Abstract
AIM:
The current study aimed to assess the practicability of a simple loop-mediated isothermal amplification (LAMP) about real-time quantitative PCR to diagnose primary toxoplasmosis among high-risk pregnant women.
METHODS:
Cloned Toxoplasma samples were used to calculate the analytical sensitivity while specificity was assessed using pooled DNA samples extracted from other parasitic stages.
RESULTS:
Both techniques showed 100% sensitivity and specificity and then applied to detect recent Toxoplasma infection in peripheral blood of 77 IgG negative women out of a total 139 women lately experienced spontaneous abortion. The 2 techniques obtained positive results in 8 samples confirming primary toxoplasmosis.
CONCLUSION:
Generally, LAMP assay is a simple, cost-effective molecular technique can be completed in less than half an hour to diagnose primary Toxoplasma infection. The technique can be applied in a minimally equipped laboratory by ordinary workers to screen the vulnerable groups. Further analysis using larger samples with the quantitative approach is recommended to confirm the sensitivity of this emergent molecular technique.
Keywords: Isothermal, LAMP, Toxoplasma, qPCR, Abortion
Introduction
Toxoplasma gondii (T. gondii) is a worldwide zoonotic protozoal parasite that causes harmless infection in a huge number of human population, yet it causes serious illness in merely immunocompromised hosts. Also, Toxoplasma trophozoites may cross the placental barriers, causing variable degrees of damage to fetuses resulting in stillbirth or spontaneous abortion [1]. Critical ocular and neurological signs plus other congenital manifestations may also arise as a result of congenital infection. These dangerous sequels typically depend on gestational age, virulence of the organism and immune status of the mother [2].
Association of Toxoplasma infection with spontaneous abortion is frequently studied with variable approaches. Toxoplasma infection among Mexican was recorded in women suffered from spontaneous abortion by detecting either specific antibodies or circulating parasitic DNA among 55% of their study group [3]. The authors recommended further studies to ascertain diagnostic policies to be trailed, to prevent congenital toxoplasmosis in the populations at risk. Toxoplasmosis was reported in 15.2% women with spontaneous abortion as well, using serological methods [4]. Based on the genomic detection of circulating Toxoplasma DNA up to 14.7% positive results were recorded regarding abortion in association with Toxoplasma gondii infection [5].
Based on serological methods, Saki et al., [6] detected IgM antibody in women who had an abortion and advised not to neglect the role of Toxoplasma gondii in the occurrence of abortion as a result of primary infection. However, the result of serological assays should be interpreted with caution, IgM antibodies may persist up to 1 year after Toxoplasma infection, so it is not appropriate to diagnose recent infection. Hence, to improve the diagnosis of primary infection with T. gondii during pregnancy, other techniques are suggested as anti-Toxoplasma IgG avidity and molecular detection by the nucleic acid polymerisation techniques to discriminate between recent and prior infection [7].
In fact, Toxoplasma can circulate at low concentrations, or intermittently, thus might be detected in earlier phases of infection as circulating genomic materials which need a sensitive and specific technique to confirm infection in such critical time. Molecular methods are reported to be effective enough to detect low concentrations of circulating genomic materials as low as 10 tachyzoites/ml which was reported in about 40% of cases in a study applied in France [8].
Real-time visualisation in addition to the precise digital quantitation of the amplified DNA products without risk of contamination, greatly direct scientists to replace the traditional PCR multi-instruments by a single quantitative PCR machine. However, the relatively high cost and the need for highly equipped laboratories still limit the wide use of such advanced technologies for diagnostic purposes of some infectious agents including Toxoplasma parasite [7]. Consequently, it is appropriate to develop simple, rapid, sensitive, cost-effective and less time- consuming diagnostic molecular method to complement the limitations of conventional and real-time PCR to diagnose such infection, especially in vulnerable groups [9].
At present, variable isothermal approaches targeting nucleic acid augmentation have been established, possibly terminating the need of expensive, sophisticated devices and complex laboratories especially in developing countries where inappropriate infrastructure is still the usual situation. One of the most verified isothermal DNA amplification methods is a LAMP (Loop-mediated isothermal amplification) which was originally described by Notomi et al., [10].
