Abstract
BACKGROUND:
Preclinical studies have demonstrated that renin-angiotensin system (RAS) signalling has strong tumour-promoting effects and RAS inhibition was associated with improvement in the overall survival in some cancer types including hepatocellular carcinoma (HCC).
OBJECTIVE:
We aimed to investigate the effect of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-II-receptor blockers (ARBs) on the survival of mice with diethylnitrosamine (DEN) induced HCC.
METHODS:
HCC was induced by weekly i.p. administration of DEN. Mice were treated with sorafenib (SO) (30 mg/kg), perindopril (PE) (1 mg/kg), fosinopril (FO) (2 mg/kg), losartan (LO) (10 mg/kg), PE (1 mg/kg) + SO (30 mg/kg), FO (2 mg/kg) + SO (30 mg/kg), or LO (10 mg/kg) + SO (30 mg/kg). Survival analysis was done using the Kaplan-Meier method, and the log-rank test was used for assessing the significance of difference between groups.
RESULTS:
The administration of PE, FO and LO as monotherapy or as combined with SO resulted in marked improvement in the liver histologic picture with no impact on overall survival of mice.
CONCLUSION:
Interfering the RAS either through the inhibition of ACE or the blockade of angiotensin II type 1 (AT1) receptors has similar effects on the liver of DEN-induced HCC mice and is not associated with longer survival due to detrimental effects of DEN on other organs. Hence, repetitive administration of DEN in such models of HCC is not suitable for mortality assessment studies.
Keywords: Diethylnitrosamine, Hepatocellular carcinoma, Renin-angiotensin system, Survival analysis
Introduction
Hepatocellular carcinoma (HCC), a major health problem is representing about 75% of primary liver cancers [1] [2], is considered the second most common cause of cancer-related mortality worldwide [3] and is characterised by poor prognosis. Risk factors for HCC have been described including infections with chronic viral hepatitis, non-alcoholic steatohepatitis (NASH) and alcohol consumption [4] [5] [6].
Treatment options of HCC are still limited and depend on liver function and patient condition [7]. Patients are usually diagnosed at advanced tumour stages as HCC patients are frequently asymptomatic. The oral multikinase inhibitor sorafenib, remains the only efficacious treatment, currently showing a certain degree of benefit [8] [9] and provides a modest prolongation of the median overall survival (OS) (2.8 months) [10]. Regorafenib, a closely related tyrosine kinase inhibitor demonstrated an increase in OS as a second-line (next to failure of sorafenib) [11]. None of the other agents tested showed a survival benefit versus sorafenib or placebo [12]. Therefore, a critical need exists to evaluate possible alternative strategies for the effective improvement in the survival of HCC patients.
There is emerging evidence that Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have beneficial effects in patients with HCC [13]. De Paepe, Verstraeten [14] reported that angiotensin II (Ang II) type 1 (AT1) receptor may be an essential step in the development of breast cancer, also it was reported that RAS inhibition was associated with reduced risk of breast cancer recurrence [15]. Furthermore, other studies revealed that ACEIs or ARBs might decrease the risk of esophageal [16] and keratinocyte [17] carcinoma. Their use was also associated with increased response in rectal cancer [18] and longer OS in patients with renal cell, pancreatic, brain, and lung cancer [19] [20]. However, little is known about the impact of RAS inhibition on the OS of HCC on the experimental and clinical sides.
Therefore, the present study was conducted to examine beneficial effects of RAS inhibition on liver histology and to assess the association between RAS inhibition and survival using the ACEIs, perindopril (PE) and fosinopril (FO) and the ARB, losartan (LO) by comparing their effects to sorafenib (SO) using a model of diethylnitrosamine (DEN)-induced HCC in mice.
Material and Methods
A total of 270 male CD-1 mice weighing 12 g were used in the current study. They were purchased from Schistosome Biological Supply Program at Theodor Bilharz Research Institute (SBSP-TBRI), Egypt and housed in polycarbonate cages in an animal facility certified by the association for assessment and accreditation of laboratory animal care and maintained in accordance with the National Institute of Health guide for the care and use of laboratory animals, Egypt. All experimental procedures were approved by local authorities at the SBSP-TBRI and by the Ethical Committee for Animal Handling at Zagazig University (ECAHZU), Egypt. All of the mice were fed rodent chow (23% protein and 4% fat) and received water ad libitum. They were kept under standard laboratory conditions of (21 Co, 45-55% humidity) and exposed to a 12/12 light-dark cycle. The animals were allowed to acclimate for 1 week before the experiments.
