Abstract
BACKGROUND:
Cryptosporidium is an important waterborne protozoan.
AIM:
The aim of this study was to investigate the effect of sunlight being the natural source of UV and artificial UV irradiation on Cryptosporidium oocysts versus the effect of chlorination, being the traditional method of water disinfection and to provide an insight into the viability and degree of infectivity of Cryptosporidium oocysts, using an animal model.
METHODS:
An experimental study including 300 neonatal mice was carried out to investigate the effect of artificial ultraviolet (UV) irradiation and sunlight being the natural source of UV irradiation versus chlorine, the traditionally used water disinfectant on the infectivity of Cryptosporidium oocysts present in water. For each item, nine different exposure times were investigated. Parasitological assessment (Modified Ziehl Neelsen stained stool smears) and histopathological assessment of the excised segments of the small intestine (stained by both Haematoxylin & Eosin and ZN stain) of mice were used to verify the inactivation of oocysts.
RESULTS:
Cryptosporidium oocysts failed to induce any noticeable infection after 4 hours of artificial UV exposure that provided a UV dose of 10mJ/cm2 and after an 8 hours exposure to sunlight, whereas they showed resistance to disinfection by chlorine.
CONCLUSION:
The results of the study demonstrate the important role of an 8 hours sunlight exposure of potable water in plastic bottles in achieving complete inactivation of any contaminating Cryptosporidium oocysts, thus offering an applicable, economical and convenient method for the control of cryptosporidiosis especially in developing countries.
Keywords: Cryptosporidium, Sunlight, Ultraviolet, Chlorine, Inactivation
Introduction
Cryptosporidium is a protozoan that may lead to fatal illness in immunocompromised persons [1]. It inhabited intestines of humans and animals and distributed through bowel motions into the environment [2]. During the last 20 years, waterborne diseases were frequently caused by Cryptosporidium. Its presence in surface, ground and potable water supplies is of particular concern as it disseminates rapidly in addition to its resistance to chlorine and other commonly used chemical disinfectants [2]. The questionable efficacy of chlorine, its safety, and potential health hazards, had led to consideration of other alternative disinfectants [3]. A recent addition to the panel of water disinfectants for protozoan parasites is UV irradiation [4]. Studies concerning such an issue may lead to improve water quality and help in designing water and wastewater treatment processes offering safe drinking water and protection of public health [5].
This study aimed to investigate the effect of sunlight being the natural source of UV and artificial UV irradiation on Cryptosporidium oocysts versus the effect of chlorination, being the traditional method of water disinfection and to provide an insight into the viability and degree of infectivity of Cryptosporidium oocysts, using an animal model.
Methods
The study was conducted according to the national guidelines for animal research; 300 neonatal albino mice were divided into 4 groups, one receiving untreated Cryptosporidium oocysts to be used as a control group (30 mice), and 3 groups infected with Cryptosporidium oocysts previously exposed to different disinfectants, in terms of chlorine (the traditional water disinfectant), sunlight (containing UVA and UVB) and artificial UV irradiation (representing UVC). Each of these 3 test groups was further subdivided into 9 subgroups (10 mice/ subgroup). Each of the 3 tested groups of mice were infected after exposure of Cryptosporidium oocysts to the tested disinfectants for variable periods of time, starting from 15 minutes (min), 30 minutes, one hour (h), 2 hours, 4 hours, 8 hours, then for 1 day(d), 2 day and 4 days (Table 1).
Table 1.
Categorization of different groups and subgroups of mice included in the present experimental study
| Groups | No. of mice | Subgroups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control group | 30 | - | ||||||||
| Mice received chlorine treated oocysts | 90 | 9 different exposure time (10 mice/each) = 90 | ||||||||
| Mice received artificial UV rays exposed oocysts | 90 | 15 min | 30 min | 1 hour | 2 ours | 4 hours | 8 h hours | 1 day | 2 days | 4 days |
| Mice received sunlight exposed oocysts | 90 | |||||||||
| Total number of mice | 300 | |||||||||
Cryptosporidium oocysts were isolated from diarrheic calves, mixed with 10 ml of distilled water and then filtered through sterile gauze. The homogenate was successively passed through two metal sieves of pore size 125µm & 75µm, followed by centrifugation at 2500 x g for 15 min. The supernatant fluid was discarded, and the sediment was then washed twice with 1ml of phosphate buffer saline (PBS) (pH was adjusted to 7.2-7.6) with centrifugation at 13,000 x g for 15 minutes each. After repeated washing, faecal debris was eliminated and thus purified. Cryptosporidium oocysts were obtained [6]. The oocysts were then preserved by mixing with an equal volume of 2.5% potassium dichromate (K2Cr2O7) and stored at 4ºC until being used for animal inoculation. Just before use, the oocysts were washed for at least three times in distilled water to remove the potassium dichromate and centrifuged at 1500 x g for 15 min until it became clear [7]. For counting oocysts, a smear was prepared on 3 slides formed of about 50 mg from each sample and stained with modified Ziehl-Neelsen stain (ZN). Oocysts were counted in each slide, and the mean number of the oocysts was calculated to get the mean number/mg of the sample [8].
