Abstract
Background
The antioxidant effects of Panax ginseng have been reported in several articles; however, little is known about the antimelanogenesis effect, skin-protective effect, and cellular mechanism of Panax ginseng, especially of P. ginseng calyx. To understand how an ethanol extract of P. ginseng berry calyx (Pg-C-EE) exerts skin-protective effects, we studied its activities in activated melanocytes and reactive oxygen species (ROS)–induced keratinocytes.
Methods
To confirm the antimelanogenesis effect of Pg-C-EE, we analyzed melanin synthesis and secretion and messenger RNA and protein expression levels of related genes. Ultraviolet B (UVB) and hydrogen peroxide (H2O2) were used to induce cell damage by ROS generation. To examine whether this damage is inhibited by Pg-C-EE, we performed cell viability assays and gene expression and transcriptional activation analyses.
Results
Pg-C-EE inhibited melanin synthesis and secretion by blocking activator protein 1 regulatory enzymes such as p38, extracellular signal-regulated kinases (ERKs), and cyclic adenosine monophosphate response element–binding protein. Pg-C-EE also suppressed ROS generation induced by H2O2 and UVB. Treatment with Pg-C-EE decreased the expression of matrix metalloproteinases, mitogen-activated protein kinases, and hyaluronidases and increased the cell survival rate.
Conclusion
These results suggest that Pg-C-EE may have antimelanogenesis properties and skin-protective properties through regulation of activator protein 1 and cyclic adenosine monophosphate response element–binding protein signaling. Pg-C-EE may be used as a skin-improving agent, with moisture retention and whitening effects.
Keywords: Antimelanogenesis, Calyx of berry, Matrix metalloproteinases, Panax ginseng, Skin protective
1. Introduction
Our body surfaces are defended by epithelia, which impose a physical barrier between the internal milieu and the external world that contains pathogens. Epithelial cells are held by tight junctions, which effectively form a seal against the external environment including infection with pathogens and excessive water loss [1]. In humans, the skin is the largest organ of the integumentary system. The skin has three layers of ectodermal tissue: epidermis, dermis, and subcutaneous tissue (hypodermis). The epidermis layer is the outer layer of the skin and contains merkel cells, keratinocytes, melanocytes, and langerhans cells. This layer plays an important role in maintaining the body temperature. The dermis layer, located beneath the epidermis layer, contains many nerve endings, hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels, and blood vessels. The subcutaneous tissue lies beneath the dermis layer and consists of loose connective tissue, adipose tissue, elastin, fibroblasts, macrophages, and adipocytes [2]. Melanin in the basal layer of the epidermis plays an important role in the determination of skin color and melanogenesis. Melanin is produced within the skin in cells called melanocytes. Although melanogenesis is not fully understood, substances that stimulate melanin production are known, such as melanocyte-stimulating hormone, forskolin, cholera toxin, isobutylmethylxanthine, diacylglycerol analogs, ultraviolet (UV) irradiation, and vitamin D metabolites [2], which trigger melanogenesis and severe diseases such as Cushing's disease and melanocytic tumor. Tyrosinase, a melanogenesis-related enzyme, is an oxidase that controls melanin production. This enzyme is involved in the hydroxylation of a monophenol and the conversion of an o-diphenol to the corresponding o-quinone, which in turn is converted to melanin through several reactions. Tyrosinase is found inside melanosomes together with tyrosinase-related protein-1 (TRP-1) and tyrosinase-related protein-2 (TRP-2). Genes associated with melanogenesis include microphthalmia-associated transcription factor (MITF), melanophilin (MLPH), ras-related protein Rab27a, and myosin-5A (Myo5A). Tyrosinase, TRP-1, and TRP-2 are involved in melanin synthesis, whereas MLPH, Rab27a, and Myo5A play a role in melanin secretion [3], [4], [5].
UV irradiation induces photoaging and reactive oxygen species (ROS) generation in addition to melanogenesis. UV irradiation damages the human skin and induces the synthesis of matrix metalloproteinases (MMPs) in fibroblasts and keratinocytes. MMPs mainly play a role in augmentation of dermal collagen degradation during UV exposure. Increased expression of MMPs causes severe problems including dermal photoaging and carcinogenesis [6], [7]. Types of MMPs expressed in skin cells are MMP-1 (interstitial collagenase), MMP-2 and -9 (gelatinase-A and -B), and MMP-3 (stromelysin 1) [8]. In addition, exogenous ROS can be produced by exposure to hydrogen peroxide (H2O2) and UV irradiation. ROS stimulate the transcription of genes encoding proinflammatory cytokines such as interleukin (IL)-1β, IL-6, IL-8, and cyclooxygenase-2 (COX-2) as well as MMPs via mitogen-activated protein kinases (MAPKs) [9], [10]. In particular, H2O2 regulates heme oxygenase 1 and nuclear factor 2 via oxidative stress and damage [11]. As mentioned previously, moisture retention plays important roles in the skin. Natural moisture factors (NMF) such as hyaluronic acid (HA) are involved in skin moisture retention and are an essential component of the extracellular matrix (ECM). Genes related to NMF production are filaggrin (FLG, epidermal barrier protein), transglutaminase-1, and hyaluronic acid synthase (HAS)-1, -2, and -3 [12]. In contrast to HAS genes, hyaluronidase (HYAL)-1, -2, -3, and -4 catalyze the degradation of HA. Hyaluronidase is known to be overexpressed during UV photoaging [13].
Panax ginseng has been used as an herbal medicine in Korea, China, and Japan for a very long time. The value of ginseng as a medicinal herb is attributed to an effective group of compounds called ginsenosides. Ginsenosides consist of protopanaxadiol and protopanaxatriol, which have known pharmacological features and physiological activities including antioxidant, immunomodulation, antidiabetic, and anticancer effects [14], [15]. P. ginseng calyx is the peduncle between the berry and root of ginseng. Ginseng calyx is not usually used in ginseng industries; therefore, it is mostly removed in large quantities during the process of harvest of ginseng roots. Nonetheless, because various kinds of ginsenosides were found to be highly existing in the ginseng calyx part, its industrial application has been proposed. Indeed, previous studies have shown that P. ginseng calyx contains the highest amount of G-Re (6%) among protopanaxatriol compounds [16] with skin barrier functions [17]. The antioxidant effects of P. ginseng have been reported in several articles [18], [19]. However, little is known about its antimelanogenesis and skin-protective effects or the cellular mechanism, especially for P. ginseng calyx.
2. Materials and methods
2.1. Materials
B16F10, HaCaT, and HEK293 cells were purchased from the American Type Culture Collection (Rockville, MD, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, phosphate-buffered saline (PBS), and penicillin-streptomycin were purchased from HyClone Laboratories Inc., (Logan, UT, USA). 3-(4-5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amresco (Brisbane, Australia). α-Melanocyte-stimulating hormone (a-MSH), arbutin, L-dopa-(phenyl-d3) (L-DOPA), mushroom tyrosinase (tyrosinase), kojic acid, retinol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), L-ascorbic acid, phorbol 12-myristate 13-acetate (PMA), forskolin from Coleus forskohlii (forskolin), and polyethylenimine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Hydrogen peroxide (H2O2; 35%) was purchased from JUNSEI (Chuo-ku, Tokyo, Japan). SB203580 (SB), SP600125 (SP), and U0126 were purchased from Calbiochem (La Jolla, CA, USA). TRIzol reagent was purchased from MRCgene (OH, USA), and the cDNA synthesis kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The primer sets (forward and reverse) for polymerase chain reaction (PCR) were synthesized by Macrogen (Seoul, Korea), and PCR premix was purchased from Bio-D Inc. (Seoul, Korea). The luciferase assay system was purchased from Promega (Madison, WI, USA). Polyvinylidenedifluoride membrane was from Merck Millipore (Billerica, MA, USA). Antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA) and Abcam (Cambridge, MA, USA). Pg-C-EE was prepared as described in a previous report [16].
2.2. Cell culture
B16F10 and HaCaT cells were cultured in DMEM with 10% fetal bovine serum and 1% penicillin-streptomycin at 37ºC in a 5% CO2 humidified incubator.
