Abstract
BACKGROUND:
The role of the immune system in the control of tumour progression has been stressed, recently. Many studies indicate the fact that the immune system can prevent tumour progression in several types of human malignant neoplasms including colorectal cancer. According to some authors, a higher density of “tumour-associated lymphocytes” (TAL), in malignant neoplasms, correlate with prolonged survival of patients.
AIM:
This study aims to determine the structure and the influence of the immune cells, TAL, in the progression of colorectal cancer (CRC).
PATIENTS AND METHODS:
The study included 103 patients with CRC operated at the University Clinic of Digestive Surgery in Skopje, whose operative material was analysed at the Institute of Pathology, Medical Faculty in Skopje. The structure of tumor-associated cells and their density were determined and were correlated with neoplasm’s grade, local growth (T), positive lymph nodes, lymphatic invasion and stage of the disease.
RESULTS:
CD4+, CD8+ and CD20+ lymphocytes (Ly) were found in TAL. The density of TAL was significantly different in neoplasms with different T status, lymphatic invasion, patients with and without nodal metastasis and patients with a different stage of the disease. The density of CD4+, CD8+, and CD20+ cells were significantly different in neoplasms with different T. The density of CD8+ and CD20+ lymphocytes was lower in patients with nodal metastasis and higher stage.
CONCLUSION:
The density of tumor-associated lymphocytes can anticipate the disease progression in patients with colorectal cancer, and the density of TAL influences the control of tumour progression.
Keywords: colorectal cancer, tumour-associated lymphocytes, grade, stage, immunohistochemistry
Introduction
Colorectal cancer (CRC) is the third most common malignant disease in the humans, with 570,000 new cases in the world each year. It is the second most common cause for the lethal outcome of malignancies in the West European countries and eighth in the developing countries. Despite the new surgical techniques and treatment with neoadjuvant and adjuvant chemotherapy, colorectal cancer is the most common tumour of the gastrointestinal tract which incidence and mortality continually increase [1] [2]. Prognosis of colorectal cancer mostly depends on the stage of disease (local growth, positive lymph nodes and distant metastasis-TNM classification) and depends on many other factors such as lymphovascular invasion, histological grade, positive surgical margins, preoperative serum values of carcinoembryonic antigen (CEA) bowel obstruction and many others [3] [4] [5] [6]. It is considered that new biomarkers such as APC, K-ras, DDS, p53, BRAF, TGF- β, CIMP and others can give more pieces of information for disease progression. The finding that some patients with certain mutations (KRAS), who have metastatic disease, do not respond to the treatment with monoclonal antibodies represents the basis in the efforts for discovering personalised therapies [7] [8] [9] [10] [11]. This is a motivation for continued investigation of new prognostic factors to determine the disease progression and patient outcome as well as to improve the knowledge for developing new therapies. The role of the immune system in the control of disease progression is the basis for the development of immunotherapy in patients with malignant diseases and is a current topic for research in the field of oncology [9] [10] [11].
The idea that the immune system can recognise a neoplasm was introduced by Paul Ehrlich and was supported by Lewis Thomas and Macfarlane Burnet. Over the course of time, this idea was turned into a concept in which it is considered that the immune cells are capable of eliminating malignant cells. This is how the theory for “immune surveillance” was born. This theory supports hypotheses that the immune system is capable of surveillance of the newly appeared malignant cells and their elimination [12] [13] [14] [15] [16].
It is well known that colorectal cancer progression is influenced by inter-reaction between cancer cells and tumour microenvironment belonging to the patient [12] [13] [14].
It is considered that the immune cells present in tumour-associated stroma are in correlation with tumour progression and patient outcome. According to some authors, the high density of memory T-cells and cytotoxic T-cells in malignant neoplasms (tumour-associated lymphocytes-TAL) is in correlation with prolonged survival of patients [17] [18] [19].
Analysis of stromal inflammatory infiltrate, and tumour-infiltrating lymphocytes in CRC has not attracted the attention of researchers in the Republic of Macedonia recently, but new therapeutic approaches in the treatment of patients with CRC need new basic investigations to provide better management of these patients. According to that, we aimed to determine the density of TAL, the type of some immune cells, “tumour-associated lymphocytes”, and their influence on CRC progression.
