Abstract
INTRODUCTION:
The detection of estrogen, progesterone and HER-2 neu receptors on the surface of the tumour cell is a significant prognostic factor, alone or in combination. The presence or absence of receptors on the surface of the tumour cell is associated with the conditional gene expression in the tumour cell itself. Based on these genetically determined expressions of the tumour cell, five molecular subtypes of breast cancer have been classified on the St. Gallen International Expert Consensus in 2011 that can be immunohistochemically detected, with each subtype manifesting certain prognosis and aggression.
AIM:
Analyzing the presentation of molecular subtypes of breast cancer that are immunohistochemically detected in surgically treated patients at the Clinic for Thoracic and Vascular Surgery.
MATERIAL AND METHODS:
We used the international classification on molecular subtypes of breast cancer which divides them into: Luminal A (ER+ and/or PR+, HER-2 negative, Ki-67 < 14%), Luminal B with HER-2 negative (ER+ and/or PR+, HER-2 negative, Ki-67 ≥ 14%), Luminal B with HER-2 positive (ER+ and/or PR+, HER-2+, any Ki-67), HER-2 enriched (ER-, PR-, HER-2+), and basal-like (triple negative) (ER-, PR-, HER-2 negative, CK5/6+ and/or EGFR+). A total of 290 patients, surgically treated for breast cancer, were analysed during 2014.
RESULTS:
In our analysis, we found that Luminal A was present in 77 (26.55%) patients, Luminal B HER-2 negative was present in 91 (31.38%) patients, Luminal B HER-2 positive was present in 70 (24.14%) patients, HER-2 enriched was present in 25 (8.62%) patients and basal-like (or triple negative) was present in 27 (9.31%) patients.
CONCLUSION:
Detecting the subtype of breast cancer is important for evaluating the prognosis of the disease, but also for determining and providing an adequate therapy. Therefore, determining the subtype of breast cancer is necessary for the routine histopathological assay.
Keywords: Subtypes of breast cancer, Luminal A, Luminal B HER-2 negative, Luminal B HER-2 positive, HER-2 enriched, Triple-negative
Introduction
Breast cancer is the most frequent malignant disease among women worldwide, but also in the Republic of Macedonia [1] [2] [3] [4]. Also, breast cancer is the leading cause of cancer mortality [1] [2] [3] [4]. Today, many factors are listed as most important ones for determining the prognosis of breast cancer, like tumour size, histologic subtype, tumour grade, lymphovascular invasion of tumour cells and axillary lymph node status. However, the presence of hormonal receptors (estrogen and progesterone) on the surface of the tumour cell, the presence of HER-2 neu receptor and other factors have been added to this list in the last twenty years [5]. Also, the biological potential for proliferation and dividing was routinely examined, represented as Ki67 value. Today, every patient that is surgically treated undergoes routine examination with standard macroscopic and microscopic histological analysis, TNM staging, staging by immunohistochemical recognition of estrogen receptors (ER), progesterone receptors (PR), the presence of HER-2 neu receptors, and the prognostic value of Ki67 [6]. Each of these parameters, alone or in combination, determines the biology and aggression of the tumour, but also gives us the opportunity to treat the given type of breast cancer properly. The combination of these parameters gives us the opportunity to determine the genetic subtype of breast cancer. According to the new classification system for breast cancer subtypes presented in St. Gallen, which we use, breast cancer is divided in Luminal A, Luminal B with HER2 negative, Luminal B with HER2 positive, HER2 enriched and basal-like (triple negative) [7].
Analyzing the presence of breast cancer subtypes in our materials, and comparing the results with other studies to see if some subtypes in our materials differ from other studies published before, also, determining if the subtypes and the clinical stage are somehow correlated.
Material and Methods
A total of 290 patients, who were surgically treated for breast cancer at the University Clinic for Thoracic Surgery, Skopje, Republic of Macedonia were analysed during 2014, with complete history, using all parameters.
All cases underwent standard histological examination, including macroscopic and microscopic analysis with standard H&E staining. Immunohistochemical staining for ER, PgR, HER-2 and Ki-67 were performed on sections of formalin-fixed paraffin-embedded tissue from the primary tumours. Pathohistological tests were conducted in three accredited laboratories (two in the Institute of Pathology at the Medical Faculty in Skopje and one in a private laboratory).
