Abstract
The intestinal immune system is intimately connected with the vast array of microbes present within the gut and the diversity of food components that are consumed daily. The discovery of novel molecular mechanisms, which mediate host-microbe-nutrient communication, have highlighted the important roles played by microbes and dietary factors in influencing mucosal inflammatory and allergic responses. In this review, we summarize the recent important findings in this field, which are important for food allergy and particularly relevant to animal models of food allergy.
Introduction
Food allergies are a growing health problem affecting a significant proportion of the population, associated with a substantial impact on quality of life and economic burden [1, 2]. Why some individuals develop allergic reactions to specific foods, while the majority tolerates these food antigens, is largely unknown. However, it is likely that the interplay between genetic factors, microbial composition and metabolic activity, dietary factors, or timing of antigen exposure may play a crucial role. Animal models of food allergy have allowed investigators to individually modulate and test specific factors, which influence sensitization and severity of disease. This review is focused on the recent knowledge gained from animal models investigating the influence of the microbiome and diet on the development of food allergies. Table 1 shows an overview about the models discussed in this review.
Table 1.
Overview of food allergy models
| Animal model | Strain Mutation | Antigen | Adjuvant | Treatment | Reference |
|---|---|---|---|---|---|
| Mouse | C57BL/6 | peanut | cholera toxin | kanamycin (4 mg/mL), gentamicin (0.35 mg/mL), colistin (8500 U/mL), metronidazole (2.15 mg/mL), and vancomycin (0.45 mg/mL) After weaning, the Abx were administered at 50-fold dilution except for vancomycin, which was maintained at 0.5 mg/mL colonization of Clostridia |
[5] |
| Mouse | BALB/cTac WT and Il4raY709 | egg protein OVA | staphylococcal enterotoxin B | WT mice reconstitution with flora derived from OVA-sensitized WT or Il4raF709 mice | [16] |
| Mouse | BALB/c | na | na | B. breve AH1205 (Bifidobacterium AH1205), B. longum AH1206 (Bifidobacterium AH 1206) and Lactobacillus salivarius AH102 of human origin |
[20] |
| Mouse | C3H/HeN | β-lactoglobulin whey protein | cholera toxin | colonization with the infant microbiota (dominance of Bifidobacterium and Bacteroides species) | [18] |
| Mouse | BALB/c | egg protein OVA | Al(OH)3 | control diet containing 15 % casein as a protein source or an experimental diet containing 15 % of a mixture of amino acids | [22] |
| Mouse | BALB/c | egg protein OVA | Al(OH)3 | raw bovine milk, raw bovine milk heated to 87°C, raw bovine milk gamma irradiated | [24] |
| Mouse | BALB/c | egg protein OVA | aluminum potassium sulfate | Ag-free diet, amino acid diet (AAD), L-amino-acid defined AIN-93G diet, irradiated and vacuum-packed AAD ampicillin (1g/L), neomycin (1g/L), metronidazole (1g/L), and 0.5g/L of vancomycin (1g/L) |
[23] |
| Mouse | BALB/c C57BL/6 WT and CD1d−/− and Jα18−/− |
Ber e 1 | na | different lipid fractions (600 μg) from Brazil nut seeds | [26] |
| Mouse | BALB/cAnNCrl mice | Ovomucoid β-lactoglobulin | Al(OH)3 | untreated antigen, sham-nitrated antigen or nitrated antigen | [25] |
| Mouse | BALB/c | egg protein OVA | Freund's adjuvant | Oil diet | [28] |
| Mouse | C3H/HeOuJ | whey protein | cholera toxin | cows’ milk protein free AIN-93G diet (containing 7 % soyabean oil) or a 10 % soyabean oil diet (59·1 % PUFA, of which 53·1 % was LA (n-6 PUFA), 5·6 % a-linolenic acid (n-3 PUFA), 24·9 % MUFA (oleic acid) and 15·1 % SFA (palmitic acid and stearic acid)) | [29] |
| Mouse | WT and Sphk1−/−, Sphk2−/−, S1pr2−/−, and S1pr3−/− ,S1pr4−/− S1pr1loxp/loxp-Mx , WSh/WSh | DNP36-HSA ( + DNP-specific IgE ) | na | polyinosinic-polycytidylic acid, histamine, albumin | [30] |
| Mouse | C57BL/6 WT and SphK1−/− SphK2 −/− l | egg protein OVA | Al(OH)3 | proton-pump-inhibitor omeprazole, sucralfate, anti-acid medication | [4] |
| Mouse | BALB/c OlaHsd | egg protein OVA | cholera toxin | different polyphenol-enriched apple extracts, polyphenol-enriched cocoa extract or purified epicatechin | [32] |
| Rat | Brown Norway | egg protein OVA | Al(OH)3, toxin from Bordetella pertussis | diet with no polyphenols, two cocoa-enriched diets either including conventional cocoa (CC) or cocoa flavonoids from nonfermented cocoa (NFC), both containing 0.4% of polyphenols | [33] |