Material and Methods
The study included 103 patients with CRC, 68 (66.2%) male, and 35 (33.98%) female with the age of the patients ranged from 33 to 97 years, the mean age 64.57 ± 11.5 years. All of them underwent surgery in our institution, and their operative material was routinely analysed for histopathological diagnosis and pTNM staging at the Institute of Pathology and was additionally analysed for TAL.
In this study the following parameters were examined: localisation of a tumour, local growth (T status), and grade of tumour differentiation (G), the presence of positive lymph nodes (LN) as well as the stage and the density of TAL.
The type of TAL was defined by immunohistochemical staining with antibodies against CD4 (Dako Monoclonal Mouse Anti-Human CD4, Clone 4B12); CD8 (Dako Monoclonal Mouse Anti-Human CD8, Clone C8/144B); CD20 (Dako Monoclonal Mouse Anti-Human CD20, Clone L26); with a standard procedure using Immunoperoxidase LSAB + system.
The density of TAL was determined semiquantitatively. The density of all cells (TAL) was determined in the first step in 4 grades and the density of positively stained cells from the total number of TAL for each marker individually was determined in the second step in the same manner as absent (-), scanty (+), moderate (++) and abundant (+++). To confirm consistency of grading, the cases were scored independently by two investigators. Examples of grading TAL and immune cell staining and their grading are shown in Figure 1.
Figure 1.
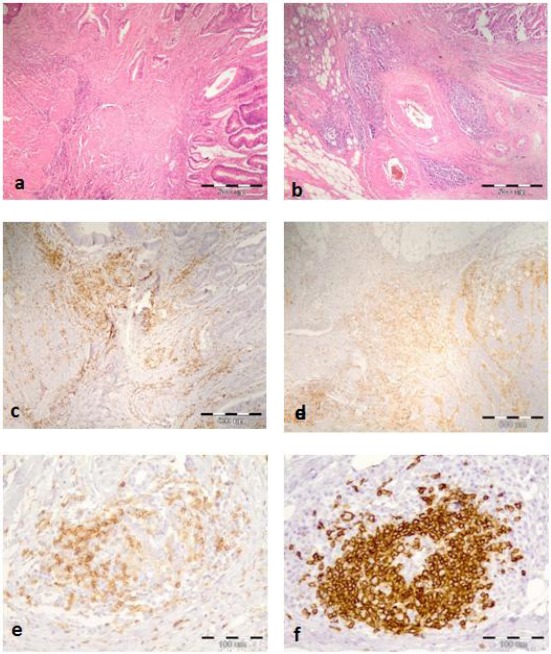
a) Scanty (+) Ly infiltration in the tumor invasive front (HEx40) b) Abundant (+++) Ly infiltration in the tumor invasive front (Hex40) c) Moderate (++) CD4 Ly infiltration (x40) d) Abundant CD4+ Ly infiltration (x40) e) Higher magnification of CD4+ cells (x200) f) Higher magnification of CD20+ cells in the tumor invasive front of CRC (x200)
Statistical analysis
Descriptive statistical methods were used for statistical analysis of the data. Categorical variables are presented with absolute and relative numbers (%). Fisher’s exact test was used for comparison of categorical variables. Spearman’s correlation coefficient was used to determine the degree of correlation between analysed parameters. The statistical program SPSS for Windows, version 19.0 was used.
Results
Density of TAL
Distribution of patients and density of TAL according to the localisation of a tumour are shown in Table 1.
Table 1.