Upon microwave-pretreated in citric acid (10-mM), monoclonal mouse antibody to ER, PgR, HER-2 or Ki-67 was applied for 30-min at room temperature using the following dilutions: anti-ER-1-100 :, anti-PgR-1-80 (DAKO laboratories, UK); pre-diluted anti-HER-2 (Hercept test, DAKO Laboratories, UK); anti-Ki-67-1-200 (DAKO Laboratories, UK). Upon three rinses in Tris-buffered saline (TBS) and incubation with the secondary antibody, positive brown staining was detected using standard avidin and biotinylated horseradish peroxidase (ABC) technique with 3, 3’-diaminobenzidine (DAB) as the chromogen. Slides were then counterstained in Mayer’s haematoxylin for 10 seconds, dehydrated in graded alcohol, mounted and scored.
Positive and negative controls were performed with each stain, and surgical specimens from the same patient were stained on the same run.
For persistence of estrogen and progesterone receptors were included all results with +, ++ or +++ on immunohistochemical examination. For persistence of HER-2 receptors were included all patients with +++ result on immunohistochemical analysis.
In cases where ICT determined HER-2 neu positive status + or ++ patients underwent FISH analyses for defining the HER2-neu gene amplification status.
Pathohistological, grading and staging criteria for breast cancer were determined by using criteria from American Join Committee (AJC) and TNM classification according to UICC (International Union for Cancer Control) [8] [9]. According to the new classification system for breast cancer subtypes presented in St. Gallen, which we use, breast cancer is divided in Luminal A, Luminal B with HER2 negative, Luminal B with HER2 positive, HER2 enriched and basal-like (triple negative) [7].
Statistical analysis was performed with Statistica 7 by using standard descriptive analyses, χ2 test and ANOVA test for analysing the variance.
Results
Patient’s age was ranged between 18-90 years, an average of 57.6 years. The mean size of a primary tumour was 30.27 + 18.3 mm. Axillary lymph nodes metastases were detected in 59% of the patients.
We used the new St. Gallen classification system for defining breast cancer subtypes into five groups. (Table 1) [7].
Table 1.
Definition of subtypes of breast cancer- St. Gallen classification
| Subtypes of breast cancer | Er and Pr | Her-2 | Ki67 |
|---|---|---|---|
| Luminal a | Er + and/or pr + | Her-2- | Ki67<14% |
| Luminal b with her-2 negative | Er+ and/or pr+ | Her-2- | Ki-67≥14% |
| Luminal b with her-2 positive | Er + and / or pr + | Her-2 + | Any ki-67 |
| Her-2 enriched | Er-, pr- | Her-2 + | Any ki-67 |
| Basal-like (triple negative) | Er-, pr- | Her-2- | Ck5/6 + and/or egfr + |
Subtypes are characterised based on tumour size, lymph nodes involvement, histologic subtype, the persistence of receptors, lymphovascular invasion, the presence of p53 and stage, and are presented in Tables 2-9.
Table 2.
Characteristics of subtypes according to the age of the patients
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | ||
|---|---|---|---|---|---|---|---|
| Number (%) | 77 (26.55%) | 91 (31.38%) | 70 (24.14%) | 25 (8.62%) | 27 (9.31%) | 290 (100%) | |
| Mean age (y) | 57.83 | 58.83 | 57.64 | 552.72 | 56.74 | 57.56 | X=57.6 y |
Table 3.
Characteristics of subtypes according to the size of the tumour
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | p | |
|---|---|---|---|---|---|---|---|
| Tumour size | |||||||
| Tis | 6 | 2 | 3 | 2 | 0 | 13 (4.48%) | |
| T1a | 11 | 11 | 12 | 1 | 2 | 37 (12.76%) | |
| T1b | 8 | 3 | 3 | 1 | 1 | 16 (5.51%) | |
| T1c | 9 | 17 | 12 | 7 | 3 | 48 (16.55%) | |
| T2 | 37 | 43 | 36 | 9 | 18 | 143 (49.31%) | |
| T3 | 3 | 6 | 2 | 2 | 2 | 15 (5.17%) | |
| T4 | 3 | 9 | 2 | 3 | 1 | 18 (6.19%) | 1.0 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 |
Table 4.
Characteristics of subtypes according to the size of a tumour and lymph nodes involvement
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | p | |
|---|---|---|---|---|---|---|---|
| Mean tumor size (mm) Axillary LN status | 29.3 | 31.8 | 27.2 | 31.3 | 35.0 | X = 30.3 | |
| N0 | 41 | 28 | 26 | 14 | 10 | 119 (41.03%) | |
| N+ | 36 | 63 | 44 | 11 | 17 | 171 (58.97%) | 0.99 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 |
Table 5.