2.3. Cell viability assay
B16F10 cells were seeded at 1 × 104 cells per well in 96-well plates for 24 h and then treated with Pg-C-EE for 48 h. HaCaT cells were seeded at 3.5 × 104 cells per well in 96-well plates for 24 h and then treated with Pg-C-EE for 24 h. Cell viability was measured using the conventional MTT assay. Cells were incubated with 10 μL/well of MTT solution for 3–4 h and then 100 μL MTT stop solution (10% sodium codicil sulfate containing 1M HCl) was added. After 8 h, solubilized formazan was measured by measuring the absorbance at 570 nm using an optical density reader (BioTek, VT, USA).
2.4. Melanin content and secretion analysis
B16F10 cells were seeded at 1 × 105 cells per well in 12-well plates for 24 h and then treated with 100 nM α-MSH, Pg-C-EE, and 1 mM arbutin for 48 h. For the melanin secretion assay, absorbance of culture media was measured using an optical density reader at 475 nm. The cells used for the melanin secretion assay were lysed with 100 mL cell lysis buffer and pelleted by centrifugation (12,000 rpm, 5 min). The pellets were dissolved in 100 mL dissolving buffer (1 M NaOH, 10% dimethyl sulfoxide (DMSO)) and melted at 55°C for 30 min. Absorbance was measured at 405 nm using an optical density reader.
2.5. Tyrosinase assay
For the tyrosinase assay, 50 mL of 2 mM L-dopamine, 50 mL of Pg-C-EE at different doses (0 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL), or 300 mM kojic acid was mixed with mushroom tyrosinase (100 U/mL) for 15 min at room temperature. Absorbance of each sample was measured at 475 nm using an optical density reader.
2.6. messenger RNA analysis by reverse transcription polymerase chain reaction
For messenger RNA (mRNA) analysis of melanogenesis-related genes, B16F10 cell pellets were used under the same conditions as for the melanin secretion experiment. All mRNA analyses using HaCaT cells were conducted in 6-well plates. HaCaT cells were exposed to UVB irradiation and hydrogen peroxide and treated with Pg-C-EE (0 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL) or retinol (10 μg/mL). Total mRNA was precipitated using TRIzol reagent according to the manufacturer's instructions. Complementary DNA was synthesized using a cDNA synthesis kit. Reverse transcription polymerase chain reaction was conducted using specific forward and reverse primers. All sequences of primer sets are listed in Table 1 (mouse) and Table 2 (human).
Table 1.
List of primers used in this study (mouse species)
| Name | Sequences (5′ to 3′) | |
|---|---|---|
| MITF | F | 5′- AACTCATGCGTGAGCAGATG -3′ |
| R | 5′- TACCTGGTGCCTCTGAGCTT -3′ | |
| TYRP-1 | F | 5′- ATGGAACGGGAGGACAAACC -3′ |
| R | 5′- TCCTGACCTGGCCATTGAAC -3′ | |
| TYRP-2 | F | 5′- CAGTTTCCCCGAGTCTGCAT -3′ |
| R | 5′- GTCTAAGGCGCCCAAGAACT -3′ | |
| Tyrosinase | F | 5′- GTCCACTCACAGGGATAGCAG -3′ |
| R | 5′- AGAGTCTCTGTTATGGCCGA -3′ | |
| MLPH | F | 5′- ATCTTCGCGAAACCCGTGAT -3′ |
| R | 5′- AGAATCACAGCGAGATGGCTT -3′ | |
| Myo5a | F | 5′- AAGCCATCCAACCCAAGGAT -3′ |
| R | 5′- GCTGTTGCCGGTTGTTTTCT -3′ | |
| Rab27a | F | 5′- TCTGGGGTAGGGAAGACCAG -3′ |
| R | 5′- AGCAGGAAACCCATAGCGTC -3′ | |
| GAPDH | F | 5′- CAATGAATACGGCTACAGCA -3′ |
| R | 5′- AGGGAGATGCTCAGTGTTGG -3′ | |
F, forward; MITF, microphthalmia-associated transcription factor; MLPH, melanophilin; Myo5A, myosin-5A; R, reverse; TYRP, tyrosinase-related protein
Table 2.
List of primers used in this study (human species)
| Name | Sequences (5′ to 3′) | |
|---|---|---|
| MMP-1 | F | 5′- CACAGCTTCCCAGCGACTC -3′ |
| R | 5′- GTCCCGATGATCTCCCCTGA -3′ | |
| MMP-2 | F | 5′- CCATGAAGCCTTGTTTACCA -3′ |
| R | 5′- TCCATACTTCTTATCCCGGT -3′ | |
| MMP-3 | F | 5′- ATCCTACTGTTGCTGTGCGT -3′ |
| R | 5′- CATCACCTCCAGAGTGTCGG -3′ | |
| MMP-9 | F | 5′- TCTTCCCCAAAGACCTGAAA -3′ |
| R | 5′- TGATGTTATGATGGTCCCAC -3′ | |
| COX-2 | F | 5′- CAAAAGCTGGGAAGCCTTCT -3′ |
| R | 5′- CCATCCTTCAAAAGGCGCAG -3′ | |
| Sirt-1 | F | 5′- CAGTGTCATGGTTCCTTTGC -3′ |
| R | 5′- CACCGAGGAACTACCTGAT -3′ | |
| IL-6 | F | 5′- TACCCCCAGGAGAAGATTCC -3′ |
| R | 5′- TTTTCTGCCAGTGCCTCTTT -3′ | |
| IL-8 | F | 5′- AAGGTGCAGTTTTGCCAAGG -3′ |
| R | 5′- CAACCCTCTGCACCCAGTTT -3′ | |
| HO-1 | F | 5′- GCCATGAACTTTGTCCGGTG -3′ |
| R | 5′- TTTCGTTGGGGAAGATGCCA -3′ | |
| NRF-2 | F | 5′- CAGTCAGCGACGGAAAGAGT -3′ |
| R | 5′- AAGTGACTGAAACGTAGCCGA -3′ | |
| FLG | F | 5′- AAGGAACTTCTGGAAAAGGAATTTC -3′ |
| R | 5′- TTGTGGTCTATATCCAAGTGATCCAT -3′ | |
| TGM-1 | F | 5′- CCCCCGCAATGAGATCTACA -3′ |
| R | 5′- ATCCTCATGGTCCACGTACACA -3′ | |
| HAS-1 | F | 5′- CCACCCAGTACAGCGTCAAC -3′ |
| R | 5′- CATGGTGCTTCTGTCGCTCT -3′ | |
| HAS-2 | F | 5′- TCTTTATGTGACTCATCTGTCTCACCG -3′ |
| R | 5′- ATTGTTGGCTACCAGTTTATCCAAACG -3′ | |
| HAS-3 | F | 5′- TATACCGCGCGCTCCAA -3′ |
| R | 5′- GCCACTCCCGGAAGTAAGACT -3′ | |
| HYAL-1 | F | 5′- CAGAATGCCAGCCTGATTGC -3′ |
| R | 5′- CCGGTGTAGTTGGGGCTTAG -3′ | |
| HYAL-2 | F | 5′- TACACCACAAGCACGGAGAC -3′ |
| R | 5′- ATGCAGGAAGGTACTGGCAC -3′ | |
| HYAL-3 | F | 5′- CCAGGATGACCTTGTGCAGT -3′ |
| R | 5′- CCATCTGTCCTGGATCTCGC -3′ | |
| HYAL-4 | F | 5′- TGAGCTCTCTTGGCTCTGGA -3′ |
| R | 5′- AGGCAGCACTTTCTCCTATGG -3′ | |
| Col1A1 | F | 5′- CAGGTACCATGACCGAGACG -3′ |
| R | 5′- AGCACCATCATTTCCACGAG -3′ | |
| Col2A1 | F | 5′- GCAACGTGGTGAGAGAGGAT -3′ |
| R | 5′- CCTGTCGTCCGGGTTCAC -3′ | |
| GAPDH | F | 5′- GCACCGTCAAGGCTGAGAAC-3′ |
| R | 5′- ATGGTGGTGAAGACGCCAGT-3′ |
Col1A1, collagen, type I, alpha 1; Col2A1, collagen, type II, alpha 1; COX-2, cyclooxygenase 2; FLG, filaggrin; F, forward; HAS, hyaluronic acid synthase; HO, heme oxygenase; HYAL, hyaluronidase; IL, interleukin; MMP, matrix metalloproteinase; NRF2, nuclear factor 2; R, reverse; Sirt1, NAD-dependent protein deacetylase sirtuin-1; TGM, transglutaminase
2.7. Preparation of cell lysates and immunoblot analysis
B16F10 and HaCaT cells were treated at the indicated time points with Pg-C-EE. The treated cells were washed, harvested in PBS, centrifuged, and lysed in cell lysis buffer (1 M Tris-HCl pH 7.5, 0.5 M NaF, 1 M β-glycerolphosphate pH 7.5, 4 M NaCl, 100% NP-40, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 2 μg/mL pepstatin A, 0.1 mM Na3VO4, 1 mM benzamide, 0.1 mM phenylmethanesulfonyl fluoride (PMSF), and 1.6 mM pervanadate) for 1 h at 4°C. Total protein lysates were pelleted by centrifugation (12,000 rpm, 10 min, 4°C), and the protein content of the supernatants was measured using the Bradford assay [20] and adjusted for concentration differences among the samples. Proteins were analyzed by immunoblotting. Proteins were separated on 10% sodium dodecyl sulfate–polyacrylamide gels and transferred to polyvinylidenedifluoride membranes. Levels of phospho or total p38, extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK), and cyclic adenosine monophosphate response element–binding protein (CREB) and levels of tyrosinase, TRP1, and 2, and MITF were determined using previously published methods [21]. β-Actin was used as an immunoblotting loading control.