| Mouse | BALB/c | Celery proteins | Al(OH)3 | acid-suppressed by proton pump inhibitor, followed by application of the celery extract mixed with 2 mg sucralfate. Other groups: celery extract alone. | [35] |
| Mouse | BALB/c | egg protein OVA | Al(OH)3 | Diets containing either 0.08, 0.25, or 2.7 ppm Se. | [38] |
| Mouse | BALB/c WT and H2R-/- | na | na | L. saerimneri 30a, famotidine | [41] |
| Mouse | BALB/c WT and H2R-/- | na | na | L. rhamnosus | [42] |
| Mouse | C57Bl/6 WT and Gpr43-/- | acute DSS colitis, chronic DSS colitis, TNBS-induced colitis, K/BxN inflammatory arthritis model, allergic airway disease (OVA/alum) | [43] | ||
| Mouse | C57BL/6J | na | na | diet-induced obesity (high fat diet) | [46] |
| Mouse | C57BL/6 | na | na |
S. flexneri 5a (M90T), IpaB4 deletion mutant S. flexneri 5a (M90TΔIpaB4), wild-type Salmonella typhimurium (UK-1), and non-invasive Shigella strains (BS176) sphingosine-1-phosphate |
[47] |
| Mouse | C57BL/6 WT and CD1d-/- | na | na | KRN7000 (1 µg/ml), bacterial lipid GSL-Bf717, PE-Cers | [48] |
| Mouse | C57BL/6 | dextran sodium sulfate (DSS) colitis model B. infantis | [49] | ||
| Mouse | BALB/c | wheat-deaminated gliadins (pups) | Al(OH)3 (pups) | diet supplemented with 4% galacto-oligosaccharides and inulin in a 9 : 1 ratio, during pregnancy and breastfeeding | [57] |
| Mouse | C57BL/6 GPR41-/- and GPR43-/- |
HDM, OVA | na | low-fiber diet, high-fiber diet (normal chow supplemented with 30% cellulose or 30% pectin) sodium propionate, sodium acetate |
[52] |
| Mouse | BALB/c | egg protein OVA | Al(OH)3 | standard-fiber chow (4% content) or a low-fiber chow (1.75% content), an extra fiber supplementation of soluble pectin or insoluble cellulose | [53] |
| Mouse | BALB/c and DO.11.10 transgenic mice | na | na |
L. rhamnosus (JB-1), L. salivarius UCC118 heme oxygenase inhibitor (Chromium(III) Mesoporphyrin IX chloride) |
[55] |
| Mouse | C3H/HeJ | Shrimp tropomyosin | cholera toxin | probiotic VSL#3 (lyophilized mixture of Lactobacillus acidophilus, L. delbrueckii subsp.bulgaricus, L. casei, L. plantarum, Bifidobacterium longum, B. infantis, B. breve, Streptococcus salivarius subsp. thermophilus) | [56] |
| Mouse | BALB/cByJ | cow's milk | cholera toxin | GF mice were orally inoculated with a 1:100 dilution of fecal homogenate freshly prepared from CV mice | [61] |
| Mouse | BALB/c, Swiss–Webster, C57BL/6, Rag1−/− BaS-TRECK Csf2rb−/− Csf2rb−/− Igh–7−/− IL–4/eGFP reporter Il4∓/− Myd88−/− Nod1−/− Tslp−/− |
ampicillin (0.5 mg ml–1), gentamicin (0.5 mg ml–1), metronidazole (0.5 mg ml–1), neomycin (0.5 mg ml–1), and vancomycin (0.25 mg ml–1) Papain, antibodies, CpG, diphtheria toxin |
[12] |
Food allergy models overview
In animal models, adjuvants are usually required to induce sensitization to food allergens and are typically applied in parallel with the allergen. Experimental adjuvants include cholera toxin, staphylococcal enterotoxin B (also known to play a role in human allergic diseases), or aluminum hydroxide. They generally induce a strong T helper cell type-2 response via their influence on dendritic cell or macrophage phenotypes or can also inhibit regulatory T cells [1, 3]. While sensitization can be induced via different routes such as oral, intranasal, sublingual, or cutaneous, the presence of adjuvants or danger signals (e.g. tape stripping the skin prior to cutaneous exposure), or the impairment of physiological gastric digestion[4, 5] is crucial. In addition to measuring sensitization (e.g. IgE induction), allergen challenge can result in anaphylaxis, which is assessed by symptoms (scratching, diarrhea, piloerection, labored respiration, cyanosis around mouth and tail, reduced activity, tremors, convulsion or death) and drop in body temperature [6]. Besides murine and rat food allergy models, there are also food allergy models in pigs, dogs or sheep. The advantages and disadvantages of different food allergy animal model design parameters have been reviewed extensively elsewhere [7]
Influence of microbiota on food allergy
There has been an increase in the number of individuals suffering from allergic and inflammatory diseases over the last decades, particularly in Western, developed countries [8]. The hygiene hypothesis suggests that altered exposure to environmental factors may play a part in this phenomenon. It suggests that excessive hygiene practices and limited contact with microorganisms may contribute to allergic sensitization, including sensitization to food allergens [9]. Other factors have also been linked with alterations in the gut microbiome and increased risk of food allergy, such as excessive antibiotic use (especially during infancy), high fat diet and mode of delivery [10, 11].