Purified oocysts were added to 0.5 ml of distilled water in ordinary transparent polyethene terephthalate bottles and then exposed to sunlight for variable durations as previously mentioned. Eight hours exposure was chosen from 8 am to 4 pm (maximum intensity of solar irradiation). For one day exposure, the previously mentioned inoculum was exposed to sunlight from 8 am to 4 pm, and then was covered with aluminium foil until next day 8 am, and then mice inoculation was done to investigate the possibility of the occurrence of dark repair [9]. The same procedure was followed for 2 days and 4 days exposure. The temperature inside the bottles was measured each hour with a thermometer [9]. For artificial UV irradiation, 10ml of PBS containing the purified suspension of oocysts was placed in a petri dish (56mm) and then placed under a 5-W low-pressure mercury lamp (QCGL5W-1497D; Iwasaki Electronic Co. Ltd., Tokyo, Japan). Using UV dose rate meter, the intensity of the UV light at a wavelength of 253.7 nm was measured. Exposure was completed throughout the mentioned durations under regular mixing using an electromagnetic stirrer [10]. Chlorine was used as Sodium hypochlorite solution (NaOCl), 4 ppm at 25ºC and applied for the same variable durations and as described by Carpenter et al., [11]. The inoculum dose was prepared to be 2.5 × 104 oocysts/mouse [9].
Neonatal mice were orally infected with a dose of 2.5 × 104 Cryptosporidium oocysts/mouse [9] using oesophagal tube [12]. According to Rossi et al., [13], all mice were sacrificed at 14 days post infection after giving intra-peritoneal anaesthesia. The terminal ileum was removed, fixed and prepared for histopathological examination.
Faecal samples were collected daily from infected mice until 14 days post infection. Parasitological examination was done using Modified ZN stain (cold method) for detection and grading of the intensity [14]. Haematoxylin & Eosin and modified ZN stained tissue sections were examined [15] to detect endogenous oocysts, assess inflammatory intensity as represented by inflammatory cells and activity represented by neutrophils [16]. While intensity of infection was determined by counting the parasite in 10 villous crypt units according to Healey et al., [17], then the mean oocysts number/single unit/mouse in each group was calculated.
All data were analysed using Statistical Package for the Social Sciences (SPSS) version 16 for Windows (SPSS Inc., Chicago, IL, USA). Data were reported as mean values ± SD for the quantitative variables. To compare the mean numbers of oocysts obtained after different time exposures in each group, we used Paired t test. To compare the mean numbers of oocysts between the three tested groups we used Analysis of variance (ANOVA) followed by pair wise analysis (Bonferroni test). P values ≤ 0.05 were considered statistically significant.
Results
Concerning the control group, shedding of Cryptosporidium oocysts was first observed on the fourth-day post infection. The mean number of oocysts was 2 ± 1.5/mg stool, and then gradually increased to report mean of 7.65 ± 0.4/ mg stool on day 8 to day 11. The maximum shedding of oocysts was reported from day 12 up to the end of the experiment with a mean value of 17.8 ± 2.3/mg stools. This difference was statistically significant (p < 0.05). Therefore, the mean oocysts count in the control group from day 12 to day 14 was used to compare the results of the other tested groups. As regards the temperature inside the bottle exposed to sunlight, a peak of 42°C was recorded. The mean number of oocysts in collected stool samples was 13.2, 12.9, 6.6, and 4.9 oocysts/mg stools when Cryptosporidium isolates were exposed to sunlight before mice inoculation for 15 min, 30 min, 1 h, and 2 h respectively. On exposure for 4 h, 9 mice out of 10 became infected in this subgroup whereas one mouse was found free of infection with a mean count 2.1 oocysts/mg stool. No Cryptosporidium oocysts were observed in the stool of any of the mice inoculated by oocysts exposed to sunlight 8 hours or longer, achieving 100% reduction rate in infectivity. Artificial UV radiation exposed oocysts showed mean counts of 11.12, 8.1, 2.8 and 1.4 when Cryptosporidium isolates were exposed to UV rays for 15 min, 30 min, 1 h, and 2 h respectively before mice inoculation. In 2 hours artificial UV exposure subgroup, the stool of 7 out of 10 mice showed Cryptosporidium oocysts while stool of 3 mice proved to be negative with a mean count 1.4 of oocysts/mg stool. Four hours artificial UV exposure for Cryptosporidium isolates subgroup showed 100% inactivation. Negative stool samples for Cryptosporidium were encountered in mice inoculated with Cryptosporidium isolates that were exposed to UV radiation for 4 hours or longer. Regarding results of chlorine-treated oocysts group, all stool samples of mice in all subgroups were positive for Cryptosporidium oocysts.