Characteristics of subtypes according to histologic subtype
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | p | |
|---|---|---|---|---|---|---|---|
| Histologic subtype | |||||||
| Ductal | 59 | 76 | 58 | 20 | 24 | 237(81.72%) | |
| Lobular | 11 | 6 | 7 | 2 | 1 | 27 (9.31%) | |
| Other | 7 | 9 | 5 | 3 | 2 | 26 (8.97%) | 0.99 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 |
Table 6.
Characteristics of subtypes according to histological grade
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | p | |
|---|---|---|---|---|---|---|---|
| Histologic grade | |||||||
| 1 | 6 | 3 | 4 | 0 | 3 | 16 (5.52%) | |
| 2 | 58 | 56 | 49 | 19 | 20 | 202 (69.65%) | |
| 3 | 13 | 32 | 17 | 6 | 4 | 72 (24.82%) | 0.99 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 |
Table 7.
Characteristics of subtypes according to the persistence of receptors (estrogen, progesterone and HER2 neu)
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | p | |
|---|---|---|---|---|---|---|---|
| Estrogen receptors | |||||||
| Positive | 72 | 86 | 57 | 0 | 0 | 215 (74.14%) | |
| Negative | 5 | 5 | 13 | 25 | 27 | 75 (25.86%) | 1.0 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 | |
| Progesterone receptors | |||||||
| Positive | 73 | 88 | 65 | 0 | 0 | 226 (77.93%) | |
| Negative | 4 | 3 | 5 | 25 | 27 | 64 (22.07%) | 1.0 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 | |
| Her 2 neu receptors | |||||||
| Positive | 0 | 0 | 70 | 25 | 0 | 95 (32.76%) | |
| Negative | 77 | 91 | 0 | 0 | 27 | 195 (67.24%) | 1.0 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 |
Table 8.
Characteristics of subtypes according to the persistence of p53, LVI (lymphovascular invasion) and values of Ki67
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | p | |
|---|---|---|---|---|---|---|---|
| P53 | |||||||
| Positive | 27 | 49 | 29 | 11 | 15 | 131(45.18%) | |
| Negative | 50 | 42 | 41 | 14 | 12 | 159 (54.82%) | 0.99 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 | |
| LVI | |||||||
| Positive | 22 | 45 | 27 | 11 | 12 | 117 (40.34%) | |
| Negative | 55 | 46 | 43 | 14 | 15 | 173 (59.65%) | 0.99 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 | |
| Ki67 | |||||||
| Up to 14% | 77 | 0 | 36 | 4 | 9 | 126 (43.44%) | |
| More than 14% | 0 | 91 | 34 | 21 | 18 | 164 (56.56%) | 1.0 |
| Number | 77 | 91 | 70 | 25 | 27 | 290 |
Table 9.
Characteristics of subtypes according to the stage of the disease
| LA | LB-Her2- | LB-Her2+ | HER2+ | TN | Total | p | |
|---|---|---|---|---|---|---|---|
| Stage | |||||||
| 0 | 1 | 2 | 0 | 0 | 0 | 3 (1.03%) | |
| IA | 16 | 6 | 11 | 7 | 3 | 43 (14.83%) | |
| IB | 3 | 4 | 2 | 0 | 0 | 9 (3.10%) | |
| IIA | 26 | 26 | 18 | 6 | 7 | 83 (28.62%) | |
| IIB | 13 | 15 | 18 | 6 | 8 | 60 (20.69%) | |
| IIIA | 7 | 17 | 13 | 2 | 4 | 43 (14.83%) | |
| IIIB | 4 | 5 | 2 | 2 | 2 | 15 (5.17%) | |
| IIIC | 7 | 16 | 6 | 2 | 3 | 34 (11.72%) | 1. |
| Number | 77 | 91 | 70 | 25 | 27 | 290 |
From our analysis, we found that:
- Luminal A was present in 77 (26.55%),
- Luminal B HER2 negative was present in 91 (31.38%),
- Luminal B HER2 positive was present in 70 (24.14%),
- HER2 enriched was present in 25 (8.62%) and
- Basal-like (or triple negative) was present in 27 (9.31%) patients.
Discussion
Breast carcinoma is a heterogeneous disease with several clinical and histopathological presentations, which present different gene expressions in several subtypes and molecular profiles, hence giving different predictive and prognostic characteristics for the patients. Gene expressions were analysed using DNA microarrays. Due to the cost of DNA analysis, the use of immunohistochemical analysis of markers, which have been used as surrogate tools for defining subtypes of breast cancer, was generally accepted. According to the 2011 St. Gallen consensus conference, 5 subtypes of breast cancer were defined using the presence of receptors on the surface of the tumour cell, and the measuring values of Ki67 [7] [10] [11].