The importance of the host microbiota on immune system in mice models was investigated with germ-free mice and broad-spectrum antibiotic treated mice. In both cases serum IgE level as well as basophil numbers were increased and mice displayed exaggerated allergic responses. Moreover, signals derived from commensal bacteria regulated bone marrow basophils development, which shows that the microbiota can influence hematopoetic programs in addition to regulation of immune cells in the mucosa. These studies are relevant to human diseases, especially in the context of children’s exposure to antibiotics early in life. [12]
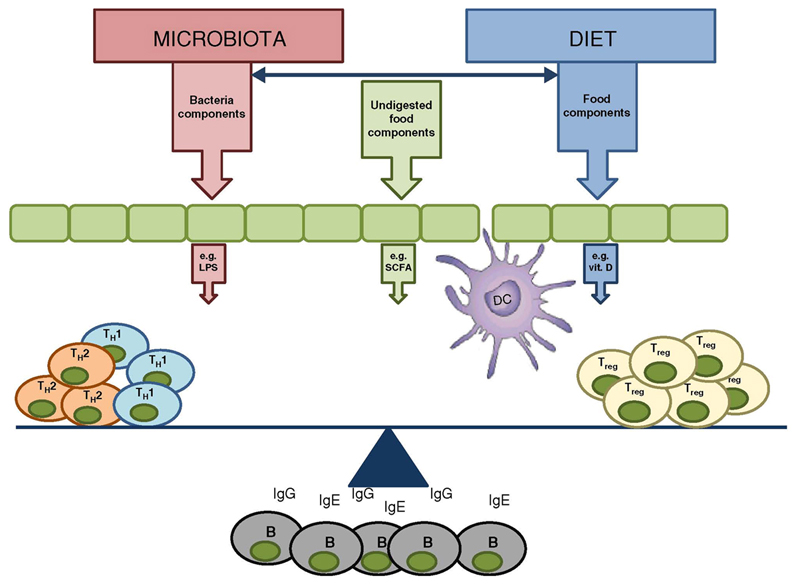
The human gut is colonized with approximately 1014 bacteria, which represents approximately 1,500 different species, [13] typically dominated by two phyla, the Bacteroidetes and the Firmicutes [14]. Many human studies and animal models have now demonstrated that appropriate host-microbiota interactions are essential for immunological development and oral tolerance. An overview of host-microbiota interactions is illustrated in Figure 1.
Figure 1.
Microbiome and diet influences mucosal immune responses. Bacterial components, dietary components and metabolites released by bacterial metabolism of undigested dietary components impact on epithelial cells, innate immune cells and adaptive immune cell polarisation. Exposure of the immune system to bacterial components or dietary factors can either promote allergic responses (e.g. LPS) or dampen allergic responses (e.g. SCFA). Allergic responses are characterized by an increase in IgE, IgG and Th2 cell numbers and a decrease of Treg cell numbers or activity.
Neonatal mice treated with broad-spectrum antibiotics became more prone to peanut allergy, as evidenced by increased levels of circulating peanut specific IgE and IgG1 antibodies [15]. In addition, another study examining the influence of a dysbiotic microbiota on food allergy was investigated in Il4raF709 mice [16]. Il4raF709 mice carry a mutation in the IL-4 receptor chain. This gain-of-function mutation results in augmented signal transducer and activator of transcription 6 activation by IL-4 and IL-13, which promotes allergy responses by increasing of IgE and mast cells level, after antigen sensitization [17]. The study clearly demonstrated that the microbiome composition differed between allergic (Il4raF709 mice) OVA-sensitized mice and wild type food allergic mice. Moreover, when the microbiome from allergic mice was transplanted into germ-free wild-type mice, these mice developed higher OVA-specific IgE antibody titres and more severe allergic reactions, suggesting that allergic sensitization may be influenced by microbiome composition. In this animal model, allergic responses were associated with a decreased abundance of Firmicutes and increased abundance of Proteobacteria [16].
Colonization of gnotobiotic mice with Clostridia (Firmicutes pylum) suggested a food allergy-protective effect. Moreover, when Clostridia were reintroduced to antibiotic treated mice, the sensitization to food allergen was decreased. The reason might be connected with the fact that Clostridia induce IL-22 production which reduces uptake of antigens from food into the systemic circulation [15]. Germ free mice colonized with microbiota from healthy human infants exhibited milder allergic symptoms after sensitization with whey protein in comparison to mice that remained germ free. The healthy infant gut microbiome is typically dominated by Bifidobacteria and Bacteroides, which are known to have anti-inflammatory properties[18]. Decreased diversity of the Bacteroidetes phylum was also reported in a separate study examining infants with atopic eczema [19]. However, not all Bifidobacteria species are equally effective in the generation of mucosal regulatory T cells or in their protective effects in food allergy models [20]. Interestingly, germ free mice colonized with microbes from healthy humans were characterized by lower plasma level of antigen specific IgG1, with no significant changes in IgE levels, suggesting that the protective effects on allergic responses are IgE-independent in this model [18].