In the present study, a trial was made to apply LAMP technique in comparison to quantitative real-time PCR (qPCR) to detect primary toxoplasmosis in women suffered from spontaneous abortion for more feasible molecular implementation in our relatively low equipped laboratory.
Patients and Methods
Concerning ethical considerations that were approved by the local ethical committee, Faculty of Medicine, Cairo University, this cross-sectional study was conducted from January 2014 to December 2016. Women included in the study were from the outpatient’s clinic of Obstetrics and Gynecology Department, Kasr Alainy Hospital and suffered from spontaneous abortion during the first trimester of the first pregnancy. The total enrolled number was 139 cases; their age range was within 21 to 34 with no major demographic variability. They were almost of the same educational and socioeconomic level being attending governmental, free of charge hospital. Diagnosis of spontaneous abortion was performed following history taking and clinical examination plus findings of ultrasonography to exclude medical causes of abortion, women with Rh-incompatibility, threatened abortion, incompetent cervix, fibroid or any uterine abnormalities. Blood samples were collected from included cases immediately after abortion and sera were separated and were tested for anti-Toxoplasma IgG and IgM antibodies using a commercial ELISA Kit (Cal biotech Inc., CA). Serological tests were performed according to the manufacturer’s instructions. Samples showed positive IgG results were excluded from the study. Cases were advised to return in follow up visits for normal checking and to inform them about their results. Positive cases were managed according to the currently approved protocols in the Obstetrics and Gynecology Department, Faculty of Medicine, Cairo University. The women who did not show any positive results were at risk of primary Toxoplasma infection at any time and were instructed to take care of their next pregnancy to avoid such serious infection.
Genomic DNA from peripheral blood samples was extracted using the DNeasy® Blood and Tissue Kits (Qiagen, CA, USA). Quality control and computing procedure were applied to rule out false positives in molecular techniques.
Four Toxoplasma-specific primer sets were used for LAMP technique to detect B1 gene of 35- fold repeats; B3: ACGTGACAGTGAAGAGAGGA, F3: CAGATGTGCTAAAGGCGTCA, BIP: TGTTCGCTGTCTGTCTAGGGCAGGTGGTCGACTTCATGGGA and FIP AGGCGGAACCAACGGAAATCCTTGCTGTTCTGTCCTATCGC. Twenty five µl LAMP mixture was prepared containing 2 µl of the extracted nucleic acid template, 40 pmol for each of FIP and BIP primers, 5 pmol for the other primers B3 and F3, 1 µl of Bst DNA polymerase (NEB) in 2.5 µl of buffer [20 mM Tris-HCl (pH 8.8), 8 mM MgSO4, 10 mM (NH4)2SO4, 0.1% Tween 20], 10 mM KCl, 0.8 M betaine, in addition to 1.4 mM (dNTP). Using a heater block, the reaction of the isothermal technique LAMP was applied for 30 min at 65°C and then was inactivated for 2 min at 80°C. The positive amplification results were visually detected by adding 2µL of 1:10 diluted 10,000 x concentration fluorescent dye SYBR Green I (Invitrogen) to the reaction tubes. Green fluorescence was observed in a successful LAMP reaction, whereas it remained the original pinkish-orange in the negative one [11].
Light Cycler® instrument (Roche Diagnostics, Hoffmann-La Roche Ltd, USA) was used, applying SYBR Green technology. Primers from bases (5´-CCG TTGGTT CCG CCT CCT TC- 3´) and (5´-GCAAAA CAG CGG CAG CGT CT-3´) were used to amplify Toxoplasma B1 gene. The resulting PCR fragment of T. gondii was analysed using the Light Cycler® Red 640 (detected in channel 640). The reaction mixture (20 µl; Master SYBR Green kit; Roche Diagnostic) contained 0.5 µM of each primer, 5 mM MgCl2 and 5 µl template DNA. Amplification was performed for 50 cycles: 5s denaturation at 95°C, 10s annealing at 61°C and 15s extensions at 72°C, with an overall ramp rate of 20°C/s [12].