Diethylnitrosamine (DEN) was diluted (1:100 v/v) in saline. Perindopril, fosinopril and losartan were freshly prepared immediately before use by suspending in distilled water.
Mice were divided into groups as follows: Group 1: normal mice received i.p. injections of normal saline once a week (n = 6). Group 2: DEN induced HCC mice (DIHCC) received i.p. injections of DEN (Sigma-Aldrich CAS: 55-18-5, St. Louis, MO, USA) (n = 60) once a week according to the following schedule (week1 (30 mg/kg), w2 (50 mg/kg), w3 (50 mg/kg), w4 (70 mg/kg), w5 (100 mg/kg), w6→w16 (50 mg/kg)). Group 3: DIHCC mice received 30 mg/kg sorafenib (Bayer Pharmaceuticals, AG, Berlin, Germany) (n = 30). Group 4: DIHCC mice received 1 mg/kg perindopril (Servier Pharmaceutical Company, Suresnes, France) (n = 30). Group 5: DIHCC mice received 2mg/kg fosinopril (Bristol-Myers Squibb Pharmaceutical Company, New York, NY, USA) (n = 30). Group 6: DIHCC mice received 10mg/kg losartan (Merck Pharmaceutical Company, Kenilworth, NJ, USA) (n = 30). Group 7: DIHCC mice received (1mg/kg perindopril + 30 mg/kg sorafenib) (n = 30). Group 8: DIHCC mice received (2 mg/kg fosinopril + 30 mg/kg sorafenib) (n = 30). Group 9: DIHCC mice received (10 mg/kg losartan + 30 mg/kg sorafenib) (n = 30). Mice were administered suspension of drugs in distilled water by oral gavage daily starting from day 45 of experiment and sacrificed by decapitation 16 weeks post induction.
The dose of sorafenib was selected based on previous studies [21] [22] [23]. On the other hand, the equivalent mouse doses of perindopril, fosinopril, and losartan were calculated by interpolating from the corresponding lowest effective human dose using approximate dose conversion factors described by Freireich et al., [24].
Liver tissues were fixed in 10% neutral buffered formalin (pH 7.0) and embedded in paraffin. Sections (5 µm thick) from the paraffin blocks were stained with hematoxylin and eosin (H&E) for histopathological examination. HCC was graded as described by Theise, Curado [25].
Statistical analysis was performed using GraphPad Prism software version 6 (GraphPad Software Inc., La Jolla, CA, USA). For overall survival, the log-rank (Mantel-cox) test was used for assessing the significance of the difference between groups in the Kaplan-Meier analysis. P values < 0.05 were considered significant.
Results
The representative histological appearance of liver specimens from untreated normal control mice showed hepatic lobules with intact lobular architecture. Liver cells are arranged in cords of one to two cell-thick, radiating from a central vein towards the lobular periphery with blood sinusoids in-between. Hepatocytes are polyhedral with abundant granular eosinophilic cytoplasm and one spherical nucleus with dispersed chromatin. Portal tracts are of normal shape and thickness.
Sections of Liver from the DEN-treated mice showed loss of hepatic lobular architecture and enlarged hepatocytes with marked nuclear atypia, nuclear hyperchromasia, pleomorphism, increased nucleocytoplasmic ratio and appearance of tumour giant cells. Also, different sections showed HCC (grade 3) characterised by malignant hepatocytes surrounded by fibrotic stroma that were infiltrated by mononuclear inflammatory cells.
The representative histological appearance of liver tissue specimens from DIHCC mice treated with Sorafenib showed regression of malignant changes and lowering of the grade of HCC to grade 1 with almost restoration of lobular architecture
The administration of perindopril, fosinopril, or losartan either as monotherapy or combined with sorafenib improved the histological picture of liver showing moderate regression of malignant and inflammatory changes with lowering of the grade of HCC to grade 1 (Figure 1).
Figure 1.