2.8. DPPH analysis
A DPPH decoloration assay was conducted as in a previous report [22]. To measure radical-scavenging activity, 200 μL DPPH solution (0.2 mM in methanol) and 200 μL of different concentrations of Pg-C-EE (0 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL) or 200 μL of 0.5 mM ascorbic acid were mixed and shaken in the dark at room temperature for 30 min. Absorbance was measured at 517 nm. Results were expressed as percentage inhibition: DPPH scavenging effect = [(Acontrol − Asample)/Acontrol] × 100%; Acontrol is the absorbance of DPPH, and Asample is absorbance of the sample (Pg-C-EE or ascorbic acid).
2.9. UVB irradiation
HaCaT cells were seeded at 7 × 105 cells per well in 6-well plates and subjected to 24 h of starvation using serum-free MEM. Before UVB irradiation, HaCaT cells were pretreated with Pg-C-EE for 30 min. After 30 min, HaCaT cells were washed with PBS and exposed to UVB irradiation (UVB lamp: Bio-link crosslinker BLX-312; Vilber Lourmat, Collegien, France). The energy of UVB irradiation was 30 mJ/cm2 or 100 mJ/cm2. After UVB irradiation, Dulbecco's Modified Eagle's medium (DMEM) containing Pg-C-EE was added to the cells and incubated for 24 h and 48 h.
2.10. H2O2 treatment
HaCaT cells were seeded at 7 × 105 cells per well in a 6-well plate and subjected to 24 h of starvation using serum-free MEM. HaCaT cells were pretreated with Pg-C-EE for 30 min and then incubated with 1 mM H2O2 for 24 h.
2.11. Morphological changes
To observe morphological changes, HaCaT cells were plated at 7 × 105 cells per well in 6-well plates and treated with Pg-C-EE, H2O2, or UVB. After the indicated time, cell images were captured using an inverted phase contrast microscope (Olympus Co., Tokyo, Japan) attached to a video camera with National Institutes of Health (NIH)imaging software.
2.12. Luciferase reporter gene assay
HEK293 cells were seeded at 1.2 × 104 cells per well in 24-well plates. After 24 h, cells were transfected with β-galactosidase and NF-κB-Luc, AP-1-Luc, CREB-Luc, or Col1A1-Luc for 24 h. Polyethylenimine was used as a transfection reagent. The cells were treated with Pg-C-EE for 24 h except in conditions of PMA or forskolin induction when the cells were pretreated with Pg-C-EE for 30 min and then treated with PMA or forskolin for 6 h. Luciferase assay was conducted using the Luciferase Assay System (Promega).
2.13. Statistical analysis
Results were analyzed using analysis of variance and Mann–Whitney tests. A p value <0.05 was considered statistically significant. All statistical tests were performed with the SPSS software package (version 22.0, 2013, IBM Corp., Armonk, NY, USA). All experiments were carried out in triplicates.
3. Results
3.1. Antimelanogenesis effect of Pg-C-EE
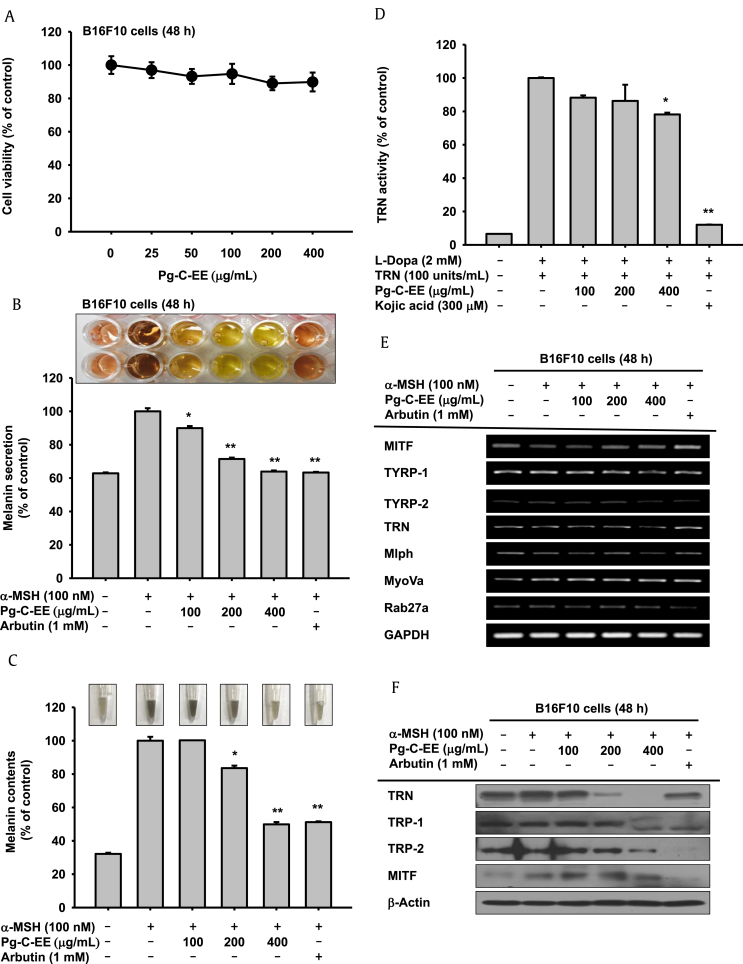
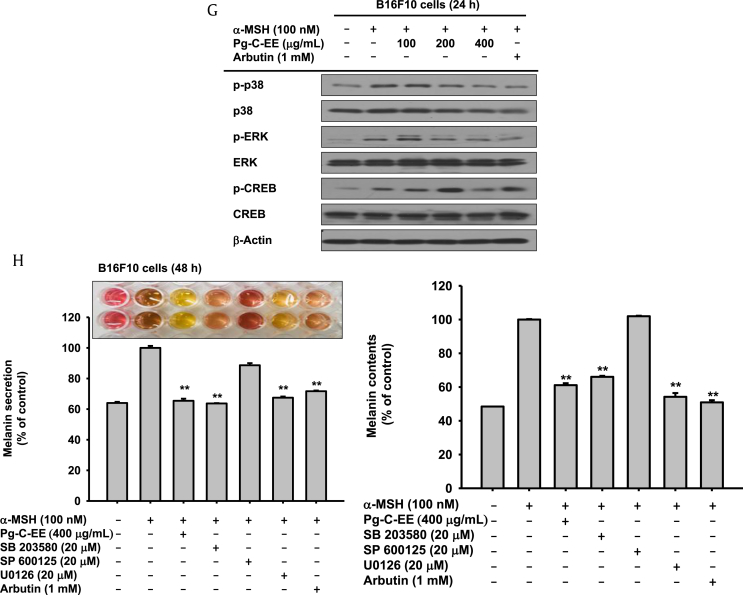
We first measured the cytotoxicity of Pg-C-EE in B16F10 cells and showed no cytotoxicity at Pg-C-EE concentrations up to 400 μg/mL (Fig. 1A). Arbutin (1 mM) was used as a positive control for antimelanogenesis activity as it is known to effectively inhibit tyrosinase activity. The inhibitory effects of Pg-C-EE on melanin secretion and synthesis were examined in α-MSH–treated B16F10 cells. The levels of inhibition of melanin secretion were 10% for Pg-C-EE 100 μg/mL, 28.5% for Pg-C-EE 200 μg/mL, 36.2% for Pg-C-EE 400 μg/mL, and 36.7% for arbutin 1 mM (Fig. 1B). The levels of inhibition of melanin content were 16.5% for Pg-C-EE 200 μg/mL, 50.2% for Pg-C-EE 400 μg/mL, and 49% for arbutin 1 mM (Fig. 1C). The photographs (upper graph) and graphs show that melanin secretion and synthesis were suppressed by Pg-C-EE treatment in a dose-dependent manner (Figs. 1B, 1C). To investigate the activity of enzymes involved in melanin production, we used mushroom tyrosinase. The inhibitory effect of Pg-C-EE on tyrosinase activity was 11.8% (100 μg/mL), 13.7% (200 μg/mL), and 21.9% (400 μg/mL), whereas kojic acid (300 μM) inhibited tyrosinase activity by up to 87.9% (Fig. 1D). In reverse transcription polymerase chain reaction analysis of the inhibitory effect of Pg-C-EE on the expression of genes related to melanin production (MITF, TYRP-1,2, tyrosinase, MLPH, MyoVa, and Rab27a), Pg-C-EE did not show a significant inhibitory effect on mRNA expression, but the mRNA expression of TYRP-2, tyrosinase, and MLPH was slightly decreased at Pg-C-EE 400 μg/mL (Fig. 1E). Although Pg-C-EE did not affect the mRNA expression of genes associated with melanin production, it showed a significant inhibitory effect at the level of protein expression. Especially, the protein expression of tyrosinase, TRP-1, and TRP-2 was dose-dependently decreased by Pg-C-EE treatment (Fig. 1F). Because Pg-C-EE inhibited the expression of melanin production–related proteins, we then examined whether Pg-C-EE blocked the upstream proteins regulating melanogenesis by measuring the phosphorylation of p38, ERK, and CREB at different time points (48–24 h). Pg-C-EE dose-dependently decreased levels of phosphorylated p38, ERK, and CREB that were stimulated under α-MSH conditions (Fig. 1G). To demonstrate that p38 and ERK inhibit melanin production, we examined the levels of melanin secretion and content in the presence of SB203580 (p38 inhibitor), SP600125 (JNK inhibitor), and U0126 (ERK inhibitor). Melanin production was suppressed with SB203580 and U0126 treatment similar to Pg-C-EE treatment but was not inhibited in the SP600125-treated group (Fig. 1H).