Many of the prognostic factors predicting the disease are very well-known, and so is their biological mode of action and how they work to spread the disease in the body. Estrogen receptors are on the surface of the tumour cell, so once estrogen binds with the estrogen receptors, it activates many processes in the cell and stimulates growth and cell division. Hence, estrogen stimulates tumour growth. Giving drugs that block estrogen receptors or drugs that block estrogen synthesis can stop the tumour growth.
The same situation applies to the presence of HER-2 neu receptors. HER-2 is a membrane tyrosine kinase and oncogene that is overexpressed and gene amplified in about 20% of the breast cancer cases. When activated it provides the cell with potent proliferative and anti-apoptosis signals, and it is the major driver of tumour development and progression of breast cancer.
Overexpression will activate many pathways in the cell, resulting in uncontrolled cell growth and division, causing the tumour to grow uncontrollably. Target drug delivery, monoclonal antibody trastuzumab (Herceptin), will block these receptors, and control a tumour. Moreover, giving chemotherapeutics that interact with the rapidly dividing cells will control a tumour. Ki67 is the factor that shows the proliferative activity of tumour cells. Ki67 correlates with the S phase of the cell cycle and with the mitotic activity. A normal breast cell has a proliferative activity of 3% (3% of the cells are in the dividing stage).
Higher Ki67 index correlates with young age, larger tumours, positive lymph nodes, negative estrogen receptors and positive HER-2 receptors [12]. An activity that is higher than 14%, at some studies large than 20%, shows aggressive tumours with poor prognosis and shorter overall survival [10] [11] [12].
Knowing the subtype:
- we can predict the biology of a tumour and its future behaviour;
- we can predict the prognosis of the disease;
- we can plan a targeted therapy for some of the subtypes.
Knowing the prevalence of subtypes in one population can help plan a general therapeutic approach [16].
Some authors define 4 subtypes: Luminal A, Luminal B, HER-2 enriched and basal cell (triple negative) (Valejos, Carey), other authors define 6 subtypes: Luminal A, Luminal B, HER2+, basal-like (triple negative), normal breast cell-like and Claudin-low (Eroles), but the most frequently used classification encompasses 5 subtypes [7] [13] [14] [17]. In practice, breast subtypes are defined by detecting the presence of estrogen, progesterone and HER-2 neu receptors on the surface of the malignant cell using immunohistochemical assays. Knowing that the presence or absence of receptors on the surface of breast cancer cell is conditioned by gene mutations and overexpression, subtypes can also be detected by assessing the gene expression. This is why the term genotype of breast cancer is cited in the literature.
The most frequent type is Luminal A which is found in 50-72% of the patients with breast cancer. Patients with this type of cancer have the best prognosis, i.e. low proliferative index, good differentiation, with the lowest risk of local recurrence and relapse [13] [15] [16] [17] [18].
However, there are different values registered in literature regarding this subtype: Italy 34%, Saudi Arabia 3.9%, China 65.3% and Japan 71% [24] [25] [26] [27].
The suggested therapy for these patients is third-generation aromatase inhibitors in postmenopausal women, selective estrogen receptor modulators (like tamoxifen) and selective estrogen receptor modulators (like fulverstone) [13] [15] [16] [17] [18].
In our examination, Luminal A type was detected only in 26.55% of the patients.
Luminal B subtype is characterised with positive estrogen receptors, with positive or negative HER2 receptors, with a higher Ki-67 value of over 14%, which in both types of Luminal B gives worse prognosis than Luminal A subtype.
Luminal B type is found in 10-20% of the patients with breast cancer, and in our examination, the HER-2 negative was detected in 31.38% and HER-2 positive in 24.14 % of the patients. Literature references are as follows: Italy 36%, Egypt 24.6% [24] [28]. This shows that most of the tumours in our group are aggressive. Luminal B subtype is much more aggressive than the Luminal A subtype and is characterised by poor differentiation, more frequent bone metastases and with a worse prognosis. Many authors suggest that patients in this subtype are younger patients with bigger tumours, with positive nodal status and higher N stage [16]. Given the presence of larger tumours and the advanced stage of the disease, Luminal B findings are more frequent in our study. Regarding patient’s age, there was no difference found between Luminal A and Luminal B types; however, in our study, Luminal B prevailed in older patients [13] [15] [16] [17] [18].
The suggested treatment is with tamoxifen, but also chemotherapy in neoadjuvant and adjuvant courses [13] [15] [16] [17] [18].