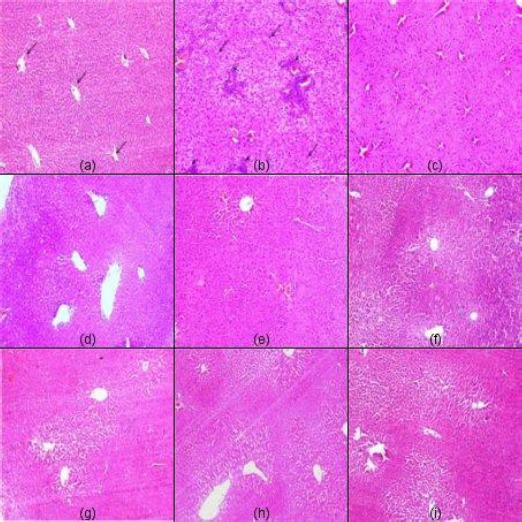
Representative light micrographs from: (a) normal control showing normal liver histology with radial arrangement of hepatocyte rays around central veins (arrows) that are separated by sinusoids; (b) DEN-treated mice showing HCC of grade (3) with disturbed architecture and loss of lobular pattern, hepatocellular atypia, pleomorphism, intralobular inflammatory infiltrate (arrowheads), malignant hepatocytes with moderate to marked nuclear anaplasia (arrows). Moreover, intralobular inflammatory infiltrates, fibrous tissue deposition (arrowheads) and congested blood vessels are evident. Micrographs from mice treated with sorafenib, (c), perindopril (d), fosinopril (e), losartan (f), perindopril plus sorafenib (g), fosinopril plus sorafenib (h), and losartan plus sorafenib (i) showing regression of malignant changes with almost restoration of lobular architecture and lowering of the grade of HCC to grade 1 (H&E x100)
Kaplan-Meier survival curves are represented in Figure 2, 3 and 4. Normal mice had no mortality at the end of the experimental period (Figure 2a). On the other hand, DEN-treated mice had a higher mortality rate with a median survival time of 86 days (95% CI of ratio: 0.02 to 0.16) vs normal control (Figure 2a). The administration of sorafenib (Figure 2, 3 and 4), perindopril (Fig. 2b), fosinopril (Figure 2c), or losartan (Figure 2d) resulted in slight increases in the median survival time as follows: 96 days (95% CI of ratio: 0.29 to 1.4), 113 days (95% CI of ratio: 0.29 to 1.4), 100 days (95% CI of ratio: 0.41 to 1.5), and 106 days (95% CI of ratio: 0.35 to 1.46), respectively.
Figure 2.
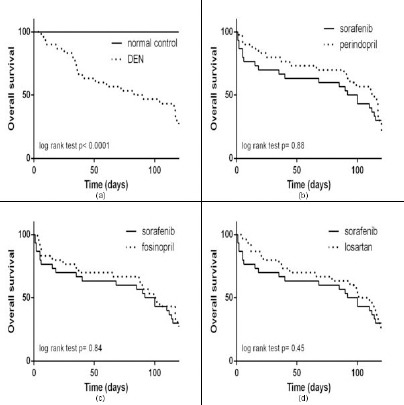
Kaplan-Meier survival curves of (a) DEN vs normal control; (b) perindopril vs sorafenib; (c) fosinopril vs sorafenib; (d) losartan vs sorafenib. Statistical analysis was performed using log-rank test (Mantel-cox method). P values <0.05 were considered significant
Figure 3.
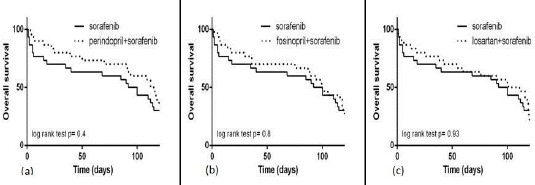
Kaplan-Meier survival curves of (a) (perindopril+sorafenib) vs sorafenib; (b) (fosinopril+sorafenib) vs sorafenib; (c) (losartan+sorafenib) vs sorafenib. Statistical analysis was performed using log-rank test (Mantel-cox method). P values <0.05 were considered significant
Figure 4.