Distribution of patients and density of TAL according to the cancer localisation
| Localization | Quantity of TAL | |||
|---|---|---|---|---|
| No of patients | Scanty (No = 59) | Moderate (No = 32) | Abundant (No = 12) | |
| Coecum | 12 (11.65%) | 8 (66.67) | 3 (25) | 1 (8.33) |
| Colon asc | 9 (8.74%) | 6 (66.67) | 3 (33.33) | 0 |
| Flex hepat | 3 (2.91%) | 1 (33.330 | 2 (66.67) | 0 |
| Colon trans | 5 (4.85%) | 2 (40) | 2 (40) | 1 (20) |
| Flex lienlis | 3 (2.91%) | 2 (66.67) | 1 (33.33) | 0 |
| Colon des | 3 (2.91%) | 3 (100) | 0 | 0 |
| Sigma | 29 (28.16%) | 17 (58.62) | 7 (24.14) | 5 (17.24) |
| Rectosig | 8 (7.77%) | 5 (62.5) | 2 (25) | 1 (12.5) |
| Rectum | 31 (30.09%) | 15 (48.39) | 12 (38.71) | 4 (12.9) |
Stage of the disease and local growth of CRC, i.e. T category according to the TNM classification for colorectal cancer (AJCC, 2010), number of patients with negative and positive lymph nodes, number of cases with and without lymphatic invasion, and the grade of differentiation in the analysed series are illustrated in Table 2. The largest number of examined patients was with a tumour in T3 and T4 local growth without metastatic deposits in the local lymph nodes, without lymphatic invasion and with moderately differentiated neoplasms. The density of tumor-associated lymphocytes about the parameters mentioned above is presented in the same Table.
Table 2.
Density of TAL according to the stage of disease, T category and nodal status and lymphatic invasion
| Stage | Quantity (Density) of TAL | P-value | |||
|---|---|---|---|---|---|
| No of patients | Scanty | Moderate | Abundant | ||
| I | 11 | 1 (9.09) | 5 (45.45) | 5 (45.45) | <0.0001** |
| II | 45 | 15 (35.71) | 23 (22.33) | 7 (16.67) | |
| III | 41 | 37 (35.92) | 4 (9.09) | 0 | |
| IV | 6 | 3 (50) | 3 (50) | 0 | |
| T category | |||||
| 1 | 5 | 0 | 4 (80) | 1 (20) | 0.0018** |
| 2 | 7 | 1 (14.29) | 2 (28.57) | 4 (57.14) | |
| 3 | 60 | 35 (58.33) | 20 (33.33) | 5 (8.33) | |
| 4 | 31 | 23 (74.19) | 6 (19.35) | 2 (6.45) | |
| N category | |||||
| N0 | 56 | 17 (30.36) | 27 (48.21) | 12 (21.43) | 0.0001** |
| N1/N2 | 47 | 42 (89.36) | 5 (10.64) | 0 | |
| L category | |||||
| L0 | 54 | 20 (37.04) | 26 (48.15) | 8 (14.81) | 0.00005** |
| L1 | 49 | 39 (79.59) | 6 (12.24) | 4 (8.16) | |
P (Kruskal-Wallis test) *P < 0.05;
P < 0.01.
The density of TAL was higher in patients with lower stage I and II of the disease, in tumours with less local invasion, in patients without nodal metastasis, and in tumours with no lymphatic invasion. On the contrary, in stage III and IV of the disease, the density of TAL was low in the most of the analysed cases and the tumour infiltrating lymphocytes was scanty in patients with advanced local growth, with nodal metastasis and with lymphatic invasion.
Statistical analysis did not show statistical differentiation in the quantity of TAL according to tumour differentiation (P > 0.05).
Different cell types of TAL and their density in CRC
CD20, CD4 and CD8 positive cells in different quantity were found in all analysed cases.
CD20+ Lymphocytes
The density of CD20+ tumour-associated lymphocytes about the stage of disease, T and N category, and lymphatic invasion is presented in Table 3.
Table 3.
Density of CD20+ cells according to the tumour stage, T and N category, and lymphatic invasion
| Stage | The quantity of CD20+ Ly | P-value | ||||
|---|---|---|---|---|---|---|
| N° of patients | CD20+ Ly not present | Scanty | Moderate | Abundant | ||
| I | 11 | 0 | 0 | 6 (54.55) | 5 (45.45) | <0.0001** |
| II | 45 | 1 (2.38) | 24 (23.30) | 16 (38.1) | 4 (9.52) | |
| III | 41 | 0 | 36 (34.95) | 4 (9.09) | 1 (2.27) | |
| IV | 6 | 0 | 6 (100) | 0 | 0 | |
| T category | ||||||
| 1 | 5 | 0 | 0 | 3 (60) | 2 (40) | 0.0002** |
| 2 | 7 | 0 | 0 | 4 (57.14) | 3 (42.86) | |
| 3 | 60 | 1 (1.67) | 41 (68.33) | 13 (21.67) | 5 (8.33) | |
| 4 | 31 | 0 | 25 (80.65) | 6 (19.35) | 0 | |
| N category | ||||||
| N0 | 56 | 1 (1.79) | 25 (44.64) | 21 (37.5) | 9 (16.07) | <0.0001** |
| N1/N2 | 47 | 0 | 41 (87.23) | 5 (10.64) | 1 (2.13) | |
| L category | ||||||
| L0 | 54 | 1 (1.85) | 26 (48.15) | 18 (33.33) | 9 (16.67) | 0.001** |
| L1 | 49 | 0 | 40 (81.63) | 8 (16.33) | 1 (2.04) | |
P (Kruskal-Wallis test) *P < 0.05;
P < 0.01.