Fig. 1.
Antimelanogenesis effect of Pg-C-EE on B16F10 cells. (A) B16F10 cell viability was measured by MTT assay in the Pg-C-EE–treated group. (B and C) B16F10 cells were treated with α-MSH (100 nM) and various concentrations of Pg-C-EE (100, 200, and 400 μg/mL), or arbutin (1 mM) for 48 h. Melanin secretion was measured by absorbance of cell supernatant (O.D) at 475, nm and melanin content was measured by absorbance of cell pellets (O.D) at 405 nm. (D) The effects of Pg-C-EE (100-400 μg/mL) or kojic acid (300 μM) on tyrosinase enzyme activity were confirmed by treatment with L-DOPA and detection of tyrosinase at 475 nm. (E) mRNA expression of genes related to melanin synthesis (MITF, TYRP-1, TYRP-2, and tyrosinase) and melanin secretion (MLPH, MyoVa, and Rab27a) were determined in B16F10 cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL), arbutin (1 mM), and α-MSH (100 nM) using RT-PCR. (F) The levels of melanogenesis-related protein (tyrosinase, TRP-1, TRP-2, and MITF) were confirmed by immunoblot analysis of B16F10 cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL), arbutin (1 mM), and α-MSH (100 nM) for 48 h. (G) Upstream proteins associated with melanogenesis were identified by treatment of B16F10 cells with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL), arbutin (1 mM), and α-MSH (100 nM) for 24 h. (H) Levels of melanin secretion and content were determined from supernatants and pellets of B16F10 cells treated with Pg-C-EE (400 μg/mL), SB203580 (p38 inhibitor, 20 μM), SP600125 (JNK inhibitor, 20 μM), U0126 (ERK inhibitor, 20 μM), or arbutin (1 mM) with α-MSH (100 nM) for 48 h. *p < 0.05 and **p < 0.01 compared with control.
α-MSH, α-melanocyte–stimulating hormone; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element binding; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; L-DOPA, L-dopamine; MITF, microphthalmia-associated transcription factor; mRNA, messenger RNA; MLPH, melanophilin; MTT, 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MyoVa, myosin Va; Pg-C-EE, Panax ginseng calyx ethanol extract; RT-PCR, reverse transcription polymerase chain reaction; TRN, tyrosinase; TYRP, tyrosinase-related protein.
3.2. Skin-protective activity of Pg-C-EE against UVB irradiation
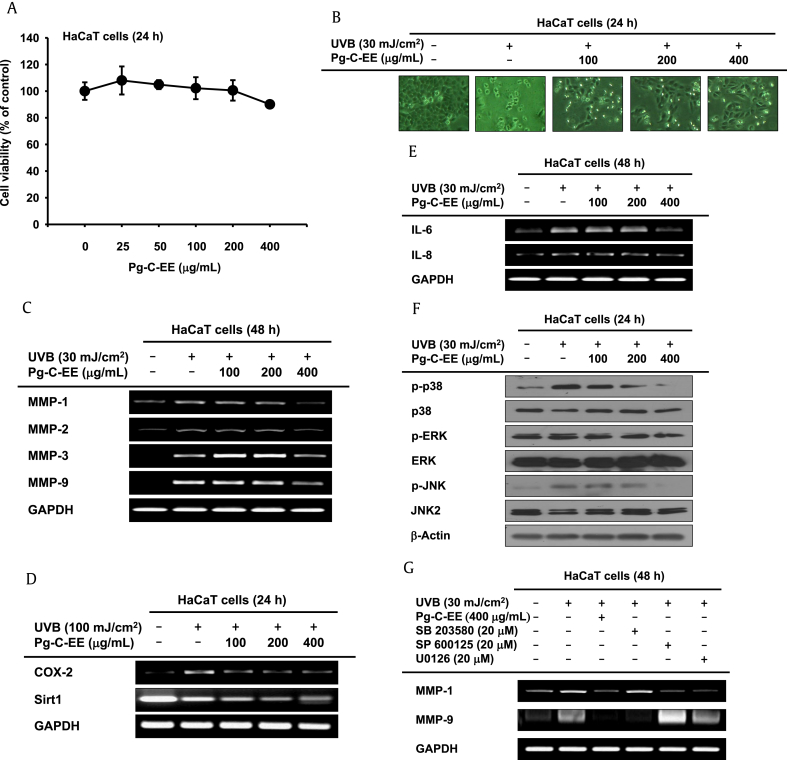
To investigate the skin-protective activity against UVB irradiation using HaCaT cells, the cytotoxicity of Pg-C-EE was measured by MTT assay. Pg-C-EE did not show cytotoxicity in HaCaT cells up to a concentration of 400 μg/mL (Fig. 2A). Images of cells captured with a camera attached to the microscope showed that Pg-C-EE protected against UVB-irradiated HaCaT cell damage (Fig. 2B). To elucidate the UVB-mediated skin damage–protective mechanisms of Pg-C-EE, the expression levels of MMP-1, -2, -3, and -9 were measured under conditions of UVB irradiation, which is known to increase skin aging through upregulation of MMPs. In UVB-irradiated HaCaT cells, Pg-C-EE at 400 μg/mL reduced the mRNA expression of MMP-1, MMP-2, MMP-3, and MMP-9 (Fig. 2C). Pg-C-EE also decreased the expression of inflammation regulatory genes COX-2, IL-6, and IL-8. After UVB irradiation with 100 mJ/cm2, COX-2 expression was decreased in the Pg-C-EE-treated group, whereas Pg-C-EE did not affect the Sirt1 gene (Fig. 2D). After UVB irradiation with 30 mJ/cm2, Pg-C-EE reduced the gene expression of IL-6 and IL-8, the most important inflammatory genes in skin cells (Fig. 2E). To investigate the regulatory mechanisms of proteins involved in UVB-mediated cell damage, such as MMPs, COX-2, and IL-6, we performed the Western blot analysis of MAPK proteins (p38, JNK, and ERK), which are upstream of the activator protein 1 (AP-1) signal. Expression of phospho-p38, phospho-JNK, and phospho-ERK decreased after Pg-C-EE treatment under UVB induction conditions (Fig. 2F). These results were confirmed by examining the expression of MMP-1 and MMP-9 in UVB irradiation conditions using MAPK inhibitors (SB203580, SP600125, and U0125). SP600125 and U0126 reduced the expression of MMP-1, and SB203580 decreased the expression of MMP-9, to the same extent as Pg-C-EE treatment (Fig. 2G).