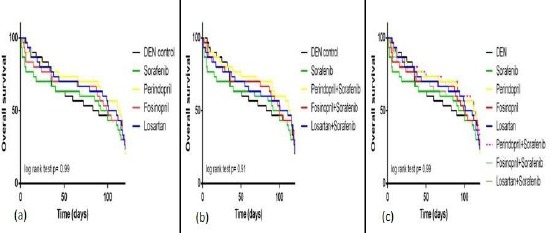
Kaplan-Meier survival curves of (a) monotherapy; (b) combination drug therapy; (c) monotherapy and combination therapy in DEN-treated mice. Statistical analysis was performed using log-rank test (Mantel-cox method). P values <0.05 were considered significant
Moreover, the administration of (perindopril + sorafenib, Figure 3a), (fosinopril + sorafenib, Figure 3b), or (losartan + sorafenib, Figure 3c) resulted in slight increases in the median survival time as follows: 114 days (95% CI of ratio: 0.3 to 1.38), 100 days (95% CI of ratio: 0.41 to 1.51) and 106 days (95% CI of ratio: 0.34 to 1.46). However, log-rank analysis for comparison of survival times revealed no significant differences in the lifespan of mice treated with the used drugs either as monotherapy (Figure 4a and 4c) or as adjunctive to sorafenib (Figure 4b and 4c) compared with DEN-treated mice.
Discussion
The discovery of new agents or repurposing current medications is vital to improving the survival and prognosis of HCC patients. RAS inhibition is an interesting prospective target for the chemoprevention of HCC. Therefore, we aimed to determine whether RAS inhibitors improve the overall survival of HCC in mice making a direct comparison for their effects with sorafenib which is the only efficacious drug that was found to prolong survival only for (2.8 months) [10] in HCC patients.
It is well documented that RAS plays an important role in promoting tumour growth [26] [27]. The use of ACEIs or ARBs has been found to inhibit different types of cancer including lung, breast, pancreatic, ovarian, prostatic, brain, colon, and liver cancer [28]. Furthermore, our lab demonstrated that the use of the ACEIs, perindopril and fosinopril and the ARB, losartan was found to decrease alpha-fetoprotein significantly and affect regression of malignant changes with improvement in liver histology in HCC mice through multifaceted mechanisms including the inhibition of angiogenesis, profibrotic mediators and inflammatory pathways (unpublished data)
Also, in the current study, combination therapy was evaluated to examine the potential augmenting effects of such safe agents when co-administered with sorafenib experimentally. Notably, a similar combination was recently evaluated clinically in which authors stated that patients treated with sorafenib plus RAS inhibition had a better median overall survival (19.5 mo) compared to those treated with either sorafenib (10.9 mo) or RAS inhibition (9.7 mo) alone (p = 0.043) [29]. Another observational study found that the use of ARBs during erlotinib treatment may prolong OS of patients with metastatic non-small cell lung cancer (NSCLC) [30].
The selection of perindopril (a high tissue affinity ACEI) and fosinopril (a low tissue affinity ACEI) [31] [32] was planned to evaluate the concept of class effect or interchangeability regarding therapeutic and side effects of ACEIs [33] [34]. Moreover, we evaluated the effect of blocking RAS signalling through antagonising Ang II via blocking its AT1 receptors using the ARB, losartan.
HCC in DEN-treated mice was evident by the appearance of malignant enlarged hepatocytes upon histologic examination. The histologic picture of HCC in mice treated with DEN was improved after treatment with the selected ACEIs, perindopril and fosinopril and the ARB, losartan either as monotherapy or when combined with sorafenib. This was manifested as a regression of HCC from grade (3) to grade (1) indicating the inhibition of malignant transformation and lower tumour production rates.
In the current study, DEN was used to induce HCC. Mice mainly develop liver tumours, but also gastrointestinal [35], skin, respiratory and haematopoietic tumours. The carcinogenic capacity of DEN is situated in its capability of alkylating DNA structures.
The DEN-induced tumorigenesis is mediated through its metabolites formed through its bioactivation by cytochrome P450 (CYP) enzymes [36]. An ethyldiazonium ion is formed and causes DNA damage by reacting with DNA-bases (nucleophiles). DEN works in a dose-dependent manner [37] to induce HCC after a period of latency. Genetically, the DEN-model verifies to be a good representation of HCC connected with poor prognosis [38]. When adult mice are exposed to a short time weekly administration of DEN, it leads to a higher incidence of a tumour in a shorter time span. For example, administration of 35 mg⁄kg DEN to mice weekly leads to HCC after 20-35 weeks [39].