Influence of dietary components on food allergy
The accurate assessment of food allergy in animal models requires careful control of dietary factors, in addition to the microbiome itself. It is important to establish stabilized animal model, as both diet and microbiota play an important role in food allergy and the response of immune system may be influenced by microbiome-diet interactions.in addition, when attempting to translate food allergy model across different laboratories, it is important to consider the effect of microbiota and diet on the study results. When establishing a food allergy animal model, the composition of the diet has to be considered specifically, as dietary antigens are the triggering factor for eliciting an immune response in these model systems. Dietary pre-exposure to the test antigen must be avoided, not only in experimental animals but also from parental generations due to the potential antigen transfer in utero [21]. Moreover, selection of suitable, relevant antigens for immunization and deciding on using whole foods (including food matrixes and related component) versus single purified allergens are crucial considerations for sensitization and challenge outcomes.
Timing of exposure and the nature of proteins themselves can contribute to immune activation and maturation, as the absence of dietary proteins until adulthood was associated with milder allergen-specific immune responses in mice following sensitization and paradoxically also hampered oral tolerance induction [22]. A recent study reported that depletion of dietary antigens by feeding an elemental diet was associated with decreased numbers of regulatory T cells developing extra-thymically in the intestine from conventional T cells and was associated with less severe mucosal inflammatory and allergic responses [23]. In addition, protein denaturation, e.g. milk heat treatment or milk gamma sterilization, or modification of the amino acids, e.g. tyrosine nitration, have a substantial influence on the immune outcome in food allergy models [24, 25].
Lipid-containing food matrixes influence the allergic response, as specific allergen bound lipid fractions were revealed to be essential for induction of Brazil-nut specific IgE and IgG1 antibodies in mice after intraperitoneal administration [26]. Lipids as matrix components not only influence food allergy development by interaction with allergenic proteins, but also have intrinsic immunomodulating properties. Polyunsaturated fatty acids (PUFA) and short chain fatty acids (SCFA) have been extensively investigated in this regard. Intake of n-6 PUFA rich soyabean oil increased the allergic response towards whey proteins in a concentration dependent manner and hindered tolerance induction when feeding partial whey hydrolysate before sensitization [27]. In contrast, the anti-inflammatory effects of n-3 PUFA containing linseed oil were mediated by conversion of dietary n-3 α-linolenic acid to 17,18-epoxyeicosatetraenoic acid in the gut [28]. Feeding of fish oil rich in n-3 PUFA was associated with prevention of cow’s milk sensitization and protection was transferred by injection of CD25+ T regulatory cells into naive recipient animals [29]. Other lipid components are also important contributors to food allergy. Sphingolipids are essential constituents of the outer cellular membranes but also have bioactive functions, e.g. activation of immune cells. Sphingosine-1-phospate (S1P) is produced by mast cells and signals back to these cells in an autocrine manner. Even though the S1P converting enzyme Sphingosine Kinase (SphK) 1 as well as the S1P receptor 2 were reported to be essential for recovery from severe anaphylactic reactions [30], it was demonstrated that intrinsic S1P production via both SphK 1 and 2 was essential for food allergen sensitization and effector cell activation in a oral mouse food allergy model, potentially via impaired intestinal epithelial barrier function [4].
In addition to the food compounds mentioned above, there is a large number of micronutrients that influence food allergy outcome by direct immune modulation, such as vitamins, trace elements and plants polyphenols. For example, vitamin D can be taken up via the diet, even though the major part is produced in the skin upon UV exposure. The inverse correlation of vitamin D levels with food allergy development has been extensively studied in human as well as animal studies underlining its modulatory effects on the innate as well the adaptive immune system [31]. Polyphenols also show immunomodulating properties. In mouse as well as rat food allergy models, polyphenols from plant sources such as cocoa were associated with reduced Th2 antibody response and an overall anti-allergic protective effect [32, 33]. Trace elements support physical barriers (skin/mucosa), cellular immunity and antibody production, and modulate immune cell function by regulating redox-sensitive transcription factors, thus affecting production of cytokines and prostaglandins [34]. Both iron and zinc serum levels were significantly reduced in aged animals when compared to younger adult mice, however food allergy could be induced equally in both groups under acid-suppressing conditions [35]. Novel in vitro data suggest an effect of low iron levels on allergy induction. The milk allergen Bos d 5 as well as the aeroallergen Bet v 1 from birch induced higher CD4+ T cell numbers and Th2-cytokine responses in addition to IFN-γ in human PBMC from healthy as well as from allergic patients only when not-loaded with iron [36, 37]. Selenium has important functions in lymphocyte activity, and protects immune cells against oxidative damage as component of selenoproteins. Selenium deficiencies reduce antibody production, lymphocyte proliferation and cytotoxicity of immune-competent cells, whereas synthesis of proinflammatory eicosanoids increases. In a murine study, high dietary selenium prevented the induction of asthma in OVA-sensitized mice [38].