HER-2 enriched subtype is found in 15-20% of the patients. In our group, it was detected in 8.62% of the patients. This subtype is characterized by high proliferative index and poor differentiation in most of the patients, and p53 mutations are very often detected. This is a very aggressive type of a tumour, with only 12% of the patients surviving 10 years [13] [14] [16] [17] [18].
The suggested treatment requires target HER-2 therapy with monoclonal antigen trastuzumab (Herceptin) which changes the prognosis. This therapy needs to be combined with chemotherapy in the neoadjuvant and adjuvant protocol. Treatment with trastuzumab in combination with DM1 is also possible [13] [15] [16] [17] [18].
Subtype Basal-like (triple negative) is characterised with larger tumours, poor differentiation, high mitotic index and tumour necrosis. This type is found in 10-20% of the patients. A similar subgroup of this subtype is Claudin-low, where the only differentiation is the difference in EGFR and the fact that this type is found in 12-14% of the patients. Both subgroups have bad prognosis, poor differentiation and high mitotic index. Very often metastases in visceral organs, lungs and CNS are detected. This type has the worst prognosis, and very often the disease relapses in the first three years, and p53 mutations are very often detected [13] [14] [15] [16] [17] [18] [34].
In our study, the triple negative was detected in 9.31% of the cases.
The suggested therapy is chemotherapy, but also PARP inhibitors (poly ADP ribosome polymerase inhibitors) like olaparib in detected BRCA1 or BRCA2 mutations [14] [15].
The normal breast cell-like subtype that is defined by some authors is found in 5-10% of the patients. This type doesn’t respond to neoadjuvant therapy, only to adjuvant chemotherapy protocol. Often, it is well-differentiated with low proliferation index and is characterised by median overall survival [14].
We registered the difference in frequencies among our subgroups and those cited in the literature. This explains the heterogeneity of breast cancer across the world. It is cited in the literature that some subtypes are more frequent in certain races (triple negative is more frequent in African-American women) [13] [19]. Knowing the prevalence of subtypes in one population, we can plan a general therapeutic approach [16].
There is no significant difference in age regarding subgroups of breast cancer, patients range between 52,72 and 58,83 years, except in Luminal B HER negative and HER enriched subgroups (P = 0.0255). Patient’s age in subgroups of breast cancer is shown in Figure 1.
Figure 1.
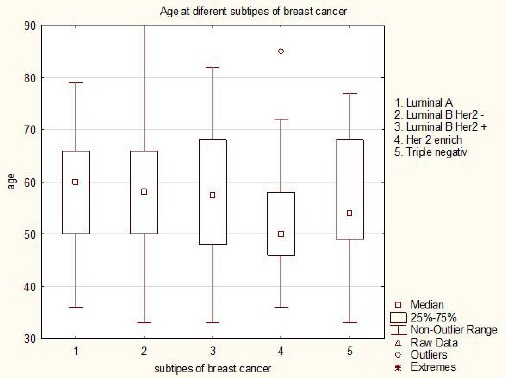
Patients age according to subgroups of breast cancer
The mean size of tumours in different subgroups ranges between 27.18 and 35 mm. The biggest tumour diameter was found in the triple negative subgroup, but there were no significant differences in the whole group, and also between subgroups. The same situation applies to the tumour diameter defined as T stage with no difference in the position between subgroups.
High differentiation values (G3-low differentiation) of the malignant cell in Luminal B subtype of breast cancer were detected in 35.16% of the patients. In triple negative subtype, G3 was detected only in 14.8% of the patients, which is contrary to the case results reported in many studies in the literature [13] [14] [17].
Regarding lymphovascular invasion, the highest values were detected in Luminal B HER-2 negative, i.e. in 49.45% of the patients, which suggested invasive and aggressive subtype of breast cancer, with a tendency for lymphatic spread and higher frequency of axillary lymph node involvement (69.23%). The relatively low values of lymphovascular invasion in triple negative subtype are interesting to comment (44.44%), with low axillary lymph node involvement (62.96%), which correlates with the findings in the literature. Knowing the aggression of triple negative subtype of breast cancer, we can conclude that the spreading of malignant cells follows other pathways other than lymphatic [14] [17] [30] [31] [32] [33] [34] [35].
Regarding the values of Ki67, the factor that suggests the proliferative activity of the tumour, its aggression and biology, values higher than 14% were detected in Luminal B HER negative subtype (in all patients), but also in HER-2 enriched and triple-negative subtypes. On the contrary, no values higher than 14% were detected in Luminal A subtype, which suggests that this subtype is less aggressive [13] [14] [15] [16] [17] [18].