Positive and negative control: To assess the analytical sensitivity of the molecular techniques, cloned and purified Toxoplasma genomic materials (Roche Diagnostics), with serial dilutions were used as control positive samples. Also, the standard curve created by these cloned DNA samples were used to allow the absolute quantification of the unknown samples. The concentrations of the standard samples were prepared to be from 101 to 106 genomic equivalents/reaction of T. gondii DNA. Data analysis was done as described in the Light Cycler® instrument operator’s manual. Specificity was approved by defining their melting curves and melting temperatures (Tm) (95°C, 4.40°C/s ramp rate; 40°C, 2.20°C/s ramp rate; 65°C, 4.40°C/s ramp rate; 95°C, 0.02°C/s ramp rate continuous measurement). In addition to 10 pooled DNA samples extracted from other parasitic stages; Giardia, Cryptosporidium, Blastocystis, Leishmania spp. And Plasmodium spp. To confirm the specificity of the molecular techniques, a negative control was applied by replacing the template DNA with ultrapure water. The standard row originated from variable Toxoplasma genomic concentrations showed Crossing Points (CPs) were automatically recorded.
Results
To confirm primary recent Toxoplasma infection, detection of Toxoplasma B1 gene in the peripheral blood samples was done by both qPCRs applying SYBR Green technology and LAMP. Initial serological screening demonstrated positive IgG results in 62 out of the 139 cases (44.6%). Therefore, the subsequent molecular investigation was done for the remaining 77 samples. IgM was detected in 4 serum samples out of the 77 cases. Concerning the sensitivity of LAMP technique and qPCR, both techniques equally succeeded to polymerise all positive control samples denoting equal analytic sensitivity for the 2 molecular techniques. Whereas, control negative samples did not show any amplification signs. Turbidities resulted by the positive reaction due to the accumulation of magnesium pyrophosphate as a by-product of the LAMP reactions were easily observed visually. Positive reactions in LAMP turned green on the addition of SYBR Green I to the PCR tubes. A standard curve was generated by qPCR using the cloned positive samples to enable absolute quantitation of the possible positive samples within the 77 samples related to the immunologically negative IgG women.
To diagnose recent Toxoplasma infection in the 77 samples cases included in the present work, DNA was extracted from the collected samples and tested using both LAMP and Quantitative real-time PCR (qPCR), applying SYBR® Green detection protocol and different primer sets which targeted T. gondii B1 gene. Green fluorescence was observed in 8 samples out of the 77 samples. The same samples generated fluorescence signals (positive results) when analysed by SYBR® Green qPCR. For qPCR, as the amount of accumulated DNA in the PCR reactions increases, the amount of fluorescence from the dye increases consistently. Quantitative genomic estimation of these 8 positive samples in qPCR ranged from 1.2 x 101 to 6 x 107 genomic equivalents. Crossing points (Cps) showed different values ranging from 16.94 to 31.02 reflecting the different DNA quantities in the 8 samples (Figure 1). To confirm specificity and true positivity of these 8 samples, gene scanning option and high resolution melting curve analysis were applied using the software of the programmed Light Cycler® 1.x/2.0/480 instrument. Positive samples showed almost the same values of Tm (84.2 ± 2.43) confirming their specific amplification. The mean value of melting temperature related to the positive control was 85.55 ± 0.53 SD. No significant difference was found between the 8 positive samples and the control positive samples. Moreover, no significant difference was reported between the 4 IgM positive samples and the other 4 samples which proved positive only by molecular techniques, putting in mind that all the 8 samples did not suffer from any manifestations related to acute toxoplasmosis. Nucleic acid amplification and visualization of the amplicons were completed within less than ½ an hour in the LAMP and in one hour using the real-time PCR assays, in addition to extra ½ an hour to confirm specificity by high resolution melting curve analysis. Clinically, these 8 women did not suffer from any manifestations of acute toxoplasmosis.
Figure 1.