Apart from these beneficial constituents of the diet, also detrimental components such as toxins can influence the immune response. Mediators such as histamine, which is produced by bacterial metabolism of the amino acid histidine and is associated with ageing of certain foods, have direct effects on the immune system via interaction with receptors on immune cells. These receptors are expressed by mucosal cells and receptor expression is altered during mucosal inflammatory responses [39, 40]. Binding of histamine to its receptor 2 on immune cells such as T cells, B cells and dendritic cells (DC) was reported to influence mucosal immunity and response to microbial ligands [41, 42].
Interaction between diet, microbiota, metabolism and the immune system
Dietary factors not only directly influence immune signaling, but also indirectly affect the microbiome composition and metabolic activity of the host. SCFA derived from intestinal microbes are important for mucosal homeostasis. The SCFA butyrate is an important energy source for colonocytes, and regulates the assembly and organization of tight junctions. In addition, SCFA bind G-protein coupled receptors (GPCRs), such as GPR41 and 43, thereby suppressing inflammation. Similarly to germ-free mice, mice deficient in GPR43 showed increased inflammatory responses in models of colitis, arthritis and asthma [43].
While a number of studies have shown SCFA-protective effects in murine asthma models, similar effects in murine food allergy models are less well described. However, the beneficial effect of a high fiber diet and SCFA production on gut inflammation has been demonstrated [44]. A high fiber diet or oral administration of SCFA increases regulatory T cells in the lamina propria of GF or antibiotic-treated mice. Moreover, tolerance to cow’s milk was improved in cow’s milk allergic infants following treatment with a probiotic formula that expanded butyrate-producing bacteria within the gut [45].
In a mouse model, feeding a high-fat diet (HFD) resulted in a (reversible) altered microbiota composition and bacterial diversity significantly declined in the HFD group after only 2 weeks of feeding. Furthermore, a gradual and significant increase of the relative abundance of Firmicutes and Proteobacteria, paralleled by a decrease in Bacteroidetes was observed [46]. Moreover, intestinal microbes release lipid mediators such as glycosphingolipids or modulate S1P-related genes of the host intestinal tissue resulting in attenuated intestinal inflammation and regulated natural killer T cell homeostasis [47, 48].
In addition to diet influencing microbiome activities, specific microbes can alter the host metabolism of dietary components. For example, vitamin A is metabolized by gut dendritic cells resulting in the secretion of retinoic acid, and retinoic acid is important for modulating mucosal inflammatory and tolerogenic responses. Specific bifidobacterial strains can upregulate expression of the enzyme that converts vitamin A into retinoic acid, thereby maximizing the anti-inflammatory effects of this vitamin [49]
Despite significant interest in this topic, a limited number of studies have been published linking the influence of diet on allergic responses via alterations of the microbiome (reviewed in [50]). Studies by Bouchaud et al. demonstrated that mice fed with the prebiotics galacto-oligosaccharides and inulin during pregnancy and breastfeeding, and their offspring were sensitized to wheat-gliadin after weaning [51]. Young animals showed a significantly reduced clinical and cellular Th2 response while T-regulatory responses increased and the intestinal barrier was preserved. Importantly, in this study the intestinal microbiota in feces were investigated in parallel in the offspring before sensitization, and showed that the supplemented maternal diet was associated with a higher total bacterial load, higher proportions of Lactobacillus and Clostridium leptum, and lower abundance of Clostridium coccoides in the offspring. However, similar changes in microbiota composition were observed after allergy induction in both offspring groups [51]. In another study, where diet and microbiome and allergy induction were evaluated in parallel, mice were fed a low-fiber diet before nasal sensitization with house dust mite extract. These animals developed higher local Th2 responses associated with increased mucus and goblet cell hyperplasia. In parallel the composition of the microbiome changed, with increased Erysipelotrichaceae in the low-fiber group, while a high-fiber diet promoted Bateroidaceae and Bifidobacteriaceae [52]. The latter diet increased circulating levels of SCFA and administration of the SCFA propionate enhanced generation of macrophage and DC precursors from bone marrow and subsequent presence of dendritic cells with high phagocytic capacity in lung tissue, associated with an impaired ability to induce Th2 effector cell functions. These effects were shown to depend on GPR41, but not GPR43 [52]. In another allergic OVA asthma mouse model, dietary fiber intake significantly prevented clinical symptoms, lowered eosinophil infiltration and goblet cell metaplasia in nasal and lung mucosa, reduced serum OVA-specific IgE levels as well as Th2 cytokines in NALF and BALF, which was paralleled by increased Bacteroidetes and Actinobacteria, whereas Firmicutes and Proteobacteria were reduced in fecal samples [53].
Development of novel dietary and microbiome approaches to protect against food allergy
Clearly dysbiosis of the gut microbiome can negatively influence intestinal homeostasis. Thus, novel immunotherapeutic strategies, mostly prebiotic and probiotic, but also fecal transplantation approaches are being examined to modify bacterial composition and metabolic activity and consequently improve tolerance and regulatory responses within the mucosa [9].
Probiotics are defined as live microorganisms which when administered in adequate amounts confer a health benefit on the host [54]. This is a relatively recent definition, however hypothesis relating to the beneficial effects associated with the consumption of live microbes was initially proposed at the beginning of 20th century by Metchnikoff. He observed a connection between health and longevity of Bulgarian peasants with their daily diet, which contained fermented milk products [44].