Fig. 2.
Antiphotoaging effect of Pg-C-EE against UVB irradiation in HaCaT cells. (A) Viability of HaCaT cells was measured by MTT assay in Pg-C-EE–treated group. (B) HaCaT cells were treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and UVB irradiation (30 mJ/cm2) for 24 h. Cytoprotective effects of Pg-C-EE against UVB were observed. (C) mRNA expression of MMP-1, MMP-2, MMP-3, and MMP-9 in HaCaT cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and UVB (30 mJ/cm2) was determined by RT-PCR. (D) mRNA expression of COX-2 and Sirt1 in HaCaT cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and UVB (30 mJ/cm2) was determined by RT-PCR. (E) mRNA expression of IL-6 and IL-8 in HaCaT cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and UVB (30 mJ/cm2) was determined by RT-PCR. (F) The levels of phospho and total forms of MAPK (p38, ERK, and JNK) in whole cell lysates were determined by immunoblot analysis after treatment of HaCaT cells with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and UVB (30 mJ/cm2) for 48 h. (G) The effects of MAPK inhibitors SB203580 (20 μM), SP600125 (20 μM), and U0126 (20 μM) on expression of MMP-9 in UVB-irradiated HaCaT cells were identified by RT-PCR.
COX-2, cyclooxygenase-2; ERK, extracellular signal-regulated kinase; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; mRNA, messenger RNA; MTT, 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Pg-C-EE, Panax ginseng calyx ethanol extract; RT-PCR, reverse transcription polymerase chain reaction; Sirt1, NAD-dependent protein deacetylase sirtuin-1; UVB, ultraviolet B.
3.3. Skin-protective activity of Pg-C-EE against hydrogen peroxide
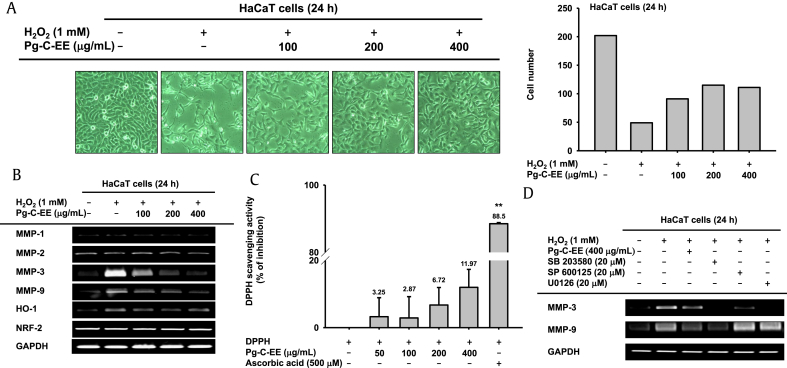
To examine whether Pg-C-EE protects against H2O2-induced damage, HaCaT cells were treated with H2O2 with and without Pg-C-EE and photographed (Fig. 3A) The number of HaCaT cells under each condition was 202 for the untreated group, 49 for 1 mM H2O2, 91 for 1 mM H2O2 plus 100 μg/mL Pg-C-EE, 115 for 1 mM H2O2 plus 200 μg/mL Pg-C-EE, and 111 for 1 mM H2O2 plus 100 μg/mL Pg-C-EE (Fig. 3A, right panel). These data confirmed that Pg-C-EE inhibited H2O2-induced cell damage. In general, H2O2 is involved in the production of ROS in cells. ROS promote skin aging through MMPs. Treatment with Pg-C-EE under H2O2-induced conditions decreased the expression levels of MMP-1, MMP-3, MMP-9, and heme oxygenase-1 in a dose-dependent manner. There was no change in the expression of nuclear factor 2 (Fig. 3B). To study the radical-scavenging activity of Pg-C-EE, we conducted a DPPH assay using ascorbic acid (500 μM) as a radical-scavenging positive control. The scavenging levels of Pg-C-EE were 3.25% (50 μg/mL), 2.87% (100 μg/mL), 6.72% (200 μg/mL), and 11.97% (400 μg/mL), whereas ascorbic acid showed a scavenging effect of 88.5% (Fig. 3C). To clarify previous results, the expression levels of MMP-3 and MMP-9 under H2O2 treatment conditions were measured in the presence of MAPK inhibitors (SB203580, SP600125, and U0125). SB203580, SP600125, and U0126 reduced the expression of MMP-3 to the same extent as Pg-C-EE treatment. However, the expression of MMP-9 was inhibited only by SB203580 among the MAPK inhibitors (Fig. 3D).
Fig. 3.
Antioxidant effect of Pg-C-EE against H2O2-induced damage in HaCaT cells. (A) Images of HaCaT cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and H2O2 (1 mM) for 24 h were obtained with a camera attached to the microscope. The number of cells in each image was counted (right panel). (B) Expression of MMP-1, MMP-2, MMP-3, MMP-9, HO-1, and NRF2 was confirmed by RT-PCR analysis of HaCaT cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and H2O2 (1 mM) for 24 h. (C) The antioxidative activity of Pg-C-EE (50 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL) and ascorbic acid (500 μM) was measured by DPPH assay. (D) The effects of MAPK inhibitors SB203580 (20 μM), SP600125 (20 μM), and U0126 (20 μM) on expression of MMP-3 in H2O2 (1 mM)-treated HaCaT cells were identified by RT-PCR. MAPK inhibitors were used to confirm the cytoprotective effect by MTT assay. DPPH, 2,2-diphenyl-1-picrylhydrazyl; H2O2, hydrogen peroxide; HO-1, heme oxygenase-1; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; MTT, 3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NRF-2, nuclear factor (erythroid-derived 2)-like 2; Pg-C-EE, Panax ginseng calyx ethanol extract; RT-PCR, reverse transcription polymerase chain reaction.