We found a significant difference in the density of CD20+ lymphocyte (Ly) in all analysed parameters.
Scanty CD20+ Ly infiltrates found in patients with advanced local growth, with nodal metastases, lymphatic invasion and with a higher stage of the disease (100% Stage IV, 88.64% Stage III, 50% Stage II and 0% of patients in Stage I). The density of CD20+ Ly infiltrate lower (scanty) in patients with poorly differentiated tumours but without a statistically significant difference (P = 0.089).
CD4+ Lymphocytes
The density of CD4+ tumour-associated lymphocytes about the stage of disease, T and N category, and lymphatic invasion is presented in Table 4.
Table 4.
Density of CD4+ tumour-associated lymphocytes about the stage of disease, T and N category, and lymphatic invasion
| Stage | The quantity of CD4+ Lymphocytes | P-value | |||
|---|---|---|---|---|---|
| No of patients | Scanty | Moderate | Abundant | ||
| I | 11 | 0 | 10 (90.91) | 1 (9.09) | P = 0.11 ns |
| II | 45 | 10 (23.81) | 26 (25.24) | 9 (21.43) | |
| III | 41 | 4 (9.09) | 34 (33.00) | 3 (6.82) | |
| IV | 6 | 0 | 6 (100) | 0 | |
| T category | |||||
| 1 | 5 | 0 | 4 (80) | 1 (20) | P = 0.034* |
| 2 | 7 | 0 | 7 (100) | 0 | |
| 3 | 60 | 6 (10) | 46 (76.67) | 8 (13.33) | |
| 4 | 31 | 8 (25.81) | 19 (61.29) | 4 (12.9) | |
| N category | |||||
| N0 | 56 | 10 (17.86) | 36 (64.29) | 10 (17.86) | P = 0.055 ns |
| N1/N2 | 47 | 4 (8.51) | 40 (85.11) | 3 (6.38) | |
| L category | |||||
| L0 | 54 | 11 (20.37) | 37 (68.52) | 6 (11.11) | P = 0.11 ns |
| L1 | 49 | 3 (6.12) | 39 (79.59) | 7 (14.29) | |
P (Kruskal-Wallis test)
P < 0.05;
**P < 0.01.
The low density of CD4+ Ly was detected in 100% of tumours with advanced local tumour growth (T4 category) and on contrary CD4+ low Ly density was not detected in tumours with T1. The statistical analysis confirmed that the density of CD4+ lymphocytes in the infiltrative front of a tumour significantly depended on the local growth of the disease.
Patients with regional lymph nodes metastasis had a lower density of CD4+ Ly infiltrate than patients without metastasis, but there was no a statistically significant difference in the density of CD4+ Ly according to the presence of lymph nodes metastasis. No statistical significance was found in the density of CD4+ Ly infiltrate according to the Stage, lymphatic invasion and grade also.
CD8 Lymphocytes
The density of CD8+ tumour-associated lymphocytes about the stage of disease, T and N category, and lymphatic invasion is presented in Table 5.
Table 5.