The patterns of distribution of cells positive for estrogen, progesterone and HER-2 neu receptors were significantly different in subgroups, which is normal because we know that these receptors are main factors for defining the different subtypes of breast cancer [7] [14].
Knowing the subtype of breast cancer, in addition to the histological type and TNM stage, can suggest further prognosis of the disease, detect the spreading and find where metastases can appear later, but can also suggest further therapeutic approach [15].
Also knowing that all factors that determine breast cancer subtypes can be evaluated from core biopsy materials before starting treatment, some subtypes (the more aggressive ones) can be treated with adequate drugs in neoadjuvant protocol [14].
In conclusion, detecting the subtype of breast cancer is important for disease prognosis, but also for determining and providing an adequate therapy. Hence, the molecular subtype of breast cancer needs to be determined in a routine histopathological assay.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Justo N, Wilking N, Jönsson B, Luciani S, Cazap E. A Review of Breast Cancer Care and Outcomes in Latin America. Oncologist. 2013;18(3):248–256. doi: 10.1634/theoncologist.2012-0373. https://doi.org/10.1634/theoncologist.2012-0373 PMid:23442305 PMCid: PMC3607519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhikoo R, Srinivasa S, Tzu-Chieh Yu, Moss D, Hill G A. Systematic Review of Breast Cancer Biology in Developing Countries (Part 1): Africa, the Middle East, Eastern Europe, Mexico, the Caribbean and South America. Cancers (Basel) 2011;3(2):2358–2381. doi: 10.3390/cancers3022358. https://doi.org/10.3390/cancers3022358 PMid:24212814 PMCid: PMC3757422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac J Cancer Prev. 2016;17(Spec No):43–6. doi: 10.7314/apjcp.2016.17.s3.43. [DOI] [PubMed] [Google Scholar]
- 4.Global cancer observatory. www.gco.iarc.fr. [Google Scholar]
- 5.Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, Díez M, Viladot M, Arance A, Mu-oz M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–35. doi: 10.1016/j.breast.2015.07.008. https://doi.org/10.1016/j.breast.2015.07.008 PMid:26253814. [DOI] [PubMed] [Google Scholar]
- 6.Bagaria SP, Ray PS, Sim MS, Ye X, Shamonki JM, Cui X, Giuliano AE. Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg. 2014;149(2):125–9. doi: 10.1001/jamasurg.2013.3181. https://doi.org/10.1001/jamasurg.2013.3181 PMid:24306257. [DOI] [PubMed] [Google Scholar]
- 7.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ. Panel members- Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Annals of Oncology. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. https://doi.org/10.1093/annonc/mdt303 PMid:23917950 PMCid: PMC3755334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. doi: 10.1245/s10434-010-0985-4. https://doi.org/10.1245/s10434-010-0985-4 PMid:20180029. [DOI] [PubMed] [Google Scholar]
- 9.Uehiro N, Horii R, Iwase T, Tanabe M, Sakai T, Morizono H, Kimura K, Iijima K, Miyagi Y, Nishimura S, Makita M, Ito Y, Akiyama F. Validation study of the UICC TNM classification of malignant tumors, seventh edition, in breast cancer. Breast Cancer. 2014;21(6):748–53. doi: 10.1007/s12282-013-0453-7. https://doi.org/10.1007/s12282-013-0453-7 PMid:23435963. [DOI] [PubMed] [Google Scholar]
- 10.Benis S, Abbass F, Akasbi Y, Znati K, Joutel KA, Mesbahi O, Amarati A. Prevalence of molecular subtypes and prognosis of invasive breast cancer in north-east of Morocco. BMC Reasrch notes. 2012;5:436. doi: 10.1186/1756-0500-5-436. https://doi.org/10.1186/1756-0500-5-436 PMid:22889054 PMCid: PMC3532150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Rodríguez G. Prevalence of breast cancer sub-types by immunohistochemistry in patients in the Regional General Hospital 72, Instituto Mexicano del Seguro Social. Cir Cir. 2015;83(3):193–8. doi: 10.1016/j.circir.2015.05.003. https://doi.org/10.1016/j.circen.