The lifespan of DEN-treated mice was significantly decreased compared with normal mice that showed no mortality. This is consistent with DEN-induced systemic toxicity. The lifespan of mice did not significantly change by drug treatment compared with DEN-treated mice. The recent retrospective study published in 2017 by Pinter, Weinmann [29] demonstrated that the overall survival was increased in patients with HCC by the inhibition of RAS. The discrepancy between our results and the results of this study might be attributed to the repetitive administration of DEN that can have a detrimental impact on animals’ survival because DEN can affect the development of aggressive metastatic lesions in the lungs [40] [41]. Also, we noticed that some animals developed solid metastatic tumours distributed all over the body particularly in the thigh.
The current study unveiled some interesting findings. First, the histologic picture of liver tissues was improved upon treatment with perindopril, fosinopril, or losartan as monotherapy or as adjunctive therapy to sorafenib and was comparable to each other and to that of sorafenib. These results demonstrate that the tissue affinity of the ACEI has no impact on its hepatoprotective effect in this model of HCC, and that is interfering the RAS either through the inhibition of ACE or the blockade of AT1 receptors has the same histologic benefit. However, perindopril seems more promising because perindopril-treated mice showed the highest survival either as monotherapy or when combined to sorafenib despite statistical insignificance. Hence, perindopril needs further evaluation with changing doses and therapy duration.
Second, despite the promising effects of perindopril, fosinopril, or losartan for managing HCC as monotherapy, they failed to produce an additive pronounced improvement from the histopathological view when administered in combination with sorafenib. Based on the current data, it is not imaginable to explain the lack of the additive effect of the drugs used and sorafenib. Therefore, a pharmacokinetic study is needed to examine whether inhibitory effects of sorafenib on phase I enzymatic reactions might affect the metabolic activation of the prodrugs, perindopril, fosinopril and losartan.
Some studies described that sorafenib could induce mild liver dysfunction [42] [43] or severe hepatitis [44]. Metabolic dysfunction has been suggested as a mechanism for sorafenib-induced liver injury [45]. Consequently, another study is needed to examine the effect of RAS inhibitors on the sorafenib-induced liver injury, suggesting augmentation of the deleterious effects of sorafenib on the liver tissue by concomitant administration of RAS inhibitors providing an alternative explanation for the lack of more improvement upon histological examination.
In conclusion, the present results provide a potential for the therapeutic benefits of ACEIs and ARBs in liver tissues suggesting the prospective use of ACEIs or ARBs in managing patients with HCC either alone or in combination with other interventions. Furthermore, a long-term repetitive administration of DEN is not suitable to study survival in experimental animals due to detrimental effects on other organs particularly respiratory and haematopoietic systems. Alternatively, single dose or short-term administration of DEN with a longer latency time is the most suitable model for performing survival analysis in rodents.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–55. doi: 10.1016/S0140-6736(11)61347-0. https://doi.org/10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA: a cancer journal for clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. https://doi.org/10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Research. 2016:5. doi: 10.12688/f1000research.6946.1. https://doi.org/10.12688/f1000research.6946.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shlomai A, de Jong YP, Rice CM. Virus associated malignancies: the role of viral hepatitis in hepatocellular carcinoma. InSeminars in cancer biology. 2014;26:78–88. doi: 10.1016/j.semcancer.2014.01.004. https://doi.org/10.1016/j.semcancer.2014.01.004 PMid:24457013 PMCid: PMC4048791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. Journal of hepatology. 2012;56(6):1384–91. doi: 10.1016/j.jhep.2011.10.027. https://doi.org/10.1016/j.jhep.2011.10.027 PMid:22326465. [DOI] [PubMed] [Google Scholar]