3.4. Effect of Pg-C-EE on skin moisture retention activity and collagen synthesis
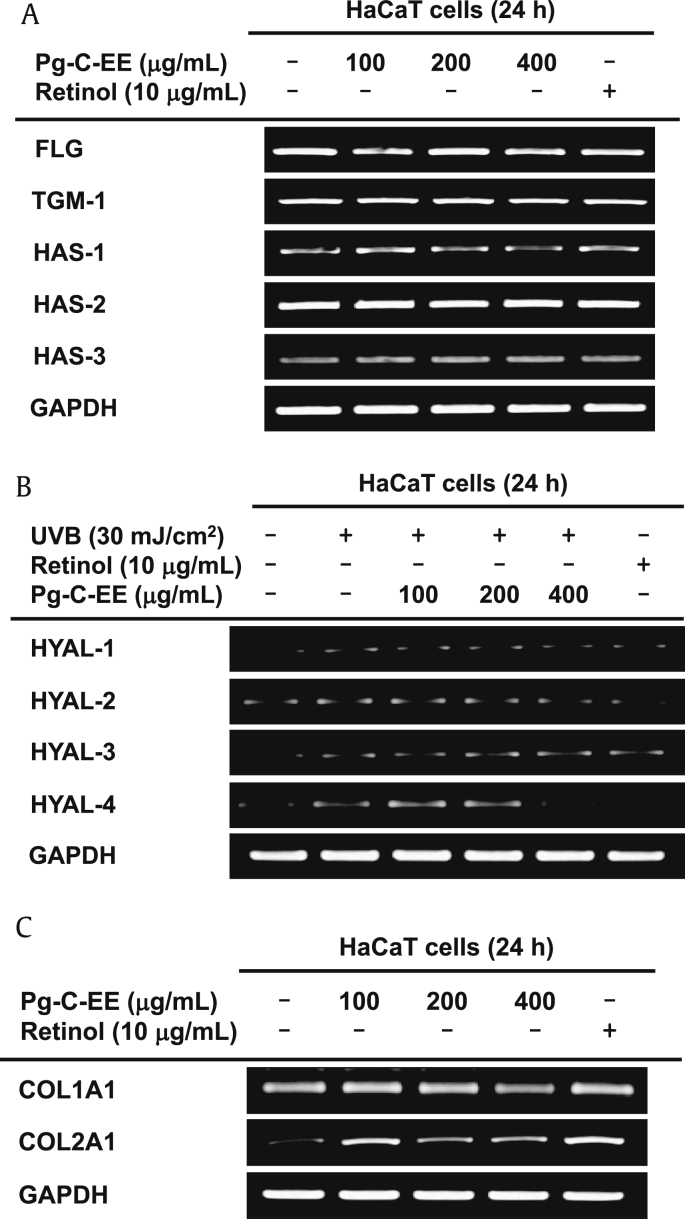
A natural moisturizing factor (NMF) that enhances skin hydration is HA. HAS is widely known as a gene that synthesizes NMF. The mRNA levels of genes related to NMF production, filaggrin (FLG), transglutaminase-1, and HAS-1, 2, 3 were not altered by Pg-C-EE treatment (Fig. 4A). However, expression of hyaluronidase (HYAL), which is known to degrade HA, was decreased by Pg-C-EE. Especially, HYAL-4 was significantly reduced to the same extent as retinol (as a positive control) (Fig. 4B). Col1A1 (collagen, type I, alpha 1) had no effect on Pg-C-EE treatment; however, Col2A1 (collagen, type II, alpha 1) tended to increase the effects of Pg-C-EE (Fig. 4C).
Fig. 4.
Effect of Pg-C-EE on moisture retention and collagen synthesis in HaCaT cells. (A) Expression of NMF synthesis–related genes (FLG, TGM-1, and HAS-1, -2, and -3) was measured by RT-PCR in HaCaT cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and retinol (10 μg/mL) for 24 h. (B) mRNA expression of HYAL-1, -2, -3, and 4 was determined by RT-PCR in HaCaT cells after irradiation with UVB (30 mJ/cm2) for 24 h and treatment with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and retinol (10 μg/mL). (C) Col1A1 and Col2A1 mRNA expression was analyzed using RT-PCR in HaCaT cells treated with Pg-C-EE (100 μg/mL, 200 μg/mL, and 400 μg/mL) and retinol (10 μg/mL) for 24 h. Col1A1, collagen, type I, alpha 1; Col2A1, collagen, type II, alpha 1; FLG, filaggrin; HAS, hyaluronic acid synthase; HYAL, hyaluronidase; NMF, natural moisturizing factor; Pg-C-EE, Panax ginseng calyx ethanol extract; RT-PCR, reverse transcription polymerase chain reaction; TGM-1, transglutaminase-1; UVB, ultraviolet B.
3.5. Effect of Pg-C-EE on transcription factor regulation
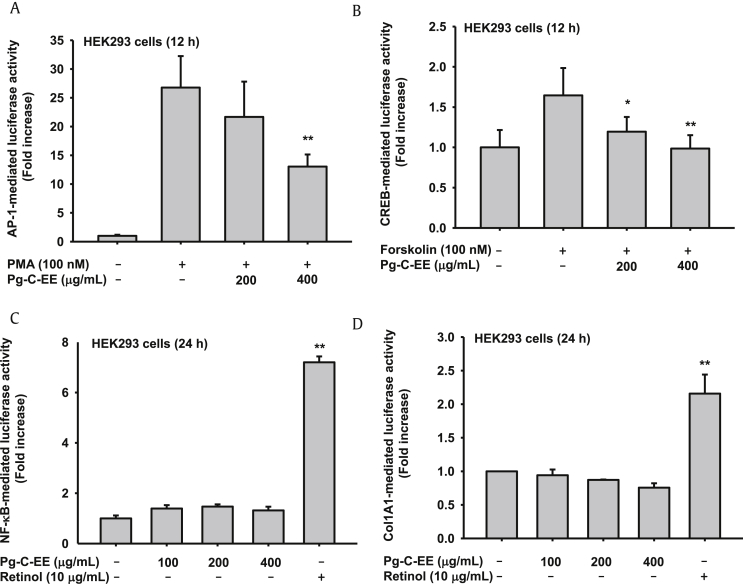
Melanogenesis and ROS-induced cell damage are associated with AP-1 signaling proteins, such as MAPKs. To support the aforementioned results, we measured luciferase activity using an AP-1-Luc plasmid construct. Interestingly, Pg-C-EE dose-dependently inhibited AP-1–mediated luciferase activities triggered by PMA (100 nM) (Fig. 5A). CREB signaling associated with melanogenesis was analyzed using a CREB-Luc plasmid. The forskolin-triggered CREB-mediated luciferase activity was decreased by Pg-C-EE in a dose-dependent manner (Fig. 5B). Without stimulation, HEK293 cells were transfected with each luciferase plasmid [Nuclear factor-κB (NF-κB) and (Col1A1)] and treated with Pg-C-EE. NF-κB- and Col1A1-mediated luciferase activities showed no difference according to the absence or presence of Pg-C-EE (Figs. 5C, 5D).
Fig. 5.
The effect of Pg-C-EE on transcription factor–mediated luciferase activity in Human embryonic kidney (HEK) HEK293 cells. (A) HEK293 cells were transfected with AP-1-Luc (1 μg/mL) and β-gal plasmid in the presence or absence of PMA (100 nM) and Pg-C-EE (200 μg/mL and 400 μg/mL) for 12 h. (B) CREB promoter binding activity was determined by a reporter gene assay with CREB-Luc construct (1 μg/mL) and β-gal plasmid (as a transfection control) in the presence or absence of forskolin (100 nM) and Pg-C-EE (200 μg/mL and 400 μg/mL) for 12 h. (C) HEK cells were transfected with NF-κB-Luc and β-gal plasmid construct with Pg-C-EE (0 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL) and retinol (10 μg/mL) for 24 h. (D) HEK cells were transfected with Col1A1-Luc and β-gal plasmid construct with Pg-C-EE (0 μg/mL, 100 μg/mL, 200 μg/mL, and 400 μg/mL) and retinol (10 μg/mL) for 24 h. *p < 0.05 compared with control. **p < 0.01 compared with control. AP-1, activator protein 1; β-gal, β-galactosidase; cAMP, cyclic adenosine monophosphate; Col1A1, collagen, type I, alpha 1; CREB, cAMP response element binding; Pg-C-EE, Panax ginseng calyx ethanol extract; PMA, phorbol 12-myristate 13-acetate; NF-κB, nuclear factor-κB.