Density of CD8+ tumour-associated lymphocytes about the stage of disease, T and N category, and lymphatic invasion
| Stage | The quantity of CD8+ Lymphocytes | p-value | ||||
|---|---|---|---|---|---|---|
| No of patients | CD8+ Ly not present | scanty | moderate | abundant | ||
| I | 11 | 0 | 0 | 11 (100) | 0 | 0.0055** |
| II | 45 | 1 (2.38) | 12 (28.57) | 28 (66.67) | 1 (2.38) | |
| III | 41 | 0 | 24 (54.55) | 20 (45.45) | 0 | |
| IV | 6 | 0 | 3 (50) | 3 (50) | 0 | |
| T category | ||||||
| 1 | 5 | 0 | 0 | 5 (100) | 0 | 0.006** |
| 2 | 7 | 0 | 0 | 7 (100) | 0 | |
| 3 | 60 | 1 (1.67) | 22 (36.67) | 37 (61.67) | 0 | |
| 4 | 31 | 0 | 17 (54.84) | 13 (41.94) | 1 (3.23) | |
| N category | ||||||
| N0 | 56 | 1 (1.79) | 15 (26.79) | 39 (69.64) | 1 (1.79) | 0.024* |
| N1/N2 | 47 | 0 | 24 (51.06) | 23 (48.94) | 0 | |
| L category | ||||||
| L0 | 54 | 0 | 18 (33.33) | 35 (64.81) | 1 (1.85) | 0.36 ns |
| L1 | 49 | 1 (2.04) | 21 (42.86) | 27 (55.1) | 0 | |
P (Kruskal-Wallis test)
P < 0.05;
P < 0.01.
We found a significant difference in the quantity of CD8+ Ly in all analysed parameters except lymphatic invasion.
The density of CD8+ Ly significantly differs according to the local tumour growth. The low density of CD8+ Ly infiltrate significantly more often was present in patients with T4 and T3 status (54.84%, 36.67% respectively); there were no patients with T1 and T2 with a low density of CD8+ Ly infiltrate.
The density of CD8+ Ly was significantly lower in patients with nodal metastasis, lymphatic invasion and with an advanced stage of the disease.
We did not find a statistical difference in the density of CD8+ Ly according to the histological differentiation of a tumour.
The moderate density of CD8+ lymphocytes decreased when the grade of the differentiation decreased, but the difference was not significant.
Correlations
The correlations of the density of tumour-associated lymphocytes with T tumour status, the involvement of the regional lymph nodes, lymphatic invasion, stage and the grade of differentiation were done using the Spearman’s correlation test. Correlations were determined between the different T cell types also.
The results showed a significant negative correlation between the density of CD20+ Ly and T status, stage and G, i.e. the density of CD20+ Ly was lower in less differentiated tumours with advanced local growth and higher stage. The density of CD20+ Ly was in positive correlation with the density of CD8+ Ly and the density of TAL (Table 6).
Table 6.
Correlations of the quantity of TAL and all other analysed parameters
| CD20 | Spearman R | t-test, P-level |
|---|---|---|
| CD20 / CD8 | 0.367 | 3.97, P = 0.00013** |
| CD20 / TAL | 0.436 | 4.87, P = 0.000004** |
| CD20 / T | -0.407 | 4.48, P = 0.00002** |
| CD20 / Stage | -0.564 | 6.87, P < 0.0001** |
| CD20 / G | -0.220 | 2.27, P = 0.025* |
| CD4 | ||
| CD4 / CD 8 | 0.387 | 4.21, P = 0.000055** |
| CD4 / CD 20 | 0.016 | 0.16, P = 0.87 |
| CD4 / TAL | 0.342 | 3.66, P = 0.00041** |
| CD4 / T | -0.155 | 1.57, P = 0.12 |
| CD4 / Stage | -0.034 | 0.34, P = 0.74 |
| CD4 / G | -0.091 | 0.92, P = 0.36 |
| CD8 | ||
| CD8 / TAL | 0.381 | 4.14, P = 0.000073** |
| CD8 / T | -0.271 | 2.83, P = 0.0056** |
| CD8 / Stage | -0.324 | 3.44, P = 0.00084** |
| CD8 / G | -0.107 | 1.08, P = 0.28 |
P < 0.05;
P < 0.01.
The density of CD8+ Ly was lower in tumours with advanced local growth and higher stage of the disease. Correlations were not statistically significant except for the density of CD8+ Ly and the stage of the disease (Table 6).
Statistically, a significant correlation was found between CD4+ Ly and CD8+ Ly and the total amount of TAL (Table 6). The density of CD8+ Ly increased when the density of Total amount of TAL increased and when the density of CD4+ Ly increased (Table 6). There was also a significant positive correlation between CD20+ cells and CD8+cells.