- 6.Pinter M, Trauner M, Peck-Radosavljevic M, Sieghart W. Cancer and liver cirrhosis: implications on prognosis and management. Esmo Open. 2016;1(2):e000042. doi: 10.1136/esmoopen-2016-000042. https://doi.org/10.1136/esmoopen-2016-000042 PMid:27843598 PMCid: PMC5070280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The Liver. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology. 2012;56(4):908–43. doi: 10.1016/j.jhep.2011.12.001. https://doi.org/10.1016/j.jhep.2011.12.001 PMid:22424438. [DOI] [PubMed] [Google Scholar]
- 8.Di Marco V, De Vita F, Koskinas J, Semela D, Toniutto P, Verslype C. Sorafenib: from literature to clinical practice. Annals of oncology. 2013;24(suppl_2):ii30–7. doi: 10.1093/annonc/mdt055. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140(5):1410–26. doi: 10.1053/j.gastro.2011.03.006. https://doi.org/10.1053/j.gastro.2011.03.006 PMid:21406195 PMCid: PMC3682501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M. Sorafenib in advanced hepatocellular carcinoma. New England journal of medicine. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. https://doi.org/10.1056/NEJMoa0708857 PMid:18650514. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Merle P, Granito A, Huang YH, Bodoky G, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross Paul J. LBA-03 Efficacy and safety of regorafenib versus placebo in patients with hepatocellular carcinoma (HCC) progressing on sorafenib: results of the international, randomized phase 3 RESORCE trial. Annals of Oncology. 2016;27(suppl_2):ii140–1. [Google Scholar]
- 12.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clinical cancer research. 2014;20(8):2072–9. doi: 10.1158/1078-0432.CCR-13-0547. https://doi.org/10.1158/1078-0432.CCR-13-0547 PMid:24589894. [DOI] [PubMed] [Google Scholar]
- 13.Facciorusso A, Del Prete V, Crucinio N, Muscatiello N, Carr BI, Di Leo A, Barone M. Angiotensin receptor blockers improve survival outcomes after radiofrequency ablation in hepatocarcinoma patients. Journal of gastroenterology and hepatology. 2015;30(11):1643–50. doi: 10.1111/jgh.12988. https://doi.org/10.1111/jgh.12988 PMid:25974743. [DOI] [PubMed] [Google Scholar]
- 14.De Paepe B, Verstraeten VL, De Potter CR, Bullock GR. Increased angiotensin II type-2 receptor density in hyperplasia, DCIS and invasive carcinoma of the breast is paralleled with increased iNOS expression. Histochemistry and cell biology. 2002;117(1):13–9. doi: 10.1007/s00418-001-0356-0. https://doi.org/10.1007/s00418-001-0356-0 PMid:11819093. [DOI] [PubMed] [Google Scholar]
- 15.Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, Tester W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer investigation. 2011;29(9):585–93. doi: 10.3109/07357907.2011.616252. https://doi.org/10.3109/07357907.2011.616252 PMid:21936625. [DOI] [PubMed] [Google Scholar]
- 16.Sjöberg T, Rodríguez LA, Lindblad M. Angiotensin-converting enzyme inhibitors and risk of esophageal and gastric cancer: a nested case-control study. Clinical Gastroenterology and Hepatology. 2007;5(10):1160–6. doi: 10.1016/j.cgh.2007.08.005. https://doi.org/10.1016/j.cgh.2007.08.005 PMid:17916544. [DOI] [PubMed] [Google Scholar]
- 17.Christian JB, Lapane KL, Hume AL, Eaton CB, Weinstock MA. Association of ACE inhibitors and angiotensin receptor blockers with keratinocyte cancer prevention in the randomized VATTC trial. Journal of the National Cancer Institute. 2008;100(17):1223–32. doi: 10.1093/jnci/djn262. https://doi.org/10.1093/jnci/djn262 PMid:18728281. [DOI] [PubMed] [Google Scholar]