4. Discussion
Skin cells are the cell type that is mostly affected by external environmental factors and are important at all ages throughout life. This study was carried out to examine skin cell protection and maintenance in a healthy state. External stimuli can be divided into physical or chemical stimuli. Among physical stimuli, UV irradiation is known to be involved in melanogenesis and wrinkle formation. Wrinkle formation is associated with the degradation of the ECM [23], and UV irradiation easily destroys the ECM. Among the UV spectrum, UVB with a wavelength of 280–315 nm is known to penetrate the upper dermis layer. The penetrative power of UVB is more effective than that of UVA of 315–400 nm wavelengths and UVC of 100–280 nm wavelengths [24]. UVB penetrates the dermis layer and degrades the ECM, resulting in photoaging of the skin. The photoaged skin layer shows various consequences of this damage, including inhibition of collagen synthesis, degradation of collagen, upregulation of MMP expression, DNA mutations, production of melanin, and local immunosuppression [25]. The DNA damage caused by UV irradiation leads directly to DNA single-strand breaks via formation of thymidine dimers [26] and indirectly to ROS generation via depletion of cellular antioxidants and antioxidant enzymes (superoxide dismutase, catalase) [27]. Consistent with the increase in MMPs with UV irradiation, MMPs function as collagenases, gelatinases, stromelysins, and membrane-type MMPs (mt-MMP) in skin cells. The MMPs can be subdivided according to functional differences. The collagenases are able to degrade triple-helical fibrillar collagens and include MMP-1 (interstitial collagenase), MMP-8 (neutrophil collagenase), and MMP-13 (collagenase 3). The gelatinases are distinguished by the presence of an additional domain inserted into the catalytic domain and include MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B). The stromelysins, including MMP-3 (stromelysin 1), MMP-10 (stromelysin 2), and MMP-11 (stromelysin 3), show a broad ability to cleave extracellular matrix proteins; however, these enzymes are unable to cleave the triple-helical fibrillar collagens. Therefore, regulation of MMP expression is a very important factor in aged cells such as those with photoaging. UV irradiation increases ROS production and the expression of MMPs in the cells. The roles of ROS in the cells include host defense [28], wound repair, maintenance of blood homeostasis (to recruit platelets), damage of DNA or RNA, oxidation of amino acids, oxidative deactivation of specific enzymes, accelerated aging [29], and induction of cancer [30], [31], [32]. In addition, exogenous ROS can be produced from hydroxyl radical, hydrogen peroxide, superoxide radical, and ultimately oxygen. Above all, hydrogen peroxide is formed in mammals as a short-lived product in biochemical processes and is toxic to cells. Hydrogen peroxide can cause irritation and allergic reactions and in high concentrations can cause blisters, redness, and other skin damage. The cause of cytotoxicity is oxidative stress processes in skin cells. Oxidative stress is involved in many human diseases, such as heart disease and Alzheimer's disease, in addition to the aging process. Cytotoxicity results from oxidation of proteins, membrane lipids, and DNA by the peroxide ions. For these reasons, it is important to maintain antioxidant levels in skin cells.
UV irradiation induced melanin production through the activation of melanocytes and ROS generation. Initiation of melanogenesis by exposure to UV irradiation causes skin tanning. The melanin is able to disperse approximately 99.9% of absorbed UV radiation [33]; therefore, melanin has skin-protective activity against UV radiation damage. However, melanin is associated with an increased risk of malignant melanoma, a cancer of melanocytes. The synthesis of melanin involves a chain of enzyme-catalyzed chemical reactions and nonenzyme-catalyzed reactions [34], [35], [36]. The main precursor of melanin is the conversion of L-tyrosinase to L-DOPA, which is catalyzed by the enzyme tyrosinase [36], [37]. Different kinds of enzymes also involved in melanin synthesis include tyrosinase-related protein 1 (TRP-1 and TYRP1 and tyrosinase-related protein 2 (TRP-2 and TYRP2). According to Kegg pathways, melanogenesis is under complex regulatory control by multiple agents. The most important positive regulator of melanogenesis is the MC1 receptor with its ligands melanocortic peptides. MC1 receptor activates the CREB [38], [39]. Increased expression of MITF and its activation by phosphorylation stimulate the transcription of tyrosinase (TYR), TRP-1, and dopachrome tautomerase, which produce melanin. Melanin synthesis occurs within intracellular organelles called melanosomes [40], [41], [42] and is regulated through this pathway. Melanin overproduction can cause skin spots and some diseases. Recently, women have shown considerable interest in skin whitening, and many companies are studying agents that inhibit melanin production.
Many studies have indicated that Korean ginseng and Korean Red Ginseng have beneficial activity [43]. However, to date, only inflammatory regulatory activity or phytochemical ingredients of Pg-C-EE have been studied. In this study, we examined the effects of Pg-C-EE on melanogenesis, photoaging, oxidative stress, moisture retention, and wrinkle formation using α-MSH–induced B16F10 cells and hydrogen peroxide– and UVB-induced HaCaT cells. We determined that Pg-C-EE can be used as skin-protective agent because it suppresses melanin secretion and melanin synthesis as effectively as arbutin (Figs. 1B, 1C) [44]. We also determined that activities of mushroom tyrosinase, which is the most important enzyme in the production of melanin, were decreased in the Pg-C-EE–treated group to the same extent as by kojic acid (Fig. 1D). Then, we examined the mRNA levels of melanogensis-related genes and found that mRNA levels were not significantly changed in the Pg-C-EE–treated group (Fig. 1E). We therefore confirmed the protein levels of tyrosinase, TRP-1, 2, and MITF under 48-h Pg-C-EE treatment conditions and phospho- and total p38, ERK, and CREB under 24-h Pg-C-EE treatment conditions. All protein levels were decreased dose dependently in the Pg-C-EE–treated group (Figs. 1F, 1G). Therefore, we have confidence that Pg-C-EE has antimelanogensis activity in B16F10 cells. In our study, Pg-C-EE was found to have effective antioxidant and antiphotoaging activity. Interestingly, the mRNA levels of MMPs were attenuated dose dependently in the Pg-C-EE–treated group under both UVB- and hydrogen peroxide–induction conditions (Fig. 2, Fig. 3B). Under UVB induction conditions, Pg-C-EE also reduced the expression of IL-6, the most important factor in inflammatory skin cells (Fig. 2E). Even more surprisingly, Pg-C-EE inhibited cell death due to hydrogen peroxide treatment (Fig. 3A). In the DPPH assay, Pg-C-EE showed similar antioxidative effects to other P. ginseng extracts (Fig. 3C) [45]. We determined that the mRNA levels of HYAL2 and HYAL4 in UVB induction conditions were attenuated in cells treated with Pg-C-EE (400 μg/mL) (Fig. 4B), indicating that Pg-C-EE can regulate moisture retention in skin cells. Surprisingly, we also found that Pg-C-EE can help the formation of collagen (Fig. 4C). We examined which transcription factors regulated the aforementioned process through the luciferase system. By transfection with NF-κB and Col1A1 luciferase, respectively, and treatment with Pg-C-EE, we confirmed that NF-κB- and Col1A1-driven luciferase activities were affected by Pg-C-EE (Figs. 5C, 5D). We also showed that AP-1 mediated luciferase activity with PMA induction and CREB mediated luciferase activity with forskolin induction. Both luciferase activities were decreased with Pg-C-EE treatment (Figs. 5A, 5B). These results show that Pg-C-EE has a skin-protective effect through AP-1 and CREB. Several experiments carried out using AP-1 inhibitors (MAPK inhibitor; SB203580, SP600125, and U0126) [46], [47], [48] verified the results from Fig. 1, Fig. 2, Fig. 3 (Figs. 1H, 2G and 3D).
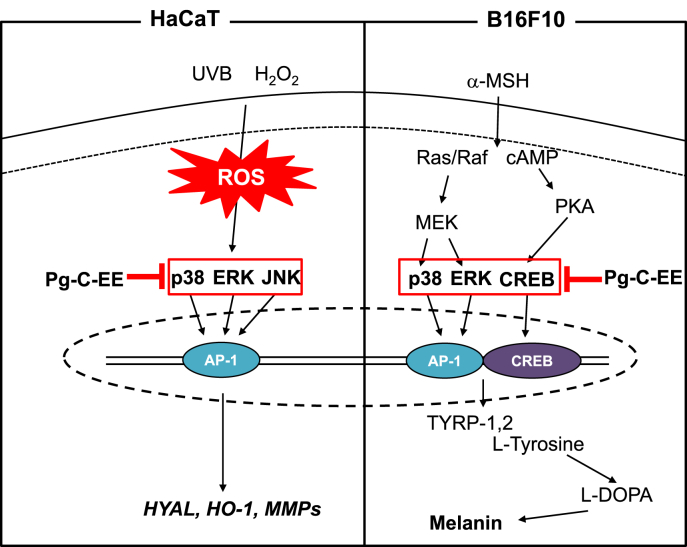
In summary, we found that Pg-C-EE exerted skin-protective effects through antimelanogenesis activity in α-MSH–induced B16F10 cells, antiphotoaging activity in UVB-irradiated HaCaT cells, and antioxidant activity in hydrogen peroxide–induced HaCaT cells. In addition, Pg-C-EE inhibited AP-1 and CREB transcription factors and their upstream activation pathway (MAPK and CREB). A brief summary of each activity is shown in Fig. 6. These results suggest that Pg-C-EE can be helpful as a skin-improving ingredient through blockade of AP-1 and CREB signaling proteins.
Fig. 6.