Discussion
Rudolf Virchow, 150 years ago, was the first who described inflammatory cells in tumours and established the theory that tumours arise at chronic inflammation sites. It is known that the risk of developing colorectal cancer is greater in patients with ulcerative colitis and those with Crohn’s disease. Despite the knowledge that chronic inflammation is a risk for development of a malignant neoplasm, many clinical and experimental studies stress the protective role of immune cells in cancer progression, hence, the statements and cognitions that immune response plays a key role in fighting tumour growth and its dissemination [7].
Some authors consider that the type, density and location of the immune cells in colorectal cancer can be superior and independent prognostic factor for tumour progression [7].
Cancer progression is a complex process that is influenced by genetic, epigenetic and environmental factors, but at the same time, it is a process of interaction between cancer cells and their microenvironment, that is, stroma. Tumour stroma is built of different cells among which are inflammatory cells that belong to congenital and inherited immune system [20] [21] [22]. Former investigations have shown that lymphocytes that infiltrate the tumour-tumour infiltrating lymphocytes (TIL/TAL) are predominantly T lymphocytes among which two subtypes are identified CD4+ and CD8+ T lymphocytes. CD4+ T lymphocytes are T helper cells, whereas CD8+ T lymphocytes are cytotoxic cells capable of killing the “target cell”, i.e. tumour cell which exposed antigens CD8+ T lymphocytes are capable of recognising. They are able to destroy tumor cells in direct cell interaction by releasing lytic components [22] [23] [24] [25] [26] [27]. CD4+ T lymphocytes are helper cells that regulate their proliferation and coordinate the immune response by stimulating the other immune cells. They are stratified in subgroups T helper 1 (Th1) and T helper 2 (Th2) cells. Th1 CD4+ T lymphocytes are responsible for the proliferation of cytotoxic T lymphocytes that participate in the killing of tumour cells.
In addition to T lymphocytes, B CD20+ lymphocytes arranged in aggregates have been found among TAL together with other immune cells. In lymph glands and the remaining lymphoid tissue, B lymphocytes receive signals for growth, differentiation and affinity maturation from follicular T helper immune cells. They differentiate into plasma cells and memory cells with long lifespan [28]. The process of lymphoid neogenesis is described in infectious diseases, chronic inflammation processes, autoimmune diseases and chronic graft rejection [29] [30] [31]. In these conditions, T and B cells cooperate to create a strong immune response when B cells enhance T cell response by antigen presentation, costimulation and modulation of migration and function of dendritic cells [28], 29].
Lymphoid neogenesis is also described in malignant neoplasms, while the role of CD20+ lymphocytes in tertiary lymphoid structures that are found in these neoplasms has not been completely clarified and it is still in the phase of research [29]. Nowadays many patients with malignant neoplasms develop tumour-specific antibodies, and B cells have a significant role in the immune response to neoplasm both by exacerbating cancer-associated inflammation and by the effect they have on T lymphocytes, macrophages and myeloid stem cells.
We found a statistically significant correlation between the examined subtypes of lymphocytes with a negative trend between CD4+ and CD20+ cells and CD8+, CD20+ cells and also a positive trend between CD4+ and CD8+. The correlation implies mutual connection in the function of these cells in one common immune response to tumour antigens.
TAL-tumour infiltrating lymphocytes are concentrated in the stroma of an invasive tumour front. Many authors examined the role of TAL in tumor progression in relation to survival of patients with colorectal cancer [23] [24] [25] [26] [32] [33].
Even though for a long time it was considered that colorectal cancer is a bad immunogenic tumor, the new cognitions suggest that there is a substantial immune response to this disease and that the presence of the immune response is connected with a better prognosis of patients with colorectal cancer, that is, the increased presence of T cytotoxic lymphocytes is associated with absence of early metastases and absence of early recurrence, as well as that the type of density and location of the immune cells have prognostic significance in colorectal cancer [7] [33] [34].
Therefore, tumour-associated lymphocytes can provide significant prognostic information about patients with colorectal cancer, which could be used for treatment with adjuvant therapy in a different stage of cancer [29].
