zhang M., Xu M., Tang W., Zhang Y. Intranasal delivery of microspheres loaded with 20 (R)-ginsenoside Rg3 enhances anti-fatigue effect in mice. Curr Drug Deliv. 2016 doi: 10.2174/1567201814666161109121151. [DOI] [PubMed] [Google Scholar]
- 13.Kim T.W., Joh E.H., Kim B., Kim D.H. Ginsenoside Rg5 ameliorates lung inflammation in mice by inhibiting the binding of LPS to toll-like receptor-4 on macrophages. Int Immunopharmacol. 2012;12:110–116. doi: 10.1016/j.intimp.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Choi S., Kim T.W., Singh S.V. Ginsenoside Rh2-mediated G(1) phase cell cycle arrest in human breast cancer cells is caused by p15 (Ink4B) and p27 (Kip1) -dependent inhibition of cyclin-dependent kinases. Pharm Res-dordr. 2009;26:2280–2288. doi: 10.1007/s11095-009-9944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong A., Lee H.J., Jeong S.J., Lee H.J., Lee E.O., Bae H. Compound K inhibits basic fibroblast growth factor-induced angiogenesis via regulation of p38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Biol Pharm Bull. 2010;33:945–950. doi: 10.1248/bpb.33.945. [DOI] [PubMed] [Google Scholar]
- 16.Ming Y., Chen Z., Chen L., Lin D., Tong Q., Zheng Z. Ginsenoside compound K attenuates metastatic growth of hepatocellular carcinoma, which is associated with the translocation of nuclear factor-kappaB p65 and reduction of matrix metalloproteinase-2/9. Planta Med. 2011;77:428–433. doi: 10.1055/s-0030-1250454. [DOI] [PubMed] [Google Scholar]
- 17.Lee B.H., Lee S.J., Hur J.H., Lee S., Sung J.H., Huh J.D. In vitro antigenotoxic activity of novel ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1998;64:500–503. doi: 10.1055/s-2006-957501. [DOI] [PubMed] [Google Scholar]
- 18.Li B., Zhao J., Wang C.Z., Searle J., He T.C., Yuan C.S. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011;301:185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benishin C.G., Lee R., Wang L.C., Liu H.J. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology. 1991;42:223–229. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- 20.Baek J.S., Yeon W.G., Lee C.A., Hwang S.J., Park J.S., Kim D.C. Preparation and characterization of mucoadhesive enteric-coating ginsenoside-loaded microparticles. Arch Pharm Res. 2015;38:761–768. doi: 10.1007/s12272-014-0395-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q.H., Wu C.F., Duan L., Yang J.Y. Protective effects of ginsenoside Rg(3) against cyclophosphamide-induced DNA damage and cell apoptosis in mice. Arch Toxicol. 2008;82:117–123. doi: 10.1007/s00204-007-0224-3. [DOI] [PubMed] [Google Scholar]
- 22.Cai B.X., Jin S.L., Luo D., Lin X.F., Gao J. Ginsenoside Rb1 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Biol Pharm Bull. 2009;32:837–841. doi: 10.1248/bpb.32.837. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X., Chen G., Wen L., Yang F., Shao A.L., Li X. Novel multiple agents loaded PLGA nanoparticles for brain delivery via inner ear administration: in vitro and in vivo evaluation. Eur J Pharm Sci. 2013;48:595–603. doi: 10.1016/j.ejps.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Li H., Ye M., Guo H., Tian Y., Zhang J., Zhou J. Biotransformation of 20(S)-protopanaxadiol by mucor spinosus. Phytochemistry. 2009;70:1416–1420. doi: 10.1016/j.phytochem.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Paek I.P., Moon Y., Kim J., Ji H.Y., Kim S.A., Sohn D.H. Pharmacokinetics of a ginseng saponin metabolite compound K in rats. Biopharm Drug Dispos. 2006;27:39–45. doi: 10.1002/bdd.481. [DOI] [PubMed] [Google Scholar]