T regulatory cells play a crucial role in blocking allergic reactions. In vitro, it was found that probiotics such as Lactobacillus rhamnosus can influence Foxp3 expression by Treg cells [55]. In murine models, oral treatment with a probiotic mixture VSL#3 protected against shrimp tropomyosin-induced anaphylaxis [56]. Decreased IL-4, IL-5 and IL-13 secretion was observed in parallel with increased levels of IFN-γ, IL-10, TGF-β, and IL-27 [56]. In humans, probiotic studies have given mixed results, although oral administration of Bifidobacterium longum 35624 resulted in increased circulating levels of Foxp3+ lymphocytes, elevated ex vivo IL-10 secretion and reduced serum proinflammatory biomarkers such as CRP in patients with psoriasis, irritable bowel syndrome and ulcerative colitis [57, 58]. A double-blind, randomized, placebo controlled trial in 119 infants with cow milk allergy whose diet was supplemented with combination of Lactobacillus casei CRL431 and Bifidobacterium lactis Bb-12 did show significant beneficial effects. The percentage of tolerance to cow milk at 6 and 12 months was 77% in the probiotics group versus 81% in the placebo group [59]. Another study showed that maternal consumption of Lactobacillus rhamnosus or Bifidobacterium lactis probiotics can influence fetal immune parameters and increase protective factors in breast milk [60]. This and many other findings (including mouse models, where colonization of germ free offsprings resulted in reduced production of specific antibodies, compared to germ free controls [61]) suggest that food supplementation with probiotics may be most effective during pregnancy or during the first months of life.
Prebiotics are food components which have beneficial influence on composition and activity of human gut microbiota, such as fiber. Fiber metabolism by colonic bacteria results in the production of metabolites, such as short chain fatty acids [62], the beneficial effects of which are described above.
A novel approach as a potential microbiome therapy against food allergy is microbiota fecal transplantation. Fecal material from a healthy non-allergic donor is administrated to the upper gastrointestinal tract and proximal colon of a patient with dysbiosis [63]. There are many studies ongoing examining fecal transplantation in the treatment of IBD, IBS, obesity and Clostridium difficile infection [64], however no data is currently available on the usefulness of fecal microbiome transplantation therapy in humans with food allergy.
Conclusions
Animal models of food allergy are an important tool in deciphering the complex in vivo molecular and cellular interactions between the diet and microbiome, which protect against, or promote, mucosal allergic responses. In addition, investigators should carefully control for dietary and microbiome parameters in their experimental models. These parameters should be taken into account when attempting to translate food allergy model results across different laboratories.
Text box.
When establishing a food allergy animal model, the composition of the diet and the microbiome has to be considered specifically, as dietary antigens are the triggering factor for eliciting an immune response in this model system. It is important to establish a stabilized animal model, as both diet and microbiota play an important role in food allergy and the response of immune system may be influenced by microbiome–diet interactions.
Funding
During research for this article, partial support was obtained by the Austrian Science Fund grants KLI284, WKP039 and SFB F4606-B28.
Abbreviations
- AAD
amino acid diet
- CRP
C-reactive protein
- GP(C)R
G-Protein coupled receptor
- GF
germ free
- HFD
high fat diet
- IFN-γ
interferon gamma
- IL
interleukin
- na
not applicable
- PBMC
peripheral blood mononuclear cell
- PUFA
polyunsaturated fatty acids
- S1P
sphingosine-1-phosphate
- SCFA
short chain fatty acids
- SphK
sphingosine kinase
- TGF-β
transforming growth factor beta
- OVA
ovalbumin
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Pelz BJ, Bryce PJ. Pathophysiology of Food Allergy. Pediatr Clin North Am. 2015;62(6):1363–75. doi: 10.1016/j.pcl.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Van Gramberg JL, dV M, O'Hehir RE, Meeusen EN, Bischof RJ. Use of animal models to investigate major allergens associated with food allergy. J Allergy. 2013 doi: 10.1155/2013/635695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston LK, C K, Bryce PJ. The immunology of food allergy. J Immunol. 2014;192(6):2529–34. doi: 10.4049/jimmunol.1303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diesner SC, et al. Sphingosine-kinase 1 and 2 contribute to oral sensitization and effector phase in a mouse model of food allergy. Immunol Lett. 2012;141(2):210–9. doi: 10.1016/j.imlet.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pali-Schöll I, J-J E. Anti-acid medication as a risk factor for food allergy. Allergy. 2011;66(4) doi: 10.1111/j.1398-9995.2010.02511.x. [DOI] [PubMed] [Google Scholar]
- 6.Dunkin D, B M, Mayer L. Allergic sensitization can be induced via multiple physiologic routes in an adjuvant-dependent manner. J Allergy Clin Immunol. 2011;128(6):1251–1258. doi: 10.1016/j.jaci.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindholm Bøgh K, u r. Current challenges facing the assessment of the allergenic capacity of food allergens in animal models. doi: 10.1186/s13601-016-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorburn AN, M L, Mackay CR. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity. 2014;40(6):833–42. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Frei R, et al. Microbiota and dietary interactions: an update to the hygiene hypothesis? Allergy. 2012;67(4):451–61. doi: 10.1111/j.1398-9995.2011.02783.x. [DOI] [PubMed] [Google Scholar]