Even after 4 days of chlorine treatment of Cryptosporidium oocysts, the reduction rate of infectivity was only 58.42%. The mean no. of oocysts/ mg stool for different exposure times and reduction rates of infectivity in different groups are presented in Table 2 and Figure 1.
Table 2.
Effect of exposure of Cryptosporidium oocysts to sunlight, artificial UV rays and chlorine on their infectivity to mice
| Time of exposure | Group Sunlight | Group of artificial UV rays | Group chlorine | ANOVA | P value | |||
|---|---|---|---|---|---|---|---|---|
| oocysts/mg stool Mean ± S.D | Reduction rate (%) | oocysts/mg stool Mean ± S.D | Reduction rate (%) | oocysts/mg stool Mean ± S.D | Reduction rate (%) | |||
| 15 min | 13.20 ± 1.68 | 25.84 | 11.12 ± 1.31 | 37.52 | 11.20 ± 1.31 | 37.07 | 6.33 | 0.006* |
| 30 min | 12.90 ± 1.19 | 27.52 | 8.1 ± 0.73 | 54.49 | 11.80 ± 1.31 | 33.70 | 51.11 | 0.000* |
| 1 hour | 6.60 ± 1.07 | 62.92 | 2.8 ± 0.78 | 84.26 | 11.10 ± 1.19 | 37.64 | 161.28 | 0.000* |
| 2 hours | 4.90 ± 0.87 | 72.47 | 1.4 ± 0.51 | 92.13 | 10.70 ± 1.33 | 39.88 | 234.53 | 0.000* |
| 4 hours | 2.1 ± 0.73 | 88.2 | 0 | 100 | 10.20 ± 0.91 | 42.69 | 626.61 | 0.000* |
| 8 hours | 0 | 100 | 0 | 100 | 9.60 ± 0.96 | 46.06 | 987.42 | 0.000* |
| 1 day | 0 | 100 | 0 | 100 | 9.90 ± 0.99 | 44.38 | 991.11 | 0.000* |
| 2 days | 0 | 100 | 0 | 100 | 10.00 ± 1.05 | 43.82 | 900.00 | 0.000* |
| 4 days | 0 | 100 | 0 | 100 | 7.40 ± 0.84 | 58.42 | 770.06 | 0.000* |
Significant p-value.
Figure 1.
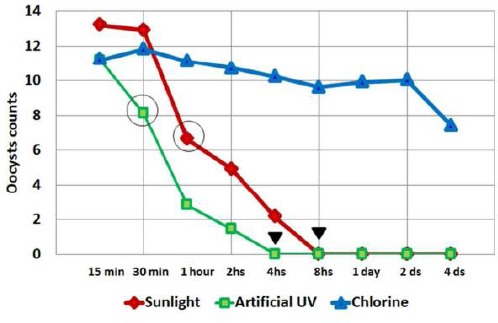
Line chart showing mean Cryptosporidium oocysts count/mg of stool in each subgroup of mice infected with oocysts previously exposed for various durations of time to sunlight, artificial UV irradiation and chlorine treatment. Circles denote the start of the significant differences and arrowheads denote full inactivation of artificial UV rays at 4 hours while absolute sunlight inactivation occurs at 8 hours
Using Paired t-test to compare between subgroups, the mean number of oocysts/mg stool at different exposure times within each group showed that, for the sunlight group, there was no statistical difference between the mean number in the 15 min and 30 min while the significant statistical difference was begun between 15 min and 60 min and extended to all other durations that showed significantly declining values. While for artificial UV rays group, the difference was statistically significant from the start between the 15 min and 30 min exposure and onward. As for the chlorine group, there was no statistical difference between 15 min and 60 min, 1 hour, 2 hours, 4 hours and onward. Comparing the results of the three groups, ANOVA test showed a significant difference between the mean numbers of oocysts among the three tested groups in all-time exposures. Bonferroni test showed a significant difference between the 3 individual groups except at 15 min where there was no significant difference between groups of artificial UV radiation and chlorine, and at 30 min there was no significant difference between sunlight and chlorine groups. Moreover, there was no statistical difference between sunlight and artificial UV radiation groups starting from 8 hours and onwards.