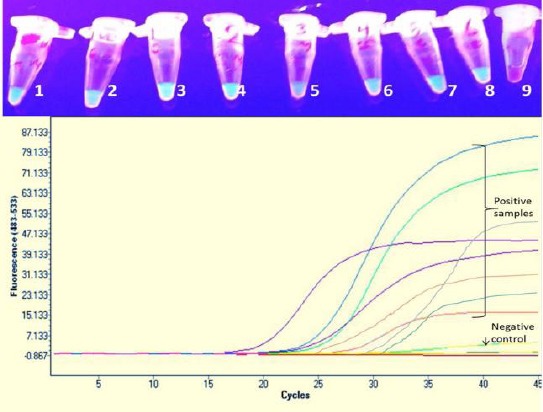
Samples in the upper photo show positive isothermal amplification for 8 samples (from 1 to 8) the sample number 9 is the negative control. The lower photo represents the real-time quantitative curves for the 8 positive samples with different crossing point at different cycles according to their genomic equivalent
Discussion
Diagnosis of primary toxoplasmosis in pregnancy is vital for the early management of this serious infection either by medical treatment or additional intervention to inhibit congenital transmission to the fetuses or unnecessary termination of the pregnancy [13]. The main target of the present study was to assess the usefulness of isothermal molecular technique that can be practised simply in our relatively low equipped laboratory. Also, to spotlight on primary toxoplasmosis about spontaneous abortion among our study group. In general, the risk of silent maternal Toxoplasma infection which may be transmitted to the fetuses is usually high [14]. And the risk of morbidity and mortality of the developing fetuses increases when primary Toxoplasma infection happens during the first trimester. While late transmission (last trimester), can cause ocular or neurological signs during the first 2 decades of life [15].
Commonly, diagnosis of acute primary infection is extremely difficult. A residual IgM antibody limits its use to detect early acute infection. The IgG avidity assay is now used to distinguish between acute and chronic toxoplasmosis, yet necessitates the presence of IgG antibodies. Low avidity means that the infection was acquired within the last 3 months [16]. However, the previous author criticized the method being not suitable for immunocompromised patients, plus the vital need of another assay as IgM ELISA to confirm the exact phases of infection. This certainly will raise the cost of the investigations. Specific Toxoplasma IgM typically develops in the early phase of the primary infection, within 1 to 2 weeks and reaches the highest level from 4 to 8 weeks after infection. The factors affecting immune response and antibody production depends on variable influences including the virulence of the organisms, inoculum volume, infective form plus the analytical sensitivity of the diagnostic method used to confirm the infection [17]. This may explain the negative results of IgM ELISA among 4 out of the 8 cases proved positive by molecular assays. These 8 cases possibly got the infection recently after pregnancy and not in the prenatal period. Possibly, the circulating parasitic stages that affected the placenta were enough to cause the evidenced damage and resulting in termination of the pregnancy.
Concerning the molecular techniques, using a simple heater, LAMP technique was completed within less than half an hour and successfully obtained the same results achieved by qPCR that was performed on a highly advanced instrument. Both obtained positive results for the same 8 samples, in addition to the equal analytical sensitivity which was proved following screening for serially purified cloned specific genomic materials (from 101 to 106). Such sensitivity of LAMP may be explained by the nature of the technique which is based on using a larger number of primers, (4 primers in this study) instead of 2 in qPCR to target different internal regions on the same target DNA [18]. A higher sensitivity of isothermal amplification technique than the ordinary multi-thermal PCR assays was also reported with other parasitic infections as Babesia spp., Trypanosoma spp. and Theileria spp [19] [20] [21]. The amplified products can be easily visualised in LAMP technique using simple UV light or even by direct turbidity inspection of the end product in the tubes. Hence, these give the technique an additional benefit and eliminate the necessity for gel electrophoresis and minimise the hazard of contamination. However, further work should be continued to apply a simple quantitative method for the amplified products.