- 26.Ren H.C., Sun J.G., Wang G.J., A J.Y., Xie H.T., Zha W.B. Sensitive determination of 20(S)-protopanaxadiol in rat plasma using HPLC-APCI-MS: application of pharmacokinetic study in rats. J Pharmaceut Biomed. 2008;48:1476–1480. doi: 10.1016/j.jpba.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Han M.H., Chen J., Chen S.L., Wang X.T. Development of a UPLC-ESI-MS/MS assay for 20(S)-protopanaxadiol and pharmacokinetic application of its two formulations in rats. Anal Sci. 2010;26:749–753. doi: 10.2116/analsci.26.749. [DOI] [PubMed] [Google Scholar]
- 28.Xu Q.F., Fang X.L., Chen D.F. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–192. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 29.Li X., Wang G., Sun J., Hao H., Xiong Y., Yan B. Pharmacokinetic and absolute bioavailability study of total panax notoginsenoside, a typical multiple constituent traditional Chinese medicine (TCM) in rats. Biol Pharm Bull. 2007;30:847–851. doi: 10.1248/bpb.30.847. [DOI] [PubMed] [Google Scholar]
- 30.Joo K.M., Lee J.H., Jeon H.Y., Park C.W., Hong D.K., Jeong H.J. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J Pharm Biomed Anal. 2010;51:278–283. doi: 10.1016/j.jpba.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Lee P.S., Song T.W., Sung J.H., Moon D.C., Song S., Chung Y.B. Pharmacokinetic characteristics and hepatic distribution of IH-901, a novel intestinal metabolite of ginseng saponin, in rats. Planta Med. 2006;72:204–210. doi: 10.1055/s-2005-916201. [DOI] [PubMed] [Google Scholar]
- 32.Qian T., Cai Z., Wong R.N., Mak N.K., Jiang Z.H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;816:223–232. doi: 10.1016/j.jchromb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Xie H.T., Wang G.J., Sun J.G., Tucker I., Zhao X.C., Xie Y.Y. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:167–173. doi: 10.1016/j.jchromb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Qi L.W., Wang C.Z., Yuan C.S. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Qian T., Cai Z., Wong R.N., Jiang Z.H. Liquid chromatography/mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun Mass Spectrom. 2005;19:3549–3554. doi: 10.1002/rcm.2232. [DOI] [PubMed] [Google Scholar]
- 36.Li L., Chen X., Li D., Zhong D. Identification of 20(S)-protopanaxadiol metabolites in human liver microsomes and human hepatocytes. Drug Metab Dispos. 2011;39:472–483. doi: 10.1124/dmd.110.036723. [DOI] [PubMed] [Google Scholar]
- 37.Hao H., Lai L., Zheng C., Wang Q., Yu G., Zhou X. Microsomal cytochrome p450-mediated metabolism of protopanaxatriol ginsenosides: metabolite profile, reaction phenotyping, and structure-metabolism relationship. Drug Metab Dispos. 2010;38:1731–1739. doi: 10.1124/dmd.110.033845. [DOI] [PubMed] [Google Scholar]
- 38.Mason T.G., Wilking J.N., Meleson K., Chang C.B., Graves S.M. Nanoemulsions: formation, structure, and physical properties. J Phys-Condens Mat. 2006;18:R635–R666. [Google Scholar]
- 39.Singh R., Lillard J.W., Jr. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbarzadeh A., Rezaei-Sadabady R., Davaran S., Joo S.W., Zarghami N., Hanifehpour Y. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touitou E., Dayan N., Bergelson L., Godin B., Eliaz M. Ethosomes – novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65:403–418. doi: 10.1016/s0168-3659(99)00222-9. [DOI] [PubMed] [Google Scholar]
- 42.Garg V., Singh H., Bimbrawh S., Singh S.K., Gulati M., Vaidya Y. Ethosomes and transfersomes: principles, perspectives and practices. Curr Drug Deliv. 2017;14:613–633. doi: 10.2174/1567201813666160520114436. [DOI] [PubMed] [Google Scholar]
- 43.Lin D.D.L., Chuanling S., Luying W., Jing L., Jing H., Jiandu L. Ginsenoside nanoparticle: a new green drug delivery system. J Nater Chem B. 2016;4:529–538. doi: 10.1039/c5tb02305j. [DOI] [PubMed] [Google Scholar]