The ability of treated oocysts to induce infection in mice was determined by counting the endogenous stages in histopathological preparations, then calculating the mean number per single villous crypt unit for each animal and each group of animals, thus determining the intensity of infection (Figure 2). The mean number of endogenous stages was calculated in the control group to be 26 ± 5.3. All mice in the control group showed severe to moderate inflammatory intensity, while the inflammatory activity was rated as moderate. A significant difference between the mean numbers of oocysts among the 3 tested groups at all durations was recorded (P ≤ 0.05). Bonferroni test showed a significant difference between the endogenous stages counts in the 3 groups. However, there was no statistical difference between subgroups sunlight and artificial UV radiation starting from 8 hours and onwards. In the chlorine-treated group, all histopathological samples showed Cryptosporidium oocysts regardless of variation in exposure time.
Figure 2.
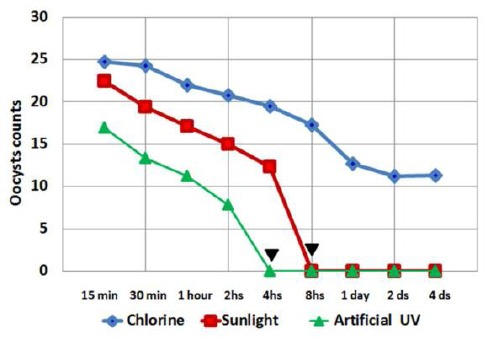
Line chart showing mean Cryptosporidium oocysts/villus crypt unit in each subgroup of mice infected with oocysts previously exposed for various durations of time to sunlight, artificial UV irradiation and chlorine treatment. Arrowheads denote complete inactivation caused by artificial UV rays at 4 hours while full sunlight inactivation appears at 8 hours
Figure 3.
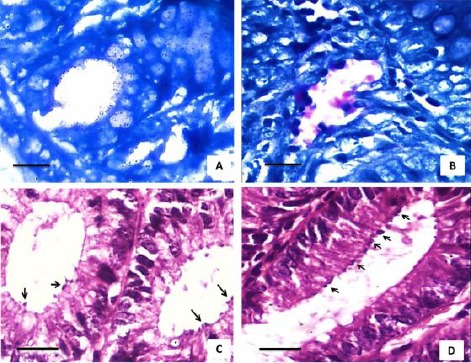
Photomicrographs for different histopathological sections prepared from mice intestines. A) Histopathological section stained with Modified ZN stain shows negative result for Cryptosporidium infection; B) Histopathological section showing pink spherical structures about 2-5 µm attached to microvillus surface of epithelium, confirming Cryptosporidium intestinal infection (scale = 25 µm); C), D) Histopathological cross sections for the intestine stained with Haematoxylin & Eosin show many Cryptosporidium oocysts (black arrows) attached to microvillus surface of epithelium (Scale = 50 µm)
Severe inflammatory reaction with moderate neutrophils infiltration was reported in intestinal sections of mice infected by samples exposed to chlorine for 2 and 4 days. No oocysts were detected in the subgroups exposed to sunlight for 8 hours, 2 days and 4 days. The moderate inflammatory reaction was observed up to the subgroup exposed to sunlight for 8 hours with slight neutrophil infiltration indicating a mild inflammatory activity.
The slight inflammatory reaction, though without neutrophils was observed in the subgroup exposed for 2 days to sunlight. Histopathological sections of subgroups exposed to artificial UV rays for 4 hours or more proved to be free from Cryptosporidium. Moderate inflammatory reaction and moderate inflammatory activity were reported in the subgroups exposed for one hour or two hours while severe inflammatory reaction with moderate neutrophil infiltration was recorded with the subgroups exposed to artificial UV irradiation for 15 min and 30 min. Those exposed for 4 hours reported only slight inflammatory reaction with minor neutrophil activity.