Blood samples were used in the present study to detect the circulating Toxoplasma parasites in the peripheral blood. Molecular diagnosis of ocular toxoplasmosis was done using blood samples as well and produced the same results obtained with aqueous humor samples [22]. The authors recommended the use of blood samples to avoid invasive techniques. Thus, in this study, we evaluate the clinical sensitivities and specificities of a LAMP assay based on blood samples and compare the results with those of real-time qPCR. The specificity of the molecular technique in this study was confirmed by the highly advanced melting curve analysis, resulting in 100% specificity for both of them. Moreover, no positive results were initiated from the 10 pooled samples extracted from other protozoal infections. Indeed to comment on the sensitivity, more studies are recommended using larger standardized samples, better to be performed on experimentally infected animals with different intensities of infection. Hence, the apparently 100% sensitivity of LAMP achieved in the present study in relation to (qPCR) should be further assessed to calculate the definite sensitivity. In other studies, sensitivity ranged between 80 to 87.5% was reported using LAMP to detect Toxoplasma infection in blood samples positive for IgM [11]. However, such reported sensitivity perhaps was undervalued by the previous authors due to its direct connection with the results of IgM. It seems unfair to link the results of a molecular technique necessitated the presence of circulating DNA to diagnose recent Toxoplasma infection to IgM antibodies which may be residual for years after infection. In fact, spontaneous abortion was not the target group in the previous study.
On the other hand, the quantitative real-time PCR assay has been successfully used to detect and accurately quantify Toxoplasma DNA in human body fluids including blood. With the use of B1 gene, the technique is considered as the best-performing technique for the diagnosis of congenital toxoplasmosis, compared with conventional PCR and nested-PCR [23]. The real-time PCR is also used to evaluate toxoplasmosis progression and treatment efficacy since it can estimate the intensity of T. gondii infection [24]. For this reason, qPCR was used in a comparative approach in this study to assess sensitivity and specificity of LAMP assay for simple, cost-effective diagnosis of primary toxoplasmosis.
In conclusion, molecular assays seem to be able to diagnose initial phases of Toxoplasma infection in earlier stages of gestation when the fetus is not yet immunocompetent. LAMP assay is a simple, cost-effective molecular technique with a detection limit of 10 specific genomic equivalents and can be completed in less than half an hour to diagnose primary Toxoplasma infection using peripheral blood samples. The ability to apply the technique in a minimally equipped laboratory by ordinary workers gives the technique great potential to be used in developing countries where toxoplasmosis is endemic, specifically for high-risk groups.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Lopes FM, Gonçalves DD, Mitsuka-Breganó R, Freire RL, Navarr IT. Toxoplasma gondii infection in pregnancy. Braz J Infect Dis. 2007;11:496–506. doi: 10.1590/s1413-86702007000500011. https://doi.org/10.1590/S1413-86702007000500011 PMid:17962877. [DOI] [PubMed] [Google Scholar]