The results from the analysis of TAL in the invasive front of cancer in our group of patients with colorectal cancer showed the presence of T (CD4+ and CD8+) and B (CD20+) lymphocytes and their density significantly correlated with clinically-pathologic parameters for disease progression. The density of TAL was higher in patients with lower stage (I and II) of the disease, in tumours with less local invasion, in patients without nodal metastasis, and in tumours with no lymphatic invasion. The density of CD8+ and CD20+ lymphocytes in a neoplasm was higher in patients without metastases in lymph nodes and with a lower stage of the disease. Lower density of CD20+ lymphocytes was found in neoplasms of patients with advanced local growth and lymphatic tumor invasion. Thus, it can be concluded that the density of tumor-associated lymphocytes can be a parameter for neoplasm progression in patients with colorectal cancer and the density of tumor-associated lymphocytes influences the control of tumor progression.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Nicholls J, Dozois R. Section IV: Colon and Rectum: neoplasma in Surgery of the colon & rectum. Churchil Livingstone. 1997 [Google Scholar]
- 2.Johnson R, Marsh R, Carson J, Seymour K. A comparison of two metods of palliation of large bowel obstruction due to irremovable colon cancer. Ann R Coll Surg Engl. 2004;86:99–103. doi: 10.1308/003588404322827473. https://doi.org/10.1308/003588404322827473 PMid:15005927 PMCid: PMC1964159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudy DR, Zdon MJ. Update on colorectal cancer. Am Fam Physician. 2000;61(6):1759–70. PMid:10750881. [PubMed] [Google Scholar]
- 4.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–5. doi: 10.1093/jnci/djh275. https://doi.org/10.1093/jnci/djh275 PMid:15467030. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th edition. France: Springer; 2017. [Google Scholar]
- 6.Roscher R, Frank R, Wagner R, Safi F, Veger HG. Surgical treatment results in colonic obstruction. Chirurg. 1991;62(3):201–5. PMid:2036896. [PubMed] [Google Scholar]
- 7.Di Caro G, Marchesi F, Laghi L, Grizzi F. Immune cells: plastic players along colorectal cancer. Progression J Cell Mol Med. 2013;17(9) doi: 10.1111/jcmm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinzler K. Vogelstein Hereditary Colorectal Cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. https://doi.org/10.1016/S0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 9.Redston MS, Papadopoulos N, Caldas C. Common occurrence of APC and K-raS gene mutation in the spectrum of colitis-associated neoplasias. Gastroenterology. 1995;108:383–392. doi: 10.1016/0016-5085(95)90064-0. https://doi.org/10.1016/0016-5085(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 10.Fishel R, Lescoe MK, Rao MRS, et al. The human mutator gene homolog msh2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. https://doi.org/10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 11.Levine AJ. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. https://doi.org/10.1146/annurev.bi.62.070193.003203 PMid:8394683. [DOI] [PubMed] [Google Scholar]
- 12.Page`s F, Galon J, Dieu-Nosjean M-C, Tartour E, Saute`s-Fridman C, Fridman W-H. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. https://doi.org/10.1038/onc.2009.416 PMid:19946335. [DOI] [PubMed] [Google Scholar]
- 13.Pаge's F, Berger A, Camus M, et al. (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. New England J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. https://doi.org/10.1056/NEJMoa051424 PMid:16371631. [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predicts clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. https://doi.org/10.1126/science.1129139 PMid:17008531. [DOI] [PubMed] [Google Scholar]