Discussion
Concerning exposure to sunlight, the results of the present study showed that at least 8 hours were needed to achieve infection inhibitory effect with zero Cryptosporidium oocysts in the stool samples of the infected mice. This nil effect was achieved as well in case of one day, 2 days and 4 days exposure to direct sunlight. This may be due to the helping effect of the sunlight which reached 42°C. Gomez-Couso et al., [9] recorded slightly higher temperature 45°C to achieve significant reduction in their murine model. Using a batch-process solar disinfection system (SODIS), exposure times of more than 10 hours was recorded to simulate solar irradiation rendering Cryptosporidium oocysts non infective [18]. On the other hand, the effect of sunlight on the survival of Cryptosporidium oocysts in a waste stabilization pond system in the northwestern of Spain was studied [19], reporting only a 40% reduction after 4 days exposure. However, these dissimilar results may be related to the difference in the nature of water used. In addition, most of the previously cited studies were performed in Europe, where the sun rays may be masked by a lot of clouds and the temperature is usually lower than that recorded in this experiment. In fact, the portion of sun rays that reaches us is made up of two types of rays: long wave ultraviolet A (UVA) and short wave ultraviolet B (UVB) and basically, there is no UVC [20]. The shorter bands of UVC, as well as the more energetic UV radiation produced by the sun, are absorbed by oxygen and generate the ozone in the ozone layer [21]. The fraction of UVB which remains in UV radiation after passing through the atmosphere is heavily dependent on cloud cover and atmospheric conditions. Thick clouds block UVB effectively; but in “partly cloudy” days, patches of blue sky showing between clouds are also sources of (scattered) UVA and UVB, which are produced. The underlying mechanism by which sunlight irradiation inactivates microorganisms is possibly through damaging their DNA. Dark repair can occur after exposure of these inactivated microorganisms to darkness. Dark repair is a phenomenon in which damaged DNA of these inactivated microorganisms can be repaired and regain its activity in the absence of light. Thus, the resulted inactivation might not be permanent inactivation [9]. For this reason, in the present study, the complete inactivation for Cryptosporidium oocysts that is occurred in case of one day sunlight subgroup which is the same effect obtained on 8 hours sunlight subgroup denoted that no resuming of infectivity had occurred for these oocysts upon absence of light, resulting in permanent inactivation.
Concerning chlorine as a disinfectant, it was extensively studied using different formulas, concentrations and in different conditions. Therefore, it is difficult to find identical results among variable scientific works. In this study, chlorine was used in a solution form (Sodium hypochlorite) in a concentration of 4 ppm at 25oC. Regardless the variable reduction rate reported in the current study which ranged from 33.07 to 58.42, even after 4 days of treatment, chlorine failed to inhibit Cryptosporidium infection. Instead, the small intestine of infected animals showed signs of inflammation. This inflammation was severe in animals infected with sampled exposed to 2 and 4 days exposure, indicating the irritant effect of such chemical disinfectant with time, which was not only dose-dependent but also time dependent. Using sodium hypochlorite as well, Fayer et al., [22] tested its effect with different concentration (5.25, 2.63 and 1.31%). Using an in vivo animal model they reported lower oocysts counts in histopathological examination of the infected small intestine, whereas the number of parasitic stages identified in the animals’ stool samples was more or less similar to our results, confirming the reported data. Relatively higher reduction rate was recorded by Barbee et al., [23] who showed a decrease in infectivity of Cryptosporidium by 90% after exposure to Clorox (NaOCl, 5.25 %). While, Weir et al., [24] described a poor effect of sodium hypochlorite (6%) up to an exposure time of 33 min which was undoubtedly an insufficient duration compared to the other recorded data including our study.
Furthermore, Venczel et al., [3], using another formula of chlorine found that 5 mg/ litre dose of free chlorine at 25°C produced no measurable inactivation of Cryptosporidium oocysts after 4 or 24 hours. Two years earlier, Finch et al., [25] did not find as well any effect of free chlorine in inactivation of Cryptosporidium oocysts. Despite the previously mentioned negative reports about chlorine, Delling et al., [26] used different substances; one of them was sodium hypochlorite (NaOCl) at different concentrations for several exposure times. Unexpectedly, their results showed an inactivation over 99 % by using 3 and 6 % NaOCl after 12 hours exposure.