- 10.Blaser M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature. 2011;476(7361):393–4. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 11.Koplin J, A K, Gurrin L, Osborne N, Tang ML, Dharmage S. Is caesarean delivery associated with sensitization to food allergens and IgE-mediated food allergy: a systematic review. Pediatr Allergy Immunol. 2008;8:682–7. doi: 10.1111/j.1399-3038.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 12.Hill DA, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18(4):538–46. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YK, M S. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–73. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley RE, et al. Worlds within worlds: evolution of the vertebrate gut microbiota. Nat Rev Microbiol. 2008;6(10):776–88. doi: 10.1038/nrmicro1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefka AT, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U S A. 2014;111(36):13145–50. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noval Rivas M, et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J Allergy Clin Immunol. 2013;131(1):201–12. doi: 10.1016/j.jaci.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noval Rivas M, et al. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez B, P G, Hacini-Rachinel F, Moine D, Bruttin A, Ngom-Bru C, Labellie C, Nicolis I, Berger B, Mercenier A, Butel MJ, et al. Infant gut microbiota is protective against cow's milk allergy in mice despite immature ileal T-cell response. FEMS Microbiol Ecol. 2012;79(1):192–202. doi: 10.1111/j.1574-6941.2011.01207.x. [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsson TR, et al. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2):434–40, 440 e1-2. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Lyons A, OM D, O'Brien F, MacSharry J, Sheil B, Ceddia M, Russell WM, Forsythe P, Bienenstock J, Kiely B, Shanahan F, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy. 2010;40(5):811–9. doi: 10.1111/j.1365-2222.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernard H, et al. Peanut allergens are rapidly transferred in human breast milk and can prevent sensitization in mice. Allergy. 2014;69(7):888–97. doi: 10.1111/all.12411. [DOI] [PubMed] [Google Scholar]
- 22.Paula-Silva J, et al. Effect of a protein-free diet in the development of food allergy and oral tolerance in BALB/c mice. Br J Nutr. 2015;113(6):935–43. doi: 10.1017/S0007114515000173. [DOI] [PubMed] [Google Scholar]
- 23.Kim KS, et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science. 2016;351(6275):858–63. doi: 10.1126/science.aac5560. [DOI] [PubMed] [Google Scholar]
- 24.Hodgkinson AJ, McDonald NA, Hine B. Effect of raw milk on allergic responses in a murine model of gastrointestinal allergy. Br J Nutr. 2014;112(3):390–7. doi: 10.1017/S0007114514001044. [DOI] [PubMed] [Google Scholar]
- 25.Diesner SC, et al. Nitration of beta-Lactoglobulin but Not of Ovomucoid Enhances Anaphylactic Responses in Food Allergic Mice. PLoS One. 2015;10(5):e0126279. doi: 10.1371/journal.pone.0126279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirotti L, et al. Lipids are required for the development of Brazil nut allergy: the role of mouse and human iNKT cells. Allergy. 2013;68(1):74–83. doi: 10.1111/all.12057. [DOI] [PubMed] [Google Scholar]
- 27.van den Elsen LW, et al. Increased intake of vegetable oil rich in n-6 PUFA enhances allergic symptoms and prevents oral tolerance induction in whey-allergic mice. Br J Nutr. 2015;114(4):577–85. doi: 10.1017/S0007114515002007. [DOI] [PubMed] [Google Scholar]
- 28.Kunisawa J, et al. Dietary omega3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci Rep. 2015;5:9750. doi: 10.1038/srep09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Elsen LW, et al. CD25+ regulatory T cells transfer n-3 long chain polyunsaturated fatty acids-induced tolerance in mice allergic to cow's milk protein. Allergy. 2013;68(12):1562–70. doi: 10.1111/all.12300. [DOI] [PubMed] [Google Scholar]
- 30.Olivera A, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120(5):1429–40. doi: 10.1172/JCI40659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suaini NH, et al. Immune Modulation by Vitamin D and Its Relevance to Food Allergy. Nutrients. 2015;7(8):6088–108. doi: 10.3390/nu7085271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A, et al. Identification of epicatechin as one of the key bioactive constituents of polyphenol-enriched extracts that demonstrate an anti-allergic effect in a murine model of food allergy. Br J Nutr. 2014;112(3):358–68. doi: 10.1017/S0007114514000877. [DOI] [PubMed] [Google Scholar]