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Inic Z, Zegarac M, Inic M, Markovic I, Kozomara Z, Djurisic I, Inic I, Pupic G, Jancic S. Difference between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin Med Insights Oncol. 2014;8:107–11. doi: 10.4137/CMO.S18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams C, Chin-Yo Lin. Oestrogen receptors in breast cancer: basic mechanisms and clinical implicastions. Ecancermedicalscience. 2013;7:370. doi: 10.3332/ecancer.2013.370. PMid:24222786 PMCid: PMC3816846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez C, Schiff R. HER 2: Biolgy, Detection, and Clinical Implications. Arch Pathol Lab Med. 2011;135(1):55–62. doi: 10.1043/2010-0454-RAR.1. PMid:21204711 PMCid: PMC3242418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Diest PJ, van der Wall E, Baak JPA. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol. 2004;57(7):675–681. doi: 10.1136/jcp.2003.010777. https://doi.org/10.1136/jcp.2003.010777 PMid:15220356 PMCid: PMC1770351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashmi AA, Aijaz S, Khan SM, Mahboob R, Irfan M, Zafar NI, Nisar M, Siddiqui M, Edhi MM, Faridi N, Khan A. Prognostic parameters of luminal A and luminal B intrinsic breast cancer subtypes of Pakistani patients. World J Surg Oncol. 2018;16(1):1. doi: 10.1186/s12957-017-1299-9. https://doi.org/10.1186/s12957-017-1299-9 PMid:29291744 PMCid: PMC5749004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. https://doi.org/10.1158/1078-0432.CCR-06-1109 PMid:17438091. [DOI] [PubMed] [Google Scholar]
- 18.Vallejos CS, Gómez HL, Cruz WR, Pinto JA, Dyer RR, Velarde R, Suazo JF, Neciosup SP, León M, de la Cruz MA, Vigil CE. Breast cancer classification according to immunohistochemistry markers: subtypes and association with clinicopathologic variables in a peruvian hospital database. Clin Breast Cancer. 2010;10(4):294–300. doi: 10.3816/CBC.2010.n.038. https://doi.org/10.3816/CBC.2010.n.038 PMid:20705562. [DOI] [PubMed] [Google Scholar]
- 19.Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treatment Reviews. 2012;38(6):698–707. doi: 10.1016/j.ctrv.2011.11.005. https://doi.org/10.1016/j.ctrv.2011.11.005 PMid:22178455. [DOI] [PubMed] [Google Scholar]
- 20.Liedtke C, Kiesel L. Breast cancer molecular subtypes- Modern therapeutic concepts for targeted therapy of a heterogeneous entity. Maturitas. 2012;73(4):288–294. doi: 10.1016/j.maturitas.2012.08.006. https://doi.org/10.1016/j.maturitas.2012.08.006 PMid:23020990. [DOI] [PubMed] [Google Scholar]
- 21.Del Casar JM, Martin A, Garcia C, Corte MD, Alvarez A, Junquera S, Gonzalez LO, Bongera M, Garcia-Muniz JL, Allende MT, Vizoso F. Characterization of breast cancer subtypes by quantitative assessment of biological parameters: Relationship with clinicopathological characteristics, biological features and prognosis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2008;141(2):147–152. doi: 10.1016/j.ejogrb.2008.07.021. https://doi.org/10.1016/j.ejogrb.2008.07.021 PMid:18768247. [DOI] [PubMed] [Google Scholar]
- 22.Morrow M. Personalizing extent of breast cancer surgery according to molecular subtypes. Breast. 2013;22:S106–S109. doi: 10.1016/j.breast.2013.07.020. https://doi.org/10.1016/j.breast.2013.07.020 PMid:24074769. [DOI] [PubMed] [Google Scholar]
- 23.García Fernández A, Giménez N, Fraile M, González S, Chabrera C, Torras T, González C, Salas A, Barco I, Cirera L, Cambra MJ, Veloso E. Survival and clinicopathological characteristics of breast cancer patient according to different tumour subtypes as determined by hormone receptor and Her2 immunohistochemistry. A single institution survey spanning 1998 to 2010. Breast. 2012;21(3):366–373. doi: 10.1016/j.breast.2012.03.004. https://doi.org/10.1016/j.breast.2012.03.004 PMid:22487206. [DOI] [PubMed] [Google Scholar]
- 24.Caldarella A, Buzzoni C, Crocetti E, Bianchi S, Vezzosi V, Apicella P, Biancalani M, Giannini A, Urso C, Zolfanelli F, Paci E. Invasive breast cancer: a significant correlation between histological types and molecular subgroups. J Cancer Res Clin Oncol. 2013;139(4):617–623. doi: 10.1007/s00432-012-1365-1. https://doi.org/10.1007/s00432-012-1365-1 PMid:23269487. [DOI] [PubMed] [Google Scholar]