Concerning the effect of artificial UV irradiation to inactivate Cryptosporidium oocysts, in the current study an exposure to low pressure (LP) UV lamp for 4 hours at 25°C that was equivalent to 10 mJ/cm2, proved to be effective in aborting Cryptosporidium infection, resulting in a 100% reduction rate. Nearly similar, Clancy et al., [4] noticed that low doses of UV radiation from either low or medium pressure lamps were effective in achieving an inactivation level of greater than three logs (99.9%) for Cryptosporidium oocysts in waste water effluent as measured by cell culture. UV collimated-beam apparatus was used to focus parallel rays and expose suspensions of purified Cryptosporidium oocysts in phosphate-buffered saline at 25°C to various doses of monochromatic LP UV rays [10]. The former workers using in vitro cell culture assay reported Cryptosporidium parvum infectivity reductions at a dose of 3 mJ/cm2 (530 J/m2). These results indicate that Cryptosporidium oocysts are very sensitive to inactivation by low doses of monochromatic LP UV radiation [10] [27]. In the present study, the effect of temperature on artificial UV irradiation had not been investigated because UV ray is temperature independent according to Craik et al., [28]. Also, the previous authors [28] found that the conventional low-pressure mercury arc lamp and the medium-pressure lamp were equally effective at inactivation of Cryptosporidium oocysts so that there was no apparent biocidal benefit arising from the broad emission spectrum or higher irradiance of the medium-pressure UV lamp. The choice of the lamp to be used for a given application should, therefore, be based on economic considerations. Low-pressure lamps have about twice the germicidal efficiency (UV dose delivery per watt of input power) compared to medium-pressure lamps, and thus low-pressure lamps will be the choice for smaller systems, so we used LP UV lamps in the experimental setting. Certainly, there is a very beneficial point favouring the use of UV over the other disinfectant methods [10] [27]. It is a physical process that does not rely on the use of chemical additions, it has been shown to be highly effective in the inactivation of protozoa, it requires relatively short contact times, and no UV disinfection by-products have been currently identified [29].
Finally, another important result in this study is that exposing plastic bottled water for 8 hours to sunlight, the chosen time to be that of maximum intensity of UV radiation, completely aborts the ability of Cryptosporidium oocysts to produce infection, thus an excellent method for Cryptosporidium inactivation in summer months. Still, there is an urgent need for different systematical investigations for the solar inactivation mechanisms for Cryptosporidium oocysts under a wide range of environmental conditions. Such studies will offer an economic and convenient method for Cryptosporidium oocysts inactivation especially in developing countries even in household applications. Furthermore, the possibility of synergistic effects from mixtures of disinfectants is recommended to be investigated in further studies.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All ethical standards applied in the animal house of Kasr-Alainy school of Medicine, Cairo University was followed.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. Journal of water and health. 2007;5(1):1–38. doi: 10.2166/wh.2006.002. https://doi.org/10.2166/wh.2006.002 PMid:17402277. [DOI] [PubMed] [Google Scholar]
- 2.Li N, Xiao L, Alderisio K, Elwin K, Cebelinski E, Chalmers R, Santin M, Fayer R, Kvac M, Ryan U, Sak B. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerging infectious diseases. 2014;20(2):217. doi: 10.3201/eid2002.121797. https://doi.org/10.3201/eid2002.121797 PMid:24447504 PMCid: PMC3901490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venczel LV, Arrowood M, Hurd M, Sobsey MD. Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Applied and Environmental Microbiology. 1997;63(4):1598–601. doi: 10.1128/aem.63.4.1598-1601.1997. PMid:9097455 PMCid: PMC168452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaefer FW, Marshall MM, Clancy JL. Inactivation and Removal of Enteric Protozoa in Water BT - The Pathogenic Enteric Protozoa: Giardia, Entamoeba, Cryptosporidium and Cyclospora. In: Sterling Charles R, Adam Rodney D, editors. Boston, MA: Springer US;; 2004. pp. 117–27. PMid:15255221. [Google Scholar]