- 33.Abril-Gil M, et al. Effect of a cocoa-enriched diet on immune response and anaphylaxis in a food allergy model in Brown Norway rats. J Nutr Biochem. 2016;27:317–26. doi: 10.1016/j.jnutbio.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Maggini S, et al. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(Suppl 1):S29–35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 35.Untersmayr E, et al. Characterization of intrinsic and extrinsic risk factors for celery allergy in immunosenescence. Mech Ageing Dev. 2008;129(3):120–8. doi: 10.1016/j.mad.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Roth-Walter F, et al. The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron. PLoS One. 2014;9(8):e104803. doi: 10.1371/journal.pone.0104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth-Walter F, et al. Bet v 1 from birch pollen is a lipocalin-like protein acting as allergen only when devoid of iron by promoting Th2 lymphocytes. J Biol Chem. 2014;289(25):17416–21. doi: 10.1074/jbc.M114.567875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann PR, et al. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol. 2007;179(5):3258–67. doi: 10.4049/jimmunol.179.5.3258. [DOI] [PubMed] [Google Scholar]
- 39.al SSe. Histamine Receptor 2 is Required to Suppress Innate Immune Responses to Bacterial Ligands in Inflammatory Bowel Disease Patients. Inflammatory Bowel Diseases. doi: 10.1097/MIB.0000000000000825. in press. [DOI] [PubMed] [Google Scholar]
- 40.Smolinska S, et al. Histamine and gut mucosal immune regulation. Allergy. 2014;69(3):273–81. doi: 10.1111/all.12330. [DOI] [PubMed] [Google Scholar]
- 41.Ferstl R, F R, Schiavi E, Konieczna P, Barcik W, Ziegler M, Lauener RP, Chassard C, Lacroix C, Akdis CA, O'Mahony L. Histamine receptor 2 is a key influence in immune responses to intestinal histamine-secreting microbes. J Allergy Clin Immunol. 2014;134(3) doi: 10.1016/j.jaci.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 42.Frei R, et al. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J Allergy Clin Immunol. 2013;132(1):194–204. doi: 10.1016/j.jaci.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Maslowski KM, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feehley T, et al. Microbial regulation of allergic responses to food. Semin Immunopathol. 2012;34(5):671–88. doi: 10.1007/s00281-012-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feehley T, Nagler CR. Cellular and molecular pathways through which commensal bacteria modulate sensitization to dietary antigens. Curr Opin Immunol. 2014;31:79–86. doi: 10.1016/j.coi.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang C, et al. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012;6(10):1848–57. doi: 10.1038/ismej.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim YI, et al. Shigella flexneri Inhibits Intestinal Inflammation by Modulation of Host Sphingosine-1-Phosphate in Mice. Immune Netw. 2014;14(2):100–6. doi: 10.4110/in.2014.14.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156(1–2):123–33. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konieczna P, et al. Immunomodulation by Bifidobacterium infantis 35624 in the murine lamina propria requires retinoic acid-dependent and independent mechanisms. PLoS One. 2013;8(5):e62617. doi: 10.1371/journal.pone.0062617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 51.Bouchaud G, et al. Maternal exposure to GOS/inulin mixture prevents food allergies and promotes tolerance in offspring in mice. Allergy. 2016;71(1):68–76. doi: 10.1111/all.12777. [DOI] [PubMed] [Google Scholar]
- 52.Trompette A, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, et al. Dietary Fiber Intake Regulates Intestinal Microflora and Inhibits Ovalbumin-Induced Allergic Airway Inflammation in a Mouse Model. PLoS One. 2016;11(2):e0147778. doi: 10.1371/journal.pone.0147778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. 2002.
- 55.Karimi K, et al. A Lactobacillus rhamnosus strain induces a heme oxygenase dependent increase in Foxp3+ regulatory T cells. PLoS One. 2012;7(10):e47556. doi: 10.1371/journal.pone.0047556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schiavi E, B B, Butteroni C, Corinti S, Boirivant M, Di Felice G. Oral therapeutic administration of a probiotic mixture suppresses established Th2 responses and systemic anaphylaxis in a murine model of food allergy. Allergy. 2011;66(4):499–508. doi: 10.1111/j.1398-9995.2010.02501.x. [DOI] [PubMed] [Google Scholar]
- 57.Konieczna P, et al. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61(3):354–66. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 58.Groeger D, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4(4):325–39. doi: 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hol J, v L E, Elink Schuurman BE, de Ruiter LF, Samsom JN, Hop W, Neijens HJ, de Jongste JC, Nieuwenhuis EE, Cow's Milk Allergy Modified by Elimination and Lactobacilli study group The acquisition of tolerance toward cow's milk through probiotic supplementation: a randomized, controlled trial. J Allergy Clin Immunol. 2008;121(6):1448–54. doi: 10.1016/j.jaci.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 60.Romano-Keeler J, Weitkamp JH. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. 2015;77(1–2):189–95. doi: 10.1038/pr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morin S, et al. Delayed bacterial colonization of the gut alters the host immune response to oral sensitization against cow's milk proteins. Mol Nutr Food Res. 2012;56(12):1838–47. doi: 10.1002/mnfr.201200412. [DOI] [PubMed] [Google Scholar]
- 62.Frei R, Akdis M, O'Mahony L. Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr Opin Gastroenterol. 2015;31(2):153–8. doi: 10.1097/MOG.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 63.Kelly CR, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015;149(1):223–37. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.ClinicalTrials.gov. Clinical trials for fecal transplant