- 15.Di Caro G, Marchesi F, Laghi L, Grizzi F. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med. 2013;17(9):1088–109. doi: 10.1111/jcmm.12117. https://doi.org/10.1111/jcmm.12117 PMid:24151976 PMCid: PMC4118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.aQiang Zhou, Rui-Qing Peng, Xiao-Jun Wu, Qing Xia, Jing-Hui Hou, Ya Ding, Qi-Ming Zhou, Xing Zhang, Zhi-Zhong Pang, De-Sen Wan, Yi-Xin Zeng, Xiao-Shi Zhang. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. Journal of Translational Medicine. 2010;8:13. doi: 10.1186/1479-5876-8-13. https://doi.org/10.1186/1479-5876-8-13 PMid:20141634 PMCid: PMC2841127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valerie C. Han Chong T, Jean-Pierre A. Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. Journal of Oncology. 2012;2012:608406. doi: 10.1155/2012/608406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzivenu KO, O'Donnell-Tormey J. Cancer and the immune system: The Vital Connection. Cancer Research Institute. 2003 [Google Scholar]
- 19.Dieu-Nosjean MC, Goc J, Girlado NA, et al. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–80. doi: 10.1016/j.it.2014.09.006. https://doi.org/10.1016/j.it.2014.09.006 PMid:25443495. [DOI] [PubMed] [Google Scholar]
- 20.Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology. 2010;6:2059–2072. doi: 10.1053/j.gastro.2009.12.065. https://doi.org/10.1053/j.gastro.2009.12.065 PMid:20420946 PMCid: PMC4243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pages F, Berger A, Camus M, Sanchez Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc P, Trajanoski Z, Fridman W, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;25:2654–2666. doi: 10.1056/NEJMoa051424. https://doi.org/10.1056/NEJMoa051424 PMid:16371631. [DOI] [PubMed] [Google Scholar]
- 22.Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;4:981–987. doi: 10.1189/jlb.1107773. https://doi.org/10.1189/jlb.1107773 PMid:18559950. [DOI] [PubMed] [Google Scholar]
- 23.Shunyakov L, Ryan CK, Sahasrabudhe DM, Khorana AA. The influence of host response on colorectal cancer prognosis. Clin Colorectal Cancer. 2004;1:38–45. doi: 10.3816/ccc.2004.n.008. https://doi.org/10.3816/CCC.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 24.Loose D, Van de Wiele C. The immune system and cancer. Cancer Biother Radiopharm. 2009;3:369–376. doi: 10.1089/cbr.2008.0593. https://doi.org/10.1089/cbr.2008.0593 PMid:19538060. [DOI] [PubMed] [Google Scholar]
- 25.Watson NF, Ramage JM, Madjd Z, Spendlove I, Ellis IO, Scholefield JH, Durrant LG. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;1:6–10. doi: 10.1002/ijc.21303. https://doi.org/10.1002/ijc.21303 PMid:16003753. [DOI] [PubMed] [Google Scholar]
- 26.Titu LV, Monson JR, Greenman J. The role of CD8(+) T cells in immune responses to colorectal cancer. Cancer Immunol Immunother. 2002;5:235–247. doi: 10.1007/s00262-002-0276-4. https://doi.org/10.1007/s00262-002-0276-4 PMid:12070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson EA, Bleackley RC. Mechanisms of lysis by cytotoxic T cells. Crit Rev Immunol. 1995;3–4:359–38438. doi: 10.1615/critrevimmunol.v15.i3-4.90. https://doi.org/10.1615/CritRevImmunol.v15.i3-4.90. [DOI] [PubMed] [Google Scholar]
- 28.Nelson BH. CD20+B Cells: The Other Tumor-Infiltrating Lymphocytes. J Immunol. 2010;185(9):4977–4982. doi: 10.4049/jimmunol.1001323. https://doi.org/10.4049/jimmunol.1001323 PMid:20962266. [DOI] [PubMed] [Google Scholar]
- 29.Bergomas F, Grizzi F, Doni A, Pesce S, Laghi L, Allavena P, Mantovani A, Marchesi F. Tertiary Intratumor Lymphoid Tissue in Colo-Rectal Cancer. Cancers (Basel) 2012;4(1):1–10. doi: 10.3390/cancers4010001. https://doi.org/10.3390/cancers4010001 PMid:24213222 PMCid: PMC3712686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51:364–371. doi: 10.1136/gut.51.3.364. https://doi.org/10.1136/gut.51.3.364 PMid:12171958 PMCid: PMC1773345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. https://doi.org/10.1084/jem.20080752 PMid:19139167 PMCid: PMC2626665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;3:318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. https://doi.org/10.1002/(SICI)1096-9896(199707)182:3<318:: AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;5795:1960–1964. doi: 10.1126/science.1129139. https://doi.org/10.1126/science.1129139 PMid:17008531. [DOI] [PubMed] [Google Scholar]
- 34.Deschoolmeester V, Baay M, Lardon F, Pauwels P, Peeters M. Immune Cells in Colorectal Cancer: Prognostic Relevance and Role of MSI. Cancer Microenvironment. 2011;4:377–392. doi: 10.1007/s12307-011-0068-5. https://doi.org/10.1007/s12307-011-0068-5 PMid:21618031 PMCid: PMC3234325. [DOI] [PMC free article] [PubMed] [Google Scholar]