- 18.Morris ZS, Saha S, Magnuson WJ, Morris BA, Borkenhagen JF, Ching A, Hirose G, McMurry V, Francis DM, Harari PM, Chappell R. Increased tumor response to neoadjuvant therapy among rectal cancer patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Cancer. 2016;122(16):2487–95. doi: 10.1002/cncr.30079. https://doi.org/10.1002/cncr.30079 PMid:27203227 PMCid: PMC4998053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keizman D, Huang P, Eisenberger MA, Pili R, Kim JJ, Antonarakis ES, Hammers H, Carducci MA. Angiotensin system inhibitors and outcome of sunitinib treatment in patients with metastatic renal cell carcinoma: a retrospective examination. European journal of cancer. 2011;47(13):1955–61. doi: 10.1016/j.ejca.2011.04.019. https://doi.org/10.1016/j.ejca.2011.04.019 PMid:21600760 PMCid: PMC3175366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menter AR, Carroll NM, Sakoda LC, Delate T, Hornbrook MC, Jain RK, Kushi LH, Quinn VP, Ritzwoller DP. Effect of angiotensin system inhibitors on survival in patients receiving chemotherapy for advanced non–small-cell lung cancer. Clinical lung cancer. 2017;18(2):189–97. doi: 10.1016/j.cllc.2016.07.008. https://doi.org/10.1016/j.cllc.2016.07.008 PMid:27637408 PMCid: PMC5424707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczynski EA, Lee CR, Man S, Chen E, Kerbel RS. Effects of sorafenib dose on acquired reversible resistance and toxicity in hepatocellular carcinoma. Cancer research. 2015;75(12):2510–9. doi: 10.1158/0008-5472.CAN-14-3687. https://doi.org/10.1158/0008-5472.CAN-14-3687 PMid:25908587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd-Alhaseeb MM, Zaitone SA, Abou-El-Ela SH, Moustafa YM. Olmesartan Potentiates the Anti-Angiogenic Effect of Sorafenib in Mice Bearing Ehrlich's Ascites Carcinoma: Role of Angiotensin (1–7) PLoS One. 2014;9(1):e85891. doi: 10.1371/journal.pone.0085891. https://doi.org/10.1371/journal.pone.0085891 PMid:24465768 PMCid: PMC3899087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang TC, Man S, Xu P, Francia G, Hashimoto K, Emmenegger U, Kerbel RS. Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia. 2010;12(11):928–40. doi: 10.1593/neo.10804. https://doi.org/10.1593/neo.10804 PMid:21076618 PMCid: PMC2978915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freireich EJ, Gehan EA, Rall D, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer chemotherapy reports. 1966;50(4):219. PMid:4957125. [PubMed] [Google Scholar]
- 25.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. World Health Organization. 2010 [Google Scholar]
- 26.Fujita M, Hayashi I, Yamashina S, Fukamizu A, Itoman M, Majima M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26(2):271–9. doi: 10.1093/carcin/bgh324. https://doi.org/10.1093/carcin/bgh324 PMid:15637093. [DOI] [PubMed] [Google Scholar]
- 27.Tamarat R, Silvestre JS, Durie M, Levy BI. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor-and inflammation-related pathways. Laboratory investigation. 2002;82(6):747. doi: 10.1097/01.lab.0000017372.76297.eb. https://doi.org/10.1097/01.LAB.0000017372.76297.EB PMid:12065685. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal T, Gavras I. Angiotensin inhibition and malignancies: a review. Journal of human hypertension. 2009;23(10):623. doi: 10.1038/jhh.2009.21. https://doi.org/10.1038/jhh.2009.21 PMid:19339998. [DOI] [PubMed] [Google Scholar]
- 29.Pinter M, Weinmann A, Wörns MA, Hucke F, Bota S, Marquardt JU, Duda DG, Jain RK, Galle PR, Trauner M, Peck-Radosavljevic M. Use of inhibitors of the renin–angiotensin system is associated with longer survival in patients with hepatocellular carcinoma. United European gastroenterology journal. 2017;5(7):987–96. doi: 10.1177/2050640617695698. https://doi.org/10.1177/2050640617695698 PMid:29163965 PMCid: PMC5676550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aydiner A, Ciftci R, Sen F. Renin-Angiotensin system blockers may prolong survival of metastatic non-small cell lung cancer patients receiving erlotinib. Medicine. 2015;94(22) doi: 10.1097/MD.0000000000000887. https://doi.org/10.1097/MD.0000000000000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabris B, Yamada H, Cubela R, Jackson B, Mendelsohn FA, Johnston CI. Characterization of cardiac angiotensin converting enzyme (ACE) and in vivo inhibition following oral quinapril to rats. British journal of pharmacology. 1990;100(3):651–5. doi: 10.1111/j.1476-5381.1990.tb15862.x. https://doi.org/10.1111/j.1476-5381.1990.tb15862.x PMid:2167741 PMCid: PMC1917808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer WH, Baer JT, Berlin JA, Kimmel SE. Class effect of angiotensin-converting enzyme inhibitors on prevention of myocardial infarction. American Journal of Cardiology. 2004;94(9):1171–3. doi: 10.1016/j.amjcard.2004.07.087. https://doi.org/10.1016/j.amjcard.2004.07.087 PMid 15518614. [DOI] [PubMed] [Google Scholar]