Pathway by which Pg-C-EE inhibits melanogenesis and ROS generation. α-MSH, α-melanocyte–stimulating hormone; AP-1, activator protein 1; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element binding; ERK, extracellular signal-regulated kinase; HO-1, heme oxygenase-1; HYAL, hyaluronidase; JNK, c-Jun N-terminal kinase; L-DOPA, L-dopamine; MMP, matrix metalloproteinase; Pg-C-EE, Panax ginseng calyx ethanol extract; PKA, Protein kinase A; ROS, reactive oxygen species; TYRP, tyrosinase-related protein; UVB, ultraviolet B.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This article was supported by Konkuk University in 2016.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2018.02.007.
Contributor Information
Young-Jin Son, Email: sony@sunchon.ac.kr.
Sang Yun Han, Email: dangsukr@naver.com.
Jae Youl Cho, Email: jaecho@skku.edu.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Murphy K., Weaver C. Janeway's immunobiology. Garland Science. 2016 [Google Scholar]
- 2.Wilkinson P., Millington R. Cambridge university press; Cambridge (GB)[etc.]: 1983. Skin (Digitally printed version ed.) [Google Scholar]
- 3.Kumar C.M., Sathisha U., Dharmesh S., Rao A.A., Singh S.A. Interaction of sesamol (3, 4-methylenedioxyphenol) with tyrosinase and its effect on melanin synthesis. Biochimie. 2011;93:562–569. doi: 10.1016/j.biochi.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Laskin J.D., Piccinini L., Engelhardt D.L., Weinstein I.B. Control of melanin synthesis and secretion by B16/C3 melanoma cells. J Cell Physiol. 1982;113:481–486. doi: 10.1002/jcp.1041130318. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.S., Kim M.-J., Choi Y.H., Kim B.K., Kim K.S., Park K.J., Park S.M., Lee N.H., Hyun C.-G. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16 F10 melanoma. Asian Pac J Trop Biomed. 2013;3:617–622. doi: 10.1016/S2221-1691(13)60125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kähäri V.M., Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 7.Curran S., Murray G.I. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Verma R.P., Hansch C. Matrix metalloproteinases (MMPs): chemical–biological functions and (Q) SARs. Bioorg Med Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Senftleben U., Karin M. The IKK/NF-κB pathway. Crit Care Med. 2002;30:S18–S26. [PubMed] [Google Scholar]
- 10.Chung J.H., Kang S., Varani J., Lin J., Fisher G.J., Voorhees J.J. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J Invest Dermatol. 2000;115:177–182. doi: 10.1046/j.1523-1747.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim E., Kim D., Yoo S., Hong Y.H., Han S.Y., Jeong S., Jeong D., Kim J.-H., Cho J.Y., Park J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J Ginseng Res. 2018;42:218–224. doi: 10.1016/j.jgr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papakonstantinou E., Roth M., Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4:253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen L.P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2008;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 15.Qi L.-W., Wang C.-Z., Yuan C.-S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S.Y., Kim J., Kim E., Kim S.H., Seo D.B., Kim J.-H., Shin S.S., Cho J.Y. AKT-targeted anti-inflammatory activity of Panax ginseng calyx ethanolic extract. J Ginseng Res. 2017 doi: 10.1016/j.jgr.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh Y., Lim H.-W., Kim K., Lim C.-J. Ginsenoside Re improves skin barrier function in HaCaT keratinocytes under normal growth conditions. Biosci Biotechnol Biochem. 2016;80:2165–2167. doi: 10.1080/09168451.2016.1206808. [DOI] [PubMed] [Google Scholar]
- 18.Ramesh T., Kim S.-W., Hwang S.-Y., Sohn S.-H., Yoo S.-K., Kim S.-K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012;32:718–726. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Kim H.-G., Yoo S.-R., Park H.-J., Lee N.-H., Shin J.-W., Sathyanath R., Cho J.-H., Son C.-G. Antioxidant effects of Panax ginseng CA Meyer in healthy subjects: a randomized, placebo-controlled clinical trial. Food Chem Toxicol. 2011;49:2229–2235. doi: 10.1016/j.fct.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Park J.G., Kang W.-S., Park K.T., Park D.J., Aravinthan A., Kim J.-H., Cho J.Y. Anticancer effect of joboksansam, Korean wild ginseng germinated from bird feces. J Ginseng Res. 2016;40:304–308. doi: 10.1016/j.jgr.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- 23.Rittié L., Fisher G.J. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 24.Mainster M.A. Violet and blue light blocking intraocular lenses: photoprotection versus photoreception. Br J Ophthalmol. 2006;90:784–792. doi: 10.1136/bjo.2005.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giudice G.J., Fuchs E.V. Vitamin A-mediated regulation of keratinocyte differentiation. Methods Enzymol. 1990;190:18–29. doi: 10.1016/0076-6879(90)90005-l. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein C., Bernstein H., Payne C.M., Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res Rev Mutat Res. 2002;511:145–178. doi: 10.1016/s1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 27.Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation–a review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 28.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 29.Muller F.L., Lustgarten M.S., Jang Y., Richardson A., Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 30.Irani K., Xia Y., Zweier J.L., Sollott S.J., Der C.J., Fearon E.R., Sundaresan M., Finkel T., Goldschmidt-Clermont P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey M.R., Sharpless N.E. ROS as a tumour suppressor? Nat Cell Biol. 2006;8:1213–1215. doi: 10.1038/ncb1106-1213. [DOI] [PubMed] [Google Scholar]
- 32.Renschler M.F. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Meredith P., Riesz J. Radiative relaxation quantum yields for synthetic eumelanin. Photochem Photobiol. 2004;79:211–216. doi: 10.1562/0031-8655(2004)079<0211:rcrqyf>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Kondo T., Hearing V.J. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011;6:97–108. doi: 10.1586/edm.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang T.-S. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ebanks J.P., Wickett R.R., Boissy R.E. Mechanisms regulating skin pigmentation: the rise and fall of complexion coloration. Int J Mol Sci. 2009;10:4066–4087. doi: 10.3390/ijms10094066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leyden J., Wallo W. The mechanism of action and clinical benefits of soy for the treatment of hyperpigmentation. Int J Dermatol. 2011;50:470–477. doi: 10.1111/j.1365-4632.2010.04765.x. [DOI] [PubMed] [Google Scholar]
- 39.Kundu J.K., SURH Y.J. Epigallocatechin gallate inhibits phorbol ester-induced activation of NF-κB and CREB in mouse skin. Ann N Y Acad Sci. 2007;1095:504–512. doi: 10.1196/annals.1397.054. [DOI] [PubMed] [Google Scholar]
- 40.Busca R., Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Melanoma Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 41.Park H., Gilchrest B. Signaling pathways mediating melanogenesis. Cell Mol Biol (Noisy-le-grand) 1999;45:919–930. [PubMed] [Google Scholar]
- 42.HEARING V.J. The melanosome: the perfect model for cellular responses to the environment. Pigment Cell Melanoma Res. 2000;13:23–34. doi: 10.1034/j.1600-0749.13.s8.7.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim K. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. J Ginseng Res. 2015;39:1–6. doi: 10.1016/j.jgr.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeda K., Fukuda M. Arbutin: mechanism of its depigmenting action in human melanocyte culture. J Pharmacol Exp Ther. 1996;276:765–769. [PubMed] [Google Scholar]
- 45.Kim S.-J., Murthy H.N., Hahn E.-J., Lee H.L., Paek K.-Y. Parameters affecting the extraction of ginsenosides from the adventitious roots of ginseng (Panax ginseng CA Meyer) Sep Purif Technol. 2007;56:401–406. [Google Scholar]
- 46.Wong V.W., Rustad K.C., Akaishi S., Sorkin M., Glotzbach J.P., Januszyk M., Nelson E.R., Levi K., Paterno J., Vial I.N. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chun K.-S., Keum Y.-S., Han S.S., Song Y.-S., Kim S.-H., Surh Y.-J. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-κB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 48.Gangnuss S., Cowin A.J., Daehn I.S., Hatzirodos N., Rothnagel J.A., Varelias A., Rayner T.E. Regulation of MAPK activation, AP-1 transcription factor expression and keratinocyte differentiation in wounded fetal skin. J Invest Dermatol. 2004;122:791–804. doi: 10.1111/j.0022-202X.2004.22319.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.