- 5.Liu Y, Dong S, Kuhlenschmidt MS, Kuhlenschmidt TB, Drnevich J, Nguyen TH. Inactivation mechanisms of Cryptosporidium parvum oocysts by solar ultraviolet irradiation. Environmental Science: Water Research & Technology. 2015;1(2):188–98. https://doi.org/10.1039/C4EW00079J. [Google Scholar]
- 6.Lumb R, Swift J, James C, Papanaoum K, Mukherjee T. Identification of the Microsporidian parasite, Enterocytozoon bieneusi in faecal samples and intestinal biopsies from an aids patient. Int J Parasitol. 1993;23(6):793–801. doi: 10.1016/0020-7519(93)90077-c. https://doi.org/10.1016/0020-7519(93)90077-C. [DOI] [PubMed] [Google Scholar]
- 7.Gaafar Maha R. Effect of solar disinfection on viability of intestinal protozoa in drinking water. J Egypt Soc Parasitol. 2007;37(1):65–86. PMid:17580569. [PubMed] [Google Scholar]
- 8.Garcia LS, Bruckner DA. Diagnostic Med. Parasitol. 5th ed. Washington D.C: ASM press; 1997. Macroscopic and microscopic examination of fecal specimens; pp. 608–49. [Google Scholar]
- 9.Gómez-Couso H, Fontán-Sainz M, Ares-Mazás E. Thermal contribution to the inactivation of Cryptosporidium in plastic bottles during solar water disinfection procedures. The American journal of tropical medicine and hygiene. 2010;82(1):35–9. doi: 10.4269/ajtmh.2010.09-0284. https://doi.org/10.4269/ajtmh.2010.09-0284 PMid:20064992 PMCid: PMC2803506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morita S, Namikoshi A, Hirata T, Oguma K, Katayama H, Ohgaki S, Motoyama N, Fujiwara M. Efficacy of UV irradiation in inactivating Cryptosporidium parvum oocysts. Applied and environmental microbiology. 2002;68(11):5387–93. doi: 10.1128/AEM.68.11.5387-5393.2002. https://doi.org/10.1128/AEM.68.11.5387-5393.2002 PMid:12406729 PMCid: PMC129916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter C, Fayer R, Trout J, Beach MJ. Chlorine disinfection of recreational water for Cryptosporidium parvum. Emerg Infect Dis. 1999;5(4):579–84. doi: 10.3201/eid0504.990425. https://doi.org/10.3201/eid0504.990425 PMid:10458969 PMCid: PMC2627758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benamrouz S, Guyot K, Gazzola S, Mouray A, Chassat T, Delaire B, Chabé M, Gosset P, Viscogliosi E, Dei-Cas E, Creusy C. Cryptosporidium parvum infection in SCID mice infected with only one oocyst: qPCR assessment of parasite replication in tissues and development of digestive cancer. PLoS One. 2012;7(12):e51232. doi: 10.1371/journal.pone.0051232. https://doi.org/10.1371/journal.pone.0051232 PMid:23272093 PMCid: PMC3521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi P, Pozio E, Besse MG, Gomez Morales MA, La Rosa G. Experimental cryptosporidiosis in hamsters. J Clin. 1990;28(2):356–7. doi: 10.1128/jcm.28.2.356-357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia LS. Diagnostic Med. Parasitol. 5th ed. Washington D.C: ASM press; 2007. Clinically important human parasites: Intestinal protozoa: Cryptosporidium spp; pp. 771–812. [Google Scholar]
- 15.Certad G, Ngouanesavanh T, Guyot K, Gantois N, Chassat T, Mouray A, Fleurisse L, Pinon A, Cailliez JC, Dei-Cas E, Creusy C. Cryptosporidium parvum, a potential cause of colic adenocarcinoma. Infectious agents and cancer. 2007;2(1):22. doi: 10.1186/1750-9378-2-22. https://doi.org/10.1186/1750-9378-2-22 PMid:18031572 PMCid: PMC2217515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rychlik A, Nieradka R, Kander M, Nowicki M, Wdowiak M, Kolodziejska-Sawerska A. A correlation between the canine Inflammatory Bowel Disease Activity Index score and the histopathological evaluation of the small intestinal mucosa in canine inflammatory bowel disease. Pol J Vet Sci. 2012;15(2):315–21. doi: 10.2478/v10181-012-0093-4. https://doi.org/10.2478/v10181-012-0093-4 PMid:22844710. [DOI] [PubMed] [Google Scholar]