- 25.Al Tamimi DM, Shawarby MA, Ahmed A, Hassan AK, AlOdaini AA. Protein expression profile and prevalence pattern of the molecular classes of breast cancer—a Saudi population based study. BMC Cancer. 2010;10(1):223. doi: 10.1186/1471-2407-10-223. https://doi.org/10.1186/1471-2407-10-223 PMid:20492711 PMCid: PMC2880995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Ying J, Wang F, Wang J, Yang H. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status in invasive breast cancer: a 3,198 cases study at National Cancer Center, China. Breast Cancer Res Treat. 2014;147(3):551–555. doi: 10.1007/s10549-014-3136-y. https://doi.org/10.1007/s10549-014-3136-y PMid:25234844. [DOI] [PubMed] [Google Scholar]
- 27.Shibuta K, Ueo H, Furusawa H, Komaki K, Rai Y, Sagara Y, Kamada Y, Tamaki N. The relevance of intrinsic subtype to clinicopathological features and prognosis in 4,266 Japanese women with breast cancer. Breast Cancer. 2011;18(4):292–298. doi: 10.1007/s12282-010-0209-6. https://doi.org/10.1007/s12282-010-0209-6 PMid:20571962. [DOI] [PubMed] [Google Scholar]
- 28.Salhia B, Tapia C, Ishak EA, Gaber S, Berghuis B, Hussain KH, DuQuette RA, Resau J, Carpten J. Molecular subtype analysis determines the association of advanced breast cancer in Egypt with favorable biology. BMC Womens Health. 2011;11(1):44. doi: 10.1186/1472-6874-11-44. https://doi.org/10.1186/1472-6874-11-44 PMid:21961708 PMCid: PMC3204283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kan C, Patel RB, Biswas T. Distribution of Newly Diagnosed Breast Cancers by Subtype and Race Using the SEER Database. International Journal of Radiation Oncology, Biology, Physics. 2015;93(3):E24–E24. https://doi.org/10.1016/j.ijrobp.2015.07.605. [Google Scholar]
- 30.Marrazzo A, Boscaino G, Marrazzo E, Taormina P, Toesca A. Breast cancer subtypes can be determinant in the decision making process to avoid surgical axillary staging: A retrospective cohort study. International Journal of Surgery. 2015;21:156–161. doi: 10.1016/j.ijsu.2015.07.702. https://doi.org/10.1016/j.ijsu.2015.07.702 PMid:26253849. [DOI] [PubMed] [Google Scholar]
- 31.Crabb SJ, Cheang MCU, Leung S, Immonen T, Nielsen TO, Huntsman DD, Bajdik CD, Chia SK. Basal Breast Cancer Molecular Subtype Predicts for Lower Incidence of Axillary Lymph Node Metastases in Primary Breast Cancer. Clinical Breast Cancer. 2008;8(3):249–256. doi: 10.3816/CBC.2008.n.028. https://doi.org/10.3816/CBC.2008.n.028 PMid:18650155. [DOI] [PubMed] [Google Scholar]
- 32.Van Calster B, Vanden Bempt I, Drijkoningen M, Pochet N, Cheng J, Van Huffel S, Hendrickx W, Decock J, Huang HJ, Leunen K, Amant F, Berteloot P, Paridaens R, Wildiers H, Van Limbergen E, Weltens C, Timmerman D, Van Gorp T, Smeets A, Van den Bogaert W, Vergote I, Christiaens MR, Neven P. Axillary lymph node status of operable breast cancers by combined steroid receptor and HER-2 status: triple positive tumours are more likely lymph node positive. Breast Cancer Res Treat. 2009;113(1):181–7. doi: 10.1007/s10549-008-9914-7. https://doi.org/10.1007/s10549-008-9914-7 PMid:18264760. [DOI] [PubMed] [Google Scholar]
- 33.Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37(9):1217–26. doi: 10.1016/j.humpath.2006.04.015. https://doi.org/10.1016/j.humpath.2006.04.015 PMid:16938528. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Suh YJ, Shim BY, Kim SH. The incidence and predictor of lymph node metastasis for patients with T1mi breast cancer that underwent axillary dissection and breast irradiation: an institutional analysis. Jpn J Clin Oncol. 2011;41(10):1162–7. doi: 10.1093/jjco/hyr128. https://doi.org/10.1093/jjco/hyr128 PMid:21903706. [DOI] [PubMed] [Google Scholar]
- 35.Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–41. doi: 10.1007/s10549-011-1891-6. https://doi.org/10.1007/s10549-011-1891-6 PMid:22147079. [DOI] [PubMed] [Google Scholar]