- 33.Furberg CD, Herrington DM, Psaty BM. Are drugs within a class interchangeable? The Lancet. 1999;354(9185):1202–4. doi: 10.1016/S0140-6736(99)03190-6. https://doi.org/10.1016/S0140-6736(99)03190-6. [DOI] [PubMed] [Google Scholar]
- 34.Lala A, McLaughlin MA. Do ACE inhibitors all provide the same outcomes benefits in high-risk cardiovascular patients? Current hypertension reports. 2008;10(4):286–92. doi: 10.1007/s11906-008-0053-7. https://doi.org/10.1007/s11906-008-0053-7 PMid:18625157. [DOI] [PubMed] [Google Scholar]
- 35.Binato M, Schmidt MK, Volkweis BS, Ribeiro GB, Edelweiss MI, Gurski RR. Mouse model of diethylnitrosamine-induced gastric cancer. Journal of Surgical Research. 2008;148(2):152–7. doi: 10.1016/j.jss.2007.12.748. https://doi.org/10.1016/j.jss.2007.12.748 PMid:18456281. [DOI] [PubMed] [Google Scholar]
- 36.Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacology & therapeutics. 1996;71(1-2):57–81. doi: 10.1016/0163-7258(96)00062-9. https://doi.org/10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 37.Williams GM, Iatropoulos MJ, Jeffrey AM. Mechanistic basis for nonlinearities and thresholds in rat liver carcinogenesis by the DNA-reactive carcinogens 2-acetylaminofluorene and diethylnitrosamine. Toxicologic pathology. 2000;28(3):388–95. doi: 10.1177/019262330002800306. https://doi.org/10.1177/019262330002800306 PMid:10862555. [DOI] [PubMed] [Google Scholar]
- 38.Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nature genetics. 2004;36(12):1306. doi: 10.1038/ng1481. https://doi.org/10.1038/ng1481 PMid:15565109. [DOI] [PubMed] [Google Scholar]
- 39.Finnberg N, Stenius U, Högberg J. Heterozygous p53-deficient (+/-) mice develop fewer p53-negative preneoplastic focal liver lesions in response to treatment with diethylnitrosamine than do wild-type (+/+) mice. Cancer letters. 2004;207(2):149–55. doi: 10.1016/j.canlet.2003.11.013. https://doi.org/10.1016/j.canlet.2003.11.013 PMid:15072823. [DOI] [PubMed] [Google Scholar]
- 40.Yoshino H, Futakuchi M, Cho YM, Ogawa K, Takeshita F, Imai N, Tamano S, Shirai T. Modification of an in vivo lung metastasis model of hepatocellular carcinoma by low dose N-nitrosomorpholine and diethylnitrosamine. Clinical & experimental metastasis. 2005;22(5):441–7. doi: 10.1007/s10585-005-2807-9. https://doi.org/10.1007/s10585-005-2807-9 PMid:16283487. [DOI] [PubMed] [Google Scholar]
- 41.Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. International journal of experimental pathology. 2009;90(4):367–86. doi: 10.1111/j.1365-2613.2009.00656.x. https://doi.org/10.1111/j.1365-2613.2009.00656.x PMid:19659896 PMCid: PMC2741148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M. Sorafenib in advanced hepatocellular carcinoma. New England journal of medicine. 2008;359(4):378–90. doi: 10.1056/NEJMoa0708857. https://doi.org/10.1056/NEJMoa0708857 PMid:18650514. [DOI] [PubMed] [Google Scholar]
- 43.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The lancet oncology. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. https://doi.org/10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 44.Schramm C, Schuch G, Lohse AW. Sorafenib-induced liver failure. The American journal of gastroenterology. 2008;103(8):2162. doi: 10.1111/j.1572-0241.2008.01982_19.x. https://doi.org/10.1111/j.1572-0241.2008.01982_19.x PMid:18796127. [DOI] [PubMed] [Google Scholar]
- 45.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–40. doi: 10.2165/00003495-200969020-00006. https://doi.org/10.2165/00003495-200969020-00006 PMid:19228077. [DOI] [PubMed] [Google Scholar]