- 17.Healey MC, Yang S, Rasmussen KR, Jackson MK, Du C. Therapeutic efficacy of paromomycin in immunosuppressed adult mice infected with Cryptosporidium parvum. J Parasitol. 1995;81(1):114–6. https://doi.org/10.2307/3284020 PMid:7876965. [PubMed] [Google Scholar]
- 18.McGuigan KG, Mendez-Hermida F, Castro-Hermida JA, Ares-Mazas E, Kehoe SC, Boyle M, et al. Batch solar disinfection inactivates oocysts of Cryptosporidium parvum and cysts of Giardia muris in drinking water. J Appl Microbiol. 2006;101(2):453–63. doi: 10.1111/j.1365-2672.2006.02935.x. https://doi.org/10.1111/j.1365-2672.2006.02935.x PMid:16882154. [DOI] [PubMed] [Google Scholar]
- 19.Reinoso R, Bécares E. Environmental inactivation of Cryptosporidium parvum oocysts in waste stabilization ponds. Microbial ecology. 2008;56(4):585–92. doi: 10.1007/s00248-008-9378-7. https://doi.org/10.1007/s00248-008-9378-7 PMid:18345476. [DOI] [PubMed] [Google Scholar]
- 20.Halliday Gary M, Byrne Scott N, Damian Diona L. Ultraviolet A radiation: its role in immunosuppression and carcinogenesis. Semin Cutan Med Surg. 2011;30(4):214–21. doi: 10.1016/j.sder.2011.08.002. https://doi.org/10.1016/j.sder.2011.08.002 PMid:22123419. [DOI] [PubMed] [Google Scholar]
- 21.Stephens Thomas J, Herndon James H, Jr, Colon Luz E, Gottschalk Ronald W. The impact of natural sunlight exposure on the UVB-sun protection factor (UVB-SPF) and UVA protection factor (UVA-PF) of a UVA/UVB SPF 50 sunscreen. J Drugs Dermatol. 2011;10(2):150–5. PMid:21283919. [PubMed] [Google Scholar]
- 22.Fayer R. Effect of sodium hypochlorite exposure on infectivity of Cryptosporidium parvum oocysts for neonatal BALB/c mice. Appl Environ Microbiol. 1995;61(2):844–6. doi: 10.1128/aem.61.2.844-846.1995. PMid:7574626 PMCid: PMC167349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbee SL, Weber DJ, Sobsey MD, Rutala WA. Inactivation of Cryptosporidium parvum oocyst infectivity by disinfection and sterilization processes. Gastrointest Endosc. 1999;49(5):605–11. doi: 10.1016/s0016-5107(99)70389-5. https://doi.org/10.1016/S0016-5107(99)70389-5. [DOI] [PubMed] [Google Scholar]
- 24.Weir Susan C, Pokorny Nicholas J, Carreno Ramon A, Trevors Jack T, Lee Hung. Efficacy of Common Laboratory Disinfectants on the Infectivity of Cryptosporidium parvum Oocysts in Cell Culture. Appl Environ Microbiol. 2002;68(5):2576–9. doi: 10.1128/AEM.68.5.2576-2579.2002. https://doi.org/10.1128/AEM.68.5.2576-2579.2002 PMid:11976138 PMCid: PMC127548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finch GR, Black EK, Gyurek LL. Ozone and chlorine inactivation of Cryptosporidium. Water Qual Technol Conf Am Water Work Assoc Denver, Colo. 1995:1303–1318. [Google Scholar]
- 26.Delling C, Holzhausen I, Daugschies A, Lendner M. Inactivation of Cryptosporidium parvum under laboratory conditions. Parasitology research. 2016;115(2):863–6. doi: 10.1007/s00436-015-4813-4. https://doi.org/10.1007/s00436-015-4813-4 PMid:26566617. [DOI] [PubMed] [Google Scholar]
- 27.Ryu H, Gerrity D, Crittenden JC, Abbaszadegan M. Photocatalytic inactivation of Cryptosporidium parvum with TiO2 and low-pressure ultraviolet irradiation. Water research. 2008;42(6-7):1523–30. doi: 10.1016/j.watres.2007.10.037. https://doi.org/10.1016/j.watres.2007.10.037 PMid:18037465. [DOI] [PubMed] [Google Scholar]
- 28.Craik SA, Weldon D, Finch GR, Bolton JR, Belosevic M. Inactivation of Cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 2001;35(6):1387–98. doi: 10.1016/s0043-1354(00)00399-7. https://doi.org/10.1016/S0043-1354(00)00399-7. [DOI] [PubMed] [Google Scholar]
- 29.Betancourt Walter Q, Rose Joan B. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet Parasitol. 2004;126(1):219–34. doi: 10.1016/j.vetpar.2004.09.002. https://doi.org/10.1016/j.vetpar.2004.09.002 PMid:15567586. [DOI] [PubMed] [Google Scholar]