- 2.Robbins JR, Zeldovich VB, Poukchanski A, Boothroyd JC, Bakardjiev AI. Tissue Barriers of the Human Placenta to Infection with Toxoplasma gondii. Infection and Immunity. 2012;80(1):418–428. doi: 10.1128/IAI.05899-11. https://doi.org/10.1128/IAI.05899-11 PMid:22083708 PMCid: PMC3255695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vado-Solís I, Suárez-Solís V, Jiménez-Delgadillo B, Zavala-Velázquez J, Segura- Correa J. Toxoplasma gondii Presence in Women with Spontanous Abortion in Yucatan, Mexico. 2013;99(2):383–385. doi: 10.1645/GE-3189.1. [DOI] [PubMed] [Google Scholar]
- 4.Sultana M, Sazzad Hossain Md, Dewan F, Sultan J, Rashid M. Association of Toxoplasma gondii Infection with Spontaneous Abortion. Bangladesh. J Obstet Gynaecol. 2014;29(2):87–93. [Google Scholar]
- 5.Matin S, Shahbazi GH. Study on abortion associated with Toxoplasma gondii in women based on PCR detection of aborted placenta and maternal serology in Ardabil, Iran. J Med Microb Diagn. 2017;6:2. [Google Scholar]
- 6.Saki J, Mohammadpour N, Khademvatan S. Seroprevalence of Toxoplasma gondii in women who have aborted in comparison with the women with normal delivery in Ahvaz, southwest of Iran. The Scientific World Journal. 2015;2015:764369. doi: 10.1155/2015/764369. https://doi.org/10.1155/2015/764369 PMid:25699288 PMCid: PMC4325198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert-Gangneux Belaz. Molecular diagnosis of toxoplasmosis. Curr Opin Infect Dis. 2016;29:330–339. doi: 10.1097/QCO.0000000000000275. https://doi.org/10.1097/QCO.0000000000000275 PMid:27191201. [DOI] [PubMed] [Google Scholar]
- 8.Romand S, Chosson M, Franck J, Wallon M, Kieffer F, Kaiser K, Dumon H, Peyron F, Thulliez P, Picot S. Usefulness of quantitative polymerase chain reaction in amniotic fluid as early prognostic marker of fetal infection with Toxoplasma gondii. Am J Obstet Gynecol. 2004;190:797–802. doi: 10.1016/j.ajog.2003.09.039. https://doi.org/10.1016/j.ajog.2003.09.039 PMid:15042017. [DOI] [PubMed] [Google Scholar]
- 9.Liu CY, Song HQ, Zhang RL, Chen MX, Xu MJ, Ai L, Chen XG, Zhan XM, Liang SH, Yuan ZG, Lin RQ, Zhu XQ. Specific detection of Angiostrongylus cantonensis in the snail Achatina fulica using a loop-mediated isothermal amplification (LAMP) assay. Mol Cell Probes. 2011;25:164–167. doi: 10.1016/j.mcp.2011.04.002. https://doi.org/10.1016/j.mcp.2011.04.002 PMid:21515360. [DOI] [PubMed] [Google Scholar]
- 10.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63. doi: 10.1093/nar/28.12.e63. https://doi.org/10.1093/nar/28.12e63 PMid:10871386 PMCid: PMC102748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau YL, Meganathan P, Sonaimuthu P, Thiruvengadam G, Nissapatorn V, Chen Y. Specific, sensitive, and rapid diagnosis of active toxoplasmosis by a loop-mediated isothermal amplification method using blood samples from patients. J Clin Microbiol. 2010;48(10):3698–702. doi: 10.1128/JCM.00462-10. https://doi.org/10.1128/JCM.00462-10 PMid:20660217 PMCid: PMC2953084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contini C, Seraceni S, Cultrera R, Incorvaia C, Sebastiani A, Picot S. Evaluation of a real-time PCR-based assay using the Lightcycler system for detection of Toxoplasma gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int J Parasitol. 2005;35:275–283. doi: 10.1016/j.ijpara.2004.11.016. https://doi.org/10.1016/j.ijpara.2004.11.016 PMid:15722079. [DOI] [PubMed] [Google Scholar]
- 13.Reis MM, Tessaro M, DAzevedo PA. Toxoplasma IgM and IgG avidity in single samples from areas with a high infection rate can determine the risk of mother to child transmission. Rev Inst med trop Sao Paulo. 2006;48:93–98. doi: 10.1590/s0036-46652006000200007. https://doi.org/10.1590/S0036-46652006000200007 PMid:16699631. [DOI] [PubMed] [Google Scholar]
- 14.Ajzenberg D, Cogne N, Paris L, Bessières MH, Thulliez P, Filisetti D, Pelloux H, Marty P, Dardé ML. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002;186:684–689. doi: 10.1086/342663. https://doi.org/10.1086/342663 PMid:12195356. [DOI] [PubMed] [Google Scholar]