- 44.Han M., Fu S., Gao J.Q., Fang X.L. Evaluation of intestinal absorption of ginsenoside Rg1 incorporated in microemulison using parallel artificial membrane permeability assay. Biol Pharm Bull. 2009;32:1069–1074. doi: 10.1248/bpb.32.1069. [DOI] [PubMed] [Google Scholar]
- 45.Hao F., He Y., Sun Y., Zheng B., Liu Y., Wang X. Improvement of oral availability of ginseng fruit saponins by a proliposome delivery system containing sodium deoxycholate. Saudi J Biol Sci. 2016;23:S113–S125. doi: 10.1016/j.sjbs.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li T., Shu Y.J., Cheng J.Y., Liang R.C., Dian S.N., Lv X.X. Pharmacokinetics and efficiency of brain targeting of ginsenosides Rg1 and Rb1 given as Nao-Qing microemulsion. Drug Dev Ind Pharm. 2015;41:224–231. doi: 10.3109/03639045.2013.858734. [DOI] [PubMed] [Google Scholar]
- 47.Wei H.J., Yang H.H., Chen C.H., Lin W.W., Chen S.C., Lai P.H. Gelatin microspheres encapsulated with a nonpeptide angiogenic agent, ginsenoside Rg1, for intramyocardial injection in a rat model with infarcted myocardium. J Control Release. 2007;120:27–34. doi: 10.1016/j.jconrel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Xu L.Q., Yu H., Yin S.P., Zhang R.X., Zhou Y.D., Li J. Liposome-based delivery systems for ginsenoside Rh2: in vitro and in vivo comparisons. J Nanopart Res. 2015;17 [Google Scholar]
- 49.Yu H., Teng L., Meng Q., Li Y., Sun X., Lu J. Development of liposomal Ginsenoside Rg3: formulation optimization and evaluation of its anticancer effects. Int J Pharm. 2013;450:250–258. doi: 10.1016/j.ijpharm.2013.04.065. [DOI] [PubMed] [Google Scholar]
- 50.Mathiyalagan R., Subramaniyam S., Kim Y.J., Kim Y.C., Yang D.C. Ginsenoside compound K-bearing glycol chitosan conjugates: synthesis, physicochemical characterization, and in vitro biological studies. Carbohydr Polym. 2014;112:359–366. doi: 10.1016/j.carbpol.2014.05.098. [DOI] [PubMed] [Google Scholar]
- 51.Chen D., Yu H., Mu H., Li G., Shen Y. Novel multicore niosomes based on double pH-sensitive mixed micelles for Ginsenoside Rh2 delivery. Artif Cells Nanomed Biotechnol. 2014;42:205–209. doi: 10.3109/21691401.2013.794358. [DOI] [PubMed] [Google Scholar]
- 52.Zheng Y., Feng Z., You C., Jin Y., Hu X., Wang X. In vitro evaluation of Panax notoginseng Rg1 released from collagen/chitosan-gelatin microsphere scaffolds for angiogenesis. Biomed Eng Online. 2013;12:134. doi: 10.1186/1475-925X-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voruganti S., Qin J.J., Sarkar S., Nag S., Walbi I.A., Wang S. Oral nano-delivery of anticancer ginsenoside 25-OCH3-PPD, a natural inhibitor of the MDM2 oncogene: nanoparticle preparation, characterization, in vitro and in vivo anti-prostate cancer activity, and mechanisms of action. Oncotarget. 2015;6:21379–21394. doi: 10.18632/oncotarget.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J., Han X., Li X., Luo Y., Zhao H., Yang M. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomedicine. 2012;7:4299–4310. doi: 10.2147/IJN.S32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi J.H., Cho S.H., Yun J.J., Yu Y.B., Cho C.W. Ethosomes and transfersomes for topical delivery of ginsenoside Rh1 from red ginseng: characterization and in vitro evaluation. J Nanosci Nanotechno. 2015;15:5660–5662. doi: 10.1166/jnn.2015.10462. [DOI] [PubMed] [Google Scholar]
- 56.Haijiang Z., Yongjiang W., Yiyu C. Analysis of 'SHENMAI' injection by HPLC/MS/MS. J Pharm Biomed Anal. 2003;31:175–183. doi: 10.1016/s0731-7085(02)00565-4. [DOI] [PubMed] [Google Scholar]
- 57.Chang T.K., Chen J., Benetton S.A. In vitro effect of standardized ginseng extracts and individual ginsenosides on the catalytic activity of human CYP1A1, CYP1A2, and CYP1B1. Drug Metab Dispos. 2002;30:378–384. doi: 10.1124/dmd.30.4.378. [DOI] [PubMed] [Google Scholar]
- 58.Chen C.M., Alli D. Use of fluidized-bed in proliposome manufacturing. J Pharm Sci-us. 1987;76:419. doi: 10.1002/jps.2600760517. [DOI] [PubMed] [Google Scholar]
- 59.Leung K.W., Wong A.S. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. doi: 10.1186/1749-8546-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otsuka H., Nagasaki Y., Kataoka K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliver Rev. 2003;55:403–419. doi: 10.1016/s0169-409x(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 61.Li F., Feng J., Cheng Q., Zhu W., Jin Y. Delivery of 125I-cobrotoxin after intranasal administration to the brain: a microdialysis study in freely moving rats. Int J Pharm. 2007;328:161–167. doi: 10.1016/j.ijpharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Abu Lila A.S., Ishida T., Kiwada H. Targeting anticancer drugs to tumor vasculature using cationic liposomes. Pharm Res-dordr. 2010;27:1171–1183. doi: 10.1007/s11095-010-0110-1. [DOI] [PubMed] [Google Scholar]
- 63.Natarajan V., Krithica N., Madhan B., Sehgal P.K. Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J Pharm Sci. 2011;100:195–205. doi: 10.1002/jps.22266. [DOI] [PubMed] [Google Scholar]
- 64.Daeihamed M., Dadashzadeh S., Haeri A., Akhlaghi M.F. Potential of liposomes for enhancement of oral drug absorption. Curr Drug Deliv. 2017;14:289–303. doi: 10.2174/1567201813666160115125756. [DOI] [PubMed] [Google Scholar]
- 65.Yang L., Zhang Z., Hou J., Jin X., Ke Z., Liu D. Targeted delivery of ginsenoside compound K using TPGS/PEG-PCL mixed micelles for effective treatment of lung cancer. Int J Nanomedicine. 2017;12:7653–7667. doi: 10.2147/IJN.S144305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vazquez I., Aguera-Ortiz L.F. Herbal products and serious side effects: a case of ginseng-induced manic episode. Acta Psychiatr Scand. 2002;105:76–77. [PubMed] [Google Scholar]
- 67.Li W., Zhang X., Xin Y., Xuan Y., Liu J., Li P. Oral subchronic toxicity evaluation of a novel antitumor agent 25-methoxydammarane-3, 12, 20-triol from Panax notoginseng in Sprague-Dawley rats. Regul Toxicol Pharmacol. 2016;77:240–251. doi: 10.1016/j.yrtph.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 68.Jadhav K.R., Shaikh I.M., Ambade K.W., Kadam V.J. Applications of microemulsion based drug delivery system. Curr Drug Deliv. 2006;3:267–273. doi: 10.2174/156720106777731118. [DOI] [PubMed] [Google Scholar]
- 69.Jaiswal M., Dudhe R., Sharma P.K. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shruti Rao T.B., Rajesh K.S., Jha Lalit Lata . Formulation, optimization and evaluation of microemulsion based gel of Butenafine Hydrochloride for topical delivery by using simplex lattice mixture design. J Pharm Investig. 2016;46:1–12. [Google Scholar]
- 71.McClements D.J. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. [Google Scholar]
- 72.Velikov K.P., Pelan E. Colloidal delivery systems for micronutrients and nutraceuticals. Soft Matter. 2008;4:1964–1980. [Google Scholar]
- 73.Koziara J.M., Lockman P.R., Allen D.D., Mumper R.J. The blood-brain barrier and brain drug delivery. J Nanosci Nanotechnol. 2006;6:2712–2735. doi: 10.1166/jnn.2006.441. [DOI] [PubMed] [Google Scholar]
- 74.Anton N., Vandamme T.F. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res. 2011;28:978–985. doi: 10.1007/s11095-010-0309-1. [DOI] [PubMed] [Google Scholar]
- 75.Komaiko J.S., McClements D.J. formation of food-grade nanoemulsions using low-energy preparation methods: a review of available methods. Compr Rev Food Sci F. 2016;15:331–352. doi: 10.1111/1541-4337.12189. [DOI] [PubMed] [Google Scholar]