- 15.Marcolino PT, Silva DAO, Leser PG, Camargo ME, Mineo JR. Molecular Markers in Acute and Chronic Phases of Human Toxoplasmosis: Determination of Immunoglobulin G Avidity by Western Blotting. Clinical and Diagnostic Laboratory Immunology. 2000;7(3):384–389. doi: 10.1128/cdli.7.3.384-389.2000. https://doi.org/10.1128/CDLI.7.3.384-389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahbari AH, Keshavarz H, Shojaee S, Mohebali M, Rezaeian M. IgG Avidity ELISA Test for Diagnosis of Acute Toxoplasmosis in Humans. Korean J Parasitol. 2012;50(2):99–102. doi: 10.3347/kjp.2012.50.2.99. https://doi.org/10.3347/kjp.2012.50.2.99 PMid:22711919 PMCid: PMC3375462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenum PA, Stray-Pedersen B. Development of Specific Immunoglobulins G, M, and A Following Primary Toxoplasma gondii Infection in Pregnant Women. J Clin Microbiol. 1998;36(10):2907–2913. doi: 10.1128/jcm.36.10.2907-2913.1998. PMid:9738042 PMCid: PMC105086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagamine K, Hase T, Notomi T. Accelerated reaction by loop mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16:223–229. doi: 10.1006/mcpr.2002.0415. https://doi.org/10.1006/mcpr.2002.0415 PMid:12144774. [DOI] [PubMed] [Google Scholar]
- 19.Alhassan A, Govind Y, Tam NT, Thekisoe OM, Yokoyama N, Inoue N, Igarashi I. Comparative evaluation of the sensitivity of LAMP, PCR and in vitro culture methods for the diagnosis of equine piroplasmosis. Parasitol Res. 2007;100:1165–1168. doi: 10.1007/s00436-006-0430-6. https://doi.org/10.1007/s00436-006-0430-6 PMid:17216488. [DOI] [PubMed] [Google Scholar]
- 20.Thekisoe OM, Inoue N, Kuboki N, Tuntasuvan D, Bunnoy W, Borisutsuwan S, Igarashi I, Sugimoto C. Evaluation of loop-mediated isothermal amplification (LAMP), PCR and parasitological tests for detection of Trypanosoma evansi in experimentally infected pigs. Vet Parasitol. 2005;30:327–330. doi: 10.1016/j.vetpar.2005.04.019. https://doi.org/10.1016/j.vetpar.2005.04.019 PMid:15908123. [DOI] [PubMed] [Google Scholar]
- 21.Iseki H, Alhassan A, Ohta N, Thekisoe, Yokoyama N, Inoue N, Nambota A, Yasuda J, Igarashi I. Development of a multiplex loop-mediated isothermal amplification (mLAMP) method for the simultaneous detection of bovine Babesia parasites. J Microbiol Methods. 2007;71:281–287. doi: 10.1016/j.mimet.2007.09.019. https://doi.org/10.1016/j.mimet.2007.09.019 PMid:18029039. [DOI] [PubMed] [Google Scholar]
- 22.Bou G, Figueroa MS, Marti-Belda P, Navas E, Guerrero A. Value of PCR for detection of Toxoplasma gondii in aqueous humor and blood samples from immunocompetent patients with ocular toxoplasmosis. J Clin Microbiol. 1999;37:3465–3468. doi: 10.1128/jcm.37.11.3465-3468.1999. PMid:10523535 PMCid: PMC85668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teixeira LE, Kanunfre KA, Shimokawa PT, Targa LS, Rodrigues JC, Domingues W, Yamamoto L, Okay TS. The performance of four molecular methods for the laboratory diagnosis of congenital toxoplasmosis in amniotic fluid samples. Rev Soc Bras Med Trop. 2013;46(5):584–8. https://doi.org/10.1590/0037-8682-0095-2013. [PubMed] [Google Scholar]
- 24.Menotti J, Vilela G, Romand S, Garin YJ, Ades L, Gluckman E, Derouin F, Ribaud P. Comparison of PCR-enzyme-linked immunosorbent assay and real-time PCR assay for diagnosis of an unusual case of cerebral toxoplasmosis in a stem cell transplant recipient. J Clin Microbiol. 2003;41(11):5313–6. doi: 10.1128/JCM.41.11.5313-5316.2003. https://doi.org/10.1128/JCM.41.11.5313-5316.2003 PMid:14605193 PMCid: PMC262488. [DOI] [PMC free article] [PubMed] [Google Scholar]