- 76.Surjyanarayan Mandal S.D.M., Chuttani Krishna, Sawant Krutika K., Subudhi Bharat Bhushan. Neuroprotective effect of ibuprofen by intranasal application of mucoadhesive nanoemulsion in MPTP induced Parkinson model. J Pharm Investig. 2016;46:41–53. [Google Scholar]
- 77.Liu Z., Zhang Q., Ding L., Li C., Yin Z., Yan G. Preparation procedure and pharmacokinetic study of water-in-oil nanoemulsion of panax notoginseng saponins for improving the oral bioavailability. Curr Drug Deliv. 2016;13:600–610. doi: 10.2174/1567201812666150608095517. [DOI] [PubMed] [Google Scholar]
- 78.Imperiale J.C., Sosnik A. Nanoparticle-in-Microparticle delivery systems (NiMDS): production, administration routes and clinical potential. J Biomater Tiss Eng. 2013;3:22–38. [Google Scholar]
- 79.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J., Hu M., Xu H., Yu X., Ye F., Wang K. Influence of type and proportion of lyoprotectants on lyophilized ginsenoside Rg3 liposomes. J Pharm Pharmacol. 2016;68:1–13. doi: 10.1111/jphp.12489. [DOI] [PubMed] [Google Scholar]
- 81.van den Hoven J.M., Nemes R., Metselaar J.M., Nuijen B., Beijnen J.H., Storm G. Complement activation by PEGylated liposomes containing prednisolone. Eur J Pharm Sci. 2013;49:265–271. doi: 10.1016/j.ejps.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 82.Zalba S., Navarro I., Troconiz I.F., Tros de Ilarduya C., Garrido M.J. Application of different methods to formulate PEG-liposomes of oxaliplatin: evaluation in vitro and in vivo. Eur J Pharm Biopharm. 2012;81:273–280. doi: 10.1016/j.ejpb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 83.Dobrzynska I., Kotynska J., Figaszewski Z. Changes in electrical charge of phosphatidylcholine and phosphatidylserine liposomal membranes caused by adsorption of monovalent ions. Chem Anal-Warsaw. 2007;52:931–944. [Google Scholar]
- 84.Abu-Lila A., Suzuki T., Doi Y., Ishida T., Kiwada H. Oxaliplatin targeting to angiogenic vessels by PEGylated cationic liposomes suppresses the angiogenesis in a dorsal air sac mouse model. J Control Release. 2009;134:18–25. doi: 10.1016/j.jconrel.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Bao Y., Guo Y., Zhuang X., Li D., Cheng B., Tan S. D-alpha-tocopherol polyethylene glycol succinate-based redox-sensitive paclitaxel prodrug for overcoming multidrug resistance in cancer cells. Mol Pharm. 2014;11:3196–3209. doi: 10.1021/mp500384d. [DOI] [PubMed] [Google Scholar]
- 86.Zhao L.Y., Feng S.S. Enhanced oral bioavailability of paclitaxel formulated in vitamin e-TPGS emulsified nanoparticles of biodegradable polymers: in vitro and in vivo studies. J Pharm Sci-us. 2010;99:3552–3560. doi: 10.1002/jps.22113. [DOI] [PubMed] [Google Scholar]
- 87.El Zaafarany G.M., Awad G.A., Holayel S.M., Mortada N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int J Pharm. 2010;397:164–172. doi: 10.1016/j.ijpharm.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 88.Kazi K.M., Mandal A.S., Biswas N., Guha A., Chatterjee S., Behera M. Niosome: a future of targeted drug delivery systems. J Adv Pharm Technol Res. 2010;1:374–380. doi: 10.4103/0110-5558.76435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rizwana Khan R.I. Niosomes: a potential tool for novel drug delivery. J Pharm Investig. 2016;46:195–204. [Google Scholar]
- 90.Chen D., Sun K., Mu H., Tang M., Liang R., Wang A. pH and temperature dual-sensitive liposome gel based on novel cleavable mPEG-Hz-CHEMS polymeric vaginal delivery system. Int J Nanomedicine. 2012;7:2621–2630. doi: 10.2147/IJN.S31757. [DOI] [PMC free article] [PubMed] [Google Scholar]